Abstract
Impacts on forests of infrequent disturbance events such as floods are not well understood in New Zealand. An extreme flood event in February 2004, Beilschmiedia tawa (tawa Lauraceae) initiated extensive mortality, studied via control and flooded plots in McPherson's Reserve, Turakina Valley. Canopy cover in Flooded plots reduced to < 3% of the Controls by 2006, although native species' richness remained similar. The near synchronous mortality of most tawa individuals in the Flooded plots may be due to prolonged anoxia, probably caused by deposition of fine sediment over flooded soil, perhaps exacerbating fungal infection. Slower dieback of other species and adjacent surviving tawa continues, exacerbated by ongoing canopy collapse and spread of infection by root grafts. Seedlings of tawa and Dacrycarpus dacrydioides were common post-flood, but recruitment was poor, due to disturbance-facilitated invasion of exotics. No comparable flood event has apparently occurred for 200 years. Recovery of the forest and its successional processes, if achievable, will be slow.
Introduction
Forest dieback can be related to the natural demographics of the declining species. For example, when trees of an ageing cohort senesce synchronously, the widespread mortality disrupts the canopy (Mueller-Dombois Citation1987; Lusk & Ogden Citation1992). Such senescence events play an infrequent but important role in forest dynamics, allowing the recruitment of other large, even-aged cohorts of trees (Stewart Citation2002). Some species not necessarily normally prone to cohort senescence can exhibit synchronous stand-level mortality in response to large-scale disturbances, such as earthquakes (Wells et al. Citation1998, Citation2001), droughts (Jane & Green Citation1986), fires (Barlow & Peres Citation2008), frosts (Kelly Citation1987) or floods (Duncan Citation1993). For some forests, flood events may be a normal, even essential, part of forest dynamics (Wells et al. Citation2001; Stewart Citation2002). However in others, such events can have significant novel effects on mortality. Flood events 450–500 years ago opened the canopy of lowland podocarp hardwood forest in Westland, New Zealand, which initiated a brief period of re-establishment, with different species more frequent in different microsite types and under differently sized canopy gaps (Duncan Citation1993). These effects persisted into dispersion of the mature trees, cohorts being recognisable over 450 years after the initiating event. The frequency of floods in that region appears to maintain the forest in a podocarp successional phase (Duncan Citation1993). Other than this, little is known about the impacts of large, infrequent flood events on native forest in New Zealand. In February 2004, an extreme flood event occurred in the Manawatu and Rangitikei areas of the lower North Island, New Zealand, also affecting the Turakina Valley (–). Subsequently, low-lying parts of McPherson's Reserve, a small (9.2 ha) lowland forest remnant on the banks of the Turakina River (), experienced extensive mortality of tawa (Beilschmiedia tawa; Lauraceae; John Marsh, pers. comm.).

Tawa is a common endemic tree, occurring from Marlborough north, and growing up to 30 m, with unpalatable bluish-green lanceolate to elliptic evergreen leaves up to 13 cm long (Knowles & Beveridge Citation1982). Tawa produces large reddish-black drupes (to 4 × 2.5 cm; 5–6 g) that ripen and commonly fall in mid summer (Knowles & Beveridge Citation1982; Wright Citation1984; Burrows Citation1999). Fruiting levels can be heavy but fluctuate widely, with masting every seven or so years (West Citation1995). Seeds have no dormancy, and germination rates are high in any light condition, decreasing as the seeds dry (Knowles & Beveridge Citation1982). Young tawa are known to be susceptible to stressors such as drought, shade and frosts (Knowles & Beveridge Citation1982; Kelly Citation1987), but can persist in the understorey with very little growth, or even shoot loss and regrowth, for 60–80 years (Cameron Citation1963; Ogden & West Citation1981; Knowles & Beveridge Citation1982; West Citation1995). When canopy gaps open, seedlings respond to release with rapid extension rates (30 cm yr−1; Knowles & Beveridge Citation1982). Once established, regeneration is generally continuous, due to tawa's shade tolerance, with a range of age classes in undisturbed forest (Knowles & Beveridge Citation1982). Tawa is usually the most common canopy-forming species in mixed forest in lowland to montane (< 800 m) forest throughout the North Island and the uppermost part of the South Island, New Zealand. These features combine to make tawa a late successional species, establishing only after a mature canopy is in place (Cockayne Citation1928, p. 153; McKelvey Citation1954; Ogden & West Citation1981; Knowles & Beveridge Citation1982; West Citation1995). Thus tawa forest often has an overstorey of emergent podocarps, especially on flatter, sedimentary substrates and well developed soils (Knowles & Beveridge Citation1982; Wright Citation1984).
This study investigates four aspects of forest dieback in relation to the 2004 flood in the Turakina Valley for insights into the forest's dynamics. What effect did the flood have on tawa mortality? How has the vegetation responded in the subsequent decade? Are there differences in the demography and regeneration of tawa between flooded and undisturbed areas? Finally, how might tawa and the forest respond in the long term?
Methods
Study site
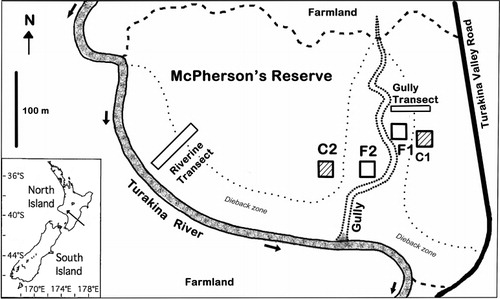
McPherson's Reserve, owned by the conservation organisation Forest and Bird, is located alongside the Turakina River near Wanganui in the lower North Island (, inset; grid ref: NZMS 260, S22 095 378), with the Turakina River forming the western boundary, and is unusual in being a relatively undisturbed remnant on riverine terraces (Royal Forest & Bird Protection Society Citation2007). It is 22 km from the coast and c. 80 m above sea level (Royal Forest & Bird Protection Society Citation2007). The reserve is spread over two old river terraces with silty alluvial sediments over a bedrock of Pliocene papa mudstone (Hancox & Wright Citation2005). A gully, orientated from north to south, splits the reserve in two (). Surrounded by farmland (), human impacts on the reserve include clearance of surrounding forest for agriculture, grazing of the understorey by farm stock until 1941 (Royal Forest & Bird Protection Society Citation2007), and possible logging within the reserve, although large millable podocarps still remain. Currently the reserve is only occasionally visited by interested individuals, educational parties, goats (Capra hircus), deer (Cervus elaphus and Rusa unicolor; David Wiessing, pers. comm.) and pest control operators targeting possums (Trichosurus vulpecula). By August 2013 a large and noisy flock of exotic sulphur-crested cockatoos (Cacatua galerita) had occupied the reserve.
The forest of McPherson's Reserve is similar to that of mesic podocarp broadleaved forest on old river terraces described by Lusk (Citation1984) for the nearby Pohangina Valley (50 km to the east), Manawatu Region, both reserves being in the Manawatu–Rangitikei Ecological Region (McEwen Citation1987). At McPherson's the forest canopy is dominated by tawa, frequent tītoki (Alectryon excelsus) and rewarewa (Knightia excelsa) with an emergent layer of podocarps (Dacrycarpus dacrydioides, Podocarpus totara and some Prumnopitys ferruginea and Dacrydium cupressinum; Royal Forest & Bird Protection Society Citation2007). Before the flood, the forest in most of the reserve appeared uniform (John Marsh, pers. comm.; Royal Forest & Bird Protection Society Citation2007), except that the higher terraces had a denser ground cover of ferns than the lower terraces.
Comparison of control and flooded stands
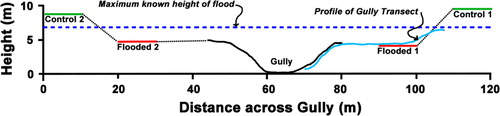
In December 2005, nearly 2 years after the flood, differences in the forest were discernible by the presence of dying trees. A pair of 20 × 20 m plots was chosen haphazardly on terraces on each side of the central gully, so that each pair was sited in a stand of predominantly healthy, unaffected tawa (called ‘Control’ or C) and in a nearby stand with predominantly flooded tawa (called ‘Flooded’ or F). To evaluate the flood's impacts, the relative heights of the plots above the gully bottom were measured using a surveyor's level and staff, averaging the corners and centre of each plot. Slopes of plots were calculated from the differences in corner heights, and aspect estimated from the approximate direction of this slope. The maximum flood level () was identified by Bill Luxton (the adjacent landholder), signs of debris and mud deposits proving to be c. 30 cm lower.
Within plots, diameter at breast height (DBH) of all tawa was measured in January–April 2006, data for multi-stemmed trees being converted to single stems of equivalent cross-sectional area. Plots were extended within stands as necessary to each include at least 20 core-able (> 10 cm DBH) trees. Density data for canopy trees were scaled to a standard plot size of 400 m2. Increment cores were extracted from 83 trees > 10 cm DBH for ageing trees and the coring height and diameter at core height recorded. Core samples were mounted and rings counted, assuming them to be annual (Ogden & West Citation1981), and correcting for missed centres using the innermost 10 arcing rings as in Duncan (Citation1989). If no arcs were visible, it was assumed that the chronological centre was identical to the geometric centre, and the age corrected as in Norton et al. (Citation1987). A linear regression was performed on DBH and age data from all cored single-stemmed trees. Two seedlings that were c. 1.5 m high were harvested at ground level to gain an estimate of age at core height, for which tree age was corrected. Two dead tawa trees with apparently unusually high ages were disked near the ground in March 2010, when on point of collapse, and ages verified.
Between 18 and 28 seedling quadrats, size 1 × 1 m, were randomly positioned within each plot and temporarily marked. Seedlings of woody species were identified and measured according to height class (< 15, 15–49 and 50–150 cm), as extended height to the apical bud, in both July 2006 and March 2010. Few seedling plots could be relocated in 2013, so no further measurements were made.
Vegetation was recorded within each plot in July–October 2006, and again in March 2010 and May 2013. Nomenclature follows the standards for the New Zealand flora, namely the Ngā Tipu website (http://nzflora.landcareresearch.co.nz/) for natives, Brownsey & Smith-Dodsworth (Citation1989) for ferns and Webb et al. (Citation1988) for exotics. Percentage covers in the canopy (> 12 m height), subcanopy (1.5–12 m) and understorey (< 1.5 m) were visually estimated as the ‘shadow’ of each species’ foliage at solar zenith. In addition, cover of dead wood, litter and bare ground was estimated. Rare species were assigned an indicative cover of 0.1%. The frequency and proportion of native and exotic species in each stand were derived. A list of exotic species invading the flooded area was compiled by the Wanganui Botanical Society in June 2005, and updated by the Manawatu Botanical Society in March 2010 and in May 2013.
Gradient responses of mortality
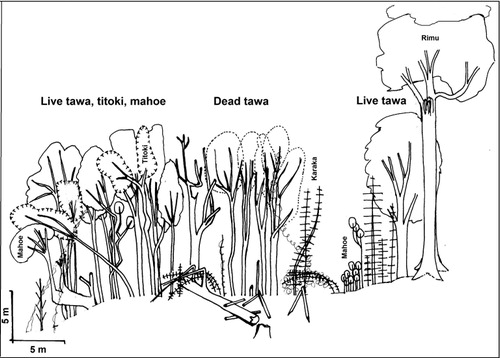
A Gully Transect (40 × 5 m) was positioned slightly upstream of the eastern pair of plots, running from the higher terrace containing the Control stand C1, across the terrace containing the Flooded stand F1, and into the gully adjacent. Topography with respect to the paired plots and tree species’ health were mapped and profiled along this transect in October 2006. Mortality was reassessed in March 2010 and in May 2013.
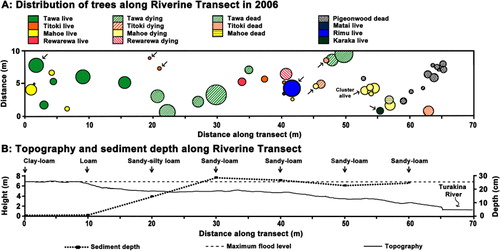
On the eastern edge of the Reserve, adjacent to the Turakina River, a 70 × 10 m Riverine Transect was laid, longwise, from within the unaffected forest across a zone of affected trees to the edge of the river in October 2006 (). Topography in relation to the maximum flood level was measured. Location, DBH, species and condition of all trees > 10 cm DBH along the transect were recorded in October 2006. Soil samples (5 × 5 × 5 cm) were taken every 10 m along the transect and texture was assessed by touch by Mr Ian Furkert (Massey University) using the ‘sausage or worm technique’ (Milne et al. Citation1995). Mortality along the transect was reassessed in March 2010 and May 2013.
Results
Flood characteristics
The February 2004 (mid-summer) flood in the Manawatu region was estimated by catchment managers to be approximately a 1 in 120-year event (Joe McGehan, Horizons Regional Council, pers. comm.), with a total damage bill of around NZ$400 million (Horizons Regional Council Citation2004). Up to 20%, and averaging around 5–10%, of the land surfaces suffered landslips, largely in the pasture-covered portion of the catchment (Hancox & Wright Citation2005). The severe storm was due to an aseasonal collision of an intense zone of low atmospheric pressure from the Antarctic with warm, moist air from a tropical cyclone. The problem was exacerbated by the relatively wet conditions over the preceding month (Fuller Citation2005). Over the 72 h from 14 to 16 February, 160–180 mm of rain fell in the Turakina catchment.
The flood in the Turakina River catchment commenced on the night of Saturday 14 February 2004 (Bill Luxton, pers. comm.; ). Peak flow was 395.13 m3 s−1 above a 13-year summer median of 1.00 m3 s−1 at Otairi, 14 km upstream of McPherson's Reserve (NIWA Citation2007, p. 158). At the maximum, flood height was 10.15 m above a baseline of 0.5 m (Horizons Regional Council Citation2004). Estimates of flood height above the normal river flow through the Reserve are 5.6–7 m, varying with channel width. Within the Reserve, the maximum flood height was at least 2.1 m above the ground height in the two flooded sites (). Water was around maximal level for 48 h, started to subside on Monday 16 February, and had largely drained by the next day (Bill Luxton, pers. comm.). At peak, the river formed a backwater (an area where water ponds separately from the main current), inundating the gully between what was to become the two pairs of plots (Bill Luxton, pers. comm.), though this would have drained as quickly as the river.
In spring 2004 (about 7 months after the flood), a dramatic leaf fall was observed in the southern part of the reserve (John Marsh, pers. comm.; ). Trees died standing, and young trees appeared to be affected earlier than bigger trees (John Marsh, pers. comm.). Over the subsequent 6 years, many standing dead trees collapsed along the Turakina River banks and in the gully, damaging remaining plants of other species and littering the terrace surface with fallen wood (GLR, pers. obs.; ). Rotten roots were visible where fallen trees had exposed root systems (John Marsh, pers. comm.). By March 2010, most fallen wood was rapidly decomposing, and only large tawa logs, a smaller number of standing stumps to 4 m tall, and a few recently fallen trees remained obvious in May 2013, though kahikatea logs were more persistent. By 2013, several large tawa in some apparently unaffected areas of the Reserve adjacent to flooded areas were dead and collapsing. By June 2014, at least four large (diameters > 0.8 m) rimu (Dacrydium cupressinum) in the Reserve were dead or dying.
Comparison of flooded and control plots
The Flooded plots exemplified the forest collapse in McPherson's Reserve, where by 2013 36% of the forest area was affected. Overall, the paired plots () were similar (), except that Control plots were found on higher terraces (9.5 and 8.8 m above the floor of the nearby gully as an arbitrary datum) than Flooded plots (4.1 and 4.7 m), and were above the maximum flood level (6.8 m in ). There was no sediment over the soil in the two Control plots. Throughout the entire flooded forest, including the Flooded plots, the sediment depth ranged from 15 to 29 cm (haphazard observations).
Table 1 Physical characteristics and species’ diversity patterns for Control and Flooded plots (n = 2) at three dates.

The Control plots () were dominated by tawa which formed 60–70% of the canopy cover (), with an average of 7.5 other species. Two years after the flood, live canopy cover in the Flooded plots was < 2%. The only live foliage remaining in the canopy stratum of the Flooded plots was of the tree Alectryon excelsus and epiphyte Collospermum hastatum. This situation persisted. In the Control plots, some canopy change had also occurred by 2010, from gap-infilling by Dacrycarpus dacrydioides and Knightia excelsa as tawa cover declined, due to unexpected thinning of tawa in C1, and loss, by 2013, of all three medium-sized tawa in the canopy of C2. Subcanopy cover differed little between plots and over the subsequent decade in the Controls, but increased in Flooded plots, largely due to a temporary seven-fold increase in Melicytus ramiflorus' cover, along with other natives, in one of the Flooded plots (). Seedlings were unimportant as cover in the ground layers of all plots. The ferns Microsorum scandens and Blechnum filiforme dominated the ground stratum of the Control plots, but were almost completely absent in the Flooded plots. Initially litter-covered, the ground layer of the Flooded plots revegetated quickly, due largely to increase of native shrubs and the exotics Holcus lanatus and Tradescantia fluminensis (), the latter despite occasional control efforts.
There were 29.1 stems (> 10 cm DBH) of tawa per plot (400 m2) in the Control plots and only 11.1 stems in the Flooded plots in 2006 (), with a narrower range of stem diameters. All trees, of all sizes, except for one seedling, died in the Flooded plots by 2006 (). In Control plots, 5% of trees died, all < 30 cm DBH.
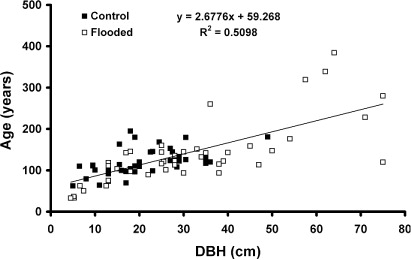
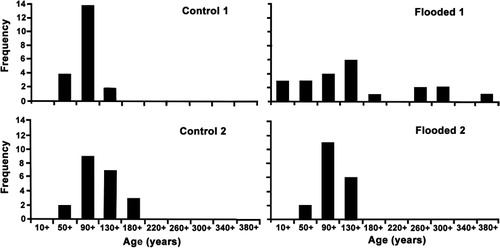
The estimated age-to-core-height of tawa was 23 years. Tree DBH was positively though weakly correlated with age (), but with an intercept of 59.3 years. There were no significant differences in the size/age correlations between plots (data not presented). Most (86%) trees were between 100 and 150 years old in 2006 (). The mean age of trees was 127 years, but was 20 years greater in the Flooded than Control plots (). Only in F1 plot was there any indications of an older cohort, averaging 316 years old, with the two oldest trees being 339 and 384 years. The diameter growth rate of all trees averaged 0.22 cm yr−1 over all sites, though it was 1.5 times greater in Flooded than Control sites over the pre-flood period.
Table 2 Density (scaled to a standard plot size of 400 m2) of tawa trees and saplings, both dead and alive, in each of the four plots in 2006, mean age of tawa trees per sampled area and diameter growth rates.
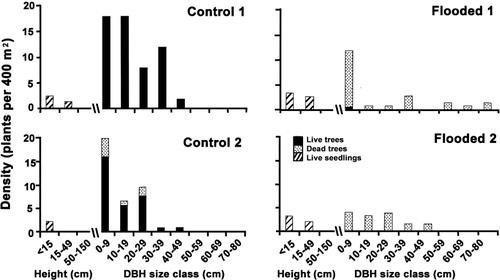
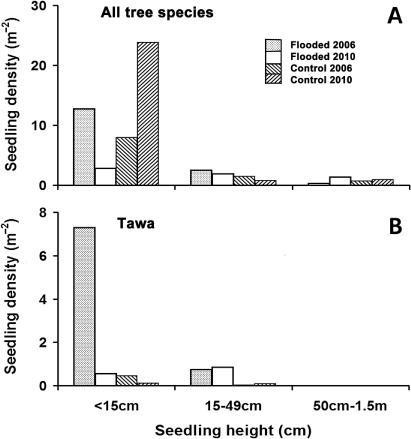
Seedling density of all species was 2.6 times higher in Flooded plots in 2006, but the reverse was found in 2010, largely due to a flush of Dacrycarpus dacrydioides seedlings (10.8 m−2) in 2010 in Control plots, and a loss of tawa in Flooded plots (). In 2006, most tawa seedlings < 15 cm tall (93%) were in Flooded plots, but by 2010 that proportion had dropped to 17%, due to falling debris (pers. obs.). By 2010, Flooded plots had very few seedlings even in the smallest size category (). There was no recruitment to the largest size class, though two seedlings of Dacrycarpus dacrydioides did recruit. Recruitment was most common for two widespread canopy tree species, Melicytus ramiflorus and, particularly in Control plots, Alectryon excelsus. Large numbers of small Knightia excelsa seedlings were present in Control plots, three times more than in Flooded plots, but none recruited over the period of the study.
Table 3 Seedling density (m−2) of forest tree species totalled over all three size categories ≤ 1.50 m at each of two dates.
A noticeable increase in cover of weeds occurred throughout the Reserve in spring 2004 (6 months after the flood; John Marsh, pers. comm.), with 21 species invading (). In June 2006 Flooded plots had 1.4 times the native species’ richness of the Control plots, but 11.4 times the number of exotic species, and over time exotic cover in Flooded plots increased to be nearly 40 times that of the Control plots (). By 2013, a total of 50 exotic species had been seen at some time. The aggressive exotic ground-covering species, Tradescantia fluminensis, not recorded in the Reserve prior to the flood (Ogle Citation2005), had by 2013 become widespread in disturbed areas despite control efforts. In addition, the natives Fuchsia excorticata, Asplenium oblongifolium, Coriaria arborea and Cortaderia toetoe were noted as present in 2010, but are not recorded by Ogle (Citation2005), though only the last two species probably represent new invasions into the disturbed habitat of the Reserve, the others being frequent forest species in the region.
Table 4 Invasion of exotic species into flooded stands. Assessment of June 2005 from Wanganui Plant List No. 86, compiled by Colin Ogle.
Gradient responses of mortality
The transects revealed spatial patterning to mortality. Most tawa trees on the main terrace of the Gully Transect were dead and leafless in 2006 (), and by March 2010 had collapsed. Alectryon excelsus trees initially surviving in the middle of the terrace () had died by 2010, perhaps due to exposure. Some live tawa trees were found amongst other surviving species on the margin of the terrace to the gully in 2006 (), and remained alive in June 2014, although the bent Melicytus ramiflorus was struggling (successfully by 2014), and the tītoki above it were dead. The very large rimu (Dacrydium cupressinum; 30 m high; DBH 1.32 m) at the upper edge of the transect, on the slope to the terrace above, was showing signs of stress by 2010; by 2012 it had died on its flooded side, and was almost completely dead by June 2014. The large live tawa near the rimu, dead by 2010, had collapsed by May 2013, as had all dead tawa on the Gully Transect.

The upper edge of the large terrace of the Riverine Transect was about the maximum height of the flood, and was only lightly covered with a fine, clay–loam sediment (). In 2004, it hosted a dense stand of tawa and Melicytus ramiflorus. Tawa was also found scattered amongst a range of other species across the terrace at 1.8 m below the maximum flood level and to within 20 m of the river. Here, sediment averaged 23 cm thick, and these trees were mostly dying in 2006, and had fallen by 2010. Initially less affected, tītoki (Alectryon excelsus) on the lower portion of the terrace collapsed before 2010, and had died by 2013 (). By May 2013, only the large Dacrydium cupressinum on the transect (x-axis = 41 m) was recognisable (DBH now 1.08 m). All other dying trees were now gone or unidentifiable, and only the live tawa at c. 1 m along the transect was still alive, with two small tītoki around 20 m along the transect resprouting from their stumps. At 60 m along the Riverine Transect (), the 2004 sediment overlaid another distinct layer of sediment, which was at least 35 cm thick (DTR, pers. obs.). In this area Hedycarya arborea was the most common tree, and though most had died by 2010, one was resprouting in 2013, as were a cluster of M. ramiflorus. Other conspicuous plants along the transect in 2013 were the exotic Buddleja davidii, and Corynocarpus laevigatus, native to northern New Zealand, but considered exotic this far south. By 2014 the large D. cupressinum was showing c. 50% loss of canopy.
Discussion
The unusually severe flood of February 2004 (mid summer) extensively affected the land, infrastructure and economy of the Rangitikei and Manawatu regions of New Zealand (Horizons Regional Council Citation2004; Westmount School Citation2004; Hancox & Wright Citation2005). One of the smaller catchments affected was the Turakina Valley, where the maximum flood level was greater than might have been expected because heavy rain during the previous fortnight had reduced soil water-holding capacity (Fuller Citation2005). Forest clearance, landsliding and the establishment of drains in the catchment area probably also increased stream discharge and sediment delivery from the slopes to the main channels, exacerbating the effects of the flood (Fuller Citation2005; Hancox & Wright Citation2005). Impacts of floods on flood-plain podocarp broadleaved forest in New Zealand are exemplified by forest mortality, especially of tawa, in McPherson's Reserve, one of the small reserves alongside the Turakina River.
The severe Mississippi floods of 1993 induced widespread mortality in the riparian forest and aquatic vegetation through a mix of scouring and deposition (Spink & Rogers Citation1996; Romme et al. Citation1998; Cosgriff et al. Citation2007). At McPherson's Reserve, physical disturbance and sediment deposition killed or removed > 70% of the vegetative cover in all layers of the Flooded plots, and were extreme in their effects on trees. Differences in flood tolerance of adult tree species are widely recorded in the literature (Spink & Rogers Citation1996; Kozlowski Citation1997; Glenz et al. Citation2006; Cosgriff et al. Citation2007), even in New Zealand (Wardle Citation1974; Duncan Citation1993). Melicytus ramiflorus, one of the species able to outcompete tawa (Knowles & Beveridge Citation1982), occupied the newly created light gaps by infilling as tawa died, showing a burst of cover in F1 (Flooded plot 1) by 2010. However, its coverage later declined, perhaps due to delayed drowning or increased exposure. Other Melicytus ramiflorus also eventually died on the outer areas of the Riverine Transect, although the large, bent plant on the edge of the Gully Transect recovered by 2014. Karaka (Corynocarpus laevigatus) appeared flood-tolerant, seedlings being abundant after the flood. Its subsequent success is hard to evaluate because it is partially managed by cutting or poisoning of adult trees and culling of seedlings; although endemic to New Zealand, it is not native in the lower North Island, even impeding regeneration of natives (Costall et al. Citation2006). Initially appearing more flood-tolerant than tawa, flooded trees of Alectryon excelsus had died in the middle of the Gully Transect by 2010, with the tree on the better-drained margin dying by 2013. The fruit of A. excelsus can germinate well and rapidly, perhaps assisted by the moisture-rich aril weighing up to 340 mg (Outred Citation2000), but takes more than a year to set. However, seedlings of A. excelsus were actually infrequent in the Flooded plots, despite the initially good survival of parent trees, implying that stress may have resulted in poor seed set. Notwithstanding these species, the major impact of the 2004 flood on the forest was the loss of tawa. Within 6 months of the flood, tawa in low-lying parts of the Reserve lost their leaves and underwent canopy collapse (John Marsh, pers. comm.). By June 2006, flooded areas were composed mainly of dead, standing tawa trunks, the smaller branches already having fallen off, landing amongst and on extensive pockets of tawa seedlings (GLR, pers. obs.). For several subsequent years, falling debris made the reserve unsafe for working in. By 2010, most large trunks had fallen, adding to the dead, rotting wood covering the plots. A decade after the flood, affected areas were a complex mix of regenerating native shrubs and rotting tawa trunks, as well as recently dead or fallen tawa, collapsing Dacrydium cupressinum forest giants and invading exotic species.
The majority of flooded trees died standing, indicating that they died from stress, rather than physical damage. Soil inundation lasting some months is the major cause of standing tree death in floodplains (Foster et al. Citation1998; Romme et al. Citation1998; Glenz et al. Citation2006). However, at McPherson's Reserve, the flood event only lasted 2 to 3 days. Fine sediment eroded from the surrounding catchment was deposited on the surfaces of terraces within McPherson's Reserve, in some places to 30 cm deep in the still margins and backwaters of the flood. These deposits may have sealed in the flood-wet soil, creating an extended period of anoxia. Alternatively, the flood and sediment deposition may have created conditions allowing fungal infections to spread and prosper. The only tawa surviving 2 years after the flood in the Gully Transect, and still alive in 2014, were on the steeper terrace margin. Despite being well beneath the maximum flood level (2.3 m), inundation effects were possibly more limited there, due to more rapid or thorough drainage, or less deposition of sediment, reducing the duration of anoxia, and hence the vulnerability to fungal infection.
Tree-falls on wet soils in otherwise intact forest are most likely due to root rot (Schaetzl et al. Citation1989). Shortly after the flood, some fallen trees at McPherson's Reserve showed rotten roots (John Marsh, pers. comm.), although it is not possible to determine if root death caused canopy death or vice versa. Tawa is a shallow-rooted species, particularly in areas with a high water table (Atkinson & Greenwood Citation1972), such as applies to the lower terraces at McPherson's Reserve. Roots form to 1–2 m deep, with the initial taproot being replaced by several diverging sinker roots interspersed with many fine roots, the complex covering a narrow radius of 3–5 m (Cameron Citation1963). Phytophthora infects roots in moist environments (Stolzy & Sojka Citation1984), and since tawa can be very susceptible to Phytophthora cinnamomi (Robertson Citation1970), infection may have contributed to mortality. Tawa stems also decay in conjunction with a range of ascomycete and basidiomycete fungi (Hood & Gardner Citation2009), and tawa heartwood is often attractively spelted. While fungal sporocarps were not noted to be unusually common on standing dead trees after the flood, tawa is susceptible to Ganoderma aff. applanatum (Hood & Gardner Citation2009), which can accelerate the rate of decay (Beets et al. Citation2008). Beets et al. (Citation2008) categorised tawa's decay rate as intermediate amongst common podocarp and beech (Fuscospora and Lophozonia) trees, with coarse woody debris of tawa taking c. 25 years to lose 50% of its biomass when wind-thrown in damp forest. By contrast, Beets et al. (Citation2008) record a rapid breakage rate for tawa stakes in a graveyard study (50% breakage within 4.7 years in a nationwide range of sites), much closer to the rate at which standing tawa at McPherson's Reserve lost branches. Rapid decay of fallen tawa may be a consequence of partial immersion in the flood and subsequent persistent anoxia. By 2013, fallen stems in the flooded zone were completely covered by exotic grass swards, creating a moist environment, possibly accelerating fungal decay.
Nearly a decade after the flood, unexpected collapse of tawa in otherwise intact forest suggested that flood damage was more pervasive than initially appeared. By 2013, three large tawa had fallen in intact forest at the head of the gully, and most tawa trees in the lower of the Control plots (C2; 2 m above maximum flood height) had died standing, an abnormal increase in Carex cover suggesting a locally heightened water table, the rest of that terrace apparently being unaffected. Tawa on the Riverine Transect that apparently survived the flood had also died by 2006, except for trees rooted very close to the upper limit of flood waters and largely unaffected by sediment deposition; however, most of these had died by 2013. These deaths were possibly due to spread of decay-causing fungi. Tawa forms extensive root grafts, both within and between individual trees (Cameron Citation1963), which may expose healthy trees to fungal pathogens from nearby collapsed stands. In non-flooded forest, all fallen tawa stems were sporting a diverse range of sporocarps in 2014, with more than 20 taxa noted, including icicle tooth fungus (Hericium coralloides).
At McPherson's Reserve, smaller trees of tawa were observed to die before bigger trees (John Marsh, pers. comm.). Flooding intolerance is often noted as being greater in seedlings than adults due to the proportionately more extensive inundation of foliage (Spink & Rogers Citation1996; Glenz et al. Citation2006; Cosgriff et al. Citation2007). The flood depths on the lower terraces at McPherson's Reserve were > 2 m, over the height of most seedlings. A seedling tawa has a tap root which, within a decade or so, develops multiple well-branched leaders (Cameron Citation1963), but these would not confer tolerance to prolonged inundation. Submergence appears the most direct cause of deaths of small tawa in the flooded zone.
Spink & Rogers (Citation1996) observed that the death of formerly light-restricting trees and the deposition of new sediment assisted plant establishment in floodplain forest following the Mississippi flood of 1993. Reproduction in plants, particularly development of fleshy fruits, is a resource expensive activity which is liable to disruption by stresses such as flooding (Kozlowski Citation1997). Streng et al. (Citation1989) noted the importance of the interaction between the timings of disturbance and phenology in flood-prone forests, since imposed stress may actually trigger an enhanced reproductive episode (Kozlowski & Pallardy Citation2002; Cosgriff et al. Citation2007). However, tawa fruit take 18 months to develop (Knowles & Beveridge Citation1982; Wright Citation1984), and trees at McPherson's Reserve did not live long enough to display enhanced fruit set post-flood. Instead, the coincidence of the flood with the period of ripening fruit from the previous year (January to March) may have increased tawa's intolerance to flooding through increased resource demand to supply reproductive effort. After the flood, plentiful seed was available for rapid colonisation of the newly opened sites. Only one extant disperser of the large tawa drupes, kererū (Hemiphaga novaeseelandiae), still operates in New Zealand (Clout & Hay Citation1989), and though kererū probably visit the Reserve, most tawa seed simply fell off the dying parent tree, and germinated underneath. Germination is rapid, and best in moist, high light conditions (Cameron Citation1963; Knowles & Beveridge Citation1982), and though able to survive burial, seeds are prone to rotting (Burrows Citation1999). In McPherson's Reserve, a flush of seedlings arose immediately after the flood (Colin Ogle, pers. comm.), and large numbers of tawa seedlings were present on freshly deposited sediment in flooded plots during the first census in 2006, confirming that tawa can establish on young mineral soils (Lusk Citation1984; Lusk et al. Citation2009). Seedlings of many New Zealand species show preferences for raised, light-rich microsites, including Fuscospora fusca (Hook. f.) Heenan & Smissen (2013) and several podocarps (June & Ogden Citation1975; Duncan Citation1993; Ebbett & Ogden Citation1998). However, there is little evidence of such a preference in tawa, which seldom occur on fallen logs. Although tawa seedlings 50–150 cm tall are common in West’s (Citation1995) data, none was recorded at McPherson's Reserve in any plot at any time, despite the presence of younger seedlings initially. The burst of young seedlings in the Flooded plots had declined by 2010, largely due to smothering by exotic grasses and other herbs, and there was no recruitment to larger size classes. Tawa seedlings seldom persist unless under a shaded, protective canopy (Knowles & Beveridge Citation1982), and the regenerating cover of the flooded zone is probably still too open for tawa saplings to recruit successfully. In the reverse trend to the Flooded plots, a higher density of seedlings, largely Dacrycarpus dacrydioides and Knightia excelsa, was observed in the Control plots in 2010 than 2006, especially C2, probably in response to gradual collapse of the tawa canopy. D. dacrydioides seedlings respond to catastrophic disturbance well, especially on mineral soils (Duncan Citation1993; Ebbett & Ogden Citation1998; Lusk et al. Citation2009). Seedlings of K. excelsa were at a high density compared with Moles & Drake’s (Citation1999) study in pine forest, but do survive litterfall better than tawa (Gillman et al. Citation2003), and so may recruit over time, though none was observed to do so during the study. The future of these seedlings is uncertain.
Tawa is known to exhibit a considerable range of diameters for any age class (Ogden & West Citation1981), and at McPherson's Reserve, size distributions were more continuous than age distributions, especially in the two flooded plots. Diameter growth rates for tawa are recorded as being as high as 2 mm yr−1 in Taranaki (Knowles & Beveridge Citation1982), very similar to our values which averaged 2.2 mm yr−1 over all sites. Our size/age correlation for tawa at McPherson's Reserve is also very similar to that reported by Ogden & West (Citation1981) for Pureora Forest (age in years = 69.7 + 2.78× diameter under bark in cm), so McPherson's tawa is not behaving atypically. However, our estimated age at core height, recorded from tawa seedlings as 23 years, compares poorly with Ogden & West’s (Citation1981) statement that seedlings may be 60–80 years old. Being largely a scalar (core heights averaged 115 cm ± 24 SD), our correction for age to core height will not affect our understanding of tawa demographics at the scale of McPherson's Reserve, but does suggest that the aged seedlings > 1.5 m high in McPherson's Reserve are atypical, and may be young for their size.
In terms of demographic structure, tawa is widely reported to undergo continuous regeneration (McKelvey Citation1954; Ogden & West Citation1981; Knowles & Beveridge Citation1982; West Citation1995), though sensitive to exposure, and slow to infill large gaps (Knowles & Beveridge Citation1982). But there was no evidence for continuous regeneration at McPherson's Reserve. Instead our demographic data indicate a single cohort of tawa, with half the trees between 100 and 150 years old, and most < 200 years. One facet of this cohort at McPherson's Reserve is the paucity of small trees < 90 years old or < 9 cm DBH. In Flooded plots, small trees may be absent because they collapsed and decomposed rapidly post flood, so that no record could be made later. But there was no evidence of the past existence of such pole trees amongst the debris on the ground, and no reports thereof. Nor is this an explanation for their absence in Control plots. Possibly eliminated due to past stock-browsing in the Reserve, tawa is actually relatively unpalatable to both cattle and deer (Knowles & Beveridge Citation1982; Smale Citation2008). However a more open, grazed understorey may have created a light and moist environment which was, at that time, inimical to tawa recruitment, and subsequent recovery of natives may have created a dense vegetation still too competitive for successful regeneration of tawa.
Control and Flooded plots were taken as being comparable prior to the flood, although there were known differences between them, that is, the zone of the Control plots had a greater initial cover of ground-layer ferns (John Marsh, pers. comm.) and a higher cover of podocarps (Prumnopitys taxifolia and Dacrycarpus dacrydioides). However, demographic differences emerged between plots, which contribute to understanding of the cohort structure and regeneration processes operating at McPherson's Reserve. Only F1 had trees > 220 years old, those five averaging 316 years, with a maximum of 384, the oldest trees being found on the gully margin. This may represent a second, older cohort, suggesting this plot has a different disturbance history to the rest of the plots. It may have been subject to a large tree-fall event around 400 years ago, creating a localised canopy gap, and initiating a more continuous distribution. Diameter growth rates are on average 1.4 times higher in F1 than in any other plot, including F2, and high growth rates may indicate release from competition. Alternatively, they may simply reflect a more favourable microsite at the time, perhaps in terms of moisture and shelter. Examination of growth widths of growth rings in the large, disked trees of F1 offers some support for the competition hypothesis, the smaller stem having apparently wider rings over its first 200 years, and the larger showing narrow rings throughout, except in the buttresses themselves. However this does not explain the absence of older trees in the other plots.
A flood might also initiate an even-aged cohort (Duncan Citation1993; Wells et al. Citation2001), but it is highly improbable that a huge flood more than 400 years ago, and affecting all terraces (and plots) in the reserve, had a lesser impact on F1, which is 60 cm lower than F2. A more minor flood event than that of 2004, which affected both Flooded plots, would be appropriate. Such a flood, c. 150 years ago at the time of early European settlement, could have eliminated the youngest cohorts in F1, while killing few large tawa. Probably carrying a much lower sediment load due to lower levels of up-catchment disturbance, anoxia-related deaths would be reduced, as perhaps would deaths due to fungal infection. Support for this scenario comes from sedimentary evidence in the Riverine Transect of a depositional event prior to 2004. But again this explanation does not address the absence of older trees in the Control plots and F2, where more continuous regeneration patterns would be expected. Another possibility for their absence is the logging and removal of large tawa from parts of the reserve around 100 years ago; however tawa is not generally the preferred timber when tall podocarps are available, and it was not commonly logged prior to the 1940s (Knowles & Beveridge Citation1982). It is possible that tawa's regeneration dynamics have been misinterpreted, and it is more of a gap-phase regenerator (sensu Lusk & Ogden Citation1992) than thought, though unable to regenerate in large gaps (West Citation1995). Regardless, in the absence of confounding factors such as weed invasion, the flooded zones at McPherson's Reserve should eventually host a shady, frost-free native canopy, under which tawa, aside from limitations to seed dispersal, will once again be able to regenerate according to normal successional processes.
Increasing richness and cover by invasive aliens species at McPherson's Reserve appears to have been accelerated by the increase in available light and by deposition of fresh sediment. Similarly, Romme et al. (Citation1998) noted that the 1993 Mississippi floods were followed within a year by occupation by opportunistic herbaceous weed species. Some invaders of McPherson's Reserve are noxious weeds, for example, Tradescantia fluminensis (wandering willy, a sward-forming clone; Kelly & Skipworth Citation1984; Standish et al. Citation2001) and Phytolacca octandra (Indian ink weed, readily spread by birds; Ferguson & Drake Citation1999), which may prevent regeneration of native seedlings. The abundance of weeds in the flooded plots suggests that future colonisation and establishment of native woody species will be inhibited, though where natives do establish or persist, a dense cover is rapidly formed (GLR, pers. obs., June 2014).
The fate of McPherson's Reserve exemplifies the difficulties experienced by small forest remnants facing ongoing or intermittent or novel stresses. Considered management may be essential to perpetuate forest remnants which are exposed to stresses outside their capacity to respond to or recover from, whether spatially, demographically or successionally. Future monitoring of the responses of the forest may be illuminating, and the impending deaths of some large podocarps will provide an opportunity to examine growth patterns of discs for tree ring-width evidence of release events. Better understanding of the range of demographics of tawa in the region would assist in interpretation of the history of McPherson's and the nature of past disturbance events. Autecological experiments on tawa and other common forest trees would be of benefit (following Cook et al. Citation1980) in understanding their limits to such stressors, as little is known about differences in response of most New Zealand tree species to floods, or their vulnerability to increasing flood regimes.
Conclusion
While flood episodes may be a normal part of some New Zealand forest dynamics (Wells et al. Citation2001; Stewart Citation2002), particularly for forest dominated by Dacrycarpus dacrydioides and Laurelia novae-zelandiae, this is not the case for tawa, which does not thrive in areas with poor drainage (Wright Citation1984). The dieback episode at McPherson's Reserve was rapid compared with other reported dieback events (Jane & Green Citation1983). This is probably a result of a threshold response as per Romme et al. (Citation1998), where the severity of a disturbance (e.g. duration or depth of flooding, or level of anoxia) exceeds the tolerance level of species, as in the Mississippi River event (Spink & Rogers Citation1996). Manion (Citation1991) categorised dieback pathology as inciting (sometimes called triggering; Mueller-Dombois Citation1987) factors, such as our flood, contributing factors (anoxia and the presence of pathogens), and predisposing factors (in our case the site and demographic structure of the tawa populations). This model fits the McPherson's Reserve event well.
The extreme flood event adversely affecting McPherson's Reserve was the result of heavy rain from an unseasonable storm falling on already wet soil. Extensive landsliding contributed large sediment loads to the flood waters and anoxia was probably caused by water-borne deposition of that sediment over saturated soil. Subsequent mortality of tawa was both extensive and rapid. The flood occurred during the growing and fruiting season, when tree susceptibility may have been heightened. The extensive canopy dieback and sediment deposition allowed the abundant establishment of native seedlings, although most did not recruit in flooded areas due to swamping by adventive herbaceous weeds. Demographic evidence suggests that no similar flood event has happened within at least the last 400 years, so that land clearance and other anthropogenic landscape modifications have exacerbated the impacts of the flood. If the frequency of similar events is increased, due to human impacts and possibly global warming (Fuller Citation2005), then the demography and dynamics of tawa will change, and it may be excluded from flood-susceptible environments. The future of the forest is uncertain.
Acknowledgements
Thanks to the Rangitikei Branch of Forest & Bird for permission to work in their reserve, and to Colin Ogle and John Marsh for bringing its condition to our attention; to Paul Barrett, Rebecca Bennick, Lorraine Cook, Carol Nicholson, Anne Redpath, Andrew Thomas and Cleland Wallace for research assistance; to Ian Furkert for performing soil assessments; to Bill Luxton, John Marsh, Joe McGehan, Shaun Nielsen, Colin Ogle, Alan Palmer and Paul Stock for helpful discussion; to Bill Luxton for the flood image and for seeing us home; to Bruce Clarkson, Rebecca Bylsma, Lorraine Cook, Shaun Nielsen, Natasha Petrove, Andrew Thomas and an anonymous referee for comment on drafts; to Hugh Stewart for field assistance with the re-survey; and finally to Massey University for financial and logistic support.
References
- Atkinson IAE, Greenwood RM 1972. Efffects of the 1969–70 drought on two remnants of indigenous lowland forest in the Manawatu district. Proceedings of the New Zealand Ecological Society 19: 34–42.
- Barlow J, Peres PA 2008. Fire-mediated dieback and compositional cascade in an Amazonian forest. Philosophical Transactions of the Royal Society London B 363: 1787–1794. 10.1038/35106547
- Beets PN, Hood IA, Kimberley MO, Oliver GR, Pearce SH, Gardner JF 2008. Coarse woody debris decay rates for seven indigenous tree species in the central North Island of New Zealand. Forest Ecology and Management 256: 548–557. 10.1016/j.foreco.2008.05.036
- Brownsey PJ, Smith-Dodsworth JC 1989. New Zealand ferns and allied plants. Auckland, Bateman.
- Burrows CJ 1999. Germination behaviour of seeds of the New Zealand woody species Beilschmiedia tawa, Dysoxylum spectabile, Griselinia lucida, and Weinmannia racemosa. New Zealand Journal of Botany 37: 95–105. 10.1080/0028825X.1999.9512616
- Cameron RJ 1963. A study of the rooting habits of rimu and tawa in pumice soils. New Zealand Journal of Forestry 8: 771–785.
- Clout MN, Hay JR 1989. The importance of birds as browsers, pollinators and seed dispersers in New Zealand forests. New Zealand Journal of Ecology 12: 27–33.
- Cockayne L 1928. The vegetation of New Zealand. 2nd edition. Leipzig, Engleman.
- Cook JM, Mark AF, Shore BF 1980. Responses of Leptospermum scoparium and L. ericoides (Myrtaceae) to waterlogging. New Zealand Journal of Botany 18: 233–246. 10.1080/0028825X.1980.10426922
- Cosgriff RJ, Nelson JC, Yin Y 2007. Floodplain forest response to large-scale flood disturbance. Transactions of the Illinois State Academy of Science 100: 47–70.
- Costall JA, Carter RJ, Shimada Y, Anthony D, Rapson GL. 2006. The endemic tree Corynocarpus laevigatus (karaka) as a weedy invader in forest remnants of the southern North Island, New Zealand. New Zealand Journal of Botany 44: 5–22. 10.1080/0028825X.2006.9513002
- Duncan RP 1989. An evaluation of errors in tree age estimates based on increment cores in kahikatea (Dacrycarpus dacrydioides). New Zealand Natural Sciences 16: 31–37.
- Duncan RP 1993. Flood disturbance and the coexistence of species in a Lowland Podocarp Forest, South Westland, New Zealand. Journal of Ecology 81: 403–416. 10.2307/2261519
- Ebbett RL, Ogden J 1998. Comparative seedling growth of five endemic New Zealand podocarp species under different light regimes. New Zealand Journal of Botany 36: 189–201. 10.1080/0028825X.1998.9638781
- Ferguson RN, Drake DR 1999. Influence of vegetation structure on spatial patterns of seed deposition by birds. New Zealand Journal of Botany 37: 671–677. 10.1080/0028825X.1999.9512661
- Foster DR, Knight DH, Franklin JF 1998. Landscape patterns and legacies resulting from large, infrequent forest disturbances. Ecosystems 1: 497–510. 10.1007/s100219900046
- Fuller IC 2005. February floods in the lower North Island, 2004: catastrophe – causes and consequences. New Zealand Geographer 61: 40–50. 10.1111/j.1745-7939.2005.00014.x
- Gillman LN, Wright SD, Ogden J 2003. Response of forest tree seedlings to simulated litterfall damage. Plant Ecology 169: 53–60. 10.1023/A:1026288306932
- Glenz C, Schlaepfer R, Iorgulescu I, Kienast F 2006. Flooding tolerance of Central European tree and shrub species. Forest Ecology and Management 235: 1–13. 10.1016/j.foreco.2006.05.065
- Hancox GT, Wright K 2005. Analysis of landsliding caused by the 15–17 February 2004 rainstorm in the Wanganui-Manawatu Hill Country, southern North Island, New Zealand. Institute of Geological and Nuclear Sciences Science Report 2005/11. Lower Hutt, Institute of Geological and Nuclear Sciences Science. 64 p.
- Hood IA, Gardner JF 2009. Fungi decaying stems of fallen tawa (Beilschmiedia tawa) trees in the central North Island of New Zealand. New Zealand Journal of Botany 47: 115–119. 10.1080/00288250909509797
- Horizons Regional Council 2004. Storm: civil emergency storm and flood report, February 2004. Horizons Report 2004/EXT/591. Palmerston North, HRC.
- Jane GT, Green TGA 1983. Episodic forest mortality in the Kaimai Ranges, North Island, New Zealand. New Zealand Journal of Botany 21: 21–31.
- Jane GT, Green TGA 1986. Etiology of forest dieback areas within the Kaimai Range, North Island, New Zealand. New Zealand Journal of Botany 24: 513–527. 10.1080/0028825X.1986.10409939
- June SR, Ogden J 1975. Studies on the vegetation of Mount Colenso, New Zealand. 3. The population dynamics of red beech seedlings. Proceedings of the New Zealand Ecological Society 22: 61–66.
- Kelly D 1987. Slow recovery of Beilschmiedia tawa after severe frosts in inland Taranaki, New Zealand. New Zealand Journal of Ecology 10: 137–140.
- Kelly D, Skipworth JP 1984. Tradescantia fluminensis in a Manawatu (New Zealand) forest: I. Growth and effects on regeneration. New Zealand Journal of Botany 22: 393–397. 10.1080/0028825X.1984.10425270
- Knowles B, Beveridge AE 1982. Biological flora of New Zealand 9: Beilschmiedia tawa (Lauraceae) Tawa. New Zealand Journal of Botany: 20: 37–54. 10.1080/0028825X.1982.10426403
- Kozlowski TT 1997. Responses of woody plants to flooding and salinity. Tree Physiology Monograph 1: 1–29.
- Kozlowski TT, Pallardy SG 2002. Acclimation and adaptive responses of woody plants to environmental stresses. The Botanical Review 68: 270–334. 10.1663/0006-8101(2002)068[0270:AAAROW]2.0.CO;2
- Lusk CH 1984. Structure and dynamics of alluvial floodplain forest at Totara reserve, Pohangina Valley. MSc thesis. Palmerston North, Massey University.
- Lusk CH, Duncan RP, Bellingham PJ 2009. Light environments occupied by conifer and angiosperm seedlings in a New Zealand podocarp-broadleaved forest. New Zealand Journal of Ecology 33: 83–89.
- Lusk CH, Ogden JO 1992. Age structure and dynamics of a podocarp-broadleaf forest in Tongariro National Park, New Zealand. Journal of Ecology 80: 379–393. 10.2307/2260684
- Manion PD 1991. Tree disease concepts. 2nd edition. Upper Saddle River, NJ, Prentice-Hall.
- McEwen WM 1987. Ecological regions and districts of New Zealand. 3rd edition. NZ Biological Resources Centre Publication No. 5. Wellington, Department of Conservation.
- McKelvey PJ 1954. Some ideas on tawa (Beilschmiedia tawa) management in Central North Island forests. New Zealand Journal of Forestry 7: 68–74.
- Milne JDG, Clayden B, Singleton PL, Wilson AD 1995. Soil description handbook. Canterbury, Manaaki Whenua Press.
- Moles AT, Drake DR 1999. Potential contributions of the seed rain and seed bank to regeneration of native forest under plantation pine in New Zealand. New Zealand Journal of Botany 37: 83–93. 10.1080/0028825X.1999.9512615
- Mueller-Dombois D 1987. Natural dieback in forests. Bioscience 37: 575–583. 10.2307/1310668
- NIWA 2007. Statisical analysis of river flow data in the Horizons region. NIWA Client Report CHC2006-154. Christchurch, National Institute of Water and Atmospheric Research Ltd.
- Norton DA, Palmer JG, Ogden J 1987. Dendroecological studies in New Zealand 1. An evaluation of tree age estimates based on increment cores. New Zealand Journal of Botany 25: 373–383. 10.1080/0028825X.1987.10413355
- Ogden J, West CJ 1981. Annual rings in Beilschmiedia tawa (Lauraceae). New Zealand Journal of Botany 19: 397–400. 10.1080/0028825X.1981.10426397
- Ogle C 2005. Wanganui plant list no. 86 (modified): indigenous higher plants of Mcpherson Bush and Sutherland Bush. Wanganui, Wanganui Botanical Society.
- Outred HA 2000. Arillate seeds, with special reference to titoki (Alectryon excelsus Gaernt. (Sapindaceae)). Agronomy Society of New Zealand Special Publication No. 12: 15–24.
- Robertson GI 1970. Susceptibility of exotic and indigenous trees and shrubs to Phytophthora cinnamomi Rands. New Zealand Journal of Agricultural Research 13: 297–307. 10.1080/00288233.1970.10425403
- Romme WH, Everham EH, Frelich LE, Moritz MA, Sparks RE 1998. Are large, infrequent disturbances qualitatively different from small, frequent disturbances? Ecosystems 1: 524–534. 10.1007/s100219900048
- Royal Forest & Bird Protection Society 2007. McPherson's reserve. http://www.forestandbird.org.nz/enjoy_nature/reserves/mcpherson/mcpherson.asp ( accessed 20 March 2007).
- Schaetzl RJ, Johnson DL, Burns SF, Small TW 1989. Tree uprooting: review of terminology, process, and environmental implications. Canadian Journal of Forest Research 19: 1–11. 10.1139/x89-001
- Smale MC 2008. Deer impacts on tawa (Beilschmiedia tawa) regeneration. DOC Research and Development Series 200. Wellington, Department of Conservation.
- Spink A, Rogers S 1996. The effects of a record flood on the aquatic vegetation of the Upper Mississippi River system: some preliminary findings. Hydrobiologia 340: 51–57. 10.1007/BF00012734
- Standish RA, Robertson AW, Williams PA 2001. The impact of an invasive weed Tradescantia fluminensis on native forest regeneration. Journal of Applied Ecology 38: 1253–1263. 10.1046/j.0021-8901.2001.00673.x
- Stewart GH 2002. Structure and canopy tree species regeneration requirements in indigenous forests, Westland, New Zealand. DOC Science Internal Series 66. Wellington, Department of Conservation. 33 p.
- Stolzy LH, Sojka RE 1984. Effects of flooding on plant disease. In: Kozlowski TT ed. Flooding and plant growth. Orlando, FL, Academic Press. Pp. 221–264.
- Streng DR, Glitzenstein JS, Harcombe PA 1989. Woody seedling dynamics in an east Texas floodplain forest. Ecological Monographs 59: 177–204. 10.2307/2937285
- Wardle P 1974. The kahikatea (Dacrycarpus dacrydioides) forest of south Westland. Proceedings of the New Zealand Ecological Society 21: 62–71.
- Webb CJ, Sykes WR, Garnock-Jones PJ 1988. Flora of New Zealand. Vol. IV. Naturalised pteridophytes, gymnosperms, dicotyledons. Christchurch, Botany Division, Department of Scientific and Industrial Research.
- Wells A, Duncan RP, Stewart GH 2001. Forest dynamics in Westland, New Zealand: the importance of large, infrequent earthquake-induced disturbance. Journal of Ecology 89: 1006–1018. 10.1111/j.1365-2745.2001.00594.x
- Wells A, Stewart GH, Duncan RP 1998. Evidence of widespread, synchronous, disturbance-initiated forest establishment in Westland, New Zealand. Journal of the Royal Society of New Zealand 28: 333–345. 10.1080/03014223.1998.9517569
- West CJ 1995. Sustainability of Beilschmiedia tawa-dominated forest in New Zealand: population predictions based on transition matrix model analysis. Australian Journal of Botany 43: 51–71. 10.1071/BT9950051
- Westmount School 2004. Destructive deluge: a pictorial coverage of February floods 2004. Palmerston North, Westmount School.
- Wright AE 1984. Beilschmiedia Nees (Lauracae) in New Zealand. New Zealand Journal of Botany 22: 109–125. 10.1080/0028825X.1984.10425238
