ABSTRACT
We examined the phylogenetic relationships within Sophora sect. Edwardsia using DNA sequences from the chloroplast trnQ-5′rps16 and trnHGUG-psbA intergenic spacers and the nuclear-encoded chloroplast-expressed glutamine synthetase gene. Sequences were analysed with median-joining networks and phylogenetic approaches. Low sequence diversity was detected, which is consistent with past genetic studies of the section. Chloroplast and nuclear sequences are shared across large geographic distances. The New Zealand species did not form a monophyletic group, nor did the species from Chile. However, species on some Pacific Islands (Lord Howe Island, Easter Island, Hawaii and French Polynesia) and Réunion Island in the Indian Ocean appear to have unique chloroplast haplotypes, indicating isolation. The S. chrysophylla chloroplast haplotype derives from the French Polynesian haplotype rather than S. denudata from Réunion Island, with which it shares morphological characters. In the nuclear phylogeny Sophora macrocarpa was sister to the remaining species that were sequenced in the section, a relationship that has been previously suggested from morphological analysis.
Introduction
Sophora sect. Edwardsia Salisb. is a monophyletic group (Mitchell & Heenan Citation2002) of small trees or shrubs mostly distributed around the Southern Hemisphere oceans (). Of the 19 species all are restricted to single islands or countries, apart from S. cassioides (Phil.) Sparre. New Zealand has the highest species diversity with eight species (Heenan et al. Citation2001). Chile and the Juan Fernandez Islands each have two species, with one of the Chilean species, S. cassioides, also found on Gough Island in the Atlantic Ocean. Six species occur on islands or archipelagos in the Pacific Ocean and a single species occurs on Réunion Island in the Indian Ocean. A number of species in the section are of conservation concern (http://www.iucnredlist.org), with one species, the Easter Island endemic S. toromiro, extinct in the wild.
Figure 1 Map of the distribution of Sophora sect. Edwardsia. Species sampled for this study are underlined.
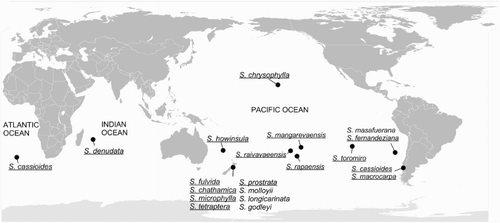
The biogeography of Sophora sect. Edwardsia has attracted considerable interest (e.g. Ridley Citation1930; Sykes & Godley Citation1967; Hurr et al. Citation1999; Peña et al. Citation2000; Mitchell & Heenan Citation2002). Its circum-Antarctic distribution is thought to result from the oceanic dispersal of its buoyant seeds (Heenan et al. Citation2001), which can remain afloat and viable in seawater for years (Sykes & Godley Citation1967). More recently seabirds have been suggested as an additional dispersal vector for species of sect. Edwardsia (Smith Citation2012). Two hypotheses have been proposed regarding the origin of sect. Edwardsia. Peña et al. (Citation1993, Citation2000) argued for a South American origin of the section, based on morphological and chemical analyses, followed by dispersal westward across the Pacific Ocean. Hurr et al. (Citation1999) and Mitchell & Heenan (Citation2002) suggested a west or northwest Pacific origin with eastward dispersal across the Pacific. Testing of these hypotheses has been hindered by a lack of a well-supported phylogeny for sect. Edwardsia (Markham & Godley Citation1972; Peña & Cassels Citation1996; Hurr et al. Citation1999; Ruiz et al. Citation1999; Peña et al. Citation2000). Previous DNA sequencing in the section found low variation in ITS (Mitchell & Heenan Citation2002) and chloroplast atpB-rbcL spacer (Hurr et al. Citation1999) sequences.
Recently, Shepherd et al. (Citation2017) published a phylogeographic study of the New Zealand species of sect. Edwardsia using DNA sequences of the chloroplast trnQ-5′rps16 intergenic spacer. This locus proved much more variable in the section than the previously sequenced genetic markers, with 22 haplotypes detected from the New Zealand species. The chloroplast variation was not partitioned by species boundaries, which likely indicates a high level of hybridisation and introgression between the New Zealand species (Shepherd et al. Citation2017). Here we sequence the trnQ-5′rps16 intergenic spacer, along with another chloroplast intergenic spacer and a nuclear locus, from most of the species of sect. Edwardsia in an attempt to reconstruct their phylogenetic relationships. Specifically, we wanted to examine whether geographically-close species are more closely related genetically. For example, do the New Zealand species form a monophyletic group?
Methods
Owing to the lack of chloroplast partitioning by species in New Zealand Sophora (Shepherd et al. Citation2017), a representative specimen of each of the 22 New Zealand haplotypes (haplotypes A to V) previously detected for the trnQ-5′rps16 intergenic spacer was selected for sequencing of additional loci (Table S1), rather than a specimen of each of the New Zealand species. DNA sequences were obtained for another 11 species of sect. Edwardsia from outside New Zealand plus an outgroup, S. tomentosa L., the type species of the genus Sophora and shown to be sister to sect. Edwardsia (Heenan et al. Citation2004).
For some specimens in cultivation, fresh Sophora leaf tissue was collected into silica gel. For these specimens, genomic DNA was obtained using a modified-CTAB extraction method (steps 1, 3–7 from table 1 in Shepherd & McLay Citation2011). For the remaining specimens, leaf tissue was removed from herbarium vouchers (Table S1). These were processed in a dedicated ancient DNA laboratory physically isolated from the modern DNA laboratory (Shepherd & Perrie Citation2014). DNA was extracted from herbarium leaf tissue with a Qiagen DNeasy plant mini kit (QIAGEN), following the manufacturer’s instructions but with a final elution volume of 45 µl.
Two chloroplast loci and one nuclear locus were amplified and sequenced. The chloroplast trnQ-5′rps16 intergenic spacer of the Large Single Copy region was initially amplified from samples using the trnQ(UUG) and rpS16 × 1 primers from Shaw et al. (Citation2007). For samples that did not initially amplify, internal primers were newly designed using Primer 3 (Untergasser et al. Citation2012) to amplify short fragments < 200 base pairs (bp) in length (trnQR 5′-TTTTATGCAATTATTATCCACAGTGA with trnQ(UUG), trnQintF 5′-TCACTGTGGATAATAATTGCATAAAAA with trnQintR 5′-ATTTATAAAGTGAAGGAGTCTCAAAAA and rpS16F 5′-TTTATAAACTATCCAAATCGATTCAA with rpS16 × 1). The high level of variation and a number of repetitive DNA regions within the trnQ-5′rps16 intergenic spacer restricted options for primer design therefore the trnQR and trnQintF primers overlapped.
The chloroplast trnHGUG -psbA intergenic spacer was amplified with the primers trnHGUG (Tate & Simpson Citation2003) and psbA (Sang et al. Citation1997). Internal primers were designed with Primer 3 (Untergasser et al. Citation2012) to amplify two short overlapping fragments in the herbarium samples: psbAIntF (5′-TACATTGCTTGCGAAGTCGT) with trnHGUG, and psbAIntR (5′-CATACGACTTCGCAAGCAAT) with psbA.
The chloroplast-expressed nuclear gene glutamine synthetase (ncpGS) was amplified, where possible, using Emshwiller & Doyle’s (Citation1999) primers GScp687f and GScp994r. Based on these sequences an internal primer (GScpshort1F 5′-TTGACAGTATTTCTTCTCGTGTGTT) was designed to amplify a short fragment (194 bp in length) with GScp994r for samples that could not be amplified for the full-length sequence.
All PCRs were performed in 12 µl reactions with 1 × Mytaq reagent buffer (Bioline), 5 pmol of each primer and 1 M betaine. PCR thermocycling followed the ‘slow and cold’ programme (Shaw et al. Citation2007).
PCR products were visualised by agarose gel electrophoresis then purified by digestion with 0.5 U shrimp alkaline phosphatase (SAP; USB Corp.) and 2.5 U exonuclease I (ExoI; USB Corp.) at 37 °C for 15 min, followed by inactivation of the enzymes at 80 °C for 15 min. PCR fragments were sequenced in both directions with the ABI Prism Big Dye Terminator cycle sequencing kit v3.1 on an ABI 3730 DNA sequencer (Massey University Genome Service).
Sequence files were edited with Sequencer v5.2 (Gene Codes Corp.). Some of the ncpGS sequences exhibited double peaks at certain nucleotide sites, indicating the presence of two alleles. Where possible, the identities of the alleles were determined using haplotype subtraction (Clark Citation1990; Fan et al. Citation2013). The sequences from S. chrysophylla Seem. and S. macrocarpa Sm. each contained two nucleotide sites with double peaks. These four positions were constant in all other sequences so were excluded from further analyses because it could not be determined to which allele they belonged.
Sequences were aligned with MUSCLE (Edgar Citation2004) with default parameters and manual alignment of large indels. For phylogenetic analysis, mononucleotide repeats of varying length were excluded, since preliminary analyses of our data indicated considerable reticulation and these regions are prone to homoplasy at larger geographic scales (Ingvarsson et al. Citation2003). Also, indels (insertions and deletions) longer than 1 bp were recoded as single events and a 6 bp inversion in the trnHGUG -psbA intergenic spacer in sample S. microphylla 3 (Table S1) was recoded as a single event. In the nuclear dataset the shorter DNA sequences obtained from several of the herbarium specimens were excluded from phylogenetic analyses but are reported in the results.
The genealogical relationships between haplotypes were determined using median-joining networks (Bandelt et al. Citation1999) constructed in Network v5.0.0.0 (www.fluxus-engineering.com). Networks have the advantage over phylogenetic trees of displaying reticulation, multificate relationships and the co-existence of ancestral and descendent sequences. Separate networks were produced for the concatenated chloroplast loci and the nuclear locus. Transitions and transversions were equally weighted.
Separate phylogenetic analyses of the nuclear sequences and concatenated chloroplast sequences were conducted with PAUP* 4.0a150 (Swofford Citation2002) for maximum parsimony (MP), the PhyML v3.0 web server (www.atgc-montpellier.fr/phyml/; Guindon et al. Citation2010) with maximum likelihood (ML) and MrBayes v3.2.1 (Huelsenbeck & Ronquist Citation2001) for Bayesian analyses (BA).
For the MP and ML analyses heuristic searches were performed with 10 (ML) or 100 (MP) random addition sequence replicates and tree bisection reconnection branch-swapping. Branch support was assessed with 1000 bootstrap pseudoreplicates. For the ML analyses the best-fit models of sequence evolution (F81 + G for the chloroplast alignment and TPM1uf for the nuclear alignment) were determined using the Akaike information criterion (AIC) in jModelTest v2.1.7 (Darriba et al. Citation2012).
For the BA two concurrent analyses were run, each with four Markov chains of 10 million generations and sampling every 1000 generations. The chloroplast dataset was partitioned by locus and the best-fitting model for each data partition determined using jModelTest v2.1.7 (TPM1uf + G for the trnHGUG -psbA intergenic spacer and TPM3uf + I for the trnQ-5′rps16 intergenic spacer). The first 25% of samples were discarded as ‘burn-in’, after this point the standard deviation of split frequencies was below 0.01. Tracer v1.6 (Rambaut & Drummond Citation2009) was used to confirm that stationarity had been reached.
Results
Chloroplast data
The trnQ-5′rps16 and trnHGUG -psbA intergenic spacer alignments had lengths of 552 bp and 353 bp, respectively. The trnQ-5′rps16 intergenic spacer contained seven indels in mononucleotide runs (which were excluded from subsequent analyses), five simple indels (length 4 bp to 13 bp), three indels in microsatellite regions and 17 substitutions. The trnHGUG -psbA intergenic spacer exhibited 20 substitutions and eight indel events, six of which were in mononucleotide runs, and therefore excluded from subsequent analyses, and two simple indels (length 5 bp and 7 bp). Combined, the aligned sequences of the two loci, with mononucleotide runs excluded, distinguished 20 haplotypes in the sect. Edwardsia samples.
The relationships between the concatenated chloroplast sequences are shown in the median-joining network (). Despite the removal of mononucleotide runs from the analyses, some reticulation was still evident. The outgroup S. tomentosa joined the network at either a haplotype found in both New Zealand and the Juan Fernandez Islands (haplotype 3; ) or at the haplotype found in the French Polynesian species (haplotype 11; ). The topologies of the ML and Bayesian phylogenies were identical. The MP analysis resulted in 180 MP trees (score = 59). The MP, ML and Bayesian phylogenies of the chloroplast sequences all showed poor resolution among the species, with few relationships receiving support (Figure S1).
Figure 2 Median-joining haplotype networks for the chloroplast haplotypes of Sophora sect. Edwardsia indicating (A) species and (B) geography. The size of each circle is proportional to haplotype frequency. Solid circles correspond to missing intermediate haplotypes. The two possible positions of the root, where the outgroup S. tomentosa joins the network, are indicated by arrows.
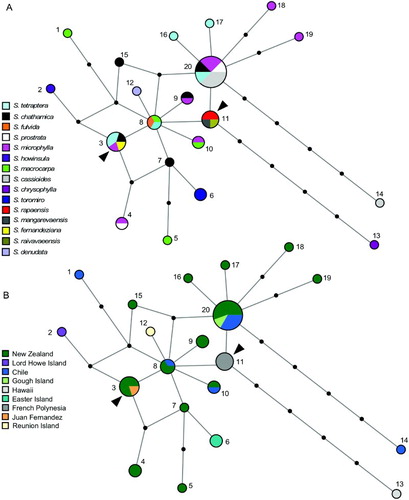
The chloroplast haplotypes from the New Zealand species did not form a monophyletic group in the chloroplast network or the phylogenies. Similarly, the sequences from the four samples of Chilean S. macrocarpa did not group together and one of them, S. macrocarpa 3, grouped with strong support in the Bayesian analysis (0.98 PP) with sample S. microphylla 6 from New Zealand (Figure S1). Sophora cassioides exhibited two haplotypes, one of which was found on Chile and Gough Island (haplotype 20) and was also shared with four New Zealand species. The other S. cassioides haplotype (haplotype 14) was unique and differed from the first by five mutational changes ().
Sophora mangarevaensis H.St.John, S. raivavaeensis H.St.John and S. rapaensis H.St.John, which are each restricted to different islands in French Polynesia (as indicated by the species epithets), exhibited the same chloroplast haplotype (haplotype 11) (). The closest relative of this chloroplast haplotype is either haplotype 8 or 20, both of which were found in New Zealand and Chile (). The chloroplast haplotype in the Hawaiian species S. chrysophylla (haplotype 13) derives from this French Polynesian haplotype (). The two samples of the Easter Island species S. toromiro Skottsb. had a unique haplotype (haplotype 6), which is most closely related to a haplotype found in New Zealand (haplotype 7; ). The samples of S. howinsula (W.R.B.Oliv.) P.S.Green, from Lord Howe Island, and S. denudata Bory, from Réunion Island, also each exhibited unique haplotypes. The haplotype detected in S. howinsula differed by two mutational events to haplotype 3, found in New Zealand. Sophora denudata was most closely related to haplotype 8, which was found in New Zealand and Chile. The sample of S. fernandeziana Skottsb. from the Juan Fernandez Islands, which are located near South America, had a chloroplast haplotype found in New Zealand (haplotype 3) but not detected in our sampling from Chile ().
Nuclear data
Full-length ncpGS sequences could not be amplified from any of the herbarium samples. For those samples that failed to amplify with the full-length primers, amplification of the shorter fragment with the internal primer was only possible for one S. toromiro, one S. macrocarpa, the S. denudata and two S. cassioides. Within the full-length ncpGS sequences a long mononucleotide run was present that could not be sequenced from most individuals. Excluding this region, the full-length ncpGS alignment of 41 sequences was 1077 bp long and contained 34 substitutions and no indels. Four substitutions, two each in S. chrysophylla and S. macrocarpa, were excluded from further analyses because they were only found in heterozygotes and could not be phased into alleles. The alignment of the shorter ncpGS fragment across all sequences contained three substitutions.
In the median-joining network of the full-length ncpGS sequences, S. macrocarpa differed from the remaining sect. Edwardsia sequences by four substitutions. The outgroup S. tomentosa joined the branch leading to S. macrocarpa (). As with the chloroplast haplotypes, many of the ncpGS alleles were shared across species. However, one of the alleles detected in S. cassioides was not found in any other species. Additionally, the two ambiguous sites detected in S. chrysophylla, which were excluded from the network analysis because they could not be phased into alleles, indicated the presence of at least one unique ncpGS variant in this species.
Figure 3 Median-joining haplotype networks for the chloroplast-expressed nuclear gene glutamine synthetase (ncpGS) sequences obtained from Sophora sect. Edwardsia indicating (A) species and (B) geography. The size of each circle is proportional to haplotype frequency. Solid circles correspond to missing intermediate haplotypes. The position of the root, where the outgroup S. tomentosa joins the network, is indicated by an arrow.
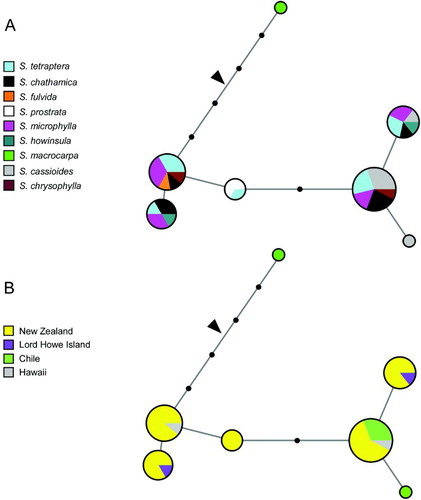
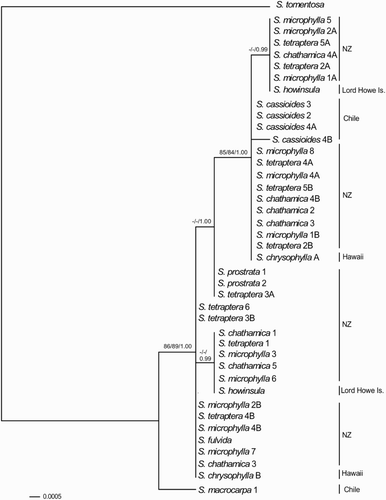
In the BA of the full-length ncpGS sequences, the sample S. macrocarpa 1 was strongly-supported (1.00 PP) as sister to the remaining species in the section (). Two synapomorphies were shared by all sect. Edwardsia ncpGS sequences, except for that from S. macrocarpa. The short ncpGS fragment included one of these synapomorphies. The short ncpGS sequence obtained from a second S. macrocarpa sample (S. macrocarpa 4) was identical to the first and lacked this synapomorphy. The additional S. cassioides, S. toromiro and S. denudata samples sequenced for the short ncpGS fragment exhibited the synapomorphy.
Figure 4 Bayesian phylogeny of the nuclear gene glutamine synthetase (ncpGS) sequences in Sophora sect. Edwardsia. Support values (maximum parsimony bootstrap, maximum likelihood bootstrap and Bayesian posterior probability) are shown for internal nodes where they exceed 70% MP and ML BS and 0.95 PP.
Discussion
Overall we found low genetic variation within Sophora sect. Edwardsia, consistent with previous studies (Hurr et al. Citation1999; Mitchell & Heenan Citation2002). However, much of the variation that we did detect was not restricted to species. Sequences from the New Zealand species were not recovered as monophyletic in the chloroplast or nuclear analyses. Haplotype sharing across the southern Pacific Ocean was notable, with three chloroplast haplotypes shared between New Zealand and Chilean species (S. cassioides or S. macrocarpa), with one of them also found on Gough Island in the Atlantic Ocean. A fourth chloroplast haplotype was shared between New Zealand and S. fernandeziana from the Juan Fernandez Islands, which are close to South America (). Nuclear alleles were also shared between New Zealand and South America. Oceanic currents linking New Zealand and South America (see figure 1 in Gillespie et al. Citation2012) are likely to have facilitated long distance seed dispersal in the section, possibly with the assistance of seabirds (petrels are known to swallow objects floating at sea, such as Sophora seeds; Tennyson Citation1995).
In contrast to the haplotype sharing in the southern Pacific Ocean, species of sect. Edwardsia on many islands further north in the Pacific Ocean (S. chrysophylla, S. toromiro, S. howinsula and the French Polynesian species) and S. denudata from Réunion Island in the Indian Ocean appear to have been isolated for sufficient time to allow the evolution of unique chloroplast haplotypes. Similarities in morphological, palynological, germination and chemical characters have been noted between S. denudata and the Hawaiian S. chrysophylla (Peña et al. Citation2000), but our analyses did not show a close relationship between them. Instead the chloroplast network indicates that S. chrysophylla likely originates from French Polynesia and S. denudata is derived from the southern Pacific region (New Zealand or Chile). Colonisation of Hawaii from French Polynesia has been suggested for a number of other species including Coprosma (Cantley et al Citation2014) and Melicope (Harbaugh et al. Citation2009).
The French Polynesian species (S. raivavaeensis, S. rapaensis and S. mangarevaensis), which are each restricted to a different island, shared a chloroplast haplotype that was not found in any other species. This result indicates that these three species represent a French Polynesian radiation, as has been found for a number of animal species (Gillespie et al. Citation2008). Sophora mangarevaensis and S. raivavaeensis are classified as endangered by the IUCN Red List (Florence Citation1998a, Citation1998b) and S. rapaensis is data deficient (Florence Citation1998c). These three species are distinguished from each other by a number of morphological characters including the number of leaflets, width of the standard petal and the length of the calyx teeth (St John Citation1985). Nuclear sequences were not able to be obtained from the French Polynesian species, which were only available to us as herbarium specimens whose DNA was of low quantity and quality.
The chloroplast sequence from Sophora toromiro was most closely related to a haplotype from New Zealand rather than to the geographically closer Chile and the Juan Fernandez Islands. Sophora toromiro was distinguished from all other species that were sequenced by a 3 bp insertion in the trnHGUG -psbA intergenic spacer. This marker may be of use for examining the maternal origins of cultivated lines of the endangered S. toromiro. This species is extinct in the wild but persists in cultivation in a number of botanic gardens and private collections around the world. The authenticity of some putative toromiro lines has been questioned, with either species misidentification or hybrid origins suggested (Maunder et al. Citation1999; Püschel et al. Citation2014). Conservation programmes aim to re-introduce toromiro to Easter Island (Maunder et al. Citation1999) but the accurate identification of the plants to be re-introduced is required.
In contrast to previous phylogenies, our ncpGS phylogeny found strong support that S. macrocarpa is sister to the other species in the section. Sophora macrocarpa shares a number of morphological characters with other sections of Sophora (Heenan et al. Citation2004) which, combined with palynological characters and phytochemicals, led to the suggestion of a South American origin of the section (Peña & Cassels Citation1996; Peña et al. Citation2000). Flavonoid patterns (Ruiz et al. Citation1999) and chromosome symmetry (Espejo et al. Citation2016) have also indicated the isolation of S. macrocarpa within the section. However, using the ncpGS phylogeny alone to infer the region of origin for sect. Edwardsia is inadvisable because dispersal asymmetry (Cook & Crisp Citation2005) is not taken into account. Although situations where biogeographic reconstruction is misled are likely to be rare (Sanmartín et al. Citation2007), the high level of inferred dispersal we detected across the South Pacific Ocean, combined with potential dispersal asymmetry owing to ocean current patterns, means that sect. Edwardsia is a strong candidate to mislead tree-based biogeographic interpretation (see nested ancestral area p. 745 in Cook & Crisp Citation2005).
In contrast to our nuclear phylogeny, S. macrocarpa was not sister to the rest of sect. Edwardsia in our chloroplast sequence analyses. The four samples of S. macrocarpa each exhibited a different chloroplast haplotype, two of which were shared with New Zealand species while the other two were not found in any other species. In the chloroplast network, the outgroup S. tomentosa joined either at a haplotype found in New Zealand and the Juan Fernandez Islands or a French Polynesian haplotype. The placement of the outgroup in the chloroplast phylogenies received low support. The conflicting positions of S. macrocarpa in the nuclear and chloroplast networks may result from past chloroplast introgression from S. cassioides into S. macrocarpa. Although we did not detect any chloroplast sharing between these two species with our limited sampling, hybrids have been reported between them (Donoso Citation1975). Further sampling of these two species across their ranges is required to test this hypothesis.
In conclusion, the data presented here indicates a rapid radiation for sect. Edwardsia with both chloroplast and nuclear sequences shared across large geographic distances. Future analyses using multiple unlinked DNA regions (e.g. ddRAD; Peterson et al. Citation2012), may allow recovery of a better resolved species phylogeny for the section, thus allowing examination of dispersal pathways and the exact numbers of dispersal events.
Acknowledgements
We thank Peter de Lange for collecting some of the New Zealand samples, Kathryn Hurr and David Penny for providing DNA of Sophora tomentosa, Leon Perrie for comments on a manuscript draft and Carlos Lehnebach for help obtaining S. denudata DNA. New Zealand Sophora samples were collected under Department of Conservation permits WA-23814-FLO, BOP-23814-FLO, TT-23661-FLO, NO-233360-FLO and Otari Wilton’s Bush permit 145. We would like to acknowledge all iwi and hapū who granted us permission to collect from within their rohe.
Additional information
Funding
References
- Bandelt H-J, Forster P, Rohl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol. 16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036
- Cantley JT, Swenson NG, Markey A, Keeley SC. 2014. Biogeographic insights on Pacific Coprosma (Rubiaceae) indicate two colonizations to the Hawaiian Islands. Bot J Linn Soc. 174:412–424. doi: 10.1111/boj.12130
- Clark A. 1990. Inference of haplotypes from PCR-amplified samples of diploid populations. Mol Biol Evol. 7:111–122.
- Cook LG, Crisp MD. 2005. Directional asymmetry of long-distance dispersal and colonization could mislead reconstructions of biogeography. J Biogeog. 32:741–754. doi: 10.1111/j.1365-2699.2005.01261.x
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 9:772. doi: 10.1038/nmeth.2109
- Donoso C. 1975. Un hibrido entre Sophora macrocarpa y Sophora microphylla. Facultad de Ciencias Forestales. Universidad de Chile. Boletín Técnico N. 30:11–19.
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797. doi: 10.1093/nar/gkh340
- Emshwiller E, Doyle JJ. 1999. Chloroplast-expressed glutamine synthetase (ncpGS): potential utility for phylogenetic studies with an example from Oxalis (Oxalidaceae). Mol Phylogenet Evol. 12:310–319. doi: 10.1006/mpev.1999.0613
- Espejo J, Baeza CM, Loureiro J, Santos C, Boshier D, Ruiz E. 2016. Exploratory karyological and genome size studies in Chilean Sophora species. NZ J Bot. 54:311–322. doi: 10.1080/0028825X.2016.1144622
- Fan DM, Yue JP, Nie ZL, Li ZM, Comes HP, Sun H. 2013. Phylogeography of Sophora davidii (Leguminosae) across the ‘Tanaka-Kaiyong Line’, an important phytogeographic boundary in Southwest China. Mol Ecol. 22:4270–4288. doi: 10.1111/mec.12388
- Florence J. 1998a. Sophora mangarevaensis. The IUCN Red List of Threatened Species. e.T35084A9902982. doi:10.2305/IUCN.UK.1998.RLTS.T35084A9902982.en.
- Florence J. 1998b. Sophora raivavaeensis. The IUCN Red List of Threatened Species. e.T35085A9903081. doi:10.2305/IUCN.UK.1998.RLTS.T35085A9903081.en.
- Florence J. 1998c. Sophora rapaensis. The IUCN Red List of Threatened Species. e.T35086A9903182. doi:10.2305/IUCN.UK.1998.RLTS.T35086A9903182.en.
- Gillespie RG, Baldwin BG, Waters JM, Fraser CI, Nikula R, Roderick GK. 2012. Long-distance dispersal: a framework for hypothesis testing. Trends Ecol Evol. 27:47–56. doi: 10.1016/j.tree.2011.08.009
- Gillespie RG, Claridge EM, Goodacre SL. 2008. Biogeography of the fauna of French Polynesia: diversification within and between a series of hot spot archipelagos. Phil Trans R Soc B. 363:3335–3346. doi: 10.1098/rstb.2008.0124
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 59:307–321. doi: 10.1093/sysbio/syq010
- Harbaugh DT, Wagner WL, Allan GJ, Zimmer EA, Bellwood D. 2009. The Hawaiian archipelago is a stepping stone for dispersal in the Pacific: an example from the plant genus Melicope (Rutaceae). J Biogeog. 36:230–241. doi: 10.1111/j.1365-2699.2008.02008.x
- Heenan PB, de Lange PJ, Wilton AD. 2001. Sophora (Fabaceae) in New Zealand: taxonomy, distribution, and biogeography. NZ J Bot. 39:17–53. doi: 10.1080/0028825X.2001.9512715
- Heenan PB, Dawson MI, Wagstaff SJ. 2004. The relationship of Sophora sect. Edwardsia (Fabaceae) and Sophora tomentosa, the type species of the genus Sophora, observed from DNA sequence data and morphological characters. Bot J Linn Soc. 146:439–446. doi: 10.1111/j.1095-8339.2004.00348.x
- Huelsenbeck JP, Ronquist FR. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 17:754–755. doi: 10.1093/bioinformatics/17.8.754
- Hurr KA, Lockhart PJ, Heenan PB, Penny D. 1999. Evidence for the recent dispersal of Sophora (Leguminosae) around the Southern Oceans: molecular data. J Biogeog. 26:565–577. doi: 10.1046/j.1365-2699.1999.00302.x
- Ingvarsson PK, Ribstein S, Taylor DR. 2003. Molecular evolution of insertions and deletion in the chloroplast genome of silene. Mol Biol Evol. 20:1737–1740. doi: 10.1093/molbev/msg163
- Markham KR, Godley EJ. 1972. Chemotaxonomic studies in Sophora 1. An evaluation of Sophora microphylla Aiton. NZ J Bot. 10:627–640. doi: 10.1080/0028825X.1972.10430251
- Maunder M, Culham A, Bordeu A, Allainguillaume J, Wilkinson M. 1999. Genetic diversity and pedigree for Sophora toromiro (Leguminosae): a tree extinct in the wild. Mol Ecol. 8:725–738. doi: 10.1046/j.1365-294X.1999.00609.x
- Mitchell AD, Heenan PB. 2002. Sophora sect Edwardsia (Fabaceae): further evidence from nrDNA sequence data of a recent and rapid radiation around the Southern Oceans. Bot J Linn Soc. 140:435–441. doi: 10.1046/j.1095-8339.2002.00101.x
- Peña RC, Cassels BK. 1996. Phylogenetic relationships among Chilean Sophora species. Biochem Sys Ecol. 24:725–733. doi: 10.1016/S0305-1978(96)00079-8
- Peña RC, Iturriaga L, Montenegro G, Cassels BK. 2000. Phylogenetic and biogeographic aspects of Sophora sect. Edwardsia (Papilionaceae). Pac Sci. 54:159–167.
- Peña RC, Iturriaga L, Mujica AM, Montenegro G. 1993. Amilisis micromorfologico de polen de Sophora (Papilionaceae). Gayana Bot. 50:57–65.
- Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE. 2012. Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE. 7:e37135. doi: 10.1371/journal.pone.0037135
- Püschel TA, Espejo J, Sanzana M-J, Benítez HA. 2014. Analysing the floral elements of the lost tree of Easter Island: a morphometric comparison between the remaining ex-situ lines of the endemic extinct species Sophora toromiro. PLoS ONE 9(12):e115548. doi: 10.1371/journal.pone.0115548
- Rambaut A, Drummond AJ. 2009. Tracer, version 1.6. http://tree.bio.ed.ac.uk/software/tracer/.
- Ridley HN. 1930. The dispersal of plants throughout the world. Kent: Reeve.
- Ruiz E, Donoso C, Gonzalez F, Becerra J, Marticorena C, Silva M. 1999. Phenetic relationships between Juan Fernandez and continental Chilean species of Sophora (Fabaceae) based on flavonoid patterns. Bol Soc Chil Quim. 44:351–356.
- Sang TD, Crawford J, Stuessy TF. 1997. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am J Bot. 84:1120–1136. doi: 10.2307/2446155
- Sanmartín I, Wanntorp L, Winkworth RC. 2007. West Wind Drift revisited: testing for directional dispersal in the Southern Hemisphere using event-based tree fitting. J Biogeog. 34: 398–416. doi: 10.1111/j.1365-2699.2006.01655.x
- Shaw J, Lickey EB, Schillin EE, Small RL. 2007. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Am J Bot. 94: 275–288. doi: 10.3732/ajb.94.3.275
- Shepherd LD, de Lange PJ, Perrie LR, Heenan PB. 2017. Chloroplast phylogeography of New Zealand Sophora trees (Fabaceae): extensive hybridization and widespread Last Glacial Maximum survival. J Biogeog. 44:1640–1651. doi: 10.1111/jbi.12963
- Shepherd LD, McLay TGB. 2011. Two micro-scale protocols for the isolation of DNA from polysaccharide-rich plant tissue. J. Plant Res. 124:311–314. doi: 10.1007/s10265-010-0379-5
- Shepherd LD, Perrie LR. 2014. Genetic analyses of herbarium material: is more care required? Taxon. 63:972–973. doi: 10.12705/635.2
- Smith JMB. 2012. Evidence for long-distance dispersal of Sophora microphylla to sub-Antarctic Macquarie Island. NZ J Bot. 50:83–85. doi: 10.1080/0028825X.2011.621715
- St John H. 1985. Sophora (Leguminasae) in Southeastern Polynesia. Bot Jahrb Syst. 106:115–122.
- Swofford DL. 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates: Sunderland.
- Sykes WR, Godley EG. 1967. Transoceanic dispersal in Sophora and other genera. Nature. 218:495–496. doi: 10.1038/218495a0
- Tate JA, Simpson BB. 2003. Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Syst Bot. 28:723–737.
- Tennyson AJD. 1995. Flora of Karewa Island, Bay of Plenty. Tane. 35:17–23.
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. 2012. Primer3—new capabilities and interfaces. Nucleic Acids Res. 40:e115. doi: 10.1093/nar/gks596
