Abstract
Most plants change shape as they grow. However, ontogenetic changes in morphology (i.e. heteroblasty) can differ markedly among species and the role that heteroblasty might play in plant defence is poorly resolved. We use a model selection approach to characterise heteroblasty in terminal leaf spines produced by two perennial plant species native to New Zealand. Aciphylla aurea (Apiaceae), a perennial herb that inhabits alpine scrublands, exhibited ‘progressive’ heteroblasty. Seedlings produced soft, entire leaves with sharpened tips. However, as plants matured they produced compound leaves with sharpened tips that were far more rigid, giving rise to a strong degree of spinescence at adulthood. By contrast, Podocarpus totara (Podocarpaceae), a tall tree inhabiting cool-temperate forests, exhibited ‘retrogressive’ heteroblasty. The size of terminal leaf spines decreased during development and leaf circularity increased. Furthermore, leaf rigidity peaked at intermediate heights resulting in a peak in terminal leaf spinescence at the sapling stage of development. These results indicate that the mode of spinescence heteroblasty varied between species in ways that appear to facilitate defence at life history stages when plants are most susceptible to attack from large herbivores (i.e. adult plants are better defended in scrublands while saplings are better defended in woodlands). However, results also showed that spinescence was reduced in very young plants, suggesting that the ontogenetic development of spinescence may be constrained at very early ontogenetic stages, perhaps because younger plants lack the energetic resources to structurally reinforce leaf spines.
Introduction
As plants increase in size during development, they can also change markedly in shape. Changes in the shape in plant organs during ontogeny are collectively known as heteroblasty, and previous work on heteroblasty has led to numerous important insights into plant form and function (see Zotz et al. Citation2011).
Environmental conditions can change markedly as plants grow in height and heteroblasty may help plants adjust to these changes. For example, many aquatic plants produce different leaf forms when they are submerged versus when they grow above the surface of water (Wells & Pigliucci Citation2000), and many epiphytic bromeliads produce cup-shaped leaves at maturity, which store water to mediate water stress (Meisner & Zotz Citation2012; Meisner et al. Citation2013). Exposure to herbivores might also change as plants grow in height (Burns Citation2014). However, the role heteroblasty might play in plant defence is poorly resolved.
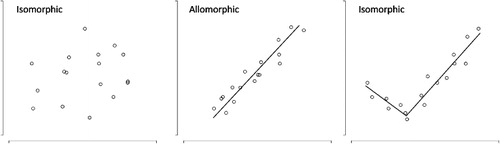
Heteroblasty can take many forms (see ). Some species show gradual shifts in morphology during ontogeny, leading to more or less continuous changes in plant shape with plant height (i.e. allomorphy; e.g. Burns Citation2005). Other plants exhibit sudden, radical changes in morphology at specific ontogenetic stages (i.e. metamorphy; e.g. Fadzly et al. Citation2009; Fadzly & Burns Citation2010). Although differences in the form of heteroblasty have been discussed widely (see Zotz et al. Citation2011 for a recent review), quantitative methods to distinguish between gradual, abrupt and other forms of heteroblasty have yet to be developed.
Burns (Citation2014) recently quantified ontogenetic changes in the production of prickles, thorns and spines in a variety of tree and shrub species inhabiting Australia, North America and several islands in the Pacific Ocean. In the scrublands of southwest Australia, structural defences increased during ontogeny, peaking in adulthood (i.e. ‘progressive’ heteroblasty). Conversely, plants inhabiting the woodlands of eastern Australia and California produced structural defences at earlier stages of development, rather than at adulthood (‘retrogressive’ heteroblasty). These differences in the modes of heteroblasty between vegetation types matched their susceptibility to large herbivores. Adult plants are more vulnerable to large mammalian herbivores in scrublands, whereas juvenile plants are more vulnerable in woodlands, where adult plants grow above the reach of mega-herbivores at maturity. Although large mammals are important herbivores in many ecosystems, they are generally absent from oceanic islands, which were instead home to large browsing birds (Whittaker & Fernández-Palacios Citation2007). However, it remains unclear whether browsing birds might select for leaf spinescence at particular life history stages.
Here, we investigate spinescence heteroblasty in two perennial plant species that are endemic to New Zealand (see ). First, we tested for progressive heteroblasty in a perennial, dicotyledonous herb that inhabits alpine scrublands (Aciphylla aurea, Apiaceae). More specifically, we predicted that terminal leaf spines in A. aurea would be produced later in its ontogeny (progressive) when adults are vulnerable to herbivory. Second, we tested for retrogressive heteroblasty in a coniferous tree that inhabits cool-temperate rainforests (Podocarpus totara, Podocarpaceae). In this species, we predicted that spinescence would be present earlier in ontogeny (retrogressive) when juveniles are susceptible to herbivory. To establish the form of heteroblasty in each species (i.e. abrupt versus gradual), we implemented a model selection approach to compare the fit of continuous and discontinuous functions to relationships between several plant traits and plant height. Overall results are used to (1) establish whether spinescence heteroblasty might occur in plants native to oceanic islands that housed avian, rather than mammalian herbivores; (2) distinguish the form of heteroblasty exhibited by multiple plant traits in each species; and (3) determine whether the two species display similar modes of heteroblasty previously delineated in scrublands and forests on continents housing mammalian rather than avian herbivores.
Materials and methods
Aciphylla aurea (Apiaceae, golden speargrass) is a native, perennial herb that grows to 1 m in height and inhabits alpine and subalpine scrublands on the South Island of New Zealand. It produces sharp, rigid leaves in a basal-rosette growth form, which gives rise to a distinctive overall appearance and hence its common name. Podocarpus totara (Podocarpaceae) is a coniferous tree that grows to 30 m tall and produces needle-like leaves that appear to be narrower on younger plants (Dawson & Lucas Citation2000).
One hundred individuals of P. totara were sampled in 2014 in Otari-Wilton’s Bush (41°14′S, 174°45′E), a forest reserve located on the southern tip of the North Island, New Zealand. The reserve is comprised of 75 hectares of mature and regenerating conifer–broadleaf forest and a detailed account of its climate, history, topography and vegetation is given elsewhere (Burns & Dawson Citation2005). The height of each tree was measured with a field tape or clinometer as the shortest distance between the highest leaf and the forest floor (Cornelissen et al. Citation2003). A single, undamaged, fully expanded leaf was collected from the top of each individual. On taller individuals whose tops were inaccessible, the specimen was taken from the outermost accessible extremity of the crown.
A digitised image of each leaf was then obtained with an Epson Perfection V30 scanner. Images were then imported into ImageJ (National Institutes of Health, Bethesda, MD, USA) to obtain leaf length and width, leaf area and spine size. The compression strength of each leaf was obtained with Motive DS2 Handy Digital Push Pull Gauge. Compression strength was obtained as an indication of terminal spine rigidity and was defined as the maximum force (N) required for the leaf to buckle. Each leaf was held 1 cm in from the tip of the petiole and was pressed against the force meter attachment. The peak force at which the leaf buckled was taken as the compression strength reading. For specimens that were less than one centimetre in length, they were given null readings (i.e. 0 N).
Thirty individuals of A. aurea were sampled above the tree line at an elevation of c. 1450 m on Mt Robert, Nelson Lakes National Park, South Island, New Zealand (41°49′S 172°48′E). Differences in sample sizes among species resulted from differences in their respective population sizes. Plant heights were measured as the distance between the ground and the highest leaf. The highest leaf from the ground was then harvested and its length, width and number of leaflets were measured. For compound leaves, the length and width of the terminal leaflet was measured. Compression strength was measured as described previously for P. totara.
Four dependent variables were obtained for A. aurea: (1) the leaf ratio was quantified as the length divided by width; (2) the size of the terminal leaf spine was measured as the size of an imaginary triangle superimposed on the tip of each leaf or leaflet. The height of the triangle was measured as the distance from the tip to the point on the leaf (or leaflet) that measured 2 mm, which then served as the triangle’s base; (3) compression strength; and (4) leaflet number. Only the first three variables are relevant to P. totara. Height was used as the independent variable in all subsequent analyses of both species.
The comparative fit of linear and piecewise (i.e. breakpoint) models was assessed to determine the mode of heteroblasty in all dependent variables in each species. For each variable, a linear model was fitted to the relationship and Akaike Information Criterion (AICc) for small sample sizes was obtained. For comparative purposes, an AICc value for a piecewise regression model was obtained with the segmented package in R (R Development Core Team Citation2008). Akaike weights (ωAICc) were obtained for each to discern which model most accurately described the relationship.
Results
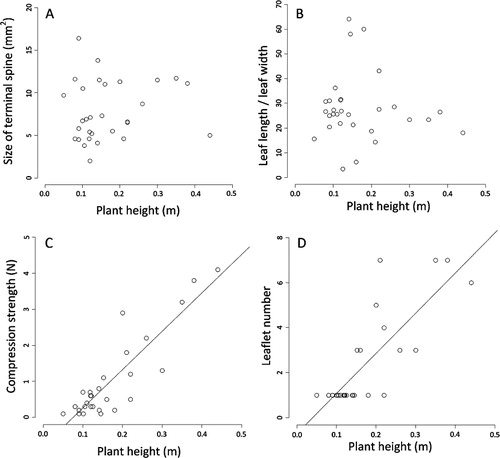
Different forms of heteroblasty were exhibited by different traits in A. aurea. The leaf ratio and size of the terminal leaf spines were clearly isomorphic (see ). The relationship between plant height and leaf ratio was poorly described by both linear (P = 0.715) and breakpoint models (P = 0.272). A linear function provided a poor fit to the relationship between plant height and the size of terminal leaf spines (P = 0.497), and breakpoint analyses failed to converge on a breakpoint parameter that separated intervals with more than one data point in each. Statistical analyses failed to discern whether compression strength was allomorphic or metamorphic (see ). Although a linear function provided the best fit between compression strength and plant height (AICc = 52.039, ∆AICc = 0.000, ωAICc = 1.000), a breakpoint model also provided a reasonable fit (AICc = 54.999, ∆AICc = 2.960, ωAICc = 0.228). Leaflet number was clearly allomorphic (see ). A linear function provided the best fit to the relationship between plant height and leaflet number (P < 0.001), and breakpoint analyses failed to converge on a breakpoint parameter that separated intervals with more than one data point in each.
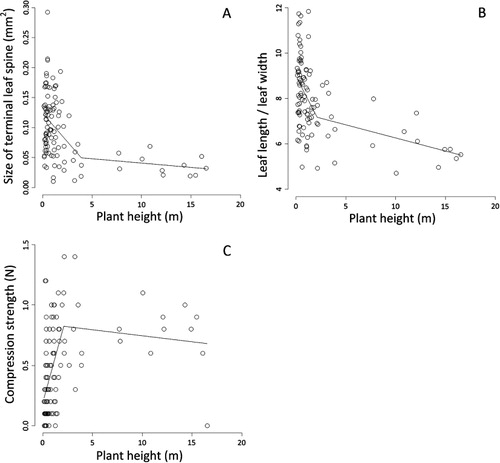
Different forms of heteroblasty were also exhibited by different leaf traits in P. totara. Statistical analyses failed to discern whether the size of terminal leaf spines was allomorphic or metamorphic (see ). A breakpoint provided the best fit between the size of terminal leaf spines and plant height (AICc = –313.586, ∆AICc = 0.000, ωAICc = 1.000). However, a linear model also provided a reasonable fit (AICc = –312.165, ∆AICc = 1.421, ωAICc = 0.491). Similar results were obtained for the leaf ratio. A breakpoint provided the best fit between leaf ratio and plant height (AICc = 359.608, ∆AICc = 0.000, ωAICc = 1.000). However, a linear model also provided a reasonable fit (AICc = 362.601, ∆AICc = 2.993, ωAICc = 0.224). Compression strength was clearly metamorphic (see ). A breakpoint model provided the best fit to the relationship between plant height and leaflet number (AICc = 61.122, ∆AICc = 0.000, ωAICc = 1.000); in comparison, a linear model did not perform as well (AICc = 80.496, ∆AICc = 19.374, ωAICc <0.001).
Discussion
Ontogenetic changes in leaf spinescence differed markedly between the two study species. In Aciphylla aurea, leaf shape and spine size were isomorphic, whereas compression strength and leaflet numbers were positively allomorphic. When interpreted jointly, these results indicate that A. aurea produces long, narrow lamina with sharp tips throughout its ontogeny. However, leaves become progressively more rigid and more divided as plants mature, ultimately resulting in a striking degree of spinescence at adulthood. Very different results were obtained in P. totora. Although compression strength was metamorphic, statistical analyses failed to discern whether the size of terminal leaf spines and leaf shape were allomorphic or metamorphic. Regardless of whether they were best described as allomorphic or metamorphic, leaves were initially long, thin and pointed, but they became much less needle-like at adulthood. Oppositely, leaves became more rigid as plants aged, reaching a peak in rigidity when plants were c. 2.5 m tall, and then dropping off sharply at adulthood. When interpreted jointly, ontogenetic trends in P. totara indicated that spinescence peaks at the sapling stage of development.
Overall, ontogenetic changes in leaf morphology of P. totara are consistent with the hypothesis that flightless browsing birds (e.g. moa, Dinornithiformes) have selected for spinescence heteroblasty in New Zealand. However, ontogenetic changes in each of the three leaf traits in P. totara did not match predictions based on the ‘moa hypothesis’ precisely. Under this hypothesis, the expression of all three leaf traits should be uniformly high across younger life history stages and then abruptly decline at c. 3 m, the height of the largest known moa species (see Atkinson & Greenwood Citation1977). Instead, leaves became progressively less needle-shaped but increasingly rigid. So although results were not entirely consistent with the moa hypothesis, when considered jointly, spinescence was higher at younger life history stages, when plants would have been susceptible to moa predation.
A spinescent structure must be both sharp and rigid for it to damage the mouthparts of herbivores and deter herbivory. Both species produced long, narrow leaves with pointed tips at early developmental stages. However, juvenile leaves were relatively soft compared to later life history stages, resulting in continuous increases in the spinescence of A. aurea, a peak in spinescence in saplings in P. totara. These results indicate that while it may be valuable to determine the mode of heteroblasty for individual leaf traits, multiple traits may have to be considered jointly to test mechanistic explanations for the evolution of heteroblasty.
Nearly all plants produce thicker, tougher leaves (i.e. high leaf mass per unit area, LMA) when they develop in direct sunlight, and thinner, papery leaves in shady conditions (see Burns Citation2004). Plastic adjustments in LMA relative to light availability help plants to maximise photosynthetic efficiency in differing light environments as they grow (i.e. the sun and shade leaf continuum). These changes will also affect the compression strength of leaves, and thereby their capacity to function as defensive structures (i.e. spinescence). Therefore, increases in the compression strength of leaves produced by both species during ontogeny may also be linked to physiological processes evolutionarily.
A model selection approach is a useful statistical platform to discern between the fit of different functions representing different modes of heteroblasty. However, results illustrate that distinguishing different modes of heteroblasty statistically might not be possible, depending on the form of the empirically derived relationship. Rather than technical limitation, this result illustrates that different modes of heteroblasty might not always be strictly categorical. Instead, different modes of heteroblasty (i.e. allomorphic versus metamorphic) might best be viewed as endpoints along a continuum of different forms of heteroblasty, and ontogenetic shifts in trait expression might defy predetermined developmental categories.
Differences in the mode of spinescence heteroblasty between species parallel previous work in other biogeographic regions. In the Mallee scrublands of southwest Australia, many plant species display progressive heteroblasty, becoming increasingly spinescent as they mature (Burns Citation2014). Conversely, in the woodlands of Africa (Eskildsen et al. Citation2004; Bond & Silander Citation2007), Europe (Crawley Citation1983), North America (Burns Citation2014) and Western Australia (Hanley et al. Citation2007), spinescent plants are often retrogressively heteroblastic and are armed only at earlier ontogenetic life history stages (i.e. as saplings).
Differences in the mode of heteroblasty between vegetation types are consistent with their susceptibility to megaherbivores (Burns Citation2014). In scrublands, mature plants are continuously exposed to megaherbivores, whereas younger plants might be protected by the spinescent canopies of adult plants above them. Conversely, in woodlands, adult plants recruit into the forest canopy when they reach a height refuge from flightless herbivores, whereas juvenile plants are exposed to megaherbivores and are therefore likely to benefit more from structural defence.
Prickles, thorns and spines have traditionally been interpreted as defensive structures. Obeso (Citation1997) provides direct evidence that European holly (Ilex aquifolium), which produce sharp lateral leaf spines, were browsed less by large ungulates than neighbouring unarmed plants (see also Cooper & Owen-Smith Citation1986). However, prickles, thorns and spines might serve other functions, including reducing radiation flux (Nobel Citation1988) or serve as climbing mechanisms (Grubb Citation1992).
In a similar study, Fadzly & Burns (Citation2010) showed that Pseudopanax crassifolius Araliaceae, a heteroblastic tree species that is endemic to New Zealand, produces lateral leaf spines that may have deterred herbivory by large vertebrates. They also show that lateral leaf spines in this species are often associated with patches of lighter-coloured leaf tissue, likely making them more conspicuous to birds (Burns Citation2010). Browsing birds have soft gums and lack teeth, the latter necessitates that they swallow food whole (Atkinson & Greenwood Citation1977); hence, herbivorous birds may have found the sharp, needle-like leaves of P. totara difficult to swallow. Support for this assertion may be found in the analysis of moa gizzard contents by Wood (Citation2007): a single leaf of P. totara was identified in comparison with numerous samples of another coniferous species, Dacrycarpus dacrydioides. Leaf spines in A. aurea may have functioned slightly differently, as their rosette growth form may have made plucking leaves difficult without damaging their facial tissues (e.g. their eyes).
The Chatham Islands, which occur 500 km of the east coast of New Zealand and lacked megaherbivores, house several endemic species of Aciphylla that are derived from New Zealand species. Leaf spinescence in these species is much reduced relative to A. aurea and other species native to New Zealand. Therefore, spinescence in these species may have been lost after arrival in the Chatham Islands, where putative selection pressures from megaherbivores is absent (see also Fadzly et al. Citation2009).
Approximately 200 species of New Zealand vascular plants in 37 families are heteroblastic, which appears to be an unusually high incidence of the phenomenon globally (Cockayne Citation1912). The high incidence of heteroblasty in New Zealand has frequently been linked to herbivory by large flightless birds named moa, of the genus Dinormis, which were driven to extinction after the arrival of humans. Given that direct experimentation is impossible, the ‘moa hypothesis’ has proved highly contentious (McGlone & Clarkson Citation1993). Alternatively, heteroblasty may have evolved in response to past climatic periods (dry soil conditions during the Pleistocene) (Cockayne Citation1912; Rattenbury Citation1962; McGlone & Webb Citation1981), interspecific hybridisation (Godley Citation1985) or to promote photosynthetic efficiency (see Gamage & Jesson Citation2007).
Overall results provide three important conclusions. First, they illustrate a statistical technique that can be used to quantitatively discern between different forms of heteroblasty, which are best viewed as endpoints along a continuous distribution of different types of heteroblasty. Second, they illustrate that leaf spinescence is the result of a combination of plant traits that may display different ontogenetic trajectories. Third, the different modes of heteroblasty exhibited by the two study species (progressive versus retrogressive) match those found in continental landmasses, suggesting that different types of megaherbivores (birds versus mammals) can select for similar modes of heteroblasty.
Acknowledgements
We would like to thank the Otari–Wilton Bush staff for their assistance and permission to conduct the study. Funding was provided by a Te Rōpū Āwhina Summer Research Scholarship, Victoria University of Wellington.
References
- Atkinson IA, Greenwood RM 1977. Evolution of divaricating plants in New Zealand in relation to moa browsing. Proceedings of the New Zealand Ecological Society 24: 21–33.
- Bond WJ, Silander JA 2007. Springs and wire plants: anachronistic defences against Madagascar’s extinct elephant birds. Proceedings of the Royal Society B: Biological Sciences 274: 1985–1992.
- Burns KC 2004. Patterns in specific leaf area and the structure of a temperate heath community. Diversity and Distributions 10: 105–112. 10.1111/j.1366-9516.2004.00058.x
- Burns KC 2005. Plastic heteroblasty in beach groundsel (Senecio lautus). New Zealand Journal of Botany 43: 665–672. 10.1080/0028825X.2005.9512983
- Burns KC 2010. The ghost of herbivory past. Australasian Science 31: 22.
- Burns KC 2014. Are there general patterns in plant defence against megaherbivores? Biological Journal of the Linnean Society 111: 38–48. 10.1111/bij.12181
- Burns KC, Dawson J. 2005. Patterns in the diversity and distribution of epiphytes and vines in a New Zealand forest. Austral Ecology 30: 883–891. 10.1111/j.1442-9993.2005.01532.x
- Cockayne L 1912. Observations concerning evolution, derived from ecological studies in New Zealand. Transactions and Proceedings of the Royal Society of New Zealand 44: 1–50.
- Cooper SM, Owen-Smith N 1986. Effects of plant spinescence on large mammalian herbivores. Oecologia 68: 446–455. 10.1007/BF01036753
- Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE et al. 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51: 335–380. 10.1071/BT02124
- Crawley MJ 1983. Herbivory: the dynamics of animal-plant interactions. Oxford, Blackwell Scientific Publications.
- Dawson J, Lucas R 2000. Nature guide to the New Zealand forest. Auckland, Godwit Publishing.
- Eskildsen LI, Olesen JM, Jones CG 2004. Feeding response of the Aldabra giant tortoise (Geochelone gigantea) to island plants showing heterophylly. Journal of Biogeography 31: 1785–1790. 10.1111/j.1365-2699.2004.01092.x
- Fadzly N, Burns KC 2010. Hiding from the ghost of herbivory past: evidence for crypsis in an insular tree species. International Journal of Plant Sciences 171: 828–833. 10.1086/654850
- Fadzly N., Jack C, Schaefer HM, Burns KC 2009. Ontogenetic colour changes in an insular tree species: signalling to extinct browsing birds? New Phytologist 184: 495–501. 10.1111/j.1469-8137.2009.02926.x
- Gamage HK, Jesson L 2007. Leaf heteroblasty is not an adaptation to shade: seedling anatomical and physiological responses to light. New Zealand Journal of Botany 31: 245–254.
- Godley EJ 1985. Paths to maturity. New Zealand Journal of Botany 23: 687–706. 10.1080/0028825X.1985.10434238
- Grubb PJ 1992. Presidential address: a positive distrust in simplicity – lessons from plant defences and from competition among plants and among animals. Journal of Ecology 80: 585–610. 10.2307/2260852
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM 2007. Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution and Systematics 8: 157–178. 10.1016/j.ppees.2007.01.001
- McGlone MS, Clarkson BD 1993. Ghost stories: moa, plant defences and evolution in New Zealand. Tuatara 32: 1–21.
- McGlone MS, Webb CJ 1981. Selective forces influencing the evolution of divaricating plants. New Zealand Journal of Ecology 4: 20–28.
- Meisner K, Winkler U, Zotz G 2013. Heteroblasty in bromeliads – anatomical, morphological and physiological changes in ontogeny are not related to the change from atmospheric to tank form. Functional Plant Biology 40: 251–262. 10.1071/FP12201
- Meisner K, Zotz G 2012. Heteroblasty in bromeliads: its frequency in a local flora and the timing of the transition from atmospheric to tank form in the field. International Journal of Plant Sciences 173: 780–788. 10.1086/666665
- Nobel PS 1988. Environmental biology of agaves and cacti. New York, Cambridge University Press.
- Obeso JR 1997. The induction of spinescence in European holly leaves by browsing ungulates. Plant Ecology 129: 149–156. 10.1023/A:1009767931817
- Rattenbury JA 1962. Cyclic hybridization as a survival mechanism in New Zealand forest flora. Evolution 16: 348–363. 10.2307/2406284
- R Development Core Team 2008. R: a language and environment for statistical computing. Vienna, R Foundation for Statistical Computing.
- Wells CL, Pigliucci M 2000. Adaptive phenotypic plasticity: the case of heterophylly in aquatic plants. Perspectives in Plant Ecology, Evolution and Systematics 3: 1–18. 10.1078/1433-8319-00001
- Wood JR 2007. Moa gizzard content analyses: further information on the diets of Dinornis robustus and Emeus crassus, and the first evidence for the diet of Pachyornis elephantopus (Aves: Dinornithiformes). Records of Canterbury Museum 21: 27–39.
- Whittaker RJ, Fernández-Palacios JM 2007. Island biogeography: ecology, evolution, and conservation. Oxford, Oxford University Press.
- Zotz G, Wilhelm K, Becker A 2011. Heteroblasty—a review. The Botanical Review 77: 109–151. 10.1007/s12229-010-9062-8