Abstract
We review the biology and ecology of Metrosideros excelsa (Myrtaceae), an endemic angiosperm evergreen tree. Metrosideros excelsa belongs to a conspicuous and widely distributed Pacific Basin genus, with centres of diversity in both New Zealand and New Caledonia. Metrosideros excelsa is an iconic tree species that forms a significant component of northern New Zealand's exposed coastal headland and cliff vegetation. Where conditions are more favourable, M. excelsa forms tall coastal forest, ranging from simple young high-density stands to diverse mature forest. Inland, M. excelsa stands are confined to the margins of lakes and rivers on the Central Volcanic Plateau, where some may originate from early Māori plantings. Metrosideros excelsa is reliant on stochastic disturbance events (e.g. landslides, volcanic eruptions) to create open sites necessary for regeneration. Mass flowering (December–January), followed by abundant production of wind-dispersed seed maximises chance colonisation of such sites. Since human settlement in New Zealand, the distribution of M. excelsa forest has declined by c. 90% and the southern limit of the species has retreated north. Natural regeneration on the mainland is limited by the infrequency of large-scale disturbances and increased anthropogenic and herbivore pressures. Consequently, M. excelsa forest has become rare and localised on the mainland; monitoring and active management are fundamental to the species' long-term conservation.
Introduction
Metrosideros excelsa Sol. ex Gaertn. (Myrtaceae) is an iconic angiosperm tree up to 25 m tall forming a notable element of the coastal vegetation in northern New Zealand. Revered in both Māori and European culture, M. excelsa is known for its sudden and spectacular annual flowering episodes, a gnarly and sprawling growth form and an ability to survive in the most precarious and inhospitable coastal environments. The species’ tendency to form young pure stands, often with low plant species richness, is also a characteristic feature of M. excelsa (Simpson Citation1994; Bylsma Citation2012). As stands develop, species richness may increase due to the incursion of mid and late successional species (Clarkson Citation1990; Bylsma Citation2012). When flowering, trees are laden with red brush flowers that yield copious amounts of nectar for consumption by endemic nectar feeders. Trees also provide nesting/roosting sites for native birds, and the flaky bark and dense leaf litter provide habitat for a wide range of host-specific indigenous insects (Hosking & Hutcheson Citation1993; Simpson Citation1994; Taylor et al. Citation2007). In the past, M. excelsa forest has undergone serious decline, initially a result of burning by Māori, with further losses arising from nineteenth century land clearance by early European immigrants, followed by the expansion of pastoral farming and logging to supply timber to the ship-building industry (Dieffenbach Citation1843; Simpson Citation2005). Currently, land clearance, coupled with pressure from introduced herbivores continues to pose a serious threat to remaining M. excelsa stands on the mainland, which represent c. 10% of their past extent (Forest Research Institute Citation1989). Further deterioration will have implications beyond M. excelsa, also affecting the associated fauna and fauna that reply on M. excelsa forest communities for food and habitat (Hosking & Hutcheson Citation1993).
Metrosideros excelsa is well represented in New Zealand literature, including forest health studies (e.g. Forest Research Institute Citation1989; Hosking & Hutcheson Citation1993), historical and cultural reviews (Conly & Conly Citation1988; Simpson Citation2005) and comprehensive accounts of the species evolution, biology and management (e.g. Schmidt-Adam et al. Citation2000; Simpson Citation2005; Bergin & Hosking Citation2006). A more limited number of studies quantitatively describe M. excelsa forest dynamics and succession (Clarkson & Clarkson Citation1994; Atkinson Citation2004; Bylsma Citation2012). Here, we review the morphology, taxonomy, ecology and other topics relevant to the biology and conservation of M. excelsa, largely from primary publications, but also supported by unpublished work.
Morphological description
Metrosideros excelsa is a dicotyledonous, evergreen woody shrub or tree. Mature trees can reach 25 m in height and trunks can be up to 2.5 m in diameter (Allan Citation1961), often with a gnarly growth habit. Trees can be multi-stemmed, particularly in open sites, and are frequently wider than they are tall due to dense and sprawling canopies. In a forest environment however, shading results in a more upward growth form (Simpson Citation2005). Branching is sympodial and spreading, branchlets are stout, and young shoots on mature trees are often densely tomentose. Trees readily produce adventitious roots, with some trees producing large quantities of these, and others producing relatively few (Simpson Citation2005). Leaves are decussate, usually opposite, and are positioned on short, stout petioles. Lamina dimensions vary, but commonly range from 5 to 10 cm in length by 2.5 to 5 cm in width. Leaf shape can range from elliptic to oblong with either acute or obtuse leaf tips. Juvenile plants have glabrous leaves, however leaves on mature trees are coriaceous, thick and clad with tomentum; as leaves develop, the tomentum wears off the adaxial surface but persists on the abaxial surface (). Inflorescences are composed of broad compound cymes terminating in groups of three flowers (Dawson Citation1968); a single inflorescence is composed of an average of 14.3 flowers (Schmidt-Adam et al. Citation1999). Pedicels are stout and tomentose (Allan Citation1961). Flowers comprise a ‘brush’ of stamens (); these are crimson to scarlet in colour or occasionally yellow (Bergin & Hosking Citation2006). Each flower comprises c. 27 stamens, however, this is highly variable. Stamens are between 20 and 37 mm in length, and positioned around a hypanthium with a single central pistil (Wootherpoon Citation1993). Petals are insignificant, oblong and persistent (Dawson Citation1968). Capsules are 7–9 mm long, tomentose, distinctly exserted and loculicidally three-valved (Allan Citation1961). Seeds range from 3.0 to 3.2 mm in length by 0.2 to 0.42 mm in width, and weigh around 0.1–0.15 mg (Schmidt-Adam et al. Citation2002).

A small M. excelsa stand located near the species southern limit at Wai-iti (Taranaki) exhibits morphological characteristics distinct from M. excelsa found further north; this stand is suspected to be natural, rather than the result of a historic planting (Benson Citation1995; Simpson Citation1997). Metrosideros excelsa leaves and flower clusters in this population are frequently smaller than those typical of M. excelsa elsewhere, with leaves ranging from 4 to 6 cm long by 1 to 1.5 cm in diameter (Simpson Citation1997). Annual shoot growth increments are also smaller, at around 2–4 cm yr−1 compared with 5–6 cm yr−1 elsewhere in New Zealand. Morphological variation may represent local adaptation to the marginal conditions present at the leading or trailing edge of migration south since the post Pleistocene climatic amelioration (Simpson Citation1997).
Anatomy
Leaf
The leaf anatomy of M. excelsa has been described by Simpson (Citation2005) and Bylsma (Citation2012). Examination of leaf cross-sections show that the tissues are notably differentiated (Bylsma Citation2012) with features that aid survival in the saline and drought-prone coastal environment (Simpson Citation2005). Leaf tissues comprise upper and lower cuticle layers, which thicken near leaf margins; upper and lower epidermal layers, from which leaf hairs protrude; a layer of hypodermis (three to four cells thick), particularly thick around the mid-rib and main veins; a mesophyll layer comprised of vertically elongated palisade cells and spongy tissue, air pockets are generally not abundant. On the abaxial leaf surface numerous stomata are protected by a dense layer of tomentum (Simpson Citation2005).
Flower stigma and style
Following the classifications of Boland & Sedgley (Citation1986), the stigma of M. excelsa is described as blunt and uniform. In cross-section the stigma is approximately circular and has an average diameter of 0.62 mm (Schmidt-Adam & Murray Citation2002). Unicellular papillae line the stigma and these lack a cuticle layer. The diameter of the style increases basipetally reaching c. 0.74 mm. Styles comprise an epidermis with outer cuticle (2–4 µm thick), parenchymous cortex and a central transmitting tissue/tract. The transmitting tract cells are loosely arranged and form a mosaic with large intercellular spaces filled with secretion products (Schmidt-Adam & Murray Citation2002).
Wood
The wood of M. excelsa has a swirled grain and is very dense. Annual growth rings are indistinct to slightly distinct (Meylan & Butterfield Citation1978). All members of the genus Metrosideros are renowned for the hardness and durability of their wood. The features of M. excelsa wood that underlie this trait include abundant parenchyma cells, wide rays arranged in radiating sheets, and a dominance of thick wall fibres comprising narrow elongated cells densely packed around water conducting vessels (Simpson Citation2005). The heartwood of M. excelsa has rich reddish-brown coloration, often with black or pinkish streaks. It has been suggested that the heartwood of M. excelsa is resistant to fungi, however, heartwood frequently has a decayed centre (Bergin & Hosking Citation2006).
Chemistry
Seven distinct anthocyanins have been identified in flower tissue of M. excelsa, where they are responsible for flower colour. These are delphinidin-3-glucoside, malvidin-3-glucoside, cyanidin-3-glucoside, petunidin-3-glucoside, peonidin-3-glucoside, cyanidin-3,5-diglucoside and malvidin-3,5-diglucoside (Andersen Citation1988). Anthocyanin pigmentation is also a feature of M. excelsa's adventitious roots. Anthocyanins in the root tissues tend to be cyanidin- and delphinidin-based and are present in cell layers of the root cap, epidermis, hypodermis and cortex. Light exposure may partially control the production of these pigments; consequently, they are more abundant in the outer-most layers, where they may protect against UV-B radiation. However, the function of anthocyanins in adventitious roots is still relatively unknown (Solangaarachchi & Gould Citation2001).
Five unusual C-methyl flavonoids with no B-ring oxidation have also been identified in M. excelsa tissues: 2′,4′-dihydroxy-3′,5-dimethyl-6-methoxychalcone; 2′,4′-dihydroxy-3′-methyl-6′-methoxychalcone; 2′,6′-dihydroxy-3′-methyl-4′-methoxychalcone; 2′-hydroxy-3′-methyl-4′,6′-dimethoxychalcone; and 5,7-dihydroxy-6,8-dimethylflavanone (Mustafa et al. Citation2005). The latter is a major constituent of M. excelsa leaves, whereas 2′,6′-dihhydroxy-3′-methl-4′-methoxychalcone is the dominant flavonoid in flowers, buds, twigs and seed. High levels of flavonoids found in flower buds may suggest that they function to defend reproductive organs from bacterial/fungal attack (Mustafa et al. Citation2005).
Changes during ontogeny
Metrosideros excelsa shows a smooth transition from juvenile to mature phenology (Clements et al. Citation1999). The vegetative phase change is characterised by a transition from juvenile plants with glabrous foliage to adult plants having leaves with dense pubescence on the abaxial surface (Kubien et al. Citation2007). Anatomical and physiological characteristics that accompany this transition from juvenile to mature phenology include: decreased photosynthetic rates, possibly due to a reduction in resources allocated to carbon gain; decreased stomatal limitation on CO2 uptake, indicating that the control of water loss shifts from a physiological to a physical basis; and increased palisade and mesophyll depth (larger cells), which may reduce light penetration and prevent sun damage. Nitrogen levels were found to be much higher in juvenile leaves, indicating a reallocation of nitrogen in adult plants to areas where it may be used for defence and reproduction rather than photosynthesis (Kubien et al. Citation2007).
Quantification of the light environments occupied by M. excelsa juveniles indicates that the minimum light requirements of M. excelsa may also decrease as juveniles develop. However, this may not indicate an increased tolerance to shade, but alternatively may relate to the over-topping of M. excelsa juveniles by faster growing species (Bylsma Citation2012). Single mature M. excelsa trees are commonly observed growing beneath a closed canopy of taller species (Atkinson Citation2004).
Cytology
The chromosome number of M. excelsa is 2n = 22 (Dawson Citation2008).
Nomenclature
The genus name Metrosideros was first used by Rumphius in 1743 to identify a group of hard-wooded timber trees in Indonesia. In 1769, the name was re-applied to Metrosideros collina, a timber tree from Tahiti. The word Metrosideros is derived from the Greek terms metra meaning core or heart, and sideron meaning iron. Thus, Metrosideros species are often referred to as iron-hearted trees, alluding to the durability and hardness of their wood (Simpson Citation2005).
Metrosideros excelsa samples were collected by Banks and Solander during Cook's first voyage to New Zealand (1769). Sample descriptions were then published by Gaertner (Citation1788), notes on the sample plate read: ‘Calyx tomentosus, quinquedentatus, capsulae ad medium usque adnatus. Capsula ovata, extra calycem prominens, pubescens, trilocularis’ (Oliver Citation1928). The term excelsa means high, lofty or outstanding, a likely reference to the species mass flowering habit.
The species has also been referred to as M. tomentosa (A. Rich.), as described in Essai d'une Flore de la Nouvelle-Zelande; a detailed account of the vegetation encountered by D'Urville on a voyage to New Zealand during the 1800s (Richard Citation1832). This name was given in reference to the densely clad underside of mature leaves (Dawson Citation1993). Metrosideros tomentosa is now considered an old synonym for M. excelsa (Oliver Citation1928), with naming authority given to Gaertner, for the first valid publication.
The Māori name ‘pōhutukawa’ is said to mean splashed by the spray, an acknowledgement of the harsh coastal environment for which this species is superbly adapted (Riley Citation1994). The name pōhutukawa is derived from the word hutukawa – a headdress of red feathers (Simpson Citation1994). Other Māori names for M. excelsa include kahika and rātā (Allan Citation1961). Metrosideros excelsa is commonly called New Zealand Christmas tree, as the flowering period coincides with Christmas and early European settlers used the flowers as a substitute for holly in their homes (Conly & Conly Citation1988).
Taxonomy and relationships
Metrosideros excelsa is a member of the genus Metrosideros, subgenus Metrosideros, and belongs to the family Myrtaceae. Other taxa within this family that are represented in New Zealand's endemic flora include members of Leptospermum, Syzygium, Kunzea, Lophomyrtus and Neomyrtus. The genus Metrosideros contains c. 50 described species and these form a conspicuous component of the Pacific Basin flora (Sreekantan et al. Citation2001; Gardner et al. Citation2004), with representatives in the Philippines, New Guinea, New Caledonia, New Zealand and many of the Pacific Islands. An outlying species is also present in South Africa (Andersen Citation1988). Metrosideros comprises two main subgenera (Dawson Citation1988); Metrosideros (the trees and shrubs) and Mearnsia (the vines). Common features of Metrosideros include the ability to occupy open rocky ground; preference for a warm temperate or subtropical climate; sensitivity to frost; the ability to form adventitious roots; and the ability to establish on young soil with a low nutrient status (Simpson Citation2005). New Zealand has 12 species of Metrosideros: six root climbing lianes (M. albiflora, M. carminea, M. colensoi, M. diffusa, M. fulgens, M. perforata), one shrub (M. parkinsonii) and five tree species (M. bartlettii, M. excelsa, M. kermadecensis, M. robusta, M. umbellata) (Dawson Citation1988).
Biogeography
The genus Metrosideros is one of the most widespread in the Pacific, with centres of diversity in the south (New Zealand; 12 spp.) and north (New Caledonia; 16 spp.). New Zealand is considered the origin and the source of the majority of Metrosideros taxa, being the only landmass where late Palaeocene/early Eocene fossil pollen grains have been recorded, and the primitive Metrosideros umbellata is considered the basal Metrosideros species (Wright et al. Citation2000). Metrosideros species fall into three monophyletic clades. The first clade includes seven New Caledonian species as well as three species in western Oceania. The second contains six taxa from Melanesia and Samoa. The third accounts for much of the total species range of Metrosideros subgenus Metrosideros, this includes New Zealand species (M. bartlettii, M. robusta and M. excelsa) as well as all of the Polynesia taxa (Wright et al. Citation2000).
Wright et al. (Citation2000) suggest a minimum of four distinct dispersal events occurred in the history of the Metrosideros subgenus Metrosideros. An initial mid/late Tertiary dispersal from New Zealand to New Caledonia; a mid/late Tertiary dispersal from New Caledonia to the western Pacific; a mid/late Tertiary dispersal from New Zealand to the southwestern Pacific; and lastly, the most recent dispersal in the Pleistocene, when the Metrosideros lineage dispersed from New Zealand to a number of islands in Oceania and remote Polynesia. The latter dispersal has been interpreted as the result of climate change during the Pleistocene glaciations, as wind-dispersed species could have taken advantage of the westerly weather systems and migrated across the marine environment (Wright et al. Citation2000).
Geographic distribution and ecological tolerance
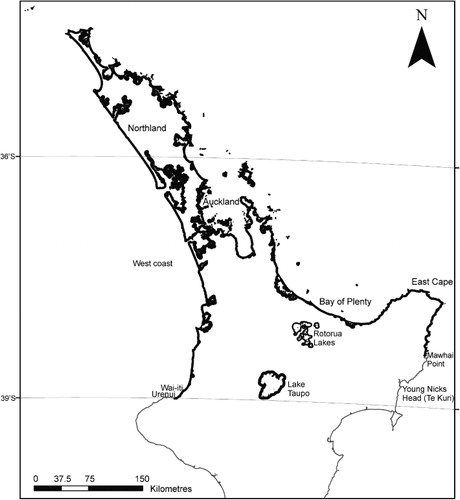
Metrosideros excelsa is primarily a coastal species (), limited to the warm temperate climate of northern New Zealand (Sakai & Wardle Citation1978; Simpson Citation2005). Metrosideros excelsa forest is currently a rare and localised ecosystem on the mainland and is rarely found more than 1000 m from the coast (Simpson Citation1994; Bylsma Citation2012). At the time of European settlement, however, the forest formed a continuous belt around the north of the North Island; stretching from Urenui on the west coast, to Gisborne or possibly Young Nick's Head (Te Kuri), on the east coast (Simpson Citation2005; Bergin & Hosking Citation2006). The southern limits have since retreated north and currently extend no further than Wai-iti on the west coast and Mawhai Point on the east coast. Metrosideros excelsa has an infrequent occurrence from Wai-iti to Manukau Harbour on the west coast, where soft highly eroded mudstone substrates dominate. Logging, accidental fire and land clearance have reduced the distribution of M. excelsa to small fragmented populations and scattered isolated trees, largely on cliff tops and coastal banks (Bergin & Hosking Citation2006).
Northern New Zealand islands provide the best examples of original M. excelsa forest, including natural plant and animal associations. Offshore islands where M. excelsa forest is prominent include Hauturu/Little Barrier Island (Hamilton & Atkinson Citation1961), the Poor Knights Island group (Simpson Citation2005), Rangitoto Island, Whakaari/White Island, Moutohora Island, Tuhua/Mayor Island and Motuotau Island (Clarkson Citation1990; Clarkson & Spring-Rice Citation1992; Clarkson & Clarkson Citation1994). Metrosideros excelsa has a strong affinity for volcanic substrates and grows naturally in proximity to geothermal activity (Clarkson et al. Citation1991; Clarkson & Clarkson Citation1994). Metrosideros excelsa forest on Whakaari/White Island is continuously disturbed by volcanic activity, including eruptions and toxic fumes. On Moutohora Island, stunted M. excelsa shrubs grow within metres of steaming springs and fumaroles (R J Bylsma pers. obs).
Inland M. excelsa stands are extant on the Central Volcanic Plateau, including on the shores of Lakes Rotorua, Ōkataina and Taupo (including Motutaiko Island), and along the Tarawera River (Simpson Citation2005; Bergin & Hosking Citation2006). In the 1840s, Colenso also recorded M. excelsa trees growing on a number of cliffs that fringed Lake Waikaremoana (Cheeseman Citation1906). Debate exists over the origins of inland stands (Clarkson et al. Citation1991). Some suggest that they originate from Māori plantings and have subsequently spread into the wider landscape. On the shores of Lake Taupo, M. excelsa co-occurs with archaeological/cultural sites and trees on Motutaiko Island are said to have been planted by Tūwharetoa ancestors (Stowe Citation2007). However, the presence of other normally coastal associates in the Rotorua Lakes area suggests that some remaining inland M. excelsa stands are natural (Clarkson et al. Citation1991). Metrosideros excelsa forest is likely to have been more widespread through inland localities, as a coloniser of new surfaces created by the late Pleistocene and early Holocene lava flows from the Ōkataina Volcanic Centre. Present-day sequences show strong competition from other broadleaved species (such as Weinmannia racemosa, Litsea calicaris and Beilschmiedia tawa), which confine M. excelsa forest to open or extreme sites (Clarkson & Clarkson Citation1994).
Metrosideros excelsa has been widely planted throughout New Zealand. In the South Island, M. excelsa is cultivated as far south as Jackson Bay on the west coast (Simpson Citation2005) and the Otago Peninsula on the east coast. There are also several M. excelsa trees on the Chatham Islands and cultivation occurs on Stewart Island (Dawson et al. Citation2010). Metrosideros excelsa has been introduced to many other countries including Australia, the USA and South Africa, where it has escaped from cultivation and is regarded as an invasive species (Yeates & Williams Citation2006; Dawson et al. Citation2010).
Genetic structure
Three studies have investigated the genetic structure of M. excelsa forests in New Zealand (Young et al. Citation2001; Gardner et al. Citation2004; Broadhurst et al. Citation2008). Using seed from 20 M. excelsa populations, Young et al. (Citation2001) assessed genetic variation and interpopulation structure. Populations were genetically diverse and showed a high level of population differentiation. Three small isolated stands (Rotoiti, Rotorua and Paparoa reef) had particularly low genetic diversity compared with elsewhere and may originate from Māori plantings. Self-fertilisation was also more prevalent in small isolated populations. Gardner et al. (Citation2004) applied chloroplast DNA sequencing to M. excelsa and other Metrosideros species from widespread New Zealand populations. Results indicated a greater diversity of haplotypes in areas identified as glacial refugia, including upper Northland, northwest Nelson and the Coromandel Peninsula. These findings are interpreted as the result of range contraction, giving rise to isolated refuges during periods of warmer climatic conditions, followed by range expansion during cooler climates. The persistence of populations in isolated refuges is suggested to have given rise to the greater haplotype diversity observed (Gardner et al. Citation2004). A later study (Broadhurst et al. Citation2008) examined the genetic structure between and within 10 populations of M. excelsa, using amplified fragment length polymorphism (AFLPs) on genomic DNA. Like Young et al.'s (Citation2001) results, this study showed genetic differentiation among and within populations, with larger populations tending to have higher levels of genetic diversity, but no evidence for a relationship between genetic structure and geographical location (Broadhurst et al. Citation2008).
Hybridisation
Hybridisation between New Zealand's Metrosideros tree species is common. Metrosideros excelsa naturally hybridises with M. robusta (early reports of this include Kirk Citation1899; Carse Citation1927; Cooper Citation1954) because the species distributions and flowering times commonly overlap. This typically occurs inland from the coast and towards the end of flowering by M. excelsa (Dawson et al. Citation2010). Hybrid populations are present in the Rotorua and Tarawera Lakes areas and also on Rangitoto Island (Cooper Citation1954; Clarkson Citation1990; Dawson et al. Citation2010). Occasionally, hybrid individuals are also found among otherwise pure populations (Simpson Citation2005). Metrosideros excelsa is also able to form hybrids with M. umbellata and M. bartlettii; however, the distributions of M. excelsa and these two species less commonly overlap (Simpson Citation2005). Metrosideros excelsa and M. kermadecensis (Kermadec Island pōhutukawa) also readily hybridise when they occur together in cultivation. Hybridisation between New Zealand's Metrosideros species has raised concern around the genetic pollution of natural stands (Simpson Citation2005), however, genetic analysis has shown that hybridisation has been an important factor in the past evolution of the species, evidenced by numerous shared DNA sequences (Gardner et al. Citation2004). The extent of hybridisation in New Zealand's Metrosideros is comparable with that reported for Hawaiian Metrosideros (Harbaugh et al. Citation2009).
Fungal associates
A total of 209 species of fungi are known to be associated with Metrosideros species in New Zealand with only a few considered primary pathogens. However, secondary pathogens, such as wood-rotting basidiomycetes, may play a role in Metrosideros species die back following severe possum browse. A change in fungal pathogenicity after possum damage has yet to be investigated for M. excelsa. The majority of fungi identified on M. excelsa are saprobes; Leptomelanconium sp. is common on M. excelsa foliage and may cause leaf spots and discoloration. Although levels of fungal infection can be high on individual trees, the effect on overall tree health is unknown (McKenzie et al. Citation1999).
Nineteen endomycorrhiza have been identified within the inner tissues of M. excelsa roots. Seedlings are rapidly colonised by these fungi, which benefit the seedlings by increasing tolerance to environmental stress (e.g. drought and low nutrient availability) and increasing seedling growth rates, particularly when growing in infertile substrates (Simpson Citation2005). Metrosideros excelsa lacks ectomycorrhiza associations.
Reproductive biology
The reproductive strategy of M. excelsa has been described by Schmidt-Adam et al. (Citation2000) as ‘wasteful but sufficient’. Metrosideros excelsa is a mass flowering species, and trees flower profusely for c. 2 wk during December to January. Each tree is thought to produce 13,000–40,000 flowers per annual flowering episode (Schmidt-Adam et al. Citation2000). Flowering episodes show high annual variation and this may promote cross-pollination between populations (Simpson Citation1994; Bergin & Hosking Citation2006). However, this annual variation is not well understood. Evidence suggests that floral induction takes place in autumn, in response to shortening day length. Following flower initiation, reduced temperatures may have an inhibitory effect on floral development (Sreekantan et al. Citation2001) and flower intensity may be affected by proximity of flower buds to developing seeds (Henriod et al. Citation2000). Six key morphological stages in M. excelsa inflorescence development have been identified and occur over a 10 wk period (Schmidt-Adam & Gould Citation2000): (1) initially, vegetative and floral buds appear identical, both covered with bud scales; (2) allometric changes result in inflorescence buds taking on an ellipsoidal shape for c. 8 d and bud scales senescing; (3) deciduous bracts subtending the secondary inflorescence axis partly cover inflorescence buds; (4) deciduous bracts abscise; (5) a pair of bracts and two pairs of bracteoles subtending lateral flower buds in each cymose inflorescence become visible; and (6) bracts and bracteoles abscise, individual flower buds become clearly separated and petals become evident (Schmidt-Adam & Gould Citation2000).
The breeding system of M. excelsa is the intermediate of facultative selfing and facultative outcrossing. Neither dichogamy nor herkogamy is important in preventing self-pollination (Schmidt-Adam et al. Citation2000). Flowers are hermaphroditic, displaying an initial female phase (mean duration 1.3 d) followed by a hermaphrodite phase (mean duration 4 d), which is then followed by a further female phase. Stigmas remain receptive for a minimum of 9 d; however in the final phase, pollination is rare because pollinator rewards are depleted. In earlier phases, individual flowers produce an average of 50 µL nectar d−1. Pollen has high levels of viability (93.6%), however, pollen tube growth is slow compared with other angiosperms; this, coupled with relatively long styles, results in a 10–15 d delay before the pollen reaches the ovary (Schmidt-Adam et al. Citation1999).
All ovules (c. 933) within the ovaries appear to be potentially fertile, although the number of ovules that develop to form fertile seeds is low (c. 10.2%), irrespective of pollen availability (Schmidt-Adam et al. Citation1999). Once ovules are successfully fertilised, seed develops and is later released in March–April (Bergin & Hosking Citation2006). Despite low seed viability, overall seed production per tree is still sufficiently high (Schmidt-Adam et al. Citation1999). Metrosideros excelsa seed is readily dispersed by wind. Light winds in the range of 5–19 km h−1 can disperse seeds over extremely long distances (Wright et al. Citation2000), providing a mechanism for gene flow between populations (Broadhurst et al. Citation2008). Metrosideros excelsa seeds lack endosperm tissue and have a thin two-layered seed coat, making them extremely vulnerable to desiccation. Consequently, M. excelsa seed can only persist in the soil for a short period and must germinate soon after dispersal (Schmidt-Adam et al. Citation2002).
Inbreeding and outbreeding
The floral biology of M. excelsa presents a paradox, the large floral display attracts pollinators and supports out-breeding and cross-pollination. However, mass flowering episodes coupled with a hermaphroditic phase and an absence of evolutionary traits to avoid pollen and stigma interference result in a high rates of self-fertilisation (Schmidt-Adam et al. Citation1999). Approximately 60% of seeds produced are self-pollinated. Controlled pollination experiments indicate that individual M. excelsa trees differ in their ability to set seed after self-fertilisation. Natural populations likely compose a mosaic of self-compatible and incompatible individuals (Schmidt-Adam et al. Citation1999). Inbreeding depression is late-acting in self-pollinated individuals of M. excelsa and has no effect on pollen tube growth, ovule/seed development or germination rate (Schmidt-Adam et al. Citation1999, Citation2000). However, following six months of seedling growth, inbreed individuals have markedly lower rates of shoot growth. It is probable that natural selection may ultimately eliminate inbred individuals before they reach reproductive maturity (Schmidt-Adam et al. Citation2000).
Plant–pollinator relationships
Metrosideros excelsa is associated with a broad range of pollinators (). The red flower coloration and copious nectar production are known to attract bird species (Godley Citation1979). Native birds are more important pollinators of M. excelsa than native bees because of their greater abundance, larger body sizes and foraging patterns which are more efficient at inducing seed set and promoting outcrossing. Similarly, introduced honeybees are more effective pollinators than native bees because their larger body size promotes stigma contact (Schmidt-Adam et al. Citation2009). Nocturnal visitation by New Zealand's endemic geckos (Whitaker Citation1987) and short-tailed bat (Mystacina tuberculata) has also been reported (Arkins et al. Citation1999; Pattemore & Wilcove Citation2012). On Hauturu/Little Barrier Island, short-tailed bats have significantly higher activity levels (indicated by echolocation calls) in the vicinity of flowering M. excelsa trees (Arkins et al. Citation1999).
Table 1 Recorded species visitation to Metrosideros excelsa flowers.
Since European settlement in New Zealand, a dramatic shift in bird pollinators has occurred (Schmidt-Adam et al. Citation2000), as many of the native nectar feeders, such as tūī (Prosthemadera novaeseelandiae), bellbird (Anthornis melanura) and hihi (Notiomystis cincta) are now locally extinct or rare on the mainland (Department of Conservation Citation2005). The original suite of pollinators, now only intact on offshore islands, has been largely replaced by non-native birds and honeybees. Owing to the ability of M. excelsa to use a wide range of pollinators, the reproductive system has been mostly resistant and the species does not suffer from enhanced inbreeding associated with pollinator shifts (Schmidt-Adam et al. Citation2000). However, Pattemore & Wilcove (Citation2012) have indicated that mainland M. excelsa populations may be suffering from a degree of pollen limitation, though the invasive ship rat (Rattus rattus) and recent colonist bird silvereye (Zosterops lateralis) at least partially maintain pollination on the mainland.
As well as providing a nectar source, M. excelsa trees also provide valuable wildlife habitat. Shags (Phalacrocorax spp.) and herons (Egretta spp.) often nest and roost in M. excelsa trees due to the close proximity of the trees to the bird feeding grounds and habitat range (Simpson Citation1994; Heather & Robertson Citation1996). Guano from roosting shags can be responsible for dieback of M. excelsa individuals, often killing a tree slowly limb by limb as leaves are scorched by solutes in the excreta (Gillham Citation1960). Holes in M. excelsa trunks provide nesting sites for birds such as the saddleback (Philesturnus carunculatus), and the flaky bark and leaf litter provides habitat for a wide range of indigenous and host specific insects including moths, weevils, beetles, flies and scale insects (Hosking & Hutcheson Citation1993; Simpson Citation1994; Taylor et al. Citation2007).
Plant communities and associations
Metrosideros excelsa forest, particularly young stands (< 60 yr), can have low species richness when compared with more widespread forest types in New Zealand (Bylsma Citation2012). The simplest M. excelsa forest is located on Whakaari/White Island (Bay of Plenty), which comprises M. excelsa, Histiopteris incisa and Phormium tenax (Clarkson Citation1990). The low species richness observed in M. excelsa forests reflects habitat severity and also the tendency of M. excelsa to retard the rate at which a diverse community can develop, primarily the result of dense shading canopies and slowly decomposing leaf litter which inhibits seedling establishment (Atkinson Citation2004). Metrosideros excelsa commonly occupies exposed, saline and drought-prone coastal sites, where only a few other plant species are capable of establishment, including Pseudopanax lessonii, Kunzea ericoides, Piper excelsum and Pittosporum crassifolium. Further inland or along coastal valleys, where soil and moisture conditions are more favourable, M. excelsa can form tall coastal forest that hosts a diverse mix of coastal and lowland plant species. Common canopy associates include Corynocarpus laevigatus, Vitex lucens, Dysoxylum spectabile, Kunzea ericoides, Litsea calicaris, Knightia excelsa and Beilschmiedia tawa (Bergin & Hosking Citation2006; Bylsma Citation2012). Metrosideros excelsa forest composition varies with topographic landform, exposure and latitudinal position (Bylsma Citation2012). Examples of compositional associations () from published and unpublished sources are summarised in six general geographic regions (Northland, Auckland, Coromandel, Bay of Plenty, East Coast and West Coast) in Supplementary file 1, to illustrate the pattern of M. excelsa dominated communities across the species natural range.
Table 2 Range of recorded vegetation types with Metrosideros excelsa as a dominant component. Metrosideros = Metrosideros excelsa.
Epiphyte associations
Epiphyte species are generally not abundant in M. excelsa forest (Bylsma Citation2012), primarily due to low humidity and high exposure. In sheltered or mature forest, epiphytes become more common. In a survey of M. excelsa forests in the Bay of Plenty, common epiphytic species included Pyrrosia eleagnifolia, Asplenium flaccidum, Microsorum scandens, Microsorum pustulatum, Blechnum filiforme, Peperomia urvilleana, Ichthyostomum pygmaeum, Astelia solandri and Collospermum hastatum. Climbers and lianas were less common but included Clematis foetida, Clematis paniculata and Ripogonum scandens (Bylsma Citation2012). Kirk (Citation1872) reported M. excelsa trees growing on the shores of Lake Tarawera to be rich with epiphytic species, including Griselinia lucida, A. solandri and Pittosporum cornifolium. Pittosporum cornifolium has also been recorded growing in coastal M. excelsa forest, in the Tamaki Ecological District (CHR 497823). On the Poor Knights Islands (Northland), Xeronema callistemon has been recorded as an epiphyte on M. excelsa; it is unknown whether these originate from plants which have fallen from cliffs above and taken hold, or have established from seed (New Zealand Plant Conservation Network Citation2013).
Establishment and succession
The germination strategy of M. excelsa is tuned-in to dynamic landscapes and stochastic disturbance; seeds do not persist in the seed bank and germination is rapid (Atkinson Citation2004). In seed trials, germination occurred between 10 and 28 d (Burrows Citation2000), thus synchrony between disturbance, seed set and seed dispersal is necessary. Successful germination is constrained to sites with high light (typically > 16% canopy openness) and free of dense leaf litter (Wotherspoon Citation1993; Bylsma Citation2012). Seedlings also have a strong affinity for fissured rock and volcanic substrate (Bylsma Citation2012), although seedling establishment on sand dunes is also possible (Bergin & Hosking Citation2006); here, seedlings are usually associated with driftwood or debris and more common on consolidated back dunes and damp swales (Simpson Citation2005; Bergin & Hosking Citation2006). Upon germination, biomass is allocated to the developing roots, which seek out and cling to moist surfaces, and emerging cotyledons (1–2 mm long) with thick waxy cuticles to prevent desiccation (Bergin & Hosking Citation2006). A lack of natural M. excelsa regeneration reported on the mainland is attributed to herbivore browse, trampling and weed invasion (Wotherspoon Citation1993; Hosking & Simpson Citation2011), and forest initiation usually requires landscape-scale disturbances (e.g. fire or volcanic eruption), which do not occur frequently. Smaller scale disturbance events, such as landslides, however, are more frequent and provide localised regeneration sites (Bylsma Citation2012).
Once established on the landscape, M. excelsa undoubtedly alter the physical environment and ultimately facilitate the establishment of later successional species. On Rangitoto Island, M. excelsa is one of the first woody species to colonise the sterile volcanic substrate. Beneath the drip zone of individual M. excelsa trees, ground temperature is reduced, humidity is increased and a humus layer is formed, and this facilitates further species establishment and the development of vegetation aggregations (Clarkson Citation1990). Aggregation development on Rangitoto Island shows that aggregation size is a strong predictor of species richness, with aggregations of 0.1 to > 100 m2 correlating with 1 to > 18 vascular taxa (BD Clarkson, University of Waikato, unpubl. data). Following canopy disturbance on White Island, however, direct succession of M. excelsa results from either the recovery and spread of an existing M. excelsa population through epicormic re-sprouting, or by colonisation of a newly emplaced surface by M. excelsa seed. Continual volcanic disturbance, isolation and extreme soil conditions prevent the incursion of later successional species here (Clarkson Citation1990).
Metrosideros excelsa forest succession and development have been quantified on the northern offshore islands (Atkinson Citation2004), Bay of Plenty islands and the Bay of Plenty mainland (Clarkson & Clarkson Citation1994; Bylsma Citation2012). Following disturbance, high-density, pure or mixed stands establish; juvenile M. excelsa densities as high as 3000 individuals ha−1 have been reported (Matata Scenic Reserve, Bylsma Citation2012). In the absence of further disturbance, no additional M. excelsa recruitment occurs, giving rise to cohort populations (). However, on sites with frequent small-scale disturbances (e.g. coastal headlands), multi-aged stands are possible, with exceptionally large stemmed individuals representing relicts of prior colonisation events (Clarkson & Clarkson Citation1994; Bylsma Citation2012).
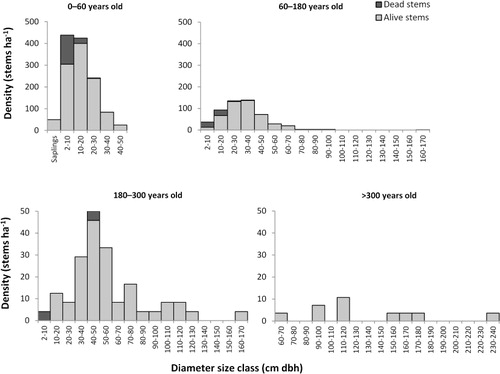
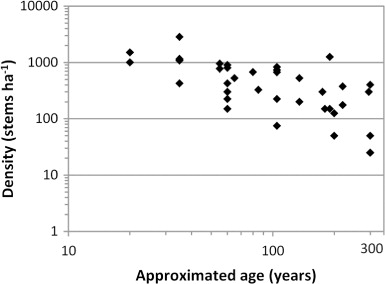
Self-thinning occurs throughout M. excelsa forest development (), resulting in mature forest (250–300 yr old) generally having < 300 M. excelsa stems ha−1. Self-thinning is most prolific in young forest (< 50 yr old), although in the early stages this may have little effect on total canopy foliage because gaps are successively filled by surviving M. excelsa. Self-thinning of mature M. excelsa forest generally involves the sequential loss of tree limbs. This creates canopy gaps and allows later successional tree species to reach the canopy and replace M. excelsa (Atkinson Citation2004; Bylsma Citation2012). The decrease of M. excelsa stems throughout stand development is initially coupled with an increase in M. excelsa basal area, reaching c. 50 m2 ha−1 in 80 yr, but does not significantly increase thereafter (Bylsma Citation2012).
Replacement strategies among key canopy species involves establishment at different stages of forest development, and this reflects species differing shade tolerances. Ranking tree species by their minimum light requirements (10th percentile of light environment occupancy) approximates their order of arrival in forest development (Metrosideros excelsa > Dysoxylum spectabile > Corynocarpus laevigatus > Litsea calicaris > Beilschmiedia tawa; Bylsma Citation2012). Early colonists that establish with M. excelsa may include K. ericoides, Leptospermum scoparium, Coriaria arborea var. arborea, Cordyline australis and Hebe stricta var. stricta. After c. 30 yr of development, a suite of shade-tolerant understory species typically establish beneath M. excelsa, including Myrsine australis, Melycytus ramiflorus, P. lessonii, P. excelsum subsp. excelsum and large leaved Coprosma species (C. robusta, C. macrocarpa, C. lucida and C. grandifolia). Mid-successional, shade-tolerant canopy species typically arrive following c. 60 yr of forest development and include C. laevigatus, V. lucens, L. calicaris, K. excelsa and D. spectabile; most of which are bird dispersed. The next stage of forest succession generally involves the arrival of late successional tree species such as Beilschmiedia tarairi and/or B. tawa (Percy Citation1956; Atkinson Citation2004; Bylsma Citation2012). In favourable locations (such as Hen and Chicken Islands, Northland), these can replace M. excelsa in the canopy after 200 yr (Atkinson Citation2004). In the Bay of Plenty, however, B. tarairi is not common, and B. tawa generally replaces M. excelsa following a minimum timespan of 300 yr (Clarkson & Clarkson Citation1994; Bylsma Citation2012), although relict individuals may be capable of surviving for 1000 yr (Simpson Citation1994). Mid-successional tree species can also contribute to M. excelsa replacement, but often to a lesser degree because mid-successional species are more reliant on canopy gaps to initiate height growth (Smale & Kimberley Citation1983; Bylsma Citation2012). Successional trends are comparable to those in other Metrosideros-dominated systems, for example, Metrosidersos polymorpha on Hawaiian lava flows (Atkinson Citation1970; Clarkson Citation1997).
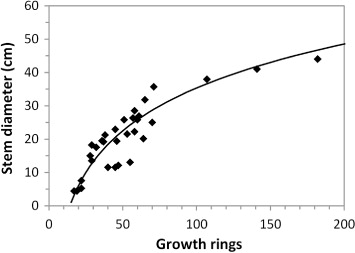
Diameter growth rates
The diameter–age relationship exhibited by M. excelsa stems in Bay of Plenty forests () suggests that trees have high initial growth rates, which can exceed 4 mm year−1 in the first 80 yr (Bylsma Citation2012). Once trees exceed 100 yr of age, diameter growth subsequently reduces to < 2 mm year−1 (Clarkson & Clarkson Citation1994; Bylsma Citation2012). Average diameter growth reported for Whakaari/White Island trees is 2.7 mm year−1 (Clarkson & Clarkson Citation1994); significantly lower than that reported for mainland trees and consistent with the harsh and frequently disturbed environment on the island (Bylsma Citation2012). Bergin & Hosking (Citation2006) reported M. excelsa diameter growth rates for planted (> 100 yr ago) trees outside the species natural range as 0.9–1.8 mm year−1. Pardy et al. (Citation1992) also assessed M. excelsa growth rates in planted stands, finding that the mean diameter growth rate was 9.7 mm year−1; much higher than that reported for natural stands.
Threats and conservation
Metrosideros excelsa is not recognised as a threatened species (de Lange et al. Citation2013). However, M. excelsa forest is a rare and localised vegetation community on the mainland (Hosking & Simpson Citation2011; Bylsma Citation2012). In the past, the greatest threat to M. excelsa forest was land clearance. Initially, this was the result of localised burning by Māori, followed by more extensive clearance by Europeans for settlement, the expansion of pastoral farming and tree felling to sustain the shipbuilding industry (Dieffenbach Citation1843; Simpson Citation2005). Fire was the primary tool used to clear land. Dense wood with a low moisture content, flaky bark, leaves containing flammable oils and dry leaf litter results in M. excelsa being extremely vulnerable to fire (Bergin & Hosking Citation2006). Despite the species having a strong ability to coppice and re-sprout epicormic shoots following damage by wind, herbivore browse or acid/toxic fumes, even a low-intensity fire can kill a mature tree (Clarkson & Clarkson Citation1994; Bergin & Hosking Citation2006). By 1989, it was estimated that 90% of the original M. excelsa forest was lost (Forest Research Institute Citation1989; Hosking Citation2000), with nearly 100% clearance in the west, where refuges from fire on steep cliffs and bluffs were infrequent. Remaining M. excelsa habitat along the northern coastline is highly desirable for residential development, thus ongoing land clearance continues to pose a serious threat (Simpson Citation2005; Bergin & Hosking Citation2006). Original M. excelsa forest on the mainland is now highly localised, isolated and fragmented (Hosking & Simpson Citation2011) and this has implications for the species’ long-term health and viability. Small, isolated populations are prone to increased rates of self-pollination and associated inbreeding depression in offspring (Schmidt-Adam et al. Citation2000), as well as increased edge effects (e.g. microclimactic changes in light, temperature and wind around the perimeter of the fragments). Contemporary pressures also include herbivore browse, trampling by stock, insect attack, accidental fire and a limited number of suitable regeneration sites (free of leaf litter, herbivore browse and competition from other vegetation; Wotherspoon Citation1993).
Possum and herbivore browse is a major cause of current stand deterioration (Simpson Citation1994; Bergin & Hosking Citation2006; Baigent-Mercer Citation2010), inhibiting the establishment and regeneration of M. excelsa and later successional species. In a Bay of Plenty survey, Orokawa and Homunga Bay stands (Waihi Beach) had severe browsing damage and a notable lack of palatable mid to late successional seedlings and saplings (e.g. L. calicaris), present in comparably aged stands elsewhere (Bylsma Citation2012). The leaves of M. excelsa are highly palatable. In winter, possums selectively browse old foliage, whereas in spring and summer the young leaves and vegetative buds are targeted. Following defoliation, trees reduce flower production and create many epicormic shoots. This alters the growth form of the tree, and as a consequence, possum-ravaged trees are more intensively grazed because of the plentiful young buds (Bergin & Hosking Citation2006). Once possums have removed c. 80% of a tree's foliage, tree death is inevitable (Wotherspoon Citation1993). Tree health can be improved if trees are protected from further possum damage, for example, by using possum control or by trunk banding which prevents possum access. Enhanced pest control, including the wider use of toxins and bait stations would undoubtedly benefit the long-term health and prosperity of M. excelsa (Wotherspoon Citation1993; Environment Bay of Plenty Citation2009; Baigent-Mercer Citation2010).
The foliage of M. excelsa is also grazed by a number of insects, including Costelytra zealandica (grass grub), Phasmatidae (stick insects), Neomycta rubida (weevil) and Eucolaspis brunneus (native bronze beetle) and inhabited by Psyllidea (sap suckers) (Bergin & Hosking Citation2006). Insect browse alone seldom causes lasting impacts on the health of mature trees (Bergin & Hosking Citation2006); however, E. brunneus has caused significant damage to M. excelsa trees on Little Barrier Island by destroying developing flower buds (G. Schmidt-Adam, pers. obs.). Psyllids can heavily infect and suppress growth of nursery-raised seedlings, however, plants rarely die and the foliage of mature trees is not targeted (Bergin & Hosking Citation2006).
During the late 1980s, widespread concern arose over the deteriorating state of New Zealand's M. excelsa forests, prompting a number of forest health studies, primarily undertaken by the Department of Conservation (DOC), Forest Research Institute (FRI) and later, Project Crimson (Hosking & Hutcheson Citation1993). The Project Crimson Trust, formed in 1990, is focused on the protection and enhancement of remaining M. excelsa forest. The trust has initiated hundreds of community-based conservation projects and funds scientific research and conservation management programmes. A re-assessment of the health of M. excelsa forests, 10 yr after the trust was established, showed that the number and extent of regeneration sites had increased, possum damage and browse had decreased and fencing around stands had increased (Bergin & Hosking Citation2006). However, re-establishment of M. excelsa through restoration plantings has had variable success, often related to a difficult establishment phase. Mortality and low growth rates of planted M. excesa seedlings are often related to plant form/size, substrate, draught, exposure and competition from faster growing vegetation (Bergin & Hosking Citation2006). A valuable set of guidelines for planting and management of M. excelsa is available (Bergin & Hosking Citation2006), with key considerations given to site selection and preparation, the use of high-quality and appropriately sourced stock and the continued maintenance and monitoring of populations. High genetic differentiation between natural populations and a complex geographic pattern suggests locally sourced material is important when using M. excelsa in restoration (Broadhurst et al. Citation2008). Where possible, seed should also be collected from larger populations, which are less prone to self-fertilisation (Young et al. Citation2001). However, if the management goal is to increase species diversity, the use of M. excelsa as a primary cover may not be appropriate due to its tendency to slow the rate at which a diverse community develops (Atkinson Citation2004). For example, M. excelsa was the main species used during initial re-vegetation of Tiritiri Matangi Island (Hauraki Gulf), although in subsequent yr, stands required extensive thinning and under-planting due to the limited nature of natural regeneration (Cooper Citation2010).
Utility, cultural and economic value
Conly & Conly (Citation1988) review the cultural significance of M. excelsa and its place in Māori tradition and medicine. The current and past use and value of M. excelsa has been described by Simpson (Citation2005) and Bergin & Hosking (Citation2006). Metrosideros excelsa is regarded by Māori as a chiefly tree, featuring strongly in legend and spiritual ceremony, as well as having many medicinal uses (Simpson Citation1994). Traditionally, an infusion made from the bark, which contains the natural antioxidant ellagic acid, was made by the tohunga (chief priest) to remedy diarrhoea (Brooker et al. Citation1961; Riley Citation1994; Seeram et al. Citation2005). Inner bark was also bound to wounds to sooth inflammation and stop bleeding (Riley Citation1994), and chewed to alleviate toothache (Bergin & Hosking Citation2006). Nectar from M. excelsa flowers also has medicinal values; traditionally this was collected in large quantities and ingested to relieve throat pain (Riley Citation1994).
Early Māori used the hard wood of M. excelsa to make implements such as paddles, weapons and mauls. The high wood density meant it was also suited for flax-softening bats and pounders used to prepare rarauhe (Pteridium esculentum) rhizomes for consumption (Bergin & Hosking Citation2006). Māori frequently used M. excelsa timber for boat building (Bergin & Hosking Citation2006), particularly in the final fitting-out stage, because the strong and curved thwarts of M. excelsa were the preferred wood to brace and strengthen long and narrow canoes (Clarke Citation2007). Metrosideros excelsa flowers are also highly valued; their red coloration is considered chiefly and worn by individuals with high rank. Consequently, trees have been widely transplanted and often mark sacred sites (Bercusson & Torrance Citation1998).
The economic values of M. excelsa are numerous (Conly & Conly Citation1988). Before glues became readily available, the strong and durable timber was used extensively for knees in shipbuilding (Kirk Citation1874). The wood is also highly decorative and is commonly used for carving and turning (Conly & Conly Citation1988). Honey produced from M. excelsa nectar is highly valued for its flavour and soothing properties and is produced on a commercial scale (Simpson Citation2005). Metrosideros excelsa is grown extensively in cultivation making it readily available for use in landscaping and gardens (Dawson et al. Citation2010).
Conclusion
Metrosideros excelsa is a New Zealand icon, intrinsic to the cultural identity of many New Zealanders. The species has many ecological adaptations that enable it to thrive along northern New Zealand's rocky, saline and drought-prone coast, and also colonise raw volcanic substrates. During the late Pleistocene–early Holocene, M. excelsa was likely more widespread inland, as a coloniser of lava fields produced from the Ōkataina Volcanic Centre (Clarkson & Clarkson Citation1994). The reproductive strategy of M. excelsa is tuned in to these dynamic landscapes and stochastic disturbance events, flowering in mass and subsequently producing thousands of small wind-dispersed seeds with rapid germination. Once established, M. excelsa can dominate forest development for centuries (Bylsma Citation2012). Harsh coastal conditions result in M. excelsa typically forming pure young stands with low plant species diversity compared with other New Zealand forest types (Bylsma Citation2012). However, further from the coast and as forests develop, they become more diverse, partially due to the incursion of shade-tolerant shrubs and mid to late successional tree species; the latter may eventually overtop and outcompete M. excelsa.
Following the arrival of people in New Zealand, M. excelsa forests became severely depleted as coastal areas were cleared for settlements and agriculture, and remaining forests were subject to logging (Hosking & Simpson Citation2011). Forest losses were accompanied by a dramatic shift in M. excelsa pollinator relationships, as many native bird species become lost from the mainland and exotic birds and bees were introduced (Schmidt-Adam et al. Citation2000). The best examples of M. excelsa forest with intact native plant and animal associations are now restricted to offshore islands. The infrequent occurrence of large-scale disturbances, coupled with human and herbivore pressure, currently limits natural regeneration on the mainland and remaining stands continue to decline. Introduced herbivores, particularly the brushtail possum, likely represent the greatest contemporary influence on M. excelsa forests and forest succession, influencing tree health and limiting regeneration of M. excelsa and later successional species (Bylsma Citation2012). Deterioration and decline of M. excelsa has implications well beyond the species itself. These forests are dynamic (Bylsma Citation2012) and strongly integrated communities; further losses of M. excelsa will reduce the habitat and resource available to plant and animal associates, including host-specific indigenous insects (Hosking & Hutcheson Citation1993). Key considerations for the conservation and restoration of M. excelsa communities include appropriate site selection, fencing and pest management, seed sourcing for local provenance and long-term monitoring of tree health (Bergin & Hosking Citation2006). Although M. excelsa has been the focus of many scientific, practical and cultural publications further research and documentation is still necessary. Future research should advance existing guidelines that assist those establishing and managing M. excelsa forest and restoration sites. Emphasis should be on reducing establishment barriers and progressing understanding of M. excelsa population genetics within a restoration context, particularly the degree to which inbreeding depression, low seed viability and pollinator shifts affect natural regeneration and the long-term success of restoration and regeneration sites.
Supplementary file
Supplementary file 1: Compositional associations in six geographic regions.
Compositional associations in six geographic regions.
Download MS Word (30.3 KB)Acknowledgements
This paper was part of an MSc project by Rebecca Bylsma at the University of Waikato and the authors thank Catherine Kirby, Dr Gabriele Schmidt-Adam and one anonymous reviewer for constructive comments on an earlier draft of the manuscript and Toni Cornes for providing the distribution map. This project was aided by funding received from the Environmental Research Institute (University of Waikato), the Whakatane Historical Society Trust and the Rotorua Botanical Society.
References
- Allan HH 1961. Flora of New Zealand. Volume 1: Indigenous Tracheophyta. Wellington, Government Printer. 1085 p.
- Andersen OM 1988. Proportions of individual anthocyanins in the genus Metrosideros. Biochemical Systematics and Ecology 16: 535–539. 10.1016/0305-1978(88)90059-2
- Anderson SH 2003. The relative importance of birds and insects as pollinators of the New Zealand flora. New Zealand Journal of Ecology 27: 83–94.
- Arkins AM, Winnington AP, Anderson S, Clout MN 1999. Diet and nectarivorous foraging behaviour of the short-tailed bat (Mystacina tuberculata). Journal of Zoology 247: 183–187. 10.1111/j.1469-7998.1999.tb00982.x
- Atkinson IAE 1970. Successional trends in the coastal and lowland forest of Mauna Loa and Kilauea volcanoes, Hawaii. Pacific Science 24: 387–400.
- Atkinson IAE 2004. Successional processes induced by fires on the northern offshore islands of New Zealand. New Zealand Journal of Ecology 28: 181–193.
- Baigent-Mercer D 2010. Seeing red. Forest and Bird May Issue: 42–45.
- Beadel SM, Shaw WB 1988. Taneatua Ecological District. Biological Survey of Reserves Series. Report no. 12. Wellington, Department of Conservation. 138 p.
- Bellingham PJ 1984. Forest regeneration on Lady Alice Island, Hen and Chickens Group. Tane 30: 31–42.
- Benson M 1995. Pohutukawa in North Taranaki. Conservation Advisory Science Notes no. 125. Wellington, Department of Conservation.
- Bercusson L, Torrance J 1998. Pohutukawa: tree of Aotearoa. Auckland, Tandem Press. 87 p.
- Bergin DO, Hosking G 2006. Pohutukawa: ecology, establishment, growth and management. New Zealand Indigenous Tree Bulletin no. 4. Rotorua, New Zealand Forest Research Institute Limited. 104 p.
- Boland DJ, Sedgley M 1986. Stigma and style morphology in relation to taxonomy and breeding system in Eucalyptus and Angophora (Myrtaceae). Australian Journal of Botany 34: 569–584. 10.1071/BT9860569
- Broadhurst LM, Young AG, Murray BG 2008. AFLPs reveal an absence of geographical genetic structure among remnant populations of pohutukawa (Metrosideros excelsa, Myrtaceae). New Zealand Journal of Botany 46: 13–21. 10.1080/00288250809509750
- Brooker SJ, Cambie RC, Cooper RC 1961. New Zealand medicinal plants. Auckland, Heinemann Publishers. 268 p.
- Burrows CJ 2000. Germination behaviour of the seeds of six species of Metrosideros (Myrtaceae). Christchurch, Rebus Publications. 20 p.
- Bylsma RJ 2012. Composition, structure and dynamics of Metrosideros excelsa (pōhutukawa) forest, Bay of Plenty, New Zealand. Unpublished MSc thesis. Hamilton, The University of Waikato.
- Carse H 1927. Botanical notes, with descriptions of new species. Transactions of the New Zealand Institute 57: 89–93.
- Cheeseman TF 1906. Contributions to a fuller knowledge of the flora of New Zealand: no. 1. Transactions of the New Zealand Institute 39: 444–445.
- Clarke A 2007. The great sacred garden of Tāne – te wao tapu nui a Tāne: a natural pre-history of Aotearoa New Zealand. New Zealand, Raupo Publishings. 520 p.
- Clarkson BD 1990. A review of vegetation development following recent (<450 years) volcanic disturbance in North Island, New Zealand. New Zealand Journal of Ecology 14: 59–71.
- Clarkson BD 1997. Vegetation succession (1967–89) on five recent montane lava flows, Mauna Loa, Hawaii. New Zealand Journal of Ecology 22: 1–9.
- Clarkson BD, Clarkson BR 1994. Vegetation decline following recent eruptions on White Island (Whakaari), Bay of Plenty, New Zealand Journal of Botany 32: 21–36. 10.1080/0028825X.1994.10410404
- Clarkson BD, Smale MC, Ecroyd CE 1991. Botany of Rotorua. New Zealand, Waikato Botanical Society. 132 p.
- Clarkson BD, Spring-Rice B 1992. Vegetation and flora of Motuotau Island, Bay of Plenty. Rotorua Botanical Society Newsletter no. 26.
- Clements J, Henriod R, Bailey D, Jameson P 1999. Vegetative phase change in Metrosideros excelsa: shoot and root restriction. Plant Growth Regulation 28: 201–214.
- Conly G, Conly M 1988. New Zealand pohutukawa. Wellington, Grantham House. 88 p.
- Cooper H 2010. Transforming the pohutukawa forest on Tiritiri Matangi. In: Dawn Chorus, Supporters of Tiritiri Matangi Inc. Bulletin 82. Pp. 4–5.
- Cooper RC 1954. Pohutukawa × rata: variation in Metrosideros (Myrtaceae) on Rangitoto Island. Records Auckland Institute and Museum 4: 205–212.
- Daniel MJ 1976. Feeding by short-tailed bat (Mystacina tuberculata) on fruit and possible nectar. New Zealand Journal of Zoology 3: 391–398. 10.1080/03014223.1976.9517927
- Dawson JW 1968. An analysis of flowers and fruits in New Zealand Metrosideros. New Zealand Journal of Botany 6: 43–55. 10.1080/0028825X.1968.10428789
- Dawson JW 1988. Forest vines to snow tussocks: the story of New Zealand plants. Wellington, Victoria University Press.
- Dawson JW 1993. Botany of the early French voyages. Tuatara 32: 64–69.
- Dawson MI 2008: Index of chromosome numbers of indigenous New Zealand vascular plants. Lincoln, New Zealand, Unpublished index held at Landcare Research.
- Dawson M, Hobbs J, Platt G, Rumbal J 2010. Metrosideros in cultivation: pōhutukawa. The first of a two part series. New Zealand Garden Journal 13: 10–22.
- de Lange PJ, Rolfe JR, Champion PD, Courtney SP, Heenan PB, Barkla JW et al. 2013. Conservation status of New Zealand indigenous vascular plants, 2012. New Zealand Threat Classification Series 3. Wellington, Department of Conservation.
- Department of Conservation 2005. Hihi/stitchbird (Notiomystis cincta) recovery plan 2004–09. Threatened Species Recovery Plan 54. Wellington, Department of Conservation. 31 p.
- Dieffenbach E 1843. Travels in New Zealand, Volume 1. London, Longman Brown.
- Environment Bay of Plenty 2009. Coastal indigenous forest canopy condition in the Bay of Plenty. Whakatane, Environment Bay of Plenty. 53 p.
- Forest Research Institute 1989. Tackling the pohutukawa health problem. What's new in Forest Research. Report no. 178. Rotorua, Forest Research Institute.
- Gaertner J 1788. De Fructibus et Seminibus Plantarum. Stuttgart, Academiae Carolina.
- Gardner RC, de Lange PJ, Keeling J, Bowala T, Brown HA, Wright SD 2004. A late Quaternary phylogeography for Metrosideros (Myrtaceae) in New Zealand inferred from chloroplast DNA haplotypes. Biological Journal of the Linnean Society 83: 399–412. 10.1111/j.1095-8312.2004.00398.x
- Gillham ME 1960. Vegetation of New Zealand shag colonies. Transactions of the Royal Society of New Zealand 88: 363–380.
- Godley EJ 1979. Flower biology in New Zealand. New Zealand Journal of Botany 17: 441–466. 10.1080/0028825X.1979.10432564
- Goldwater N, Beadel S 2010. Natural areas of Manaia Ecological District. Reconnaissance survey report for the Protected Natural Areas Programme. Whangarei, Northland Conservancy, Department of Conservation.
- Hamilton WM, Atkinson IA 1961. Vegetation. In: Hamilton WM ed. Little Barrier Island (Hauturu). Department of Scientific and Industrial Research Bulletin 137. Wellington, Government Printer. Pp. 87–121.
- Harbaugh DT, Wagner WL, Percy DM, James HF, Fleischer RC 2009. Genetic structure of the polymorphic Metrosideros (Myrtaceae) complex in the Hawaiian Islands using nuclear microsatellite data. PLoS ONE 4: e4698. 10.1371/journal.pone.0004698
- Heather BD, Robertson HA 1996. The field guide to the birds of New Zealand. Auckland, Penguin Books. 432 p.
- Henriod RE, Jameson PE, Clemens J 2000. Effects of photoperiod, temperature and bud size on flowering in Metrosideros excelsa (Myrtaceae). Journal of Horticultural Science and Biotechnology 75: 55–61.
- Hosking G 2000. ‘Measuring our success’ a reassessment of pohutukawa health ten years on. Report for an assessment commissioned by the Project Crimson Trust. Wellington, Project Crimson Trust. 8 p.
- Hosking G, Simpson P 2011. Pohutukawa: New Zealand and's Christmas tree. Wellington, Department of Conservation. 24 p.
- Hosking GP, Hutcheson JA 1993. Pohutukawa (Metrosideros excelsa) health and phenology in relation to possums (Trichosurus vulpecular) and other damaging agents. New Zealand Journal of Forestry Science 23: 49–61.
- Kirk T 1872. Notes on the flora of the Lake District of the North Island. Transactions and Proceedings of the Royal Society of New Zealand 5: 322–345.
- Kirk T 1874. Durability of New Zealand timbers. Appendix to the Journals of the House of Representatives 2 D H–25.
- Kirk T 1899. The students flora of New Zealand and outlying islands. Wellington, Government Printer. 408 p.
- Kubien DS, Jaya E, Clemens J 2007. Differences in structure and gas exchange physiology of juvenile and adult leaves in Metrosideros excelsa. International Journal of Plant Sciences 168: 563–570. 10.1086/513485
- Lux J, Martin T, Beadel S 2007. Natural areas of Waipu Ecological District. Reconnaissance survey report for the Protected Natural Areas Programme. Whangarei, Northland Conservancy, Department of Conservation.
- McKenzie EHC, Buchanan PK, Johnston PR 1999. Fungi on pohutukawa and other Metrosideros species in New Zealand. New Zealand Journal of Botany 37: 335–354. 10.1080/0028825X.1999.9512637
- Meylan BA, Butterfield JK 1978. The structure of New Zealand woods. Department of Scientific and Industrial Research Bulletin 222. Wellington, Department of Scientific and Industrial Research. 250 p.
- Mustafa KA, Perry NB, Weavers RT 2005. Lipophilic C-methylflavonoids with no B-ring oxygenation in Metrosideros excelsa (Myrtaceae). Biochemical Systematics and Ecology 33: 1049–1059. 10.1016/j.bse.2005.02.003
- New Zealand Plant Conservation Network 2013. Xeronema callistemon. http://www.nzpcn.org.nz/flora ( accessed 1 July 2013).
- Oliver WRB 1928. The New Zealand species of Metrosideros with a note on Metrosideros collina (Forst) Gray. Transactions of the New Zealand Institute 59: 419–423.
- Pardy GP, Bergin DO, Kimberley MO 1992. Survey of native tree plantations. New Zealand Ministry of Forestry, Forest Research Institute Bulletin no. 5. 24 p.
- Pattemore DE, Wilcove DS 2012. Invasive rats and recent colonist birds partially compensate for the loss of endemic New Zealand pollinators. Proceedings of the Royal Society B. doi:10.1098/rspb.2011.2036
- Percy CA 1956. A primary survey of the vegetation of Marotiri Island. Tane 7: 3–6.
- Regnier CE 1987. Coromandel Ecological Region. Protected Natural Areas Programme: phase 1. Wellington, Department of Conservation. 241 p.
- Regnier CE, Clarkson BD 1988. Tainui Ecological Region Protected Natural Areas Programme: phase 1. New Zealand. Wellington, Department of Conservation.
- Regnier CE, Courtney SP, Weissing MI 1988. Pukeamaru ecological district. Survey report for the Protected Natural Areas Programme. Wellington, Department of Conservation. 104 p.
- Richard A 1832. Voyage de Decouvertes de L'Astrolabe, Botanique. Essai d'une Flore de La Nouvelle-Zelande. Paris, Tastu.
- Riley M 1994. Māori healing and herbal. Paraparaumu, New Zealand, Viking Sevenseas New Zealand Ltd. 528 p.
- Sakai A, Wardle P 1978. Freezing resistance of New Zealand trees and shrubs. New Zealand Journal of Ecology 1: 51–61.
- Schmidt-Adam G, Gould KS 2000. Phenology of inflorescence development in pohutukawa (Metrosideros excelsa, Myrtaceae). New Zealand Journal of Botany 38: 333–337. 10.1080/0028825X.2000.9512685
- Schmidt-Adam G, Gould KS, Murray BG 1999. Floral biology and breeding system of pohutukawa (Metrosideros excelsa, Myrtaceae). New Zealand Journal of Botany 37: 687–702. 10.1080/0028825X.1999.9512663
- Schmidt-Adam G, Gould KS, Murray BG 2002. Seed biology of Metrosideros excelsa (Myrtaceae). New Zealand Journal of Botany 40: 419–425. 10.1080/0028825X.2002.9512803
- Schmidt-Adam G, Murray BG 2002. Structure and histochemistry of the stigma and style of Metrosideros excelsa. New Zealand Journal of Botany 40: 95–103. 10.1080/0028825X.2002.9512773
- Schmidt-Adam G, Murray BG, Young AG 2009. The relative importance of birds and bees in the pollination of Metrosideros excelsa (Myrtaceae). Austral Ecology 34: 490–498. 10.1111/j.1442-9993.2009.01949.x
- Schmidt-Adam G, Young AG, Murray BG 2000. Low outcrossing rates and shift in pollinators in New Zealand pohutukawa (Metrosideros excelsa; Myrtaceae). American Journal of Botany 87: 1265–1271. 10.2307/2656719
- Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair M et al. 2005. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. The Journal of Nutritional Biochemistry 16: 360–367. 10.1016/j.jnutbio.2005.01.006
- Simpson P 1997. Natural pohutukawa in Taranaki. Conservation Advisory Science Notes no. 150. Wellington, Department of Conservation. 9 p.
- Simpson PG 1994. Pohutukawa and biodiversity. Conservation Advisory Science Notes no. 100. Wellington, Department of Conservation. 12 p.
- Simpson PG 2005. Pohutukawa and rata: New Zealand's iron hearted trees. Wellington, Te Papa Press. 346 p.
- Smale MC, Clarkson BR, Clarkson BD, Floyd CG, Cornes TS, Clarkson FM et al. 2009. Natural areas of Kaipara Ecological District (Northland Conservancy). Reconnaissance Survey Report for the Protected Natural Areas Programme. Report prepared by Landcare Research New Zealand and The University of Waikato for Department of Conservation, Northland Conservancy.
- Smale MC, Kimberley MO 1983. Regeneration patterns in Beilschmiedia tawa dominant forest at Rotoehu. New Zealand Journal of Forestry Science 13: 58–71.
- Solangaarachchi SM, Gould KS 2001. Anthocyanin pigmentation in the adventitious roots of Metrosideros excelsa (Myrtaceae). New Zealand Journal of Botany 39: 161–166. 10.1080/0028825X.2001.9512724
- Sreekantan L, McKenzie MJ, Jameson PE, Clemens J 2001. Cycles of floral and vegetative development in Metrosideros excelsa (Myrtaceae). International Journal of Plant Sciences 162: 719–727. 10.1086/320779
- Stowe C 2007. A review of the endemic trees (and shrubs) cultivated by Māori in Aotearoa (New Zealand) from 1769–1840. Bay of Islands, New Zealand. Contract report prepared for Ngāti Hine, Urtica Inc.
- Taylor G, Davies K, Martin N, Crosby T 2007. First record of Fergusonina (Diptera: Fergusoninidae) and associated Fergusobia (Tylenchida: Neotylenchidae) forming galls on Metrosideros (Myrtaceae) from New Zealand. Systematic Entomology 32: 548–557. 10.1111/j.1365-3113.2007.00383.x
- Whitaker A 1987. The roles of lizards in New Zealand plant reproductive strategies. New Zealand Journal of Botany 25: 315–328. 10.1080/0028825X.1987.10410078
- Wotherspoon SH 1993. Regeneration ecology and restoration of pohutukawa (Metrosideros excelsa) in the Auckland Region. Unpublished MSc thesis. Auckland, University of Auckland.
- Wright SD, Yong CG, Dawson JW, Whittaker DJ, Gardner RC 2000. Riding the ice age El Nino? Pacific biogeography and evolution of Metrosideros subg. Metrosideros (Myrtaceae) inferred from nuclear ribosomal DNA. Proceedings of the National Academy of Sciences 97: 4118–4123. 10.1073/pnas.050351197
- Yeates GW, Williams PA 2006. Export of plant and animal species from an insular biota. In: Allen RB, Lee WG eds. Biological invasions in New Zealand. Berlin, Germany, Springer-Verlag. Pp. 85–100.
- Young AG, Schmidt-Adam G, Murray BG 2001. Genetic variation and structure of remnant stands of pohutukawa (Metrosideros excelsa, Myrtaceae). New Zealand Journal of Botany 39: 133–140. 10.1080/0028825X.2001.9512721