ABSTRACT
Non-glandular trichomes are traditionally known as acting in the physical protection of plants against biotic and abiotic stresses, forming a mechanical barrier against low humidity, high light intensity and temperatures, and feeding and oviposition activities of insects. However, detailed studies involving morphology, histochemistry and ultrastructure of these epidermal appendages are lacking. We characterised the morphology and histochemistry of the non-glandular trichomes in three Lamiaceae and four Verbenaceae species and evidenced their involvement in the production, storage and/or liberation of biologically active substances. Samples of leaves were prepared according to standard methods for scanning and transmission electron microscopy. Histochemical tests were performed using fresh material. The combination of structural and ultrastructural studies and histochemical techniques allowed us to verify that the non-glandular trichomes of all studied species are comprised by living cells able to act in the synthesis, storage and/or liberation of biologically active compounds. This is the first work showing the production of compounds in non-glandular trichomes in Lamiaceae and Verbenaceae species. Our observations indicate that more than a physical protection, the non-glandular trichomes of these species participate in the chemical interaction of the plants with the environment, supplementing the work of the typically glandular trichomes.
Introduction
Trichomes are epidermal appendages with variable morphology and functions (Werker Citation2000; Evert Citation2006). Based on their shape and function, glandular and non-glandular trichomes can be recognised and the absence of a glandular head in the non-glandular trichomes is the main morphological difference between them (Werker Citation2000).
Non-glandular trichomes can be unicellular or multicellular with a thin apex (Behnke Citation1984; Werker Citation2000), branched or not (Evert Citation2006), and can constitute a dense cover on the plant organ surfaces (Mayekiso et al. Citation2008; Dmitruk & Weryszko-Chmielewska Citation2010). In the earliest stages of development, the cells of non-glandular trichomes are metabolically active (Mayekiso et al. Citation2008) and can remain alive at maturity or die and become dry (Evert Citation2006). Unlike the glandular trichomes, the non-glandular trichomes are traditionally considered not to participate in the production, storage and/or liberation of biologically active chemical compounds (Werker Citation2000). They are known by their action exclusively on the physical protection of plants against biotic and abiotic stresses, forming a protective barrier against low humidity, high temperatures and sun radiation (Werker Citation2000), retaining air film on aquatic plant surfaces and influencing its fluctuation (Barthlott et al. Citation2009), and deterring the activities of feeding and ovipositing insects (Levin Citation1973; Baur et al. Citation1991). In this sense, a positive relation between the density of non-glandular trichomes in leaves and their resistance to herbivore attacks has been demonstrated within several plant species (Levin Citation1973; Baur et al. Citation1991). For example, Baur et al. (Citation1991) observed that the density of the non-glandular type increased in leaves of young alder trees after the attacks of alder beetles and that the higher abundance of non-glandular trichomes was positively correlated with the intensity of the defoliation in this plant.
Lamiaceae and Verbenaceae species are known by the dense pubescence of their vegetative and reproductive organs and many of these species are important in the production of compounds with aromatic and medicinal potential (Serrato-Valenti et al. Citation1997; Ascensão et al. Citation1999; Combrinck et al. Citation2007; Argyropoulou et al. Citation2010; Silva et al. Citation2016). Several studies report the morphology, histochemistry and ultrastructure of the glandular trichomes in Verbenaceae (Bonzani et al. Citation2003; Combrinck et al. Citation2007; Argyropoulou et al. Citation2010; Tozin et al. Citation2015) and the Lamiaceae (Werker Citation1993; Ascensão et al. Citation1995; Serrato-Valenti et al. Citation1997; Ascensão & Pais Citation1998; Ascensão et al. Citation1999) species. However, information concerning the non-glandular trichomes of Lamiales is scarce and restricted to a few species (Argyropoulou et al. Citation2010; Dmitruk & Weryszko-Chmielewska Citation2010) and detailed studies involving morphology, histochemistry and ultrastructure of these epidermal appendages are lacking.
In this paper, we investigated the morphological, histochemical and subcellular features of non-glandular trichomes in three Lamiaceae and four Verbenaceae species, demonstrating their involvement in the secretion of bioactive compounds.
Material and methods
Plant material
Samples of fully expanded leaves were collected from adult individuals of Aegiphila verticillata Vell., Hyptis villosa Pohl. Ex Benth. and Plectranthus barbatus Andrews belonging to Lamiaceae and from Lantana camara L., Lippia alba (Mill.) N.E. Br. ex Britton & P. Wilson, Lippia origanoides Kunth and Stachytarpheta cayennensis belonging to Verbenaceae. The plants were growing in natural areas of ‘campo cerrado’ in the District of Rubião Júnior (22°53′29.82″S, 48°29′25.81″W), Botucatu city, São Paulo State, Brazil.
We collected fully expanded leaves located in the third and fourth stem nodes from five individuals of each species.
Specimens were deposited in the Herbarium Irina Delanova Gemtchújnicov (BOTU), Department of Botany, Institute of Biosciences of Botucatu, IBB, UNESP.
Light microscopy
To identify the main chemical classes of substances in the non-glandular trichomes, samples of fresh leaves were sectioned using razor blades and the sections were treated with: Sudan IV to detect total lipids (Johansen Citation1940); Nadi’s reagent for terpenes (David & Carde Citation1964); 10% ferric chloride for phenolic compounds (Johansen Citation1940); periodic acid/Schiff reagent for non-cellulosic polysaccharides (Taboga & Vilamaior Citation2001); Dragendorff reagent for alkaloids (Yoder & Mahlberg Citation1976) and bromophenol blue for proteins (Mazia et al. Citation1953). The control tests were performed according to the protocol proposed by the author of each technique. The non-glandular trichomes in both leaf surfaces were analysed. The results were documented using a Leica DMR microscope connected to a digital system of image capture.
Scanning electron microscopy
Samples were fixed in 2.5% glutaraldehyde with 0.1 m phosphate buffer, pH 7.3 overnight at 4 °C, dehydrated in a graded acetone series, critical-point dried with Leica EM CPD030, mounted on aluminium stubs, gold-coated (20 nm) with BAL-TEC SCD 050 Sputter Coater (Robards Citation1978), and both leaf surfaces were examined with a Fei Quanta 200 scanning electron microscope. The relevant results were documented with a digital system of image capture coupled to the electron microscope.
Quantitative data
To evaluate the density of glandular and non-glandular trichomes, three fully expanded leaves were collected from four individuals of each species. The samples were processed and examined with a Fei Quanta scanning electron microscope as described above. The density was calculated in 1 mm2 using the Scandium software with an image-capture system coupled to the scanning electron microscope. We calculated the density of glandular and non-glandular trichomes in the adaxial and abaxial leaf surfaces in all species. The data were submitted to an analysis of variance followed by the Tukey’s test at the 5% probability level.
Transmission electron microscopy
For conventional transmission electron microscopy, leaf samples were fixed in 2.5% Karnovsky in 0.1 m phosphate buffer, pH 7.3 for 24 h at room temperature, post-fixed with 1% osmium tetroxide aqueous solution in the same buffer for 1 h, dehydrated in an acetone series and embedded in Araldite resin (Machado & Rodrigues Citation2004). Ultra-thin sections (70 nm) were stained with uranyl acetate and lead citrate (Reynolds Citation1963). The samples were examined using a FEI Tecnai Spirit transmission electron microscope at 80 kV. The relevant results were documented with a digital system of image capture coupled to the electron microscope.
Results
Morphology, distribution and histochemistry of the non-glandular trichomes
Live non-glandular trichomes were observed on both surfaces of the leaf blade in all the studied species (A,B) (). These epidermal appendages were more abundant on the abaxial leaf surface (A) than on the adaxial leaf surface (B) (). Moreover, the density of the non-glandular trichomes was higher than of glandular trichomes on the abaxial leaf surface in four of the studied species. In the adaxial leaf surface, the density of glandular and non-glandular trichomes is the same, except in S. cayennensis ().
Figure 1. Scanning electron micrographs of the leaf blade in Lamiaceae and Verbenaceae species. A, B, Abaxial and adaxial leaf surfaces, respectively, in Lippia origanoides showing non-glandular trichomes. C–J, Morphological features of the non-glandular trichomes in Verbenaceae and Lamiaceae species. C, Aegiphila verticillata. Detail showing the basal cells. D, Hyptis villosa. E, F, Plectranthus barbatus. G, Lantana camara. Observe accumulations of secretion (detail) on the apical cell. H, Lippia alba. I, Lippia origanoides. J, Stachytarpheta cayennensis. Scale bars: A, B = 500 µm; C, D, F, J = 50 µm; E = 200 µm; G–I = 25 µm.
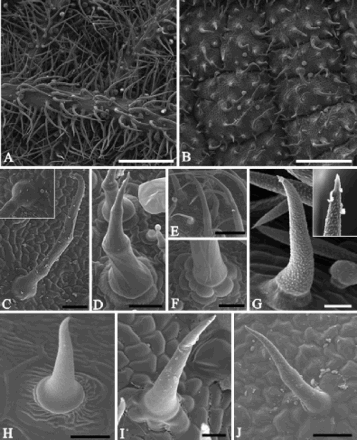
Table 1. Density of glandular and non-glandular trichomes (mm2) in leaves of Lamiaceae and Verbenaceae species.
The non-glandular trichomes showed great morphological variety. In all the plants, they exhibited a uniseriate conical body with a thin apex and a multicellular base with cells arranged in rosettes (C–J). In A. verticillata, these trichomes presented a body constituted by three to four cells and base with 8–12 cells (C). In H. villosa, the non-glandular trichomes showed a one- to four-celled body and a base with four to six cells (D); the lower cell of the body is elongated. Plectranthus barbatus possessed non-glandular trichomes with a body constituted by three to five cells (E), a whorled pedestal with 8–10 cells and a prominent basis comprising around 10 cells (F). The non-glandular trichomes of Lantana camara (G), Lippia alba (H) and Lippia origanoides (I) exhibited elongated unicellular body and four to eight basal cells; in Lantana camara the base is prominent (G). In S. cayennensis, the non-glandular trichomes presented a body with three to four cells and a base with six to eight cells (J). Accumulations of secretions were observed on the apex of the non-glandular trichomes and were more abundant in Lantana camara (G and insert). Only in Lippia alba (H), S. cayennensis (J) and mainly in Lantana camara (G) was the cuticle verrucose.
Different classes of chemical compounds were histochemically identified inside the cells of the non-glandular trichomes of the Lamiaceae (A–I) and Verbenaceae (J–R) species (). In addition, oil droplets were observed impregnated to the cell walls of the body of the non-glandular trichomes of H. villosa (D), P. barbatus (H), Lantana camara (K), Lippia origanoides and S. cayennensis.
Figure 2. Histochemical tests of non-glandular trichomes in leaves of Lamiaceae and Verbenaceae species. A–C, Aegiphila verticillata. D, E, Hyptis villosa. F–I, Plectranthus barbatus. J, K, Lantana camara. L, Lippia alba. M, N, Lippia origanoides. O–R, Stachytarpheta cayennensis. A, N, Q, Dragendorff reagent. B, G, O, Bromophenol blue. C, F, J, L, P, Ferric chloride. D, H, K, Sudan IV. Arrows indicate oil droplets on the cell walls. E, I, R, Nadi reagent. M, Periodic acid/Schiff reagent. Scale bars = 50 µm.
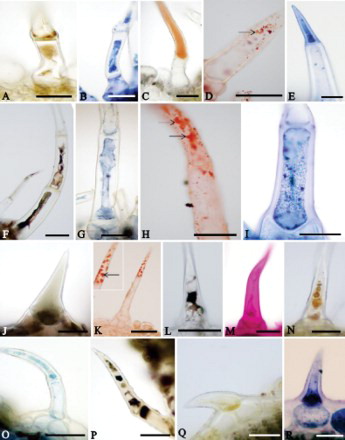
Table 2. Histochemistry of the non-glandular trichomes in leaves of Lamiaceae and Verbenaceae species.
Subcellular features of the non-glandular trichomes
In the non-glandular trichomes of Lantana camara, the body cell showed thick lateral walls with lamellar structure and electron-dense cytoplasm (A–D). The cuticle is thick (B) and presents prominent areas equivalent to the warts observed on scanning electron microscopy (G and insert). Lipophilic impregnations were observed in the cell wall, subjacent to the cuticle (B). The body cell presented active protoplast, nucleus with irregular contour (A), vacuoles with flocculate material (A) and cytoplasm with smooth endoplasmic reticulum (C), mitochondria (C, D), Golgi bodies (D), plastids (A, D), vesicles and oil drops (E). The plastids were devoid of thylakoids and contained starch grains and voluminous plastoglobuli (A, D). In the basal cell (E), the pectin-cellulosic walls were thick. The vacuoles were voluminous with membranous inclusions and the cytoplasm was reduced with numerous mitochondria, plastids without thylakoids, smooth endoplasmic reticulum cisternae, Golgi bodies, vesicles and oil drops. Plasmodesmata were visualised connecting the basal cells to the apical cell of the trichomes (E).
Figure 3. Transmission electron micrographs of the non-glandular trichomes in leaves of two Verbenaceae and one Lamiaceae species. A–E, Lantana camara. A, Apical cell showing nucleus with irregular contour and plastids (Pl) with starch grains and voluminous oil bodies (Ol) in the cytoplasm. Va: vacuole. B, Detail showing lipidic substances (arrows) impregnated in the wall of the apical cell. C, Portion of apical cell showing mitochondria (Mi), smooth endoplasmic reticulum (Sr) and vesicles (Ve). Va: vacuole. D, Detail of apical cell with large plastids containing voluminous oil drops (Ol) and starch grains (St). Gb: Golgi body; Mi: mitochondria; Va: vacuole. E, Plasmodesmata (arrowhead) connecting apical (AC) and basal (BC) cells. Ol: oil drop; Sr: smooth endoplasmic reticulum; Va: vacuole; Ve: vesicles. F, G, Lippia origanoides. F, Apical cell. Observe thick cuticle (Ct) with electron-dense ramifications. Cw: cell wall; Gb: Golgi body; Mi: mitochondria; Ol: oil; Va: vacuole. G, Portion of basal cell exhibiting thick cell wall (Cw) with lamellar aspect and accumulations of electron-dense material (*) in the outer portion. Observe flocculate cytoplasm and oil (Ol) drops. H–J, Hyptis villosa. H, Apical cell with Golgi bodies (Gb) hyperactive in the vesicle production in the cytoplasm and paramural bodies (Pb) in the wide periplasmic space. Cw: cell wall. Nu: nucleus. Va: vacuole. I, Detail of apical (AC) and basal (BC) cells. Cw: cell wall. Gb: Golgi body. Mi: mitochondria. Va: vacuole. Observe vesicles (Ve) next to the plasmalemma in the basal cells. J, Portions of apical (AC) and basal (BC) cells with polyribosomes, rough endoplasmic reticulum (Rr) and vesicles (Ve). Va: vacuole. Scale bars = 1 μm.
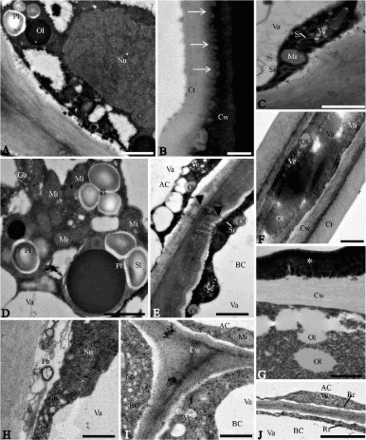
In Lippia origanoides, the body cell possessed thinner walls covered by a thick and smooth cuticle (F). The cell walls presented a lamellar structure (F, G) and electron-dense ramifications reach the cuticle (F). The cytoplasm was dense and abundant with mitochondria, smooth endoplasmic reticulum cisternae, Golgi bodies, plastids, vesicles and oil drops (F). The plastids were devoid of thylakoids and contained osmiophilic inclusions. A large central vacuole filled with lipids and fibrillar material is observed in the body cell (F). The basal cells showed thick walls with a lamellar feature (G). Accumulations of electron-dense substances were observed in the outer portion of the cell wall (G). Abundant and voluminous oil drops were observed in the cytoplasm (G).
In H. villosa, the non-glandular trichomes presented body cells with thick pectin-cellulosic walls (H). Fibrillar content and paramural bodies were observed in the periplasmic space (H). The cytoplasm was dense and rich in organelles (H–I). The nucleus was voluminous and peripherally located (H). A large and central vacuole was observed in these cells (H). Abundant Golgi bodies, proliferated rough endoplasmic reticulum, mitochondria and vesicles (H–J) were predominant in the cytoplasm. In the basal cells, the cytoplasm was dense and contained rough endoplasmic reticulum, mitochondria, vesicles and an abundance of hyperactive Golgi bodies (I, J). Vesicles near to the plasmalemma were observed in these cells (I).
Discussion
In this paper, the combination of structural and ultrastructural studies and histochemical techniques allowed us to verify that the non-glandular trichomes of all studied species comprise living cells able to act in the synthesis, storage and/or liberation of biologically active compounds.
Despite the trichomes referred to in this paper not presenting typical morphology of glandular trichomes, our results showed the presence of different classes of secondary metabolites inside their cells, histochemically identified as lipids, terpenes, polysaccharides, alkaloids and phenolic compounds. Trichomes that are typically non-glandular showed secretory activity in Heracleum sosnovskii Manden. (Apiaceae), however, their subcellular aspects were not investigated (Weryszko-Chmielewska & Chwil Citation2014). Terpenes were identified in the trichomes of all the studied species and total lipids were identified except in A. verticillata and Lippia alba. The presence of abundant smooth endoplasmic reticulum cisternae, plastids with plastoglobuli devoid of thylakoids and oil drops scattered in the cytoplasm of trichome cells in Lantana camara and Lippia origanoides corroborate their potential for the secretion of lipophilic compounds (Fahn Citation1979; Evert Citation2006; Tozin et al. Citation2015; Silva et al. Citation2016).
The association of images obtained from electron microscopy and from the histochemical tests suggests that the lipophilic portion of the secretion can be eliminated and accumulated on the surface of the trichomes in H. villosa, P. barbatus, Lantana camara, Lippia origanoides and S. cayennensis. These substances can cross the boundaries of the trichome body cells without the existence of openings in the cell walls and cuticle, like that proposed by Evert (Citation2006) for typical secretory cells producing lipophilic compounds. In addition, the verrucose cuticle of Lantana camara trichomes can enlarge the surface of the cell wall improving the release of lipophilic secretion towards the outside. These substances can play an important role in the interaction of the plants with the environment, protecting them against infections by pathogens and attacks of herbivores (Werker Citation1993; Langenheim Citation2003; Sukontason et al. Citation2004). In the same way, the alkaloids detected in trichomes of all the studied species can be associated to the chemical defence of plants against herbivores because these substances are toxic to insects (Levin Citation1973; Wagner Citation1991). Following Baur et al. (Citation1991), non-glandular trichomes are important structures to protect the plants against the oviposition activities of insects.
Non-cellulosic polysaccharides were histochemically identified in all the studied species, except in P. barbatus and S. cayennensis. In fact, hyperactive Golgi bodies were present in the trichome cells of all the species studied using transmission electron microscopy and indicate their potential to produce hydrophilic substances (Fahn Citation1979; Ascensão & Pais Citation1998; Tozin et al. Citation2015). In addition, the abundance of vesicles in the cytoplasm of H. villosa and Lippia origanoides are indexes of hydrophilic secretion (Evert Citation2006). Moreover, the proximity of vesicles to the plasmalemma in trichome cells of H. villosa indicate the granulocrine process of secretion (Fahn Citation1979; Ascensão & Pais Citation1998; Tozin et al. Citation2015), more common during the release of hydrophilic substances from the protoplast. In addition, the presence of pectin ramifications in the outer portions of the cell wall and cuticle, as observed in Lantana camara and Lippia origanoides, can represent a way of secretion release, favouring the liberation of the hydrophilic portion of the secretion (Ascensão & Pais Citation1998; Tozin et al. Citation2015) by the lateral walls of the non-glandular trichome cells. Hydrophilic substances like polysaccharides can act in the maintenance of the water potential in the cells and in the lubrication of leaf primordia facilitating the expansion of leaves and protecting the organs against dehydration (Ascensão et al. Citation1999; Werker Citation2000; Machado et al. Citation2012). These are fundamental abilities mainly to plants living in environments with low air humidity such as the campo cerrado (Machado et al. Citation2012).
Aegiphila verticillata, P. barbatus, Lantana camara, Lippia alba and S. cayennensis presented phenolic compounds in the non-glandular trichomes. These compounds can present important roles in the protection of aerial organs against intense UV-B radiation (Liakoura et al. Citation1997; Tattini et al. Citation2007). In the campo cerrado, where the plants are subjected to high levels of sun irradiance (Maroni et al. Citation2006), the occurrence of phenolic compounds in the aerial organs is important to protect them (Liakoura et al. Citation1997).
In summary, our ultrastructural results confirm the histochemical detection of different compounds in the non-glandular trichomes of selected Lamiaceae and Verbenaceae species and evidence their activity in the secretory process. So, it becomes clear that the epidermal appendages with the typical morphology of non-glandular trichomes remain alive and metabolically active even when mature and are sites of synthesis and accumulation of substances with ecological value and can act in the chemical interaction of the plants with the environment, complementing the action of the typically glandular trichomes in these species.
Acknowledgements
We thank the staff of the Centro de Microscopia Eletrônica (CME), IBB, UNESP, for helping in the sample preparation.
Associate Editor: Dr Luis Corcuera.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Argyropoulou C, Akoumianaki-loannidou A, Christodoulakis SN, Fasseas C. 2010. Leaf anatomy and histochemistry of Lippia citriodora (Verbenaceae). Aust J Bot. 58:398–409. doi: 10.1071/BT10072
- Ascensão L, Marques NE, Pais MS. 1995. Glandular trichomes on vegetative and reproductive organs of Leonotis leonurus (Lamiaceae). Ann Bot. 75:619–626. doi: 10.1006/anbo.1995.1067
- Ascensão L, Mota L, Castro MM. 1999. Glandular trichomes on the leaves and flowers of Plectranthus ornatus: morphology, distribution and histochemistry. Ann Bot. 84:437–447. doi: 10.1006/anbo.1999.0937
- Ascensão L, Pais MS. 1998. The leaf capitate trichomes of Leonotis leonurus: histochemistry, ultrastructure and secretion. Ann Bot. 81:263–271. doi: 10.1006/anbo.1997.0550
- Barthlott W, Wiersch S, Colic Z, Koch K. 2009. Classification of trichome types within species of the water fern Salvinia, and ontogeny of the egg-beater trichomes. Botany. 87:830–836. doi: 10.1139/B09-048
- Baur R, Binder S, Benz G. 1991. Nonglandular leaf trichomes as short-term inducible defense of the grey alder, Alnus incana (L.), against the chrysomelid beetle, Agelastica alni L. Oecologia. 87:219–226. doi: 10.1007/BF00325259
- Behnke HD. 1984. Plant trichomes - structure and ultrastructure: general terminology, taxonomic applications, and aspects of trichome-bacteria interaction in leaf tips of Dioscorea. In: Rodriguez E, Healey PL, Metha I, editor. Biology and chemistry of plant trichomes. New York (NY): Plenum Press; p. 95–112.
- Bonzani NE, Filippa EM, Barboza GE. 2003. Estudio anatômico comparativo de tallo em algunas espécies de Verbenaceae. Anales del Instituto de Biologia, Universidad Nacional Autónoma Del México, Serie Botánica. 74:31–45.
- Combrinck S, Plooy GW, McCrindle RI, Botha BM. 2007. Morphology and histochemistry of glandular trichomes of Lippia scaberrima (Verbenaceae). Ann Bot. 99:1111–1119. doi: 10.1093/aob/mcm064
- David R, Carde JP. 1964. Coloration differentielle dês inclusions lipidique et terpeniques des pseudophylles du pine maritime au moyen du reactif Nadi. CR Biol. 257:1338–1340.
- Dmitruk M, Weryszko-Chmielewska E. 2010. Morphological differentiation and distribution of non-glandular and glandular trichomes on Dracocephalum moldavicum L. shoots. Acta Agrobotanica. 63:11–22.
- Evert RF. 2006. Esau's plant anatomy. 3a. ed. Hoboken (NJ): Wiley-Interscience.
- Fahn A. 1979. Secretory tissues in plants. London: Academic Press.
- Johansen DA. 1940. Plant microtechnique. New York (NY): McGraw-Hill.
- Langenheim JH. 2003. Plant resins: chemistry, evolution, ecology and Ethnobotany. Portland (OR): Timber Press.
- Levin DA. 1973. The role of trichomes in plant defense. Q Rev Biol. 48:3–15. doi: 10.1086/407484
- Liakoura V, Stepfanou M, Manetas Y, Cholevas C, Karabourniotis G. 1997. Trichome density and its UV-B protective potential are affected by shading and leaf position on the canopy. Environ Exp Bot. 38:223–229. doi: 10.1016/S0098-8472(97)00005-1
- Machado SR, Barreiro DP, Rocha JF, Rodrigues TM. 2012. Dendroid colleters on vegetative and reproductive ápices in Alibertia sessilis (Rubiaceae) differ in ultrastructure and secretion. Flora. 207:868–877. doi: 10.1016/j.flora.2012.09.013
- Machado SR, Rodrigues TM. 2004. Anatomia e ultra-estrutura do pulvino primário de Pterodon pubescens Benth. (Fabaceae-Faboideae). Rev Bras Bot. 27:135–147. doi: 10.1590/S0100-84042004000100015
- Maroni BC, Di Stasi LC, Machado SR. 2006. Plantas medicinais do cerrado de Botucatu – guia ilustrado. São Paulo: Editora UNESP.
- Mayekiso B, Magwa ML, Coopoosamy R. 2008. The morphology and ultrastructure of glandular and non-glandular trichomes of Pteronia incana (Asteraceae). Afr J Plant Sci. 2:050–060.
- Mazia D, Brewer PA, Alfert M. 1953. The cytochemical staining and measurement of protein with mercuric bromphenol blue. Biol Bull. 104:57–67. doi: 10.2307/1538691
- Reynolds ES. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 17:208–212. doi: 10.1083/jcb.17.1.208
- Robards AW. 1978. An introduction to techniques for scanning electron microscopy of plant cells. In: Hall JL, editor. Electron microscopy and cytochemistry of plant cells. New York (NY): Elsevier; p. 343–403.
- Serrato-Valenti G, Bisio A, Cornara L, Ciarallo G. 1997. Structural and histochemical investigation of the glandular trichomes of Salvia aurea L. leaves, and chemical analysis of the essential oil. Ann Bot. 79:329–336. doi: 10.1006/anbo.1996.0348
- Silva, SCM, Tozin, LRS, Rodrigues TM. 2016. Morphological and histochemical characterization of secretory sites of bioactive compounds in Lantana camara L. (Verbenaceae) leaves. Botany. 94:321–336. doi: 10.1139/cjb-2015-0247
- Sukontason, Kl, Boonchu N, Sukontason K, Choochote W. 2004. Effects of eucalyptol on house fly (Diptera: Muscidae) and blow fly (Diptera: Calliphoridae). Rev Inst Med Trop São Paulo. 46:97–101. doi: 10.1590/S0036-46652004000200008
- Taboga SR, Vilamaior PSL. 2001. Citoquimica. In: A célula. Edited by Carvalho HF, SM Recco-Pimentel. Manole LTDA, Barueri.
- Tattini M, Matteini P, Saracini E, Traversi ML, Giordano C, Agati G. 2007. Morphology and biochemistry of non-glandular trichomes in Cistus salvifolius L. leavez growing in extreme habitats of Mediterranean basin. Plant Biol. 9:411–419. doi: 10.1055/s-2006-924662
- Tozin, LRS, Carvalho SF, Machado SR, Rodrigues TM. 2015. Glandular trichome diversity on leaves of Lippia origanoides Kunth and Lippia stachyoides Cham. (Verbenaceae): morphology, histochemistry and ultrastructure. Botany. 93:297–306. doi: 10.1139/cjb-2014-0251
- Wagner GJ. 1991. Secreting glandular trichomes: more than just hairs. Plant Physiol. 96:675–679. doi: 10.1104/pp.96.3.675
- Werker E. 1993. Function of essential oil-secreting glandular hairs in aromatic plants of Lamiaceae – a review. Flavour Fragr J. 8:249–255. doi: 10.1002/ffj.2730080503
- Werker E. 2000. Trichome diversity and development. Adv Bot Res. 31:1–35. doi: 10.1016/S0065-2296(00)31005-9
- Weryszko-Chmielewska E, Chwil M. 2014. Structures of Heracleum sosnovskii Manden. Stem and leaves releasing photodermatosis-causing substances. Acta Agrobot. 67:25–32. doi: 10.5586/aa.2014.057
- Yoder LR, Mahlberg PG. 1976. Reactions of alkaloid and histochemical indicators in laticifers and specialized parenchyma cells of Catharanthus roseus (Apocynaceae). Am J Bot. 63:1167–1173. doi: 10.2307/2441734
