Abstract
The nature, value and relative importance of different lines of evidence for deciding the circumscription and rank of genera are examined and discussed. First, I argue that the circumscriptions of genera (and higher taxa) should always be monophyletic, preferably with strong support from independent evidence. Second, the rank of genus is decided on more subjective grounds, but several criteria are helpful: phylogeny should be recoverable from the classification hierarchy, genera should be distinctive, ranks should not be redundant and familiar names should be retained if other criteria are met. I then apply these criteria to an evaluation of the circumscription and rank of 59 New Zealand endemic genera of seed plants accepted in two recent checklists. Fifteen genera (25.4%) were rejected and 16 (27.1%) are considered equivocal. However, my lower estimate almost certainly underestimates endemism at genus rank because the circumscriptions of several genera not evaluated here, such as Pachycladon, could change in the future to become endemic. One new combination is made: Sonchus novae-zelandiae (Hook.f.) Garn.-Jones, although work in progress by others is likely to lead to several more.
I am fully convinced that species are not immutable; but that those belonging to what are called the same genera are lineal descendants of some other and generally extinct species, in the same manner as the acknowledged varieties of any one species are the descendants of that species.
Introduction
In the 21 years since Chase et al. (Citation1993) provided the first molecular phylogeny of a large sample of flowering plants, a major reorganisation of their classification at family rank and above has been undertaken. This has largely stabilised as the APG classification (APG III Citation2009). In New Zealand, most major herbaria and databases have followed this international research (e.g. Breitwieser et al. Citation2012). Now, as DNA sequencing and phylogenetic analysis have become commonplace, attention has turned to classification at generic rank. In New Zealand, the classification of genera, and especially those that are endemic, is likely to be a focus of molecular systematics research for at least the near future.
However, in spite of the wealth of data from DNA sequencing and the relative rigour of cladistic analyses and classifications, disagreement remains about particular classifications. Competing solutions to taxonomic questions point to an evident problem for taxonomy. Differing conclusions may arise when taxonomists apply differing criteria, when they emphasise different lines of evidence or when they are not explicit about the nature and application of their evidence. Objectivity is only possible when hypotheses can potentially be falsified. Taxonomic hypotheses can be falsified only by measuring how well they accord with known facts, whether these relate to relationships or to similarity. Practitioners should at least be explicit about their methods, results and conclusions (Goldacre Citation2008). Where appropriate evidence is not presented explicitly, the conclusions drawn should be regarded as unsupported and their acceptance withheld.
In spite of the difficulties, taxonomy has been progressive in rejecting subjectivity, assertion and authoritarianism in classifications (Hennig Citation1950, Citation1966; Sokal & Sneath Citation1963; Wiley Citation1975, Citation1981; Hamilton & Wheeler Citation2008), but this progress has been incremental and is still not complete. Many authors have proposed objective criteria for scientific classifications (e.g. Hennig Citation1950, Citation1966; Wiley Citation1981; Entwisle & Weston Citation2005; Hopper Citation2009; Vences et al. Citation2013).
Goals of classification
The classification of plant species into higher ranked taxa has largely been guided by two goals that are not necessarily complimentary: natural classifications and stable classifications.
Natural classifications
Many taxonomists have sought to produce classifications that are natural, that is, classifications that reflect something real from nature (reviewed by Judd et al. Citation2008). Following Darwin (Citation1859), this has increasingly come to mean classifications that accord with evolutionary history. Natural classifications are hierarchical because of the evolutionary process, and classifications that reflect that hierarchy can be interpreted to add valuable meaning and predictions. Insofar as a classification system reflects evolutionary history, taxonomy provides a framework for the interpretation of comparative biology. Recently, rapid progress in molecular phylogenetics has led to many changes in classifications, so that classifications have arguably become more natural, but at the cost of stability, at least in the short term.
The approach to natural classifications has moved from intuitive methods, through phenetic analyses to phylogenetics. But even when phylogenies are considered the basis of natural taxonomies, there is disagreement among practitioners about whether paraphyletic groups are or are not acceptable (e.g. evolutionary vs. cladistic classifications).
Stable classifications
Both taxonomists and users of taxonomic information require plant names to be stable (e.g. Brickell et al. Citation2008). Changes in classification at the rank of genus directly affect the binomial, which can have legal and economic costs for users, in addition to the inconvenience for non-taxonomists needing to keep up with the taxonomic literature.
Conflict between naturalness and stability cannot be resolved by agreeing to abandon either. However, I believe that natural classifications based on sound evidence will increase stability in the longer term. This is borne out by the three (so far) iterations of the APG classification, where changes at the family rank in APG III (Citation2009) are relatively minor. Thus, stability is served when classifications are firmly and explicitly based on evidence, and when there is general agreement on the kinds of evidence that are most informative.
Scope of this review
In this review, I consider evidence-based objective and subjective approaches to generic classification and apply these to a consideration of the current status of New Zealand's endemic seed plant genera. In part 1, I discuss the value of several lines of evidence as they relate to common criteria for genus-level classifications, focusing on their value to decisions about both circumscription and rank. In part 2, I apply the most valuable criteria to an evaluation of the evidence for acceptance of the 50–60 New Zealand seed plant genera that are currently recognised as endemic (de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012).
Although classification criteria can be applied at all ranks, my focus is on the rank of genus, for three reasons. First, the genus is the highest rank that many individual taxonomists are able to research because taxa at family level and above often require research that is world-wide in scope, involving international collaboration and often big budgets. Second, genera are especially important because the genus is the only rank above species that affects the binomial. Thus generic names are also a shorthand way of indicating low-level relationships and, conversely, changes in generic circumscription affect nomenclature. Third, genus is often the rank at which ecological and evolutionary comparisons are conducted (e.g. Webb et al. Citation1999; McGlone et al. Citation2001; Lee et al. Citation2011), so that criteria for taxonomic decisions about genera can have major effects on research findings and on dependent policy (such as conservation priorities). Humphries & Linder (Citation2009) argue that the genus may be the most important rank ‘to get right’.
In addition, although I briefly discuss the advantages of monophyletic classifications and mention the Phylocode, these topics are discussed in detail elsewhere.
Part 1. Principles of generic classification
Properties of taxa
In biological classifications, taxa have two fundamental properties: circumscription and rank. Taxonomists who circumscribe narrowly or who tend to raise taxa to higher ranks may be characterised as ‘splitters’, whereas those who circumscribe broadly or tend to reduce the ranks of groups may be characterised as ‘lumpers’ (de Queiroz & Gauthier Citation1994; Endersby Citation2009).
The principles of classification have been widely discussed throughout history (reviewed by Judd et al. Citation2008). Here, I refer mostly to three recent papers (Entwisle & Weston Citation2005; Hopper Citation2009; Vences et al. Citation2013) that set out criteria for the taxonomic recognition of genera and higher taxa, some of which are objective, but others of which are subjective. Entwisle & Weston (Citation2005) reviewed criteria for taxonomic recognition with the aim of providing a single preferred classification of Australian plants through the Australian Virtual Herbarium (AVH). Following consultation and discussion within the Australian botanical community, they arrived at a set of recommended guidelines, of which the paramount one was that taxa should be monophyletic. Hopper (Citation2009) considered these questions with special reference to recent ‘taxonomic turmoil’ in Australasian orchids. Vences et al. (Citation2013) discussed criteria for Linnaean classifications in an attempt to limit changes within a consistent and cladistic framework; although their examples were mostly drawn from herpetology their conclusions are widely applicable.
These three papers are largely in agreement in concluding that all groups recognised should primarily be monophyletic, and then setting out a number of secondary, and more subjective, criteria for deciding which of many clades to name and rank. Here, I organise these thoughts under three headings: evidence for deciding (1) group circumscriptions, (2) formal taxonomic recognition of circumscribed groups and (3) ranks of recognised groups.
Criteria for deciding taxon circumscription
Species and higher ranks differ in the criteria used for their circumscription. Speciation is the point at which net-like relationships among individuals in a panmictic population, or panmictic populations in a metapopulation, give way to (mostly) branching relationships of species in a phylogeny. While genetic evidence about the circumscription of species falls largely in the domain of population genetics, evidence about higher taxa falls in the domain of phylogenetics.
Circumscription of genera and higher taxa concerns which species belong to a group and which are excluded. A group may be circumscribed by (1) listing the component individuals or subordinate taxa, (2) specifying some unique characteristic that members display (a character-based definition) or (3) defining relationships at its basal node or in relation to their most recent common ancestor (node- and stem-based definitions) (de Queiroz & Gauthier Citation1994).
Most modern botanists would agree that members of a taxon should be closely related to each other (Judd et al. Citation2008). However, there is disagreement about whether such within-group relationships should always be closer than any relationships to species outside the group (Brummitt Citation2002, Citation2006; Wiley Citation2009; Hörandl & Stuessy Citation2010; Schmidt-Lebuhn Citation2011).
Willi Hennig (Citation1950, Citation1966) was the first to recognise that the only evidence for historical relationships between species is provided by synapomorphies, uniquely derived character states that are shared by related species. Then, having provided a method by which clades of historically related species could be discovered, Hennig proposed that only groups that are circumscribed to be congruent with clades (monophyletic taxa) should be recognised in taxonomic classifications. Hennig's view that only synapomorphies are informative in systematics (Hennig Citation1950, Citation1966; Wiley Citation1981) contrasts with the phenetic approach of using overall differences and similarities—the full extent of character-state differences between groups—which can be misleading about relationships (Wiley Citation1981).
Ever since Hennig (Citation1950, Citation1966), the idea that taxonomic groups should be circumscribed so as to be monophyletic has been controversial, in that arguments have been raised against it, sometimes questioning the requirement for monophyly itself (Cronquist Citation1987; Brummitt Citation2002, Citation2006), and sometimes in reference to inconvenient nomenclatural changes that may result from its implementation (e.g. Brickell et al. Citation2008).
Practical importance of monophyly
Users of taxonomy are likely to assume that members of a named group, particularly a genus, are more closely related to each other than they are to members of another group. For example, the popular ecology software Phylomatic assumes that genera are monophyletic (Webb & Donoghue Citation2005). Thus, it is a desirable property of taxa that every member should be more closely related to every other member than any is to any non-member. This desirable and useful property is found only in monophyletic groups (clades).
Although alternative meanings of the term monophyly have been proposed by some (e.g. Ashlock Citation1971; Hörandl & Stuessy Citation2010), most systematists tend to follow Hennig (Citation1950, Citation1966; Wiley Citation1981) who defined a monophyletic group as comprising an ancestral species and all of its descendants. Hennig (Citation1966) also provided, more pragmatically, a clear statement of what monophyly means for a classification. In general terms, this can be restated: for any two monophyletic genera, A and B, every trio of species with at least one from each genus will demonstrate a three-taxon relationship of either ((A,A)B) or (A(B,B)). Such underlying relationships are historical, real and potentially discoverable facts.
Non-monophyletic taxa can seriously skew inferences drawn from classifications, because at least some of their relationships will be of the form ((A,B)A). Restricting taxonomic recognition to only monophyletic groups produces classifications that represent evolutionary history.
Using monophyly as a criterion for questions about circumscription has an additional advantage: monophyly is a hypothesis that can objectively be tested. Although the merits of different data sets (e.g. morphology, nuclear vs. chloroplast DNA, secondary metabolites) and the kinds of phylogenetic analyses (parsimony, likelihood, Bayesian) are debated fully elsewhere (e.g. Shaw et al. Citation2005; Hughes et al. Citation2006; Joly et al. Citation2009; Leaché & Rannala Citation2011; Straub et al. Citation2012), it is sufficient to note here that any data, considered in a phylogenetic context and capable of falsifying a phylogenetic hypothesis, are useful. Because of this objectivity and its explicit linking of systematics with evolution, many botanists now consider monophyly (sensu Hennig Citation1966) to be an essential property of higher taxa (Entwisle & Weston Citation2005; Hopper Citation2009; Schmidt-Lebuhn Citation2011).
Vences et al. (Citation2013) argued additionally that monophyletic groups should have a stronger case to be recognised and named if they have clade stability, most likely in clades that have strong, independent, and congruent support from several lines of evidence. Some data sets (e.g. nuclear DNA vs. chloroplast DNA vs. morphology) can give conflicting signals about relationships (e.g. in Anemanthele [Poaceae] and Lophomyrtus [Myrtaceae], see below). Such conflict can be informative if evolution has been reticulate (e.g. in Poaceae subf. Danthonioideae, see Linder et al. Citation2010), or it may indicate that taxonomic conclusions should not be drawn until conflicts are resolved. In either case, uncritical a priori privileging of one data set over another is unwarranted.
However, because there is only one correct solution to the question of the history of life, it is theoretically possible to determine the phylogeny and test the monophyly of all taxa. In practice, it seems unlikely at present that we can confidently discover that solution exactly. Phylogenetic analysis of DNA sequence data is a theoretically sound approach that has also been shown empirically to be powerful in spite of difficulties associated with detecting reticulate evolution, potential lack of congruence among gene trees, the inference of species trees, incomplete lineage sorting, insufficient time for evolution of informative differences, and too much time so that informative differences are obliterated. Because many of these difficulties are especially relevant to studies of the New Zealand flora, some critics suggest the requirement for monophyly may be impractical (e.g. de Lange & Rolfe Citation2010) or dogmatic (de Lange Citation2011a). Nevertheless, in spite of many potential sources of error in tree-building, tree topologies—including reticulate ones—are testable hypotheses, so that errors in topology, branch lengths and support values can be discovered and corrected. Furthermore, improvements to gene and even genome sequencing and sampling, rapid sequencing, new analytical methods and computing are continually improving the power, speed and resolution of analyses (Soltis et al. Citation2011). Already there are many deep and shallow, species-rich and species-poor branches on the tree of life that we can recognise as monophyletic taxa with a high degree of confidence (e.g. angiosperms, Onagraceae, Fuchsia).
Non-monophyletic taxa
By recognising in formal classifications only those groups that are monophyletic, taxonomists avoid naming groups from which incorrect or misleading relationships could be inferred. In such non-monophyletic groups, some group members will be more closely related to non-members than they are to other (named) group members (). Non-monophyletic groupings are of two general kinds.
First, polyphyletic groups are those that occur when distantly related but similar organisms are classified together due to a misinterpretation of the origins of their similarity. Typically, character states initially assumed to be homologous may be found to appear on multiple lineages and to be the result of convergent or parallel evolution. The dismantling and reclassification of polyphyletic groups to restore monophyly is generally non-controversial. For example, Pseudopanax (Araliaceae) as circumscribed by Philipson (Citation1965) was shown to be polyphyletic by Mitchell & Wagstaff (Citation1997) leading to the segregation and reinstatement of Raukaua (Mitchell et al. Citation1997).
Second, paraphyletic groups occur when a distinctive subclade is excluded based on its differences and in spite of its relationships. The logic of rejecting paraphyletic groups has been widely and largely uncontroversially applied in recent New Zealand research (e.g. Heenan Citation1998c, Citation2002, Citation2007, Citation2012; Heenan et al. Citation2002, Citation2008; Wagstaff & Wege Citation2002; Garnock-Jones et al. Citation2007; de Lange Citation2012). In most examples, a subclade had been segregated at the same rank from within a more inclusive clade in order to recognise its distinctiveness, leaving the name of the original clade to apply only to a paraphyletic residue. Thus the distinctive Neopaxia australasica (Montiaceae) was segregated from Montia on the basis of its morphological distinctness (Nilsson Citation1966; McNeill Citation1975). Later this monotypic genus was greatly enlarged by the description of seven new species (Heenan Citation1999a), and when shown by O'Quinn & Hufford (Citation2005) to be nested within Montia all species were returned to Montia (Heenan Citation2007).
Similarly, Lignocarpa and Scandia (Apiaceae) were segregated from Anisotome by Dawson (Citation1961, Citation1967) on the basis of distinctive morphological differences. There is little doubt that these segregate genera are distinctive and monophyletic, but if they render Anisotome paraphyletic (Mitchell et al. Citation1998; Radford et al. Citation2001; Spalik et al. Citation2010; but see Nicolas & Plunkett Citation2009) their status should be reconsidered (see below).
In other examples, newly discovered species have been described in new genera, although some have been later found to belong to clades that already have names at genus rank. Thus, Garnock-Jones & Johnson (Citation1987) proposed a new genus, Iti, for a newly discovered plant in Brassicaceae because they could find no synapomorphies that would place it in a known genus. Although they noted similarities to Cardamine, they excluded it from that genus because of its incumbent radicle and absence of rapidly coiling silique valves. Using explicitly phylogenetic methods and molecular data, Mitchell & Heenan (Citation2000) and Heenan (Citation2002) showed that Iti is nested within Cardamine (now as Cardamine lacustris) and has presumably secondarily lost the explosive fruit dehiscence (Vaughn et al. Citation2011) characteristic of that genus. Similarly, Carmichaelia (Fabaceae) has been enlarged by the inclusion of Chordospartium, Corallospartium and Notospartium (Heenan Citation1998a,Citationc; Wagstaff et al. Citation1999) because excluding them rendered its circumscription paraphyletic. In both these examples, the influential morphological characters were related to functional changes to fruit dehiscence.
When a group is recognised to be paraphyletic, cladistic taxonomists can address it in classifications in two ways. Either the paraphyletic group can be enlarged to include the excluded subclade, or it can be dismembered into smaller monophyletic groups. This is a question of rank, rather than circumscription, and is addressed below.
Methodologically, the accidental recognition of groups that are paraphyletic is a potential consequence of phenetic analyses, that is, those analyses that estimate relationship based on an assessment of overall similarity. This is so regardless of whether phenetic analysis is carried out intuitively or by an algorithm. Pioneering pheneticists (e.g. Sokal & Sneath Citation1963) sought explicit and objective methods for classification. Many initially considered that quantifying overall similarity was an effective way to infer phylogenetic relationships, and this was generally thought achievable if sufficient morphological or genetic characters were analysed. Hennig (Citation1966) and Wiley (Citation1981) demonstrated the difficulty of inferring historical relationships through overall similarity, by showing that in phenetic classifications symplesiomorphies are as influential as synapomorphies, and they make likely the inadvertent recognition of groups that turn out to be paraphyletic.
Arguments for paraphyly
The proposition that taxa above species rank should always be monophyletic is widely but not universally accepted in the botanical community (for dissenting views see, e.g. Cronquist Citation1987; Brummitt Citation2002, Citation2006; Brickell et al. Citation2008; de Lange & Rolfe Citation2010; Hörandl & Stuessy Citation2010).
During the twentieth century, many, often small, new genera were segregated from large and variable genera, for example, in Bellis and Crepis (Asteraceae) (Humphries & Linder Citation2009). These were usually delimited on the basis of morphological distinctiveness, and with hindsight this justification can be seen to have been largely phenetic. However, the advent of phylogenetic analysis has shown that commonly such distinctive clades are nested within larger and also distinctive grades, such as Embergeria and Kirkianella within Sonchus (Asteraceae) (Kim et al. Citation2007). In such cases, the distinctiveness of the basal grade results in part from symplesiomorphy, relative to the apomorphies of the segregated genus. Many recent studies of New Zealand plants (e.g. Heenan Citation1998c, Citation2007; Chung Citation2007; Wanke et al. Citation2007) have rejected generic status for such distinctive subclades and merged them with the larger clades within which they have originated.
In spite of widespread acceptance of a requirement for taxa to be monophyletic, proponents of paraphyletic taxa in classifications (e.g. Cronquist Citation1987; Brummitt Citation2002, Citation2006; Hörandl & Stuessy Citation2010) argue that grades may be meaningful, even natural, because they represent stages in evolution that may be morphologically similar and occupy similar habitats or geographic areas. Schmidt-Lebuhn (Citation2011) provided a detailed rebuttal of such arguments for taxonomic recognition of paraphyletic groups.
Further, if non-monophyletic groups are sometimes acceptable, are there any general and objective principles that could help us objectively to decide which ones to accept and name? Stuessy & König (Citation2008) and Hörandl & Stuessy (Citation2010) advocate patrocladistics, a system that uses phenetic distances and cladistic branching trees to derive classifications (see Wiley Citation2009 for rebuttal). It seems that some arbitrary amount of phenetic difference could establish a cut-off point, such that decisions might be supported by one data set but not by others, which could be a particular difficulty in fast-evolving island floras where morphological change appears to have proceeded faster than DNA sequence evolution (Winkworth et al. Citation2002a).
Criteria for deciding which clades to name
Once robustly supported clades have been discovered, an additional concern in a nomenclatural system with a limited number of available ranks is deciding which of them should be formally named. In any flow diagram or decision chart (e.g. Vences et al. Citation2013) this decision must follow circumscription of clades and precede rank assignment and naming.
Recognition
It is practical to expect named taxa to be distinctive, or at least diagnosable. This is particularly important to field biologists who might encounter organisms that they do not immediately recognise. At present, members of groups recognised only by distinctive DNA sequences cannot be identified in the field, although technology might enable this in future.
Naming only groups that are morphologically distinctive is subjective, because there are no explicit criteria for deciding which clades are distinctive enough to be named. Further, distinctiveness can be applied only after clades have been circumscribed, because it otherwise introduces the risk of recognising paraphyletic taxa (groups recognised by symplesiomorphies). For example, Norton & Molloy (Citation2009) and de Lange & Rolfe (Citation2010) argued that including southern segregates in a broad circumscription of Veronica (Plantaginaceae) (Garnock-Jones et al. Citation2007) does not draw attention to well-marked clades and does not recognise the distinctive characters that distinguish the Hebe group, and particularly Heliohebe, from a paraphyletic northern circumscription of Veronica. In this case, recognising well-marked clades as genera also dictates either the recognition at the same rank of clades that are not well-marked (e.g. Veronica clades I–IV of Albach & Chase Citation2001), or the recognition of a mostly well-marked grade (the standard 1929–2007 circumscription of Veronica). Further, if such phenetic criteria were applied strictly, it would likely indicate the separation of a number of plesiomorphic New Zealand and Australian (Oliver Citation1944; Albach & Briggs Citation2012) species from their nearest relatives. As an additional complication, however, the generic segregates of the Hebe complex are mostly neither monophyletic nor unambiguously well-marked (Garnock-Jones & Albach Citation2007); the exceptions are the sun hebe clade (Garnock-Jones Citation1993b, as Heliohebe) and the semi-whipcord hebe clade (Bayly & Kellow Citation2006, as Leonohebe).
Although it is desirable that recognised groups be distinctive, it does not necessarily make sense that distinctive groups should always be named, especially if their sister groups are not distinctive (Hopper Citation2009).
Biogeography and ecology
Vences et al. (Citation2013) discussed the desirability of groupings that have a geographically coherent distribution or inhabit a similar environment. They regarded these criteria as ambiguous and difficult to apply. In addition, they should not over-ride a requirement for monophyly; for example, Mark (Citation2012) and Norton & Molloy (Citation2009) were influenced by their southern distribution in recognising Hebe and segregate genera as separate from a paraphyletic circumscription of Veronica.
International perspective
In New Zealand, high species endemism means that most taxonomic research at species rank can be conducted with little reference to the rest of the world, but independence becomes increasingly problematic at higher ranks, especially at family rank and above where no groups are endemic. At genus rank, New Zealand botanists should inform their decisions by considering plants from Australia, the Pacific Islands, Southeast Asia and further abroad. To this end, a DSIR–CSIRO colloquium in 1983 (Department of Scientific and Industrial Research Citation1987) led to increased international collaboration, particularly with Australia, and more multi-author research. Such collaboration and the improved availability of herbarium loans, international travel and electronic communication have led to taxonomists increasingly interpreting the New Zealand flora in relation to relatives overseas (e.g. Cross et al. Citation2002; Heenan et al. Citation2002; Garnock-Jones et al. Citation2007; Pelser et al. Citation2007; Knox et al. Citation2008; Linder et al. Citation2010; Smissen et al. Citation2011; Woo et al. Citation2011). It seems likely that studies conducted overseas will increasingly include New Zealand samples, and improved sampling generally will allow identification of the close international relatives of New Zealand plants (e.g. Barker et al. Citation2007a; Chung Citation2007; Kim et al. Citation2007; Wanke et al. Citation2007; Nazaire & Hufford Citation2012; Nicolas & Plunkett Citation2012).
Criteria for assigning ranks to recognised taxa
The rank of a taxon concerns where it is placed in a hierarchical system. Although the circumscription of monophyletic groups is consistent, objective and testable, assigning rank to a circumscribed group is for the most part subjective (Entwisle & Weston Citation2005; Hopper Citation2009). The subjectivity of ranks has led to proposals for unranked systems of nomenclature (brief outline and key references were presented by Laurin & Bryant Citation2009), which are outside the scope of this review.
Constraints of hierarchical classifications
First, the traditional codes of nomenclature, excluding the rankless Phylocode (de Queiroz & Gauthier Citation1994; Laurin & Bryant Citation2009) (e.g. the International Code of Nomenclature for Algae, Fungi, and Plants [ICN], McNeill et al. Citation2012), require ranks to be hierarchical; this places some objective constraints on their use. For instance, higher ranks cannot be less inclusive than included lower ones (e.g. families within a genus would not be allowed). That requirement rules out some classification options, but does little to guide the application of ranks and names to clades. Nevertheless, the genus, as a rank between species and family in the classification hierarchy, is constrained by the application of those higher and lower ranks if taxa are to be monophyletic. Thus, Notospartium (Fabaceae) could not be recognised as a monophyletic genus within the genus Carmichaelia, but could take a lower rank, for example, as a section, so long as all other sections were also monophyletic. Under the Phylocode, however, Notospartium could be a clade included within a monophyletic circumscription of Carmichaelia because neither clade would be assigned a rank.
Second, sister taxa ideally ought to be accorded the same rank (Hennig Citation1950, Citation1966) to recognise their common origin. This quickly becomes problematical if applied to a bifurcating tree because many more ranks are needed than are available if every node is named, so pragmatic solutions must be sought (Wiley Citation1981), such as assigning the same rank (e.g. genus) to each of the major subclades of a larger clade (e.g. a family) even when they do not all originate from nodes at the same level in the tree.
Rank redundancy
While adherence to the notion of naming every clade in a phylogenetic classification may be impractical, classifications ought to maximise information content such that a classification reflects phylogeny as closely as possible and never misrepresents it (Nelson Citation1973). In addition, redundant ranks should be avoided where possible. Ranks are redundant when two taxa exist that have identical circumscription but different ranks (e.g. Nothofagus s.l. and Nothofagaceae). The elimination of redundant ranks can improve the information content of a classification. Monospecific genera and families convey no relationship information, although they might sometimes be unavoidable (Entwisle & Weston Citation2005). For example, when two sister genera are monospecific and classified together with other genera in a larger family, like Manoao and Lagarostrobos within Podocarpaceae, there is no rank in the classification hierarchy that represents their relationship (Kelch Citation2002). The alternative, recognising these two sister species within one genus Lagarostrobos, is more informative, even though, as Molloy (Citation1995) has shown, there are distinctive morphological differences between them.
Hymenanthera and Melicytus (Violaceae) were combined under the latter name by Green (Citation1970) and Garnock-Jones (in Connor & Edgar Citation1987), following Beuzenberg (Citation1961). Recent phylogenetic study (Mitchell et al. Citation2009) suggests there may be two well-supported sister clades within Melicytus that closely match the former circumscriptions of the two genera, although the position of M. lanceolatus is unclear. Mitchell et al. (Citation2009) preferred to continue to recognise a broad circumscription of Melicytus. Nevertheless, further research should test the idea that two distinctive clades could be recognised at genus or subgenus rank, a solution that would add information content if monophyly of both could be demonstrated.
The family Nothofagaceae provides a third example. The order Fagales has two subordinate clades. One of these comprises seven families and is sister to the other, which includes only the family Nothofagaceae with one genus, Nothofagus (Li et al. Citation2004). While family and, potentially, suborder ranks convey information about its sister clade (Fagaceae, Betulaceae, Myricaceae, Ticodendraceae, Rhoipteleaceae, Juglandaceae and Casuarinaceae), the traditional circumscriptions of Nothofagus and Nothofagaceae are congruent and one rank is thus redundant. The recent subdivision of Nothofagus (Heenan & Smissen Citation2013) recognises well-defined clades (previously treated as subgenera within Nothofagus) at the rank of genus. New names that result from recognition of these former subgenera at genus rank instantly convey the information that silver beech (Lophozonia menziesii) is not the closest relative of the other New Zealand southern beeches (Fuscospora).
Nomenclatural stability
There are pragmatic conflicts in the argument for continuing to recognise distinctive groupings that are not clades. Although it would suit some users of classifications, for example, horticulturalists who might have real costs in re-labelling stock for sale (Brickell et al. Citation2008), legislators who wish to prohibit drug plants or protect plant variety rights, or botanists who worry about the effects of nomenclatural changes on the interpretation of old publications, acceptance of non-monophyletic groups can introduce major distortions into research or applications that depend on evolutionary relationship, such as in ecology and drug discovery.
For this reason, Entwisle & Weston (Citation2005), Hopper (Citation2009) and Vences et al. (Citation2013) argued that although named taxa should always be monophyletic, ranks should be applied to clades in such a way that existing nomenclature is disturbed as little as possible, consistent with the above criteria.
Hybrids
Although Entwisle & Weston (Citation2005) did not believe that the existence of intergeneric hybrids is evidence for or against generic segregation, where they do occur they are an indication of close genetic similarity or functional compatibility of genomes. Although a strict application of the biological species concept would suggest that ability to interbreed indicates two plants are conspecific, most taxonomists sensibly will accept low levels of hybridisation between species, particularly where hybrids have reduced fertility. Does hybridisation between plants in different genera provide us with any useful information regarding their evolutionary history and generic classification?
An intergeneric hybrid should not be viewed as a hybrid between two genera, but as an interspecific hybrid between two plants that are classified in different genera. The further back in time the last common ancestor of two plants occurred, the lower the likelihood that they can successfully interbreed. Because the ability to hybridise indicates close genetic similarity and likely indicates recent common ancestry, the existence of intergeneric hybrids is a strong suggestion that generic boundaries might be incorrectly delineated, although it should be borne in mind that the similarity might reflect shared plesiomorphic features of the genome. Morgan-Richards et al. (Citation2009) suggested that some intergeneric hybrids in the New Zealand flora might involve species from recent species radiations.
de Lange & Rolfe (Citation2010) took a different position, arguing that the low or zero fertility of some hybrids within the New Zealand Veronica complex (Veronica sect. Hebe–Plantaginaceae) was evidence that supported their generic level separation as Chionohebe, Hebe, Hebejeebie, Heliohebe, Leonohebe and Parahebe. Further, de Lange & Rolfe (Citation2010) argued that the absence of hybrids between V. sect. Hebe and northern Veronica was evidence they should not be congeneric. In my opinion, such a generic concept is not biologically realistic. First, it would leave the defining characteristics of genera very close to those of (biological) species, because it implies that all congeneric species should be able to form fertile hybrids. Second, such a criterion would lead to a big reduction in the circumscription of most genera and many would be monotypic. Third, it is an asymmetric test, because while ability to hybridise indicates genetic similarity and compatibility and this implies some level of close (congeneric?) relationship, failure to hybridise does not falsify a hypothesis of close relationship.
Time banding
Vences et al. (Citation2013) point out that taxa of the same rank do not necessarily represent comparable levels in a hierarchy and proposed some approaches towards reducing those disparities, in particular, the use of similar ranks at similar clade ages (Hennig Citation1966), a concept they refer to as time banding. They point out that time banding cannot be applied universally, but could be very useful within clades (e.g. families).
Accessory criteria
Entwisle & Weston (Citation2005), Hopper (Citation2009) and Vences et al. (Citation2013) all took into account the disruption that name changes inflict on a broad community of users, even when well supported by evidence and leading to improved evolutionary interpretations. They advocate several similar accessory considerations that can help where criteria such as monophyly do not provide support for one or other of competing classifications. These include avoiding where possible classifications that change familiar names and circumscriptions of significant groups (e.g. Drosophila, Acacia) and seeking a consensus, or at least following a majority, of the taxonomic community.
Conclusion for this section
I support the following working criteria for genus recognition, in order of importance. In the remainder of this review, I apply these criteria to New Zealand's endemic genera.
Circumscriptions of named taxa can be objective if all are required to be monophyletic. Monophyletic taxa are objective, testable, accord with natural biological patterns and processes, facilitate the application of taxonomy in other pure and applied disciplines, and will, over time, lead to classifications that are stable. The requirement for monophyly involves ensuring not only that groups are monophyletic, but also that recognition of such groups does not render another group paraphyletic. In some cases, recognition of a distinctive clade in New Zealand (e.g. Colensoa, Embergeria, Haastia, Hebe, Kirkianella, Oreostylidium) has resulted in implied recognition of a grade in New Zealand or some other part of the world. Thus an international perspective is important.
Not all clades need to be named. Ideally, named clades should be recognisable based on morphological characters, and their monophyly should be strongly supported, preferably by independent lines of evidence. There may be circumstances in which named clades are not distinctive or distinctive clades remain unnamed.
Decisions about ranks for named taxa cannot be wholly objective, but the following principles can usefully be applied: (a) The phylogeny of the group should be represented in (and recoverable from) the classification where possible. (b) Clades recognised should be distinctive, but recognition of every distinctive clade is not necessary, especially if it mandates recognition of a non-distinctive sister clade or a distinctive grade. (c) Rank redundancy (named groups that have the same circumscription but different ranks) should be avoided where possible. (d) Existing and familiar names should be retained where possible consistent with the above guidelines.
Part 2. New Zealand endemic seed plant genera
The seed plant flora of New Zealand displays a high level of endemism at species rank. Godley (Citation1975) published figures showing that 84.6% of the 1833 species recognised at that time were considered endemic. I recalculated endemism at species rank using the data in de Lange & Rolfe (Citation2010): total endemism at species rank in New Zealand seed plants is 84.1% (Gymnosperms: 100%; Angiosperms 83.9%).
At family rank, only one endemic family, Ixerbaceae, has been recognised in recent years (Breitwieser et al. Citation2012), but the sister relationship of its sole genus Ixerba to New Caledonian Strasburgeria (Strasburgeriaceae; Sosa & Chase Citation2003; Matthews & Endress Citation2006; Carlquist Citation2007) and consequent placement in Strasburgeriaceae (Qiu et al. Citation2010) means that there are no endemic families currently recognised in New Zealand (de Lange & Rolfe Citation2010).
In contrast to high endemism at species rank, Millener (Citation1960) and Godley (Citation1975) noted that endemism at genus rank is low in New Zealand. Godley (Citation1975) listed 38 endemic seed plant genera. A recent listing (Breitwieser et al. Citation2012) recognised 52 endemic Angiosperm genera, only 27 of which were also on Godley's list. An alternative (de Lange & Rolfe Citation2010) treated 58 genera as endemic. The changes since Godley (Citation1975) reflect altered, and often less inclusive (Humphries & Linder Citation2009) circumscriptions of genera, especially in Orchidaceae (Clements & Jones Citation2002; Clements et al. Citation2002), and some altered knowledge of distribution ranges. Some genera, such as Iti and Heliohebe, have been proposed (Garnock-Jones & Johnson Citation1987; Garnock-Jones Citation1993b) and rejected (Heenan Citation2002; Garnock-Jones et al. Citation2007) in the period since Godley (Citation1975).
List of endemic genera
The starting point for this review was a list of endemic seed plant genera drawn up from two recent checklists of the New Zealand Flora (de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012) that also included information on endemism at genus rank. The two lists differ slightly, and genera considered endemic in one list but not the other were still included for consideration here. The combined list of potential endemic genera from these two sources () comprises those genera currently recognised by New Zealand's largest plant taxonomy research community at Landcare Research (Breitwieser et al. Citation2012) for their herbarium and databases and also reflects the taxonomic opinions of New Zealand's field botanists and plant conservation community (de Lange & Rolfe Citation2010). A newer list (de Lange et al. Citation2013) does not address generic endemism explicitly, but its taxonomic deviations from de Lange & Rolfe (Citation2010) have been addressed in and elsewhere.
Table 1 New Zealand endemic seed-plant genera. The list combines recent lists of endemic genera (de Lange & Rolfe Citation2010 (*), Breitwieser et al. Citation2012 (†)). Names in bold are fully accepted as genera according to the criteria outlined above and are considered to have a distribution endemic to the political boundaries of New Zealand, although for some of these the notes suggest questions for future research. Underlined names are considered to be of doubtful taxonomic status (usually this means the monophyly of the genus or its close relatives is in question). All other names are rejected (either they have been shown not to be monophyletic, to render another genus paraphyletic, or they are distributed beyond political New Zealand).
Nomenclature
The assessment of the status of New Zealand's endemic genera presented below results in some clear recommendations about taxonomy. I present a new combination in only one such case, Sonchus novae-zelandiae. For others (e.g. Rytidosperma exiguum, Sonchus grandifolius, Stylidium subulatum) binomials are already available. In other endemic genera, taxonomic status is still equivocal and awaits the conclusion of current research projects (e.g. in the Raoulia complex, I. Breitwieser pers. comm.). For a number of genera (e.g. Teucridium, Toronia, Trilepidea), there is so far insufficient research that bears on their monophyly or that of their close relatives, and so although I recommend provisional taxonomic acceptance, I indicate in the discussion that more research is needed.
Gymnosperms
Podocarpaceae
Halocarpus Quinn (3 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
The partition of traditional large genera of Podocarpaceae, Dacrydium and Podocarpus (de Laubenfels Citation1969; Quinn Citation1982), seems justified by the monophyly of morphologically divergent subclades such as Halocarpus (Wagstaff Citation2004) and by the great age of these groupings. Biffin et al. (Citation2011) used matK, trnL–F and internal transcribed spacer (ITS)2 sequence data to show the three species of Halocarpus form a well-supported clade that renders no other segregate genus paraphyletic. Burleigh et al. (Citation2012) presented a supertree, derived from previous mitochondrial, chloroplast and nuclear DNA studies, which supports monophyly of Halocarpus (). Morphology also strongly supports the relationship of the three New Zealand species assigned to Halocarpus. Divergence of Halocarpus was dated at (112–) 87 (–61) million years old by Biffin et al. (Citation2011), indicating a likely Gondwanan vicariance origin. I accept Halocarpus as a genus endemic to New Zealand.
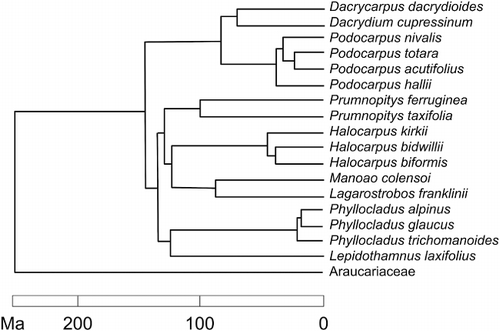
Manoao Molloy (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Manoao is a monospecific genus segregated by Molloy (Citation1995) from Lagarostrobos, consequently also monospecific. Molloy (Citation1995) documented morphological differences between M. colensoi and L. franklinii of Tasmania, and argued that their separation at generic rank is justified by the extent of their differences. A sister relationship between Manoao and Lagarostrobos has been demonstrated with 18S rDNA (Kelch Citation2002; moderate support), matK, trnL–F and ITS2 (Biffin et al. Citation2011; strong support), rbcL (Knopf et al. Citation2012; strong support), and in a supertree presented by Burleigh et al. (Citation2012; ), but using the NEEDLY intron 2, Manoao was weakly supported as sister to Halocarpus (Knopf et al. Citation2012). Thus, although most evidence supports the monophyly of Lagarostrobos (both s.l. and s.s.) and Manoao, at least with respect to extant species, there is conflict between two competing criteria for deciding rank. On the one hand, recognising two monospecific genera that are sisters makes the genus rank redundant and the classification is thus nowhere informative about the sister relationship between them (Kelch Citation2002). Kelch (Citation2002) concluded ‘A conservative approach (i.e. one avoiding unnecessary monotypes) favours the retention of L. colensoi in Lagarostrobos, pending further evidence of relationships within the group’. On the other hand, segregation of Manoao does appear to be widely accepted in New Zealand (de Lange & Rolfe Citation2010; Dawson & Lucas Citation2011; Breitwieser et al. Citation2012; but see Miller Citation2006) and the inferred late Cretaceous age of the separation of L. franklinii and M. colensoi is similar to that of other generic separations in the family (Burleigh et al. Citation2012; but see Biffin et al. Citation2011 who dated that split as Paleogene). Christenhusz et al. (Citation2011) accepted Manoao without discussion and Biffin et al. (Citation2011) concluded that the morphological differences between Manoao and Lagarostrobos justify their continued recognition. Jordan et al. (Citation2011) identified Oligocene/Miocene pollen from New Zealand as Lagarostrobos and noted that Manoao is not known in the fossil record. This suggests the possibility that Lagarostrobos would be paraphyletic if extinct species were included. In my opinion, classifying L. colensoi in the same genus as its closest living relative is preferable to a classification that reflects its phenetic distance from L. franklinii.
Angiosperms
Alseuosmiaceae
Alseuosmia A.Cunn (5 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
The monophyly of Alseuosmia has not been questioned. The genus is strongly supported as sister to Wittsteinia and more distantly related to Crispiloba and Platyspermation (Tank & Donoghue Citation2010 and references therein), based on extensive sampling of the chloroplast genome and nuclear ribosomal DNA. More extensive taxon sampling is needed to confirm monophyly, but provisionally I accept Alseuosmia as a genus endemic to New Zealand.
Apiaceae
Lignocarpa J.W.Dawson (2 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012) and Scandia J.W.Dawson (2 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Lignocarpa and Scandia were segregated from Anisotome (Dawson Citation1967), initially as Gingidium p.p. (Dawson Citation1961). Lignocarpa (two species) is a morphologically divergent clade of scree-adapted plants. Scandia (two species) is a clade of softly woody semi-climbing plants. Together with non-endemic Aciphylla, Anisotome and Gingidia, they make up the Aciphylleae (Downie et al. Citation2010). This complex is a clade of morphologically highly divergent plants adapted to differing environments, a classic adaptive radiation in New Zealand on a similar scale to those of Brachyglottis, Celmisia and Veronica.
Mitchell et al. (Citation1998), Radford et al. (Citation2001) and Spalik et al. (Citation2010) found congruent results for this group using ITS sequence data (). Gingidia decipiens and G. flabellata are nested within Anisotome, whereas the remaining species of Gingidia form a clade with Scandia and Lignocarpa that is nested within Aciphylla. Webb & Druce (Citation1984) described an intergeneric hybrid, Aciphylla squarrosa × Gingidia montana, which is supporting evidence of a close relationship. Furthermore the Aciphylla–Gingidia–Lignocarpa–Scandia clade is nested within paraphyletic Anisotome when large numbers of Aciphylla and Anisotome are included (Radford et al. Citation2001).
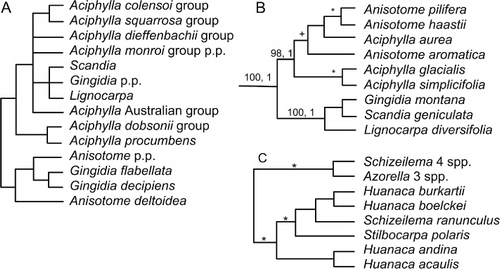
These analyses suggest the recognition of at best two genera, Anisotome (including G. flabellata and G. decipiens) and Aciphylla (including the remaining species of Gingidia, Anisotome procumbens, Lignocarpa and Scandia). Given the morphological distinctiveness of Aciphylla, Scandia and Lignocarpa, this is likely to be contentious, yet classifying them together draws attention to this spectacular adaptive radiation and puts evolutionary studies, such as the detailed descriptions and evolutionary pathways of sexual systems provided by Webb (Citation1979), in a firm phylogenetic framework. Before making the nomenclatural changes, wider sampling of species (especially in Anisotome) and genes is recommended. Caution is also indicated by the study of Nicolas & Plunkett (Citation2009). They used chloroplast DNA only and did not find the same topology as Radford et al. (Citation2001; ); here a clade of Scandia, Lignocarpa and Gingidia (one species each) was well supported as a sister to a clade that combined six species of Aciphylla and Anisotome.
Stilbocarpa (Hook.f.) Decne. & Planch. (3 spp.; de Lange & Rolfe Citation2010)
De Lange & Rolfe included Stilbocarpa as endemic because they included Macquarie Island in the New Zealand botanical region (see also Allan Citation1961). That is not the convention followed here and by Breitwieser et al. (Citation2012), for which endemism to the political region is the criterion.
Nicolas & Plunkett (Citation2009, Citation2012) showed that Stilbocarpa is nested within an otherwise South American clade containing the genus Huanaca and one species of Schizeilema (). Rearrangements of generic boundaries are needed, so that Schizeilema and Huanaca can have monophyletic circumscriptions; if treated as a single genus, the oldest genus name for the Huanaca/Stilbocarpa clade appears to be Huanaca.
Asteraceae
Asteraceae includes several large independent adaptive radiations (treated separately below) where rapid and relatively recent adaptation to different habitats has driven morphological divergence, often extreme, within clades. This in turn has encouraged recognition of distinctive small clades as genera separate from the distinctive large grades they are nested within.
1. Celmisia–Olearia complex (Tribe Astereae)
This generic complex comprises a clade that includes species currently referred to Celmisia, Damnamenia, Olearia p.p., Pachystegia and Pleurophyllum, and an additional clade that includes Australian species that are more distantly related (Olearia A + B of Cross et al. Citation2002, which includes the type of the genus). Three of these genera—Damnamenia, Pachystegia and Pleurophyllum—have been listed as endemic in recent accounts.
Damnamenia Given. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012), Pachystegia Cheeseman (3 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012) and Pleurophyllum Hook.f. (3 spp.; de Lange & Rolfe Citation2010)
Taxonomic resolution of the Celmisia–Olearia complex into monophyletic groups involves broad considerations of Olearia, Celmisia and many smaller genera throughout their ranges. It will likely require further collaborative study that includes investigators and samples from Australia, New Zealand and South America.
Recent molecular phylogenetic research (Cross et al. Citation2002; Wagstaff et al. Citation2011; ) provides evidence that Celmisia, Pleurophyllum, Damnamenia and Pachystegia are each monophyletic as currently circumscribed. However, Olearia is not monophyletic; Cross et al. (Citation2002) include species of Olearia in five major clades (they identified several smaller clades within these most inclusive ones). In that study, which analysed ITS sequences, an Australian clade (which includes the type species of Olearia as well as samples of Oritrophium and Felicia) is sister to a clade of Olearia from Australia and New Zealand (including the type species of Shawia), Celmisia, Damnamenia, Pleurophyllum and Pachystegia. Wagstaff et al. (Citation2011) used ITS, external transcribed spacer (ETS) and two chloroplast sequences to further analyse this mostly New Zealand clade. In their combined analysis, Celmisia was monophyletic and sister to a clade comprising Olearia p.p. (Shawia clade) and its sister group Pachystegia. Both studies recovered a clade comprising Damnamenia, Pleurophyllum and the six species of macrocephalous Olearia as sister to a clade comprising Olearia p.p. (Shawia), Pachystegia and Celmisia ().
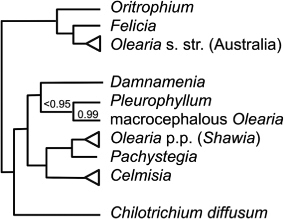
Damnamenia. Given (Citation1973) used largely phenetic criteria to raise Celmisia sect. Antarcticae (Allan Citation1961) to genus rank as Damnamenia, arguing its one species lacked character states characteristic of mainland Celmisia, yet shared a number with Pleurophyllum and macrocephalous Olearia. The phenetic similarity of Damnamenia with Pleurophyllum and macrocephalous Olearia (Drury Citation1968; Given Citation1973) suggested relationships that have since been supported by explicitly phylogenetic methods and molecular data (Wagstaff et al. Citation2011). The three species of Pleurophyllum are sister to a clade of six species of macrocephalous Olearia, with the monospecific Damnamenia as sister to these nine species (Cross et al. Citation2002; Wagstaff et al. Citation2011). The distinctiveness of these mostly southern species of macrocephalous Olearia has long been recognised (Allan Citation1961) and Drury (Citation1968) was first to ally them with Pleurophyllum.
There are several possible taxonomic solutions for this grouping following the criteria recommended here, two of which are likely outcomes. First, describing a new genus for macrocephalous Olearia would have the advantage of leaving the nomenclature of Pleurophyllum and Damnamenia unchanged. Second, Wagstaff et al. (Citation2011) argued that Damnamenia could be combined with Pleurophyllum and macrocephalous Olearia as a single near-endemic genus, for which the correct name would be Pleurophyllum. Thus Pleurophyllum would be enlarged to include Damnamenia, Olearia angustifolia, O. oporina, O. chathamica, O. semidentata, O. lyallii and O. colensoi. This wider concept of Pleurophyllum is supported by a hybrid between Damnamenia vernicosa and Pleurophyllum speciosum (as Celmisia ×campbellensis F.R.Chapman = C. ×chapmanii T.Kirk), species separated at the most basal node in the clade. Allan (Citation1961) and Given (Citation1973) referred to a hybrid plant found by W.B. Brockie on Mt Lyall, additional to Chapman's collection from about 12 plants seen at Venus Bay. On current evidence, I prefer the latter solution.
Pleurophyllum
Whatever its eventual circumscription, Pleurophyllum is not endemic to New Zealand because P. hookeri extends to Macquarie Island (Breitwieser et al. Citation2012), although Allan (Citation1961) and de Lange & Rolfe (Citation2010) included Macquarie Island in the New Zealand botanical region.
Pachystegia
This genus is weakly supported either as sister to a clade of species from Australia and New Zealand (Olearia clades E + H of Cross et al. Citation2002) or sister to a larger clade of Olearia (equivalent to clades E, F, G and H of Cross et al. Citation2002) (e.g. Wagstaff et al. Citation2011). Current evidence is thus equivocal, and future acceptance of Pachystegia will depend on the outcome of more detailed study of Olearia and resolution of the conflicts between the phylogenetic signals from ITS and ETS (Wagstaff et al. Citation2011).
Based on current limited sampling, Celmisia is monophyletic and either sister to a clade that includes O. paniculata, type species of Shawia Forst.f. (Wagstaff et al. Citation2011), or nested within a larger grade of Australian and New Zealand Olearia (Cross et al. Citation2002). The topology needs to be resolved if a monophyletic Shawia is to be circumscribed and wider sampling of Celmisia will be needed before its monophyly can be confirmed. Note also an additional intergeneric hybrid, between Olearia arborescens (Shawia clade) and Celmisia gracilenta (Clarkson Citation1988).
2. Brachyglottis complex (Tribe Senecioneae)
Dolichoglottis B.Nord. (2 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012), Haastia Hook.f. (3 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012) and Traversia Hook.f. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Nordenstam (Citation1978) extended the circumscription of Brachyglottis from a single species (Allan Citation1961) to include 30 species treated under Senecio by Allan (Citation1961), at the same time segregating Dolichoglottis (two species) and Urostemon (one species, transferred to Brachyglottis by Webb in Connor & Edgar Citation1987). For this grouping of Senecioid genera, Pelser et al. (Citation2007) using ITS and chloroplast DNA (cpDNA), recovered a well-supported Caputia–Brachyglottis clade (as Senecio medley-woodii–Brachyglottis clade: see Nordenstam & Pelser Citation2012), in which the current circumscription of Brachyglottis is paraphyletic (). The segregate genera Acrisione, Urostemon, Papuacalia, Traversia, Centropappus, Bedfordia and Haastia are all nested within Brachyglottis. Additionally, Dolichoglottis is placed either as sister to Brachyglottis (Pelser et al. Citation2007) or nested within it (Wagstaff & Breitwieser Citation2004). Of these, Dolichoglottis, Haastia and Traversia are considered endemic to New Zealand.
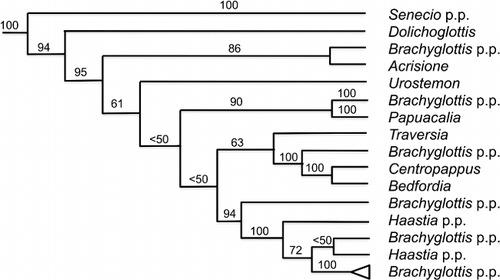
Haastia was traditionally classified in either Tribe Gnaphalieae or Tribe Astereae until Breitwieser & Ward (Citation2005) transferred it to Senecioneae based on morphology (Breitwieser & Ward Citation2005), anatomy and flavonoid chemistry (Breitwieser & Ward Citation1993; Breitwieser Citation2008), palynology (Breitwieser & Sampson Citation1997a,Citationb) and molecular systematics (Wagstaff & Breitwieser Citation2002, Citation2004). Its phylogenetic position, nested within Brachyglottis, and the lack of evidence for its monophyly have both been supported in more recent studies (e.g. Pelser et al. Citation2007; ).
Making generic circumscriptions monophyletic in this complex will have interesting nomenclatural consequences. On current evidence, if Haastia were to be retained with the current species included, it would need to be enlarged to also include several leafy shrubs such as Brachyglottis perdicioides and B. monroi. In that scenario, Brachyglottis would revert to its earlier, narrow circumscription (Allan Citation1961). Otherwise, evidence supports the inclusion of Haastia within Brachyglottis. This solution would draw attention to the close relationship of these alpine cushion plants to shrubs from lower altitude, an adaptive radiation that parallels the snow hebes within Veronica, the vegetable sheep within the Raoulia complex and pulvinate Myosotis.
At the basal nodes of the Brachyglottis clade, support values for many branches are moderate at best (Pelser et al. Citation2007), indicating that a broad circumscription of Brachyglottis is sensible. This would enlarge the genus to include not only Haastia, but also Acrisione, Urostemon, Papuacalia, Traversia, Centropappus and Bedfordia. In that circumscription, Dolichoglottis could be accepted as a genus endemic to New Zealand. A second option would be to enlarge the circumscription of Brachyglottis a little further to include Dolichoglottis. A third option would divide the complex narrowly to contain five New Zealand endemic genera, but there is currently only weak support for some of these clades (Pelser et al. Citation2007). I accept Dolichoglottis, but not Haastia and Traversia, as genera endemic to New Zealand
3. Sonchus complex (Tribe Lactuceae)
Embergeria Boulos (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012) and Kirkianella Allan (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Kim et al. (Citation2007) used ITS and cpDNA data to show the Sonchus alliance includes many small, often island, genera (including Embergeria and Kirkianella) that are nested within a paraphyletic or polyphyletic circumscription of Sonchus (). They recommended that the circumscription of Sonchus be enlarged to include these segregates and avoid breaking up that largely uniform genus. Mejías & Kim (Citation2012) transferred Dendroseris and Thamnoseris to Sonchus.
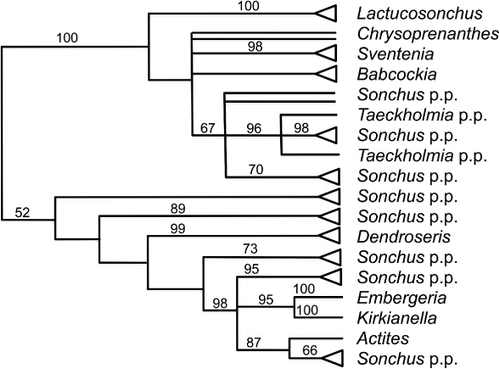
Embergeria is clearly nested within Sonchus, based on evidence from molecular data (Kim et al. Citation2007 and references therein). Apart from its larger stature and large fruits, the absence of stiff clavate barbellate bristles among the shorter slender soft pappus hairs appears to be an apomorphy perhaps associated with loss of dispersal in its island habitat (Garnock-Jones in Webb et al. Citation1988). The correct name in Sonchus for its single species is S. grandifolius Kirk. Within Sonchus it appears most closely related to S. novae-zelandiae (Kirkianella novae-zelandiae, see below); both have only soft fine pappus hairs.
Inclusion of Kirkianella within Sonchus is supported based on evidence from molecular data (Kim et al. Citation2007 and references therein) and morphology (Bentham & Hooker Citation1873). Like S. grandifolius, it appears to lack stiff barbellate pappus bristles, which supports the molecular finding (Kim et al. Citation2007) of a sister relationship between them. Allan (Citation1961) segregated the genus from its previous placement in Crepis based on its solitary capitula. Although the International Plant Names Index (www.ipni.org) cites Bentham & Hooker (Citation1873) as making the combination in Sonchus, they did not definitely associate the epithet with the genus name (ICN, Art. 35.2), but instead only gave their opinion of where the species should be classified.
Sonchus novae-zelandiae (Hook.f.) Garn.-Jones, comb. nov. ≡ Crepis novae-zelandiae Hook.f., Handbook of the New Zealand Flora: 164 (Citation1864). ≡ Kirkianella novae-zelandiae (Hook.f.) Allan, Flora of New Zealand 1: 762 (Citation1961). Lectotype: S. novae-zelandiae has not previously been formally lectotypified, although Allan (Citation1961, p. 762) recommended a Haast specimen at K should be selected. However the Haast specimens in Hooker's herbarium (at K) lack fruits, which were clearly described in the protologue, and are thus not as representative as others. Four collections were listed in the protologue, collected by Banks & Solander, Sinclair & Haast, Lindsay, and Hector & Buchanan. The last three are mounted together on one sheet in Hooker's herbarium at Kew. The Banks and Solander material is at BM, WELT, and AK. I designate the Banks & Solander material at BM as lectotype because it includes all the life history stages described in the protologue; additionally it is a high quality collection and duplicates are available in New Zealand (WELT, AK).
4. Raoulia alliance (Tribe Gnaphalieae)
Ewartiothamnus Anderb. (1 sp.; de Lange & Rolfe Citation2010), Leucogenes Beauverd (4 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012), Rachelia J.M.Ward & Breitw. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012) and Raoulia Hook.f. (23 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
The Raoulia alliance comprises a distinctive grouping of plants that includes a range of distinct morphological (e.g. cushion plants, mat plants, leafy and whipcord shrubs) and anatomical types (Breitwieser & Sampson Citation1997a, Citationb; Breitwieser & Ward Citation1998). Based on current circumscriptions, several hybrids are treated as intergeneric within the Raoulia alliance (Ward Citation1997; McKenzie et al. Citation2004; Smissen et al. Citation2007), indicating close genetic compatibility in spite of the divergent morphologies (Morgan-Richards et al. Citation2009). Monophyly of the current generic circumscriptions has not been convincingly demonstrated with molecular evidence (), probably because of this complex's history of adaptive radiation, hybridism and reticulate evolution, and divergent and possibly convergent morphological evolution (Smissen et al. Citation2011). Including all groupings (currently genera, most endemic) within one morphologically variable but genetically closely related and probably endemic genus is just one of several possible solutions, but one that would recognise the close relationship among these distinctive New Zealand plants within the wider classification of the tribe. However, that step would be premature while current research is investigating the complex evolution of the group (Breitwieser et al. Citation1999; Smissen et al. Citation2007, Citation2011). I have reflected this uncertainty here by accepting Raoulia with an uncertain circumscription, while labelling the other genera in the Raoulia complex as taxonomically equivocal ().
![Figure 7 Relationships in the Raoulia alliance. A, Nuclear DNA tree based on ITS and ETS; PP shown if > 0.95. B, Chloroplast DNA tree based on trnL intron, trnL–F, ndhF, trnK intron [incl. matK]; PP shown if > 0.95 (simplified from Smissen et al. Citation2011).](/cms/asset/8ef63537-7b7c-4950-bbc9-83ef982d7509/tnzb_a_902854_f0007_b.jpg)
Balanophoraceae
Dactylanthus Hook.f. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Nickrent (http://www.parasiticplants.siu.edu/ListParasites.html, accessed 27 September 2013) accepted Dactylanthus as a monospecific genus endemic to New Zealand. A sister relationship between Dactylanthus and Hachettia of New Caledonia, also monospecific, has been suggested (see Fay et al. Citation2010). I note that very small genera seem to be a feature of the classifications of parasitic plants throughout the world. I accept Dactylanthus as a genus endemic to New Zealand.
Boraginaceae
Myosotidium Hook. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
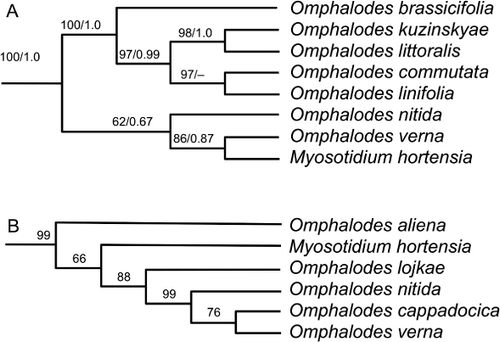
Heenan et al. (Citation2010) obtained ambiguous indications of the relationships of Myosotidium hortensiaFootnote1 in a NeighborNet comparison of DNA sequence similarities. On the one hand, analysis of ITS sequences indicated the genus has similar sequences to Omphalodes, a possible relationship previously alluded to by Hooker (Citation1859) on morphological grounds. On the other hand, its chloroplast atpB sequences were not closely similar to those of other genera, although Heenan et al. (Citation2010) discussed sequence similarities with Lappula and Trichodesma.
Nazaire & Hufford (Citation2012; ) used data from the ITS region of nuclear ribosomal DNA and ndhF, matK, rbcL and trnL–F from the chloroplast genome in a phylogenetic analysis of Boraginaceae. Khoshsokhan et al. (Citation2013) included M. hortensia in a study of Boraginaceae trib. Eritrichieae using nuclear ITS data. Cohen (Citation2013; ) used ITS and three chloroplast sequences (matK, ndhF and trnL–trnF). Their results are broadly similar to those of Heenan et al. (Citation2010) and most analyses suggest the current circumscription of Omphalodes is paraphyletic unless Myosotidium is included in its circumscription. Cohen (Citation2013) also included an analysis and discussion of morphological characters and showed that Myosotidium shares three synapomorphies with Omphalodes: secondary leaf venation (a labile character state in the family), cordate leaves and nutlets with marginal glochids or wings. Cohen recommended wider taxon sampling within Omphalodes to resolve the taxonomy within this clade. These four largely congruent results strongly indicate that even if Myosotidium is retained at genus rank it is very likely to have a wider and no longer endemic circumscription.
Brassicaceae
Notothlaspi Hook.f. (2 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Notothlaspi is a distinctive clade, for which close relatives have yet to be established. Some of its distinctive features, such as the thick grey–green leaves and disproportionately long radicle (Hooker Citation1864; Garnock-Jones Citation1991a) are likely to be adaptations to its scree and stonefield habitats, and might obscure its relationships to other genera. However, it is apparent that its nearest relatives are not in New Zealand (Mitchell & Heenan Citation2000). Recent work (Warwick et al. Citation2010; Al-Shehbaz Citation2012) placed it in its own tribe, Notothlaspideae, closest among the genera sampled to the South African Heliophila, Trib. Heliophileae (). I accept Notothlaspi as a genus endemic to New Zealand.

Campanulaceae
Colensoa Hook.f. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
The genus includes only one species, Colensoa physaloides, treated as Pratia physaloides by Allan (Citation1961). Botanists, including in New Zealand, generally are adopting a wider circumscription of Lobelia, to include Pratia, Isotoma and Hypsela (Antonelli Citation2008; Heenan et al. Citation2008; Knox et al. Citation2008; Lammers Citation2011). However, while the transfers of Pratia, Isotoma and Hypsela to Lobelia have been generally accepted, many botanists (e.g. de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012; E.B. Knox, pers. comm.) continue to treat Colensoa as a separate genus. There is good evidence (Antonelli Citation2008) that Colensoa physaloides (as Lobelia physaloides) is not closely related to the other New Zealand species of Lobelia, including those formerly classified with it under Pratia. Nevertheless, Colensoa is nested within the current circumscription of Lobelia s.l., which thus appears to be paraphyletic. Overseas, the genus is very variable in habit, flower size and shape, and fruit structure. Many characters are homoplasious, and additionally, L. physaloides has significant autapomorphies (Lammers Citation2011).
Lammers (Citation2011; following Antonelli Citation2008) provided a taxonomic treatment, in which L. physaloides was placed on its own in Lobelia sect. Colensoa, related to a large section of species (sect. Delostemon) that is found in Africa, Asia and South America and perhaps also to a smaller section (sect. Holopogon) that is endemic to Australia. Thus, if Lobelia is not to be expanded to have an identical circumscription to the subfamily Lobelioideae, L. physaloides belongs among a group of species with a good claim to generic rank (Clade C1 of Antonelli Citation2008 or some part of it, ). Since that study, E.B. Knox (pers. comm.) has increased taxon sampling in the group by including species of the Australian clade Lobelia sect. Holopogon, within which he found L. physaloides is nested.
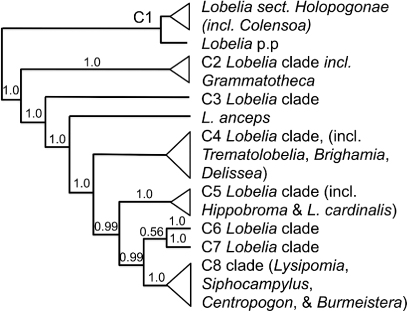
If Colensoa is to be retained as a genus, its circumscription should be reconsidered. To maintain monophyletic groups, related species could be treated as congeneric with Colensoa and the C1 clade could be treated at generic rank or further subdivided. However, only by very fine division of the C1 clade could Colensoa remain as a New Zealand endemic. Expanding the circumscription of Lobelia to have the same circumscription as subfam. Lobelioideae would be a less informative alternative.
Clianthus Lindl. (2 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012) and Montigena Heenan. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Both Clianthus and Montigena are related to Carmichaelia. Clianthus is sister clade to Carmichaelia (Heenan Citation1998a; Wagstaff et al. Citation1999; ), distinguished from it by its leafiness, large red flowers and large, many-seeded follicular fruits. Both genera appear to have apomorphic characters and there are substantial morphological differences between them. Maintaining Clianthus separate from Carmichaelia seems acceptable, and taxonomic recognition of their sister relationship at a higher rank than genus may be justified.
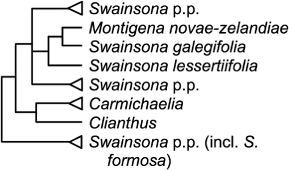
Heenan (Citation1998b) proposed the new genus Montigena to reflect significant morphological and anatomical differences from Swainsona, yet the strong support from molecular data for its inclusion in Swainsona suggests such features are autapomorphies and adaptations to its New Zealand alpine habitat. Thus, recognition of Montigena is not recommended.
Recognition of Clianthus, Carmichaelia and Montigena renders the Australian genus Swainsona paraphyletic. One clade within Swainsona (including S. formosa) is sister to a clade comprising Carmichaelia, Clianthus and the rest of Swainsona. Resolution of that situation is perhaps more likely to lead to subdivision of Swainsona than to synonymy of Carmichaelia.
Gesneriaceae
Rhabdothamnus A.Cunn. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
According to a phylogeny based on ITS, G-CYCLOIDEA and four cpDNA sequences (Woo et al. Citation2011), this monotypic endemic genus is sister to Coronanthera, which has up to 20 species in New Caledonia and one in Papua New Guinea and the Solomon Islands. Rhabdothamnus, a twiggy shrub with solitary flowers and showy orange tubular corollas with spreading lobes, is also morphologically distinct from Coronanthera, trees with cymose inflorescences and small white, green, yellow, or brownish campanulate small-lobed corollas (Woo et al. Citation2011). I accept Rhabdothamnus as a genus endemic to New Zealand.
Lamiaceae
Teucridium Hook.f. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
A molecular study based on rbcL and ndhF sequence data (Wagstaff et al. Citation1998) placed Teucridium as sister to the monospecific genus Oncinocalyx of southeast Australia (). These two small genera were shown to be sister to Teucrium fruticans, the sole representative in that study of a large and widespread genus, a relationship supported also by pollen (Abu-Asab & Cantino Citation1993) and chemical (Grayer et al. Citation2002) data. Although there is no indication that Teucridium is nested within Teucrium, that possibility has not been refuted by including a larger sample of species. Monophyly of Teucrium needs to be established before Teucridium can be unequivocally accepted.

Loranthaceae
Alepis Tiegh. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012), Peraxilla Tiegh. (2 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012) and Trilepidea Tiegh. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Allan (Citation1961) included Alepis, Peraxilla and Trilepidea in a broad circumscription of Elytranthe, following Engler (Citation1897). A sister relationship between Alepis flavida and Peraxilla tetrapetala is well-supported and these are sister to Elytranthinae (Vidal-Russell & Nickrent Citation2008; ) based on a phylogenetic analysis of chloroplast and nuclear DNA sequence data. However, because only one of the two species of Peraxilla was sampled, its monophyly has yet to be demonstrated.
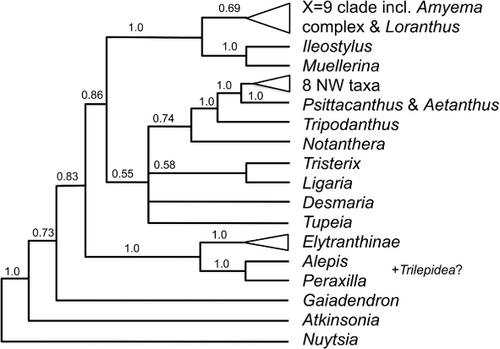
The chief qualitative characters that separate Alepis and Peraxilla (Alepis: corolla gamopetalous; anthers dorsifixed near the base; Peraxilla: corolla choripetalous; anthers basifixed) are not necessarily substantial and might relate to changes in pollinators (Vidal-Russell & Nickrent Citation2008). Furthermore, even if Peraxilla is shown to be monophyletic, its recognition as a genus distinct from Alepis obscures their close relationship; combining them would probably make a more informative classification and nomenclature.
Trilepidea is a monospecific genus and presumed extinct (de Lange et al. Citation2010); thus it has not been included in molecular phylogenetic studies, but was included in Tribe Elytrantheae by Vidal-Russell & Nickrent (Citation2008). It appears similar to Alepis and Peraxilla, differing chiefly in its 3 persistent floral bracts, 5–6-merous corolla and apiculate anthers. Of these, I suspect the first and last are likely to be autapomorphies, whereas the 5–6-merous corolla is likely to be plesiomorphic. The additional floral bracts might be related to a reduction in inflorescence flower number in Trilepidea. Thus, although different, there may be little evidence to support its generic distinction from Alepis and Peraxilla. Currently, there is not evidence that falsifies the hypothesis that Alepis, Peraxilla and Trilepidea are a single adaptive radiation in New Zealand and more research is needed before three endemic genera can be accepted with confidence.
Tupeia Cham. & Schltdl. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Tupeia and Ileostylus are both monospecific and are separate lineages from each other and from Alepis, Peraxilla and Trilepidea (Vidal-Russell & Nickrent Citation2008; ). Their relationships appear to be with South American and Australian plants (Desmaria and Muellerina) respectively (Vidal-Russell & Nickrent Citation2008), and their continued generic status depends on comparisons beyond New Zealand. I accept Tupeia as a genus endemic to New Zealand, whereas Ileostylus lost its endemic status when it apparently self-introduced to Norfolk Island in the 1930s (Mills Citation2010).
Malvaceae
Entelea R.Br. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Phylogenetic analysis of ndhF sequence data (cpDNA) strongly supported a sister relationship of Entelea arborescens with Sparrmannia africana and a more distant relationship between those two and Apeiba tibourbou (Brunken & Muellner Citation2012). In a parsimony analysis of morphological data, which included additional genera, Entelea and Clappertonia were weakly supported as a clade sister to Sparrmannia (Brunken & Muellner Citation2012). Currently, Sparrmannia is a genus of seven species, characterised by pendent flowers with a recurved corolla and irritable stamens (Mabberley Citation2008). These results are consistent with less conclusive earlier findings based on morphological (Judd & Manchester Citation1997) and pollen (Perveen et al. Citation2004) data. I accept Entelea as a genus endemic to New Zealand, although the monophyly of related genera needs to be investigated.
Hoheria A.Cunn. (7 spp., de Lange & Rolfe Citation2010; 6 spp., Breitwieser et al. Citation2012) and Plagianthus J.R. & G.Forst. (2 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
In phylogenetic studies based on ITS and cpDNA, Wagstaff et al. (Citation2010) and Wagstaff & Tate (Citation2011) found good support for the monophyly of Plagianthus and of Hoheria. I accept both Hoheria and Plagianthus as a genera endemic to New Zealand.
Montiaceae
Hectorella Hook.f. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Applequist et al. (Citation2006, using matK and rbcL data) and Nyffeler & Eggli (Citation2010, using trn K and matK data) found Hectorella to be sister to a clade comprising Lewisia, Montia and Claytonia, within a grouping recognised at family rank as Montiaceae (Nyffeler & Eggli Citation2010). Wagstaff & Hennion (Citation2007, using matK, trnK and rbcL data; ) also found this, and included Lyallia (from Kerguelen Island) in their study. Lyallia and Hectorella are sister taxa, as long suspected from morphological studies (Philipson & Skipworth Citation1961; Nyananyo & Heywood Citation1987). Both genera are monospecific, and differ in numbers of petals and stamens and their sexual systems (Hectorella is gynodioecious, whereas Lyallia is cosexual). Wagstaff & Hennion (Citation2007) and Applequist et al. (Citation2006) consider these differences sufficient to maintain the genera, contrary to the view of Nyananyo & Heywood (Citation1987), who combined them in a single genus, Lyallia. Deciding between the alternatives may seem subjective, but I prefer to follow Nyananyo & Heywood (Citation1987) because: (1) both flower merosity (Garnock-Jones Citation1993a) and sexual systems (Webb Citation1979; Garnock-Jones Citation1991b; Bayly & Kellow Citation2006) can be labile in other groups, and (2) maintaining two monospecific sister genera results in rank redundancy. The combination Lyallia caespitosa (Hook.f.) Nyananyo & Heywood is available.
Myrtaceae
Lophomyrtus Burret (2 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012) and Neomyrtus Burret (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Neomyrtus and Lophomyrtus were segregated from Myrtus by Burret (Citation1941). Lucas et al. (Citation2007) showed with moderate support from combined plastid and ITS sequence data that L. obcordata is more closely related to N. pedunculata than it is to L. bullata (). However, a phylogeny from ITS sequences alone had Neomyrtus sister to a monophyletic Lophomyrtus (Lucas et al. Citation2005). Monophyly of Lophomyrtus might be further supported by its 4-merous flowers and uniseriate ovules (Allan Citation1961), and the conflict between nuclear and chloroplast sequence analyses might indicate intergeneric chloroplast exchange. Thornhill et al. (Citation2012) reported only minor differences in pollen morphology between Lophomyrtus and Neomyrtus. Further research is needed to test reciprocal monophyly of Lophomyrtus and Neomyrtus, and even if both are monophyletic, consideration should be given to combining them to reflect their close relationship.

Nanodeaceae
Mida Endl. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Current published and unpublished knowledge on the status of Mida was summarised by de Lange (Citation2011b). A proposed congeneric, M. fernandeziana of the Juan Fernández Islands (Der & Nickrent Citation2008), is now firmly established as belonging in Santalum (Bernardello et al. Citation2006; and see discussion by de Lange Citation2011b), which leaves Mida monospecific and endemic to New Zealand. Nickrent et al. (Citation2010) place it with Nanodea of Patagonia and Tierra del Fuego in Nanodeaceae, a small clade of just two monospecific genera. Again, the issue of monospecific sister genera and redundant ranks arises, although Nickrent et al. (Citation2010) describe major morphological differences between Mida and Nanodea.
Orchidaceae
There are 6–8 currently accepted (de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012) endemic genera of orchids in New Zealand (but see de Lange et al. Citation2013); most are monospecific. Janes & Duretto (Citation2010) note that whereas Australian botanists tend to adopt wider generic circumscriptions in orchids (they particularly refer to Pterostylidinae), New Zealand botanists have tended to adopt smaller segregate genera. For some of these (e.g. Ichthyostomum), detailed phylogenies are not yet available.
Aporostylis Rupp & Hatch (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
The phylogenetic tree of Clements et al. (Citation2002, based on ITS sequence data) places A. bifolia in a large polytomy with many other Diurideae; further research is needed in order to clarify its relationships. The genus was accepted by www.e-monocot.org (accessed 27 September 2013). I accept Aporostylis as a genus endemic to New Zealand on current evidence.
Danhatchia Garay & Christenson (1 sp.; de Lange & Rolfe Citation2010, accepted but not as endemic by de Lange et al. Citation2013; Breitwieser et al. Citation2012)
Danhatchia australis was originally placed in Yoania (as Y. australis) by Hatch (Citation1963), but he recognised its differences from other species of Yoania by proposing a new section, sect. Tarairea for it. Moore (in Moore & Edgar Citation1970) and Garay & Christenson (Citation1995) elaborated the distinctive differences between this plant and Yoania, which remove it from Yoania (Tribe Cymbidieae) and instead support placement in subtribe Goodyerinae. In Goodyerinae, Garay & Christenson (Citation1995) considered it to be related to Chamaegastrodia, but sufficiently different to be segregated as a new genus Danhatchia. The genus is widely accepted, including by www.e-monocot.org (accessed 27 September 2013). However, there is a recent online record of two observations of D. australis in Australia (http://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=sp&name=Danhatchia∼australis), so Danhatchia should no longer be considered endemic.
Earina Lindl. (3 spp.; de Lange & Rolfe Citation2010)
The current circumscription of Earina includes additional species in Fiji, Vanuatu, Samoa and New Caledonia (e.g. Lehnebach & Robertson Citation2004), so Earina should not be considered endemic.
Ichthyostomum D.L.Jones, M.A.Clem. & Molloy (1 sp.; de Lange & Rolfe Citation2010, but not de Lange et al. Citation2013 who include it under Bulbophyllum)
This monospecific genus was published by Clements & Jones (Citation2002) to accommodate the species previously known as Bulbophyllum pygmaeum. They presented a diagnosis with the comment that several genera were being segregated ‘resulting from detailed morphological and molecular studies that will be published elsewhere.’ I have not found phylogenies that include B. pygmaeum and I note www.e-monocot.org (accessed 27 September 2013) and Felix & Guerra (Citation2010) do not accept Ichthyostomum. Acceptance of Ichthyostomum before the presentation of supporting evidence is not justified.
Breitwieser et al. (Citation2012) treated Ichthyostomum as non-endemic, apparently an error based on an erroneous record (Moore in Moore & Edgar Citation1970) of I. pygmaeum from Lord Howe Island (I. Schönberger pers. comm.), a record not supported by Rodd & Pickard (Citation1983).
Molloybas D.L.Jones & M.A.Clem. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012) and Singularybas Molloy, D.L.Jones & M.A.Clem. (1 sp.; de Lange & Rolfe Citation2010, but not de Lange et al. Citation2013; Breitwieser et al. Citation2012)
Clements et al. (Citation2002) combined ITS sequences and morphological data to derive relationships within Corybas s.l. They promoted numerous small clades to genus rank including Molloybas and Singularybas, both monospecific and endemic to New Zealand.
Molloybas (one species, endemic to New Zealand) and Nematoceras (seven species) were resolved as sister clades, together sister to Corysanthes (five species) (). Molloybas is morphologically distinctive, largely due to characters related to its non-photosynthetic nutrition. Clements et al. (Citation2002) presented strong support for monophyly of Nematoceras (100% bootstrap; 10 ITS and 3 morphological characters) and Molloybas (24 autapomorphies, 8 of them morphological although several are likely to be functionally or developmentally linked). The sister relationship of Nematoceras and Molloybas received 84% bootstrap support (13 supporting characters, of which 6 morphological ones were listed).
de Lange et al. (Citation2013) returned most of these segregate genera (Anzybas, Nematoceras, Singularybas) to a more inclusive circumscription of Corybas. However, without explanation, they continued to accept Molloybas. Such a wider circumscription of Corybas is not monophyletic because some species treated as Corybas, for example, C. trilobus, are more closely related to Molloybas cryptanthus than they are to Corybas aconitiflorus.
With monophyly of both the wide circumscription of Corybas, including both Molloybas and Singularybas, and of the smaller segregate genera apparently well established, the issue resolves around the perceived advantages of large or small genera in this group, as discussed by Hopper (Citation2009).
Singularybas (Corybas oblongus) has a large number of morphological autapomorphies (Clements et al. Citation2002) and its sister clade (six genera) also appears to enjoy moderate support (four synapomorphies, although none of them is unique in the wider tree). Its acceptance is similarly dependent on subjective preferences for narrow vs. wide generic circumscriptions in the Corybas complex.
Molloybas and Singularybas were treated as synonyms of Corybas by www.e-monocot.org (accessed 27 September 2013). I regard their acceptance at generic rank as equivocal on current evidence.
Waireia D.L.Jones, Molloy & M.A.Clem. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Clements et al. (Citation2002) used ITS sequence data to demonstrate that Waireia stenopetala is not closely related to Lyperanthus, where it had previously been classified (as L. antarcticus). Instead, it is well supported (93% bootstrap support) as sister to another monospecific genus, Rimacola from Australia. The Rimacola–Waireia clade is placed among a largely unresolved group of other genera in a consensus tree (Clements et al. Citation2002, based on ITS sequence data). Although classifying them together in a genus would be more information-rich and would avoid rank redundancy, they are strikingly different in appearance and there seems little reason to introduce more names by challenging the status quo, especially while its wider relationships are not resolved. The genus was accepted by www.e-monocot.org (accessed 27 September 2013).
Winika M.A.Clem., D.L.Jones, & Molloy (1 sp.; de Lange & Rolfe Citation2010, but not de Lange et al. Citation2013; Breitwieser et al. Citation2012)
Winika is a monotypic genus segregated from Dendrobium (Clements et al. Citation1997). In publishing the new genus, Clements et al. (Citation1997) described the plants in detail and compared them with Dendrobium sect. Kinetochilus from New Caledonia. However, Burke et al. (Citation2008; ) demonstrated that Winika cunninghamii is sister to the Australian Dendrobium clade and together these are sister to the Asian Dendrobium clade (which includes the type of the genus). Dendrobium sect. Kinetochilus was not included in those studies. If the Australian clade is separated from Dendrobium at generic rank, recognition of Winika might be appropriate, but there is little support from recent consensus trees (Burke et al. Citation2008; Adams Citation2011) for further subdivision of the Australian clade. Burke et al. (Citation2008) and Adams (Citation2011) discuss the options, and use the binomial Dendrobium cunninghamii in their papers. Conran et al. (Citation2009) also followed a broad circumscription of Dendrobium (incl. Winika). The genus was treated as a synonym of Dendrobium by www.e-monocot.org (accessed 27 September 2013). In addition, the cultivar Dendrobium ‘Malcolm Campbell’ is a hybrid between D. kingianum of the Australian clade and D. cunninghamii (http://apps.rhs.org.uk/horticulturaldatabase/orchidregister/orchiddetails.asp?ID=145092). Dendrobium cunninghamii appears to represent a relatively early divergent lineage within Australasian Dendrobium s.l., and future research, which should include species from New Caledonia, might support it as an endemic New Zealand genus.
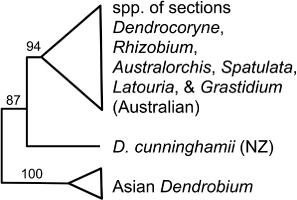
Plantaginaceae
Heliohebe Garn.-Jones (6 spp.; de Lange & Rolfe Citation2010) and Leonohebe Heads (5 spp.; de Lange & Rolfe Citation2010)
Breitwieser et al. (Citation2012) did not accept Heliohebe and Leonohebe as endemic genera because they treated the New Zealand genera Chionohebe, Hebe, Hebejeebie, Heliohebe, Leonohebe and Parahebe as part of Veronica (Garnock-Jones et al. Citation2007), whereas de Lange & Rolfe (Citation2010) did accept Heliohebe and Leonohebe as endemic genera.
Both Heliohebe (Garnock-Jones Citation1993a,Citationb; Wagstaff & Garnock-Jones Citation1998) and Leonohebe (Wagstaff et al. Citation2002; Bayly & Kellow Citation2006) are monophyletic, as circumscribed by Garnock-Jones (Citation1993b) and Bayly & Kellow (Citation2006), respectively, but other genera in the complex are not (Wagstaff et al. Citation2002; Albach & Meudt Citation2010; ). Moreover, the paraphyly of Veronica with respect to the southern hebe complex is well established (Albach & Chase Citation2001; Wagstaff et al. Citation2002; Albach & Meudt Citation2010). Despite assertions that a broad circumscription of Veronica fails to consider other evidence than DNA sequences (Gardner Citation2007; de Lange & Rolfe Citation2010; de Lange et al. Citation2010), phylogenetic inclusion of the hebe complex within Veronica is consistent with evidence from morphology (Oliver Citation1944; Raven Citation1973; Garnock-Jones Citation1975, Citation1993a; Hong Citation1984; Kampny & Dengler Citation1997; Albach & Chase Citation2001; Hufford & McMahon Citation2004; Bayly & Kellow Citation2006; Muñoz-Centeno et al. Citation2006), cytology (Hair Citation1967, Citation1970; Albach & Greilhuber Citation2004; Briggs & Ehrendorfer Citation2006b; Albach et al. Citation2008), chemistry (Taskova et al. Citation2004, Citation2008, Citation2012; Albach et al. Citation2005; Pedersen et al. Citation2007; Jensen et al. Citation2008), while their tendency to hybridise between groups (Garnock-Jones & Lloyd Citation2004; Meudt & Bayly Citation2008) is evidence against multiple genera in New Zealand.
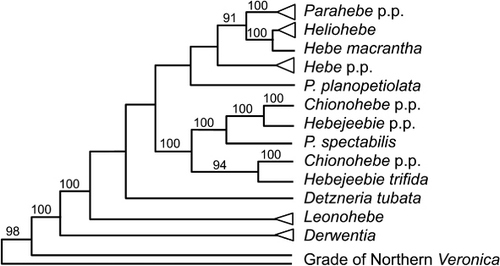
The paraphyly of Veronica and range of potential solutions, particularly with respect to Hebe and Leonohebe, were clearly discussed by Albach et al. (Citation2004) and Bayly & Kellow (Citation2006) and consequences for southern hemisphere plants were addressed nomenclaturally by Garnock-Jones et al. (Citation2007) and Heenan (Citation2012). Albach & Chase (Citation2001), Albach et al. (Citation2004), Garnock-Jones et al. (Citation2007), Mabberley (Citation2008), Albach (Citation2008) and Albach & Meudt (Citation2010) also presented arguments for including all the southern genera in a wide and monophyletic circumscription of Veronica. Briggs & Ehrendorfer (Citation2006a), Jensen et al. (Citation2008), Mabberley (Citation2008), Meudt (Citation2008), Taskova et al. (Citation2008, Citation2012), Humphries & Linder (Citation2009), Hughes et al. (Citation2010), Pufal et al. (Citation2010), Lee et al. (Citation2011), Heenan (Citation2012), Lord et al. (Citation2013), and the Landcare Research herbarium and databases (see Breitwieser et al. Citation2012) have followed this wider circumscription. Nevertheless, other New Zealand botanists have explicitly rejected an inclusive monophyletic circumscription of Veronica (Gardner Citation2007; Thorsen Citation2007; Norton & Molloy Citation2009; de Lange & Rolfe Citation2010; de Lange Citation2011a; Mark Citation2012; de Lange et al. Citation2013; Wilson Citation2013). Their arguments have mostly focused on distinctiveness of the southern segregates, while Norton & Molloy (Citation2009) and CitationMark (2012) also placed significance on their southern distribution. In my opinion, classifying them in Veronica among their northern hemisphere relatives provides a better indication of their relationships, evolution and biogeographic history, and avoids misleading inferences of relationships based on the earlier classification. While continued recognition of segregate genera (Gardner Citation2007; Thorsen Citation2007; Norton & Molloy Citation2009; de Lange & Rolfe Citation2010; de Lange Citation2011a; Mark Citation2012; de Lange et al. Citation2013; Wilson Citation2013) would preserve nomenclatural stability for New Zealand users, this is at the expense of the monophyly of Veronica, Parahebe, Chionohebe and Hebejeebie. Alternatively, if northern hemisphere segregate genera were introduced to avoid paraphyly of Veronica, this would be at the cost of introducing some that were undiagnosable with morphological characters and would result in changes to nearly 200 species names (Albach et al. Citation2004; Garnock-Jones et al. Citation2007; Garnock-Jones & Albach Citation2007). The arguments for inclusion of the hebe complex in Veronica are the same as those for inclusion of Neopaxia within Montia, Iti within Cardamine, Macropiper within Piper and Exarrhena within Myosotis, which have been widely accepted by New Zealand botanists.
Poaceae
Anemanthele Veldkamp (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Anemanthele appears to be either nested within or sister to Austrostipa, according to the molecular phylogenetic studies of Jacobs et al. (Citation2000, Citation2007) and Romaschenko et al. (Citation2012: ITS and nine plastid regions). Romaschenko et al. (Citation2012) found conflicting results on the relationship between Anemanthele and Austrostipa. First, based on cpDNA, they found Anemanthele to be sister to Austrostipa. Second, using ITS data, Anemanthele was sister to one of two Austrostipa clades. If the non-monophyly of Austrostipa found in the ITS tree is supported by further studies, two taxonomic solutions would involve including Anemanthele within either Austrostipa or a part of Austrostipa, removing it from New Zealand's endemic genera. A three-genus solution would retain a monotypic endemic Anemanthele, which would reflect its morphological differences (Entwisle & Weston Citation2005; Jacobs et al. Citation2007). I accept Anemanthele only provisionally as a genus endemic to New Zealand.
Austroderia N.P.Barker & H.P.Linder. (5 spp.; de Lange Citation2011a; Breitwieser et al. Citation2012)
The genus was proposed by Linder et al. (Citation2010) to accommodate a clade of New Zealand species previously treated under Cortaderia. The published phylogeny of Barker et al. (Citation2007a) is complex because of conflicts between the signals from nuclear and cpDNA (). However, it is clear that Austroderia is a clade and is more closely related to Plinthanthesis than it is to Cortaderia. Linder et al. (Citation2010) have recognised clades at generic rank that are all diagnosable and well supported (including Austroderia), rather than an alternative of a large and highly varied genus Danthonia. I accept Austroderia as a genus endemic to New Zealand.
Pyrrhanthera Zotov (1 sp.; de Lange & Rolfe Citation2010, but not de Lange Citation2011a, de Lange et al. Citation2013; Breitwieser et al. Citation2012)
Barker et al. (Citation2007a) analysed data from ITS and three chloroplast regions (rpoC2, trnL intron, rbcL gene) and showed the single species of Pyrrhanthera is nested within Rytidosperma. They treated it as R. exiguum (Kirk) H.P.Linder, a classification that I accept.
Simplicia Kirk (2 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
There appears to be no current doubt that Simplicia (two spp.) is acceptable as a genus and endemic to New Zealand. Gillespie et al. (Citation2009) cited a dissertation by Döring (2009, not seen) who placed it in Poinae and showed Simplicia is not nested within Poa. I accept Simplicia as a genus endemic to New Zealand.
Stenostachys Turcz. (3 spp., de Lange & Rolfe Citation2010; 4 spp., Breitwieser et al. Citation2012)
Stenostachys is a small New Zealand endemic genus with an allopolyploid origin involving Australopyrum and Hordeum, according to the molecular studies of Petersen et al. (Citation2011), who support its recognition as a genus based on analysis of two single-copy nuclear genes, four chloroplast genes and one mitochondrial gene. They support the transfer of Elymus enysii to Stenostachys (Barkworth & Jacobs Citation2011) additional to the three species accepted by Edgar & Connor (Citation2000). I accept Stenostachys as a genus endemic to New Zealand.
Zotovia Edgar & Connor (3 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Verboom et al. (Citation2003) presented a phylogeny based on combined cpDNA, ITS1 and morphology data that showed Zotovia is nested within Ehrharta as currently circumscribed (). Support for this relationship was weak, and the sister relationship of Z. colensoi and M. avenacea was supported only by the ITS data. If these relationships are supported by additional research, both Zotovia and Ehrharta could acquire monophyletic circumscriptions by the transfer of E. avenacea, Microlaena avenacea (these two names have different types and are not considered conspecific) and M. tasmanica to Zotovia (Verboom et al. Citation2003; Kellogg Citation2009). The genus would not then be endemic. Alternatively, those three species could be classified as a sister genus to Zotovia, which latter would then still be endemic. I regard generic status of Zotovia as equivocal on current evidence.
Primulaceae
Elingamita G.T.S.Baylis (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Baylis (Citation1951) compared Elingamita johnsonii with Myrsine and other genera, noting that although it matches Myrsine in its uniseriate ovules, it differs in its paniculate inflorescences, short tubular corolla, well-developed filaments and punctiform stigma. Although Stöckler (Citation2001: ITS, ndhF and two AFLP-derived markers) suggested that E. johnsonii is nested within New Zealand Myrsine, ITS sequences of E. johnsonii independently obtained by P.B. Heenan (pers. comm.) and C.E.C. Gemmill & S. Pratt (pers. comm.) did not support a close relationship of Elingamita within Myrsine. Current research is focused on placing Elingamita in relation to Myrsine and other genera in Primulaceae and testing monophyly of its relatives (C.E.C. Gemmill pers. comm.). I accept Elingamita provisionally as a genus endemic to New Zealand.
Proteaceae
Knightia R.Br. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Recent circumscriptions of Knightia have included one New Zealand and two New Caledonian species (e.g. Virot Citation1968; Jaffré et al. Citation2001). However, Weston & Barker (Citation2006) treated the New Caledonian species as Eucarpha Spach, based on a phylogenetic supertree that combined several molecular studies. In that tree, Knightia grouped with Roupala, Orites and Neorites, whereas Eucarpha grouped with Floydia and Darlingia. Sauquet et al. (Citation2009) presented a phylogeny of Proteaceae, based on nuclear ITS and chloroplast atpB, atpB–rbcL, rbcL, matK, rpl16 intron, trnL intron and trnL–F, in which Knightia was sister to a clade comprising nine genera, one of them Eucarpha (). Their study indicated a Paleogene age for Knightia. I accept Knightia as a genus endemic to New Zealand.
Plants from New Caledonia assigned to Eucarpha differ from K. excelsa in being shrubs or small trees, having smaller flowers and enlarged showy bracts that subtend each floral pair, and woody follicles that dehisce partly along the midrib as well as fully at the margins (Virot Citation1968). They differ also in their pollen: K. deplancheiFootnote2 grains differ from those of K. excelsa by having convex sides, circular and non-protruding pores, weakly developed vestibule and thinner exine with smaller and more scattered bacculae (P.J. Garnock-Jones, unpubl. data).
Toronia L.A.S.Johnson & B.Briggs (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Toronia, a monospecific genus from New Zealand, was previously included in Persoonia, as P. toru (Allan Citation1961). Weston & Barker (Citation2006), Barker et al. (Citation2007b), Hoot & Douglas (Citation1998) and Sauquet et al. (Citation2009) accepted Toronia and included it in Persoonioideae. Sauquet et al. (Citation2009; ), based on ITS and seven cpDNA regions, showed Toronia to be sister to Persoonia (100 spp.), Acidonia (1) and Garnieria (1). However, only a few samples of Persoonia have been included in those studies, and Weston & Barker (Citation2006) referred to unpublished results of Weston & Porter that suggest Persoonia is not monophyletic. Acceptance of Toronia should thus be provisional until those studies are completed.
Barker et al. (Citation2007b) calculated the divergence of Placospermum and Toronia within a range that includes the 80 million year old separation of the New Zealand land mass from Gondwana, but Sauquet et al. (Citation2009) indicate divergence of Toronia is of Miocene age.
Strasburgeriaceae
Ixerba A.Cunn. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
The relationships of Ixerba have been a long-standing enigma (Cameron Citation2003). Recently, DNA sequence data (Sosa & Chase Citation2003; Qiu et al. Citation2010; ), flower morphology (Matthews & Endress Citation2006) and wood anatomy (Patel Citation1973; Cameron Citation2003; Carlquist Citation2007) all support the sister relationship of Ixerba brexioides and Strasburgeria robusta. Although they share a base chromosome number of x = 25, Ixerba (2n = 50) and Strasburgeria (2n = 500) have very different chromosome numbers (Oginuma et al. Citation2006). Maintaining these as two monotypic families or genera appears to be a subjective question. Cameron (Citation2003) listed differences and similarities and argued that maintaining two families was justified; this has been maintained by others (Matthews & Endress Citation2006; Dickison Citation2007; Schneider Citation2007; Endress & Matthews Citation2012). New Zealand botanists (de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012) have followed the Angiosperm Phylogeny Group (APG III Citation2009) and Qiu et al. (Citation2010), including Ixerba in Strasburgeriaceae, a classification that increases information content and decreases rank redundancy. In this case, combining the two species under a single genus would neither increase information content nor decrease rank redundancy and I accept Ixerba as a genus endemic to New Zealand.
Stylidiaceae
Oreostylidium Berggr. (1 sp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Oreostylidium subulatum is nested within the Australian genus Stylidium (Wagstaff & Wege Citation2002; ). The name Stylidium subulatum Hook.f. is available and preferred, for example, by Wege (Citation2012). The New Zealand species appears to be distinctive because it lacks the irritable column and zygomorphic corolla of Stylidium in Australia, but the phylogeny presented by Wagstaff & Wege (Citation2002) would suggest these are secondary losses and not unique in the genus (Wege Citation2012).
Winteraceae
Pseudowintera Dandy (4 spp.; de Lange & Rolfe Citation2010; Breitwieser et al. Citation2012)
Doust & Drinnan (Citation2004) used ITS and trnL sequence data and morphology to study the relationships and monophyly of Tasmannia (Winteraceae). They found strong support for a monophyletic Pseudowintera (two of the four species were sampled), strongly supported as sister to a clade of Belliolum, Bubbia, Exospermum and Zygogynum. I accept Pseudowintera as a genus endemic to New Zealand.
Discussion
This re-evaluation of the taxonomic and distribution status of up to 59 seed plant genera that are treated as endemic in New Zealand has reduced the number to between 28 and 44. Fifteen genera, all angiosperms, should no longer be accepted as endemic or should not be accepted at generic rank. For these, either the monophyly of a related assemblage has been falsified (e.g. Kirkianella and Embergeria, Heliohebe and Leonohebe, Oreostylidium), or they have been shown to occur outside New Zealand (e.g. Danhatchia, Earina, Stilbocarpa). The status of a further 16 genera is equivocal and these require further reconsideration of their relationships and status. Thus, about half of the currently accepted endemic genera in New Zealand are taxonomically rejected or equivocal or they are not endemic. Even in some pairs of genera for which reciprocal monophyly has not been conclusively falsified (e.g. Lophomyrtus and Neomyrtus, Teucridium and Teucrium), currently available research indicates uncertainty, and a few of them could more informatively be classified in the same genus as close relatives in New Zealand and overseas.
This major reduction in the number of endemic genera recognised in New Zealand is a logical taxonomic outcome of recent molecular studies of relationships (Mitchell & Heenan Citation2000; Wagstaff & Wege Citation2002; Wagstaff & Breitwieser Citation2004) and a corollary of recent biogeographic research (Winkworth et al. Citation2002b) in the New Zealand flora. There has been widespread recognition in the last 15 years that the flora is mostly of Oligocene or more recent origin in New Zealand and very few groups are Mesozoic relicts. The very recent uplift of the New Zealand mountains (Heenan & McGlone Citation2012), where most of the plant species diversity is found, correlates well with the young age of the flora. In an analysis of Tasmanian endemic genera, Rozefelds (Citation2008) divided them into paleoendemics and neoendemics, and showed that members of these two groupings tended to have different distributions. In New Zealand, a few endemic genera with a Paleogene (65–23 Ma) or older advent (e.g. Ixerba, Knightia, Rhabdothamnus) are found in northern and lowland sites, whereas some of the likely neoendemics (e.g. Notothlaspi, Raoulia) are southern and alpine. However, we must await better estimates of dating before such an analysis can be completed for New Zealand endemic genera.
McGlone et al. (Citation2001) calculated that c. 10% of vascular plant genera (including ferns) in New Zealand are endemic, compared with 15% in Hawaii and 13.5% in New Caledonia. Here, I provide an estimate of 7–10.5% for the seed plants. My upper bound is higher than the estimate of McGlone et al. (Citation2001) because their figure included ferns, a group with low endemism. Such estimates are highly susceptible to taxonomic concepts and methodologies. Floras with a long history of isolation from other landmasses would be expected to have more endemism overall and more endemism at higher ranks than do young floras. However, if phenetic criteria are widely used in a young and rapidly diversifying island flora, high levels of inferred endemism might be expected, and this might be misinterpreted as a greater age for the flora. Where generic classification is partly or wholly based on phenetic considerations, it is likely that generic endemism in island floras will be overestimated.
Some families appear to have greater numbers of endemic genera than their size, or their size in New Zealand, would suggest. Thus Loranthaceae, with just 7 species in New Zealand (Breitwieser et al. Citation2012), has 3 or 4 endemic genera, compared with 84 genera for 950 species world-wide (Mabberley Citation2008). It may be that in some families, such as Loranthaceae and Balanophoraceae, our few species are geographic outliers and their adaptations to local conditions have led to their segregation at genus rank. In Brassicaceae, phylogenetic systematics has led to a reduction in the number of recognised endemic genera from four (Garnock-Jones Citation1991a) to the current one (Heenan et al. Citation2002; but see Heenan et al. Citation2012).
The previous recognition of 50–60 endemic genera is not just an artefact of an earlier view that the New Zealand flora is ancient and has evolved here in long isolation. Many of the genera rejected here display considerable morphological and ecological divergence, which encouraged their separation in a pre-phylogenetic era. However, recognising their sister relationships through phylogenetic classification enables a more realistic appraisal of their evolutionary history. For example, treating Haastia as an endemic genus gives no hint that these distinctive vegetable sheep are nested within Brachyglottis, whereas including them in Brachyglottis makes it immediately clear that this is an example of the evolution of a cushion-plant clade within a genus of shrubby plants, and is an instructive parallel to the distinctive cushion-plant clades within Raoulia, Veronica and Myosotis.
Sonchus is a good example of a widespread and otherwise coherent genus that has diverged morphologically on islands or in extreme habitats (Kim et al. Citation2007). Small distinctive locally endemic clades are nested within Sonchus in New Zealand (previously treated as Embergeria and Kirkianella), Juan Fernández Islands (treated as Dendroseris until Mejías & Kim Citation2012) and Australia (Actites), while a diverse generic complex in Macaronesia (Babcockia, Chrysoprenanthes, Lactucosonchus, Sventenia, Taeckholmia) appears to be sister to Sonchus s.l.
However, there are probably other genera that are not currently considered to be endemic, but for which changes to current circumscriptions may be needed to make them monophyletic. The approach adopted here has taken the currently recognised endemic genera and subjected them to consistent criteria based upon the primacy of clade recognition and analyses based on molecular phylogenetics. It is important to note that, by starting with such a list, a reduction was probably likely. Critical assessment of the status of all genera in the flora might, however, lead to the recognition of additional endemic genera, increasing the total for the country, as in the following three examples. First, the current circumscription of Pseudopanax (Araliaceae) may be paraphyletic (Nicolas & Plunkett Citation2009) and the authors resolve this by accepting the segregate genus Neopanax, which was considered endemic when Allan (Citation1961) first named it. Additionally, in Araliaceae, Mitchell et al. (Citation2012) showed the current circumscription of Raukaua is paraphyletic and Australian and South American species should be excluded. Potentially, an emended circumscription of Raukaua might be endemic to New Zealand, but this depends on whether species of Cheirodendron from Hawaii and the Marquesas Islands should be included.
Second, Ischnocarpus and Pachycladon (Brassiaceae), formerly endemic genera, are now combined (Heenan et al. Citation2002) with the non-endemic Cheesemania in a wider circumscription of Pachycladon. Heenan et al. (Citation2012) showed that including the Tasmanian P. radicata in the circumscription of Pachycladon makes the genus non-monophyletic. This justifies taxonomic exclusion of P. radicata, and that would make Pachycladon endemic to New Zealand. Additionally, except for P. wallii, the three former genera form morphologically distinctive endemic clades that have weak molecular support (the former C. wallii has morphological and molecular similarities to P. novae-zelandiae and P. crenatus, and shares their southern distribution—Garnock-Jones Citation1991b; Heenan et al. Citation2002). However, fertile hybrids between these clades have been produced (Heenan Citation1999b) and retention of a single endemic genus might be preferable.
Third, the current paraphyletic circumscription of Olearia (Asteraceae) will need to be revised. If Celmisia and Pachystegia are to be retained, Olearia will need to be divided into several clades (Cross et al. Citation2002). Some of these, for example, Shawia, might be endemic New Zealand genera.
Additionally, it is not immediately clear how to list genera that have lost their endemic status through recent migration (e.g. Ileostylus, Mills Citation2010) or to account for the possibility of a genus becoming endemic through extinction of a foreign population.
In any case, it is pertinent to question the significance of genera in analyses of the flora such as those of McGlone et al. (Citation2001), Webb et al. (Citation1999) and Lee et al. (Citation2011). The genus is just one of many ranks in the classification hierarchy, and some of its significance derives from the binomial system, wherein the genus name forms part of the species name. Choosing to analyse the flora at the rank of genus or of family is simply using the rank as a proxy for clades that might be equivalent in size and significance. However, one should not assume that genera, even if they are monophyletic, are in any way necessarily equivalent. Some kinds of analyses should focus instead on clades of any rank. For example, treating current genera as data points in analyses could over-represent some groups, such as the Raoulia complex (currently including four endemic genera) and under-represent others (clades within Pachycladon). Additionally, excluding from such analyses clades that originated and diversified in New Zealand, but which through dispersal are not endemic (e.g. Veronica sect. Hebe or the Aciphylla–Anisotome complex) could also be misleading. An analysis of endemism that compared the most inclusive endemic clades in terms of their size, fossil or molecular age of origin in New Zealand, degree of morphological and physiological divergence, internal disparity of morphology, physiology, habitat requirements, or sexual systems would be revealing.
Acknowledgements
Several people helped with discussions and information: Eric Knox (Campanulaceae); Peter de Lange (orchids and grasses); Steve Pratt, Chrissen Gemmill, Peter Heenan and Karin Stöckler (Elingamita); and Angela Moles. I thank Chrissen Gemmill, Heidi Meudt, and two anonymous referees who read and commented on an earlier draft.
Notes
1. The epithet hortensia is a noun in apposition (www.ipni.org/index.html). Hortensia is a synonym of Hydrangea, of which some species have dense corymbs of blue flowers like those of Myosotidium. The epithet is not to be altered to hortensium as by Heenan & Schönberger (Citation2009) and de Lange et al. (Citation2010, Citation2013).
2. It appears that although the name Eucarpha is legitimate, combinations for the two species in New Caledonia have not been made. Therefore, it is necessary to refer to them as Knightia until new combinations (Weston & Garnock-Jones in prep.) are published.
References
- Abu-Asab MS, Cantino PD 1993. Phylogenetic implications of pollen morphology in Tribe Ajugeae (Labiatae). Systematic Botany 18: 100–122. 10.2307/2419791
- Adams PB 2011. Systematics of Dendrobiinae (Orchidaceae), with special reference to Australian taxa. Botanical Journal of the Linnean Society 166: 105–126. 10.1111/j.1095-8339.2011.01141.x
- Al-Shehbaz IA 2012. A generic and tribal synopsis of the Brassicaceae (Cruciferae). Taxon 61: 931–954.
- Albach DC 2008. Further arguments for the rejection of paraphyletic taxa: Veronica subgen. Pseudolysimachium (Plantaginaceae). Taxon 57: 1–6.
- Albach DC, Briggs BG 2012. Phylogenetic analysis of Australian species of Veronica (V. section Labiatoides; Plantaginaceae). Australian Systematic Botany 25: 353–363. 10.1071/SB12014
- Albach DC, Chase MW 2001. Paraphyly of Veronica (Veroniceae: Scrophulariaceae): evidence from internal transcribed spacer (ITS) sequences of nuclear ribosomal DNA. Journal of Plant Research 114: 9–18. 10.1007/PL00013971
- Albach DC, Greilhuber J 2004. Genome size variation and evolution in Veronica. Annals of Botany 94: 897–911. 10.1093/aob/mch219
- Albach DC, Jensen SR, Özgökce F, Grayer RJ 2005. Veronica: chemical characters for the support of phylogenetic relationships based on nuclear ribosomal and plastid DNA sequence data. Biochemical Systematics and Ecology 33: 1087–1106. 10.1016/j.bse.2005.06.002
- Albach DC, Martínez-Ortega MM, Delgado L, Weiss-Schneeweiss H, Özgökce F, Fischer MA 2008. Chromosome numbers in Veroniceae (Plantaginaceae): review and several new counts. Annals of the Missouri Botanical Garden 95: 543–566. 10.3417/2006094
- Albach DC, Martínez-Ortega MM, Fischer MA, Chase MW 2004. A new classification of the tribe Veroniceae — problems and a possible solution. Taxon 53: 429–452. 10.2307/4135620
- Albach DC, Meudt HM 2010. Phylogeny of Veronica in the Southern and Northern Hemispheres based on plastid, nuclear ribosomal and nuclear low-copy DNA. Molecular Phylogenetics and Evolution 54: 457–471. 10.1016/j.ympev.2009.09.030
- Allan HH 1961. Flora of New Zealand, Vol. 1. Wellington, Government Printer.
- Antonelli A 2008. Higher level phylogeny and evolutionary trends in Campanulaceae subfam. Lobelioideae: molecular signal overshadows morphology. Molecular phylogenetics and evolution 46: 1–18. 10.1016/j.ympev.2007.06.015
- APG III 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105–121. 10.1111/j.1095-8339.2009.00996.x
- Applequist WL, Wagner WL, Zimmer EA, Nepokroeff M 2006. Molecular evidence resolving the systematic position of Hectorella (Portulacaceae). Systematic Botany 31: 310–319. 10.1600/036364406777585900
- Ashlock PD 1971. Monophyly and associated terms. Systematic Biology 20: 63–69. 10.1093/sysbio/20.1.63
- Barker NP, Galley C, Verboom GA, Mafa P, Gilbert M, Linder HP 2007a. The phylogeny of the austral grass subfamily Danthonioideae: evidence from multiple data sets. Plant Systematics and Evolution 264: 135–156. 10.1007/s00606-006-0479-9
- Barker NP, Weston PH, Rutschmann F, Sauquet H 2007b. Molecular dating of the ‘Gondwanan’ plant family Proteaceae is only partially congruent with the timing of the break-up of Gondwana. Journal of Biogeography 34: 2012–2027. 10.1111/j.1365-2699.2007.01749.x
- Barkworth ME, Jacobs SWL 2011. The Triticeae (Gramineae) in Australasia. Telopea 13: 37–56.
- Baylis GTS 1951. Elingamita (Myrsinaceae) a new monotypic genus from West Island, Three Kings. Records of the Auckland Institute and Museum 4: 99–102.
- Bayly MJ, Kellow AV 2006. An illustrated guide to New Zealand Hebes. Wellington, Te Papa Press.
- Bentham G, Hooker JD 1873. Sonchus. Genera Plantarum 2: 528.
- Bernardello G, Anderson GJ, Stuessy TF, Crawford DJ 2006. The angiosperm flora of the Archipelago Juan Fernández (Chile): origin and dispersal. Canadian Journal of Botany 84: 1266–1281. 10.1139/b06-092
- Beuzenberg EJ 1961. Observations on sex differentiation and cytotaxonomy of the New Zealand species of the Hymenantherinae (Violaceae). New Zealand Journal of Science 4: 337–349.
- Beuzenberg EJ, Hair JB 1984. Contributions to a chromosome atlas of the New Zealand flora—27. Compositae. New Zealand Journal of Botany 22: 353–356. 10.1080/0028825X.1984.10425266
- Biffin E, Conran JG, Lowe AJ 2011. Podocarp evolution: a molecular phylogenetic perspective. Smithsonian Contributions to Botany 95: 1–20. 10.5479/si.0081024X.95.1
- Breitwieser I 2008. Comparative leaf anatomy of New Zealand and Tasmanian Inuleae (Compositae). Botanical Journal of the Linnean Society 111: 183–209. 10.1111/j.1095-8339.1993.tb01898.x
- Breitwieser I, Brownsey PJ, Garnock-Jones PJ, Perrie LR, Wilton A 2012. Phylum Tracheophyta, vascular plants. In: Gordon D ed. The New Zealand inventory of biodiversity: a Species 2000 Symposium review. Christchurch, University of Canterbury Press. Pp. 411–459.
- Breitwieser I, Glenny DS, Thorne A, Wagstaff SJ 1999. Phylogenetic relationships in Australasian Gnaphalieae (Compositae) inferred from ITS sequences. New Zealand Journal of Botany 37: 399–412. 10.1080/0028825X.1999.9512644
- Breitwieser I, Sampson FB 1997a. Pollen characteristics of New Zealand Gnaphalieae (Compositae) and their taxonomic significance. I. LM and SEM. Grana 36: 65–79. 10.1080/00173139709362593
- Breitwieser I, Sampson FB 1997b. Pollen characteristics of New Zealand Gnaphalieae (Compositae) and their taxonomic significance. II. TEM. Grana 36: 80–95. 10.1080/00173139709362594
- Breitwieser I, Ward JM 1993. Systematics of New Zealand Inuleae (Compositae- Asteraceae)—3 numerical phenetic analysis of leaf anatomy and flavonoids. New Zealand Journal of Botany 31: 43–58. 10.1080/0028825X.1993.10419533
- Breitwieser I, Ward JM 1998. Leaf anatomy of Raoulia Hook.f. (Compositae, Gnaphalieae) Botanical Journal of the Linnean Society 126: 217–235.
- Breitwieser I, Ward JM 2005. Morphological evidence for the tribal position of Haastia (Asteraceae). New Zealand Journal of Botany 43: 767–777. 10.1080/0028825X.2005.9512989
- Brickell CD, Crawley M, Cullen J, Frodin DG, Gardner M, Grey-Wilson C et al. 2008. Do the views of users of taxonomic output count for anything? Taxon 57: 1047–1048.
- Briggs BG, Ehrendorfer F 2006a. New Australian species and typifications in Veronica (Plantaginaceae). Telopea 11: 276–292.
- Briggs BG, Ehrendorfer F 2006b. Chromosome numbers of Australian and New Guinea species of Veronica (Plantaginaceae). Telopea 11: 294–298.
- Brummitt RK 2002. How to chop up a tree. Taxon 51: 31–41. 10.2307/1554961
- Brummitt RK 2006. Am I a bony fish? [Letter to the editor]. Taxon 55: 268–269. 10.2307/25065576
- Brunken U, Muellner AN 2012. A new tribal classification of Grewioideae (Malvaceae) based on morphological and molecular phylogenetic evidence. Systematic Botany 37: 699–711. 10.1600/036364412X648670
- Burke JM, Bayly MJ, Adams PB, Ladiges PY 2008. Molecular phylogenetic analysis of Dendrobium (Orchidaceae), with emphasis on the Australian section Dendrocoryne, and implications for generic classification. Australian Systematic Botany 21: 1–14. 10.1071/SB07038
- Burleigh JG, Barbazuk WB, Davis JM, Morse AM, Soltis PS 2012. Exploring diversification and genome size evolution in extant gymnosperms through phylogenetic synthesis. Journal of Botany.
- Burret M 1941. Myrtaceen-Studien. Notizblatt des Botanischen Gartens und Museums zu Berlin-Dahlem 15: 479–550. 10.2307/3995084
- Cameron KM 2003. On the Phylogenetic position of the New Caledonian endemic families Paracryphiaceae, Oncothecaceae, and Strasburgeriaceae: a comparison of molecules and morphology. Botanical Review 68: 428–443. 10.1663/0006-8101(2002)068[0428:OTPPOT]2.0.CO;2
- Carlquist S 2007. Wood anatomy of Crossosomatales: patterns of wood evolution with relation to phylogeny and ecology. Aliso 24: 1–18. 10.5642/aliso.20072401.02
- Chase MW, Soltis DE, Olmstead RG, Morgan D, Les DH, Mishler BD et al. 1993. Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden 80: 528–580. 10.2307/2399846
- Christenhusz MJM, Reveal JL, Farjon A, Gardner MF, Mill RR, Chase MW 2011. A new classification and linear sequence of extant gymnosperms. Phytotaxa 19: 55–70.
- Chung K-F 2007. Inclusion of the South Pacific alpine genus Oreomyrrhis (Apiaceae) in Chaerophyllum based on nuclear and chloroplast DNA sequences. Systematic Botany 32: 671–681. 10.1600/036364407782250517
- Clarkson BD 1988. A natural intergeneric hybrid, Celmisia gracilenta × Olearia arborescens (Compositae) from Mt Tarawera, New Zealand. New Zealand Journal of Botany 26: 325–331. 10.1080/0028825X.1988.10410122
- Clements MA, Jones DL 2002. Nomenclatural changes in the Australian and New Zealand Bulbophyllinae and Eriinae (Orchidaceae). Orchadian 13: 498–501.
- Clements MA, Jones DL, Molloy BPJ 1997. Winika, a new monotypic genus for the New Zealand orchid previously known as Dendrobium cunninghamii Lindl. Orchadian 12: 214–219.
- Clements MA, Jones DL, Sharma IK, Nightingale ME, Garratt MJ, Fitzgerald KJ et al. 2002. Phylogenetics of Diurideae (Orchidaceae) based on the internal transcribed spacer (ITS) regions of nuclear ribosomal DNA. Lindleyana 17: 135–171.
- Cohen JI 2013. A phylogenetic analysis of morphological and molecular characters of Boraginaceae: evolutionary relationships, taxonomy, and patterns of character evolution. Cladistics. doi:10.1111/cla.12036
- Connor HE, Edgar E 1987. Name changes in the indigenous New Zealand flora, 1960–1986 and Nomina Nova IV, 1983–1986. New Zealand Journal of Botany 25: 115–170. 10.1080/0028825X.1987.10409961
- Conran JG, Bannister JM, Lee DE 2009. Earliest orchid macrofossils: early Miocene Dendrobium and Earina (Orchidaceae: Epidendroideae) from New Zealand. American Journal of Botany 96: 466–474. 10.3732/ajb.0800269
- Cronquist A 1987. A botanical critique of cladism. Botanical Review 53: 1–52. 10.1007/BF02858181
- Cross EW, Quinn CJ, Wagstaff SJ 2002. Molecular evidence for the polyphyly of Olearia (Astereae: Asteraceae). Plant Systematics and Evolution 235: 99–120. 10.1007/s00606-002-0198-9
- Darwin C 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. 1st edition. London, John Murray.
- Dawson JW 1961. A revision of the genus Anisotome (Umbelliferae). University of California Publications in Botany 33: 1–98.
- Dawson JW 1967. New Zealand Umbelliferae. Lignocarpa gen. nov. and Scandia gen. nov. New Zealand Journal of Botany 5: 400–417. 10.1080/0028825X.1967.10428755
- Dawson JW, Lucas R 2011. New Zealand's native trees. Nelson, Craig Potton Publishing.
- Dawson MI 2000. Index of chromosome numbers of indigenous New Zealand spermatophytes. New Zealand Journal of Botany 38: 47–150. 10.1080/0028825X.2000.9512673
- de Lange P 2011a. New endemic genus for toetoe. Trilepidea 87: 7–8.
- de Lange P 2011b. What's up in the Santalales? Trilepidea 91: 1–5.
- de Lange PJ 2012. Taxonomic notes on the New Zealand flora: new names in Piper (Piperaceae). New Zealand Journal of Botany 50: 485–487. 10.1080/0028825X.2012.708904
- de Lange P, Heenan P, Norton D, Rolfe J, Sawyer J 2010. Threatened plants of New Zealand. Christchurch, University of Canterbury Press.
- de Lange PJ, Rolfe JR 2010. New Zealand indigenous vascular plant checklist. Wellington, New Zealand Plant Conservation Network.
- de Lange PJ, Rolfe JR, Champion PD, Courtney SP, Heenan PB, Barkla JW et al. 2013. Conservation status of New Zealand indigenous vascular plants, 2012. Department of Conservation Te Papa Atawhai New Zealand Threat Classification Series 3: 1–70.
- De Laubenfels DJ 1969. A revision of the Malesian and Pacific rainforest conifers, I. Podocarpaceae, in part. Journal of the Arnold Arboretum 50: 274–369.
- de Queiroz K, Gauthier J 1994. Toward a phylogenetic system of biological nomenclature. Trends in Ecology and Evolution 9: 27–31. 10.1016/0169-5347(94)90231-3
- Department of Scientific and Industrial Research 1987. Botany division triennial report, 1982–84. Wellington, Department of Scientific and Industrial Research.
- Der JP, Nickrent DL 2008. A molecular phylogeny of Santalaceae (Santalales). Systematic Botany 33: 107–116. 10.1600/036364408783887438
- Dickison W 2007. Strasburgeriaceae. In: Kubitzki K ed. The families and genera of vascular plants, Volume 9. Flowering plants: Eudicots. Berlin, Springer-Verlag. Pp. 446–448.
- Doust AN, Drinnan AN 2004. Floral development and molecular phylogeny support the generic status of Tasmannia (Winteraceae). American Journal of Botany 91: 321–331. 10.3732/ajb.91.3.321
- Downie SR, Spalik K, Katz-Downie DS, Reduron J-P 2010. Major clades within Apiaceae subfamily Apioideae as inferred by phylogenetic analysis of nrDNA ITS sequences. Plant Diversity and Evolution 128: 111–136. 10.1127/1869-6155/2010/0128-0005
- Drury DG 1968. A clarification of the generic limits of Olearia and Pleurophyllum (Astereae-Compositae). New Zealand Journal of Botany 6: 459–466. 10.1080/0028825X.1968.10428583
- Edgar E, Connor HE 2000. Flora of New Zealand, Volume. 5: Gramineae. Lincoln, Manaaki Whenua Press.
- Endersby J 2009. Lumpers and splitters: Darwin, Hooker, and the search for order. Science 326: 1496–1499. 10.1126/science.1165915
- Endress PK, Matthews ML 2012. Progress and problems in the assessment of flower morphology in higher-level systematics. Plant Systematics and Evolution 298: 257–276. 10.1007/s00606-011-0576-2
- Engler A 1897. Loranthaceae. In: Engler A, Prantl K eds. Die Natürlichen Pflanzenfamilien Nachträge zum II–IV. Teil.: 124–140.
- Entwisle TJ, Weston PH 2005. Majority rules, when systematists disagree. Australian Systematic Botany 18: 1–6. 10.1071/SB04013
- Fay MF, Bennett JR, Dixon KW, Christenhusz MJM 2010. Parasites, their relationships and the disintegration of Scrophulariaceae sensu lato. Curtis's Botanical Magazine 26: 286–313. 10.1111/j.1467-8748.2009.01668.x
- Felix LP, Guerra M 2010. Variation in chromosome number and the basic number of subfamily Epidendroideae (Orchidaceae). Botanical Journal of the Linnean Society 163: 234–278. 10.1111/j.1095-8339.2010.01059.x
- Garay LA, Christenson EA 1995. Danhatchia: a new genus for Yoania australis. Orchadian 11: 469–471.
- Gardner RO 2007. Hebe and Hera. New Zealand Botanical Society Newsletter 89: 14–15.
- Garnock-Jones PJ 1975. A systematic study of Parahebe Oliver in New Zealand. Unpublished PhD thesis. Christchurch, New Zealand, University of Canterbury.
- Garnock-Jones PJ 1991a. Seed morphology and anatomy of the New Zealand genera Cheesemania, Ischnocarpus, Iti, Notothlaspi, and Pachycladon (Brassicaceae). New Zealand Journal of Botany 29: 71–82. 10.1080/0028825X.1991.10415544
- Garnock-Jones PJ 1991b. Gender dimorphism in Cheesemania wallii (Brassicaceae). New Zealand Journal of Botany 29: 87–90. 10.1080/0028825X.1991.10415546
- Garnock-Jones PJ 1993a. Phylogeny of the Hebe complex (Scrophulariaceae: Veroniceae). Australian Systematic Botany 6: 457–479. 10.1071/SB9930457
- Garnock-Jones PJ 1993b. Heliohebe (Scrophulariaceae – Veroniceae), a new genus segregated from Hebe. New Zealand Journal of Botany 31: 323–339. 10.1080/0028825X.1993.10419510
- Garnock-Jones PJ, Albach D 2007. Veronica and Hebe, a response to Rhys Gardner. New Zealand Botanical Society Newsletter 90: 12–14.
- Garnock-Jones PJ, Albach D, Briggs BG 2007. Botanical names in Southern Hemisphere Veronica (Plantaginaceae): sect. Detzneria, sect. Hebe, and sect. Labiatoides. Taxon 56: 571–582.
- Garnock-Jones PJ, Johnson PN 1987. Iti lacustris (Brassicaceae), a new genus and species from southern New Zealand. New Zealand Journal of Botany 25: 603–610. 10.1080/0028825X.1987.10410091
- Garnock-Jones PJ, Lloyd DG 2004. A taxonomic revision of Parahebe (Plantaginaceae) in New Zealand. New Zealand Journal of Botany 42: 181–232. 10.1080/0028825X.2004.9512899
- Gillespie LJ, Soreng RJ, Jacobs SWL 2009. Phylogenetic relationships of Australian Poa (Poaceae: Poinae), including molecular evidence for two new genera, Saxipoa and Sylvipoa. Australian Systematic Botany 22: 413–436. 10.1071/SB09016
- Given DR 1973. Damnamenia gen. nov. A new subantarctic genus allied to Celmisia Cass. (Astereae—Compositae). New Zealand Journal of Botany 11: 785–796. 10.1080/0028825X.1973.10430310
- Godley EJ 1975. Flora and vegetation. In: Kuschel G ed. Biogeography and ecology in New Zealand. The Hague, Junk. Pp. 177–229.
- Goldacre B 2008. Bad science. London, Harper Perennial.
- Grayer RJ, Veitch NC, Kite GC, Paton AJ, Garnock-Jones PJ 2002. Scutellarein 4’-methyl ether glycosides as taxonomic markers in Teucridium and Tripora (Lamiaceae, Ajugoideae). Phytochemistry 60: 727–731. 10.1016/S0031-9422(02)00192-9
- Green PS 1970. Notes relating to the floras of Norfolk and Lord Howe Islands, I. Journal of the Arnold Arboretum 51: 204–220.
- Hair JB 1967. Contributions to a chromosome atlas of the New Zealand flora —10. Hebe (Scrophulariaceae). New Zealand Journal of Botany 5: 322–352. 10.1080/0028825X.1967.10428752
- Hair JB 1970. Contributions to a chromosome atlas of the New Zealand flora — 13. Parahebe and Pygmea (Scrophulariaceae). New Zealand Journal of Botany 8: 255–259. 10.1080/0028825X.1970.10429126
- Hamilton A, Wheeler QD 2008. Taxonomy and why history of science matters for science. Isis 99: 331–340. 10.1086/588691
- Hatch ED 1963. Notes on New Zealand orchids II. Transactions of the Royal Society of New Zealand, Botany 2: 185–188.
- Heenan PB 1998a. Phylogenetic analysis of the Carmichaelia complex, Clianthus, and Swainsona (Fabaceae), from Australia and New Zealand. New Zealand Journal of Botany 36: 21–40.
- Heenan PB 1998b. Montigena (Fabaceae – Galegeae), a new genus endemic to New Zealand. New Zealand Journal of Botany 36: 41–51. 10.1080/0028825X.1998.9512545
- Heenan PB 1998c. An emended circumscription of Carmichaelia (Fabaceae – Galegeae), with new combinations, a key, and notes on hybrids. New Zealand Journal of Botany 36: 53–63. 10.1080/0028825X.1998.9512546
- Heenan PB 1999a. A taxonomic revision of Neopaxia Ö.Nilss. (Portulacaceae) in New Zealand. New Zealand Journal of Botany 37: 213–234. 10.1080/0028825X.1999.9512629
- Heenan PB 1999b. Artificial intergeneric hybrids between the New Zealand endemic Ischnocarpus and Pachycladon (Brassicaceae). New Zealand Journal of Botany 37: 595–601. 10.1080/0028825X.1999.9512656
- Heenan PB 2002. Cardamine lacustris, a new combination for Iti lacustris (Brassicaceae). New Zealand Journal of Botany 40: 563–569. 10.1080/0028825X.2002.9512816
- Heenan PB 2007. New combinations in Montia (Portulacaceae). New Zealand Journal of Botany 45: 437–439. 10.1080/00288250709509725
- Heenan PB 2012. Taxonomic notes on the New Zealand flora: new names in Veronica (Plantaginaceae). New Zealand Journal of Botany 50: 87–88. 10.1080/0028825X.2011.633534
- Heenan PB, Goeke DF, Houliston GJ, Lysák MA 2012. Phylogenetic analyses of ITS and rbcL DNA sequences for sixteen genera of Australian and New Zealand Brassicaceae result in the expansion of the tribe Microlepidieae. Taxon 61: 970–979.
- Heenan PB, Knox EB, Courtney SP, Johnson PN, Dawson MI 2008. Generic placement in Lobelia and revised taxonomy for New Zealand species previously in Hypsela and Isotoma (Lobeliaceae). New Zealand Journal of Botany 46: 87–100. 10.1080/00288250809509756
- Heenan PB, McGlone MS 2012. Evolution of New Zealand alpine and open-habitat plant species during the late Cenozoic. New Zealand Journal of Ecology 37: 105–113.
- Heenan PB, Mitchell AD, Koch M 2002. Molecular systematics of the New Zealand Pachycladon (Brassicaceae) complex: generic circumscription and relationships to Arabidopsis sens. lat. and Arabis sens. lat. New Zealand Journal of Botany 40: 543–562. 10.1080/0028825X.2002.9512815
- Heenan PB, Mitchell AD, de Lange PJ, Keeling J, Paterson AM 2010. Late-Cenozoic origin and diversification of Chatham Islands endemic plant species revealed by analyses of DNA sequence data. New Zealand Journal of Botany 48: 83–136. 10.1080/0028825X.2010.494337
- Heenan PB, Schönberger I 2009. Typification of Myosotis hortensia Decne., the basionym of Myosotidium hortensium (Decne.) Baill., and its synonym Cynoglossum nobile Hook.f. (Boraginaceae). New Zealand Journal of Botany 47: 121–125. 10.1080/00288250909509798
- Heenan PB, Smissen RD 2013. Revised circumscription of Nothofagus and recognition of the segregate genera Fuscospora, Lophozonia, and Trisyngyne (Nothofagaceae). Phytotaxa 146: 1–31. 10.11646/phytotaxa.146.1.1
- Hennig W 1950. Grundzüge einer Theorie der phylogenetischen Systematik. Berlin, Deutscher Zentralverlag.
- Hennig W 1966. Phylogenetic Systematics. Davis D, Zangerl R trans. Urbana, University of Illinois Press.
- Hong DY 1984. Taxonomy and evolution of the Veroniceae (Scrophulariaceae) with special reference to palynology. Opera Botanica 75: 1–60.
- Hooker JD 1859. Myosotidium nobile. Curtis's Botanical Magazine, 3rd series. 15: t. 5137.
- Hooker JD 1864. Handbook of the New Zealand Flora. London, Reeve.
- Hoot SB, Douglas AW 1998. Phylogeny of the Proteaceae based on atpB and atpB-rbcL intergenic spacer region sequences. Australian Systematic Botany 11: 301–320. 10.1071/SB98027
- Hopper SD 2009. Taxonomic turmoil down-under: recent developments in Australian orchid systematics. Annals of Botany 104: 447–455. 10.1093/aob/mcp090
- Hörandl E, Stuessy TF 2010. Paraphyletic groups as natural units of biological classification. Taxon 59: 1641–1653.
- Hufford L, McMahon M 2004. Morphological evolution and systematics of Synthyris and Besseya (Veroni-caceae): a phylogenetic analysis. Systematic Botany 29: 716–736. 10.1600/0363644041744310
- Hughes CE, Eastwood RJ, Bailey CD 2006. From famine to feast? Selecting nuclear DNA sequence loci for plant species-level phylogeny reconstruction. Philosophical Transactions of the Royal Society series B 361: 211–225. 10.1098/rstb.2005.1735
- Hughes NM, Smith WK, Gould KS 2010. Red (anthocyanic) leaf margins do not correspond to increased phenolic content in New Zealand Veronica spp. Annals of Botany 105: 647–654. 10.1093/aob/mcq005
- Humphries AM, Linder HP 2009. Concept versus data in delimitation of plant genera. Taxon 58: 1054–1074.
- Jacobs S, Bayer R, Everett J, Arriaga M, Barkworth M, Sabin-Badereau A et al. 2007. Systematics of the tribe Stipeae (Gramineae) using molecular data. Aliso 23: 349–361. 10.5642/aliso.20072301.28
- Jacobs SWL, Everett J, Barkworth ME, Hsiao C 2000. Relationships within the Stipoid grasses (Gramineae). In: Jacobs SWL, Everett J eds. Grasses, systematics and evolution. Melbourne, CSIRO Publishing. Pp. 75–82.
- Jaffré, T, Morat P, Veillon J-M, Rigault F, Dagostini G 2001. Composition et caracterisation de la flore indigène de Nouvelle-Calédonie. New Caledonia, Centre IRD de Noumea.
- Janes JK, Duretto MF 2010. A new classification for subtribe Pterostylidinae (Orchidaceae), reaffirming Pterostylis in the broad sense. Australian Systematic Botany 23: 260–269. 10.1071/SB09052
- Jensen SR, Gotfredsen CH, Grayer RJ 2008. Unusual iridoid glycosides in Veronica sects. Hebe and Labiatoides. Biochemical Systematics and Ecology 36: 207–215. 10.1016/j.bse.2007.09.011
- Joly S, McLenachan PA, Lockhart PJ 2009. A statistical approach for distinguishing hybridization and incomplete lineage sorting. American Naturalist 174: E54–E70. 10.1086/600082
- Jordan GJ, Carpenter RJ, Bannister JM, Mildenhall DC, Hill RS 2011. High conifer diversity in Oligo-Miocene New Zealand. Australian Systematic Botany 24:121–136. 10.1071/SB11004
- Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ 2008. Plant systematics a phylogenetic approach. Sunderland, Massachusetts, Sinauer.
- Judd WS, Manchester SR 1997. Circumscription of Malvaceae (Malvales) as determined by a preliminary cladistic analysis of morphological, anatomical, palynological, and chemical characters. Brittonia 49: 384–405. 10.2307/2807839
- Kampny CM, Dengler NG 1997. Evolution of flower shape in Veroniceae (Scrophulariaceae). Plant Systematics and Evolution 205: 1–25. 10.1007/BF00982795
- Kelch DG 2002. Phylogenetic assessment of the monotypic genera Sundacarpus and Manoao (Coniferales: Podocarpaceae) utilising evidence from 18S rDNA sequences. Australian Systematic Botany 15: 29–35. 10.1071/SB01002
- Kellogg EA 2009. The evolutionary history of Ehrhartoideae, Oryzeae, and Oryza. Rice 2: 1–14. 10.1007/s12284-009-9022-2
- Khoshsokhan MM, Kazempour Osaloo S, Oskoueiyan R, Saffar KN, Amirahmadi A 2013. Tribe Eritrichieae (Boraginaceae s.str.) in West Asia: a molecular phylogenetic perspective. Plant Systematics and Evolution 299: 197–208. 10.1007/s00606-012-0715-4
- Kim S-C, Lee C, Mejías JA 2007. Phylogenetic analysis of chloroplast DNA matK gene and ITS of nrDNA sequences reveals polyphyly of the genus Sonchus and new relationships among the subtribe Sonchinae (Asteraceae: Cichorieae). Molecular Phylogetics & Evolution 44: 578–579. 10.1016/j.ympev.2007.03.014
- Knopf P, Schulz C, Little DP, Stützel T, Stevenson DW 2012. Relationships within Podocarpaceae based on DNA sequence, anatomical, morphological, and biogeographical data. Cladistics 28: 271–299. 10.1111/j.1096-0031.2011.00381.x
- Knox EB, Heenan PB, Muasya AM, Murray BG 2008. Phylogenetic position and relationships of Lobelia glaberrima (Lobeliaceae), a new alpine species from southern South Island (New Zealand). New Zealand Journal of Botany 46: 77–85. 10.1080/00288250809509755
- Lammers TG 2011. Revision of the Infrageneric Classification of Lobelia L. (Campanulaceae: Lobelioideae). Annals of the Missouri Botanical Garden 98: 37–62. 10.3417/2007150
- Laurin M, Bryant HN 2009. Third meeting of the international society for phylogenetic nomenclature: a report. Zoologica Scripta 38: 333–337. 10.1111/j.1463-6409.2008.00379.x
- Leaché AD, Rannala B 2011. The accuracy of species tree estimation under simulation: a comparison of methods. Systematic Biology 60: 126–137. 10.1093/sysbio/syq073
- Lee WG, Tanentzap AJ, Heenan PB 2011. Plant radiation history affects community assembly: evidence from the New Zealand alpine. Biology Letters 8: 558–561. 10.1098/rsbl.2011.1210
- Lehnebach CA, Robertson AW 2004. Pollination ecology of four epiphytic orchids of New Zealand. Annals of Botany 93: 773–781. 10.1093/aob/mch097
- Li R-Q, Chen Z-D, Lu A-M, Soltis DE, Soltis PS, Manos PS 2004. Phylogenetic relationships in Fagales based on DNA sequences from three genomes. International Journal of Plant Science 165: 311–324. 10.1086/381920
- Linder HP, Baeza M, Barker NP, Galley C, Humphreys AM, Lloyd KM et al. 2010. A generic classification of Danthonioideae (Poaceae). Annals of the Missouri Botanical Garden 97: 306–364. 10.3417/2009006
- Lord JM, Huggins L, Little LM, Tomlinson VR 2013. Floral biology and flower visitors on subantarctic Campbell Island. New Zealand Journal of Botany 51: 168–180. 10.1080/0028825X.2013.801867
- Lucas EJ, Belsham SR, Nic Lughadha EM, Orlovich DA, Sakuragui CM, Chase MW et al. 2005. Phylogenetic patterns in the fleshy-fruited Myrtaceae – preliminary molecular evidence. Plant Systematics and Evolution 251: 35–51. 10.1007/s00606-004-0164-9
- Lucas EJ, Harris SA, Mazine FF, Belsham SR, Nic Lughadha EM, Telford A et al. 2007. Suprageneric Phylogenetics of Myrteae, the generically richest tribe in Myrtaceae (Myrtales). Taxon 56: 1105–1128. 10.2307/25065906
- Mabberley DJ 2008. Mabberley's plant book. 3rd edition. Cambridge, Cambridge University Press.
- Mark AF 2012. Above the treeline: a nature guide to alpine New Zealand. Nelson, Craig Potton Publishing.
- Matthews ML, Endress PK 2006. Floral structure and systematics in four orders of rosids, including a broad survey of floral mucilage cells. Plant Systematics and Evolution 260: 199–221.
- McGlone MS, Duncan RP, Heenan PB 2001. Endemism, species selection and the origin and distribution of the vascular plant flora of New Zealand. Journal of Biogeography 28: 199–216. 10.1046/j.1365-2699.2001.00525.x
- McKenzie RJ, Ward JM, Lovis JD, Breitwieser I 2004. Morphological evidence for natural intergeneric hybridization in the New Zealand Gnaphalieae (Compositae): Anaphalioides bellidioides × Ewartia sinclairii. Botanical Journal of the Linnean Society 145: 59–75. 10.1111/j.1095-8339.2003.00282.x
- McNeill J 1975. A generic revision of Portulacaceae tribe Montieae using techniques of numerical taxonomy. Canadian Journal of Botany 53: 789–809. 10.1139/b75-096
- McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, Hawksworth DL et al. 2012. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). Regnum Vegetabile 154. Königstein, Koeltz Scientific Books.
- Mejías JA, Kim S-C 2012. Taxonomic treatment of Cichorieae (Asteraceae) endemic to the Juan Fernández and Desventuradas Islands (SE Pacific). Annales Botanici Fennici 49: 171–178. 10.5735/085.049.0303
- Meudt HM 2008. Taxonomic revision of Australasian snow hebes (Veronica, Plantaginaceae). Australian Systematic Botany 21: 387–421. 10.1071/SB08034
- Meudt HM, Bayly MJ 2008. Phylogeographic patterns in the Australasian genus Chionohebe (Veronica s.l., Plantaginaceae) based on AFLP and chloroplast DNA sequences. Molecular Phylogenetics and Evolution 47: 319–338. 10.1016/j.ympev.2007.12.019
- Miller CJ 2006. Vegetation on the edge: a gradient analysis of the riparian zone, Poerua River, New Zealand. New Zealand Journal of Ecology 30: 357–370.
- Millener LH 1960. Our plant world. New Zealand Junior Encyclopaedia: 310–336. Melbourne.
- Mills K 2010. Defining indigenous plants: some problematic species from Norfolk Island. Cunninghamia 11: 407–412.
- Mitchell AD, Frodin DG, Heads MJ 1997. Reinstatement of Raukaua, a genus of the Araliaceae centred in New Zealand. New Zealand Journal of Botany 35: 309–315. 10.1080/0028825X.1997.10410156
- Mitchell AD, Heenan PB 2000. Systematic relationships of New Zealand endemic Brassicaceae inferred from nrDNA ITS sequence data. Systematic Botany 25: 98–105. 10.2307/2666676
- Mitchell AD, Heenan PB, Murray BG, Molloy BPJ, de Lange PJ 2009. Evolution of the south-western Pacific genus Melicytus (Violaceae): evidence from DNA sequence data, cytology and sex expression. Australian Systematic Botany 22: 143–157. 10.1071/SB08042
- Mitchell AD, Li R, Brown JW, Schönberger I, Wen J 2012. Ancient divergence and biogeography of Raukaua (Araliaceae) and close relatives in the southern hemisphere. Australian Systematic Botany 25: 432–446. 10.1071/SB12020
- Mitchell AD, Wagstaff SJ 1997. Phylogenetic relationships of Pseudopanax species (Araliaceae) inferred from parsimony analysis of rDNA sequence data and morphology. Plant Systematics and Evolution 208: 121–138. 10.1007/BF00985438
- Mitchell AD, Webb CJ, Wagstaff SJ 1998. Phylogenetic relationships of species of Gingidia and related genera (Apiaceae, subfamily Apioideae). New Zealand Journal of Botany 36: 417–424. 10.1080/0028825X.1998.9512580
- Molloy BPJ 1995. Manoao (Podocarpaceae), a new monotypic conifer genus endemic to New Zealand. New Zealand Journal of Botany 33: 183–201. 10.1080/0028825X.1995.10410483
- Moore LB, Edgar E 1970. Flora of New Zealand. Vol. 2. Wellington, Government Printer.
- Morgan-Richards M, Smissen RD, Shepherd LD, Wallis GP, Heyward JJ, Chan C-H et al. 2009. A review of genetic analyses of hybridisation in New Zealand. Journal of the Royal Society of New Zealand 39: 15–34. 10.1080/03014220909510561
- Muñoz-Centeno LM, Albach DC, Sánchez-Agudo JA, Martínez-Ortega MM 2006. Systematic significance of seed morphology in Veronica (Plantaginaceae): a phylogenetic perspective. Annals of Botany 98: 335–350. 10.1093/aob/mcl120
- Nazaire M, Hufford L 2012. A broad phylogenetic analysis of Boraginaceae: implications for the relationships of Mertensia. Systematic Botany 37: 758–783. 10.1600/036364412X648715
- Nelson G 1973. Classification as an expression of phylogenetic relationships. Systematic Zoology 22: 344–359. 10.2307/2412943
- Nickrent DL, Malécot V, Vidal-Russell R, Der JP 2010. A revised classification of Santalales. Taxon 59: 538–558.
- Nicolas A, Plunkett GM 2009. The demise of subfamily Hydrocotyloideae (Apiaceae) and the re-alignment of its genera across the entire order Apiales. Molecular Phylogenetics and Evolution 53: 134–151. 10.1016/j.ympev.2009.06.010
- Nicolas A, Plunkett GM 2012. Untangling generic limits in Azorella, Laretia, and Mulinum (Apiaceae: Azorelloideae): insights from phylogenetics and biogeography. Taxon 61: 826–840.
- Nilsson Ö 1966. Studies in Montia L. and Claytonia L. and allied genera, I. Correction and additions. Botaniska Notiser 119: 469.
- Nordenstam B 1978. Taxonomic studies in the tribe Senecioneae (Compositae). Opera Botanica 44: 1–83.
- Nordenstam B, Pelser P 2012. Caputia, a new genus to accommodate four succulent South African Senecioneae (Compositae) species. Compositae Newsletter 50: 56–69.
- Norton DA, Molloy BPJ 2009. Heliohebe maccaskillii (Plantaginaceae)—a new rank for a threatened limestone endemic, North Canterbury, New Zealand. New Zealand Journal of Botany 47: 405–409. 10.1080/0028825x.2009.9672715
- Nyananyo BL, Heywood VH 1987. A new combination in Lyallia (Portulacaceae). Taxon 36: 640–641. 10.2307/1221861
- Nyffeler T, Eggli U 2010. Disintegrating Portulacaceae: a new familial classification of the suborder Portulacineae (Caryophyllales) based on molecular and morphological data. Taxon 59: 227–240.
- Oginuma K, Muntzinger J, Tobe H 2006. Exceedingly high chromosome number in Strasburgeriaceae, a monotypic family endemic to New Caledonia. Plant Systematics and Evolution 262: 97–101. 10.1007/s00606-006-0451-8
- Oliver WRB 1944. The Veronica-like species of New Zealand. Records of the Dominion Museum, Wellington1: 288–231.
- O'Quinn R, Hufford L 2005. Molecular systematics of Montieae (Portulacaceae): implications for taxonomy, biogeography and ecology. Systematic Biology 30: 314–331.
- Patel RN 1973. Wood anatomy of the dicotyledons indigenous to New Zealand 2. Escalloniaceae. New Zealand Journal of Botany 11: 421–434. 10.1080/0028825X.1973.10430292
- Pedersen B, Gotfredsen CH, Wagstaff SJ, Jensen SR 2007. Chemical markers in Veronica sect. Hebe. II. Biochemical Systematics and Ecology 35: 777–784. 10.1016/j.bse.2007.06.011
- Pelser PB, Nordenstam B, Kadereit JW, Watson LE 2007. An ITS phylogeny of tribe Senecioneae (Asteraceae) and a new delimitation of Senecio L. Taxon 56: 1077–1104. 10.2307/25065905
- Perveen A, Grafström E, El-Ghazaly G 2004. World Pollen and Spore Flora 23. Malvaceae Adams. P.p. Subfamilies: Grewioideae, Tilioideae, Brownlowioideae. Grana 43: 129–155. 10.1080/00173130410000730
- Petersen G, Seberg O, Salomon B 2011. The origin of the H, St, W, and Y genomes in allotetraploid species of Elymus L. and Stenostachys Turcz. (Poaceae: Triticeae). Plant Systematics and Evolution 291: 197–210. 10.1007/s00606-010-0381-3
- Philipson WR 1965. The New Zealand genera of the Araliaceae. New Zealand Journal of Botany 3: 333–341. 10.1080/0028825X.1965.10429024
- Philipson WR, Skipworth JP 1961. Hectorellaceae: a new family of Dicotyledons. Transactions of the Royal Society of New Zealand Botany 1: 31.
- Pufal G, Ryan KG, Garnock-Jones PJ 2010. Hygrochastic capsule dehiscence in New Zealand alpine Veronica (Plantaginaceae). American Journal of Botany 97: 1413–1423. 10.3732/ajb.1000066
- Qiu Y-L, Li L, Wang B, Xue J-Y, Hendry TA, Li R-Q et al. 2010. Angiosperm phylogeny inferred from sequences of four mitochondrial genes. Journal of Systematics and Evolution 48: 391–425. 10.1111/j.1759-6831.2010.00097.x
- Quinn CJ 1982. Taxonomy of Dacrydium Sol. ex Lamb. emend. de Laub. (Podocarpaceae). Australian Journal of Botany 30: 311–320. 10.1071/BT9820311
- Radford EA, Watson MF, Preston J 2001. Phylogenetic relationships of species of Aciphylla (Apiaceae, subfamily Apioideae) and related genera using molecular, morphological, and combined data sets. New Zealand Journal of Botany 39: 183–208. 10.1080/0028825X.2001.9512730
- Raven PH 1973. Evolution of alpine and subalpine plant groups in New Zealand. New Zealand Journal of Botany 11: 177–200. 10.1080/0028825X.1973.10430272
- Rodd AN, Pickard J 1983. Census of vascular flora of Lord Howe Island. Cunninghamia 1: 267–280.
- Romaschenko K, Peterson PM, Soreng RJ, Garcia-Jacas N, Futorna O, Susanna A 2012. Systematics and evolution of the needle grasses (Poaceae: Pooideae: Stipeae) based on analysis of multiple chloroplast loci, ITS, and lemma micromorphology. Taxon 61: 18–44.
- Rozefelds AC 2008. Uniquely Tasmanian — a review of the phylogenetic and biogeographic relationships of Tasmania's endemic vascular plant genera. Kanunnah 2: 35–86.
- Sauquet H, Weston PH, Anderson CL, Barker NP, Cantrill DJ, Mast AR et al. 2009. Contrasted patterns of hyperdiversification in Mediterranean hotspots. Proceedings of the National Academy of Sciences 106: 221–225. 10.1073/pnas.0805607106
- Schmidt-Lebuhn AN 2011. Fallacies and false premises—a critical assessment of the arguments for the recognition of paraphyletic taxa in botany. Cladistics 27: 1–14. 10.1111/j.1096-0031.2010.00316.x
- Schneider JV 2007. Ixerbaceae. In: Kubitzki K ed. The families and genera of vascular plants. Volume 9. Flowering plants: Eudicots. Berlin, Springer-Verlag. Pp. 205–207.
- Shaw J, Lickey EB, Beck JT, Farmer SB, Liu W, Miller J et al. 2005. The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. American Journal of Botany 92: 142–166. 10.3732/ajb.92.1.142
- Smissen RD, Breitwieser I, Ward JM 2007. Genetic characterization of hybridization between the New Zealand everlastings Helichrysum lanceolatum and Anaphalioides bellidioides (Asteraceae: Gnaphalieae). Botanical Journal of the Linnean Society 154: 89–98. 10.1111/j.1095-8339.2007.00632.x
- Smissen RD, Galbany-Casals M, Breitwieser I 2011. Ancient allopolyploidy in the everlasting daisies (Asteraceae: Gnaphalieae): complex relationships among extant clades. Taxon 60: 649–662.
- Sokal RR, Sneath PH 1963. Principles of Numerical Taxonomy. San Francisco, WH Freeman & Co.
- Soltis DE, Smith SA, Cellinese N, Wurdack KJ, Tank DC, Brockington SF et al. 2011. Angiosperm phylogeny: 17 genes, 640 taxa. American Journal of Botany 98: 704–730. 10.3732/ajb.1000404
- Sosa V, Chase MW 2003. Phylogenetics of Crossosomataceae based on rbcL sequence data. Systematic Botany 28: 96–105.
- Spalik K, Piwczyński M, Danderson DA, Kurzyna-Mlynik R, Bone TS, Downie SR 2010. Amphitropic amphiantarctic disjunctions in Apiaceae subfamily Apioideae. Journal of Biogeography 37: 1977–1994.
- Stöckler K 2001. Origins and evolution of the New Zealand forest flora a molecular phylogenetic approach. PhD dissertation. Palmerston North, New Zealand: Massey University.
- Straub SCK, Parks M, Weitemier K, Fishbein M, Cronn RC, Liston A 2012. Navigating the tip of the genomic iceberg: next-generation sequencing for plant systematics. American Journal of Botany 99: 349–364. 10.3732/ajb.1100335
- Stuessy TF, König C 2008. Patrocladistic classification. Taxon 57: 594–601.
- Tank DC, Donoghue MJ 2010. Phylogeny and phylogenetic nomenclature of the campanulidae based on an expanded sample of genes and taxa. Systematic Botany 35: 425–441. 10.1600/036364410791638306
- Taskova RM, Albach DC, Grayer RJ 2004. Phylogeny of Veronica—a combination of molecular and chemical evidence. Plant Biology 6: 673–682. 10.1055/s-2004-830330
- Taskova RM, Kokubun T, Garnock-Jones PJ, Jensen SR 2012. Iridoid and phenylethanoid glycosides in the New Zealand sun hebes (Veronica; Plantaginaceae). Phytochemistry 36: 207–215.
- Taskova RM, Kokubun T, Grayer RJ, Ryan KG, Garnock-Jones PJ 2008. Flavonoid profiles in the Heliohebe group of New Zealand Veronica (Plantaginaceae). Biochemical Systematics and Ecology 36: 110–116. 10.1016/j.bse.2007.08.008
- Thornhill AH, Hope GS, Craven LA, Crisp MD 2012. Pollen morphology of the Myrtaceae. Part 4: tribes Kanieae, Myrteae and Tristanieae. Australian Journal of Botany 60: 260–289. 10.1071/BT11177
- Thorsen M 2007. Recent botanical literature. Otago Botanical Society Newsletter 52: 13–17.
- Vaughn KC, Bowling AJ, Ruel KJ 2011. The mechanism for explosive seed dispersal in Cardamine hirsuta (Brassicaceae). American Journal of Botany 98: 1276–1285. 10.3732/ajb.1000374
- Vences M, Guayasamin JM, Miralles A, de la Riva I 2013. To name or not to name: criteria to promote economy of change in Linnaean classification schemes. Zootaxa 3636: 201–244. 10.11646/zootaxa.3636.2.1
- Verboom GA, Linder PH, Stock WD 2003. Phylogenetics of the grass genus Ehrharta: evidence for radiation in the summer-arid zone of the South African cape. Evolution 57: 1008–1021. 10.1111/j.0014-3820.2003.tb00312.x
- Vidal-Russell R, Nickrent DL 2008. Evolutionary relationships in the showy mistletoe family (Loranthaceae). American Journal of Botany 95: 1015–1029. 10.3732/ajb.0800085
- Virot R 1968. Flore de la Nouvelle-Calédonie et Dépendances, No. 2 Proteacées. Muséum National d'Histoire Naturelle.
- Wagstaff SJ 2004. Evolution and biogeography of the austral genus Phyllocladus (Podocarpaceae). Journal of Biogeography 31: 1569–1577. 10.1111/j.1365-2699.2004.01066.x
- Wagstaff SJ, Bayly MJ, Garnock-Jones PJ, Albach DC 2002. Classification, origin, and diversification of the New Zealand hebes (Scrophulariaceae). Annals of the Missouri Botanical Garden 89: 38–63. 10.2307/3298656
- Wagstaff SJ, Breitwieser I 2002. Phylogenetic relationships of New Zealand Asteraceae inferred from ITS sequences. Plant Systematics and Evolution 231: 203–224. 10.1007/s006060200020
- Wagstaff SJ, Breitwieser I 2004. Phylogeny and classification of Brachyglottis (Senecioneae, Asteraceae): an example of a rapid species radiation in New Zealand. Systematic Botany 29: 1003–1010. 10.1600/0363644042450991
- Wagstaff SJ, Breitwieser I, Ito M 2011. Evolution and biogeography of Pleurophyllum (Astereae, Asteraceae), a small genus of megaherbs endemic to the Subantarctic Islands. American Journal of Botany 98: 62–75. 10.3732/ajb.1000238
- Wagstaff SJ, Garnock-Jones PJ 1998. Evolution and biogeography of the Hebe complex (Scrophulariaceae) inferred from ITS sequences. New Zealand Journal of Botany 36: 425–437. 10.1080/0028825X.1998.9512581
- Wagstaff SJ, Hennion F 2007. Evolution and biogeography of Lyallia and Hectorella (Portulacaceae), geographically isolated sisters from the Southern Hemisphere. Antarctic Science 19: 417–426. 10.1017/S0954102007000648
- Wagstaff SJ, Heenan PB, Sanderson MJ 1999. Classification, origins, and petterns of diversification in New Zealand Carmichaelinae (Fabaceae). American Journal of Botany 86: 1346–1356. 10.2307/2656781
- Wagstaff SJ, Hickerson L, Spangler R, Reeves PA, Olmstead RG 1998. Phylogeny in Labiatae s. l., inferred from cpDNA sequences. Plant Systematics and Evolution 209: 265–274. 10.1007/BF00985232
- Wagstaff SJ, Molloy BPJ, Tate JA 2010. Evolutionary significance of long-distance dispersal and hybridisation in the New Zealand endemic genus Hoheria (Malvaceae). Australian Systematic Botany 23: 112–130. 10.1071/SB09017
- Wagstaff SJ, Tate JA 2011. Phylogeny and character evolution in the New Zealand endemic genus Plagianthus (Malveae, Malvaceae). Systematic Botany 36: 405–418. 10.1600/036364411X569589
- Wagstaff SJ, Wege J 2002. Patterns of diversification in New Zealand Stylidiaceae. American Journal of Botany 89: 865–874. 10.3732/ajb.89.5.865
- Wanke S, Jaramillo MA, Borsch T, Samain M-S, Quandt D, Neinhuis C 2007. Evolution of Piperales—matK gene and trnK intron sequence data reveal lineage specific resolution contrast. Molecular Phylogenetics and Evolution 42: 477–497. 10.1016/j.ympev.2006.07.007
- Ward JM 1997. Raoulia and its hybrids. In: Sheppard JS ed. Southern Alpines 1996, Conference Proceedings, Christchurch, New Zealand. Pp. 40–44.
- Warwick SI, Mummenhoff K, Sauder CA, Koch MA, Al-Shehbaz IA 2010. Closing the gaps: phylogenetic relationships in the Brassicaceae based on DNA sequence data of nuclear ribosomal ITS region. Plant Systematics and Evolution 285: 209–232. 10.1007/s00606-010-0271-8
- Webb CJ 1979. Breeding systems and the evolution of dioecy in New Zealand apioid umbelliferae. Evolution 33: 662–672. 10.2307/2407789
- Webb CJ, Druce AP 1984. A natural intergeneric hybrid, Aciphylla squarrosa × Gingidia montana, and the frequency of hybrids among other New Zealand apioid Umbelliferae. New Zealand Journal of Botany 22: 403–411. 10.1080/0028825X.1984.10425272
- Webb CJ, Lloyd DG, Delph LF 1999. Gender dimorphism in indigenous New Zealand seed plants. New Zealand Journal of Botany 37: 119–130. 10.1080/0028825X.1999.9512618
- Webb CJ, Sykes WR, Garnock-Jones PJ 1988. Flora of New Zealand. Vol. IV. Christchurch, Botany Division DSIR.
- Webb CO, Donoghue MJ 2005. Phylomatic: tree assembly for applied phylogenetics. Molecular Ecology Notes 5: 181–183. 10.1111/j.1471-8286.2004.00829.x
- Wege JA 2012. Navigating the floral Milky Way: the taxonomy of the microgeophytic triggerplants (Stylidium petiolare and allies: Stylidiaceae). Australian Systematic Botany 25: 138–169. 10.1071/SB12001
- Weston PH, Barker NP 2006. A new suprageneric classification of the Proteaceae, with an annotated checklist of genera. Telopea 11: 314–344.
- Wiley EO 1975. Systematics, and classification: a reply to Walter Bock and other evolutionary taxonomists. Systematic Zoology 24: 233–243 10.2307/2412764
- Wiley EO 1981. Phylogenetics. New York, Wiley.
- Wiley EO 2009. Patrocladistics: nothing new. Taxon 58: 2–6.
- Wilson HD 2013. Plant life on Banks Peninsula. Cromwell, Manuka Press.
- Winkworth RC, Grau J, Robertson AW, Lockhart PJ 2002a. The origins and evolution of the genus Myosotis L. (Boraginaceae). Molecular Phylogenetics and Evolution 24: 180–193. 10.1016/S1055-7903(02)00210-5
- Winkworth RC, Wagstaff SJ, Glenny D, Lockhart PJ 2002b. Plant dispersal N.E.W.S. from New Zealand. Trends in Ecology and Evolution 17: 514–520. 10.1016/S0169-5347(02)02590-9
- Woo VL, Funke MM, Smith JF, Lockhart PJ, Garnock-Jones PJ 2011. New World origins of southwest Pacific Gesneriaceae: multiple movements across and within the south Pacific. International Journal of Plant Science 172: 434–457. 10.1086/658183
