ABSTRACT
The Asteraceae have a high diversity of reproductive mechanisms, as shown by studies conducted mainly with species from temperate regions. Aiming to contribute knowledge on the reproduction of tropical species, we compared the floral biology and breeding systems of the co-occurring species Adenostemma brasilianum, Bidens segetum and Grazielia intermedia, and additionally identified their pollinators. The study was conducted in a seasonal semideciduous forest fragment in southeastern Brazil. Florets opened in early morning and were protandrous. In the first day of anthesis, secondary pollen presentation occurred during the staminate phase, mainly in the morning. In the same day, the pistillate phase began, being long in A. brasilianum and B. segetum (up to 4 days) and short in G. intermedia (1 day). Pollen viability was high in all three species, except for two G. intermedia individuals which showed male sterility, thus characterising a gynodioecious population. Self-compatibility was observed in A. brasilianum and self-incompatibility in B. segetum. In G. intermedia, besides sexual reproduction, apomixis was also observed. This is the first report of apomixis in the genus Grazielia. Although the studied species show distinct reproductive mechanisms, all of them benefitted from cross-pollination, which was mostly done by butterflies.
Introduction
The Asteraceae have a cosmopolitan distribution, high species richness (c. 24,000; Funk et al. Citation2009) and high diversity of reproductive mechanisms (Werpachowski et al. Citation2004; Ferrer & Good-Avila Citation2007; Noyes Citation2007; Jeffrey Citation2009), as shown by studies conducted mainly with species from temperate regions. In Brazil, 2065 species have been reported (Nakajima et al. Citation2015), most of which are from non-forested vegetation (Funk et al. Citation2009). Only c. 1.5% of these species has available data on aspects of their reproductive biology (Fonseca et al. Citation2013). Although little investigated, the diversity of reproductive mechanisms is also high (details in Fonseca et al. Citation2013), often including novel information on flower morphology and biology (Bessa et al. Citation2010).
The study species Adenostemma brasilianum (Pers.) Cass., Bidens segetum Mart ex Colla, and Grazielia intermedia (DC.) R.M. King & H. Rob. inhabit an area of seasonal semideciduous forest, which occurs in the Atlantic Forest domain (Oliveira-Filho & Fontes Citation2000), in Viçosa municipality, southeastern Brazil. Apart from studies on seed quality and germination of A. brasilianum and its life history (Godinho et al. Citation2011; Valentin-Silva et al. Citation2016), no study addressing reproductive aspects of the other species analysed herein can be found in the literature.
All three species have a wide distribution throughout the Brazilian territory (Hattori Citation2015; Hattori & Almeida Citation2015; Mondin et al. Citation2015), but in the study area they are restricted to forest fragments (the largest one with 168.7 ha; Silva et al. Citation2014) and do not occur in open areas. Matrices surrounding the fragment are mainly agricultural in origin: pastures; monocultures; and rural constructions (Silva et al. Citation2014). Despite severe fragmentation, these asteraceans interact with 108 species of anthophilous butterflies (Cruz et al. Citation2012).
Although potentially psychophilous, it is unknown if these three asteraceans are dependent on pollinators for fruiting. In order to clarify the foregoing, we aimed to compare their floral biology and breeding systems. Additionally, we identified their capitulum visitors, highlighting the pollinators. We sought answers to the following questions: 1. Are there differences between the species related to their floral biology and breeding systems? 2. Do the different breeding systems result in differential reproductive success (production of seeds)? 3. Does fruit set depend on pollinators? 4. What are the main pollinators?
Material and methods
Study area and species
The study was conducted from March 2005 through May 2008 in a forest fragment at the Station of Research, Environmental Training and Education Mata do Paraíso (hereafter referred to as Mata do Paraíso). Mata do Paraíso is located at Viçosa municipality (20°48′07″ S, 42°51′31″ W), Minas Gerais state, southeastern Brazil, with altitudes ranging between 690 and 870 m, its vegetation being classified as seasonal semideciduous montane forest (Veloso et al. Citation1991). The climate in Viçosa is type Cwa (mesothermal with hot rainy summers and cold dry winters) according to Köppen’s classification (Alvares et al. Citation2013).
Adenostemma brasilianum (Eupatorieae) is a biannual herb (Valentin-Silva et al. Citation2016) 0.6–1.2 m in height; capitula are homogamous, discoid, with 30–35 hermaphrodite florets with white corollas. In the study area, A. brasilianum, a typical species of the forest interior (King & Robinson Citation1987), occurs in shady and moist places. Its flowering began in December and its anthesis occurred between January and June (flowering peak in February); fruiting began in February and lasted until July (Valentin-Silva et al. Citation2016). We used 25 individuals in this study.
Bidens segetum (Coreopsideae) is a decumbent subshrub c. 3 m in height; capitula are heterogamous, radiate, with 5–9 sterile ray florets and 12–44 hermaphrodite disk florets, both with yellow corollas. In the study area, B. segetum occurs in sunny places. Its flowering began in March and its anthesis occurred between March and May (flowering peak in April); fruiting began in April and lasted until September (Lelis Citation2008). We used 17 individuals in this study.
Grazielia intermedia (= Eupatorium intermedium DC., Eupatorieae) is an erect subshrub c. 2.5 m in height; capitula are homogamous, discoid, with five florets with white or lilac corollas. In the study area, G. intermedia occurs in sunny places. Its flowering began in January and its anthesis occurred in February and March (flowering peak in March); fruiting also began in February and lasted until April (Cruz Citation2009). We used 25 individuals in this study.
Voucher specimens of the studied species were deposited in the VIC Herbarium (numbers 21,530, 21,725 and 29,191). Photographs of the study species can be found in Cruz et al. (Citation2012).
Floral biology
Capitula with flower buds were tagged in the aforementioned individuals (70 capitula in A. brasilianum, 54 in B. segetum and 30 in G. intermedia) and observed on a daily basis. We registered floret longevity, time of anthesis and of secondary pollen presentation (SPP), and duration of pistillate and staminate phases in the florets.
Pollen grain viability was tested with acetic carmine (Radford et al. Citation1974) using 25 flower buds in pre-anthesis in five individuals per species. In G. intermedia, no pollen could be found in flower buds from two individuals. In order to confirm absence of pollen, we analysed 30 more flower buds from these individuals.
Stigmatic receptivity was tested with a 10% hydrogen peroxide solution (Dafni et al. Citation2005) in flowers at different stages. To verify presence of glucose in the floral secretion collected by visitors, a glucose enzyme test band (Alamar Tecno Científica Ltda) was used, as glucose is one of the main sugars found in nectar (Baker & Baker Citation1983).
Breeding system
Breeding tests (Sage et al. Citation2005) were performed with all species, taking the capitulum as the pollination unit, for: spontaneous self-pollination (protocol 2.5); hand self-pollination (protocol 2.6); apomixis (protocol 2.4); cross-pollination (protocol 2.10, step 2); and open pollination (control; protocol 2.6). For hand self-pollination and cross-pollination, florets were not emasculated and were pollinated with additional geitonogamous and xenogamous pollen, respectively. For the apomixis test, flower buds were ‘emasculated’ (i.e. the corolla, androecium and style were removed with disposable razor blades); cuts were made immediately above the inferior ovary (adapted from Richards Citation1997). In all tests, except open pollination, capitula were maintained bagged until fruiting. In one G. intermedia individual with male sterility, additional tests were performed for apomixis.
Fruit set was calculated in all breeding tests from the number of florets per capitulum (disk florets only, in B. segetum) and the number of fruits produced (each fruit having a seed with embryo involved by carbonised pericarp = reproductive success sensu Brunet Citation2005). These data were subjected to ANOVA and means were compared by Tukey’s test at 5% probability.
Capitulum visitors
We observed capitulum visitors during two consecutive flowering seasons per species, from 0700 h to 1700 h. Identification was made with the aid of specialists and voucher specimens were deposited in the collection of the Regional Museum of Entomology from the Department of Animal Biology of Federal University of Viçosa. Capitulum visitors were classified according to visiting behaviour and presence of pollen on their bodies into pollinators and thieves sensu Inouye (Citation1980). These analyses were conducted in parallel with the survey of anthophilous butterflies published in Cruz et al. (Citation2012).
Results
Floral biology
In all three species, florets (disk florets in B. segetum) opened in the early morning and were protandrous. The beginning of the staminate phase was characterised by anther dehiscence in the flower bud in pre-anthesis, at around 0600 h. At that time, SPP occurred (i.e. pollen was deposited on the sterile portion of the style branches, located above the stigmatic lines). Later on, the opening of the corolla laciniae occurred ().
Figure 1. A, Anthesis of Adenostemma brasilianum; B, Bidens segetum; C, Grazielia intermedia florets. an, anther; co, corolla; fi, filament; ne, nectary; pa, pappus; sb, style branch; sl, stigmatic line.
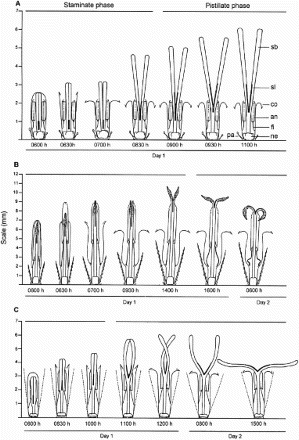
In A. brasilianum, exposure of the sterile portion of style branches above the corolla tube also resulted in pollen grain presentation for c. 2 h (from 0630 h to 0830 h; A). During this period, the anthers and juxtaposed stigmatic lines remained inside the corolla tube and the style and style branches elongated (). The pistillate phase occurred on the same morning of the staminate phase (starting at 0900 h; A) and was characterised by the exposure of four receptive stigmatic lines (positive result in the hydrogen peroxide test). At that time, there may be residual pollen on the sterile portion of branches. Florets remained in this stage for 2 more days.
In the disk florets of B. segetum, filaments and style elongated, which resulted in the partial exposure of anthers above the corolla. Because of this, pollen grains were protected by the connivent connectives of anthers, which form a cone above the juxtaposed style branches (B). Pollen exposure started at 0930 h. At 1400 h, the sterile portion of style branches, which were separated, was totally exposed. Stigmatic lines remained juxtaposed. The staminate phase ended at 1600 h and the sterile portion of style branches, which were more distant and in a horizontal position, could have residual pollen. At that time, there was retraction of filaments and the consequent partial retraction of anthers into the corolla tube (B). The pistillate phase started at 1600 h, on the same day as the staminate phase. On the next day, the receptive stigmatic lines were totally exposed and there may still be residual pollen on the sterile portion of branches. Florets remained on this phase for 2 more days (disk florets; ray florets lasted, on average, 10 days).
In G. intermedia, the exposure of the sterile portion of style branches above the corolla tube also resulted in the presentation of pollen grains for c. 2 h (from 0830 h to 1000 h; C). During this period, the anthers and juxtaposed stigmatic lines remained inside the corolla tube and there was elongation of the style and style branches (C). The beginning of the pistillate phase occurred in the same morning as the staminate phase (starting at 1100 h; C) and was characterised by exposure of the four receptive stigmatic lines. At 1200 h, the crossed style branches were totally exposed above the corolla tube (C). At that time there may be residual pollen in the sterile portion of the branches. On the next day (at 0800 h), the branches, erect and parallel to one another (C), fully exposed the stigmatic lines. At 1500 h branches were in a horizontal position. The pistillate phase ended on this same day.
Pollen viability was high in all three species, with an average 98% viable pollen in A. brasilianum, 81.5% in B. segetum and 97% in G. intermedia. In the third species, the absence of pollen was confirmed in two individuals.
No nectar was observed in florets of A. brasilianum and G. intermedia. However, the glucose enzyme test band showed a positive result at the beginning of the pistillate phase in these two species. Nectar was detected in florets of B. segetum at around 0700 h, during the staminate phase, until the end of flower anthesis. Accumulated nectar ascended by capillary action within the corolla tube.
Breeding system
The results showed that the studied species have distinct breeding systems. Self-compatibility was seen in A. brasilianum (). However, the fruit set obtained through cross- and open-pollination (81.6% and 71.3%, respectively) compared to the ones obtained through spontaneous and hand self-pollination (13.9% and 35.6%, respectively) indicate that this species is favoured by pollinator activity.
Table 1. Results of pollination tests in capitula of Adenostemma brasilianum, Bidens segetum and Grazielia intermedia in Viçosa, southeastern Brazil. Values in parentheses correspond to data from one of the two G. intermedia individuals that did not produce pollen.
On the other hand, B. segetum was self-incompatible (), and therefore depended on pollinators for fruiting. Only cross- and open-pollination resulted in fruit set (29.1% and 41.1%, respectively), which were the lowest percentages of these tests among the studied species. The fruit set obtained through hand self-pollination (1.5%) must be the result of contamination with compatible pollen.
Apomixis was verified in G. intermedia () and the high fruit sets obtained in all tests (≥ 50%) corroborated the occurrence of this mechanism, as well as the fruit set observed in the individual with male sterility (≥ 54%). Apart from this individual, the fruit set observed in the apomixis test was significantly lower than the ones observed in the other tests ().
Capitulum visitors
The capitulum visitors () were insects from orders Coleoptera (beetles), Diptera (flies), Hemiptera (chinch bugs), Hymenoptera (bees and wasps) and, mainly, Lepidoptera (butterflies, previously identified by Cruz et al. Citation2012, and diurnal moths) (). Dipterans, lepidopterans and most hymenopterans contacted style branches during visits with the ventral portion of their bodies, legs and proboscis (lepidopterans), where adhered pollen grains were observed. The number of individuals inspected for pollen varied due to the abundance of each species of floral visitor. We did not analyse the presence of pollen on the bodies of all butterflies. However, the similar visiting behaviour among them, and the location of pollen on their bodies (observed in the sampled butterflies), confirmed their role in pollination of the studied plants. The pollen grains of the study plants (and many other Asteraceae) are easily identified because they present typical echinate ornamentation. Other factors that helped to identify the pollen of each species were its colour (yellow in B. segetum and white in A. brasilianum and G. intermedium) and association with flowering peaks at different times. Therefore, these insects were classified as pollinators; the other floral visitors were classified as thieves ().
Table 2. Capitulum visitors of Adenostemma brasilianum (A), Bidens segetum (B) and Grazielia intermedia (G), and visitation categories. All butterflies (Lepidoptera, Rhopalocera) listed in Cruz et al. (Citation2012) have been observed on the study species and are considered pollinators. This table lists additional Lepidoptera and other insect visitors.
Of the 98 species of pollinators observed in G. intermedia, 93.9% were lepidopterans (88 butterflies and four moths). In B. segetum, of the 59 species of pollinators, 79.7% were lepidopterans (45 butterflies and two moths). In A. brasilianum, of the 31 species of pollinators, 83.9% were lepidopterans (19 butterflies and seven moths). Thus, the studied species are predominantly psychophilous and the main resource exploited was nectar.
Bees acted secondarily as pollinators, especially in B. segetum (), and dipterans were considered as occasional pollinators, especially in G. intermedia (); these insects collected both nectar and pollen as resources.
Discussion
Floret opening in the morning is a common feature among the Asteraceae (Proctor & Yeo Citation1978), and it usually occurs before 0800 h (Mani & Saravanan Citation1999), as observed in our study. The SPP, as reported herein, is universal among the Asteraceae, is associated with protandry (Howell et al. Citation1993; Yeo Citation1993; Lane Citation1996), and is an ancestral feature in the family (Jeffrey Citation2009). Differences between the studied species regarding the mechanism of pollen presentation are related to the dynamics of exposure of the sterile portion of style branches with pollen grains, to the period of exposure of grains, and to movements of the style branches until total exposure of the stigmatic lines. These differences are commonly observed (Cerana Citation2004; Fonseca et al. Citation2013) and depend upon style branches morphology. In A. brasilianum and G. intermedia, both from the tribe Eupatorieae, branches are long and clavate with collecting papillae. In B. segetum, from the tribe Coreopsideae, branches are shorter, triangular-shaped in the apex and covered with collecting trichomes. The tribes, as well as the subfamilies, are characterised by having style branches with distinct shapes, thicknesses and sizes. The location of stigmatic areas and collecting trichomes, when present, are also distinct (details in Bremer Citation1994).
The florets of all species had a physical barrier (herkogamy) between the site of pollen deposition and stigmatic lines, a short staminate phase (up to 7 h), and a long pistillate phase (except for G. intermedia) starting on the same day as the staminate one. This set of features was also observed in Tilesia baccata (L.f.) Pruski (Heliantheae) in a study conducted by Fonseca et al. (Citation2013) in the same area as the present study. Herkogamy favours xenogamy, and pollination must occur in the morning of the second day of floret opening, when florets are receptive (pistillate phase) and there is pollen available from other florets (first day of opening, staminate phase) in the population. However, the occurrence of geitonogamous crosses is possible due to the centripetal opening of florets in the capitulum on consecutive days (Grombone-Guaratini et al. Citation2004; Fonseca et al. Citation2013; Valentin-Silva et al. Citation2016; A. Valentin-Silva, pers. obs.).
In A. brasilianum and B. segetum, the long pistillate phase (up to 4 days) resembles that which was observed in other Asteraceae species (Dieringer & Cabrera Citation1989; Mani & Saravanan Citation1999; Cerana Citation2004; Grombone-Guaratini et al. Citation2004). This feature increases the chances of occurrence of pollination. In G. intermedia, the pistillate phase had a short duration, lasting c. 1 day. This fact may be related to the occurrence of apomixis in this species. This is the first report of apomixis in the genus Grazielia. According to Noyes (Citation2007), there are six apomictic genera in the tribe Eupatorieae: Ageratina, Campovassouria, Chromolaena, Eupatorium, Gyptis and Praxelis.
The absence of pollen in two G. intermedia individuals may be related to apomixis. Obligatory apomictic species tend not to produce pollen (Montgomery & Fairbrothers Citation1970; Sullivan Citation1976; Coleman Citation1989; Bertasso-Borges & Coleman Citation1998) or to have either low or null pollen viability (Coleman & Coleman Citation1984; Rozenblum et al. Citation1988; Bertasso-Borges & Coleman Citation2005; Lu et al. Citation2008). However, some individuals of G. intermedia showed high pollen viability, a condition that indicates facultative apomixis.
The apomixis associated with male sterility reported here in G. intermedia has also been observed in other species from different genera in the Eupatorieae (Montgomery & Fairbrothers Citation1970; Sullivan Citation1976; Coleman & Coleman Citation1984; Rozenblum et al. Citation1988; Coleman Citation1989; Bertasso-Borges & Coleman Citation1998, Citation2005; Lu et al. Citation2008). However, with the exception of G. intermedia, the species are obligate apomicts. For example, Praxelis pauciflora (Kunth) R.M. King & H. Rob. (= Eupatorium pauciflorum Kunth), besides being an obligate apomict, shows precocious fruiting (i.e. prior to anthesis; Bertasso-Borges & Coleman Citation1998), a feature that was not observed in G. intermedia.
The finding of apomixis in G. intermedia differs from the results of Bertasso-Boges & Coleman (Citation1998), which considered the species as diploid with sexual reproduction, based on cytogenetic and embryological analyses. Studies on other Eupatorieae species verified that apomixis may occur in polyploid populations, as well as sexual reproduction in diploid populations (Grant Citation1953; Sullivan Citation1976; Watanabe Citation1986; Siripun & Schilling Citation2006). Cytogenetic analyses on the population sampled in our study might clarify this issue.
In G. intermedia, the co-occurrence of functionally female individuals and hermaphrodite individuals defines the sexual expression of the population as gynodioecious sensu Yampolsky & Yampolsky (Citation1922). In this case, according to Mayer & Charlesworth (Citation1991), gynodioecy is cryptic, since pistillate flowers are difficult to identify, as their sexual organs are morphologically similar to the ones of hermaphrodite flowers. Gynodioecy is considered rare in the Asteraceae (Yampolsky & Yampolsky Citation1922), having been reported, for instance, to species of Bidens (Sun & Ganders Citation1987) and Chersodoma (Dillon & Sagástegui-Alva Citation1996).
In the two other studied species we observed only sexual reproduction, and their breeding systems were distinct: self-incompatibility in B. segetum and self-compatibility in A. brasilianum. Both systems have been reported to Bidens species (Ganders & Nagata Citation1983; Ballard Citation1986; Sun & Ganders Citation1988, Citation1990; Grombone-Guaratini et al. Citation2004; Werpachowski et al. Citation2004; Huang et al. Citation2012; Huang & Kao Citation2013) as well as to other asteracean species from several other genera (McMullen Citation1987; Armbruster & McGuire Citation1991; Cerana Citation2004; Werpachowski et al. Citation2004; Ferrer & Good-Avila Citation2007; Jeffrey Citation2009). The high pollen viability recorded in B. segetum and A. brasilianum is a common feature among species with sexual reproduction (Sun & Ganders Citation1988; Werpachowski et al. Citation2004).
Therefore, B. segetum is dependent on pollinators for fruiting. The lowest fruit set obtained through open-pollination was observed in this species (41.1%) in comparison with the other two (71.3% in A. brasilianum and 76.6% in G. intermedia), despite the high number of visiting species and the abundant nectar. In this case, there seems to be pollen limitation in the population (see Knight et al. Citation2005), possibly resulting from visitation by bees, which, besides the accumulated nectar, also collect pollen. Another possibility, besides the damage caused by pollen-eating bees, may be the incompatibility system, which might be negatively affecting the reproductive success of this species, yet that remains to be confirmed in future studies. In A. brasilianum, the high fruit set obtained through open pollination (71.3%) compared to that obtained through spontaneous self-pollination (13.9%) indicates that, despite the self-compatibility, herkogamy seems to be a limiting factor to self-pollination, and that pollinators are important to increase the species reproductive success.
The occurrence of butterflies as the main pollinators, as we report here, corroborates the importance of these insects for the sexual reproduction of asteraceans (Mani & Saravanan Citation1999). Although the studied species are psychophilous, the butterflies that visit each species tend to have distinct predominant groups, especially A. brasilianum (male Ithomiinae, Cruz et al. Citation2012).
The predominance of male Ithomiinae in A. brasilianum (Cruz et al. Citation2012) may be associated with the consumption of nectar by these insects, which in this plant is enriched with pyrrolizidine alkaloids (Brown Citation1984a; Valentin-Silva et al. Citation2016). These alkaloids are used by butterflies, for example, as precursors in the synthesis of pheromones, which are fundamental for the mating success (Brown Citation1984a, Citation1984b). As pyrrolizidine alkaloids are synthesised mainly by species from the tribes Cardueae, Eupatorieae and Senecioneae (Calabria et al. Citation2009), it is possible that G. intermedia, which is also visited by Ithomiinae butterflies, is another source of such compounds in the study area.
The studied species showed sequential or overlapping flowering periods for c. 6 months (from January through June), and therefore floral resources remained available for a long period. Such availability may be critical to supporting the local fauna of adult butterflies, as well as dipterans, hymenopterans and other anthophilous lepidopterans. In the case of anthophilous lepidopterans, 45 species of nocturnal moths (families Arctiidae, Crambidae, Geometridae and Noctuidae) have also been observed in capitula of G. intermedia (Cruz Citation2009).
Our study shows that, although the study species have distinct breeding systems (self-incompatibility in B. segetum, self-compatibility in A. brasilianum and facultative apomixis in G. intermedia), all three benefitted from cross-pollination, performed predominantly by butterflies. However, self-compatibility and apomixis, added to psychophily, are the breeding systems which resulted in greater reproductive success. The combination of gynodioecy and facultative apomixis in G. intermedia is an unreported reproductive feature in the Asteraceae.
Acknowledgements
We thank André Victor Lucci Freitas, Gabriel Melo, Keith S. Brown Jr., Lúcio Antônio de Oliveira Campos, Manoel Martins Dias Filho and Paulo Sérgio Fiúza Ferreira for identifying the floral visitors.
Associate Editor: Dr Janice Lord.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G. 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift. 22:711–728. doi: 10.1127/0941-2948/2013/0507
- Armbruster WS, McGuire AD. 1991. Experimental assessment of reproductive interactions between sympatric Aster and Erigeron (Asteraceae) in interior Alaska. Am J Bot. 78:1449–1457. doi: 10.2307/2445283
- Baker HG, Baker I. 1983. Floral nectar sugar constituents in relation to pollinator type. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold Company Inc.; p. 117–141.
- Ballard R. 1986. Bidens pilosa complex (Asteraceae) in North and Central America. Am J Bot. 73:1452–1465. doi: 10.2307/2443850
- Bertasso-Borges MS, Coleman JR. 1998. Embryology and cytogenetics of Eupatorium pauciflorum and E. intermedium (Compositae). Genet Mol Biol. 21:504–514. doi: 10.1590/S1415-47571998000400017
- Bertasso-Borges MS, Coleman JR. 2005. Cytogenetics and embryology of Eupatorium laevigatum (Compositae). Genet Mol Biol. 28:123–128. doi: 10.1590/S1415-47572005000100022
- Bessa J, Cruz KC, Vieira MF. 2010. Location of the stigmatic areas in Mutisia speciosa Aiton ex. Hook. A new floral feature in Asteraceae. Sexual Plant Reprod. 23:207–209. doi: 10.1007/s00497-010-0141-0
- Bremer K. 1994. Asteraceae—cladistics and classification. Portland: Timber Press.
- Brown Jr KS. 1984a. Adult-obtained pyrrolizidine alkaloids defend Ithomiinae butterflies against a spider predator. Nature. 309:707–709. doi: 10.1038/309707a0
- Brown Jr KS. 1984b. Chemical ecology of dehydropyrrolizidine alkaloids in adult Ithomiinae (Lepidoptera: Nymphalidae). Revista Brasileira de Biologia. 44:435–446.
- Brunet J. 2005. Plant–pollinator interactions and pollen dispersal. In: Dafni A, Kevan PG, Husband BC, editors. Practical pollination biology. Cambridge: Enviroquest; p. 56–82.
- Calabria LM, Emerenciano VP, Scotti MT, Mabry TJ. 2009. Secondary chemistry of Compositae. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ, editors. Systematics, evolution, and biogeography of Compositae. Vienna: IAPT; p. 73–88.
- Cerana MM. 2004. Flower morphology and pollination in Mikania (Asteraceae). Flora. 199:168–177. doi: 10.1078/0367-2530-00145
- Coleman JR. 1989. Embryology and cytogenetics of apomitic hexaploid Eupatorium odoratum L. (Compositae). Revista Brasileira de Genética. 12:803–817.
- Coleman JR, Coleman MA. 1984. Apomixis in two triploid Brazilian species of Eupatorium: E. bupleurifolium and E. callilepis. Revista Brasileira de Genética. 7:549–567.
- Cruz KC. 2009. Biologia reprodutiva e polinizadores de Eupatorium intermedium DC. (Asteraceae) em fragmento de Floresta Atlântica [dissertation]. Viçosa (MG): Universidade Federal de Viçosa.
- Cruz KC, Lelis SM, Godinho MAS, Fonseca RS, Ferreira PSF, Vieira MF. 2012. Species richness of anthophilous butterflies of an Atlantic Forest fragment in Southeastern Brazil. Rev Ceres. 59:571–579. doi: 10.1590/S0034-737X2012000500001
- Dafni A, Pacini E, Nepi M. 2005. Pollen and stigma biology. In: Dafni A, Kevan PG, Husband BC, editors. Practical pollination biology. Cambridge: Enviroquest; p. 83–146.
- Dieringer G, Cabrera L. 1989. Pollination ecology and stigma longevity of Liatris mucronata DC. (Compositae). Am J Bot. 76:135–141.
- Dillon MO, Sagástegui-Alva A. 1996. Revision of the dioecious genus Chersodoma Phil. (Senecioneae, Asteraceae), including a new species and status change. Brittonia. 48:582–604. doi: 10.2307/2807880
- Ferrer MM, Good-Avila SV. 2007. Macrophylogenetic analyses of the gain and loss of self-incompatibility in the Asteraceae. New Phytol. 173:401–414. doi: 10.1111/j.1469-8137.2006.01905.x
- Fonseca RS, Campos LAO, Vieira MF. 2013. Melittophily and ornithochory in Tilesia baccata (L.f.) Pruski: an Asteraceae of the Atlantic Forest understory with fleshy fruits. Flora. 208:370–380. doi: 10.1016/j.flora.2013.04.008
- Funk VA, Susanna A, Stuessy TF, Robinson H. 2009. Classification of Compositae. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ, editors. Systematics, evolution, and biogeography of Compositae. Vienna: IAPT; p. 171–189.
- Ganders FR, Nagata KM. 1983. Relationships and floral biology of Bidens cosmoides (Asteraceae). Lyonia. 2:23–31.
- Godinho MAS, Mantovani-Alvarenga E, Vieira MF. 2011. Germinação e qualidade de sementes de Adenostemma brasilianum (Pers.) Cass., Asteraceae nativa de sub-bosque de Floresta Atlântica. Revista Árvore. 35:1197–1205. doi: 10.1590/S0100-67622011000700006
- Grant WF. 1953. A cytotaxonomic study in the genus Eupatorium. Am J Bot. 40:729–742. doi: 10.2307/2439689
- Grombone-Guaratini MT, Solferini VN, Semir J. 2004. Reproductive biology in species of Bidens L. (Asteraceae). Scientia Agricola. 61:185–189. doi: 10.1590/S0103-90162004000200010
- Hattori EKO. 2015. Grazielia—Lista de espécies da flora do Brasil [Internet]. Rio de Janeiro: Jardim Botânico do Rio de Janeiro; [cited 2015 May 13]. Available from: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB27080.
- Hattori EKO, Almeida G. 2015. Adenostemma —Lista de espécies da flora do Brasil [Internet]. Rio de Janeiro: Jardim Botânico do Rio de Janeiro; [cited 2015 May 13]. Available from: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB80592.
- Howell GJ, Slater AT, Knox RB. 1993. Secondary pollen presentation in angiosperms and its biological significance. Aust J Bot. 41:417–438. doi: 10.1071/BT9930417
- Huang Y-L, Chen S-J, Kao W-Y. 2012. Floral biology of Bidens pilosa var. radiata, an invasive plant in Taiwan. Bot Stud. 53:501–507.
- Huang Y-L, Kao W-Y. 2013. Different breeding systems of three varieties of Bidens pilosa in Taiwan. Weed Res. 54:162–168. doi: 10.1111/wre.12060
- Inoye DW. 1980. The terminology of floral larceny. Ecology. 61:1251–1253. doi: 10.2307/1936841
- Jeffrey C. 2009. Evolution of Compositae flowers. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ, editors. Systematics, evolution, and biogeography of Compositae. Vienna: IAPT; p. 131–138.
- King RM, Robinson H. 1987. The genera of the Eupatorieae (Asteraceae). Monographs in Systematics Botany from the Missouri Botanical Garden 22. St. Loius: Missouri Botanical Garden.
- Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, Dudash MR, Jonhston MO, Mitchell RJ, Ashman TL. 2005. Pollen limitation of plant reproduction: pattern and process. Annu Rev Ecol Evol Syst. 36:467–497. doi: 10.1146/annurev.ecolsys.36.102403.115320
- Lane MA. 1996. Pollination biology of Compositae. In: Caligari PDS, Hind DJN, editors. Compositae: biology and utilization. London: Royal Botanical Gardens; p. 61–80.
- Lelis SM. 2008. Biologia reprodutiva de Bidens segetum Mart. ex Colla (Heliantheae, Asteraceae) [dissertation]. Viçosa (MG): Universidade Federal de Viçosa.
- Lu H, Shen J, Sang W, Zhang X, Lin J. 2008. Pollen viability, pollination, seed set, and seed germination of croftonweed (Eupatorium adenophorum) in China. Weed Sci. 56:42–51. doi: 10.1614/WS-06-210.1
- Mani MS, Saravanan JM. 1999. Pollination ecology and evolution in Compositae (Asteraceae). Enfield: Science Publishers.
- Mayer SS, Charlesworth D. 1991. Cryptic dioecy in flowering plants. Trends Ecol Evol. 6:320–325. doi: 10.1016/0169-5347(91)90039-Z
- McMullen CK. 1987. Breeding systems of selected Galápagos Islands angiosperms. Am J Bot. 74:1694–1705. doi: 10.2307/2444139
- Mondin CA, Nakajima JN, Bringel Jr JBA. 2015. Bidens —Lista de espécies da flora do Brasil [Internet]. Rio de Janeiro: Jardim Botânico do Rio de Janeiro; [cited 2015 May 13]. Available from: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB114857.
- Montgomery JD, Fairbrothers DE. 1970. A biosystematic study of the Eupatorium rotundifolium complex (Compositae). Brittonia. 22:134–150. doi: 10.2307/2805807
- Nakajima J, Loeuille B, Heiden G, Dematteis M, Hattori EKO, Magenta MAG, Ritter MR, Mondin CA, Roque N, Ferreira SC, et al. 2015. Asteraceae – Lista de espécies da flora do Brasil [Internet]. Rio de Janeiro: Jardim Botânico do Rio de Janeiro; [cited 2015 May 13]. Available from: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB55.
- Noyes RD. 2007. Apomixis in the Asteraceae: diamonds in the rough. Funct Plant Sci Biotechnol. 1:207–222.
- Oliveira-Filho AT, Fontes MAL. 2000. Patterns of floristic differentiation among Atlantic Forests in southeastern Brazil and the influence of climate. Biotropica. 32:793–810. doi: 10.1111/j.1744-7429.2000.tb00619.x
- Proctor MCF, Yeo P. 1978. The pollination of flowers. Glasgow: William Collins Sons & Co Ltd.
- Radford AE, Dickison WC, Massey JR, Bell CR. 1974. Vascular plant systematics. New York: Harper and Row.
- Richards AJ. 1997. Plant breeding systems. London: Chapman and Hall.
- Rozenblum E, Maldonado S, Waisman CE. 1988. Apomixis in Eupatorium tanacetifolium (Compositae). Am J Bot. 75:311–322. doi: 10.2307/2443978
- Sage TL, Husband BC, Routley MB. 2005. Intrinsic attributes of the breeding system. In: Dafni A, Kevan PG, Husband BC, editors. Practical pollination biology. Cambridge: Enviroquest; p. 30–55.
- Silva CA, Vieira MF, Carvalho-Okano RM, Oliveira LO. 2014. Reproductive success and genetic diversity of Psychotria hastisepala (Rubiaceae), in fragmented Atlantic forest, Southeastern Brazil. Revista de Biología Tropical. 62:309–319. doi: 10.15517/rbt.v62i1.5854
- Siripun KC, Schilling EE. 2006. Molecular confirmation of the hybrid origin of Eupatorium godfreyanum (Asteraceae). Am J Bot. 93:319–325. doi: 10.3732/ajb.93.2.319
- Sullivan VI. 1976. Diploidy, polyploidy, and agamospermy among species of Eupatorium (Compositae). Can J Bot. 54:2907–2917. doi: 10.1139/b76-313
- Sun M, Ganders FR. 1987. Microsporogenesis in male-sterile and hermaphroditic plants of nine gynodioecious taxa of Hawaiian Bidens (Asteraceae). Am J Bot. 74:209–217. doi: 10.2307/2444022
- Sun M, Ganders FR. 1988. Mixed mating systems in Hawaiian Bidens (Asteracae). Evolution. 42:516–527. doi: 10.2307/2409036
- Sun M, Ganders FR. 1990. Outcrossing rates and allozyme variation in rayed and rayless morphs of Bidens pilosa. Heredity. 64:139–143. doi: 10.1038/hdy.1990.18
- Valentin-Silva A, Godinho MAS, Vieira MF. 2016. Life history of Adenostemma brasilianum (Pers.) Cass. (Eupatorieae, Asteraceae): a psychophilous herbaceous species of the Brazilian Atlantic Forest understory. J Torrey Bot Soc. 143:87–92. doi: 10.3159/TORREY-D-14-00088.1
- Veloso HP, Rangel Filho ALR, Lima JCA. 1991. Classificação da vegetação brasileira, adaptada a um sistema universal. Rio de Janeiro: IBGE.
- Watanabe K. 1986. The cytogeography of the genus Eupatorium (Compositae). A review. Plant Species Biol. 1:99–116. doi: 10.1111/j.1442-1984.1986.tb00019.x
- Werpachowski JS, Varassin IG, Goldenberg R. 2004. Ocorrência de apomixia e partenocarpia em algumas espécies subtropicais de Asteraceae. Revista Brasileira de Botânica. 27:607–613.
- Yampolsky C, Yampolsky H. 1922. Distribution of sex forms in the phanerogamic flora. Bibliotheca Genetica. 3:1–62.
- Yeo PF. 1993. Secondary pollen presentation: form, function and evolution. Plant Syst Evol. 6 (supplement): 1–268. doi: 10.1007/978-3-7091-6670-3_1
