ABSTRACT
Within Solanaceae, Nolana L. f. and Sclerophylax Miers are two genera that have deserved special attention because of their rare anatomical characteristics. However, they are poorly known from the cytogenetic perspective. In order to discuss their chromosome features in an evolutionary context, classical staining, chromomycin A3/4′-6-diamidino-2-phenylindole (CMA/DAPI) fluorescent banding and in situ hybridisation (FISH) with probes for the 18-5.8-26S and the 5S rDNA loci were applied to root tips of germinating seeds. All the species presented the chromosome number 2n = 24. Karyotypes were highly symmetric, with most chromosomes being metacentric and with a maximum of three submetacentric pairs in N. divaricata. The CMA/DAPI banding technique, assayed for the first time in Sclerophylax, showed CMA+/DAPI− bands associated with nucleolar organiser regions (NORs) in the first metacentric chromosome pair in each species. The FISH technique (applied to four species of Sclerophylax and, for the first time, in one species of Nolana) showed that the 18-5.8-26S loci coincide with CMA+/DAPI− bands. Three Sclerophylax species presented two pairs of 5S signals, whereas S. adnatifolia showed three. The rDNA loci resulted as asyntenic in Sclerophylax, but were localised in the same chromosome in N. divaricata. Despite the morphological peculiarities of Nolana and Sclerophylax, the chromosome number and karyotype features are consistent with the position of the two genera within the ‘x = 12 clade’, while the number and position of rDNA loci established the chromosome rearrangements, suggesting different evolutionary pathways with respect to their closest relatives, Lycium and Jaborosa.
Introduction
The Solanaceae is a cosmopolitan family that includes c. 2700 species (Olmstead & Bohs Citation2007; Särkinen et al. Citation2013), some of them having great economic, ethnobotanic, experimental and ornamental value. Potato (Solanum tuberosum L.) and tomato (Solanum lycopersicum L.) are species of this family originally from the South American Andes, together with another 16 genera (Hunziker Citation2001).
The phylogeny of the Solanaceae is relatively resolved and the limits of the family have been expanded to also embrace the Sclerophylacaceae, Nolanaceae, Goetzeaceae and the Duckeodendraceae (Olmstead et al. Citation2008; Särkinen et al. Citation2013). This scheme is supported by strong evidence and it is interesting to find supplementary chromosomal synapomorphies that complement it. In spite of the relevance of cytology for the diversification and systematics of this family (e.g. Tu et al. Citation2005; Moscone et al. Citation2007; Chiarini et al. Citation2016), chromosome studies are still lacking in many of the Solanaceae. In particular, several genera and many species (e.g. two thirds of the c. 1400 species of Solanum) in the so called ‘x = 12 clade’ are cytogenetically unknown. To fill this gap we have carried out a study on two unexplored genera, Nolana L. f. and Sclerophylax Miers, in order to understand their systematic relationships and chromosome evolution.
Nolana is one of the four largest genera within Solanaceae. It embraces 89 species mainly distributed in arid regions of Chile and Peru, in coastal environments dependent for water on fog called lomas formations (Dillon et al. Citation2007, Citation2009). Nolana species are annual or perennial herbs or shrubs, with showy flowers (N. paradoxa Lindl. is cultivated as ornamental) with a gynoecium formed of five strongly lobed carpels (Tago-Nakawaza & Dillon Citation1999; Freyre et al. Citation2005). Nolana is easily recognised by the unusual sclerified fruits divided into many mericarps, each one with several seeds (Bruno Citation1994), a feature which is rare in the family (Knapp Citation2002). This character is an autapomorphy (a uniquely derived character) of the genus, and it was the principal reason why some authors have maintained the group at the family level (Nolanaceae; Hunziker Citation2001). However, according to molecular studies, Nolana is a natural group within the Solanaceae (Tu et al. Citation2008), Sclerophylax being its sister clade, and it is also closely related to Lycium L. and Jaborosa Juss. (Dillon Citation2005; Särkinen et al. Citation2013). Concerning cytogenetics, chromosome counts in five species and the karyotype of N. crassulifolia Poepp. with the formula 11m + 1sm, is the only information available for the entire genus (Bolkhovskikh et al. Citation1969; Chiarini et al. Citation2010).
Sclerophylax is a small genus that embraces 12 species endemic to dry regions in western and central Argentina (only two species occur outside Argentina: S. spinescens Miers, in Paraguay, and S. lorentzianus Hoffm., in Uruguay). Sclerophylax species are herbs, almost glabrate or pubescent, succulent and mostly annual, with breakable, winding stems of circular-triangular section. They have crystals of calcium oxalate in roots, stems and leaves. Flowers are perfect, axillary, solitary and sessile, with an asymmetrical calyx and a slightly zygomorphic corolla, generally white or blue (Di Fulvio Citation1961; Barraza Citation1994); the ovary is superior, bicarpellate, bilocular and contains two to three ovules. Sclerophylax was excluded from the Solanaceae based on its distinctive gynoecium and fruit morphology: the fruit is dry, indehiscent, with a membranous pericarp enclosing two to three small straight seeds, and it is usually embedded into the stem and crowned by the spiny calyx. Thus, the genus was alternatively included in Boraginaceae, Hydrophyllaceae or in its own family, Sclerophylacaceae, but finally studies of molecular phylogeny have demonstrated that Sclerophylax belongs in the Solanaceae (Olmstead et al. Citation2008). With respect to the cytogenetics, to date it is only known that the basic chromosome number is x = 12 and the karyotypes are constituted mostly by metacentric (m) and submetacentric (sm) chromosomes (Di Fulvio Citation1961).
Lycium and Jaborosa, two of the genera most closely related to Sclerophylax and Nolana, have been the focus of detailed chromosome studies that curiously revealed different evolutionary paths, apparently related to key geological events (Stiefkens et al. Citation2010; Blanco et al. Citation2012; Chiarini et al. Citation2016). However, no species of Nolana or Sclerophylax have been studied up to now using, for instance, fluorochrome banding or fluorescence in situ hybridisation (FISH) procedures, which are useful in detecting the chromosome rearrangements involved in speciation (e.g. Hasterok et al. Citation2006; Chacón et al. Citation2012). Chromomycin A3/4′-6-diamidino-2-phenylindole (CMA/DAPI) staining reveals heterochromatin blocks, which are one of the most remarkable chromosome components because of their apparent lack of genes, their unknown function and their differential stainability, thus constituting a source of variability for comparative purposes (Guerra Citation2000). FISH permits homologous chromosomes in a complement to be identified and allows comparison between related species, thus answering chromosomal evolutionary questions (e.g. Chacón et al. Citation2012; Chiarini et al. Citation2014). The most common FISH markers are ribosomal genes (5S and 18–5.8–26S rDNA), which are abundant and highly conserved in higher plants (Heslop-Harrison & Schwarzacher Citation2011).
Considering this background, the aim of this work was to study the mitotic chromosomes of Sclerophylax and Nolana by means of classic staining, CMA/DAPI banding and FISH with probes for the ribosomal genes 5S and 18–5.8–26S rDNA, in order to establish chromosome numbers, karyotypes, heterochromatin patterns, and the number and position of rDNA sites, as well as to discuss the resulting information in an evolutionary context.
Material and methods
Voucher specimens of the studied material are deposited at Museo Botánico de Córdoba (CORD). The provenance of the plant material is as follows:
Sclerophylax adnatifolia Di Fulvio: ARGENTINA, prov. Jujuy, dpt. Tumbaya, 23°46′18″ S, 65°56′51″ W, Barboza et al. 4382.
Sclerophylax arnottii Miers: ARGENTINA, prov. La Rioja, dpt. Gobernador Gordillo, 30°04′23″ S, 66°47′47″ W, Barboza et al. 4202.
Sclerophylax kurtzii Di Fulvio: ARGENTINA, prov. La Rioja, dpt. Famatina, 28°51′16.7″ S, 67°36′30″ W, Barboza et al. 4235.
Sclerophylax spinescens Miers: ARGENTINA, prov. Córdoba, dpt. San Justo, Miramar, Laguna Mar Chiquita, 30°55′02.45″ S, 62°40′58.88″ W, Chiarini 1267.
Nolana divaricata (Lindl.) I.M. Johnst.: CHILE, Region II Antofagasta, 25°33′16.2″ S, 70°21′34.3″ W, Barboza et al. 2971.
Nolana villosa (Phil.) I.M. Johnst.: CHILE, Region II Antofagasta, Paposo, 25°00′21.8″ S, 70°27′55.8″ W, Barboza et al. 2979.
Nolana stenophylla I.M. Johnst.: CHILE, Region II Antofagasta, Paposo, 25°00′21.8″ S, 70°27′55.8″ W, Barboza et al. 2978.
Nolana patula (Phil.) M.O. Dillon: CHILE, Region III Atacama, 26°09′14.9″ S, 70°27′43.4″ W, Barboza et al. 2970.
It was necessary to rupture the sclerified fruits of the species in both genera in order to release the seeds, which were very reluctant to germinate, especially the Nolana species. Mitotic chromosomes were examined in root tips obtained from germinating seeds. Roots were pretreated in saturated p-dichlorobenzene in water for 2 h at room temperature, fixed in 3:1 ethanol:acetic acid, washed in distilled water, digested for 45 min at 37 °C with Pectinex SP ULTRA (Novozymes) and squashed in a drop of 45% acetic acid. After coverslip removal in liquid nitrogen, the slides were stored at –20 °C.
For mitotic counts and karyotypes, slides were stained with Giemsa (Guerra Citation1983). At least 10 metaphases of each species were photographed with phase contrast in a Zeiss Axiophot microscope. Photographs were used for taking the following measurements of each chromosome pair: s (short arm); l (long arm); and c (total chromosome length). The arm ratio (r = l/s) was then calculated and used to classify the chromosomes as recognised by Levan et al. (Citation1964). In addition, the total haploid chromosome length of the karyotype (TL) based on the mean chromosome length was calculated. Karyotype asymmetry was estimated using Romero Zarco’s (Citation1986) indices (A1 = intrachromosomal asymmetry index, and A2 = interchromosomal asymmetry index). Idiograms were based on the mean values for each species.
For fluorescent banding, slides were stained with a drop of 0.5 mg/mL chromomycin A3 (CMA) in McIlvaine buffer, pH 7.0 and distilled water (1:1) containing 2.5 mM MgCl2 for 90 min, and subsequently stained with 2 μg/mL 4′-6-diamidino-2-phenylindole (DAPI) for 30 min, and finally mounted in McIlvaine’s buffer-glycerol v/v 1:1 (Schweizer Citation1976; Schweizer & Ambros Citation1994). The amount of heterochromatin was expressed as a percentage of the total length of the haploid karyotype.
The location and number of rDNA sites were determined by FISH using two probes: the pTa71 containing the 18-5.8-26S gene of wheat (Gerlach & Bedbrook Citation1979) labelled with biotin-14-dATP (BioNick, Invitrogen Carlsbad) and a 5S rDNA fragment obtained by polymerase chain reaction (PCR) from Solanum stuckertii Bitter (Chiarini et al. Citation2014), labelled with digoxigenin-11-dUTP (DigNick, Roche). The FISH protocol was according to Schwarzacher & Heslop-Harrison (Citation2000), with minor modifications. The preparations were incubated in 100 μg/mL RNAase, post-fixed in 4% (w/v) paraformaldehyde, dehydrated in a 70%–100% graded ethanol series, and air-dried. On each slide, 15 μL of hybridisation mixture was added (4–6 ng/μL of probe, 50% formamide, 10% dextran sulfate, 2× saline sodium citrate and 0.3% sodium dodecyl sulfate), previously denatured at 70 °C for 10 min. Chromosome denaturation/hybridisation was undertaken at 90 °C for 10 min, 48 °C for 10 min and 38 °C for 5 min using a thermal cycler (Mastercycler, Eppendorf), and slides were placed overnight in a humid chamber at 37 °C. The 18-5.8-26S probe was detected with avidin-FITC conjugate (Sigma-Aldrich), the 5S probe was detected with antidigoxigenin-rhodamine (Roche), and then counterstained and mounted with 25 μL antifade Vectashield (Vector Lab.) containing 1.5 μg/mL of DAPI.
At least 10 metaphases of each species from at least three different individuals were photographed with a Zeiss Axiophot microscope equipped with epifluorescence and a digital image capture system. The free software ImageJ (http://rsbweb.nih.gov/ij/) was used for merging the images.
Results
The mitotic chromosome number in all the species studied is 2n = 24. Idiograms based on average chromosome measurements are shown in , and chromosome variables are summarised in . In the general context of angiosperms (Guerra Citation2000), chromosomes here studied are relatively small to medium sized (, ), with a chromosome size varying from 3.20 to 6.77 µm. The shortest chromosome was measured in pair 12 of S. adnatifolia (2.3 µm) and the longest was in pair 1 of N. patula (8.67 µm). This species also presents the highest value for total haploid genome length (TL = 81.32 µm), while S. kurtzii presents the lowest (38.47 µm).
Figure 1. Idiograms of Nolana and Sclerophylax. A, N. stenophylla; B, N. patula; C, N. villosa; D, N. divaricata; E, S. kurtzii; F, S. arnottii; G, S. adnatifolia; H, S. spinescens. Chromosomes are ordered from the longest to the shortest within each category, from m to sm. Species only studied with classical staining are shown in grey. Black blocks are 5S signals and hatched blocks indicate the 18-5.8-26S signals plus a CMA+/DAPI– NOR associated band in the same position.
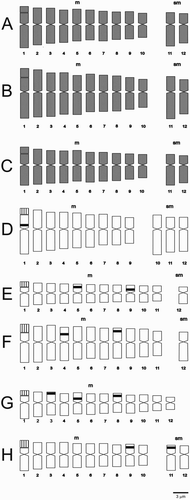
Figure 2. Photomicrographs of mitotic metaphases of Sclerophylax and Nolana stained with Giemsa. A, N. divaricata; B, N. patula; C, S. arnottii; D, N. villosa; E, N. stenophylla; F, S. adnatifolia; G, S. spinescens; H, S. kurtzii. Arrows point to satellites. All at the same scale, bar = 5 µm.
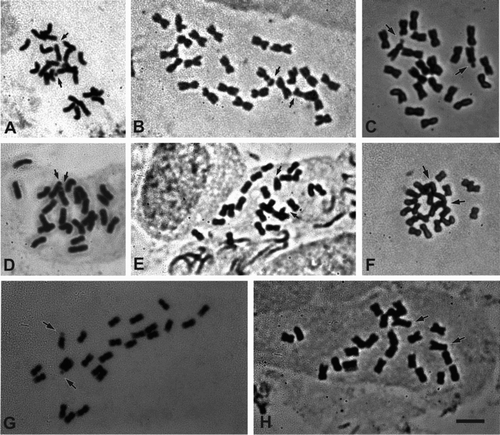
Table 1. Chromosome variables of species of Sclerophylax and Nolana. Karyotype formulae, total haploid genome length in μm (TL), average chromosome length in μm (c) ± standard deviation, average arm ratio (R) ± standard deviation, intrachromosomal asymmetry index (A1) and interchromosomal asymmetry index (A2).
All species show secondary constrictions (), which belong to the microsatellite type of Battaglia (Citation1955). Karyotype formulae are composed of nine to 12 m chromosome pairs, plus zero, one, two or three sm pairs (). According to Romero Zarco (Citation1986), karyotypes are symmetrical (), that of S. adnatifolia being the most symmetrical and that of N. divaricata the least.
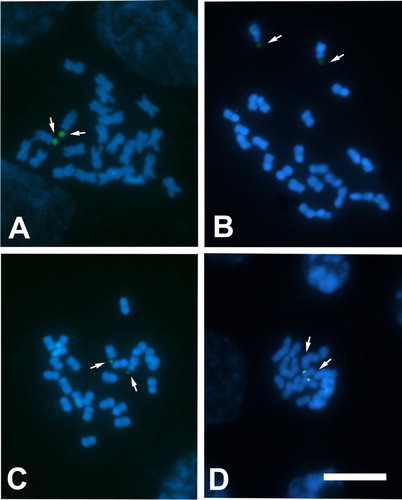
In all the species analysed with the CMA/DAPI technique (S. adnatifolia, S. arnottii, S. kurtzii and S. spinescens), CMA+/DAPI− bands associated with nucleolar organiser regions (NORs) were detected, always located in the largest m pair (). The heterochromatin percentage varied from 2.15% in S. arnottii to 2.89% in S. adnatifolia ().
Figure 3. Photomicrographs of mitotic metaphases of Sclerophylax stained with CMA/DAPI. A, S. kurtzii; B, S. arnottii; C, S. spinescens; D, S. adnatifolia. Arrows point to CMA+/DAPI– bands (green signals). All at the same scale, bar = 10 µm.
Ribosomal DNA genes were studied with FISH in five species: S. adnatifolia, S. arnottii, S. kurtzii, S. spinescens and N. divaricata. In all cases the probe for the 18-5.8-26S gene hybridised in the secondary constriction on the short arm of the largest m pair, coinciding with a CMA+/DAPI− NOR associated band. On the other hand, signals for the 5S gene varied in number and position according to the species (, ).
Figure 4. Fluorescent in situ hybridisation (FISH) in metaphasic chromosomes of Sclerophylax and Nolana. A, S. arnottii; B, S. spinescens; C, S. adnatifolia; D, N. divaricata; E, S. kurtzii. Arrows point to 18-5.8-26S signals (green) and asterisks indicate 5S signals (red). All at the same scale, bar = 10 µm. Notice that in N. divaricata the 18-5.8-26S and 5S signals are in the same chromosome but they are not embedded to each other.
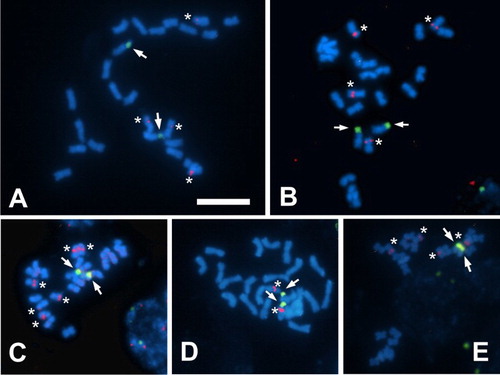
In N. divaricata, one 18-5.8-26S signal is located in the same chromosome that also bears the 5S gene, although the two signals are not co-localised (i.e. they are syntenic, contiguous in the same chromosome, but they are not embedded to each other).
Discussion
Chromosome number
In both Nolana and Sclerophylax all species presented 2n = 24, which confirm previous reports (Di Fulvio Citation1961; Chiarini et al. Citation2010) and is consistent with the data available for the Solanoideae or ‘x = 12 clade’ (Hunziker Citation1979; Olmstead et al. Citation2008). All the analysed species resulted as diploids, suggesting that neither polyploidy or aneuploidy/dysploidy have played any significant role in the diversification of these two genera. However, more species need to be counted (especially for Nolana) to confirm this assertion.
According to Särkinen et al. (Citation2013), Sclerophylax + Nolana + Lycium form a clade, whose sister clade is the Hyoscyameae (Hyoscyamus L., Atropa L., Anisodus Link. ex Spreg., Scopolia Jacq., Physochlaina G. Don and Przewalskia Maxim.). Whereas Sclerophylax and Nolana are South American, Lycium inhabits both South and North America, as well as the Old World (Miller et al. Citation2011). However, the Hyoscyameae are exclusive to the Old World, with some genera endemic to China (Tu et al. Citation2010; Sanchez-Puerta & Abbona Citation2014). This is important for comparative purposes, since the continental separation could have led to different evolutionary pathways, including at the chromosomal level. In fact, most Hyoscyameae are polyploids or have derived basic numbers (2n = 28, 34, 42, 44, 48, 68, 72, 84, 96) probably originated by dysploidy from a polyploid ancestor (Tu et al. Citation2005), whereas Lycium, Jaborosa, Sclerophylax and Nolana are mostly diploids with 2n = 24 (Stiefkens & Bernardello Citation1996; Bernardello et al. Citation2008; Chiarini & Barboza Citation2008; Chiarini et al. Citation2010; Stiefkens et al. Citation2010).
Karyotypes
In the species studied, the chromosomes are small compared with other angiosperms in general (Guerra Citation2000), but medium sized with respect to the Solanaceae (Badr et al. Citation1997). Chromosomes of Nolana are larger than those of Sclerophylax, and also larger than those of closely related genera, such as Lycium (Stiefkens & Bernardello Citation1996, Citation2000, Citation2002; Stiefkens Citation2001; Bernardello et al. Citation2008; Stiefkens et al. Citation2009, Citation2010; Blanco et al. Citation2012) and Jaborosa (Chiarini & Barboza Citation2008). The four species of Nolana studied here all presented symmetrical karyotypes, similar to that previously reported for N. crassulifolia (Chiarini et al. Citation2010), and Sclerophylax also has low asymmetry indices. Symmetrical karyotypes are the rule in Lycium where most species have complements with 10–12 m chromosomes. On the contrary, Jaborosa displays a wider karyotype diversity, with some species having up to six sm pairs and two to three st pairs (Chiarini & Barboza Citation2008; Chiarini et al. Citation2016). Another similarity between Nolana, Sclerophylax and Lycium is the possession of satellites positioned in the short arm of the largest m chromosome pair, which differs from Jaborosa species that present more than one pair and in variable positions (e.g. Stiefkens & Bernardello Citation1996; Bernardello et al. Citation2008; Chiarini & Barboza Citation2008; Chiarini et al. Citation2010; Stiefkens et al. Citation2010; Blanco et al. Citation2012). All these chromosome features bring Nolana and Sclerophylax closer to Lycium and separate them from Jaborosa, which would have followed a different evolutionary path at the chromosomal level, perhaps caused by a different biogeographic history.
Concerning heterochromatin, in the Solanaceae the number and the size of bands are variable, but the overall pattern is relatively preserved, such as in Lycium (Stiefkens et al. Citation2010; Blanco et al. Citation2012). The species studied of Sclerophylax are all similar in having a low percentage of heterochromatin, with only one CMA+/DAPI− band associated with NOR located at the first m pair, which is co-localised with the 18-5.8-26S loci. The same pattern has been observed in Lycium (Stiefkens et al. Citation2009, Citation2010; Blanco et al. Citation2012), as well as in other Solanaceae of the x = 12 clade not so closely related, such as Solanum L. (Chiarini et al. Citation2014), Capsicum L. (Moscone et al. Citation1996, Citation2007), Lycianthes (Dunal) Hassl. and Vassobia Rusby (Rego et al. Citation2009). On the contrary, the heterochromatin percentage is more variable in Jaborosa species, with many of them having additional CMA+/DAPI− bands that are not associated with NORs.
It has been observed that asymmetric karyotypes seem to be associated with a greater amount of heterochromatin in species of the Solanum sect. Acanthophora (Chiarini et al. Citation2013). Coincidently, in the species studied here, the karyotypes are symmetrical and the amount of heterochromatin is low. Contrary cases have been found only in species of the Cactaceae (Las Peñas et al. Citation2008).
The rDNA 18-5.8-26S loci are part of the structure of NORs, which are generally in the secondary constriction of chromosomes (Dubcovsky & Dvorak Citation1995) and consist of tandem repeat units. The FISH technique, conducted in four species of Sclerophylax and, for the first time, in Nolana, revealed that the 18-5.8-26S site is located in the first m pair and co-localised with a CMA+/DAPI− band in both genera. This localisation is usual for the Solanaceae and for most plant species where 18-5.8-26S always occurs in the terminal regions of the chromosomes (Lim et al. Citation2000; Fregonezi et al. Citation2006; Kwon & Kim Citation2009). Meanwhile, 5S rDNA units are composed of sequences of 120 highly conserved base pairs organised in tandem at specific locations of the genome (Appels & Honeycutt Citation1986). Sclerophylax species differ from each other in terms of the location and number of sites of 5S rDNA loci. The probe hybridised in two chromosome pairs in S. kurtzii, S. spinescens and S. arnottii, and in three in S. adnatifolia. This is different from what happens in Lycium where only a single pair of signals per complement has been seen (Blanco et al. Citation2012). Concerning N. divaricata, the synteny of the rDNA loci 18-5.8-26S and 5S is remarkable. One hypothesis to explain this situation would be an accidental insertion of 5S rDNA within or near the 18-5.8-26S by means of retrotransposons or other mobile elements (Drouin & De Sa Citation1995; Lönnig & Saedler Citation2002; Altinkut et al. Citation2006). This phenomenon has also been observed in other species such as some Asteraceae (Garcia et al. Citation2007) and Amaryllidaceae (Chang et al. Citation2009). The presence of both rDNA signals on the same chromosome can be interpreted as evidence of a chromosomal rearrangement (translocation) since the 18-5.8-26S and 5S signals are located on different chromosomes in the sister clades (Lycium and Sclerophylax) (Tang et al. Citation2008). The synteny in N. divaricata can be considered as a synapomorphy, although studies on a larger number of species would be needed to establish whether this is a characteristic of the genus or if it is particular to the species.
Morphological diversification and chromosome differentiation
In Sclerophylax, both morphological and karyotype features are fairly uniform, whereas in Nolana, although morphological differences exist, such as the habit, corolla shape, leaf type, presence or absence of glandular hairs, etc (Johnston Citation1936; Di Fulvio Citation1961), they are not accompanied by any remarkable karyotype changes (Chiarini et al. Citation2010; this study), although in this case it would be necessary to analyse a greater number of species cytogenetically in order to establish a general pattern.
In contrast, Lycium shows a vast morphological variability (e.g. the fruit can be a berry or a drupe, the corollas can be rotate or tubular, the leaves are fleshy or membranous, orbicular, lanceolate or linear; Bernardello Citation1986; Bernardello & Chiang Cabrera Citation1998), but the karyotypes are very similar (Stiefkens & Bernardello Citation1996, Citation2002; Stiefkens et al. Citation2010; Blanco et al. Citation2012) which has been called ‘karyotypic orthoselection’. Likewise, Jaborosa presents notable morphological variability (especially floral characters), but in this case it is accompanied by a karyotype diversification (Chiarini & Barboza Citation2008; Chiarini et al. Citation2013).
One possible explanation for these karyotype variations may arise from establishing the divergence times among the genera. According to Särkinen et al. (Citation2013), the clade that embraces Lycium, Sclerophylax and Nolana originated about 16 million years ago (mya), a period during which the chromosome number and karyotypes have remained more or less constant (2n = 24, karyotypes with most m chromosomes), unlike what happened in Hyoscyameae where polyploidy and disploidy have occurred (Tu et al. Citation2005, Citation2010), or Jaborosa where karyotypes are much more asymmetric (Chiarini & Barboza Citation2008; Chiarini et al. Citation2013). Sclerophylax split from Nolana and Lycium about 12.16 mya whereas the latter two genera separated from each other at ±10.57 mya (Särkinen et al. Citation2013). This would explain the fact that both Nolana and Lycium present only one 5S rDNA site (Blanco et al. Citation2012), whereas Sclerophylax, which diverged earlier, has two or three sites. Since in most of the Lycium species studied so far the two rDNA loci are asyntenic, it is presumed that this would be the ancestral state, and during the last 10 million years the rearrangement that led to the synteny in N. divaricata would have occurred.
Moreover, it is noticeable that S. adnatifolia has recently separated (± 4 mya) from the other species of the genus (Särkinen et al. Citation2013) suggesting that the change in number and position of the 5S rDNA loci is relatively fast, and the times of variation of this locus are different from those of the 18-5.8-26S locus, which would be more stable. A similar situation, where the two rDNA loci vary differentially, has been described in Aloe L. (Asphodelaceae) where the karyotype formula is retained, but in this genus the pattern of the 5S signal is similar between the species and the 18-5.8-26S sites are more variable (Adams et al. Citation2000).
The data provided by this study (chromosome number, karyotypes, heterochromatin patterns, number and position of rDNA sites) reinforce the position of Nolana and Sclerophylax within the ‘x = 12 clade’, but point to a different evolutionary pathway with respect to their closest relatives, Lycium, Jaborosa and the Hyoscyameae. The features examined here allow the differentiation of Nolana, Sclerophylax and Lycium; however, studies on a larger number of species (especially in Nolana) would be required in order to propose patterns that would definitely separate them from other genera of the Solanaceae. However, all the variables analysed do enable eight species to be singled out clearly.
Acknowledgements
Associate Editor: Dr Gary Houliston.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adams SP, Leitch IJ, Bennett MD, Chase MW, Leitch AR. 2000. Ribosomal DNA evolution and phylogeny in Aloe (Asphodelaceae). American Journal of Botany. 87:1578–1583. doi: 10.2307/2656733
- Altinkut A, Kotseruba V, Kirzhner VM, Nevo E, Raskina O, Belyayev A. 2006. Ac-like transposons in populations of wild diploid Triticeae species: comparative analysis of chromosomal distribution. Chromosome Research. 14:307–317. doi: 10.1007/s10577-006-1048-3
- Appels R, Honeycutt RL. 1986. Datta SK, editor. rDNA: evolution over a billon years. Boca Raton, Florida: DNA systematics CRC Press Inc.
- Badr A, Khalifa SF, Aboel-Atta AI, Abou-El-Enain MM. 1997. Chromosomal criteria and taxonomic relationships in the Solanaceae. Cytologia. 62:103–113. doi: 10.1508/cytologia.62.103
- Barraza O. 1994. Flora del Valle de Lerma. Aportes botánicos de Salta – Ser. Flora. Buenos Aires 177 - 4400 Salta – República Argentina. 25:1–9.
- Battaglia E. 1955. Chromosome morphology and terminology. Caryologia. 8:179–187. doi: 10.1080/00087114.1955.10797556
- Bernardello G, Stiefkens L, Las Peñas ML. 2008. Karyotype studies in Grabowskia and Phrodus (Solanaceae). Plant Systematics and Evolution. 275:265–269. doi: 10.1007/s00606-008-0060-9
- Bernardello L. 1986. Revisión taxonómica de las especies sudamericanas de Lycium (Solanaceae). Boletín de la Academia Nacional de Ciencias de Córdoba. 57:173–356.
- Bernardello L, Chiang-Cabrera F. 1998. A cladistic study in the American species of Lycium (Solanaceae) based on morphological variation. Monographs in Systematic Botany of the Missouri Botanical Garden. 68:33–46.
- Blanco S, Las Peñas ML, Bernardello G, Stiefkens L. 2012. Mapeo de genes ribosómicos y heterocromatina en seis especies de Lycium de sudamérica (Solanaceae). Boletín de la Sociedad Argentina de Botánica. 47:389–399.
- Bolkhovskikh Z, Grif V, Matvejeva T, Zakharyeva O. 1969. Solanaceae Juss. In: Fedorov A, editor. Chromosome numbers of flowering plants (Reprint 1974). Koenigstein: O. Koeltz; p. 685–703.
- Bruno GB. 1994. Organizacion y vasculatura del gineceo de Nolana crassulifolia y N. rostrata (Nolanaceae). Boletín de la Sociedad Argentina de Botánica. 30:51–57.
- Chacón J, Sousa A, Baeza CM, Renner SS. 2012. Ribosomal DNA distribution and a genus-wide phylogeny reveal patterns of chromosomal evolution in Alstroemeria (Alstroemeriaceae). American Journal of Botany. 99:1501–1512. doi: 10.3732/ajb.1200104
- Chang YC, Shii CT, Chung MC. 2009. Variations in ribosomal RNA gene loci in spider lily (Lycoris spp.). Journal of the American Society for Horticultural Science. 134:567–573.
- Chiarini FE, Barboza GE. 2008. Karyological studies in Jaborosa (Solanaceae). Botanical Journal of the Linnean Society. 156:467–478. doi: 10.1111/j.1095-8339.2007.00734.x
- Chiarini FE, Moré M, Urdampilleta JD, Barboza GE. 2013. Phylogeny and chromosome evolution in Jaborosa (Solanaceae). 3° Reunião Brasileira de Citogenética - IV Simposio Latino Americano de Citogenética y Evolución. 26 al 29 de Mayo de 2013. Guarujá, São Paulo, Brasil.
- Chiarini FE, Moreno N, Barboza GE, Bernardello G. 2010. Karyotype characterization of Andean Solanoideae (Solanaceae). Caryologia. 63:278–291. doi: 10.1080/00087114.2010.589738
- Chiarini FE, Moreno N, Moré M, Barboza G. 2016. Chromosomal changes and recent diversification in the Andean genus Jaborosa (Solanaceae). Botanical Journal of the Linnean Society (on-line). doi:10.1111/boj.12493.
- Chiarini FE, Santiñaque FF, Urdampilleta JD, Las Peñas ML. 2014. Genome size and karyotype diversity in Solanum sect. Acanthophora (Solanaceae). Plant Systematics and Evolution. 300:113–125. doi: 10.1007/s00606-013-0864-0
- Di Fulvio TE. 1961. El género Sclerophylax (Solanaceae), estudio anatómico, embriológico y cariológico con especial referencia a la taxonomía. Museo Botánico, FCEFyN. Córdoba, Argentina. Kurtziana. 1:9–103.
- Dillon MO. 2005. The Solanaceae of the lomas formations of coastal Peru and Chile. Monographs in Systematic Botany. 104:131–156.
- Dillon MO, Tu T, Soejima A, Yi T, Nie Z, Tye A, Wen J. 2007. Phylogeny of Nolana (Nolaneae, Solanoideae, Solanaceae) as inferred from granule-bound starch synthase I (GBSSI) sequences. Taxon. 56:1000–1011. doi: 10.2307/25065900
- Dillon MO, Tu T, Xie L, Quipuscoa Silvestre V, Wen J. 2009. Biogeographic diversification in Nolana (Solanaceae), a ubiquitous member of the Atacama and Peruvian Deserts along the western coast of South America. Journal of Systematics and Evolution. 47:457–476. doi: 10.1111/j.1759-6831.2009.00040.x
- Drouin G, De Sa MM. 1995. The concerted evolution of 5S ribosomal genes linked to the repeat units of other multigene families. Molecular Biology and Evolution. 12:481–493.
- Dubcovsky J, Dvorák J. 1995. Ribosomal RNA multigene loci: nomads of the triticeae genomes. Genetics. 140:1367–1377.
- Fregonezi JN, Fernandes T, Torezan JMD, Vieira AOS, Vanzela ALL. 2006. Karyotype differentiation of four Cestrum species (Solanaceae) based on the physical mapping of repetitive DNA. Genetics and Molecular Biology. 29:97–104. doi: 10.1590/S1415-47572006000100019
- Freyre R, Douglas AC, Dillon MO. 2005. Artificial hybridizations in five species of Chilean Nolana (Solanaceae). HortScience. 40:532–536.
- Garcia S, Garnatje T, Hidalgo O, McArthur ED, Siljak-Yakovlev S, Vallès J. 2007. Extensive ribosomal DNA (18S-5.8S-26S and 5S) colocalization in the North American endemic sagebrushes (subgenus Tridentatae, Artemisia, Asteraceae) revealed by FISH. Plant Systematics and Evolution. 267:79–92. doi: 10.1007/s00606-007-0558-6
- Gerlach WL, Bedbrook JL. 1979. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Research. 7:1869–1885. doi: 10.1093/nar/7.7.1869
- Guerra M. 1983. O uso da Giemsa na citogenética vegetal. Ciencias Culturales. 35:190–193.
- Guerra M. 2000. Patterns of heterochromatin distribution in plant chromosomes. Genetics and Molecular Biology. 23:1029–1041. doi: 10.1590/S1415-47572000000400049
- Hasterok R, Marasek A, Donnison IS, Armstead I, Thomas A, King IP, Jenkins G. 2006. Alignment of the genomes of Brachypodium distachyon and temperate cereals and grasses using bacterial artificial chromosome landing with fluorescence in situ hybridization. Genetics. 173:349–362. doi: 10.1534/genetics.105.049726
- Heslop-Harrison JS, Schwarzacher T. 2011. Organisation of the plant genome in chromosomes. The Plant Journal. 66:18–33. doi: 10.1111/j.1365-313X.2011.04544.x
- Hunziker AT. 1979. South American Solanaceae: a synoptic survey. Linnean Society Symposium Series. 7:49–85.
- Hunziker AT. 2001. The genera of Solanaceae illustrated, arranged according to a new system. Ruggell: A.R.G. Gantner Verlag K. G.; p. 1–500.
- Johnston IM. 1936. A study of the Nolanaceae. Proceedings of the American Academy of Arts and Sciences. 71:1–83. doi: 10.2307/20023213
- Knapp S. 2002. Tobacco to tomatoes: a phylogenetic perspective on fruit diversity in the Solanaceae. Journal of Experimental Botany. 53:2001–2022. doi: 10.1093/jxb/erf068
- Kwon JK, Kim BD. 2009. Localization of 5S and 25S rRNA genes on somatic and meiotic chromosomes in Capsicum species of chili pepper. Molecules and Cells. 27:205–209. doi: 10.1007/s10059-009-0025-z
- Las Peñas ML, Bernardello G, Kiesling R. 2008. Karyotypes and fluorescent chromosome banding in Pyrrhocactus (Cactaceae). Plant Systematics and Evolution. 272:211–222. doi: 10.1007/s00606-007-0611-5
- Levan A, Fredga K, Sandberg A. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52:201–220. doi: 10.1111/j.1601-5223.1964.tb01953.x
- Lim KY, Matyášek R, Lichtenstein CP, Leitch AR. 2000. Molecular cytogenetic analyses and phylogenetic studies in the Nicotiana section Tomentosae. Chromosoma. 109:245–258. doi: 10.1007/s004120000074
- Lönnig WE, Saedler H. 2002. Chromosome rearrangements and transposable elements. Annual Review of Genetics. 36:389–410. doi: 10.1146/annurev.genet.36.040202.092802
- Miller JS, Kamath A, Damashek J, Levin RA. 2011. Out of America to Africa or Asia: inference of dispersal histories using nuclear and plastid DNA and the SRNase self-incompatibility locus. Molecular Phylogenetics and Evolution. 28:793–801.
- Moscone EA, Lambrou M, Ehrendorfer F. 1996. Fluorescent chromosome banding in the cultivated species of Capsicum (Solanaceae). Plant Systematics and Evolution. 202:37–63. doi: 10.1007/BF00985817
- Moscone EA, Scaldaferro MA, Grabiele M, Cecchini NM, Sánchez García Y, Jarret R, Daviña JR, Ducasse DA, Barboza GE, Ehrendorfer F. 2007. The evolution of chili peppers (Capsicum – Solanaceae): a cytogenetic perspective. Acta Horticulturae. 745:137–170. doi: 10.17660/ActaHortic.2007.745.5
- Olmstead RG, Bohs L. 2007. A summary of molecular systematic research in Solanaceae: 1982–2006. Acta Horticulturae. 745:255–268. doi: 10.17660/ActaHortic.2007.745.11
- Olmstead RG, Bohs L, Migid HA, Santiago-Valentin E, Garcia VF, Collier SM. 2008. A molecular phylogeny of the Solanaceae. Taxon. 57:1159–1181.
- Rego LNAA, Da Silva CRM, Torezan JMD, Gaeta ML, Vanzela AL. 2009. Cytotaxonomical study in Brazilian species of Solanum, Lycianthes and Vassobia (Solanaceae). Plant Systematics and Evolution. 279:93–102. doi: 10.1007/s00606-009-0149-9
- Romero Zarco C. 1986. Report of the committee for Spermatophyta: 30. Taxon. 35:556–530. doi: 10.2307/1221918
- Sanchez-Puerta MV, Abbona CC. 2014. The chloroplast genome of Hyoscyamus niger and a phylogenetic study of the tribe Hyoscyameae (Solanaceae). PloS One. 9:e98353. doi: 10.1371/journal.pone.0098353
- Särkinen T, Bohs L, Olmstead RG, Knapp S. 2013. A phylogenetic framework for evolutionary study of the nightshades (Solanaceae): a dated 1000-tip tree. BMC Evolutionary Biology. 13:214–0. doi: 10.1186/1471-2148-13-214
- Schwarzacher T, Heslop-Harrison P. 2000. Practical in situ hybridization. Oxford: Bios Scientific Publishers Limited.
- Schweizer D. 1976. Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma. 58:307–324. doi: 10.1007/BF00292840
- Schweizer D, Ambros P. 1994. Chromosome banding. In: Gosden JR, editor. Methods in molecular biology, chromosome analysis protocols. Totowa: Humana Press.
- Stiefkens L, Bernardello L. 1996. Karyotypic studies in South American Lycium (Solanaceae). Cytologia. 61:395–402. doi: 10.1508/cytologia.61.395
- Stiefkens L, Bernardello G. 2000. Karyotypes and DNA content in diploid and polyploid Lycium (Solanaceae). Boletín de la Sociedad Argentina de Botánica. 35:237–244.
- Stiefkens L, Bernardello G. 2002. Karyotypic studies in Lycium section Mesocope (Solanaceae) from South America. Caryologia. 55:199–206. doi: 10.1080/00087114.2002.10589278
- Stiefkens L. 2001. Estudios cariotípicos y de contenido de ADN en la tribu Lycieae (Solanaceae). Tesis Doctoral. Universidad Nacional de Córdoba.
- Stiefkens L, Las Peñas ML, Bernardello G. 2009. Cariotipos y bandeo cromosómico fluorescente en seis especies norteamericanas de Lycium (Solanaceae). Boletín de la Sociedad Argentina de Botánica. 44:26.
- Stiefkens L, Las Peñas ML, Bernardello G, Levin RA, Miller JS. 2010. Karyotypes and fluorescent chromosome banding patterns in southern African Lycium (Solanaceae). Caryologia. 63:50–61. doi: 10.1080/00087114.2010.10589708
- Tago-Nakawaza M, Dillon MO. 1999. Biogeografia y evolución en el clado Nolana (Solaneae-Solanaceae). Arnaldoa. 6:81–116.
- Tang H, Bowers JE, Wang X, Ming R, Alam M, Paterson AH. 2008. Synteny and collinearity in plant genomes. Science. 320:486–488. doi: 10.1126/science.1153917
- Tu T, Dillon MO, Sun H, Wen J. 2008. Phylogeny of Nolana (Solanaceae) of the Atacama and Peruvian deserts inferred from sequences of four plastid markers and the nuclear LEAFY second intron. Molecular Phylogenetics and Evolution. 49:561–573. doi: 10.1016/j.ympev.2008.07.018
- Tu T, Volis S, Dillon MO, Sun H, Wen J. 2010. Dispersals of Hyoscyameae and Mandragoreae (Solanaceae) from the New World to Eurasia in the early Miocene and their biogeographic diversification within Eurasia. Molecular Phylogenetics and Evolution. 57:1226–1237. doi: 10.1016/j.ympev.2010.09.007
- Tu TY, Sun H, Gu ZJ, Yue JP. 2005. Cytological studies on the Sino-Himalayan endemic Anisodus and four related genera from the tribe Hyoscyameae (Solanaceae) and their systematic and evolutionary implications. Botanical Journal of the Linnean Society. 147:457–468. doi: 10.1111/j.1095-8339.2005.00384.x
