Abstract
Vascular epiphytes live non-parasitically on other plants and are a distinctive and intergral component of tropical forests. There is a general lack of studies examining epiphyte diversity in urban settings. The aim of this study was to document the diversity of epiphytes on host trees in the eThekwini Metropolitan Area (EMA). In addition, the number of individuals of each epiphyte, host tree circumference at breast height and height were recorded. In total, 30 epiphyte species from 12 families were recorded, with most epiphyte species belonging to the Moraceae (n = 8) and Araliaceae (n = 5). A total of 34 host species from 15 families were recorded. These numbers did not increase when considering herbarium material within the EMA sensu stricto. The highest epiphyte richness (n = 13) was reported on the palm Raphia australis. The high number of both alien host and epiphytic taxa are of concern. More studies are needed to understand how epiphyte/host interactions in the urban landscape are established and maintained.
Introduction
Epiphytes are plants that grow on other plants for structural support and anchorage, and not for water or nutrient supplies (i.e. non-parasitic) (Laube & Zotz Citation2006). Approximately 84 vascular plant families have evolved an epiphytic strategy and at least 876 genera have one epiphytic species (Gentry & Dodson Citation1987; Kress Citation1989). The number of epiphytes is estimated to be c. 29,000 species (Gentry & Dodson Citation1987). Epiphytes are considered an important component of global plant diversity and represent c. 10% of the world’s vascular plant species (Gentry & Dodson Citation1987; Nieder et al. Citation2001).
Araceae, Bromeliaceae, Orchidaceae and several fern families have a substantial number of epiphytes (Fernandez Monteiro et al. Citation2009). Epiphytes can be broadly classified as holoepiphytes (spending their entire life cycle in the canopy) and hemiepiphytes (spending some stage of their life rooted in terrestrial soil). Some epiphytes start life in the canopy and send roots to the ground (primary hemiepiphytes), whereas others start on the ground, grow to the canopy and lose terrestrial connections (secondary hemiepiphytes) (Benzing Citation2004; Lowman & Rinker Citation2004). Epiphytes are ecologically important, playing a significant role in forest ecosystem processes and interactions, and contribute to species richness (Cummings et al. Citation2006; Burns & Zotz Citation2010). Epiphytes are considered to be indicators of ecosystem diversity, health and productivity (Jovan & McCune Citation2006; Bartels & Chen Citation2012). They provide nutrients for other flora and fauna, and are also very sensitive to shifts in microclimate (Gradstein Citation2008). Epiphytes play an important role in tropical ecosystem functioning (Madison Citation1977), by producing relatively high amounts of organic matter on host trees and fixing atmospheric nitrogen (Nadkarni Citation1984). Epiphytes are also considered an integral part of some urban ecosystems and constitute an important structural and functional component (Ellis & Coppins Citation2007). Epiphytic plants provide water, food (nectar, fruits), habitat and nesting materials for invertebrates and birds (Affeld Citation2008; Alvarenga et al. Citation2010). Díaz et al. (Citation2012) have shown that trees with epiphytes have greater invertebrate species richness and abundance compared with trees with epiphytes removed.
Epiphyte distributions are influenced by stand characteristics, such as stand age and tree species composition, as well as by dispersal limitation (Woods Citation2013). A host tree species may contain a diverse community of epiphytes that is often different from other host tree species (Laube & Zotz Citation2006). Host-specific differences in epiphyte assemblages suggest that epiphyte diversity may be related to variation in microhabitats within individual host trees (Cardelús & Chazdon Citation2005). Some authors have reported that epiphyte growth on particular host trees is also affected by several physical and chemical host traits, and environmental factors (Vanderpoorten et al. Citation2004). The preference of a specific epiphyte species for a particular host tree species has been reported repeatedly (Benzing Citation1990; Male & Roberts Citation2005; Laube & Zotz Citation2006). Several studies have examined epiphytic flora on single host species (Affeld et al. Citation2008; Werner & Gradstein Citation2009; Larreaa & Werner Citation2010). A total of 126 morphospecies (52 genera and 21 plant families) were found growing epiphytically on a single mature host tree (phorophyte) in Costa Rica (Schuettpelz & Trapnell Citation2006). Other studies have examined a limited number of phorophytes. Freiberg (Citation1996) recorded a total of 77 species (17 families) from three phorophyte tree species in French Guiana.
Epiphytes contribute to species richness and play a substantial role in the processes and interactions that make a forest function (Cummings et al. Citation2006). They constitute a large proportion of photosynthetically active material (Hofstede et al. Citation2001), and significantly influence important processes, particularly mineral and hydrological cycles (Benzing Citation1998). Besides that, epiphytes play key roles in forest dynamic processes and they are a major contributor to local, regional and global plant diversity (Batke Citation2012).
Urban green spaces (parks, avenues, greenways, etc.) provide an important contribution to quality of life by providing habitats for a range of species (Bullock Citation2008; Savard et al. Citation2000). Ecological studies on epiphytes have been conducted in several urban settings across the globe (Fudali Citation2007, Citation2012; Richter et al. Citation2009; Adhikari et al. Citation2012). However, limited studies have examined epiphytes in South Africa (Zotz Citation2005), and no studies have examined epiphytic plants in an urban setting. Information on epiphytes is required in order to develop potential conservation and management strategies. Hence, this study was carried out to investigate diversity of epiphytes on host trees in the eThekwini Municipal Area (EMA), which will provide baseline information that could guide conservation and management of urban epiphyte communities.
Material and methods
Study area
The study was performed within the EMA from March to July 2012. Selected localities within the EMA, viz. Che Guevara Road (Moore), Clark Road, Helen Joseph Road, Florida Road, and Randles Road (), which have a notable presence of epiphytes, were investigated. The sites were based on the extensive field experience of one of the authors (YG). These sites have trees primarily planted and maintained by the eThekwini Municipality. Mean annual rainfall of the study area is between 1000 and 1250 mm. The mean annual temperature is 20.5°C with a variance of 8.3°C. The study region has a subtropical and humid climate, with rainfall mostly falling in the summer months. However, there is some form of precipitation in all seasons (Roberts Citation1993), which allows continuous plant growth throughout the year. The natural vegetation is a mosaic of coastal forest, savanna, grassland, thicket and mangroves (Roberts Citation1993). Municipal authorities manage trees along roadsides, parks and other green spaces (eThekwini Municipality Citation2007). It is important to note that there is no active planting of epiphytes on street or park trees within the municipality.
Table 1 Sites surveyed within the eThekwini Municipal Area (EMA) during March–July 2012.
Data collection and analysis
Visual searches were made for all epiphyte species on trees along public roads included in this study. The field survey was carried out using a ladder. Binoculars were used to detect epiphyte individuals occurring on distant branches. The number of host trees was recorded. The growth habit of all epiphytes and hosts (phorophtyes) was documented. Phorophytes with fewer than 20 individual trees across the entire study site were recorded, but excluded from further analysis, because some of these were difficult to sample with hampered access to the canopy for examination of epiphytes. Consequently, only nine frequent host species comprising 254 trees were found to be appropriate for sampling (i.e. with unhampered canopy assess for examination of epiphytes) (). The circumference at breast height (CBH, i.e. 1.37 m from the ground), height, bark characteristics and number of epiphyte species on phorophytes were recorded. The height of each host tree was measured with a clinometer, or estimated visually. Three different rugosity classes (smooth, medium and rough) were delimited based on the roughness of the host tree bark. All taxa were identified using published accounts and with the aid of herbarium material at the Ward Herbarium (UDW) and KwaZulu-Natal Herbarium (NH). The epiphytic taxa were catagorised into their typical life forms as well as epiphytic type. Epiphytes were divided into two types following Kersten et al. (Citation2009) namely specialist (found in only one species of host tree) and selective (frequently found on few species of host trees and rarely on other species).
Herbarium material and existing literature for all taxa surveyed (phorophytes and epiphytes of the present study) were checked in order to determine if epiphytes had a wider pool of host species in the study area. Records of epiphytism (both herbarium and literature) within and beyond the EMA were noted. These cases represent potential epiphyte–phrorphyte interactions of taxa known to occur within the EMA, but were not detected.
Results
Host taxa
Thirty-four phorophyte tree species from 15 families were observed. Twenty species were alien to South Africa, with 14 indigenous South African species (Appendix 1). Nine species were considered the most frequent phorophytes (i.e. housed more than 20 epiphytic individuals), and included Brachychiton acerifolium, Casuarina equisitifolia, Colvillea racemosa, Delonix regia, Jacaranda mimosifolia, Phoenix reclinata, Pleiogynium timorense, Raphia australis and Trichilia dregeana. The remaining 25 phorophyte species had a poor representation of epiphytes (fewer than 20 individuals).
Epiphytic taxa
Epiphyte species composition varied substantially among host species. A total of 30 epiphyte species from 12 families were recorded, with most epiphyte species belonging to the Moraceae (n = 8) and Araliaceae (n = 5). In terms of life forms, there were 17 trees, 2 shrubs and 11 herbs (forbs) for the epiphytic taxa. A total of 13 epiphytic species are found to be specialist (found on only one host species), whereas 17 are selective (epiphyte species found on more than one host species) (Appendix 1). Only 13 of the 30 epiphytic species observed were native to South Africa (, Appendix 1).
Table 2 List of the epiphyte species (n = 30) recorded during study.
In most cases, herbarium label data of both host and epiphyte taxa (recorded in this study) did not specify either host or epiphyte details. When combined with the taxa of the present study, the herbarium and literature survey (Appendix 2) revealed 52 phorophytes and 33 epiphytes.
In an effort to facilate reporting, major epiphytic taxa are grouped based on phylogenetic affinities: pteridophytes (ferns and allies), Moraceae (viz. Ficus), Araliaceae (viz. Schefflera) and Orchidaceae. Ferns are well known for epiphytism (Roux Citation2001). Stenochlaena tenuifolia is known to be epiphytic when mature (Roux Citation2001; Crouch et al. Citation2011) (see Appendix 2 for herbarium and literature records).The genus Platycerium is known to be epiphytic (Kreier & Schneider Citation2006). Platycerium bifurcatum is a mid- to high-level epiphyte sometimes observed on concrete bridges (Roux Citation2001; Crouch et al. Citation2011). It has escaped from cultivation and is naturalised in the greater Durban region (Crouch et al. Citation2011). Polystichum is known to be epilithic or epiphytic (Roux Citation2001). Polystichum minitum has been recorded as epiphytic (Williams & Sillett Citation2007). Pleopeltis macrocarpa is either epilithic or epiphytic (Roux Citation2001), and regarded by Crouch et al. (Citation2011) as one of the most widespread epiphytes in southern Africa. Rumohra adiantiformis is recorded as being terrestrial, epilithic or low level epiphytic (Roux Citation2001).
The genus Ficus (Moraceae) is well documented to be epiphytic (Burrows & Burrows Citation2003). Several South African figs are known to have a strangling habit. Representatives of the Ficus thonningii complex (F. rokko, F. psilopoga, F. persicifolia, F. petersii, F. burkei and F. natalensis) have a strangler/hemiepiphytic habit. Ficus lutea, F. polita and F. natalensis are hemiepiphytic or epiphytic at first before becoming free-standing trees (Burrows & Burrows Citation2003). Ficus burtt-davyi is known to be an occasional hemiepiphyte or strangler. Ficus bubu can be hemiepiphytic. Ficus benghalensis is not known to be epiphytic (Burrows & Burrows Citation2003). It is interesting to note that F. benghalensis can potentially be a host and epiphyte (Appendix 1). A detailed record of epiphytic figs from the herbarium records are provided in Appendix 2.
No herbarium records were found to suggest that Schefflera taxa are epiphytic within the EMA. However, Schefflera actinophylla has been observed to become a strangler in Durban. Several Schefflera taxa (S. abyssinica, S. goetzenii, S. myriantha) have an epiphytic habit (Cannon Citation1978). Schefflera actinophylla is a canopy epiphyte in the lowland rainforests of Viti Levu. This species can start out as an epiphyte but turn into a strangler (Keppel & Watling Citation2011). Both S. actinophylla and S. arboricola are sometimes hemiepiphytes (Tomlinson et al. Citation2005). Schefflera venulosa is known to be a climber (Wyatt-Smith Citation1953).
There are numerous epiphytic Orchidaceae species in South African including the EMA (Harrison Citation1981). Ansellia africana, Cyrtorchis arcuata and Mystacidium venosum were found in the study area. All three species are known to be epiphytic (Harrison Citation1981; Linder & Kurzweil Citation1999; McMurtry et al. Citation2008). No herbarium records were found within the EMA, however, several herbarium records were found for KwaZulu-Natal (Appendix 2). A summary of additional phorophytes of the of these orchids obtained from McMurtry et al. (Citation2008) is given in Appendix 2.
Literature checks were carried out on all the remaining epiphytes (both genera and species) found in this study, and are summarised below. Ageratum houstonianum is not epiphytic; however, Ageratum conyzoides is known to be epiphytic (Akinsoji Citation1990). Asystasia gangetica is reported to be an epiphyte (Akinsoji Citation1990). Chromolaena odorata has been described as a diffuse scrambling shrub (Hilliard Citation1977), and is reported to be an epiphyte (Akinsoji Citation1990). The following taxa (n = 11) recorded in this study were not previously known to be epiphytic: Ageratum houstonianum, Amaranthus tricolor, Arecastrum romanzoffiana, Cassia multijuga, Chrysophyllum oliviforme, Cussonia spicata, F. benghalensis, Livistona chinensis, Morus alba, S. venulosa and Solanum mauritianum.
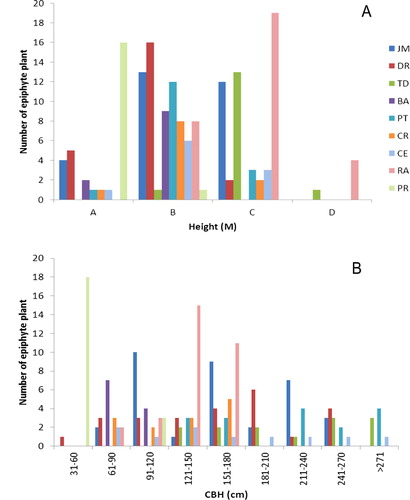
The specificity analysis among epiphyte and host trees revealed that 13 epiphytic species are specialist (epiphyte species found on only one host species), whereas 17 are selective (epiphyte species found on more than one host species) (Appendix 1). In our study area, most of the epiphyte species seem to grow on tree forks of the host tree (). Epiphyte diversity on host trees with different CBH and height classes are presented in . In general, most epiphytes were recorded on trees with a CBH of 61–180 cm (n = 92) and heights of 11–20 m (n = 74). ANCOVA showed no significant relationships between epiphyte diversity and tree CBH (P > 0.05) and height (P > 0.05). This suggests that host tree CBH and height are not suitable predictors of epiphyte diversity. However, the epiphyte diversity showed a significant relationship with host tree species (P < 0.05) ().

Discussion
The present study was conducted in an urban South African setting. Elsewhere, studies in urban settings mainly focused on epiphytic bryophytes and lichens (Fudali Citation2007, Citation2012; Richter et al. Citation2009). In total 34 host species were found, with 20 species being non-native to South Africa. Seventeen of the 30 epiphytes recorded were non-native to South Africa. The epiphytes associated with the highest number of phorophytes were F. natalensis, S. actinophylla, F. thonningii, F. lutea and Platycerium bifurcatum. Schefflera actinophylla and Platycerium bifurcatum are alien epiphytes.
The high number of alien epiphytes and phoropytes is concerning. Platycerium bifurcatum has become naturalised in Florida but is unlikely to become invasive there (Pemberton Citation2003). Schefflera actinophylla is of more concern. This species can start out as an epiphyte but turn into a strangler. There has been an expansion of this species in some urban areas of Fiji, and it may become problematic (Keppel & Watling Citation2011). Keppel & Watling (Citation2011) have also noted the concerning spread of Spathodea campanulata in Fiji, a species also found in the current study. Both native and non-native host species have almost the same number of epiphytes, including native and alien epiphytes. Our findings suggest that urban settings are promoting facilitative interactions of both alien phorophytes and epiphytes. These preliminary findings are indicative of a scenario supporting invasional meltdown (Simberloff & von Holle Citation1999) in phorophytes and epiphytes. At present, it is difficult to test this hypothesis further without additional sampling and rigous testing. Host–epiphytes interactions are poorly documented for native and alien South Africa taxa. Therefore, such data will increased the understanding of the role of epiphytism in biological invasions.
Epiphytes depend on their host tree mainly for support, whereby individual host trees provide the substrate for the establishment of epiphyte species. The successful establishment of epiphytes on their hosts depends on several host tree traits such as size, age, branch quality and bark texture (Lie et al. Citation2009; Bartels & Chen Citation2012). Brown (Citation1990) found that variations in microclimate and substrate diameter are the main factors responsible for epiphyte distribution patterns. Tree size and bark characteristics have been suggested to explain the presence of epiphytes on trees (Benzing Citation1990; Nieder et al. Citation2001; Cascante-Marin et al. Citation2009). It is generally expected that large adult trees should harbour more epiphytes than small hosts (Lopez-Villalobos et al. Citation2008). These results are based on studies conducted in different natural forest types and remnant forest patches. The ANCOVA results for epiphyte diversity and host tree characteristics (viz. tree CBH and height) did not show such relationships in the present study. Our results for this aspect are best treated as inclonclusive, possibly due to limited sampling. Brown (Citation1990) found that variations in microclimate and substrate diameter are the main factors responsible for epiphyte distribution patterns. It is generally expected that large adult trees should harbour more epiphytes than small hosts (Lopez-Villalobos et al. Citation2008). Hence, host attributes like CBH and height did not appear important determinants or predictors of epiphyte diversity in urban settings.
The greatest epiphyte richness in this study was found on the palm R. australis, which has smooth bark. In general, smooth surfaces of host trunks prevent seeds lodging directly on the bark, as well as humus or water accumulation and retention (Wyse & Burns Citation2011). Akinsoji (Citation1990) found that smooth-barked hosts (phorophytes) had poor epiphyte representation because they are unable to accumulate dust, debris and moisture for the germination and growth of the epiphytes. However, R. australis has persistent leaf stalks in a more or less rossete arrangement, that transverse almost the entire length of the main trunk. The trunk has numerous leaf joints that can trap debris and facilitate epiphyte establishment. This might explain why palms with a smooth bark host a high epiphyte richness. Thus, specific traits such as the architecture (e.g. R. australis) and microhabitat resource availability of hosts are presumed to be important in the establishment of resident epiphytes. The occurence and density of epiphytes are influenced by host characteristics (host bark, pH, water holding capacity of bark and bark rugosity), which play importent role in determining aggregation and abundance of epiphyte species (Adhikari Citation2013). It is also reported that some epiphyte species show a preference for particular host and host traits (Hirata et al. Citation2009). A more comprehensive study is required in wild host populations to confirm this assertion.
It is worth noting that figs are an important indigenous component of epiphytic diversity in the EMA. This is possibly due to the fact that many figs can have a scrambling habit making them capable of colonising a very wide variety of host taxa (Harrison et al. Citation2003). It seems probable the habitat preferences of hemiepiphytic figs in the present study reflects similar microsite conditions and requirements for germination and seedling establishment among host species (Harrison et al. Citation2003).
This study represents the first South African survey of epiphyte diversity in an urban setting, and recovered some interesting findings. There is a general paucity of data in the study area, perhaps because urban ecology is an emerging discipline in South Africa (Cilliers & Siebert Citation2012). More comprehensive documentation of epiphyte diversity on host trees within natural ranges is needed. This will help understand how epiphyte/host interactions in the urban landscape are established and maintained.
Acknowledgement
SG and AB thank UKZN for postdoctoral fellowships. YG thanks eThekwini Municipality PRC for support. HB and SR thank UKZN for research funding and support. Preshen Banwari is thanked for the map of the EMA.
References
- Adhikari YP 2013. Distribution pattern, micro-site conditions, host tree characteristics and utilization of epiphytic orchids in the central Himalayas. PhD thesis. München, Germany, Technical University of Munich.
- Adhikari YP, Fischer SH, Fischer A 2012. Host tree utilization by epiphytic orchids in different land-use intensities in Kathmandu Valley, Nepal. Plant Ecology 213: 1393–1412.
- Affeld K 2008. Spatial complexity and microclimatic responses of epiphyte communities and their invertebrate fauna in the canopy of northern rata (Metrosideros robusta A. Cunn.: Myrtaceae) on the West Coast of the South Island, New Zealand. PhD thesis. Christchurch, Lincoln University.
- Affeld K, Sullivan J, Worner SP, Didham RK 2008. Can spatial variation in epiphyte diversity and community structure be predicted from sampling vascular epiphytes alone? Journal of Biogeography 35: 2274–2288.
- Akinsoji A 1990. Studies on epiphytic flora of a tropical rain forest in Southwestern Nigeria I: the vascular epiphytes. Vegetatio 88: 87–92.
- Alvarenga LDP, Porto KC, De Oliveira J 2010. Habitat loss effects on spatial distribution of non-vascular epiphytes in a Brazilian Atlantic forest. Biodiversity and Conservation 19: 619–635.
- Bartels SF, Chen HYH 2012. Mechanisms regulating epiphytic plant diversity. Critical Reviews in Plant Sciences 31: 391–400.
- Batke S 2012. Epiphytes: a study of the history of forest canopy research. The Plymouth Student Scientist 5: 253–268.
- Benzing DH 1990. Vascular epiphytes. Cambridge, Cambridge University Press. 354 p.
- Benzing DH 1998. Vulnerabilities of tropical forests to climate change: the significance of resident epiphytes. Climatic Change 39: 519–540.
- Benzing DH 2004. Vascular epiphytes. In: Lowman MD, Rinker HB eds. Forest canopies. 2nd edition. London, Elsevier Academic Press. Pp. 175–211.
- Brown DA 1990. El epifitismo en las selvas montanas del Parque Nacional “El Rey” Argentina: Composición florística y padrón de distribución. Revista de Biologia Tropical 38: 155–166.
- Bullock CH 2008. Valuing urban green space: hypothetical alternatives and the status quo. Journal of Environmental Planning and Management 51: 15–35.
- Burns KC, Zotz G 2010. A hierarchical framework for investigating epiphyte assemblages: networks, meta-communities, and scale. Ecology 91: 377–385.
- Burrows J, Burrows S 2003. Figs of Southern and South-Central Africa. Hatfield, South Africa, Umdaus Press.
- Cannon JFM 1978. Araliaceae. In: Robert LE ed. Flora Zambesiaca. Volume 4. Glasgow, MacLehose and Co. Ltd. Pp. 62–632.
- Cardelús CL, Chazdon RL 2005. Inner-crown microenvironments of two emergent tree species in a lowland wet forest. Biotropica 37: 238–244.
- Cascante-Marin AJ, Von Mijenfeldt N, Hanneke MH, De Leeuwa JHD, Wolf J, Oostermeijera GB et al. 2009. Dispersal limitation in epiphytic bromeliad communities in a Costa Rican fragmented Montane landscape. Journal of Tropical Ecology 25: 63–73.
- Cilliers SS, Siebert J 2012. Urban ecology in Cape Town: South African comparisons and reflections. Ecology and Society 17: 33.
- Crouch NR, Klopper RR, Burrows JE, Burrows SM 2011. Ferns of Southern Africa, a comprehensive guide. Cape Town, South Africa, Struik Nature.
- Cummings J, Martin M, Rogers A 2006. Quantifying the abundance of four large epiphytic fern species in remnant complex notophyll vine forest on the Atherton Tableland, north Queensland, Australia. Cunninghamia 9: 521–527.
- Díaz IA, Sieving KE, Pena-Foxon M, Armesto JJ 2012. A field experiment links forest structure and biodiversity: epiphytes enhance canopy invertebrates in Chilean forests. Ecosphere 3: 1–17. 10.1890/ES11-00168.1
- Ellis CJ, Coppins BJ 2007. 19th century woodland structure controls stand-scale epiphyte diversity in present-day Scotland. Diversity and Distributions 13: 84–91.
- Ethekwini Municipality 2007. Durban biodiverity report. Durban, South Africa, eThekwini Municipality.
- Fernandez Monteiro JA, Zotz G, Körner C 2009. Tropical epiphytes in a CO2-rich atmosphere. Acta Oecologica 35: 60–68.
- Freiberg M 1996. Spatial distribution of vascular epiphytes on three emergent canopy trees in French Guiana. Biotropica 28: 345–355. 10.2307/2389198
- Fudali E 2007. Bryophyte dynamic tendencies in urban parks—a case study of Wrocław. Annales Silesiae 35: 11–20.
- Fudali E 2012. Recent tendencies in distribution of epiphytic bryophytes in urban areas: a Wrocław case study (south-west Poland). Polish Botanical Journal 57: 231–241.
- Gentry AH, Dodson CH 1987. Diversity and biogeography of Neotropical vascular epiphytes. Annals of the Missouri Botanical Garden 74: 205–233.
- Gradstein SR 2008. Epiphytes of tropical montane forests—impact of deforestation and climate change. Biodiversity Ecological Series 2: 51–65.
- Harrison ER 1981. Epiphytic orchids of Southern Africa. Durban, South Africa, Wildlife Society of Southern Africa.
- Harrison RD, Hamid AA, Kenta T, Lafrankie J, Lee HS, Nagamasu H et al. 2003. The diversity of hemi-epiphytic figs (Ficus; Moraceae) in a Bornean lowland rain forest. Biological Journal of the Linnean Society 78: 439–455.
- Hilliard OM 1977. Compositae in Natal. Pietermaritzberg, University of Natal Press.
- Hirata A, Kamijo T, Saito S 2009. Host trait preferences and distribution of vascular epiphytes in a warm-temperate forest. Plant Ecology 201: 247–254.
- Hofstede RGM, Dickinson KJM, Mark AF 2001. Distribution, abundance and biomass of epiphyte-lianoid communities in a New Zealand lowland Nothofagus-podocarp temperate rain forest: tropical comparisons. Journal of Biogeography 28: 1033–1049.
- Jovan S, McCune B 2006. Using epiphytic macrolichen communities for biomonitoring ammonia in forests of the greater Sierra Nevada, California. Water, Air, & Soil Pollution 170: 69–93.
- Keppel G, Watling D 2011. Ticking time bombs—current and potential future impacts of four invasive plant species on the biodiversity of lowland tropical rainforests in south-east Viti Levu, Fiji. The South Pacific Journal of Natural and Applied Sciences 29: 43–45.
- Kersten RA, Borgo M, Silva SM 2009. Diversity and distribution of vascular epiphytes in an insular Brazilian coastal forest. Revista de Biologia Tropical 57: 749–759.
- Kreier HP, Schneider H 2006. Phylogeny and biogeography of the staghorn fern genus Platycerium (Polypodiaceae, Polypodiidae). American Journal of Botany 93: 217–225.
- Kress WJ 1989. The systematic distribution of vascular epiphytes. In: Lüttge U ed. Vascular plants as epiphytes, evolution and ecophysiology. Ecological studies. Heidelberg, Springer Verlag. Pp. 234–261.
- Larreaa ML, Werner FA 2010. Response of vascular epiphyte diversity to different land-use intensities in a neotropical montane wet forest. Forest Ecology and Management 260: 1950–1955.
- Laube S, Zotz G 2006. Neither host-specific nor random: vascular epiphytes on three tree species in a Panamanian lowland forest. Annals of Botany 97: 1103–1114.
- Lie MH, Arup U, Grytnes JA, Ohlson M 2009. The importance of host tree age, size and growth rate as determinants of epiphytic lichen diversity in boreal spruce forests. Biodiversity and Conservation 18: 3579–3596.
- Linder HP, Kurzweil H 1999. Orchids of Southern Africa. Rotterdam, AA Balkema Publishers.
- Lopez-Villalobos A, Flores-Palacios A, Ortiz-Pulido R 2008. The relationship between bark peeling rate and the distribution and mortality of two epiphyte species. Plant Ecology 198: 265–274.
- Lowman MD, Rinker HB 2004. Forest canopies. 2nd edition. London, Elsevier Academic Press.
- Madison M 1977. Vascular epiphytes: their systematic occurrence and salient features. Selbyana 2: 1–13.
- Male TD, Roberts GE 2005. Host associations of the strangler fig Ficus watkinsiana in a subtropical Queensland rain forest. Austral Ecology 30: 229–236.
- McMurtry D, Grobler L, Grobler J, Burns, S 2008. Field guide to the orchids of Northern South Africa and Swaziland. Hatfield, Umdaus Press.
- Nadkarni NM 1984. Biomass and mineral capital of epiphytes in an Acer macrophyllum community of a temperate moist coniferous forest, Olympic Peninsula, Washington State. Canadian Journal of Botany 62: 2223–2228.
- Nieder J, Prosperi J, Michaloud G 2001. Epiphytes and their contribution to canopy diversity. Plant Ecology 153: 51–63.
- Pemberton RW 2003. The common staghorn fern, Platycerium bifurcatum, naturalizes in Southern Florida. American Fern Journal 93: 203–206.
- Richter S, Schütze P, Bruelheide H 2009. Modelling epiphytic bryophyte vegetation in a urban landscape. Journal of Bryology 31: 159–168.
- Roberts DC 1993. The vegetation ecology of municipal Durban, Natal. Floristic classification. Bothalia 23: 271–326.
- Roux JP 2001. Conspectus of southern African Pteridophyta. South African Botanical Diversity Network Report No. 13. Pretoria, South Africa, SABONET.
- Savard JPL, Clergeau P, Mennechez G 2000. Biodiversity concepts and urban ecosystems. Landscape and Urban Planning 48: 131–142.
- Schuettpelz E, Trapnell DW 2006. Exceptional epiphyte diversity on a single tree in Costa Rica. Selbyana 27: 65–71.
- Simberloff D, Von Holle B 1999. Positive interactions of nonindigenous species: invasional meltdown? Biological Invasions 1: 21–32.
- Tomlinson PB, Fisher JB, Halle F, Villalobos I 2005. Development of woody branch attachments in Schefflera (Araliaceae or Apiaceae). American Journal of Botany 92: 1765–1773.
- Vanderpoorten A, Sotiaux A, Engels P 2004. Trends in diversity and abundance of obligate epiphytic bryophytes in a highly managed landscape. Ecography 27: 1–10.
- Werner FA, Gradstein SR 2009. Diversity of dry forest epiphytes along a gradient of human disturbance in the tropical Andes. Journal of Vegetation Science 20: 59–68.
- Williams CB, Sillett SC 2007. Epiphyte communities on redwood (Sequoia sempervirens) in northwestern California. The Bryologist 110: 420–452.
- Woods C 2013. Factors influencing the distribution and structure of tropical vascular epiphyte communities at multiple scales. PhD thesis. Clemson, SC, Clemson University.
- Wyatt-Smith J 1953. The vegetation of Jarak Island, Straits of Malacca. Journal of Ecology 41: 207–225.
- Wyse S, Burns B 2011. Do host bark traits influence trunk epiphyte communities? New Zealand Journal of Ecology 35: 296–301.
- Zotz G 2005. Vascular epiphytes in the temperate zones—a review. Plant Ecology 176: 173–183.
