ABSTRACT
The pollen of the informal bracteate-prostrate group of Southern Hemisphere Myosotis sect. Exarrhena (Boraginaceae: Cynogossoideae: Myosotideae) was investigated using scanning electron microscopy (SEM) to obtain taxonomically useful characters for delimiting species and species groups. Pollen grains of one to four individuals of each of 30 different taxonomic entities were imaged using SEM, scored for 16 different morphological characters, described and analysed. Pollen grains are small, heterocolpate, with 8–12 apertures, oblate spheroidal to prolate in equatorial view, with linear pseudocolpi which alternate with the colpori and can be partially to fully fused (‘anastomosing’) to form an apocopial field (‘polar cap’). Colpori ektoapertures are wider and often shorter than pseudocolpi, rhombic or narrowly rhombic with often circular or elliptical endoapertures. The exine is usually verrucate on the endoaperture membranes but densely and evenly granulate elsewhere. Statistical analyses of the pollen morphological dataset recovered three significant clusters, two of which contained the majority of individuals and coincided with the previously categorised Myosotis australis type pollen, while individuals in the third cluster had M. uniflora type pollen. Myosotis brevis (M. discolor type) and M. glabrescens (M. angustata type) represented two additional pollen types and thus had the most distinctive pollen of the whole dataset, whereas M. albiflora and M. tenericaulis showed high intraspecific variability. Important pollen characters separating the clusters included aperture number, polar cap presence, and P:E ratio (shape). Although most species could not be identified based solely on pollen morphology, species with similar habits clustered together, and pollen characters will be useful for delimiting species groups or species when combined with additional morphological, genetic or other datasets. Additional pollen studies of the remaining Myosotis species is warranted, including those from sect. Exarrhena with M. discolor type pollen and the ebracteate-erect species, as well as Northern Hemisphere sect. Myosotis.
Introduction
Myosotis L. (Boraginaceae) comprises c. 100 species, commonly called forget-me-nots, which are found in two main centres of diversity, Eurasia and New Zealand. Boraginaceae (Chacón et al. Citation2016; Luebert et al. Citation2016) are monophyletic, the generic relationships among the major lineages within it are now relatively well understood (Weigend et al. Citation2013, Citation2016; Chacón et al. Citation2016), and the infrafamilial classification has recently been updated (Chacón et al. Citation2016). In the most up-to-date treatment, c. 90 genera are classified into 11 tribes in three subfamilies: Boraginoideae Arn.; Cynoglossoideae Weigend; and Echiochiloideae Weigend (Chacón et al. Citation2016). Myosotis belongs to Cynoglossoideae—which is the largest and taxonomically most complex of these subfamilies—and is placed in the tribe Myosotideae Rchb.f. with three largely Asian genera: its sister genus Decalepidanthus Riedl, plus Trigonotis Steven and Brachybotris Maxim. ex Oliv. (Chacón et al. Citation2016; Weigend et al. Citation2016).
Boraginaceae have unusually high pollen morphological diversity (i.e. the family is eurypalynous; Díez & Valdés Citation1991; Weigend et al. Citation2016), which is taxonomically useful for delimiting tribes, genera and even species (Díez & Valdés Citation1991; Hargrove & Simpson Citation2003; Weigend et al. Citation2016). Each of the three subfamilies has unique pollen characters which unite most genera and species (Weigend et al. Citation2016). Most Cynoglossoideae including Myosotis have heterocolpate pollen (i.e. grains with two types of apertures: true apertures [colpori] alternating with pseudoapertures [pseudocolpi]; ) (El Ghazali & Krzywinski Citation1989; Hargrove & Simpson Citation2003; Weigend et al. Citation2016). Furthermore, the colpori are compound apertures comprised of smaller endoapertures inside larger ektoapertures (D). Heterocolpate pollen is not found in the other subfamilies of Boraginaceae and is in fact very rare among angiosperms (El Ghazali & Krzywinski Citation1989; Díez & Valdés Citation1991). The ultrastructure and exine morphology of Cynoglossoideae heterocolpate pollen has been studied and described in detail by Hargrove and Simpson (Citation2003; eight species of Cryptantha Lehm. ex G.Don., tribe Cynoglosseae) and Volkova et al. (Citation2013; two species each of Cryptantha and Rindera Pall. [also Cynoglosseae], plus Myosotis palustris Hill). Another scanning electron microscopy (SEM) study of pollen showed the taxonomic utility of pollen morphological characters among nine Iberian Cynoglossoideae genera (including Myosotis) and also among some species (Díez & Valdés Citation1991). Taxonomically useful characters and three main pollen types were found in Omphalodes Mill. (Omphalodeae) using SEM, with the aperture margin sculpturing and presence of a ring-like aperture being particularly important (Coutinho et al. Citation2012).
Figure 1. Scanning electron microscope (SEM) images of Southern Hemisphere bracteate-prostrate Myosotis pollen showing different pollen types, features and terminology in equatorial view (A–G) and polar view (H–I). A, Myosotis discolor type pollen of M. brevis (WELT SP090543/A) with distinctive lack of granula on most of exine (psilate; white arrow) and alternating colpori (c) and pseudocolpi (pc); B, Myosotis angustata type pollen of M. glabrescens (WELT SP044913) with distinctive oblong (rectangular) shape; C, Myosotis australis type pollen of M. sp. “Volcanic Plateau” (CHR 310610) with wide and psilate pseudocolpus (white arrow); D, close up of M. brevis (WELT SP090550/A) pollen with granulate endoaperture (en) and ektoaperture (ek) membranes of the colporus (c), and psilate membrane of the pseudocolpus (pc), as well as granulate aperture margins (white arrows); E, Myosotis australis type pollen of M. lyallii (CHR 207153) with partially anastomosing pseudocolpi (pc; white arrow); F, Myosotis uniflora type pollen of M. matthewsii (AK 46608) with distinctive polar cap (p cap); G, close up of M. colensoi × M australis (WELT SP090554) colporus with granula (g) on exine and ektoaperture (ek) membrane, and verruca (v) on endoaperture (en) membrane; H, Myosotis australis type pollen of M. glauca (CHR 191750) in polar view, which lacks a polar cap (white arrow); I, Myosotis uniflora type pollen of M. pulvinaris (OTA 031024) in polar view, with fully anastomosing pseudocolpi (pc; white arrow) forming a distinctive polar cap (p cap). pc, pseudocolpus; c, colporus (a compound aperture comprised of ektoaperture [ek] and endoaperture [en]); p cap, polar cap; v, verruca; g, graunula. Scale bar = 5 μm.
![Figure 1. Scanning electron microscope (SEM) images of Southern Hemisphere bracteate-prostrate Myosotis pollen showing different pollen types, features and terminology in equatorial view (A–G) and polar view (H–I). A, Myosotis discolor type pollen of M. brevis (WELT SP090543/A) with distinctive lack of granula on most of exine (psilate; white arrow) and alternating colpori (c) and pseudocolpi (pc); B, Myosotis angustata type pollen of M. glabrescens (WELT SP044913) with distinctive oblong (rectangular) shape; C, Myosotis australis type pollen of M. sp. “Volcanic Plateau” (CHR 310610) with wide and psilate pseudocolpus (white arrow); D, close up of M. brevis (WELT SP090550/A) pollen with granulate endoaperture (en) and ektoaperture (ek) membranes of the colporus (c), and psilate membrane of the pseudocolpus (pc), as well as granulate aperture margins (white arrows); E, Myosotis australis type pollen of M. lyallii (CHR 207153) with partially anastomosing pseudocolpi (pc; white arrow); F, Myosotis uniflora type pollen of M. matthewsii (AK 46608) with distinctive polar cap (p cap); G, close up of M. colensoi × M australis (WELT SP090554) colporus with granula (g) on exine and ektoaperture (ek) membrane, and verruca (v) on endoaperture (en) membrane; H, Myosotis australis type pollen of M. glauca (CHR 191750) in polar view, which lacks a polar cap (white arrow); I, Myosotis uniflora type pollen of M. pulvinaris (OTA 031024) in polar view, with fully anastomosing pseudocolpi (pc; white arrow) forming a distinctive polar cap (p cap). pc, pseudocolpus; c, colporus (a compound aperture comprised of ektoaperture [ek] and endoaperture [en]); p cap, polar cap; v, verruca; g, graunula. Scale bar = 5 μm.](/cms/asset/09e4f470-6315-4266-bfce-66eab6799854/tnzb_a_1229343_f0001_b.gif)
The morphological variability of pollen was also found to be taxonomically useful in Myosotis (Grau & Leins Citation1968; Grau & Schwab Citation1982; Díez & Valdés Citation1991). Seven different pollen types were distinguished based on light microscopy observations of pollen size, shape, number of apertures, and other characters based on a sample of 28 individuals representing 28 species and varieties. Four of the seven pollen types—M. angustata, M. australis, M. exarrhena and M. uniflora types—were exclusive to the Southern Hemisphere species sampled, including 10 from New Zealand (Grau & Leins Citation1968). The seven pollen types were categorised into two main geographic groups. The first main group is largely Southern Hemisphere, includes all four Southern Hemisphere types plus the M. discolor type from a few European and one East African species, and comprises large pollen grains with more apertures and morphological diversity. The second main group is largely Northern Hemisphere, includes two types—M. arvensis and M. verna types—including all other Eurasian, African and North American species, and comprises small, uniform hourglass-shaped pollen grains. Neither of these two main groups nor their corresponding seven pollen types coincided with the previous sectional treatment based on flower and fruit characters (de Candolle Citation1846).
In a complementary study, Grau & Schwab (Citation1982) increased their sampling to one to two samples each of 82 species and varieties of Myosotis (including 11 Southern Hemisphere species). They used both SEM and light microscopy to further study the pollen, stigmas, faucal scales and anther appendages. Their data corroborated the findings of Grau & Leins (Citation1968), leading the authors to propose a new sectional classification of Myosotis in which Southern Hemisphere species (plus those with M. discolor type pollen, which were considered to be a closely-allied subgroup) were placed in Myosotis sect. Exarrhena (R.Br.) Hook.f. whereas the remaining largely Northern Hemisphere species were placed in sect. Myosotis. Grau & Schwab (Citation1982) also reiterated that pollen surface characters were highly variable among the 11 Southern Hemisphere species studied, noting several differences among them. More recent studies have also found that certain pollen characters observed using SEM (e.g. pollen size and number of apertures) were found to be useful for distinguishing some species of European Myosotis (Díez & Valdés Citation1991; Nikiforova Citation2002).
Moar (Citation1993) further characterised the Myosotis australis, M. angustata and M. uniflora types of Grau & Leins (Citation1968) in New Zealand Myosotis by studying the pollen of M. angustata Cheeseman (one individual sampled), M. australis R.Br. (four) and M. uniflora Hook.f. (one; voucher specimens were listed in the appendix), as well as individuals of 24 other species and varieties of New Zealand Myosotis (no vouchers specimens listed). Although that study is valuable as the first and only survey of New Zealand Myosotis pollen, it mostly relied on bright field microscopy (as opposed to SEM), voucher specimens were not listed for the majority of the specimens, and it was noted that the variation and irregularity of pollen characteristics observed were due to incorrect names on herbarium labels and lack of an up-to-date taxonomic revision (Moar Citation1993).
In summary, pollen morphology, particularly when assessed using SEM, has been shown to be taxonomically useful at the family (Boraginaceae), subfamilial, tribal (e.g. Cynoglosseae), generic and species levels (including the genus Myosotis). Although previous studies on Myosotis pollen have been promising, sampling among the Southern Hemisphere (and particularly the New Zealand) species has been uneven and incomplete both within and among species. Therefore, a new study on the taxonomic utility of pollen morphology in New Zealand Myosotis was undertaken. The emphasis was on the ‘bracteate-prostrate’ group (Robertson Citation1989; Meudt et al. Citation2015), as it is the current focus of forget-me-not taxonomic research (J. Prebble et al. Citation2015, unpubl. data; H. Meudt et al., unpubl. data). The bracteate-prostrate group comprises 19 described species and varieties, together with c. nine tag-named entities, all endemic to New Zealand except M. albiflora Banks & Sol. ex Hook.f. (endemic to southern Chile and Argentina) and M. antarctica Hook.f. (also native to Chile; ). As the name suggests, plants in this group are largely prostrate rosette plants whose inflorescences have cauline leaves (sometimes called bracts) subtending the flowers, with habits ranging from very tightly compacted cushions with solitary flowers to creeping herbs with long, trailing branches with multiple flowers. By contrast, the remaining 33 described ebracteate-erect species and varieties comprise larger rosette plants whose inflorescences are erect and do not have cauline leaves subtending the flowers (see Meudt et al. Citation2015, ).
Table 1. Voucher information for all 78 Myosotis specimens sampled in this study.
The main aim of this study was to observe and measure morphological characters from SEM images of pollen from multiple individuals of all species and tag-named entities to obtain taxonomically useful characters for delimiting species and species groups within the bracteate-prostrate group of New Zealand Myosotis. This pollen study is part of a larger research project currently underway that aims to fully revise all species of New Zealand Myosotis using an integrative taxonomic framework.
Methods
Sampling
Five pollen grains from each of one to four individuals were sampled from 30 different taxonomic entities in the Myosotis bracteate-prostrate group (i.e. each of 19 species and varieties, nine tag-named entities, one putative hybrid and one unknown Myosotis sp.). The aim was for representative geographical and morphological sampling among specimens that were also sampled for additional morphological and genetic studies (H. Meudt et al., unpubl. data; J. Prebble et al., unpubl. data). Although measuring 30 pollen grains per species would be ideal from a statistical point of view (Wrońska-Pilarek et al. Citation2015), this was unrealistic for herbarium specimens of Myosotis due to lack of suitable flowering specimens with sufficient flowers containing adequate pollen of good quality. In some cases, it was not even possible to sample three individuals and/or measure five pollen grains per individual. Permission for destructive sampling was obtained from AK, CHR, CONC, OTA, S and WELT herbaria (acronyms follow Index Herbariorum; Thiers, continuously Citationupdated); voucher information for the 78 herbarium specimens sampled for this study, collected between 1898–2016 (age range: 0–118 years old, mean: 28, median: 22) are listed in . For each specimen, one to two flowers or buds were removed with tweezers and partially dissected under a stereomicroscope at WELT to check that they contained pollen. Pollen-containing flowers were placed in a packet and transferred with permits to the Manawatu Microscopy and Imaging Centre (MMIC), Massey University, Palmerston North, New Zealand for preparation and imaging on the SEM.
SEM imaging
Several different preparation and drying methods have been used in previous studies of Boraginaceae pollen (Díez & Valdés Citation1991; Hargrove & Simpson Citation2003; Coutinho et al. Citation2012; Volkova et al. Citation2013). Myosotis pollen grains have a very thin exine (Moar Citation1993) and have collapsed following acetolysis (Díez & Valdés Citation1991). After trialling several different preparation methods, the following methodology was chosen. For each sample, anthers containing pollen were placed into a solution of 1:400 Triton X-100 solution for 1–5 min and then rinsed twice with RO water and placed into FAA solution (90 mL of 70% ethanol, 5 mL of glacial acetic acid, 5 mL of 40% formaldehyde). They were fixed overnight and washed twice with 25% ethanol (10 min each) and dehydrated (15 min each: 50%, 75%, 95%, 100% ethanol) with a final 100% ethanol step for 1 h. The samples were then critical point dried with liquid carbon dioxide using ethanol as the transition fluid (Polaron E3000 series II critical point drying apparatus), mounted onto a stub and coated with c. 100 nm of gold (BAL-TEC SCD005 sputter coater) for imaging on the FEI Quanta 200 SEM (Hillsboro).
Data collection and analyses
At least five images per specimen were taken of several different pollen grains in polar and equatorial views at different magnifications. In total, 16 different characters were measured (eight characters), calculated (six) or scored (two) for each specimen (Table S1) following Díez & Valdés (Citation1991), Moar (Citation1993), Hargrove & Simpson (Citation2003), Coutinho et al. (Citation2012) and Volkova et al. (Citation2013). Five pollen grains per specimen (occasionally only three or four) were measured and scored using the software ImageJ (Rasband Citation1997–Citation2016). The image scale bar was measured using the line tool and the ImageJ ‘Set Scale’ function. The eight measurements (polar axis length [P], equatorial axis length [E], pseudocolpus length, pseudocolpus width, colporus ektoaperture length, colporus ektoaperture width, colporus endoaperture length and colporus endoaperture width) were made on grains in equatorial view using the ImageJ line tool and ‘Measure’ function. These were entered into an Excel spreadsheet and used to calculate six ratios (Table S1). In addition, two qualitative characters were also scored from the images. One multistate character, total number of apertures (colpori + pseudocolpi), was scored as being 8, 10 or 12. As some individuals were polymorphic, this character was subsequently scored as 0, 1, 2, 3 or 4 for 8, 8/10, 10, 10/12 and 12, respectively, and was treated as an ordered character for multidimensional scaling. One binary character, presence/absence of a ‘polar cap’ (I), was also scored. Other descriptive features were also noted. Pollen terminology (see ) follows Hesse et al. (Citation2009), and pollen type names follow Moar et al. (Citation2011).
For each of the 14 quantitative characters (measurements and ratios), a mean value from all (3–)5 pollen grains measured per specimen was calculated and used in downstream analyses. Statistical analyses were conducted in R (RCore Team Citation2015) using RStudio (RStudio Team Citation2015). All 16 characters (14 means plus two binary/multistate characters) were compared using the Morphotools (Koutecky Citation2015) functions cormat.p and cormat.s (Pearson’s and Spearman’s coefficient, respectively). High correlations (Pearson’s or Spearman’s coefficient > 0.8) were found between four pairs of characters: P + pseudocolpus length; pseudocolpus width + pseudocolpus length:width ratio; colporus ektoaperture length + colporus ektoaperture length:P ratio; and colporus ektoaperture width + colporus endoaperture width. Therefore, the second character listed in each pair above was excluded from downstream analyses, as were three other characters (E, pseudocolpus length:P ratio and colporus ektoaperture length:width ratio) so as to avoid having a ratio character and both of its component measurements included.
After excluding those seven characters, the remaining nine character dataset () was analysed using Ward clustering (clust.ward function, Morphotools) and then was transformed into a distance matrix using Gower’s coefficient (daisy function, ‘cluster’ package; Maechler et al. Citation2015). The distance matrix was analysed using non-metric multidimensional scaling (nMDS; metaMDS function, ‘vegan’ package; Oksanen et al. Citation2015) and Bayesian model-based clustering, which identifies the number of clusters present in the data using Bayesian information criteria (BIC), and assesses the amount of uncertainty of each individual regarding its cluster classification (Mclust function, ‘mclust’ package; Fraley & Raftery Citation2002; Fraley et al. Citation2012).
Table 2. Summary of the nine quantitative and qualitative pollen morphological characters analysed in the nMDS and Mclust analyses from the Myosotis bracteate-prostrate group taxonomic entities.
Results
In total, 69/78 herbarium specimens sampled were successfully imaged on the SEM. Pollen grains from nine herbarium specimens failed to rehydrate properly after multiple attempts, or did not contain sufficient pollen in the anthers. The 69 specimens represent 30 different taxonomic entities, and thus on average 2.3 individuals per entity were sampled (range 1–4, median 2.5). In general, flowers with dehisced anthers on Myosotis herbarium specimens contained very little pollen, and usually only between 5–30 grains were able to be imaged per specimen. Given these constraints, the aim was to measure five pollen grains for each of the 69 specimens. In total, 335 pollen grains were measured, which was on average 4.96 pollen grains per specimen (range 3–5, median 5.0) and 11.2 per taxonomic entity (range 3–20, median 10.5).
The New Zealand Myosotis pollen grains examined here (–) are monad, radially symmetrical, isopolar, heterocolpate with eight (e.g. A–B), 10 (e.g. M–N) or 12 (e.g. A, B, F) median apertures, lacking a median constriction and a polar pseudoaperture, small (P 10.6–16.5 × E 8.5–14.9 μm), with a P:E ratio of 0.94–1.41, oblate spheroidal, spheroidal or prolate spheroidal (rarely prolate, A, or oblong, B) in equatorial view with rounded ends (rarely flat, B), and spheroidal, quadrilateral, pentagonal or hexagonal in polar view. Pseudocolpi are linear (rarely narrowly elliptic), alternating with and parallel to the colpori, 6.1–12.5 × 0.2–1.3 μm (58%–88% the polar axis length P), sometimes partially anastomosing (E), or even fully anastomosing (I) near the poles to form an apocopial field (or ‘polar cap’; I, A–T). Colporus ektoapertures (D) are wider and often shorter than pseudocolpi, rhombic or narrowly rhombic, 4.4–10.3 × 2.2–5.3 μm (36%–75% P and 1.5–3.3 length:width ratio). Colporus endoapertures (D) are 1.7–4.5 × 1.6–3.9 μm, generally longer than wide (0.7–2.2 length:width ratio), and often circular or elliptical (rarely lalongate). The exine is usually granulate (rarely largely psilate in M. brevis; A,D, Q–R), with granula densely and homogeneously distributed on the exine as well as pseudocolpus and colporus ektoaperture margins and membranes (rarely on margins only in M. brevis; A, D, Q–R), and verrucate on the endoaperture membranes, with sparsely to densely distributed verruca (e.g. G, A, K). Sometimes pseudocolpi are wide (≥ 0.8 μm) and largely lacking granula (i.e. psilate; M. brevis, D, Q; M. sp. “Tapuae-o-Uenuku”, O; M. sp. “Volcanic Plateau”, C, I; M. sp. (unknown), M; and sometimes also individuals of other species; note M. glabrescens pseudocolpi are psilate but not wide, B, S).
Figure 2. Scanning electron microscope (SEM) images of Southern Hemisphere bracteate-prostrate Myosotis pollen from nMDS Cluster 1 () in equatorial view (A, C, E, G, I, K, M, O, Q, S), polar view (B, D, F, H, J, L, P, R, T) or close up of endoaperture (N) showing Myosotis australis type (A–P), M. discolor type (Q–R) and M. angustata type pollen (S–T). A–B, Myosotis antarctica (WELT SP102775, WELT SP102779, respectively); C–D, M. drucei (CHR 386879, WELT SP102783); E–F, M. glauca (CHR 191750, WELT SP100497); G–H, M. pygmaea (CHR 245912, AK 231694); I–J, M. sp. “Volcanic Plateau” (WELT SP089738, CHR 310610); K–L, M. aff. tenericaulis (WELT SP103811, CHR 439290); M–N, M. sp. (unknown) (both WELT SP103892); O–P, M. albiflora (S15-37506, CONC 73040); Q–R, M. brevis (WELT SP090550/A, WELT SP102761); S–T, M. glabrescens (both WELT SP044913). Scale bar = 5 μm. These and additional pollen images are available on Te Papa’s Collections Online (http://collections.tepapa.govt.nz/Topic/10487).
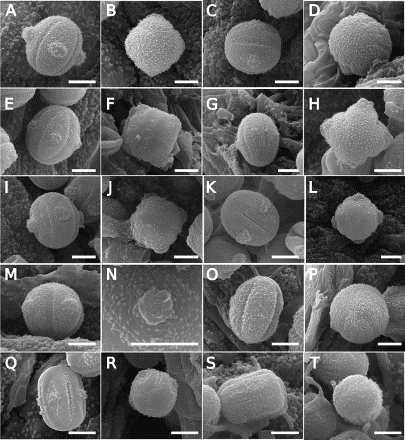
Figure 3. Scanning electron microscope (SEM) images of Southern Hemisphere bracteate-prostrate Myosotis pollen from nMDS Cluster 2 () in equatorial view (A, C, E, G, I, K, M, O, Q, S) or polar view (B, D, F, H, J, L, N, P, R, T) showing Myosotis australis type pollen (A–T). A–B, Myosotis colensoi (WELT SP095578, CHR 96401, respectively); C–D, M. sp. “Rock and Pillar” (OTA 002703, WELT SP089763/A); E–F, M. elderi (CHR 132818, OTA 031559); G–H, M. lyallii var. lyallii (CHR 207153, WELT SP039638); I–J, M. lyallii var. townsonii (WELT SP104488/A, WELT SP091837); K–L, M. sp. “Fiordland” (both CHR 338108); M–N, M. sp. “serpentine” (both CHR 320240); O–P, M. sp. “Tapuae-o-Uenuku” (from two different plants on CHR 386966); Q–R, M. sp. “intermedia” (WELT SP089911, WELT SP089909); S–T, M. aff. glauca (WELT SP093282, WELT SP089898). Scale bar = 5 μm. These and additional pollen images are available on Te Papa’s Collections Online (http://collections.tepapa.govt.nz/Topic/10487).
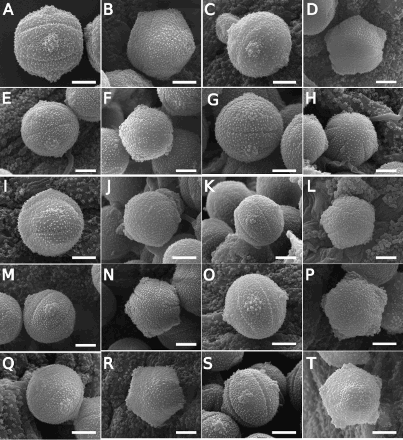
Figure 4. Scanning electron microscope (SEM) images of Southern Hemisphere bracteate-prostrate Myosotis pollen from nMDS Cluster 3 () in equatorial view (A, C, E, G, I, K, M, O, Q, S) or polar view (B, D, F, H, J, L, N, P, R, T) showing Myosotis uniflora type pollen (A–R) and M. australis type pollen (S–T). A–B, Myosotis cheesemanii (CHR 475919, OTA 62709, respectively); C–D, M. uniflora (both CHR 499326); E–F, M. pulvinaris (WELT SP103819, WELT SP089842); G–H, M. aff. pulvinaris (WELT SP002699, CHR 624106); I–J, M. chaffeyorum (both CHR 310255); K–L, M. matthewsii (WELT SP002571, AK 46608); M–N, M. spathulata var. radicata (both AK174020); O–P, M. spathulata var. spathulata (CHR 210782, AK 303980); Q–T, M. tenericaulis (WELT SP095613 x2, WELT SP002691, WELT SP089834/A), showing high intraspecific variation with both M. uniflora (Q–R) and M. australis type pollen (S–T) represented. Scale bar = 5 μm. These and additional pollen images are available on Te Papa’s Collections Online (http://collections.tepapa.govt.nz/Topic/10487).
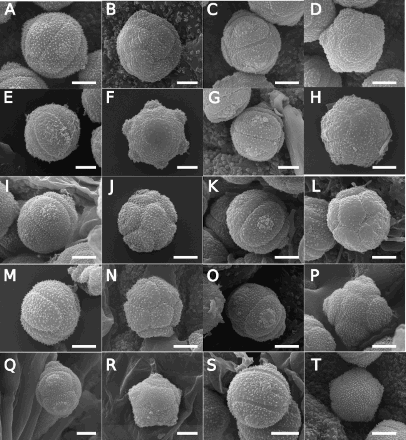
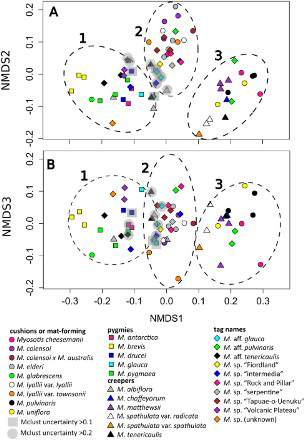
Table S1 summarises the 16 quantitative and qualitative pollen characters examined in this study for each of the 30 taxonomic entities; summarises only the nine characters analysed using nMDS. Fifteen entities were polymorphic for aperture number (, S1). Twelve of these were based on polymorphic individuals, whereas three had different individuals with differing numbers of apertures. Myosotis tenericaulis Petrie and M. aff. pulvinaris were polymorphic for presence of a polar cap (, S1). Multidimensional scaling and Mclust analyses of 69 individuals and the nine included morphological characters showed that there were three significant clusters in the data () and this result was congruent with the Ward clustering analysis (data not shown). There is good separation of Cluster 3 from Clusters 1 and 2, but there are 10 individuals with high uncertainty scores (> 0.1) between Clusters 1 and 2 (; ). In general, individuals from a taxonomic entity clustered together, or were at least found in the same Mclust cluster, with the exception of M. albiflora, M. sp. “intermedia”, M. glauca (G.Simpson & J.S.Thomson) de Lange & Barkla and M. aff. glauca (each with individuals in Clusters 1 and 2, the latter two taxa with high uncertainty), M. aff. pulvinaris (Clusters 2 and 3) and M. tenericaulis (Clusters 1, 2 and 3, one of these with high uncertainty; ).
Figure 5. Non-metric multidimensional scaling (nMDS) analysis of a Gower’s distance matrix of nine pollen morphological characters for 69 individuals representing 30 taxonomic entities of Southern Hemisphere bracteate-prostrate Myosotis. Shapes refer to major habitat groups (circles, cushions or mat-forming plants with compact rosettes and branches; squares, pygmy plants belonging to the M. pygmaea complex; triangles, creeping or trailing rosette plants with long branches; diamonds, tag-named entities). Colours indicate different species within each habit group. The three significant clusters found in Bayesian clustering analyses using Mclust are shown as dashed lines (left to right, Clusters 1 to 3); individuals with uncertainty > 0.1 or > 0.2 are enclosed with shaded squares or circles, respectively. A, nMDS 1 vs. nMDS 2; B, nMDS 1 vs. nMDS 3.
Discussion
Of the 27 Southern Hemisphere species that have been included over three previous studies of Myosotis pollen mostly using light microscopy (Grau & Leins Citation1968; Grau & Schwab Citation1982; Moar Citation1993), only nine of these were from the bracteate-prostrate group, and were generally represented by one individual each. The present study uses SEM and increases sampling to multiple individuals of 30 taxonomic entities. It both confirms the findings of those previous studies that pollen surface morphology is variable both among and occasionally within New Zealand species of Myosotis, and provides new insights regarding the taxonomic utility of pollen morphology.
Of the two main Myosotis pollen groups identified in previous studies, all pollen investigated here clearly belongs in the Southern Hemisphere + M. discolor type pollen group of Grau & Leins (Citation1968), that is Myosotis sect. Exarrhena of Grau & Schwab (Citation1982). According to molecular phylogenetic studies, sect. Exarrhena is derived within sect. Myosotis, and the Southern Hemisphere species are a highly-supported lineage. The grade immediately leading up to the Southern Hemisphere lineage comprises mostly species with M. discolor type pollen including the annuals M. abyssinica Boiss. & Reut., M. congesta Shuttlew., M. discolor Pers. and M. persoonii Rouy, the latter of which is the sister species to the Southern Hemisphere lineage (Northern Hemisphere species sampling is incomplete; Winkworth et al. Citation2002; Meudt et al. Citation2015). The phylogeny of the Southern Hemisphere species is unresolved (Meudt et al. Citation2015), and thus no insights can be gained from it regarding pollen evolutionary trends. Relative to sect. Myosotis, sect. Exarrhena has larger pollen grains which lack both a median constriction and a triangular polar pseudoaperture (vs. small pollen grains with a median constriction and a polar pseudoaperture), more apertures (8–12 vs. 6–8), and exines that are usually densely and evenly granulate (vs. psilate but granulate on the aperture margins only). Although some species in sect. Myosotis have some of the smallest known pollen grains among angiosperms (P = 5.5–13 μm, Grau & Leins Citation1968; 6–14 μm, Díez & Valdés Citation1991; 5.6–10.5 um, Nikiforova Citation2002; Weigend et al. Citation2016), species of sect. Exarrhena have larger grains (P = 12–20, Cranwell Citation1942; 14–20 μm, Grau & Leins Citation1968; 14–25 μm, Díez & Valdés Citation1991; 16–22 μm, Moar Citation1993). The range of P found here using SEM for Southern Hemisphere Myosotis (P = 10.6–16.5 μm) was smaller than in previous studies using light microscopy, but because pollen size is affected by treatment, mounting material and microscopic methodology (Reitsma Citation1969; Moar Citation1993), all of which differed greatly among these studies, direct comparisons of size are not possible.
The three pollen groups found in the nMDS analysis () do not correspond exactly to the M. angustata, M. australis and M. uniflora types of Grau & Leins (Citation1968) and Moar (Citation1993), but there is some congruence. In the nMDS, the most important characters distinguishing the clusters were number of apertures, P:E ratio (shape) and presence of a polar cap. Although pollen grains with more apertures were generally larger (as was suggested previously for Myosotis; Hargrove & Simpson Citation2003) and had a lower P:E ratio, these characters were not strongly associated (Pearson’s/Spearman’ coefficients c. 0.5 and −0.5, respectively).
All species in nMDS Clusters 1 and 2 (with two notable exceptions, A–B,D, see below) correspond to M. australis type pollen, particularly due to the lack of both a polar cap and median constriction, as well as being rounded at the poles and having longer pseudocolpi, some of which may sometimes partially anastomose near the pole (C,E,H, –). All individuals in nMDS Cluster 3 correspond to M. uniflora type pollen, especially due to the presence of a polar cap (F,I, ). Thus, these analyses agree with Moar (Citation1993) in that, of the species that were sampled for both studies, all individuals of M. antarctica (A–B), M. colensoi (Kirk) J.F.Macbr. (A–B), and M. elderi L.B.Moore (E–F; Clusters 1 and 2, ) have M. australis type pollen, and those of M. cheesemanii Petrie (A–B), M. pulvinaris Hook.f. (E–F) and M. uniflora (C–D; Cluster 3; ) have M. uniflora type pollen. Furthermore, the results—which are based on statistical analysis of data and increased sampling relative to previous studies—suggest there is additional differentiation within M. australis type pollen among bracteate-prostrate species; two separate clusters (1 and 2) are able to be distinguished within it in nMDS analyses ().
The clusters found in the nMDS analysis () also roughly correspond with different habits, suggesting that pollen characteristics may be taxonomically useful for distinguishing species groups within the bracteate-prostrate forget-me-nots. Due to very low genetic divergence among New Zealand Myosotis species (Winkworth et al. Citation2002; Meudt et al. Citation2015), it is unknown whether different habits (or pollen types) are a particularly good indicator of evolutionary relatedness, how pollen has evolved over evolutionary time, or even whether the bracteate-prostrate and ebracteate-erect groups are monophyletic. Nevertheless, studying these informal habit- and inflorescence-based groups of morphologically similar species (Robertson Citation1989; Meudt et al. Citation2015) is currently the most useful way to systematically revise the taxonomy of the Southern Hemisphere Myosotis sect. Exarrhena. Although the pollen morphological data do not support separation of the bracteate-prostrate and ebracteate-erect groups (as M. angustata, M. australis and M. uniflora type pollen is found in both; Moar Citation1993; this study), the data do provide additional independent support for some groupings within the bracteate-prostrate group.
Accordingly, pollen characteristics support the overall morphological similarity of all individuals which make up nMDS Cluster 1 () (i.e. all five species of pygmy forget-me-nots: M. antarctica [Campbell Island only; three sampled individuals]; M. brevis de Lange & Barkla [three]; M. drucei (L.B.Moore) de Lange & Barkla [two]; M. glauca [one of two]; and M. pygmaea [three]), plus individuals of M. glabrescens L.B.Moore (one), M. albiflora (two of three) and the tag-named M. aff. glauca (one of three, with high uncertainty), M. sp. “intermedia” (one of three), M. aff. tenericaulis (three), M. sp. “Volcanic Plateau” (two), and M. sp. (unknown) (one) (). All individuals in this cluster have pollen grains that are spheroidal to prolate (P:E ratio of 1.0–1.4), lack a polar cap, and have eight apertures only (), except for M. albiflora (O–P), M. glauca (E–F), M. sp. “intermedia” (Q–R), M. sp. “Volcanic Plateau” (I–J) and M. aff. tenericaulis (K–L), which can also have 10 apertures. Based on habit and other characteristics, the placement in Cluster 1 of M. glabrescens (a cushion plant with exserted stamens) and some M. albiflora (a creeping plant from southern South America with included stamens) are perhaps the most surprising. Most of the other taxonomic entities in this cluster are pygmy forget-me-nots, that is small rosette plants with included stamens, small corollas (< 4 mm diameter) and other plant parts (Moore Citation1961; J. Prebble, unpubl. data), and likely have a selfing breeding system (Moore Citation1961; Robertson & Lloyd Citation1991; Brandon Citation2001). With few exceptions (see below), Cluster 1 species have very similar pollen and cannot be distinguished from one another based on pollen characteristics alone.
The pollen grains of the Cluster 1 cushion species M. glabrescens and the pygmy forget-me-not M. brevis, have unique characteristics that further distinguish them from pollen of all other bracteate-prostrate species. Individuals of both M. brevis and M. glabrescens have relatively short pseudocolpi, and were the sample species with prolate pollen (P:E ≥ 1.4; A–B,D, Q–T). The sole M. glabrescens individual sampled here has pollen with a distinctive oblong shape in equatorial view (B, S–T). Although Moar (Citation1993) included M. glabrescens (no voucher given) under M. australis type, the M. glabrescens specimen sampled here best matches Moar’s description of M. angustata type pollen (B, S–T). The pollen grains of M. brevis are even more distinctive, as they are largely psilate with granula densely distributed only around the aperture margins and the colporus membranes (A,D, Q–R). They are perhaps best placed in the M. discolor type (which was not known in New Zealand species previously), as they are quite similar to pollen grains of M. persoonii and, particularly, M. abyssinica (compare A,D, Q–R to Grau & Schwab Citation1982, p. 16, and 7). As M. brevis clearly belongs to the Southern Hemisphere lineage (Meudt et al. Citation2015), this could represent parallel evolution due to shared annual habit: M. brevis is the only annual Southern Hemisphere species, and all other M. discolor type species are also annuals. Similar granulate margins were also found to be positively correlated with annual habits in Omphalodes pollen (Coutinho et al. Citation2012).
The pollen grains in nMDS Cluster 2 usually have 10 apertures (sometimes eight or 12), are generally spheroidal to prolate spheroidal (P:E ratio usually 1.0–1.3) and lack a polar cap (E,H, ). Cluster 2 comprises all individuals of the mat-forming M. colensoi (three), M. colensoi x M. australis (one), M. elderi (four) and M. lyallii Hook.f. (four), the creeping M. albiflora (one of three, with high uncertainty) and M. tenericaulis (one of three), as well as the tag-named M. aff. glauca (two of three, with high uncertainty), M. aff. pulvinaris (one of three), M. sp. “Fiordland” (one), M. sp. “Rock and Pillar” (three), M. sp. “intermedia” (two of three, one with high uncertainty), M. sp. “serpentine” (one), and M. sp. “Tapuae-o-Uenuku” (three) (). Of these species, all have fully or mostly included anthers, except M. lyallii, which has wholly exserted anthers. Further separation of individuals or species/tag-names within this cluster was not apparent in the nMDS analysis, as the pollen grains were very similar to one another.
The pollen grains in nMDS Cluster 3 can have eight, 10 or 12 apertures, are generally oblate spheroidal to spheroidal (P:E ratio usually 0.9–1.0), and have a polar cap (F,I, ). Cluster 3 comprises individuals from two morphologically dissimilar groups: cushion or mat-forming plants with mostly included anthers (M. cheesemanii, two sampled; M. pulvinaris, three: M. uniflora, two; M. aff. pulvinaris, two of three) as well as creeping plants with either included anthers (M. chaffeyorum Lehnebach [Lehnebach Citation2012], one; M. tenericaulis, one of three) or wholly exserted anthers (M. matthewsii L.B.Moore, three; M. spathulata G.Forst, three) (). The two habit types form two overlapping subclusters in (cushions: triangles and diamonds vs. creepers: circles). Individuals of M. spathulata (both varieties; M–P) are visibly separated from the other individuals and species in both nMDS 1 vs. 2 and 1 vs. 3, but it is unclear which specific pollen characters separate them from the other individuals in this group. The absence from Cluster 3 of all but one of the sampled individuals of the two other species with a creeping habit—M. albiflora from Chile/Argentina and M. tenericaulis from New Zealand—is notable, as is their intraspecific diversity (see Q–T for pollen of all three sampled individuals of M. tenericaulis). These are two of the six species for which individuals are found in multiple clusters in the nMDS. Four of the six sampled individuals of M. albiflora and M. tenericaulis, which are very similar to one another morphologically (H. Meudt, unpubl. data), are found in the area where Clusters 1 and 2 meet (with some uncertainty, ; M. albiflora, O–P; M. tenericaulis, S–T), whereas one M. tenericaulis is in Cluster 3 (presence of polar cap, Q–R) and one M. albiflora is in Cluster 2 (pollen not shown). Grau & Schwab (Citation1982) noted that M. albiflora pollen forms a transition (Übergang) between M. uniflora and M. angustata types (the pollen of M. tenericaulis has not been studied previously).
In conclusion, and as expected, pollen of the 30 taxonomic entities of the bracteate-prostrate group studied using SEM have the morphological characters of sect. Exarrhena. As in many other Boraginaceae genera, Myosotis pollen grains were found to be heterocolpate and morphologically variable (–), and were described in detail based on measurements and observations of 16 morphological characters. When nine of these characters were analysed using nMDS and Mclust, three significant clusters were found, corresponding to M. uniflora type pollen (Cluster 3; F,I, –) and M. australis type pollen (Clusters 1 and 2; C,E,G–H, –, ), distinguished by number of apertures, P:E ratio (shape) and presence of a polar cap. Further subgrouping of individual species or groups of species based on pollen characteristics within Clusters 1 and 3 (but not Cluster 2) could be seen, and was partly congruent with habit characteristics. Myosotis brevis and M. glabrescens (Cluster 1) had the most distinctive pollen among the bracteate-ebracteate species (A–B,D, Q–T), likely comprising examples of M. discolor and M. angustata type pollen, respectively. Pollen morphology may be taxonomically useful for delimiting certain species or species groups in the bracteate-prostrate group when used in conjunction with additional data. The SEM methodology outlined here was very useful for observing pollen characteristics, as in previous Boraginaceae studies (e.g. Hargrove & Simpson Citation2003; Coutinho et al. Citation2012), with the caveat that obtaining sufficient herbarium material was often difficult. Based on these results, a similar study of the M. sect. Exarrhena Southern Hemisphere ebracteate-erect species and affiliated species from the M. discolor group, and indeed of the Northern Hemisphere M. sect. Myosotis species is warranted to complete the picture of comparative pollen morphology within and among Myosotis species. The ability to examine pollen evolution in a phylogenetic context would also prove insightful but will only be possible once a more resolved phylogeny is available. Light microscopy studies may also be worth pursuing in Myosotis, building upon the work of Cranwell (Citation1942) and Moar (Citation1993), to assemble a complete reference set of pollen from all species for comparing to fossil pollen records.
Supplementary data
Table S1. Summary of quantitative and qualitative pollen morphological characters from the Myosotis bracteate-prostrate group taxonomic entities examined.
Table S1.
Download MS Excel (19.6 KB)Acknowledgements
I thank Niki Murray at the Manawatu Microscopy and Imaging Centre for preparing and imaging the pollen, staff at MPN and WELT for facilitating the transfer of material, Jessie Prebble (Massey University and WELT) for help with choosing pygmy forget-me-not samples and conducting analyses in R, and staff at AK, CHR, CONC, OTA, S and WELT for facilitating specimen loans and allowing destructive sampling for this pollen study. I also thank Jessie Prebble and Pat Brownsey (WELT) for critically commenting on a previous version of this article, and referees Dallas Mildenhall and Andrew Thornhill for their helpful reviews.
Associate editor: Dr Peter de Lange
Disclosure statement
No potential conflict of interest was reported by the author.
ORCiD
HM Meudt http://orcid.org/0000-0002-2433-9071
Additional information
Funding
References
- Brandon AM. 2001. Breeding systems and rarity in New Zealand Myosotis [Unpublished PhD thesis]. Palmerston North, New Zealand: Massey University. 183 pp.
- Chacón J, Luebert F, Hilger HH, Ovchinnikova S, Selvi F, Cecchi L, Guilliams CM, Hasenstab-Lehman K, Sutorý K, Simpson MG, Weigend M. 2016. The borage family (Boraginaceae s. str.): a revised infrafamilial classification based on new phylogenetic evidence, with emphasis on the placement of some enigmatic genera. Taxon. 65:523–546. doi: 10.12705/653.6
- Coutinho AP, Castro S, Carbajal R, Ortiz S, Serrano M. 2012. Pollen morphology of the genus Omphalodes Mill (Cynoglosseae, Boraginaceae). Grana. 51:194–205. doi: 10.1080/00173134.2012.665943
- Cranwell LM. 1942. New Zealand pollen studies: 1. Key to the pollen grains of families and genera in the native flora. Rec of Ak Inst Mus. 2:280–308.
- de Candolle AP. 1846. Prodromus Systematis Naturalis Regni Vegetabilis. Vol. 10. Paris: Treuttel et Würtz.
- Díez MJ, Valdés B. 1991. Pollen morphology of the tribes Eritrichieae and Cynoglosseae (Boraginaceae) in the Iberian Peninsula and its taxonomic significance. Bot J Linn Soc. 107:49–66. doi: 10.1111/j.1095-8339.1991.tb00214.x
- El Ghazali GE, Krzywinski K. 1989. An attempt to clarify the term heterocolpate. Grana. 28:179–186. doi: 10.1080/00173138909427429
- Fraley C, Raftery AE. 2002. Model-based clustering, discriminant analysis and density estimation. J Am Stat Assoc. 97:611–631. doi: 10.1198/016214502760047131
- Fraley C, Raftery AE, Murphy TB, Scrucca L. 2012. Mclust version 4 for R: Normal mixture modeling for model-based clustering, classification, and density estimation. Technical Report no. 597, Department of Statistics, University of Washington.
- Grau J, Leins P. 1968. Pollenkorntypen und Sektionegliederung der Gattung Myosotis. Berichte der Deutschen Botanischen Gesellschaft. 81:107–115.
- Grau J, Schwab A. 1982. Mikromerkmale der Blüte zur Gliederung der Gattung Myosotis. Mitteilungen der Botanischen Staatssammlung München. 18:9–58.
- Hargrove L, Simpson MG. 2003. Ultrastructure of heterocolpate pollen in Cryptantha (Boraginaceae). Int J Plant Sci. 164:137–151. doi: 10.1086/344548
- Hesse M, Halbritter H, Zetter R, et al. 2009. Pollen terminology: an illustrated handbook. Vienna, Austria: Springer.
- Koutecky P. 2015. MorphTools: a set of R functions for morphometric analysis. Plant Syst Evol. 301:1115–1121. doi: 10.1007/s00606-014-1153-2
- Lehnebach C. 2012. Two new species of forget-me-nots (Myosotis, Boraginaceae) from New Zealand. Phytokeys. 16:53–64. doi: 10.3897/phytokeys.16.3602
- Luebert F, Cecchi L, Frohlich MW, Gottschling M, Guilliams CM, Hasenstab-Lehman KE, Hilger HH, Miller JS, Mittelbach M, Nazaire M, et al. 2016. Familial classification of the Boraginales. Taxon. 65:502–522. doi: 10.12705/653.5
- Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K. 2015. Cluster: cluster analysis basics and extensions. R package version 2.0.1.
- Meudt HM, Prebble JM, Lehnebach CL. 2015. Native New Zealand forget-me-nots (Myosotis, Boraginaceae) comprise a Pleistocene species radiation with very low genetic divergence. Plant Syst Evol. 301:1455–1471. doi: 10.1007/s00606-014-1166-x
- Moar NT. 1993. Pollen grains of New Zealand Dicotyledonous Plants. Lincoln, New Zealand: Maanaki Whenua Press. 200 pp.
- Moar NT, Wilmshurst JM, McGlone MS. 2011. Standardizing names applied to pollen and spores in New Zealand Quaternary palynology. N Z J Bot. 49:201–229. doi: 10.1080/0028825X.2010.526617
- Moore LB. 1961. Boraginaceae. In: Allan H, editor. Flora of New Zealand. Vol. 1. Wellington, New Zealand: PD Hasselberg, Government Printer; p. 806–833.
- Nikiforova OD. 2002. Palynomorphological study of the genus Myosotis and some related genera (Boraginaceae). Botanicheskii Zhurnal. 87:44–53.
- Oksanen J, Blanchet FG, Kindt R, et al. 2015. vegan: Community Ecology Package. R package version 2.3–0. Available from: http://CRAN.R-project.org/package=vegan
- Prebble JM, Tate JA, Meudt HM, Symonds VV. 2015. Microsatellite markers for the New Zealand endemic Myosotis pygmaea species group (Boraginaceae) amplify across species. Appl Plant Sci. 3(6):1500027. doi:10.3732/apps.1500027.
- Rasband, WS. 1997–2016. ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/.
- RCore Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: http://www.R-project.org/
- Reitsma TJ. 1969. Size modification of recent pollen grains under different treatments. Rev Palaeobot Palyno. 9:175–202. doi: 10.1016/0034-6667(69)90003-7
- Robertson A. 1989. Evolution and pollination of New Zealand Myosotis (Boraginaceae) [Unpublished PhD thesis]. Christchurch, New Zealand: University of Canterbury. 160 pp.
- Robertson AW, Lloyd DG. 1991. Herkogamy, dichogamy and self-pollination in six species of Myosotis (Boraginaceae). Evol Trend Plant. 5:53–63.
- RStudio Team. 2015. RStudio: Integrated Development for R. RStudio, Inc., Boston, MA URL Available from: http://www.rstudio.com/
- Thiers B. [continuously updated]. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. Available from: http://sweetgum.nybg.org/science/ih/
- Volkova OA, Severova EE, Polevova SV. 2013. Structural basis of harmomegathy: evidence from Boraginaceae pollen. Plant Syst Evol. 299:1769–1779. doi: 10.1007/s00606-013-0832-8
- Weigend M, Luebert F, Selvi F, Brokamp G, Hilger HH. 2013. Multiple origins for hound’s tongues (Cynoglossum L.) and navel seeds (Omphalodes Mill.)–the phylogeny of the borage family (Boraginaceae s. str.). Mol Phylogenet Evol. 68:604–618. doi: 10.1016/j.ympev.2013.04.009
- Weigend M, Selvi F, Thomas DC, Hilger HH. 2016. Boraginaceae. In: Kadereit JW, Bittrich V, editors. Flowering plants. Eudicots. The families and genera of vascular plants. Vol. 14. Cham, Switzerland: Springer International Publishing; p. 41–102.
- Winkworth RC, Grau J, Robertson AW, Lockhart PJ. 2002. The origins and evolution of the genus Myosotis L.(Boraginaceae). Mol Phylogenet Evol. 24:180–193. doi: 10.1016/S1055-7903(02)00210-5
- Wrońska-Pilarek D, Jagodziński AM, Bocianowski J, Janyszek M. 2015. The optimal sample size in pollen morphological studies using the example of Rosa canina L. (Rosaceae). Palynology. 39:56–75. doi: 10.1080/01916122.2014.933748
