ABSTRACT
Synechococcus sp. PCC 7002 is a euryhaline cyanobacterium that serves as a model organism for studies of photosynthesis and cellular metabolism. At high light intensity Synechococcus sp. PCC 7002 exhibits a fast growth rate and rapid biomass accumulation, making it an ideal candidate for the production of biofuels and commercially valuable secondary metabolites. To fully realise the production potential for this cyanobacterium a system is required to maintain axenic cultures under optimal growth conditions. We have designed and constructed two sizes of modular culture vessel systems, designed specifically to meet the abnormally high light penetration requirements of Synechococcus sp. PCC 7002. The smaller volume culture vessels are best suited to phenotype analysis, allowing multiple candidate strains to be screened simultaneously, whereas the larger vessels are designed to facilitate higher biomass production.
Introduction
Many strains of cyanobacteria are cultured in laboratory settings, each possessing their own specific growth requirements, including nutrient, temperature and light conditions. Provided that no other resource is limiting, Synechococcus sp. PCC 7002 (hereafter Synechococcus 7002) can grow at much higher light intensities than is common for most cyanobacteria, resulting in rapid growth and biomass production (Van Baalen Citation1962; Lambert & Stevens Citation1986; Nomura et al. Citation2006). This unique tolerance to light and other characteristics, such as its highly efficient natural genetic transformability, make Synechococcus 7002 an ideal candidate to engineer for the photoautotrophic production of biofuels or secondary metabolites (Stevens & Porter Citation1980; Ducat et al. Citation2011; Vu et al. Citation2013). However, to fulfil the production potential of this cyanobacterium, cultures must be grown in vessels that maximise light exposure relative to volume. In this article, we present two tubular vessels that have been designed to grow Synechococcus 7002 cultures under optimal lighting conditions in a laboratory setting. The two vessels differ primarily in volume. The first is a low culture volume vessel for use in phenotypic screening and characterisation of different growth conditions or mutant strains, and the second is a larger volume vessel for scale-up production or preparative isolation of biomass. Both sizes of vessel are modular and have been designed to allow sterilisation by steam autoclaving. In addition, we present a syringe-based method to facilitate withdrawal of culture samples whilst maintaining the aseptic integrity of the culture. The primary advantages of these vessels are their comparatively low construction cost and that their modular designs provide flexibility and ease of expansion in capacity.
Materials and methods
Cyanobacteria strains and physiology measurements
The Synechococcus 7002 strain referred to throughout as wild type is a sub-cultivar of Donald Bryant group’s strain (Penn State University, PA, USA) (Frigaard et al. Citation2004), obtained from Fiona Davies (Colorado School of Mines, CO, USA). The growth media used was based on modified A+ media (Stevens et al. Citation1973; Stevens & Porter Citation1980) with the following alterations to nutrient concentrations: 10 mM Tris-HCl pH 8.2, 2.5 µM ZnCl2, 0.2 µM MoO3, 0.01 µM CuSO4, 0.04 µM CoCl2 and 8 µg/mL vitamin B12. Strains were maintained on plate or in liquid culture, both at 37°C. Measurements of the optical density at 730 nm (OD730) were made using an Evolution 201 UV/Vis spectrophotometer (Thermo Scientific), and samples were diluted using A+ media to ensure a reading below 0.4 was obtained. The concentration of chlorophyll a was determined by the absorption at 663 nm of cell extracts in 90% methanol (MacKinney Citation1941). Room temperature fluorescence measurements, used to determine the variable chlorophyll a fluorescence, were made using a FL-3300 fluorometer (PSI Instruments) equipped with blue light emitting diodes (455 nm) that were used for the actinic and measuring flashes. Samples were diluted using A+ media to an OD730 of less than 2.0, dark adapted for 5 min and then measured using a fluorescence relaxation protocol as described in Jackson et al. (Citation2014). For the measurements of dry cell mass (DCM), 10 mL of culture were harvested by centrifugation, washed twice with water and transferred to a pre-weighed microcentrifuge tube. The cells were pelleted again, dried in a centrifugal speed vacuum unit (SC110, Savant) and the tube plus cell pellet weighed; the weight was recorded with an accuracy of 0.1 mg.
Small volume culture modules
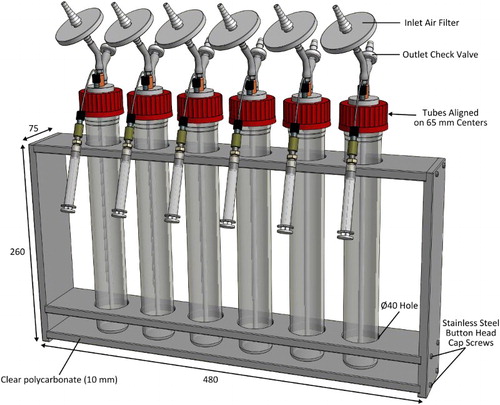
The small volume culture system is based on a vertical airlift setup as described in . Each unit consists of a vessel capped with a custom designed and constructed tubing interface that contains ports for aeration and sample removal (A). The vessels used in this instance are glass tubes, commonly referred to as roller or hybridisation bottles, which are commercially available or can be produced by a glassblower. Each has an internal diameter (ID) of 35 mm and outer diameter (OD) of 40 mm, with a GL45-type thread at the open end. The length specified herein is 300 mm, although tubes of varying dimensions can be utilised, depending on volume requirements. A GL45 aperture cap (Schott) made of poly(1,1,2,2-tetrafluorobutane-1,4-diyl) (ETFE) is used to secure the tubing interface to the top of the culture vessel. The tubing interface consists of a piece of machined poly(1,1,2,2-tetrafluoroethylene) (PTFE) rod with a press-fitted polyetheretherketone (PEEK) insert (B). Threaded ports (1/4–28 unified national fine [UNF], flat bottomed) are used for connection of fittings (IDEX Corporation and Value Plastics, Nordson Medical) to attach tubing (Cole-Parmer) as required.
Figure 1. Small volume culture vessels. A, Basic overview of the system without additional fittings, including a cross section view showing the aeration dip tube attachment; B, upper: the custom designed adaptor consisting of an insert press-fit into a base; lower: side view of the adaptor with dimensions annotated; C, a singular small volume growth vessel complete with fittings, including the sample withdrawal assembly and syringe. All dimensions given are in mm unless otherwise noted. The 1/4–28 specified refers to UNF thread type. The 1/16″ (OD) tubing is made of fluorinated ethylene propylene (FEP) with an ID of 1/32″. The 1/4″ (OD) silicone tubing (96400–25) was obtained from Cole-Parmer.
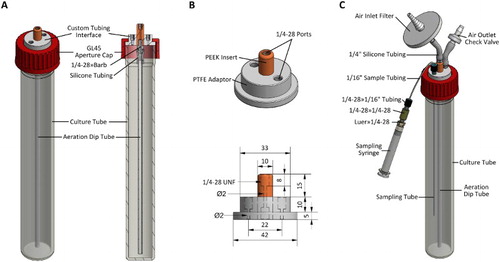
Each complete unit holds a nominal working volume of c. 200 mL (allowing for c. 6 cm headspace), and when fitted with accessories such as an inlet air filter (Millex-FG, 50 mm, 0.2 µm, PTFE vent filter, Millipore), glass aeration dip tube (6 mm OD, 2 mm ID, 275 mm long) and outlet check-valve (Value Plastics, Nordson Medical), is essentially a standalone culture module (C). A key feature of the setup is the addition of a tube through which samples can be withdrawn from the culture using a luer-lock syringe, negating the need to remove the lid. This enables withdrawal of samples in situ without compromising the sterile environment inside the growth vessel. Typically, a 3 mL syringe (Becton Dickinson) is used, and it is important to purge the tube by withdrawing and discarding a small aliquot immediately prior to taking a sample for assay. Multiple small volume growth vessel units can be mounted in a custom-made rack as shown in . The rack is constructed of 10 mm thick clear polycarbonate sheet and stainless steel button head cap screws and can be autoclaved with the growth vessels in place.
Large volume culture modules
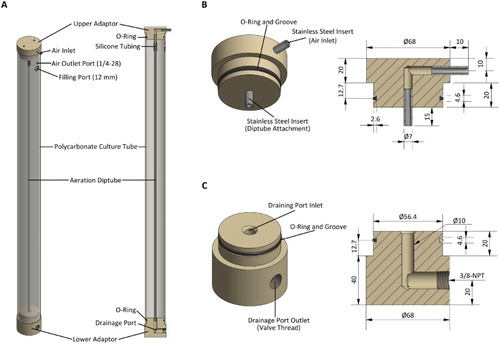
The large volume growth vessel system is also a vertical airlift setup but uses polycarbonate, instead of glass, tubes (A). The tubes (unspecified manufacturer) supplied by Amazon, USA have a nominal ID of 57.2 mm, OD of 63.5 mm and length of 914.4 mm. Each tube is drilled with access ports and fitted with custom designed and constructed end pieces. The end pieces serve to seal the tubes and facilitate connection of additional componentry for filling, draining and aeration (B–C). These end pieces are machined from stock polyoxymethylene (POM) (acetal) rod and contain O-ring (ethylene propylene diene monomer (EPDM), 3.54 mm × 50.4 mm/57.48 mm) grooves to provide a static seal against the inside of the polycarbonate tube: POM was used because of material availability restrictions; ideally PEEK, PTFE or a similar biocompatible thermoplastic would be used. Stainless steel inserts are press-fitted into the upper end piece, allowing attachment of tubing necessary for culture aeration, and the lower end piece contains a threaded drainage port for harvesting cultures.
Figure 3. Construction of the large volume growth vessels. A, Each growth vessel consists of a polycarbonate tube with two end-piece adaptors held in place by the friction of O-rings; B, upper end-piece adaptor has press-fitted stainless steel inserts for the aeration system; C, lower end piece contains a right angle drainage port. All dimensions given are in mm unless otherwise noted.
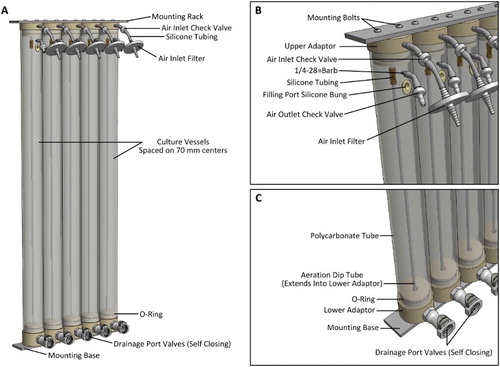
Each complete large volume growth vessel unit is 1010 mm long, has a nominal working volume of 2 L and is essentially a standalone culture vessel. Five of these units are mounted in a modular rack system, providing a total volume of 10 L (A). The upper and lower end pieces are bolted to the aluminium alloy rack using button head cap screws (B). Inlet air filters (Millex-FG, 50 mm, 0.2 µm, PTFE vent filter, Millipore) and outlet check-valves (Value Plastics, Nordson Medical) are added, (to maintain sterility, yet still facilitate aeration venting), as well as silicone bungs (SubaSeal, ACE Glass Inc.) to plug the filling ports when not in use. The addition of a 3/8-national pipe thread (NPT) threaded quick-connect type fitting (Colder Products Co.) to the lower end piece allows for harvesting of the culture (C). These drainage port valves are self-closing; flow is enabled upon connection of a mating fitting, so no additional valves are required.
Figure 4. The large scale growth system. A, Five tube modules mounted to aluminium alloy racks; B, close-up of the upper adaptor and fittings; C, close-up of the lower adaptor and fittings. The aeration tubes (6 mm OD, 2 mm ID, 850 mm long) extend partially into the opening of the vertical section of the drainage port to prevent cell sedimentation in this area.
Growth conditions
Purpose built climate-controlled growth rooms or cabinets are ideal for laboratory scale culturing of cyanobacteria. For the smaller volume growth system, we used the tube rack described in housed inside a modified commercial growth cabinet (Model 3744, Thermo Electron Corp.). Illumination was provided by an array of six horizontal fluorescent tubes (865 Phosphor 15W T8; NARVA) at a distance of 75 mm from the front plane of the culture vessels. The incident illumination at the front of the vessels was 300 µmol photons m−2 s−1 (determined using a LI-189 metre with a Quantum probe; LI-COR Biosciences). A fan was used to improve airflow within the cabinet and was necessary to ensure the temperature of the culture vessels remained in equilibrium with the set temperature (37°C) of the chamber. Aeration of the cultures was provided by an aquarium pump running through a pre-humidifier (150 mm water-height equivalent), into a distribution valve block and then into the culture vessels. The concentration of carbon dioxide inside the growth chamber was maintained at 1% using a carbon dioxide control module (Portamatic CO2 controller, Thermo Electron Corp.).
The large volume growth vessel array was housed inside a commercial growth cabinet (MLR-351, Sanyo Electric Co., Ltd.) set at 37°C. Illumination was provided by a vertical array of five fluorescent tubes (865 Phosphor 36W T8; Radium Lampenwerk GmbH), spaced at 70 mm centres, 100 mm from the front plane of the tube module, resulting in a light intensity of 250 µmol photons m−2 s−1. For aeration of these growth vessels we combined air from an aquarium pump with carbon dioxide from a gas cylinder, each run through adjustable gas flow metres (Cole-Parmer), allowing continuous mixing. The combined gas stream (0.1% v/v CO2) was run through a humidifier and distribution valve arrangement, as described for the small tube setup.
Cleaning and sterilisation
For the small growth vessels complete disinfection, disassembly and cleaning of the units is expediently accomplished by unscrewing or removing all components, then washing by means ordinarily applied to laboratory glassware. All the plastics used are fully compatible with most common cleaning products, including bleach, ethanol and Virkon (Du Pont). Disassembly and reassembly of the large tube modules is more laborious, so these are usually cleaned whilst mostly assembled; we do occasionally disassemble the large tubes for a more thorough cleaning, as per the small tubes, but with the exception that bleach cannot be used. For decontamination without complete disassembly the air inlet filters, sampling port components and filling port bung are first removed. Next, the tubes are filled with a Virkon (Du Pont) solution, inverted briefly to ensure all internal surfaces are wetted, and left to soak for several hours. The decontamination solution is then drained and the tubes are rinsed several times with water (it is important to ensure liquid flow through the aeration tube and out of the air inlet is achieved to fully rinse away the disinfectant solution). Use of a high-flow peristaltic pump is recommended to accelerate the filling and draining procedures. Our laboratory uses a Masterflex I/P pump module (Cole-Parmer), which also forms an integral part of a tangential flow filtration system for harvesting cyanobacteria from large volume cultures. The entire modular growth vessel rack can be sterilised using a steam autoclave (121°C, 15 min) and fits inside a standard model laminar flow hood (Clyde-Apac, Clean Air HWS Series, AES Environmental); the working area of which is 1180 mm wide, 580 mm high and 480 mm deep.
Results
Growth characteristics in the small vessels
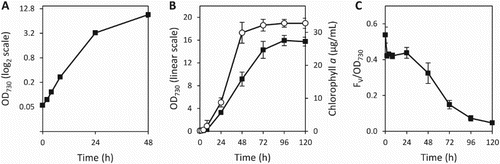
The small volume growth vessels were inoculated with Synechococcus 7002 at an OD730 of 0.05 and monitored for 120 h (). The cells initially displayed logarithmic growth (0–24 h) with an apparent doubling time of 4.1 ± 0.2 h (A); the OD730 value can be influenced by multiple factors including cell size, so provides a proxy for overall growth but does not necessarily correlate with cell number. As the OD730 increased growth became linear (24–72 h) before plateauing as the cultures entered stationary phase (72–120 h) (B). During the first 48 h the chlorophyll a content of the culture increased rapidly, reaching c. 30 µg mL−1, but after this point plateaued and no further significant increases were observed (B). Photosynthetic activity of the culture was assessed by measuring the variable fluorescence (FV; equal to the maximal fluorescence following a single turnover actinic flash [FM] minus the dark-adapted background fluorescence [FO]) over the time course (C). The FV/OD730 was highest at the beginning of the experiment and during the logarithmic phase of growth and exhibited a sigmoidal decline as cells transitioned from linear growth to stationary phase.
Figure 5. Growth characteristics of Synechococcus 7002 in the small vessels. A, Photoautotrophic growth, measured by the OD730, over the first 48 h displayed on a logarithmic (base 2) scale; B, extended growth out to 120 h displayed on a linear scale, showing the OD730 (▪) and chlorophyll a concentration (○); C, variable chlorophyll a fluorescence (FV) (normalised to OD730) over the 120 h time course. Data presented are the mean of three independent experiments, and the error bars represent the standard deviation.
Growth characteristics in the large vessels
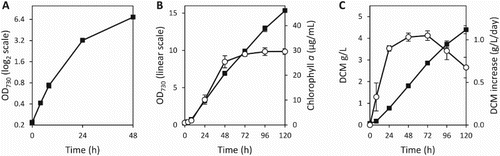
To examine the growth characteristics of Synechococcus 7002 in the large volume system we inoculated these vessels at an OD730 of 0.2. Exponential growth was observed for the first 8 h with a doubling time of 4.6 ± 0.1 h, after which time linear growth ensued for the remainder of the experiment (A–B). The chlorophyll a concentration correlated with cell growth up to 48 h, at which point the rate of increase slowed significantly and only a minor increase was observed out to the 120 h time point (B). For the large tubes we determined biomass accumulation by measuring the DCM at each time point, and subsequently calculated the gain in DCM for each 24 h period (C). Over the course of the experiment DCM correlated well with the OD730, and at 120 h the DCM of the cultures had reached 4.40 ± 0.19 g L−1. The rate of biomass accumulation increased rapidly during the exponential growth phase and up to the 24 h time point, where the rate of accumulation corresponded to 0.90 ± 0.03 g L−1 day−1. From 24 h onwards biomass accumulation gradually increased out to the 72 h time point, peaking at 1.05 ± 0.06 g L−1 day−1, before declining between 72 h and 120 h.
Figure 6. Growth characteristics of Synechococcus 7002 in the large vessels. A, Photoautotrophic growth, measured by the OD730, over the first 48 h displayed on a logarithmic (base 2) scale; B, extended growth out to 120 h displayed on a linear scale, showing the OD730 (▪) and chlorophyll a concentration (○); C, DCM, normalised to the volume of culture (▪), and rate of DCM increase between measurements, normalised to a 24 h period (○). Data presented are the mean of three independent experiments, and the error bars represent the standard deviation.
Discussion
A standardised design for growth of Synechococcus 7002
We have demonstrated that both sizes of modular growth vessels detailed herein are well suited to the photoautotrophic growth of Synechococcus 7002. The smaller vessels were able to support the logarithmic growth of low inoculum cultures for up to 24 h, resulting in an increase in OD730 from 0.05 to greater than 3 over this period. The growth rate observed is comparable to maximum reported values obtained using this growth medium, but greater rates have been observed by providing alternative nitrogen sources (Nomura et al. Citation2006; Ludwig & Bryant Citation2012a; Ludwig & Bryant Citation2012b). These vessels are best suited to phenotypic characterisation of different strains or experimental variables. We have utilised the setup described herein for a detailed study of photosynthetic activity in both wild-type Synechococcus 7002 and a mutant strain that secretes elevated levels of organic acids (Jackson et al. Citation2015). The nominal working volume of the vessels (200 mL) should generally be sufficient for most physiology experiments, but longer glass tubes can be used to increase the volume; each centimetre of vessel length corresponds to approximately 9.6 mL of volume capacity. Standardisation of growth conditions is an important consideration for comparisons of physiological data, particularly the surface to volume ratio of growth vessels which affects the average light penetration. The design we have presented is intended to be easily replicated, and it is hoped that it will be straightforward to commission machining of the tubing adaptors necessary to construct the growth vessels from either an in-house workshop or a commercial entity.
For the large growth vessels, exponential growth from a starting OD730 of 0.2 was supported for the first 8 h. The early transition to a linear growth phase (compared to the small tubes where the exponential growth was maintained for 24 h) is not surprising given that we began the growth experiments in the large tubes with higher starting inoculums—to mimic a commercial scale-up seeding process. Furthermore, the larger growth modules are a compromise on the light penetration to volume ratio, designed to facilitate higher total biomass production. If more optimal light saturation is required then the light intensity could be increased, or the culture vessels illuminated from both sides. Light emitting diodes would be an attractive alternative light source, enabling higher photon flux densities and more spectral options than fluorescent tubes.
The autoclave compatibility of the vessels, combined with proper aseptic technique, will allow growth and maintenance of axenic cyanobacterial cultures (or algae), either photoautotrophically, mixotrophically or heterotrophically by the addition of suitable nutrient sources. It is also possible to grow up to five separate strains simultaneously rather than 10 L of a single strain, thereby facilitating screening and phenotype analysis of candidate strains on a mid-scale production basis. The modular nature of both size systems means more growth vessels can easily be added to expand capacity, enable growth of more strains or test a wider range of growth conditions simultaneously.
Observations from extended use in our laboratory
Both sizes of growth vessel described in this report have been used extensively by our research group for the cultivation of cyanobacteria. The sample withdrawal syringe system on the smaller tube modules has proved particularly convenient, and adaption of the same system to the large scale vessels could easily be accomplished through the addition of another 1/4–28 UNF port. The sampling system would also be useful in instances where cells are to be grown under anaerobic conditions; addition of a non-return valve on the sampling tube would ensure no ingress of outside atmosphere. For cultivation of Synechococcus 7002 we have noted different growth characteristics and photosynthetic activity for cells grown using fluorescent tubes with different phosphor coatings, even though two products we acquired were marketed as ‘cool white, 6500 K’; we, therefore, advise meticulous care in the selection of fluorescent tubes (data not shown). In addition, we have found application of the large tubes in physiological studies of Photosystem II assembly in the cyanobacterium Synechocystis sp. PCC 6803. For this we employ a lower light intensity than used for growth of Synechococcus 7002, and have found these growth vessels to avoid complications relating to light shading and decreases in the Photosystem II to Photosystem I ratio that occur when growing Synechocystis sp. PCC 6803 cultures in large volume glass carboys.
Acknowledgements
We thank Gary Shriffer (University of Otago, New Zealand) for assistance with mechanical aspects of this project, and Dr Fiona Davies (Colorado School of Mines, USA) for the gift of the Synechococcus sp. PCC 7002 wild-type cultivar.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ducat DC, Way JC, Silver PA. 2011. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 29:95–103. doi: 10.1016/j.tibtech.2010.12.003
- Frigaard NU, Sakuragi Y, Bryant DA. 2004. Gene inactivation in the cyanobacterium Synechococcus sp. PCC 7002 and the green sulfur bacterium Chlorobium tepidum using in vitro-made DNA constructs and natural transformation. Methods Mol Biol. 274:325–340.
- Jackson SA, Eaton-Rye JJ, Bryant DA, Posewitz MC, Davies FK. 2015. Dynamics of photosynthesis in a glycogen-deficient glgC mutant of Synechococcus sp. strain PCC 7002. Appl Environ Microbiol. 81:6210–6222. doi: 10.1128/AEM.01751-15
- Jackson SA, Hervey JR, Dale AJ, Eaton-Rye JJ. 2014. Removal of both Ycf48 and Psb27 in Synechocystis sp. PCC 6803 disrupts Photosystem II assembly and alters QA− oxidation in the mature complex. FEBS Lett. 588:3751–3760. doi: 10.1016/j.febslet.2014.08.024
- Lambert DH, Stevens SE, Jr. 1986. Photoheterotrophic growth of Agmenellum quadruplicatum PR-6. J Bacteriol. 165:654–656. doi: 10.1128/jb.165.2.654-656.1986
- Ludwig M, Bryant DA. 2012a. Acclimation of the global transcriptome of the cyanobacterium Synechococcus sp. strain PCC 7002 to nutrient limitations and different nitrogen sources. Front Microbiol. 3:145. doi:10.3389/fmicb.2012.00145.
- Ludwig M, Bryant DA. 2012b. Synechococcus sp. strain PCC 7002 transcriptome: acclimation to temperature, salinity, oxidative stress, and mixotrophic growth conditions. Front Microbiol. 3:354. doi:10.3389/fmicb.2012.00354.
- MacKinney G. 1941. Absortion of light by chlorophyll solutions. J Biol Chem. 140:315–322.
- Nomura CT, Sakamoto T, Bryant DA. 2006. Roles for heme-copper oxidases in extreme high-light and oxidative stress response in the cyanobacterium Synechococcus sp. PCC 7002. Arch Microbiol. 185:471–479. doi: 10.1007/s00203-006-0107-7
- Stevens SE, Patterson COP, Myers J. 1973. The production of hydrogen peroxide by blue-green algae: a survey. J Phycol. 9:427–430.
- Stevens SE, Porter RD. 1980. Transformation in Agmenellum quadruplicatum. Proc Natl Acad Sci USA. 77:6052–6056. doi: 10.1073/pnas.77.10.6052
- Van Baalen C. 1962. Studies on marine blue-green algae. Bot Mar. 4:129–139. doi: 10.1515/botm.1962.4.1-2.129
- Vu TT, Hill EA, Kucek LA, Konopka AE, Beliaev AS, Reed JL. 2013. Computational evaluation of Synechococcus sp. PCC 7002 metabolism for chemical production. Biotechnol J. 8:619–630. doi: 10.1002/biot.201200315