ABSTRACT
Chemotaxonomic analysis of kōwhai leaf extracts in New Zealand are limited with previous reports of Sophora tetraptera having a flavonoid profile distinct from S. microphylla sensu lato and S. prostrata. Eight Sophora species are now recognised in New Zealand: S. chathamica, S. fulvida, S. godleyi, S. longicarinata, S. microphylla, S. molloyi, S. prostrata and S. tetraptera. We now report liquid chromatography-ultraviolet-mass spectrometry (LC-UV-MS) analyses of leaf and seed extracts of individual plants (2–16) from each of these eight species, plus the Chilean S. cassioides. All of the S. tetraptera leaf extracts had similar LC-UV-MS profiles, different from all of the other Sophora samples, consisting of four predominant compounds, characterised by MS and nuclear magnetic resonance (NMR) spectroscopy as: luteolin-7-O-rutinoside 1, luteolin-7-O-glucoside 2, apigenin-7-O-rutinoside 3, and apigenin-7-O-glucoside 4. The other Sophora leaf extracts showed complex flavonoid compositions, with no clear distinction between species. Most of the Sophora seed samples, including those of S. tetraptera, showed one major phenolic compound, but a few had a related compound. These were purified and characterised by MS and NMR spectroscopy as 3′,4′,7-trihydroxyisoflavone 5 (in most seeds) and its 7-O-glucoside 6 (in a few seeds), neither of which has been previously reported from these Sophora species.
Introduction
There are over 50 species of trees and shrubs of the genus Sophora (family Fabaceae) known worldwide (Heenan et al. Citation2001; Krishna et al. Citation2012). All of the New Zealand representatives have bright yellow flowers (Dawson & Lucas Citation2011) and are known locally as kōwhai (pronounced kō-faī), the Māori word for yellow. Kōwhai leaves, roots and especially bark have been used by Māori to prepare medicines for a range of ailments (Riley Citation1994). The latest taxonomic treatment of New Zealand Sophora recognises eight closely related endemic species () based largely on growth habit and leaf morphology (Heenan et al. Citation2001), compared with two to four species in earlier treatments (e.g. Allan Citation1961).
Table 1. New Zealand Sophora species including taxonomic authorities (Heenan et al. Citation2001) and literature reports of leaf flavonoids.
We have previously reported a chemotaxonomic study on the alkaloids in these New Zealand Sophora species, plus the Chilean S. cassioides (F. Phil.) Sparre (McDougal et al. Citation2015). We did not find any consistent qualitative or quantitative differences for this class of secondary metabolite between any of the species, in either leaf or seed. Sophora are also rich in flavonoids (Krishna et al. Citation2012) which have proved to be useful taxonomic markers in other Fabaceae (Hegnauer & Gpayer-Barkmeijer Citation1993). A range of flavonoids have been reported for the previously defined New Zealand Sophora species: S. tetraptera; S. prostrata; and S. microphylla (see and S1). We chose to analyse leaves and seeds because of the ease of non-destructive sampling, following up Markham’s detailed study (Markham Citation1973). He used paper chromatography (PC) comparisons with reference compounds, plus 1H nuclear magnetic resonance (NMR) spectroscopy of some isolated compounds, to identify 17 flavonoids from leaves of these three species (sensu lato, Table S1), including the 7-O-rutinosides and -glucosides of luteolin and apigenin (compounds 1–4, ). Markham & Godley (1972, p. 632) stated that PC analysis of leaf extracts ‘ … is an ideal method for distinguishing S. microphylla or S. prostrata from S. tetraptera, but only of limited value in distinguishing S. microphylla from S. prostrata … ’. The compounds distinguishing S. tetraptera were the 7-O-glucosides of luteolin and apigenin (compounds 2 and 4, ), plus three other flavone glycosides (Table S1). Several different individual plants from each taxon (species) were collected from around New Zealand, but there was no explicit comment of intraspecific variation of leaf flavonoids. Markham & Godley (1972, p. 632) further stated that PC analysis of seed extracts gave ‘A well-defined distinction of S. microphylla from S. prostrata … ’, but ‘The chemistry of the seed coat constituents has not, as yet, been investigated’.
The combination of modern analytical instrumentation with new taxonomic revisions (Heenan et al. Citation2001), and an experimental garden containing the eight New Zealand Sophora growing alongside a Chilean Sophora species, provided an opportunity to re-examine the chemotaxonomy of kōwhai. There have been no previous reports of the flavonoids from the Chilean S. cassioides (Heenan et al. Citation2001), also known by the names of pelū and pilo-pilo, so it was included in the study to provide a context for interpreting variation among the New Zealand species. We now report a liquid chromatography-ultraviolet-mass spectrometry (LC-UV-MS) investigation of flavonoids in leaves and seeds from the eight New Zealand Sophora species and from Chilean S. cassioides, using the same study population as our alkaloid work (McDougal et al. Citation2015).
Materials and methods
Sophora samples
Leaf and seed were harvested from trees in the experimental garden, Landcare Research, Lincoln in September/October/November 2013 (McDougal et al. Citation2015). Table S2 lists sample codes, original plant provenance and voucher identifiers. These trees were 40 to 50 years old and had been used to differentiate the New Zealand Sophora by morphometric analyses (Heenan et al. Citation2001).
Extraction
Leaf samples were frozen in liquid N2 and ground to fine powders by mortar and pestle. Seed samples were ground to fine powders in a coffee grinder. Subsamples (50 ± 3 mg) were shaken overnight at room temperature in EtOH (1.0 mL) containing 0.2 mg/mL C12-anilide as an internal standard (Perry et al. Citation1996). Samples were then centrifuged (10 min at 10,000 g) and the supernatant extracts stored at −20 °C.
LC-UV-MS analysis
Extracts were analysed on a Bruker MicrOTOF-Q instrument equipped with a Phenomenex Luna 5 µm C18(2) 100 Å column (part no. 00G-4252-Y0, 250 × 3 mm, 5 µm particle size). The mobile phase consisted of Buffer A (99.9% H2O: 0.1% formic acid) and Buffer B (99.9% MeCN: 0.1% formic acid) run linearly from 90% A:10% B to 100% B over 40 min, giving a C12-anilide internal standard a retention time of 37 min. Sample injection size was 5 µL and column temperature was 30 °C. UV detection was at 280 nm. MS data were collected in the negative ion mode (appropriate for phenolics), with MS settings: 180 °C, drying N2 flow 5 L/min, nebuliser N2 0.5 Bar, end plate offset −500 V, capillary voltage 3000 V, mass range 500–1000 Da. Internal mass calibration used sodium formate clusters. MS data were processed using the Compass Data Analysis software package (Bruker Corporation). The LC 280 nm chromatograms were exported as ASCII files using the Bruker HyStar Post Processing software. Chromatograms were normalised based on the peak height and retention time of the internal standard (C12-anilide) providing a qualitative and semi-quantitative comparison of sample peaks. For statistical analyses, data were opened and processed using UnScrambler multivariate analysis software (CAMO Software).
Sophora tetraptera leaf flavonoid isolation and characterisation
Dried, ground S. tetraptera t2 leaf (52 g) was extracted with EtOH (1 L) by shaking overnight at room temperature. Filtering and solvent removal under vacuum gave crude extract (5.1 g). A subsample (1.0 g) was dissolved by sonication in MeCN (1.0 mL) plus H2O (9.0 mL) containing 0.1% trifluroacetic acid (TFA). Flash vacuum chromatography used an Isolute C18 cartridge (10 g) started with 9:1 (v/v) H2O:MeCN (with 0.1% TFA) and ended at 3:7 H2O:MeCN. Analytical HPLC showed the four major compounds concentrated in two fractions that were combined and solvent removed (134 mg). A second round of purification through Isolute C18 cartridge used 5% to 25% MeCN in H2O further concentrated these compounds (83 mg). Final LC purification using a C18 column (Phenomenex, 250 × 10 mm), flow rate 5 mL/min, isocratic mobile phase 75:25 H2O:MeCN (0.1% TFA), column at 30 °C, injection volume 75 µL, gave: 1 (8 mg, analytical LC-MS retention time 10.0 min); 2 (9.1 mg, 10.8 min); 3 (4 mg, 11.3 min); and 4 (3 mg, 12.0 min). The HR-LC-MS data were supported by MSn data obtained from analyses on a LTQ 2D linear ion-trap API-MS (Thermo-Finnigan) instrument with electrospray ionisation (ESI) in the negative and positive mode. Data were acquired for precursor masses from m/z 120 to m/z 2000 with collision-induced dissociation (CID) fragmentation at 35 arbitrary units to allow interpretation of data. The purified components were characterised by NMR spectroscopy on a Varian INOVA-500 spectrometer at 500 MHz for 1H, and 125 MHz for 13C. Chemical shifts are given in ppm on the δ scale. Spectra in DMSO-D6 were referenced to residual solvent signals at 2.50 ppm (1H) and 39.5 ppm (13C), while spectra in acetone-D6 were referenced to 2.06 ppm (1H) and to 29.8 ppm (13C). Standard 2D experiments (COSY, NOESY, HSQC and HMBC) were employed for assignment of proton and carbon resonances of isoflavone 5.
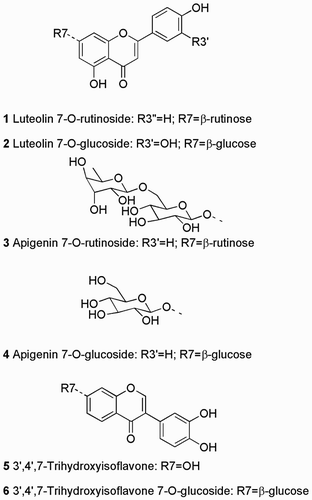
Luteolin-7-O-β-rutinoside 1 [Registry Number 20633-84-5]: Yellow gum; 1H NMR see Figure S1, matches Wang et al. (Citation2003); HRESIMS m/z 595.1628 [M + H]+ (calcd for C27H31O15 595.1663).
Luteolin-7-O-β-glucoside 2 [5373-11-5]: Yellow gum; 1H NMR see Figure S2, matches Wang et al. (Citation2003); HRESIMS m/z 449.1068 [M + H]+ (calcd for C21H21O12 449.1084).
Apigenin-7-O-β-rutinoside 3 [552-57-8]: Yellow gum; 1H NMR see Figure S3, matches Wang et al. (Citation2003); HRESIMS m/z 579.1678 [M + H]+ (calcd for C27H31O14 579.1714).
Apigenin-7-O-β-glucoside 4 [578-74-5]: Yellow gum; 1H NMR see Figure S4, matches Bennini et al. (Citation1992); HRESIMS m/z 433.1136 [M + H]+ (calcd for C21H21O11 433.1135).
Sophora microphylla seed flavonoid isolation and characterisation
Two separate dried, ground S. microphylla seed samples (m3, 0.3 g and m18, 1.0 g) were extracted and fractionated by the methods described above. The main compound in the m3 extract eluted from a C18 cartridge in 8:2 H2O:MeCN (8 mg, one main compound 6), and the main m18 compound eluted (from a separate cartridge) in 1:1 H2O:MeCN (80 mg, still a mixture). Final HPLC purification was as above, but used 60:40 H2O:MeCN. The main peak (retention time of 3.7 min) was collected and dried to isoflavone 5 (8 mg).
3′,4′,7-Trihydroxyisoflavone 5 [485-63-2]: Yellow powder; full NMR data in , matches Goto et al. (Citation2009); 1H NMR spectrum see Figure S5; HRESIMS m/z 271.0620 [M + H]+ (calcd for C15H11O5 271.0606).
Table 2. NMR data for 3′,4′,7-trihydroxyisoflavone 5.a
3′,4′,7-Trihydroxyisoflavone-7-O-β-glucoside 6 [1439934-73-2]: Yellow gum; 1H NMR see Figure S6, matches Yang et al. (Citation2015); HRESIMS m/z 433.1136 [M + H]+ (calcd for C21H21O10 433.1135).
Results
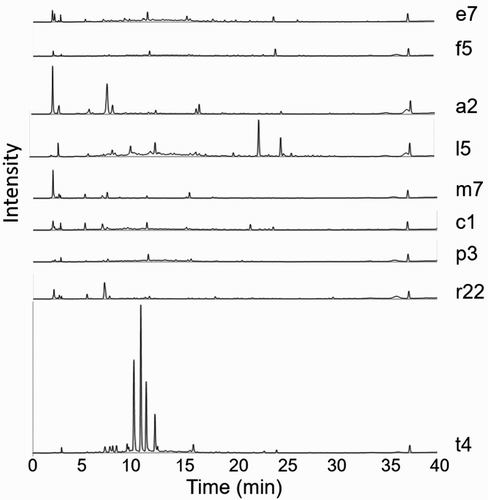
Ethanol extracts of 53 Sophora leaf extracts were analysed by LC-UV-MS, with individual plant chromatograms presented in Figures S7–S15. A wide range of compounds absorbing at 280 nm were resolved, showing complex mixtures of flavonoids and other phenolic compounds for most of the extracts (). A principal component analysis (not presented) of these LC peak data separated the four S. tetraptera leaf extracts from all the others by the presence of four major phenolic constituents (). The MS of these S. tetraptera signature phenolic compounds suggested that they were glucosides and rutinosides of the flavones apigenin and luteolin, perhaps among those identified by Markham (Citation1973) (Table S1). These compounds were purified and identified as luteolin-7-O-β-rutinoside 1, luteolin-7-O-β-glucoside 2, apigenin-7-O-β-rutinoside 3 and apigenin-7-O-β-glucoside 4 (structures in ) by their 1H NMR spectra (Figures S1–S3) showing good matches to literature data (Bennini et al. Citation1992; Wang et al. Citation2003).
Figure 2. Representative leaf extract chromatograms (UV detection at 280 nm) for Sophora spp. (from top to bottom): S. chathamica, S. fulvida, S. godleyi, S. longicarinata, S. microphylla, S. molloyi, S. prostrata, S. cassioides and S. tetraptera. All chromatograms are normalised to the C12-anilide internal standard at TR = 37 min.
Ethanol extracts of 41 Sophora seed extracts were also analysed by LC-UV-MS, with individual plant chromatograms presented in Figures S16–S25. These seed extracts showed fewer flavonoids than the leaf extracts, and there was the same dominant peak (retention time 12.4 min) in almost all of them. The high resolution MS for this peak was consistent with the formula C15H10O5, matching 3′,4′,7-trihydroxy flavone previously purified from New Zealand Sophora leaf samples (Markham Citation1973) (Table S1). However, the purified compound giving the main peak in these Sophora seed samples had a 1H NMR spectrum (Figure S6) with a low field singlet at 8.13 ppm typical of H2 of isoflavones (Chen et al. Citation2005), rather than the singlet around 6.8 ppm typical of H3 of flavones (e.g. 1–4, see Figures S1–S3). 1H and 13C 1D and 2D NMR results were consistent with the structure 3′,4′,7-trihydroxyisoflavone 5 () and very closely matched the NMR data (Table S3) for this compound produced by total synthesis (Goto et al. Citation2009).
Three of the S. microphylla seed extracts (m3, m6 and m7, Figure S20) stood out for having different LC-UV traces: instead of the major peak at 12.4 min assigned to 3′,4′,7-trihydroxyisoflavone 5, they had a major peak at retention time 7.2 min. The MS data for this peak suggested a molecular formula C21H20O10, with fragmentation by loss of a glycoside moiety to give an ion corresponding to a trihydroxy flavone or isoflavone. The compound giving this 7.2 min peak was purified from the m3 S. microphylla seed sample, and gave a 1H NMR spectrum (Figure S6) with a low field singlet at 8.2 ppm typical of H2 of isoflavones (Chen et al. Citation2005), plus other signals with chemical shift and couplings very similar to those of 3′,4′,7-trihydroxyisoflavone 5 (). The 1H NMR spectrum also showed an anomeric proton signal characteristic of a β-glucoside: a 7 Hz doublet at 5.2 ppm. The only hexoside of 3′,4′,7-trihydroxyisoflavone that we could find reported in the literature was the 7-O-β-glucoside 6 (structure in ), which has been isolated from roots of S. flavescens (Yang et al. Citation2015) and from seeds of S. alopecuroides (Reziguli et al. Citation2012). Allowing for differences in solvents, the 13C NMR data for the compound from S. microphylla showed signals matching those in the literature (Yang et al. Citation2015), so we tentatively identify this compound as 3′,4′,7-trihydroxyisoflavone-7-O-β-glucoside 6.
Discussion
The advantage of having the majority of Sophora trees used in this study growing together at one site was minimisation of potential environmentally-induced biosynthetic variation. This study has built on the earlier work of Markham & Godley (Citation1972) who provided much new information on the chemistry of New Zealand Sophora. The samples used by Markham & Godley (Citation1972) () and assigned to S. prostrata and S. tetraptera can be considered to be correct. However, samples assigned to S. microphylla by Markham & Godley (Citation1972) () are problematic as some will be S. microphylla sensu stricto, but, following the taxonomic revision of Heenan et al. (Citation2001), others would now be placed in S. cassioides, S. chathamica, S. fulvida, S. godleyi, S. longicarinata or S. molloyi.
Leaf flavonoid profiles distinguished S. tetraptera, with high concentrations of compounds 1–4, from the other seven New Zealand Sophora species and from S. cassioides. Sophora tetraptera is morphologically distinct as it has the largest leaves of the New Zealand species and it has been shown to be genetically distinct (Heenan et al. Citation2018). This chemotaxonomic distinction by foliage flavonoids contrasts with our results on the alkaloids from these samples, which showed no consistent distinctions between the species (McDougal et al. Citation2015). This foliage flavonoid distinction confirms and extends the results of Markham, who found that flavonoids, including glucosides 2 and 4, distinguished S. tetraptera from S. microphylla sensu lato and S. prostrata by PC (Markham Citation1973). Markham reported rutinosides 1 and 3 in all three of these taxa. We did find peaks corresponding to 3 (but not to 1, 2 or 4) in the LC-UV-MS traces of some of the leaf extracts of the other species (e.g. ), but at much lower absolute concentrations than in the S. tetraptera extracts. LC-UV-MS analyses have the advantage over PC analyses of being quantitative, especially when an internal standard is added as in our work.
Seed flavonoid profiles did not clearly distinguish between any of the nine Sophora species examined, but we did identify 3′,4′,7-trihydroxyisoflavone 5 in these species for the first time, as a major component in almost all of the extracts. Markham & Godley (Citation1972, p. 627) reported that S. microphylla sensu lato and S. prostrata ‘ … are readily separated by the phenolic constituents of their seed coats … ’. This separation was based on PC patterns, but the identification of these flavonoids was not reported. As stated above, our identification of the major Sophora seed phenolic as 3′,4′,7-trihydroxyisoflavone 5 was based on purification of this compound, 2D NMR structure assignments (), and matching the NMR data for this compound produced by total synthesis () (Goto et al. Citation2009). Our NMR data also largely matched (allowing for solvent differences) the data reported for a compound isolated from a fermentation broth of a Streptomyces sp. (Funayama et al. Citation1989) and from a soybean food fermented with Aspergillus sp. (Chen et al. Citation2005)(Table S3). However, a compound produced synthetically and reported to have this same structure 5 showed distinctly different NMR data (Matin et al. Citation2009), most noticeably in the 1H NMR signals of H2′, H5′ and H6′ (Table S3). These strongly shifted 1H NMR signals of H2′, H5′ and H6′ have also been reported for compounds reported as 5 for several sources (Table S3): 7-hydroxyisoflavone transformed by Burkholderia sp. (Seeger et al. Citation2003); 4′,7-dihydroxyisoflavone transformed by Streptomyces avermitilis (Roh et al. Citation2009); and from another fermented soybean food (Park et al. Citation2008). We cannot explain these two distinct sets of NMR data for supposedly the same compound from very diverse sources. We did isolate 5 from S. microphylla seeds twice and obtained the same spectra, and the differences between some of the published data could not be attributable to the NMR solvent used (Table S3).
The 7-O-β-glucoside 6 was the main phenolic in three S. microphylla seed extracts, instead of its aglycone 5. Markham & Godley (1972, p. 634) noted that ‘ … the pattern of seed phenolics can differ between trees of the same population … ’ of S. microphylla sensu lato, but their qualitative PC suggested more complexity than we found by quantitative LC.
An interesting range of biological activities has been reported for 3′,4′,7-trihydroxyisoflavone 5 (Lee et al. Citation2010; Chang Citation2014). However, kōwhai seeds are not recorded as traditional Māori medicines (Riley Citation1994). This could be because seeds also contain higher concentrations of toxic alkaloids than do other kōwhai plant parts (McDougal et al. Citation2015) such as leaves, which were used medicinally (Riley Citation1994).
To conclude, the leaf phenolic profiles of New Zealand Sophora species and Chilean S. cassioides were found to be complex and non-distinctive, except for the profiles of S. tetraptera leaves, which were rich in four luteolin and apigenin glycosides. The seed phenolic profiles of New Zealand Sophora species were similar to those of Chilean S. cassiodes and could not be used to distinguish species.
Supplemental Material
Download MS Word (2.3 MB)Acknowledgements
We thank N. Joyce and J. Pentelow for LC-MS analyses; I. Stewart for help with NMR spectroscopy; J. Steel and B. Smallfield for sample collections; S. Basu for data presentation; D. Killeen for principal component analysis; and L. Larsen and E. Burgess for assisting with compound purifications.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Owen Michael McDougal http://orcid.org/0000-0002-1502-3462
Peter Brian Heenan http://orcid.org/0000-0001-8229-7466
Nigel Brian Perry http://orcid.org/0000-0003-3196-3945
John William van Klink http://orcid.org/0000-0002-0843-567X
Additional information
Funding
References
- Allan HH. 1961. Flora of New Zealand. Indigenous Tracheophyta. Psilopsida, Lycopsida, Filicopsida, Gymnospermae, Dicotyledons. Wellington: DSIR.
- Bennini B, Chulia AJ, Kaouadji M, Thomasson F. 1992. Phytochemistry of the Ericaceae. Part 2. Flavonoid glycosides from Erica cinera. Phytochemistry. 31:2483–2486. doi: 10.1016/0031-9422(92)83305-I
- Chang T-S. 2014. Isolation, bioactivity, and production of ortho-hydroxydaidzein and ortho-hydroxygenistein. Int J Mol Sci. 15:5699–5716. 18. doi: 10.3390/ijms15045699
- Chen Y-C, Sugiyama Y, Abe N, Kuruto-Niwa R, Nozawa R, Hirota A. 2005. DPPH radical-scavenging compounds from dou-chi, a soybean fermented food. Biosci Biotechnol Biochem. 69:999–1006. doi: 10.1271/bbb.69.999
- Dawson J, Lucas R. 2011. New Zealand’s native trees. Nelson: Craig Potton.
- Funayama S, Anraku Y, Mita A, Komiyama K, Omura S. 1989. Structural study of isoflavonoids possessing antioxidant activity isolated from the fermentation broth of Streptomyces sp. J Antibiot. 42:1350–1355. doi: 10.7164/antibiotics.42.1350
- Goto H, Terao Y, Akai S. 2009. Synthesis of various kinds of isoflavones, isoflavanes, and biphenyl-ketones and their 1,1-diphenyl-2-picrylhydrazyl radical-scavenging activities. Chem Pharm Bull. 57:346–360. doi: 10.1248/cpb.57.346
- Heenan P, Mitchell C, Houliston G. 2018. Genetic variation and hybridisation among eight species of kōwhai (Sophora: Fabaceae) from New Zealand revealed by microsatellite markers. Genes. 9:111. doi: 10.3390/genes9020111
- Heenan PB, de Lange PJ, Wilton AD. 2001. Sophora (Fabaceae) in New Zealand: taxonomy, distribution, and biogeography. NZ J Bot. 39:17–53. doi: 10.1080/0028825X.2001.9512715
- Hegnauer R, Gpayer-Barkmeijer RJ. 1993. Relevance of seed polysaccharides and flavonoids for the classification of the Leguminosae: a chemotaxonomic approach. Phytochemistry. 34:3–16. doi: 10.1016/S0031-9422(00)90776-3
- Krishna PM, Rao KNV, Sandhya S, Banji D. 2012. A review on phytochemical, ethnomedical and pharmacological studies on genus Sophora, Fabaceae. Rev Bras Farmacogn. 22:1145–1154. doi: 10.1590/S0102-695X2012005000043
- Lee DE, Lee KW, Song NR, Seo SK, Heo Y-S, Kang NJ, Bode AM, Lee HJ, Dong Z. 2010. 7,3′,4′-Trihydroxyisoflavone inhibits epidermal growth factor-induced proliferation and transformation of JB6 P+ mouse epidermal cells by suppressing cyclin-dependent kinases and phosphatidylinositol 3-kinase. J Biol Chem. 285:21458–21466. doi: 10.1074/jbc.M109.094797
- Markham KR. 1973. Chemotaxonomic studies in Sophora 2. Flavonoids and chemotaxonomy of 3 Sophora species. Phytochemistry. 12:1091–1094. doi: 10.1016/0031-9422(73)85021-6
- Markham KR, Godley EJ. 1972. Chemotaxonomic studies in Sophora 1. An evaluation of Sophora microphylla Ait. NZ J Bot. 10:627–640. doi: 10.1080/0028825X.1972.10430251
- Matin A, Gavande N, Kim MS, Yang NX, Salam NK, Hanrahan JR, Roubin RH, Hibbs DE. 2009. 7-Hydroxy-benzopyran-4-one derivatives: a novel pharmacophore of peroxisome proliferator-activated receptor α and -γ (PPARα and γ) dual agonists. J Med Chem. 52:6835–6850. doi: 10.1021/jm900964r
- McDougal OM, Heenan PB, Jaksons P, Sansom CE, Smallfield BM, Perry NB, van Klink JW. 2015. Alkaloid variation in New Zealand kōwhai, Sophora species. Phytochemistry. 118:9–16. doi: 10.1016/j.phytochem.2015.07.019
- Park J-S, Park HY, Kim DH, Kim DH, Kim HK. 2008. Ortho-dihydroxyisoflavone derivatives from aged doenjang (Korean fermented soypaste) and its radical scavenging activity. Bioorg Med Chem Lett. 18:5006–5009. doi: 10.1016/j.bmcl.2008.08.016
- Perry NB, Burgess EJ, Lorimer SD, van Klink JW. 1996. Fatty acid anilides as internal standards for high performance liquid chromatographic analyses of Valeriana officinalis L. and other medicinal plants. Phytochemical Analysis. 7:263–268. doi: 10.1002/(SICI)1099-1565(199609)7:5<263::AID-PCA311>3.0.CO;2-S
- Reyes A, Miranda N, Martinez R. 1988. Constituents of Sophora tetraptera sensu Reiche. Rev Latinoam Quim. 19:32.
- Reziguli K, Li M, Rena K, Muaitaer N. 2012. Flavonoids from the seeds of Sophora alopecuroides L. in Xinjiang. Xinjiang Yike Daxue Xuebao. 35:591–593.
- Riley M. 1994. Maori healing and herbal. Paraparaumu: Viking Sevenseas N.Z. Ltd.
- Roh C, Seo S-H, Choi K-Y, Cha M, Pandey BP, Kim J-H, Park J-S, Kim DH, Chang IS, Kim B-G. 2009. Regioselective hydroxylation of isoflavones by Streptomyces avermitilis MA-4680. J Biosci Bioeng. 108:41–46. doi: 10.1016/j.jbiosc.2009.02.021
- Ruiz E, Donoso C, Gonzalez F, Becerra J, Marticorena C, Silva M. 1999. Phenetic relationships between Juan Fernandez and continental Chilean species of Sophora (Fabaceae) based on flavonoid patterns. Bol Soc Chil Quim. 44:351–356.
- Seeger M, Gonzalez M, Camara B, Munoz L, Ponce E, Mejias L, Mascayano C, Vasquez Y, Sepulveda-Boza S. 2003. Biotransformation of natural and synthetic isoflavonoids by two recombinant microbial enzymes. Appl Environ Microbiol. 69:5045–5050. doi: 10.1128/AEM.69.9.5045-5050.2003
- Wang M, Simon JE, Aviles IF, He K, Zheng Q-Y, Tadmor Y. 2003. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J Agric Food Chem. 51:601–608. doi: 10.1021/jf020792b
- Yang J, Yang X, Wang C, Lin Q, Mei Z, Zhao P. 2015. Sodium-glucose-linked transporter 2 inhibitors from Sophora flavescens. Med Chem Res. 24:1265–1271. doi: 10.1007/s00044-014-1200-0