ABSTRACT
Mucidosphaerium sphagnale, a new record for New Zealand, is reported from Awarua Wetland. The genus Mucidosphaerium has recently been separated from the well-known Dictyosphaerium, a colonial green alga with cells connected by thin, branching stalks, to encompass those forms with near-spherical adult cells. The previous name for this taxon, Dictyosphaerium sphagnale, is not recorded from New Zealand; however, Mucidosphaerium pulchellum (syn. Dictyosphaerium pulchellum), which has been reported here many times and is very difficult to distinguish without DNA sequences, may represent previously misidentified instances of M. sphagnale. The M. sphagnale is reported here from a Sphagnum bog, as is typical of its European range, whereas M. pulchellum in New Zealand appears to have been reported exclusively from lakes and rivers. The organism reported here is therefore quite likely a genuine new occurrence. Co-introduction to New Zealand with foreign Sphagnum is a possibility.
Introduction
The genus Dictyosphaerium Nӓgeli comprises colonial green algae in which the cells are united by thin, branching stalks originating at the centre of an approximately spherical, mucilaginous colony (Komárek & Perman Citation1978). Dictyosphaerium has been widely reported in New Zealand, mostly from lakes. Two species are relatively common, according to Cassie (Citation1984): Dictyosphaerium ehrenbergianum Nӓgeli (12 sites) and Dictyosphaerium pulchellum H.C.Wood (20 sites); other species reported from New Zealand are Dictyosphaerium planktonicum Tiffany & Ahlstrom (one site) and Dictyosphaerium primarium Skuja (two sites). The most recent checklist by Broady et al. (Citation2012) included no additional records but noted the transfer of D. planktonicum to Lobocystis planctonica (Tiffany & Ahlstrom) Fott, and D. primarium to Dictyosphaerium subsolitarium Van Goor.
Dictyosphaerium has undergone a prolonged series of taxonomic revisions. Those incorporating DNA sequence data (which are perhaps more reliable) have led to the establishment of the genus Hindakia C.Bock, Prӧschold & Krienitz for forms belonging to the Chlorella clade (Bock et al. Citation2010; Krienitz et al. Citation2010), and more recently the splitting of the remaining forms in the Parachlorella clade into those with ellipsoidal mature cells (Dictyosphaerium) from those with near-spherical mature cells: the genus Mucidosphaerium C. Bock, Prӧschold & Krienitz (Bock et al. Citation2011). One of the species previously reported from New Zealand, D. pulchellum, has been transferred to this genus.
Here the occurrence of a further species, Mucidosphaerium sphagnale (Hindák) C. Bock, Prӧschold & Krienitz (syn. Dictyosphaerium sphagnale Hindák), previously known only from Europe, is reported from New Zealand for the first time.
Materials and methods
Taxon sampling, culturing and observation
Water was squeezed from Sphagnum collected from a pool in the Awarua Wetland complex, 46.553364°S, 168.637025°E, into a sterile container on 7 November 2011. The sample was kept on ice until transferred to the laboratory after 3 days in the field, when it was used to inoculate sterile MLA medium (Bolch & Blackburn Citation1996) containing 0.5% agarose. Cells were grown at room temperature under natural light and repeatedly subcultured using standard aseptic techniques to obtain a unialgal strain. Specimens were examined using a Leica DMLB compound microscope with Nomarksi differential interference contrast and a Canon DS126271 digital camera.
Electron microscopy
Cells were prepared for the transmission electron microscope by fixing in 3% glutaraldehyde, 0.1 m cacodylate buffer, for 3 h. After washing for 1 h in three changes of 0.1 m cacodylate buffer, specimens were post-fixed in 1% osmium tetroxide, 0.1 m cacodylate buffer, for 2 h. The specimens were dehydrated in ethanol and embedded in Spurr’s resin. Sections were cut, stained in uranyl acetate and lead citrate, and examined with a JEOL 1200 EX microscope.
DNA extraction, PCR amplification and sequencing
A Petri plate containing an approximately 3-week-old culture of strain LCR-Awa6/2 was verified microscopically to be unialgal. Algae were scraped from the plate into an Eppendorf tube, manually disrupted using a sterile plastic pestle in lysis buffer from a Maxwell extraction cartridge, and the resulting mixture was transferred back to the cartridge and subjected to extraction in an automated Maxwell®16 instrument (Promega Corporation). The resulting samples were processed using Zymo Clean and Concentrate columns, according to the manufacturer’s instructions (Zymo Research). The resulting genomic DNA was used as a template in polymerase chain reactions (PCR) incorporating the primers and conditions of Mikhailyuk et al. (Citation2008) for the nuclear ITS1-5.8S-ITS2 region. Sequencing of these products was carried out by Landcare Research New Zealand Ltd. Electropherograms were checked using Sequencher 4.8 (Gene Codes Corporation). The sequence of strain LCR-Awa6/2 is available at https://www.ncbi.nlm.nih.gov/, accession number KX812540.
Phylogenetic analysis
The sequence was aligned to the internal transcribed spacer (ITS) alignment of Bock et al. (Citation2011; submission 10727 at https://treebase.org) using Profile Alignment Mode in ClustalX 1.8 (Thompson et al. Citation1997) and checked by eye. The resulting data set contained 843 base positions, 371 of which were variable, including 295 parsimony-informative sites.
The aligned data set was used in two phylogenetic analyses. 1. Bayesian inference in MrBayes v3.2 (Ronquist & Huelsenbeck Citation2003), using the GTR + G substitution model selected using MEGA6 (Tamura et al. Citation2013): two parallel runs were completed, each of 2.5 million cycles, with default burn in, and parameter estimates were compared with their associated variances to assess the effectiveness of modelling. 2. Maximum Parsimony heuristic bootstrap analysis in PAUP*4.0b10 (Swofford Citation2002) was carried out using the following settings: branches collapsed if maximum length = 0, DELTRAN character state optimisation, Maxtrees set at 10,000, tree bisection and reconnection branch-swapping algorithm, 10 random sequence additions, and assignment of character states not observed in terminal taxa allowed at internal nodes. Non-parametric bootstrap values for nodes were calculated on the basis of 1000 replicates.
Results
Illustrative material is provided in , and the results of molecular analyses are shown in .
Figure 1. Mucidosphaerium sphagnale strain LCR-Awa6/2, viewed by light and electron microscopy. A–G, Light microscopy. A, Mature colony with large, near-spherical cells. B, Colony with a single autosporangium (arrow), showing the elongated shape of autospores. C, Colony enlargement: several of the mature cells have formed autosporangia; on release the autospores remain attached. D–G, Formation of a new colony: on initial release, the four autospores remain attached at their narrower ends; lengthening of the stalks follows, and usually rotation of the cell such that the pyrenoid (arrows) moves from a lateral to a basal position. H, Electron microscopy of the pyrenoid, which is surrounded by a bilenticular starch sheath and bisected by a single thylakoid (arrow), as observed in other members of the Parachlorella clade. Scale in G = 10 µm. Scale in H = 0.5 µm. Use scale in G for A–G.
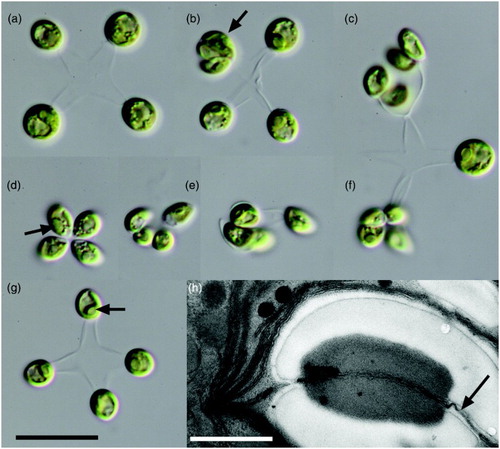
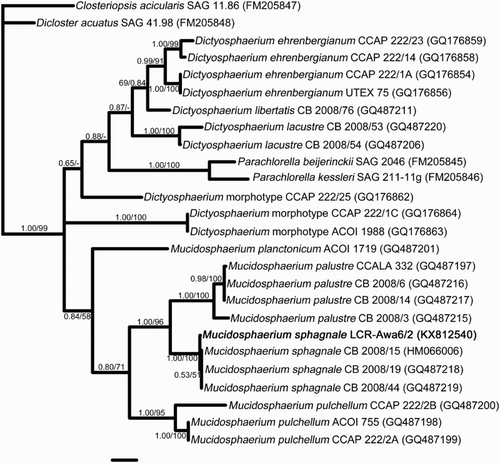
Figure 2. Bayesian phylogeny of the ITS1-5.8S-ITS2 region based on the secondary structure alignment of Bock et al. (Citation2011) with Mucidosphaerium sphagnale LCR-Awa6/2 added (shown in bold). Strain numbers follow the names, with GenBank Accession numbers in brackets. The species of Mucidosphaerium established by Bock et al. are clearly resolved. Support values above branches are Bayesian Posterior Probabilities / Maximum Parsimony bootstrap percentages. Scale bar represents 0.1 substitutions per site.
Mucidosphaerium sphagnale (Hindák) C. Bock, Pröschold & Krienitz
()
Reference: Bock et al. (Citation2011), p. 642, fig. 6G.
Basionym: Dictyosphaerium sphagnale Hindák
Specimens examined
Cultured strain LCR-Awa6/2, isolated from material collected at Awarua Wetland, 46.553364°S, 168.637025°E, pond water containing Sphagnum sp., 7 November 2011.
Colonies approximately spherical, 7–30 µm diameter, containing cells united by thin dichotomously or tetrachotomously branching stalks originating from the colony centre; unicells occasionally observed. Cells ellipsoidal to pyriform, fusiform or semilunate when young, broadly ovoid to spherical when mature, 2.1–4.9 µm wide, 3.6–5.5 µm long, length : width approximately 1.7 at autospore release, reducing to approximately 1.1 at maturity. Chloroplast parietal, cup-shaped, with a single pyrenoid surrounded by two hemispherical starch grains. Pyrenoid matrix bisected by a single thylakoid. Pyrenoid variably positioned (although often lateral) in young cells, which are joined at their narrow ends by very short mucilaginous stalks that lengthen with age, at which time the pyrenoid is usually located at the side of the cell to which the stalk is connected. Colonies 4–16 celled, up to approximately 30 µm in diameter.
DNA data
The ITS sequence of this strain was closest to two strains of M. sphagnale (CB 2008/19 and CB 2008/44, p-distance = 0.005 in each case; p-distance to CB 2008/15 = 0.008). Both methods of analysis placed the strain in a clade comprising M. sphagnale with high support (). The phylogeny was largely congruent with that of Bock et al. (Citation2011), despite the use of shorter sequences (ITS region only).
Distribution
A new record for New Zealand. This appears to be the first record of the species outside Europe.
Discussion
The recently established genus Mucidosphaerium is readily distinguished from Dictyosphaerium morphotypes by its near-spherical mature cells (Bock et al. Citation2011). Spherical-celled species in New Zealand have previously been identified as D. pulchellum H.C.Wood (now Mucidosphaerium pulchellum (H.C.Wood) C. Bock, Pröschold & Krienitz) and D. primarium Skuja (since subsumed into D. subsolitarium Van Goor; Cassie Cooper Citation2001). Cells of the latter are spherical (suggesting that this species will prove to belong in Mucidosphaerium should molecular data become available), but considerably smaller at maturity than those found in the present material; Lineham (Citation1983) noted a maximum diameter of 3 µm in specimens from Te Waihora/Lake Ellesmere. New Zealand records of D. primarium/subsolitarium can be excluded by size as possible previous observations of M. sphagnale.
Records of M. pulchellum are more difficult to distinguish and could represent misidentified records of M. sphagnale. According to the original description of Wood (Citation1873), the cell diameter of M. pulchellum is 0.00025″, or approximately 6 µm. This is outside the size range of the specimens reported here; however, later authors state size ranges such as 5–8 µm (Komárek & Perman Citation1978), 6–8 µm (Bock et al. Citation2011), or 5 µm for cells in colonies (Lineham Citation1983) for this species; the latter measurement is the upper size limit observed in the Awarua strain. As far as can be determined, only two records of M. pulchellum from New Zealand contain descriptions and/or illustrations. Cells in the image by Kloos (Citation1976) correspond to approximately 5–9 µm at the stated scale, and those of Lineham (Citation1983) are 5–7 µm. There seems to be only slight overlap between these ranges and those of the Awarua material, which is in fair agreement with the description of Bock et al. (Citation2011) for M. sphagnale (adult cells 4–6(–7.5) µm in diameter; confirmed with online images of strain CCAP 222/13 at www.ccap.ac.uk). It seems safest to conclude that morphology does not reliably discriminate these two species, a view shared by Bock et al. (Citation2011), who stated that the size overlap between all species of Mucidosphaerium means that DNA sequence data are necessary to separate them; DNA data unequivocally place the New Zealand strain in M. sphagnale (). The use of morphology at the level of light microscopy does not, therefore, rule out previous records of M. pulchellum being misidentified collections of M. sphagnale.
Pyrenoid ultrastructure can sometimes be used to distinguish close relatives (e.g. Friedl Citation1989), but ultrastructural features of these species do not yet appear to have been widely studied. However, pyrenoid ultrastructure of strain LCR-Awa6/2, in which a single bisection of the pyrenoid by thylakoids occurs, appears the same as that observed in other members of the Parachlorella clade, such as Parachlorella kessleri (Fott & Nováková) Krienitz, EH Hegewald, Hepperle, V Huss, T Rohr & M Wolf (Juárez et al. Citation2011) and Planktochlorella nurekis Škaloud & Nĕmcová (Škaloud et al. Citation2014). This character state therefore seems likely to be present throughout the group, and may offer little further taxonomic information.
However, these species may be distinguished by their ecology. Mucidosphaerium pulchellum (as D. pulchellum) has been recorded from at least 19 sites in New Zealand (Cassie Citation1984). All of these are lakes, apart from records from the Waikato River (Hill Citation1970; Lam Citation1977; Waikato Valley Authority Citation1979). In contrast, M. sphagnale (as D. sphagnale) has historically been recorded from bog habitats (Komárek & Fott Citation1983; Bock et al. Citation2011). The record of M. sphagnale reported here, from a wetland, is therefore consistent for a first observation of this species in New Zealand. The species does not appear to be common: in a wide-ranging survey of algal diversity in 21 North Island and 17 South Island wetland habitats, D. ehrenbergianum was the only species of Dictyosphaeriaceae recorded, and that only once in the North Island and four times in the South Island (Kilroy & Sorrel Citation2013). A re-examination of images collected during the survey of that material (C. Kilroy, NIWA, pers. comm. 2016) indicates that the cells were near-spherical rather than ellipsoidal, and not connected at short axes. However, identifications were undertaken on frozen samples, and the effect of freezing on the ellipsoidal cell morphology of D. ehrenbergianum is presently unknown. Consequently, the identity of the Dictyosphaeriaceae in the survey by Kilroy & Sorrel (Citation2013) would require further investigation to resolve.
The species Mucidosphaerium planctonicum C. Bock, Pröschold & Krienitz () has not been found in New Zealand to date. This name should not be confused with Dictyosphaerium planktonicum, a species that has been recorded in New Zealand from Albany (Chapman et al. Citation1957) but has since been transferred to the genus Lobocystis (Fott Citation1975). Examination of the original illustration of Tiffany & Ahlstrohm (Citation1931) shows strongly elongate cells, quite different from M. sphagnale.
The new record of M. sphagnale raises the possibility of a recent introduction of this species to New Zealand. If that is the case, how might it have arrived? The presence of introduced Sphagnum in New Zealand, such as Sphagnum subnitens Russ & Warnst., offers a possible route through co-invasion. Although many microbes are associated with Sphagnum (Hingley Citation1993; Kreutz & Foissner Citation2006), associations with particular species of the moss appear to be little studied (Kostka et al. Citation2016). Images of Sphagnum taken during collection of M. sphagnale from Awarua Wetland represent the widespread indigenous species Sphagnum falcatulum Besch. (A. Fife and D. Glenny, Landcare Research, pers. comm.). Sphagnum subnitens is restricted to Westland (Fife Citation1996), and the distribution of M. sphagnale in New Zealand beyond Awarua Wetland is unknown, so there is currently no evidence for an association. However, it is certainly possible for algae, such as Didymosphenia geminata (Lyngbye) Schmidt, which is sensitive to salt, drying, freezing and the low pH characteristic of a bird gut (Kilroy et al. Citation2007), to disperse widely following successful introduction (Kilroy & Unwin Citation2011). Therefore, lack of demonstrated overlap in distributions between introduced Sphagnum and introduced algae does not necessarily negate the former as an invasion vector for the latter.
Acknowledgements
The author thanks M. Schallenberg (University of Otago) for field assistance, R Cole (Department of Conservation) for helpful advice regarding collecting sites, and R Smissen, D Glenny, A Fife, I Breitwieser, R Prebble and two anonymous reviewers for helpful comments on the manuscript. Associate editor: Dr Roberta D'Archino.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Bock C, Pröschold T, Krienitz L. 2010. Two new Dictyosphaerium-morphotype lineages of the Chlorellaceae (Trebouxiophyceae): Heynigia gen. nov. and Hindakia gen. nov. European Journal of Phycology 45:267–77. doi: 10.1080/09670262.2010.487920
- Bock C, Pröschold T, Krienitz L. 2011. Updating the genus Dictyosphaerium and description of Mucidosphaerium gen. nov. (Trebouxiophyceae) based on morphological and molecular data. Journal of Phycology 47:638–652. doi: 10.1111/j.1529-8817.2011.00989.x
- Bolch CJS, Blackburn SI. 1996. Isolation and purification of Australian isolates of the toxic cyanobacterium Microcystis aeruginosa Kütz. Journal of Applied Phycology 8:5–13. doi: 10.1007/BF02186215
- Broady PA, Flint EA, Nelson WA, Cassie Cooper V, De Winton MD, Novis PM. 2012. Phyla Chlorophyta and Charophyta: green algae. In: Gordon DP, editor. New Zealand inventory of biodiversity volume three. Kingdoms Bacteria, Protozoa, Chromista, Plantae, Fungi. Christchurch: Canterbury University Press; p. 347–381.
- Cassie V. 1984. Revised checklist of the freshwater algae of New Zealand (excluding diatoms and charophytes) Part I (Water and Soil Technical Publication 25). Wellington: National Water and Soil Conservation Authority.
- Cassie Cooper V. 2001. Recent name changes in eukaryotic freshwater algae of New Zealand. New Zealand Journal of Botany 39:601–616. doi: 10.1080/0028825X.2001.9512763
- Chapman VJ, Thompson RH, Segar ECM. 1957. Check list of the freshwater algae of New Zealand. Transactions of the Royal Society of New Zealand 84: 695–747.
- Fife AJ. 1996. A synopsis of New Zealand Sphagna, with a description of S. simplex sp. nov. New Zealand Journal of Botany 34:309–328. doi: 10.1080/0028825X.1996.10410697
- Fott B. 1975. Übersicht der Familie der Characiaceae (Chlorococcales) mit taxonomischen Namensänderungen und Neubeschreibungen. Preslia 47:211–231.
- Friedl T. 1989. Comparative ultrastructure of pyrenoids in Trebouxia (Microthamniales, Chlorophyta). Plant Systematics and Evolution 164:145–159. doi: 10.1007/BF00940435
- Hill CF. 1970. Phyto- and zooplankton recorded from the Waikato River and hydroelectric lakes between Taupo control gates and the Meremere power station. Hamilton: New Zealand Electricity Department.
- Hingley M. 1993. Microscopic life in Sphagnum. In: Corbet SA, Disney RHL, editors. Naturalists Handbooks 20. Slough: The Richmond Publishing Company; 64 p.
- Juárez ÁB, Vélez CG, Iñiguez AR, Martínez DE, Rodríguez MC, Vigna MS, de Molina MCR. 2011. A Parachlorella kessleri (Trebouxiophyceae, Chlorophyta) strain from an extremely acidic geothermal pond in Argentina. Phycologia 50:413–421. doi: 10.2216/10-79.1
- Kilroy C, Lagerstedt MA, Robinson K. 2007. Studies on the survival of the invasive diatom Didymosphenia geminata under a range of environmental and chemical conditions. NIWA Client Report 2006-095 for Biosecurity New Zealand, 15 p.
- Kilroy C, Sorrel B. 2013. Algal biodiversity of New Zealand wetlands: distribution patterns and environmental linkages (Science for Conservation 324). Wellington: Department of Conservation. Available from: http://www.doc.govt.nz/Documents/science-and-technical/sfc324entire.pdf
- Kilroy C, Unwin M. 2011. The arrival and spread of the bloom-forming, freshwater diatom Didymosphenia geminata, in New Zealand. Aquatic Invasions 6:249–262. doi: 10.3391/ai.2011.6.3.02
- Kloos JA. 1976. Phytoplankton in Lake Rotorua and Lake Okareka, and its interaction with aquatic macrophytes [MSc thesis]. University of Waikato, Hamilton.
- Komárek J, Fott B. 1983. Chlorophyceae (Grünalgen) Ordnung: Chlorococcales. In: Huber-Pestalozzi G, editor. Das Phytoplankton des Süßwassers 7. Teil, 1. Stuttgart, Germany: Hälfte. E. Schweizerbartsche Verlagsbuchhandlung (Nägele u. Obermiller); p. 1–1044.
- Komárek J, Perman J. 1978. Review of the genus Dictyosphaerium (Chlorococcales). Algological Studies 20:233–297.
- Kostka JE, Weston DJ, Glass JB, Lilleskov EA, Shaw AJ, Turetsky MR. 2016. The Sphagnum microbiome: new insights from an ancient plant lineage. New Phytologist 211:57–64. doi: 10.1111/nph.13993
- Krienitz L, Bock C, Luo W, Pröschold T. 2010. Polyphyletic origin of the Dictyosphaerium morphotype within Chlorellaceae (Trebouxiophyceae). Journal of Phycology 46:559–63. doi: 10.1111/j.1529-8817.2010.00813.x
- Kreutz M, Foissner W. 2006. The Sphagnum ponds of Simmelried in Germany: a biodiversity hot-spot for microscopic organisms. In: Foissner W, editor. Protozoological monographs 3. Aachen: Shaker Verlag; 267 p.
- Lam CWY. 1977. Blue-green algae in the Waikato River [PhD thesis]. Auckland University, Auckland.
- Lineham IW. 1983. Eutrophication of Lake Ellesmere: a study of phytoplankton [PhD thesis]. University of Canterbury, Christchurch.
- Mikhailyuk TI, Sluiman HJ, Massalski A, Mudimu O, Demchenko EM, Kondratyuk SY, Friedl T. 2008. New streptophyte green algae from terrestrial habitats and an assessment of the genus Interfilum (Klebsormidiophyceae, Streptophyta). Journal of Phycology 44:1586–1603. doi: 10.1111/j.1529-8817.2008.00606.x
- Ronquist F, Huelsenbeck JP. 2003. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi: 10.1093/bioinformatics/btg180
- Škaloud P, Nĕmcová Y, Pytela J, Bogdanov NI, Bock C, Pickinpaugh SH. 2014. Planktochlorella nurekis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta), a novel coccoid green alga carrying significant biotechnological potential. Fottea 14: 53–62. doi: 10.5507/fot.2014.004
- Swofford DL. 2002. PAUP*: phylogenetic analysis using parsimony (* and other methods), version 4.0b10. Sunderland, MA: Sinauer Associates.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30:2725–2729. doi: 10.1093/molbev/mst197
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25:4876–4882.
- Tiffany LH, Ahlstrohm EH. 1931. New and interesting plankton algae from Lake Erie. Ohio Journal of Science 31:455–467.
- Waikato Valley Authority. 1979. The Waikato River: a water resources study (Water and Soil Technical Publication 11). Wellington: Water and Soil Division, Ministry of Works and Development.
- Wood HC. 1873. A contribution to the history of the fresh-water algae of North America (Smithsonian Contributions to Knowledge 19). Washington, DC: Smithsonian Institution.
