ABSTRACT
The cyanobacterial genus Chroococcus is well documented in freshwater habitats; however, less is known about the distribution, ecology and taxonomy of marine strains. In this study, we characterise six Chroococcus-like strains isolated from Tahiti, Hawaii, Cook Islands and New Zealand through sequencing of the 16S ribosomal RNA gene. We also assessed their toxin producing potential by screening for genes known to be involved in cyanotoxin production. The cell sizes (> 27 × 38 µm) of all strains were notably larger than those described previously. Phylogenetic analysis of a partial (c. 700 bp) region of the 16S rRNA gene showed that the six strains formed a single well-supported group (85% bootstrap support), despite their origins from several distantly separated Pacific locations. The strains were genetically most closely related to the recently proposed genus Limnococcus. The phylogeny also revealed the polyphyletic nature of the genus Chroococcus when using 16S rRNA sequence data, and we suggest further analysis is required. In a previous study, the Tahitian strain exhibited low toxicity to mice; however, none of four genes involved in cyanotoxin-production (mcyE, sxtA, cyrJ and anaC) that we tested for were detected. To increase knowledge on the ecology of marine Chroococcus-like strains, and to further delineate the taxonomic position of this group, a larger study using strains sourced from additional Pacific locations, as well as sites in the Indian and Atlantic Oceans, is recommended.
Introduction
The Cawthron Institute Culture Collection of Micro-algae (CICCM) is a nationally significant collection of micro-algae and cyanobacteria. Cyanobacteria form half of the collection, with 99% of the isolates being cryopreserved (Rhodes et al. Citation2015). One cyanobacterial genus that has been difficult to classify, due to limited morphological features, is Chroococcus, which belongs to the order Chroococcales (Komárek & Anagnostidis Citation1999). Cells are single or clustered into small colonies. When cells are closely packed in colonies they are hemispherical. Cells have homogeneous or granular content, sometimes with several prominent granules visible using a light microscope (Komárek & Anagnostidis Citation1999). Chroococcales are well described from freshwater habitats (Komárek & Anagnostidis Citation1999); however, little is known about their distribution, taxonomy or phylogeny in marine habitats (León-Tejera et al. Citation2011). They primarily occur as a component of metaphyton in fresh and marine waters, with the name Limnococcus suggested for a subgenus of phytoplanktonic Chroococcus species (Komárek & Anagnostidis Citation1999). Phylogenetic analyses of 16S rRNA gene sequences from 10 freshwater isolates of Chroococcus-like cells showed that they formed four distinct clusters or groups (Komárková et al. Citation2010).
Recently, as part of a study to identify the producer of pinnatoxin in Rangaunu Harbour in Northland (New Zealand), a strain CAWBG101, tentatively identified as Chroococcus cf. giganteus was established (Rhodes et al. Citation2010). A toxicity study conducted using mice bioassays (Munday Citation2009) and subsequent chemical analysis initially suggested that the strain was the pinnatoxin-producer (Rhodes et al. Citation2010). However, following intensive investigations, it was determined that the pinnatoxins were produced by a small, typically non-motile, dinoflagellate, Vulcanodinium rugosum (Rhodes et al. Citation2011) and it appeared that the cyanobacterium had sequestered the toxin. Further culturing and toxin analysis of CAWBG101 supported this as no pinnatoxin was detected or toxicity identified in further batch cultures (L. Rhodes, unpubl. data). Since the isolation of CAWBG101, a further five Chroococcus-like strains from two other New Zealand harbours and three Pacific island lagoons have been established. Extracts of a Tahitian strain (CAWBG103) tested positive in a mouse bioassay, but with low toxicity and the effects were minor (Munday Citation2010).
The aims of this study were to undertake a polyphasic investigation of the six Chroococcus-like species held in the CICCM, and to use a molecular approach to determine whether any of the isolates had the potential to produce cyanotoxins (microcystins, nodularins, saxitoxins, cylindrospermopsin and anatoxins).
Materials and methods
Sample collection, isolation and culture
Surface sediment samples were collected from three harbours located in the North Island of New Zealand (Rangaunu, 34°58′ S, 173°16′ E; Hokianga, 35°31′ S, 173°22′ E; and Motuarohia, 35°13′ S, 174°10′ E), and three selected lagoons from Pacific islands (Cook Islands, 21°11′ S, 159°4′ W; Tahiti, 17°31′ S, 149°46′ W; and Hawaii, 21°18′ N, 157°50′ W). Single Chroococcus-like cells were isolated from samples by micro-pipetting and were transferred to 24-well plates (Becton Dickinson) containing 500 µL F2 medium (Guillard & Ryther Citation1962) per well. Successfully isolated strains were incubated under standard conditions (100 ± 20 µ mol photons m−2.s−1; 12:12 hour light:dark; 18 ± 1 °C for New Zealand and Cook Island strains or 25 ± 1 °C for Hawaiian and Tahitian strains) and maintained in 50 mL plastic bottles (ThermoFisher Scientific). The strains can be ordered from the CICCM website: http://cultures.cawthron.org.
Morphological and molecular analyses
Four week old cultures (stationary phase) of each isolate were identified using microscopy (Olympus light microscope BX51), and the software Analysis LS Research (Soft Imaging System). For each isolate, the length and width of 50 randomly selected cells (both single and in colonies) were measured.
Subsamples (c. 10 mL) of each isolate were harvested by centrifugation (13 000 × g, 1 min). DNA was extracted from pellets using a PureLink Genomic DNA kit (Life Technologies) according to the Gram-negative bacteria protocol supplied by the manufacturer. Polymerase chain reaction (PCR) amplification of an approximately 700 bp region of the 16S rRNA gene was performed using primers 27F and 806R (Jungblut et al. Citation2005) following the PCR conditions described in Wood et al. (Citation2014). Amplicons of the correct size were purified with the AxyPrep PCR Clean-up Kit (Axygen Biosciences) and sequenced bi-directionally using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems).
To assess the toxin-producing potential of each isolate, amplification of the following genes was carried out: mcyE (for microcystins and nodularins using the HEPF/HEPR primers; Jungblut & Neilan Citation2006); sxtA (for saxitoxins using the sxtaf/sxtar primers; Ballot et al. Citation2010); cyrJ (for cylindrospermopsin using the CylsulfF/CylnamR primers; Mihali et al. Citation2008); and anaC (for anatoxins using the Ana-c-F/Ana-c-R primers; Rantala-Ylinen et al. Citation2011). PCR conditions described followed those used in Wood et al. (Citation2014).
DNA sequences were edited and assembled using Geneious v6.1.4 (Biomatters Ltd), and aligned with previously published Chroococcus-like sequences (Komárková et al. Citation2010; ) for phylogenetic analyses using BioEdit v5.0.9 (Hall Citation1999). The DNA alignment contained 17 Chroococcales sequences and two Synechococcales sequences, including Synechococcus sp. (NR074309) which was used as an outgroup. The alignment was 742 bp long (including gaps), of which 214 sites were variable and 140 sites were parsimony-informative. The best-fit model of evolution was estimated for the sequence alignment using the Smart Model Selection (SMS) implemented in the program PhyML 3.0 (Guindon et al. Citation2010). The General Time-Reversible (GTR) model (Lanave et al. Citation1984), with a discrete Gamma distribution (G) and a proportion of Invariant sites (I), was used to model evolutionary rate differences among sites (six categories, G = 0.438, I = 0.403). Initial trees for the heuristic search were obtained automatically by applying the BioNJ algorithm to a matrix of pairwise distances estimated using the Maximum Likelihood (ML) approach, and then selecting the topology with superior log likelihood value. Topologies were optimised with subtree pruning-regrafting branch swapping. The reliability of internal branches was assessed using 100 bootstraps. Sequences were deposited in GenBank under accessions KU513781-513786.
Table 1. List of strains and 16S rRNA gene sequences included in the phylogenetic analyses.
Results and discussion
Average cell sizes for the six isolates ranged from 27–43.1 µm long to 38 to 63.4 µm wide (). The strains all shared some morphological characteristics with species in the subgenus Limnococcus (Komárek & Anagnostidis Citation1999) and with two species that Komárková et al. (Citation2010) suggested should be placed in this group. For example, mucilaginous envelopes were enlarged in most strains and not lamellate. They usually attained a spherical form before cell division (). However, the cell sizes of the strains examined in this study were notably larger than those previously documented. For example, Komárková et al. (Citation2010) indicated that the Limnococcus cells they observed were up to 22 µm in diameter.
Figure 1. Light micrographs of stationary phase cells of Chroococcus-like species isolated from A, Rangaunu Harbour, New Zealand (CAWBG101; B, Cook Islands (CAWBG102); C, Tahiti (CAWBG103); D, Hawaii (CAWBG110); E, Hokianga Harbour, New Zealand (CAWBG113); F, Motuarohia Island, New Zealand (CAWBG126). Scale bar = 20 µm.
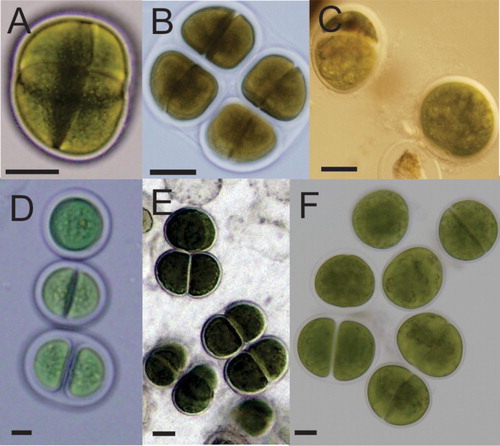
Table 2. Mean cell sizes (n = 50) of Chroococcus spp. strains investigated in this study.
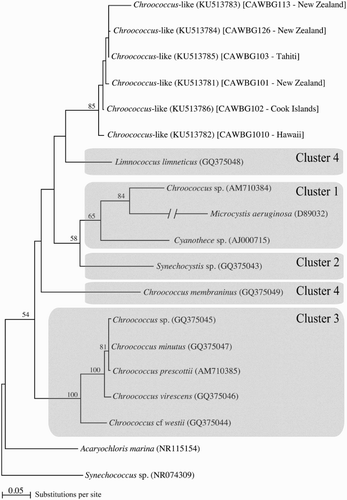
To our knowledge only two studies have examined phylogenetic relationships (16S rRNA) within the genus Chroococcus (Komárková et al. Citation2010; Roldán et al. Citation2013). Komárková et al. (Citation2010) grouped species of Chroococcus into four distinct clusters. Strains within Clusters 1 and 2 were very small in size, and the structure of colonies and phylogenetic position indicated that they were not ‘typical’ Chroococcus, as defined using the closest suite of features to the original type description of Chroococcus rufescens (Kützing) Nägeli, 1849. Cluster 3 grouped ‘typical’ morphospecies of Chroococcus including: C. virescens; C. cf. westii; C. prescottii; and C. minutus. Finally, Cluster 4 grouped some planktonic forms of Chroococcus-like cells, including Chroococcus limneticus and Chroococcus cf. membraninus, but their affiliation to the ‘typical’ Chroococcus phylogenetic Cluster 3 was questioned. For example, based on their phylogenetic, morphological and ecological observations, Komárková et al. (Citation2010) proposed that C. limneticus (GQ375048) in Cluster 4 did not belong to Chroococcus and should be elevated to a new genus Limnococcus and the strain be renamed L. limneticus. Furthermore, Komárková et al. (Citation2010) noted that C. cf. membraninus neither corresponded to the original description of C. membraninus (Meneghini) Nägeli, 1849 nor could be designated to the genus Chroococcus. Later, Roldán et al. (Citation2013) used 50 chroococcalean cyanobacterial 16S rRNA sequences and showed that C. cf. membraninus was phylogenetically most closely related to C. minutus, and was separated from L. limneticus. The latter observation is consistent with our phylogenetic analysis (). Cluster 4 as defined in Komárková et al. (Citation2010) was split, with C. cf. membraninus positioned as sister to Cluster 3 and L. limneticus positioned basally as sister to the six Chroococcus-like strains investigated in the present study. The six strains formed a well-supported group (85% bootstrap support), despite their origins from several distantly separated Pacific locations. Nevertheless, the topology () is also characterised by an overall lack of branch support, most branch nodes yielding less than 70% bootstrap values. This situation implies that resulting topologies are sensitive to alignment editing and taxon inclusion (data not shown), and also explains the contrasting results from this and previous studies. Additionally, there are 51 Chroococcus-like 16S rRNA sequences available in GenBank to date, only five of which represent isolates from marine environments, further limiting phylogenetic investigations. This highlights the difficulty in interpreting evolutionary relationships within this cyanobacterial group using 16S rRNA sequences. Because of the limitations detailed above, it is not possible to conclude on whether the six Chroococcus-like strains studied here belong to the genus Limnococcus or if they represent a single species that has successfully spread around the Pacific. Any biogeographic conclusions will require a much more detailed study using additional isolates from a greater range of Pacific locations. Additional phylogenetic analysis using fast-evolving genes/regions, such as intergenic spacer region, together with investigations into morphological plasticity, are also required to validate the status of Limnococcus and to establish the identity of our strains.
Figure 2. Phylogenetic reconstruction of Chroococcus-like strains based on 16S rRNA sequences. Clusters 1–4 are those used in Komárková et al. (Citation2010). Numbers at nodes correspond to the Maximum Likelihood bootstrap support; values < 50% are not shown. Species names are indicated in italics followed by the GenBank Accession numbers in parentheses. Isolate numbers and original references for all sequences are detailed in . Chroococcus spp. sequences that were in Cluster 2 in Komárková et al. (Citation2010), were not available on GenBank.
An increasing number of marine cyanobacteria have been shown to produce cyanotoxins, including toxins also produced by freshwater species (Frãzao et al. Citation2010; Méjean et al. Citation2010) and novel compounds such as trichotoxin (Schock et al. Citation2011). None of the six isolates used in this study contained genes (mcyE, sxtA, cyrJ and anaC) known to be involved in cyanotoxin production. The identification of low levels of toxicity from the Tahitian strain (CAWBG103; Munday Citation2010) suggests that further investigation into the toxicity of these strains is warranted. The ability of strain CAWBG101 to sequester pinnatoxins in its mucilage poses interesting ecological questions. Exogenous cyanotoxins have been shown to be sequestered intra-cellularly by the nontoxic cyanobacterium Synechocystis (PCC6803), and it may be using the toxins to enhance its survival (Phelan & Downing Citation2014). CAWBG101 can have an extensive mucilage sheath and it may be that these species have evolved to enable uptake and use toxins produced by other algae for their own benefit. Pinnatoxin has been shown to be lethal to a range of marine larvae (Rhodes et al. Citation2013) and acquiring it may reduce predation, without the energetic expenses related to producing it.
In conclusion, this analysis has shown that the marine Chroococcus-like strains held in the CICCM have similar morphological features. One notable difference from those included in previous polyphasic studies was the large mean (> 27 × 38 µm) cell size. The phylogenetic analysis showed that the six strains formed a single well-supported group (85% bootstrap support), despite their origins from several distantly separated Pacific sites. The strains clustered most closely with the recently proposed genus Limnococcus, although the weakly supported phylogenetic relationships combined with the polyphyletic nature of 16S rRNA sequence data for this cyanobacterial group prevents any meaningful taxonomic and biogeographic conclusions. We suggest a more robust study, for example using multiple-genes, and measuring growth and morphology under different environmental conditions, using many more strains from the Pacific region. This would provide valuable insights into the evolution, distribution and taxonomy of the Chroococcus–Limnococcus species complex. Although none of the genes involved in production of the common cyanotoxins were detected, the low toxicity (to mice) of the Tahitian strain suggests further investigation is warranted.
Acknowledgements
The authors thank Janet Adamson and Sarah Challenger (Cawthron Institute) for technical assistance in maintaining the culture collection. Associate Editor: Professor Julian Eaton-Rye.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ballot A, Fastner J, Wiedner C. 2010. Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in Northeast Germany. Appl Environ Microb. 76:1173–1180. doi: 10.1128/AEM.02285-09
- Frãzao B, Martins R, Vasconcelos V. 2010. Are known cyanotoxins involved in the toxicity of picoplanktonic and filamentous North Atlantic marine cyanobacteria? Mar Drug. 8:1908–1919. doi: 10.3390/md8061908
- Guillard RR, Ryther JH. 1962. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Micriob. 8:229–239. doi: 10.1139/m62-029
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 59:307–321. doi: 10.1093/sysbio/syq010
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Sym Series. 41:95–98.
- Jezberová J. 2006. Phenotypic diversity and phylogeny of pico-cyanobacteria in mesotrophic and eutrophic freshwater reservoirs investigated by a cultivation-dependent polyphasic approach. PhD Thesis. Faculty of Biological Science, University of South Bohemia, Czech Republic.
- Jungblut AD, Hawes I, Mountfort D, Hitzfeld B, Dietrich DR, Burns BP, Neilan BA. 2005. Diversity within cyanobacterial mat communities in variable salinity meltwater ponds of McMurdo Ice Shelf, Antarctica. Environ Microb. 7:519–529. doi: 10.1111/j.1462-2920.2005.00717.x
- Jungblut AD, Neilan BA. 2006. Molecular identification and evolution of the cyclic peptide hepatotoxins, microcystin and nodularin, synthetase genes in three orders of cyanobacteria. Arch Microb. 185:107–114. doi: 10.1007/s00203-005-0073-5
- Komárek J, Anagnostidis K. 1999. Süßwasserflora von Mitteleuropa: Cyanoprokaryota 19/1. Teil: Chroococcales. Berlin: Spektrum Akaddemischer Verlag.
- Komárková J, Jezberová J, Komárek O, Zapomělová E. 2010. Variability of Chroococcus (Cyanobacteria) morphospecies with regard to phylogenetic relationships. Hydrobiologia. 639:69–83. doi: 10.1007/s10750-009-0015-3
- Kondo R, Komura M, Hiroishi S, Hata Y. 1998. Detection and 16S rDNA sequence analysis of a bloom-forming cyanobacterial genus Microcystis. Fisheries Sci. 64:840–841.
- Lanave C, Preparata G, Saccone C, Serio G. 1984. A new method for calculating evolutionary substitution rates. J Mol Evol. 20:86–93. doi: 10.1007/BF02101990
- León-Tejera H, Pérez-Estrada CJ, Montejano G, Serviere-Zaragoza E. 2011. Biodiversity and temporal distribution of Chroococcales (Cyanoprokaryota) of an arid mangrove on the east coast of Baja California Sur Mexico. Fottea. 11:235–244. doi: 10.5507/fot.2011.022
- Méjean A, Peyraud-Thomas C, Kerbrat AS, Golubic S, Pauillac S, Chinain M, Laurent D. 2010. First identification of the neurotoxin homoanatoxin-a from mats of Hydrocoleum lyngbyaceum (marine cyanobacterium) possibly linked to giant clam poisoning in New Caledonia. Toxicon. 56:829–835. doi: 10.1016/j.toxicon.2009.10.029
- Mihali TK, Kellmann R, Muenchhoff J, Barrow KD, Neilan A. 2008. Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis. Appl Environ Microb. 74:716–722. doi: 10.1128/AEM.01988-07
- Munday R. 2009. Preliminary toxicity study on an extract of a culture of Chroococcus giganteus. Report number DST89125, February 2009, Hamilton, New Zealand: AgResearch; 2 p.
- Munday R. 2010. Toxicity of extracts of Chroococcus CAWBG103 ex Tahiti. Report number DST910082, February 2009, Hamilton, New Zealand: AgResearch; 3 p.
- Nubel U, Garcia-Pichel F, Muyzer G. 1997. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl Environ Microb. 63:3327–3332.
- Phelan RR, Downing TG. 2014. The localization of exogenous microcystin-LR taken up by a non-microcystin producing cyanobacterium. Toxicon. 89:87–90. doi: 10.1016/j.toxicon.2014.07.007
- Rantala-Ylinen A, Kana S, Wang H, Rouhiainen L, Wahlsten M, Rizzi E, Berg K, Gugger M, Sivonen K. 2011. Anatoxin-a synthetase gene cluster of the cyanobacterium Anabaena sp. strain 37 and molecular methods to detect potential producers. Appl Environ Microb. 77:7271–7278. doi: 10.1128/AEM.06022-11
- Rhodes L, Selwood A, McNabb P, Smith K. 2010. Pinnatoxin in Rangaunu Harbour, Northland, New Zealand. In: Ho K-C, Qi YI, Harmful Algae. 2008. Proc. 13th Int. Conf. on Harmful Algae, Hong Kong, China, Nov. 2008. ISSHA and Environmental Pub. House Hong Kong; p. 151–154.
- Rhodes L, Smith K, MacKenzie L, Wood L, Ponikla K, Harwood T, Packer M, Munday R. 2015. A significant national collection—the Cawthron Institute culture collection of micro-algae. J Mar Fresh Res. doi:10.1080/00288330.2015.1116450.
- Rhodes L, Smith K, Munday R, Hallegraeff GM, Selwood A, Molenaar S, McNabb P, Adamson J, Wilkinson C. 2013. Potency of pinnatoxins produced by dinoflagellates isolated from New Zealand and South Australia. Proc. 14th Int. Conf. on Harmful Algae; Nov. 2010; Greece: Hersonissos-Crete; p. 209–211.
- Rhodes L, Smith K, Selwood A, McNabb P, Munday R, Suda S, Molenaar S, Hallegraeff G. 2011. Dinoflagellate Vulcanodinium rugosum Nézan et Chomérat newly identified as the causative organism of pinnatoxins in Australia, New Zealand and Japan. Phycologia. 50:624–628. doi: 10.2216/11-19.1
- Roldán M, Ramírez M, del Campo J, Hernández-Mariné M, Komárek J. 2013. Chalicogloea cavernicola gen. nov., sp. nov. (Chroococcales, Cyanobacteria), from low-light aerophytic environments: combined molecular, phenotypic and ecological criteria. Int J Syst Evol Micr. 63:2326–2333. doi: 10.1099/ijs.0.045468-0
- Schock TB, Huncik K, Beauchesne KR, Villareal TA, Moeller PDR. 2011. Identification of Trichotoxin, a novel chlorinated compound associated with the bloom forming Cyanobacterium, Trichodesmium thiebautii. Env Sci Tec. 45:7503–7509. doi: 10.1021/es201034r
- Sugita C, Ogata K, Shikata M, Jikuya H, Takano J, Furumichi M, Kanehisa M, Omata T, Sugiura M, Sugita M. 2007. Complete nucleotide sequence of the freshwater unicellular cyanobacterium Synechococcus elongatus PCC 6301 chromosome: gene content and organization. Photosynth Res. 93:55–67. doi: 10.1007/s11120-006-9122-4
- Wood SA, Pochon X, Luttringer-Plu L, Vant BN, Hamilton DP. 2014. Recent invader or indicator of environmental change? A phylogenetic and ecological study of Cylindrospermopsis raciborskii in New Zealand. Harmful Algae. 39:64–74. doi: 10.1016/j.hal.2014.06.013
