Abstract
The behaviour and risk associated with wood-inhabiting saprobic fungi introduced through trade is poorly understood. We used the cosmopolitan species Schizophyllum commune, considered a biosecurity risk in New Zealand, as a case study to test the potential for naturalization of alien strains/populations of wood-inhabiting saprobic fungi to occur. Phylogenetic analysis of the intergenic spacer 1 region was undertaken to determine the origin of New Zealand samples and one Cook Islands sample from the three known globally geographically distinct clades: North/Central America (NAM); South America (SAM); European/Asia/Australia (EAS). All New Zealand S. commune sequences fall within the EAS clade in two distinct groups with four haplotypes (Ia, Ib, IIb and IIa), with no evidence of naturalization of individuals from the NAM and SAM clades. The diversity of the haplotypes can be explained by two natural dispersal events in pre-human times. Ensuring timber is sufficiently treated will prevent alien populations possibly outcompeting native populations.
Introduction
Schizophyllum commune is found in every continent except Antarctica. It has been reported from the dead wood of at least 150 different plant genera (Raper et al. Citation1958) usually as a white rot but occasionally as a pathogen of both humans and trees (Schmidt & Liese Citation1980). It is known to a number of indigenous peoples world-wide as a food source, especially in Africa and Asia, with approximately 2.5 million tons produced annually (Kothe Citation2001), and in some countries as a type of chewing gum (Cooke Citation1961). So far there has been no evidence that this species has been used by indigenous people of New Zealand or elsewhere in the Pacific.
As a model organism, S. commune has been used to test ideas of phylogeography and biogeography in fungi (James et al. Citation1999, Citation2001). Lumbsch et al. (Citation2008) suggest two alternative explanations for the global distribution of fungal species, either vicariance resulting in geographically distinct populations or frequent long-distance dispersal events resulting in more heterogeneous globally distributed populations. Phylogenetic analysis of the intergenic spacer (IGS) from 195 samples of S. commune from global populations revealed three geographically distinct clades: one with most of the North and Central America isolates (NAM clade); a second with most of the South America isolates (SAM clade); and a third with most of the European and Asia/Australia isolates (EAS clade) (James et al. Citation2001; ). The global distribution of S. commune and the heterogeneity of the populations in many regions of the world indicates that long distance and relatively frequent dispersal events have occurred (James et al. Citation2001). James et al. (Citation2001) also note a few geographic outliers in their clades and raise the possibility that these represent very recent, human-mediated, range expansions. Since this study, the validity of using the IGS region to determine haplotype groups has come under scrutiny. High variability exists in some species of fungi (Diaz et al. Citation2005) and not in others (Kauserud & Schumacher Citation2003). The variation has been also attributed to polymerase chain reaction (PCR)-mediated errors in the form of sequence errors and haplotype chimeras for example (Keirle et al. Citation2011). However, the IGS region is still considered to be useful in determining haplotype groups when used with caution (Keirle et al. Citation2011). Schizophyllum commune, although thought to be native to New Zealand (Anon. Citation2001–2012), is also found as a contaminant on imported poles, piles, rounds and railway sleepers (Biosecurity New Zealand Citation2010). As S. commune is often found in disturbed sites and secondary forest growth, or on processed wood products (Biosecurity New Zealand Citation2010), it seems to be ideally suited to transportation through trade. In New Zealand, newly introduced organisms are recognized as having the potential to ‘seriously damage our natural resources, threaten our economy and undermine our way of life’ (Biosecurity New Zealand Citation2012), and threaten the conservation of native fungal species (Buchanan & May Citation2003). In contrast with the well-documented movement of plant pathogens following the earlier introduction of their hosts (e.g. Wingfield et al. Citation2001), the behaviour of wood-inhabiting saprobic fungi following their introduction, and the risk of naturalization of these species in native habitats, is not well known. One example is Agaricus bisporus where the invasion of European germ plasm, probably through cultivation, has begun to displace indigenous coastal populations in California (Kerrigan Citation1995). The only well-documented example of a naturalized, recent introduction of a wood-inhabiting saprobic fungus into New Zealand is the orange pore fungus, Favolaschia calocera, now widely naturalized in native forests (Johnston et al. Citation2006).
In this study we use S. commune as a case investigation to test the potential for the introduction of alien strains of saprobic, wood-inhabiting fungi and the likelihood that these fungi will become naturalized in native forests. The three New Zealand isolates included in the James et al. (Citation2001) study are located firmly in the EAS clade, consistent with patterns of natural dispersal and long-term geographic isolation of many of New Zealand's indigenous fungi (e.g. Johnston Citation1997; Hibbett Citation2001; Weinstein et al. Citation2002; Zhong & Pfister Citation2004). At the same time, S. commune is often detected on imported wooden products (Biosecurity New Zealand Citation2010). We surveyed New Zealand isolates of S. commune to test whether members of the exotic SAM and NAM clades have become naturalized in New Zealand forests as a result of recent, human-mediated dispersal and discussed the potential for introduced strains to become naturalized.
Materials and methods
Fungal samples
Sources of S. commune samples included: 25 of the best preserved and most recently collected fruiting body specimens from New Zealand, from the New Zealand Fungal and Plant Disease Collection (PDD) at Landcare Research, four mycelial cultures from the International Collection of Micro-organisms Landcare Research (ICMP), six fresh specimens collected in New Zealand during 2010 and 2011, plus one specimen collected from the Cook Islands in 2008.
DNA extraction
Tissue for DNA extraction was obtained from mycelial cultures by scraping mycelium from 1-week-old cultures on Nobel's agar (2% agar + 1.3% malt extract in water). Up to 0.1 g of mycelium was ground to a powder in liquid nitrogen using a mortar and pestle. For dried and fresh fruiting body specimens, up to 0.1 g of gill tissue was disrupted using a Tissue Lyser with buffer (Qiagen, Düsseldorf, Germany). Total DNA was extracted using the QIAGEN DNeasy Plant Mini Kit (Qiagen) or Zymo Research Fungal/Bacterial DNA MicroPrep kit (Zymo Research Corporation, Irvine, CA, USA).
Polymerase chain reaction
The IGS1 region was amplified using the LR20R and 5sRNA primers of James et al. (Citation2001). In addition, forward primer IGS1 1–22 and reverse primer IGS1 493–516 were designed to a sub-set of 50 of the S. commune sequences used by James et al. (Citation2001), that were most closely related to the published New Zealand sequences (). The sequences were aligned using Clustal W (Thompson et al. Citation1994) in BIOEDIT (Tom Hall, Ibis Therapeutics, Carlsbad, CA, USA) and the primers were designed using Primer3 (Steve Rozen and Helen Skaletsky, Whitehead Institute, Cambridge, MA, USA and Howard Hughes Medical Institute, Chevy Chase, MD, USA). Primers with the least overlap to prevent self-annealing and a dgt value of > 3 were chosen.
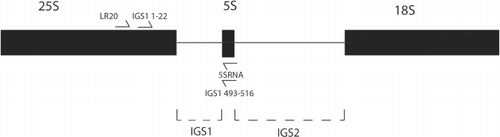
Amplification of the IGS1 loci using the primers LR20R and 5sRNA was performed using the reaction conditions and thermocycling parameters described in Vilgalys & Hester (Citation1990). For the IGS1 1–22 and IGS1 493–516 primer amplification cycles the annealing step was altered to 53 °C for 30 seconds and the extension to 68 °C for 30 seconds. Dimethyl sulphoxide was also added to all PCR to a final concentration of 1.1%.
PCR products were then electrophoresed in a 1% Tris-borate EDTA agarose gel, stained with ethidium bromide at a concentration of 0.5 µg/mL, visualized under UV light and the band size was measured against a 1 kb + DNA ladder (Life Technologies, Carlsbad, CA, USA). Controls of no DNA template were used in every set of amplifications. DNA bands were cut from the gel and purified using Invisorb Fragment clean up by Invitrogen (Life Technologies) and sequenced. Where possible sequences were obtained directly from purified PCR products, but if not, usually due to multiple IGS1 haplotypes within heterogeneous PCR, the PCR product was cloned before sequencing.
PCR products were cloned using the pGEM®-T Easy Vector (Promega, Madison, WI, USA) following the manufacturer's instructions, using Escherichia coli DH5α competent cells (Life Technologies). Clones were screened using the T7 and SP6 primers and only clones containing an insert of between 650 and 850 base pairs (bp) were sequenced. Plasmids were extracted using the QIAGEN Spin Miniprep Kit (QIAGEN) and the inserts were sequenced by Macrogen Inc. (Seoul, Korea).
Forward and reverse reads of the IGS1 region were assembled and contigs were created using BIOEDIT (Tom Hall, Ibis Biosciences, Carlsbad, CA, USA). Sequences for the IGS1 were deposited in GenBank under accession numbers KF312255–KF312275.
Phylogenetic analysis
Our newly generated sequences, plus 194 IGS1 sequences used in James et al. (Citation2001) were imported into BIOEDIT (Tom Hall, Ibis Therapeutics) and aligned using Clustal W (Thompson et al. Citation1994). The alignment of 520 bp was imported into Mega5 (Tamura et al. Citation2011) and a phylogeny was constructed using the neighbour-joining method (maximum composite likelihood model). The branch support was evaluated with 10,000 bootstrap random addition replicates to create a consensus tree. To outline the main topology and reduce redundancy with previous work (James et al. Citation2001) another analysis was run with 34 selected sequences from James et al. (Citation2001) together with our newly generated sequences. To highlight the overall topology a haplotype network was constructed of our newly generated sequences and all of James et al. (Citation2001) sequences based on single nucleotide polymorphisms, insertions and deletions.
Results
PCR and sequencing
Polymerase chain reaction products were obtained from 18 of the original 36 DNA extractions ( and ). The PCR failures were primarily attributed to the low quality of the DNA extracted from the herbarium samples collected before the year 2000. Successful amplification of the IGS1 region was completed by first using the LR20R and 5sRNA primers and then the IGS1 1–22 Forward and IGS1 493–516 primers. Intense bands of between 650 and 850 bp were observed for the LR20R and 5sRNA primers and between the 400 and 500 bp for the IGS1 1–22 Forward and IGS1 493–516 primers.
Table 1 Specimens with newly generated DNA sequences. Haplotype groups indicated for all specimens.
Phylogenetic analysis
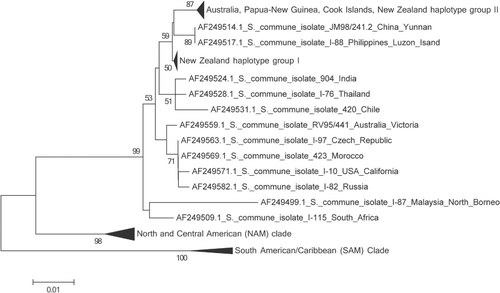
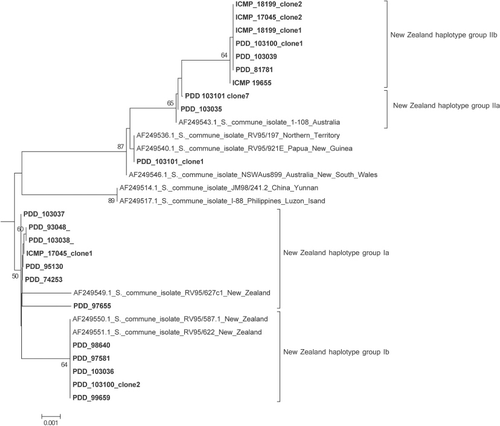
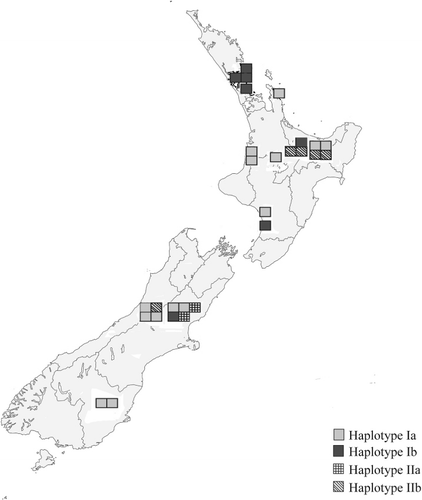
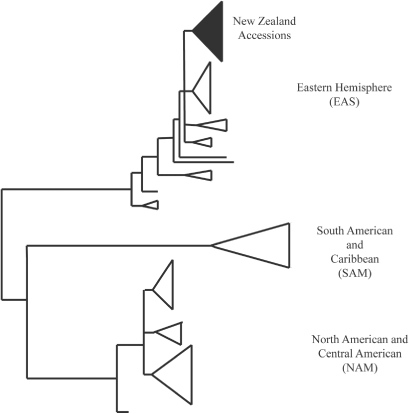
A neighbour-joining tree () shows the same topology as that described in (James et al. Citation2001) with three main clades: NAM, SAM and EAS. All of the New Zealand specimens fall into the EAS clade, as did the single Cook Island specimen. However, within the EAS clade the New Zealand specimens split into two groups discussed here as Haplotype Group I and Haplotype Group II (). There was no evidence of haplotype chimeras and only a small number of sequence errors within the well-conserved areas, indicating the amount of variation attributed to PCR-mediated errors was negligible. Sequences from isolate specimens PDD 93048, PDD 95130, PDD 74253, PDD 97655, PDD 98640, PDD 97581, PDD 99659, PDD 103036, PDD 103037, PDD 103038, PDD 103100 clone 2 and ICMP 17045 clone 1, group with the previously published New Zealand sequences, AF249550, AF249551 and AF249549, while specimens PDD 81781, PDD 103035, PDD 103039, PDD 103100 clone 1, ICMP 17045, ICMP18199 clones 1 and 2, ICMP 19655, ICMP 17045 clone 2 and the Cook Islands specimen PDD 103101 clone 7 cluster with the Australian and Papua New Guinea isolates sampled earlier by James et al. (Citation2001). PDD 103101 clone 1 is from the Cook Islands and lies outside the four groups and falls with specimens from Papua New Guinea and the Northern Territory of Australia and is not placed in a haplotype subgroup on the table. Sequences generated from clones from specimens PDD 103100, PDD 103101 and ICMP 17045 represented more than one haplotype group. The geographic pattern in shows that Haplotypes Ia, Ib and IIb were found throughout New Zealand associated with both indigenous and exotic habitats while Haplotype IIa was found only in the South Island associated with an indigenous forest. A haplotype network was constructed manually for the New Zealand isolates plus their closest sister taxa from the alignment containing all of the James et al. (Citation2001) sequences, based on shared single nucleotide polymorphisms and insertions and deletions.
Figure 3 A, Intergenic spacer 1 (IGS1) neighbour-joining tree showing the European/Asia/Australia (EAS) clade with New Zealand samples showing the Australia, Papua-New Guinea, Cook Islands and New Zealand haplotype group II clade and the New Zealand haplotype group I clade. Numbers indicate bootstrap values over 50 at 10,000 replicates. B, Sub-tree of intergenic spacer 1 (IGS1) neighbour-joining tree showing the European/Asia/Australia (EAS) clade with all New Zealand and Cook Island samples highlighted in bold. The four haplotype groups can be seen, New Zealand Ia, Ib, IIa and IIb. PDD 103101 clone 1 is from the Cook Islands and lies outside these four groups. Numbers indicate bootstrap values over 50 at 10,000 replicates.Discussion
Diversity in the New Zealand population
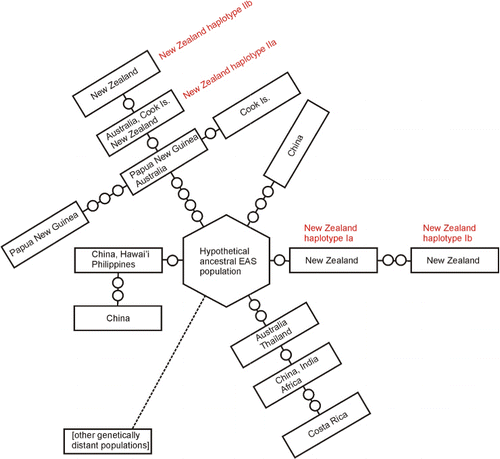
While all of the New Zealand S. commune sequences obtained during this study fall within the previously identified EAS clade (James et al. Citation2001) it is evident that the New Zealand population is more diverse than was previously thought. The sequences fall into two distinct groups, with four haplotypes Ia, Ib and IIa and IIb, those sequences that cluster with the previously published New Zealand sequences (James et al. Citation2001) and those that cluster with Australian and Papua New Guinean sequences. This indicates that there have been at least two distinct naturalization events from introductions of S. commune into New Zealand as illustrated in . The first can be seen as coming from the hypothetical ancestral EAS population, the origin of which is unknown and the second from Australia. Haplotype Ia, Ib and IIb have the widest distributions, making Haplotype I the most commonly collected during this study. Haplotype IIa has the smallest distribution in New Zealand only being found in the South Island. There was no distinctive haplotype distribution pattern related to indigenous and modified habitats. However, this haplotype was shared with the sample from the Cook Islands suggesting that it could have been a more recent introduction.
In two cases, the fruit bodies that we sampled contained DNA from both of the New Zealand haplotype groups. The directly sequenced signal from these specimens was mixed and a cloning step was needed to get clean sequences. The sequences from these specimens were then found to represent the two haplotype groups, presumably the dikaryon containing a nucleus from each of the haplotype lineages. We assume that the tissue from which we extracted DNA for all of the specimens that we sampled was dikaryotic. In all cases the tissue was from fruiting bodies or from cultures grown from fruit body tissue, rather than from single basidiospores. Although S. commune monokaryons can form fruiting bodies in some conditions, those found in nature are typically dikaryotic (Leslie & Leonard Citation1980). Dikaryotic hyphae of S. commune have been found to be more fit than monokaryons, more able to adapt to changing environmental conditions (Clark & Anderson Citation2004). Although many parts of the country had populations with individuals from both haplotype lineages, few mixed individuals were found. This perhaps supports earlier findings that dikaryons generated from more distantly related populations form a less well adapted mycelium, reflecting evolution of co-adapted gene complexes within lineages (Simchen Citation1967; Clark & Anderson Citation2004). Further research is needed to look at the distribution of S. commune haplotypes in the Pacific, for example the islands of Fiji, Tonga and the Solomon Islands. This would provide a clearer picture of the movements of haplotypes in the Pacific and provide further insight into the dispersal of fungi across the Pacific.
Origin of New Zealand's populations
Two possibilities exist for the two introduction and subsequent naturalization events of S. commune in New Zealand; either long-distance dispersal by means of wind-blown spores or human-mediated dispersal by the movement of timber or other wooden items. We found no evidence of individuals from the NAM and SAM clades becoming naturalized in New Zealand, despite S. commune commonly being intercepted at the border on products imported from parts of the world where these clades are common. In addition, Australia is the closest large land mass to New Zealand and the prevailing winds are westerly from Australia to New Zealand, bringing not only introductions of fungi but insects, seeds and pollen (Close et al. Citation1978). Previous studies on New Zealand species of fungi provide several examples of natural dispersal from Australia. For example genetic studies on the genus Pisolithus (Moyersoen et al. Citation2003) suggest recent trans-Tasman dispersal events of at least three species between Australia and New Zealand. Introductions of plant pathogens from Australia have occurred by wind-blown spores for rust species, two examples are wheat stripe rust (Puccinia striiformis Westend. f. sp. tritici) and blackberry rust (Phragmidium violaceum (Shultz) Winter) (Viljanen-Rollinson & Cromey Citation2002). Wood-decay fungi are not reliant on the distribution by urediospores; however, long-distance spore dispersal events are known to occur for wood-decay species of the genus Armillaria. These events in Armillaria prevent genetic differentiation of subpopulations hundreds of kilometres apart, similar to other generalist wood-decay fungi (Baumgartner et al. Citation2010). In New Zealand, individuals of the species Armillaria hinnulea in the South Island are the same as those in Australia, but are different to those of the North Island (Coetzee et al. Citation2001). In terms of S. commune, research in the Caribbean (James & Vilgalys Citation2001) shows the potential for wind distribution of S. commune with an abundance of spores in the air, as well as a distribution of IGS1 haplotypes agreeing with the prevailing regional wind patterns. Based on the capability of long-distance wind dispersal by S. commune and knowledge of the distribution of other New Zealand fungi, both introductions could have been through natural dispersal in pre-human times.
Risk from other S. commune from other clades
The fact that Australian-like isolates are the only non-New Zealand specific isolates indicates that naturalization from human-mediated dispersal events is not common. This could be because of reduced ability to mate, as is present in Panellus stypticus collections between the northern hemisphere and Oceania (Jin et al. Citation2001), preventing the naturalization of individuals from other clades. In S. commune this is not the case with Raper et al. (1958) demonstrating no connection between mating allele distribution and geographic location. However, this does not exclude the possibility that historic introductions of S. commune from Australia were human mediated, or that future introductions from Australia or elsewhere followed by naturalization could occur in the future. Schizophyllum commune has previously been identified as a pest species on imported poles, piles, rounds and railway sleepers (Biosecurity New Zealand Citation2010) and recently, railway sleepers imported from Peru to New Zealand have been reported to have degraded at elevated rates (resulting in structural concerns), with S. commune considered as a causal agent. The Ministry for Primary Industries suggested that the fungus of concern was a species of Schizophyllum and it was not on the unwanted organisms register and poses no biosecurity risk (Radio New Zealand News Citation2012). However, two other species of Schizophyllum are on the register, S. alneum and S. radicata (Biosecurity New Zealand Citation2013). Schizophyllum alneum is a synonym for S. commune and raises issues with the data held on the unwanted organisms register. It also raises the question whether some strains of S. commune may be more destructive than others and whether this should be considered when determining biosecurity risk. Potentially, more aggressive strains absent from New Zealand could become naturalized and out-compete what is here already. Making sure that timber has been sufficiently treated before entering the country will prevent the spread of overseas populations in New Zealand. As another example of a fungal species capable of long-distance dispersal this mode of introduction needs to be taken into account when developing biosecurity regulations and protocols. However, if dispersal is by natural means then very little can be done.
Conclusions
The origin of one of New Zealand's populations of S. commune is Australia and it is not clear as to the origin of the other. These results suggest long-distance trans-oceanic dispersal events that have occurred on at least two occasions; one is the distant past (haplotypes Ia, Ib, IIb) and one more recently (haplotype IIa). Although, there is no evidence of individuals from the NAM and SAM clades becoming naturalized in New Zealand, suggesting that there has been no human-mediated dispersal, it might still occur in the future, with the possibility of recent introductions out-competing existing populations. Concern has been recently raised that S. commune as the causal agent in degraded railway sleepers. Ensuring timber has been sufficiently treated before entering the country will prevent decay and the spread of overseas populations of S. commune in New Zealand.
Acknowledgements
We thank the New Zealand Department of Conservation and the Cook Islands National Research Council for providing permits to allow collection of many of the specimens. RJMF was funded by the Te Wheke A Toi Post-Doctoral Fellowship thanks to the Tertiary Education Commission. PRJ was funded through the Landcare Research Systematics Portfolio, with Core funding support from the Science and Innovation Group of the New Zealand Ministry of Business, Innovation and Employment.
References
- Anon. 2001–2012. NZ Fungi database of New Zealand fungi. Landcare Research, New Zealand. http://nzfungi2.landcareresearch.co.nz (accessed 4 November 2012).
- Baumgartner K, Travadon R, Bruhn J, Bergemann SE 2010. Contrasting patterns of genetic diversity and population structure of Armillaria mellea sensu stricto in the eastern and western United States. Phytopathology 100: 708–718.10.1094/PHYTO-100-7-0708
- Biosecurity New Zealand 2010. Import health standards poles, piles, rounds, and sleepers from all countries. Wellington, New Zealand: 22. Ministry for Primary Industries, New Zealand Government. http://www.biosecurity.govt.nz/files/ihs/poles-piles-rounds-sleepers.pdf (accessed 17 January 2013).
- Biosecurity New Zealand 2012. Biosecurity New Zealand Profile. Ministry for Primary Industries, New Zealand Government. http://www.biosecurity.govt.nz/files/biosec/org/profile.pdf (accessed 5 June 2012).
- Biosecurity New Zealand 2013. Unwanted Organisms Database. Ministry for Primary Industries, New Zealand Government. http://www1.maf.govt.nz/uor/searchframe.htm (accessed 31 January 2013).
- Buchanan PK, May TW 2003. Conservation of New Zealand and Australian fungi. New Zealand Journal of Botany 41: 407–421.10.1080/0028825X.2003.9512859
- Clark TA, Anderson JB 2004. Dikaryons of the basidiomycete fungus Schizophyllum commune: evolution in long-term culture. Genetics 167: 1663–1675.10.1534/genetics.104.027235
- Close RC, Moar NT, Tomlinson AI, Lowe AD 1978. Aerial dispersal of biological material from Australia to New Zealand. International Journal of Biometeorology 22: 1–19.10.1007/BF01553136
- Coetzee MPA, Wingfield BD, Bloomer P, Ridley GS, Kile GA, Wingfield MJ 2001. Phylogenetic relationships of Australian and New Zealand Armillaria species. Mycologia 93: 887–896.10.2307/3761754
- Cooke WB 1961. The genus Schizophyllum. Mycologia 53: 575–599.10.2307/3756459
- Diaz MR, Boekhout T, Kiesling T, Fell JW 2005. Comparative analysis of the intergenic spacer regions and population structure of the species complex of the pathogenic yeast Cryptococcus neoformans. FEMS Yeast Research 5: 1129–1140.10.1016/j.femsyr.2005.05.005
- Hibbett DS 2001. Shiitake mushrooms and molecular clocks: historical biogeography of Lentinula. Journal of Biogeography 28: 231–241.10.1046/j.1365-2699.2001.00528.x
- James TY, Moncalvo JL, Li S, Vilgalys R 2001. Polymorphism at the ribosomal DNA spacers and its relation to breeding structure of the widespread mushroom Schizophyllum commune. Genetics 157: 149–161.
- James TY, Porter D, Hamrick JL, Vilgalys R 1999. Evidence for limited intercontinental gene flow in the cosmopolitan mushroom, Schizophyllum commune. Evolution 53: 1665–1677.10.2307/2640430
- James TY, Vilgalys R 2001. Abundance and diversity of Schizophyllum commune spore clouds in the Caribbean detected by selective sampling. Molecular Ecology 10: 471–479.10.1046/j.1365-294x.2001.01224.x
- Jin J, Hughes KW, Petersen RH 2001. Biogeographical patterns in Panellus stypticus. Mycologia 93: 309–316.10.2307/3761652
- Johnston PR 1997. Tropical rhytismatales. In: Hyde KD ed. Biodiversity of tropical microfungi. Hong Kong, Hong Kong University Press. Pp. 241–254.
- Johnston PR, Whitton SR, Buchanan PK, Park D, Pennycook SR, Johnson JE , et al. 2006. The basidiomycete genus Favolaschia in New Zealand. New Zealand Journal of Botany 44: 65–87.10.1080/0028825X.2006.9513007
- Kauserud H, Schumacher T 2003. Genetic structure of Fennoscandian populations of the threatened wood-decay fungus Fomitopsis rosea (Basidiomycota). Mycological Research 107: 155–163.10.1017/S0953756203007214
- Keirle MR, Avis PG, Hemmes DE, Mueller GM 2011. Variability in the IGS1 region of Rhodocollybia laulaha: is it allelic, genomic or artificial? Fungal Biology 115: 310–316.10.1016/j.funbio.2011.01.002
- Kerrigan RW 1995. Global genetic resources for Agaricus breeding and cultivation. Canadian Journal of Botany 73: S973–S979.10.1139/b95-347
- Kothe E 2001. Mating-type genes for basidiomycete strain improvement in mushroom farming. Applied Microbiology and Biotechnology 56: 602–612.10.1007/s002530100763
- Leslie JF, Leonard TJ 1980. Monkaryotic fruiting in Schizophyllum commune: survey of a population from Wisconsin. American Midland Naturalist 103: 367–374.10.2307/2424636
- Lumbsch HT, Buchanan PK, May TW, Mueller GM 2008. Phylogeography and biogeography of fungi. Mycological Research 112: 423–424.10.1016/j.mycres.2008.02.002
- Moyersoen B, Beever RE, Martin F 2003. Genetic diversity of Pisolithus in New Zealand indicates multiple long-distance dispersal from Australia. New Phytologist 160: 569–579.10.1046/j.1469-8137.2003.00908.x
- Radio New Zealand News 2012. Risk from sleeper fungus nil –ministry. Posted online 4 August 2012. http://www.radionz.co.nz/news/national/112422/risk-from-sleeper-fungus-nil-ministry (accessed 31 January 2013).
- Raper JR, Krongelb GS, Baxter MG 1958. The number and distribution of incompatibility factors in Schizophyllum. The American Naturalist 92: 221–232.10.1086/282030
- Schmidt O, Liese W 1980. Variability of wood degrading enzymes of Schizophyllum commune. Holzforschung 34: 67–72.10.1515/hfsg.1980.34.2.67
- Simchen G 1967. Independent evolution of a polygenic system in isolated populations of the fungus Schizophyllum commune. Evolution 21: 310–315.10.2307/2406679
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739.10.1093/molbev/msr121
- Thompson JD, Higgins DG, Gibson TJ 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680.10.1093/nar/22.22.4673
- Vilgalys R, Hester M 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246.
- Viljanen-Rollinson SLH, Cromey MG 2002. Pathways of entry and spread of rust pathogens: implications for New Zealand biosecurity. New Zealand Plant Protection 55: 42–48.
- Weinstein RN, Pfister DH, Iturriaga T 2002. A phylogenetic study of the genus Cookeina. Mycologia 94: 673–682.
- Wingfield MJ, Slippers B, Roux J, Wingfield BD 2001. Worldwide movement of exotic forest fungi, especially in the tropics and the southern hemisphere. BioScience 51: 134–140.
- Zhong Z, Pfister DH 2004. Phylogenetic relationships among species of Leotia (Leotiales) based on ITS and RPB2 sequences. Mycological Progress 3): 237–246.