ABSTRACT
The species Ranunculus acris L. (giant buttercup) is native to Europe but has a very large naturalised range in several countries. In New Zealand it is a serious weed of pasture, particularly in high rainfall areas. Biological control options are being sought as chemical control has proven insufficient and is leading to resistance. To find efficient pathogens or natural enemies the search needs to focus on areas of origin of the material present in New Zealand. The target range of biocontrol agents needs to be broad enough to include all R. acris haplotypes found in New Zealand but also host specific enough to not attack unintended targets. A previous study found that R. acris in New Zealand has very high chloroplast diversity, with mixed chloroplast haplotypes occurring in single populations. Haplotype-specific genetic data are therefore essential for the identification of the origin and for sourcing effective biocontrol agents. The aim of this study was to identify putative origins of the material found in New Zealand. We compared chloroplast diversity in New Zealand populations of R. acris to parts of the native and naturalised ranges, including areas of the putative origin of the New Zealand plants. The non-coding chloroplast regions trnK and psbJ-petA were sequenced to identify haplotypes. We found that the native range also had high chloroplast diversity, and that most New Zealand chloroplast haplotypes were common in northern Europe and the United Kingdom. Further sampling would be required to trace the remaining two New Zealand haplotypes to their origin, however the results suggest that R. acris was introduced from several regions in Europe.
Introduction
Giant buttercup (Ranunculus acris L.) is a pan-European species naturalised in several countries, including the United States of America, Canada, South Africa and New Zealand (Coles Citation1971). It has become an economically significant weed in cattle-grazed pastures in Canada and New Zealand. In New Zealand, R. acris is a serious pasture weed in areas of relative high rainfall in summer, where its low palatability reduces dairy production values by up to 33% (Bourdôt et al. Citation2003).
Ranunculus acris was most likely introduced to New Zealand with early European settlers in the nineteenth century as a contaminant of pasture grasses imported for agriculture. Since then it has spread throughout both main islands. It was first noticed in Taranaki and Golden Bay, and has now established in Horowhenua, Hawke’s Bay, Wairarapa, South Auckland, Southland and Canterbury (Bourdôt and Saville Citation2010). Ranunculus acris is characterised by its deeply palmate leaves and a plant height of up to 100 cm. Its roots form a stout rhizome from which it will regrow if mown or grazed. It is known to comprise several subspecies and chromosome races, although a high degree of plasticity makes these classifications difficult to apply consistently (Harper Citation1957). The species is a very good disperser, with small seeds that often stick to surfaces, such as farm machinery and equipment. Control of this species is problematic, with reports of possible herbicide resistance to the selective herbicides favoured for cleaning pasture (Lusk et al. Citation2011). Attempts at control with the pathogenic fungus Sclerotinia sclerotiorum (Lib.) de Bary as a bioherbicide have resulted in mixed success (Bourdôt et al. Citation2007), and it does not seem to be a candidate for widespread control, despite promising results in some circumstances. Finding the origin of the weed is paramount to ensure the development of efficient biological control programmes. Biocontrol agents sourced from regions with populations that match the genetic material in the invasive range have higher chances of being adapted to the specific weed genotype and to displaying required host-specificity (Sutton et al. Citation2017).
An earlier study on R. acris in New Zealand using flow cytometry and trnK and psbJ-petA sequences found remarkably high levels of chloroplast haplotype diversity, including multiple haplotypes in single populations (Houliston et al. Citation2015). Chloroplast haplotypes are often not variable within species, or even within genera (Gielly and Taberlet Citation1994), so to find multiple types within an introduced species at single sites is unusual. This would indicate that there have been multiple introductions of R. acris into New Zealand from several localities (Houliston et al. Citation2015). Initial comparison to a very limited number of samples from overseas showed little similarity between the chloroplast types in New Zealand and those from the Netherlands, Japan, Montenegro and Canada. Despite the literature referring to multiple chromosome lines being present in R. acris (Sorokin Citation1927a, Citation1927b; Coles Citation1971), only two different genome sizes were detected in the earlier study, with one belonging to a locally rare haplotype (Houliston et al. Citation2015).
Non-coding chloroplast regions have been successfully used in plant phylogeny studies at different taxonomic levels, usually at a genus or species level. This study presents an extension of this method for resolving origins of the invasive weed species by looking at the variability within a species. As the chloroplast diversity within a species is generally low, studies on plant species with high chloroplast diversity are very rare and utilise other methods, such as restriction site analysis (Golden and Bain Citation2000) or chloroplast microsatellite markers (Hatziskakis et al. Citation2009).
This study was a continuation of the previous work (Houliston et al. Citation2015) and describes the findings of a more comprehensive survey of the native range, including additional regions/countries (Belgium/Luxembourg, France, England and Wales), in combination with the earlier findings. The aim of this study was to identify the putative origin of R. acris in New Zealand by comparing chloroplast haplotypes from New Zealand with native and naturalised regions. Two fast evolving non-coding chloroplast regions–trnK and psbJ-petA–were used, as in previous study. The trnK and psbJ-petA regions are two of the most informative noncoding cpDNA regions found within angiosperms that offer high levels of variability (Shaw et al. 2007) and therefore were chosen as the best suited candidates to investigate the chloroplast diversity within R. acris. The results of this study will assist with the search for biocontrol agents for R. acris in the area of origin of the haplotypes found in New Zealand.
Materials and methods
Plant material and DNA extraction
Plant material was collected from 57 R. acris plants from seven countries (). Two leaves per plant were collected and placed in a sealed plastic bag with 50 g of silica gel. DNA was extracted from dried plant samples using the iNtRON plant DNA extraction kit (iNtRON Biotechnology, Seongnam, South Korea), following the manufacturer’s instructions. DNA quality and quantity were determined using a Nanodrop® ND-1000 (Nanodrop Technologies, Wilmington, Kentucky, USA).
Table 1. Collection data for the Ranunculus acris samples.
PCR amplification
The chloroplast trnK intron (Paun et al. Citation2005) and psbJ-petA non-coding region (Shaw et al. Citation2007) were amplified and sequenced as described in Houliston et al. (Citation2015), using primers trnK345f and trnK3r, and psbJ and petA, respectively.
Sequence data analysis
Sequence data for the two chloroplast regions were edited in Sequencher 5.1 (Gene Codes Corporation, Ann Arbour, MI, USA). The two regions were concatenated and the sequences from 57 samples generated in this study were aligned to sequences of 119 samples from New Zealand, three from the Netherlands, one from Canada, one from Montenegro and 15 from Japan, generated in the earlier study (Houliston et al. Citation2015) using ClustalW as implemented in MEGA7 (Kumar et al. Citation2016). Haplotypes were identified and the haplotype-specific trnK and psbJ-petA sequences accessioned to NCBI GenBank ().
Table 2. Name and GenBank® accession numbers of psbJ-petA and trnK sequences and haplotype labels for their combinations.
MEGA7 was also used to search for the best fitting substitution model for concatenated haplotype sequences of all detected R. acris haplotypes and one outgroup (Ranunculus glabriusculus HQ338353 and HQ338207, Emadzade et al. Citation2011). A Bayesian phylogeny was constructed with MrBayes version 3.2.6 (Ronquist et al. Citation2012), as implemented in Geneious 10.1.3 (Biomatters Ltd., Auckland, New Zealand), using the best fitting substitution model, gamma rate variation and unconstrained branch lengths. The model was run for 1,100,000 iterations, with a burn-in length of 100,000. A majority rule phylogram was constructed with Consensus Tree Builder, implemented in Geneious 10.1.3 using the raw trees output from MrBayes support threshold = 50%, Majority greedy clustering method.
For comparison and to estimate the relationships between haplotypes, Statistical Parsimony analysis for the haplotypes and Ranunculus glabriusculus as outgroup was performed with MEGA7 Phylogeny/Maximum Parsimony Tree(s), using the following parameters: Bootstrap method with 500 replicates, using all sites, Subtree-Pruning-Regrafting (SPR) as MP search method, 10 initial trees (random addition), MP search level 1 and a maximum of 100 trees to retain.
Results
All new 57 samples from overseas produced sequence data for the two chloroplast regions (GenBank accession numbers in ). The combined alignment was 1740bp long, consisting of 1275 bp for the trnK and 455–465 bp for the psbJ-petA part. The trnK sequences had seven variable sites (five single and one 2 bp substitutions) and no gaps, resulting in seven different sequences (). The psbJ-petA region had 15 variable sites (one single and two 2 bp substitutions and two 5 bp insertions/deletions), resulting in five different sequences (). There was no data missing in psbJ-petA but the insertions/deletions led to two gaps in the alignment.
Table 3. Concatenated alignments of variable sites in the two chloroplast regions of Ranunculus acris.
We identified the new haplotype K from Belgium/Luxembourg and the Netherlands. The list of all 11 to date identified chloroplast haplotypes and their frequencies per country/region can be seen in (graphical representation is shown in ). In total, 8 haplotypes were found in Europe and 7 in New Zealand. These two regions had five haplotypes in common (A, C, D, G and J). Two chloroplast haplotypes from central Europe were not found anywhere else (B and K) and two haplotypes from New Zealand were not found anywhere else (E and F). New Zealand was dominated by haplotype F (50% of all haplotypes found there) while the most common haplotypes in central Europe were A, B and C. All three haplotypes found in Canada were also found in Europe and New Zealand (A, G and J).
Figure 1. Visualisation of haplotype frequencies in New Zealand and overseas. Numbers in parentheses represent sample sizes. The sizes of the pie charts are visual guides for sample sizes and are not to scale.
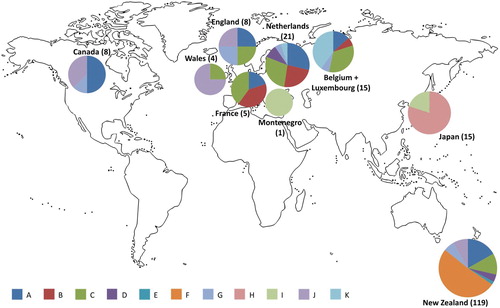
Table 4. Frequencies of the different chloroplast haplotypes from the combined trnK intron and psbJ-petA sequences for Ranunculus acris from New Zealand and overseas.
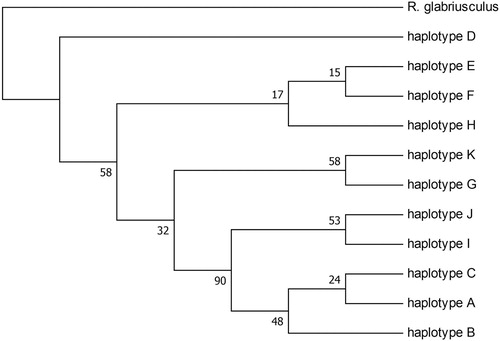
A Bayesian consensus tree (the Majority rule phylogram) for all 11 detected R. acris haplotypes and one outgroup species was constructed using HKY85 as the best fitting substitution model. The phylogram is shown in . The parameter estimates of the posterior output for tree likelihood and prior probabilities were ESS(TL) > 4,700 and ESS(alpha) > 2,400, respectively. The maximum parsimony consensus tree is shown in . Both tree analyses had similar results. Haplotype D was found to be distinct from the other 10 haplotypes and the A, B, C, J and I clade had good consensus support. However, D was found only in the Netherlands and New Zealand and B only in central Europe.
Figure 2. Majority rule phylogram for concatenated haplotype sequences. Bayesian consensus tree for all Ranunculus acris chloroplast haplotypes (concatenated trnK and psbJ-petA sequences, 1740bp), rooted on the outgroup species R. glabriusculus. Scale bar indicates genetic distance expressed as number of substitutions per site, branch labels show the consensus support (%).
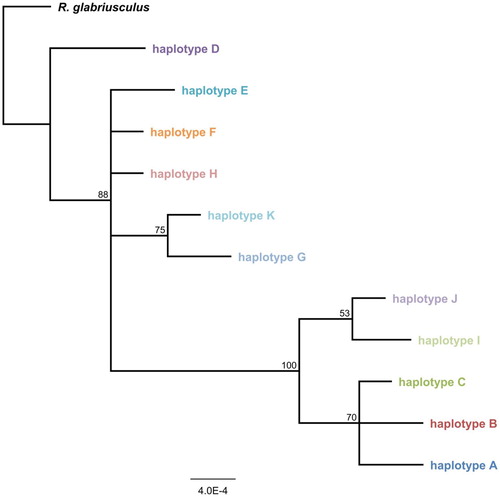
Figure 3. Bootstrap consensus tree for 11 haplotypes and outgroup. The evolutionary history was inferred using the Maximum Parsimony method. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches. The analysis involved 12 nucleotide sequences. There were a total of 1740 positions in the final dataset.
Discussion
The samples from Europe were a good match with most of those found in New Zealand, and also in Canada, which is also part of the naturalised range (). Although there were only a limited number of samples from Canada, it was notable that all of the haplotypes found in Canada are also present in New Zealand (A, G and J). These haplotypes are widespread in Europe, with A and G found in both the United Kingdom and mainland Europe, while J is present in Wales and England (). That England and Wales were the only European countries with haplotype J indicates that some of the introduced R. acris in New Zealand and in Canada might have come from the United Kingdom. We found two haplotypes in Europe that were not present in New Zealand or Canada (B and K), and two haplotypes in New Zealand that were not present in Europe (E and F). Haplotypes E and F could be results of mutations after material arrived in New Zealand. However, the timescale of R. acris invasion of New Zealand might have been too short for this to have happened. While natural invasions occur over large time frames (millions of years) and enable the application of neutral theory of evolution, the timescales for human-mediated invasions are smaller than for mutation and genetic drift (Cristescu Citation2015). Given the high diversity within populations it is more likely that these haplotypes are present in the native range but were not sampled. . Further sampling from Europe and New Zealand would be required to uncover all of the chloroplast diversity in R. acris and to trace all haplotypes to their origin.
Haplotype D was found to be distinct from the other 10 haplotypes by Bayesian and Maximum Parsimony analyses (). Plants with this haplotype also have a significantly larger genome size than the other six analysed by Houliston et al. (Citation2015). However, neither the worldwide distribution of haplotype D nor of any other haplotypes correlated with the phylograms, making it impossible to resolve the evolutionary relationships between the R. acris haplotypes. Despite this, the results show high genetic diversity present in both the native and introduced range of R. acris (). It was notable that the variation in New Zealand is even higher than in many other European regions (except for the Netherlands). Alleles found in the introduced populations that do not co-occur in native populations suggest multiple introductions (Johnson et al. Citation2011). This finding supports the conclusion from the previous study (Houliston et al. Citation2015) that there have been introductions of R. acris from multiple locations within Europe. As mentioned before, the possibility that certain haplotypes had not been sampled should be considered.
The high haplotype diversity in both Europe and New Zealand raises the question as to how this has been maintained over time without lineage sorting or drift reducing the number of chloroplast haplotypes present. This is particularly pertinent in New Zealand, where this diversity is present following a reasonably recent introduction, with a larger potential for founder effects. Ranunculus is a large and diverse genus (Hӧrandl and Emadzade Citation2012), but that does not explain the intraspecific variation we observed in chloroplast sequences. Founder effects, such as genetic drift and lineage sorting, lead to loss of genetic variation in an invading population. However, if the invading population has large amounts of genetic variation and expanded quickly after invasion or there are ongoing invasions, these effects might not be efficient in removing genetic variation from the population (Dlugosch and Parker Citation2008, Fitzpatrick et al. Citation2012, Cristescu Citation2015).
High genetic diversity in the native range, multiple introductions into New Zealand and extremely large populations in several areas of New Zealand indicate that founder effects did not occur and variation has been maintained after invasion.
In Europe this explanation is insufficient. Earlier work (Sorokin Citation1927b; Coles Citation1971) has identified morphologically and geographically different subspecies of R. acris, with further variation also present. Whether these subspecies are correlated with different haplotypes, and whether they have different ecologies, is not known. The sympatric distribution of the different haplotypes in New Zealand would indicate that they do not have substantially different ecological niches, although the situation in the naturalised range may not reflect what is happening in their home environment, particularly given other challenges, such as natural enemies. This variation is possibly of high importance if options for the potential biocontrol of R. acris in New Zealand are to be considered (Houliston et al. Citation2015). Bioprospecting for control agents will be more profitable in areas of the origin of the haplotypes found in New Zealand.
Although chloroplast diversity is high in R. acris, variation in the nuclear genome is not as readily assessed due to the presence of polyploidy in the species (Houliston et al. Citation2015). Sequencing of nuclear phylogenetic regions is problematic due to multiple copies of commonly used regions, such as ribosomal ITS (internal transcribed spacer) and ETS (external transcribed spacer). Development of nuclear markers such as SSRs (simple sequence repeats) or whole genome approaches may be useful to further examine variation within the R. acris complex.
Amplification and sequencing of several fast evolving non-coding chloroplast regions is a cost-effective way of estimating chloroplast diversity within a genus or, as this study has shown–a species, especially when sequencing of nuclear regions is problematic due to polyploidy. As a continuation of this study, it also would be interesting to investigate the levels of chloroplast diversity in other related Ranunculaceae species worldwide as well as other New Zealand weed species.
This study provided the first molecular comparison of New Zealand R. acris to field collected material overseas. It provides evidence for the potential origins of this naturalised serious pasture weed species in New Zealand and will inform the downstream research on the best geographical regions to search for a potential biocontrol agents. It also presents an unusual case of high chloroplast diversity in plants and highlighted the benefits of utilising non-coding chloroplast regions for investigating the chloroplast diversity on a species level.
Acknowledgements
We would like to thank the numerous landowners in the Netherlands who allowed access to their properties for sampling. Samples from Canada were supplied by Marian Jones, ESRD Resource Management, Alberta, Canada.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bourdôt GW, Hurrell GA, Saville DJ. 2007. Variation in the efficacy of a mycoherbicide and two synthetic herbicide alternatives. In: Julien HH, Sforza R, Bon MC, Evans H, Hatcher PE, Hinz H, Rector BG, editors. XIIth International Symposium on Biological Control of Weeds, La Grand Motte, Montpellier, France. Wallingford, UK: CAB International.
- Bourdôt GW, Saville DJ. 2010. Giant buttercup – a threat to sustainable dairy farming in New Zealand. Proceedings of the 4th Australasian Dairy Science Symposium, Melbourne, Australia.
- Bourdôt GW, Saville DJ, Crone D. 2003. Dairy production revenue losses in New Zealand due to giant buttercup (Ranunculus acris). New Zealand Journal of Agricultural Research. 46:295–303. doi: 10.1080/00288233.2003.9513557
- Coles SM. 1971. The Ranunculus acris L. complex in Europe. Watsonia. 8:237–261.
- Cristescu ME. 2015. Genetic reconstructions of invasion history. Molecular Ecology. 24(9):2212–2225. doi: 10.1111/mec.13117
- Dlugosch KM, Parker IM. 2008. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Molecular Ecology. 17:431–449. doi: 10.1111/j.1365-294X.2007.03538.x
- Emadzade K, Gehrke B, Linder HP, Hörandl E. 2011. The biogeographical history of the cosmopolitan genus Ranunculus L.(Ranunculaceae) in the temperate to meridional zones. Molecular Phylogenetics and Evolution. 58:4–21. doi: 10.1016/j.ympev.2010.11.002
- Fitzpatrick BM, Fordyce JA, Niemiller ML, Reynolds RG. 2012. What can DNA tell us about biological invasions? Biological Invasions. 14(2):245–253. doi: 10.1007/s10530-011-0064-1
- Gielly L, Taberlet P. 1994. The use of chloroplast DNA to resolve plant phylogenies: noncoding versus rbcL sequences. Molecular Biology and Evolution. 11:769–777.
- Golden JL, Bain JF. 2000. Phylogeographic patterns and high levels of chloroplast DNA diversity in four Packera (Asteraceae) species in southwestern Alberta. Evolution. 54(5):1566–1579. doi: 10.1111/j.0014-3820.2000.tb00702.x
- Harper JL. 1957. Ranunculus acris L. The Journal of Ecology. 45:289–342. doi: 10.2307/2257092
- Hatziskakis S, Papageorgiou AC, Gailing O, Finkeldey R. 2009. High chloroplast haplotype diversity in Green population of beech (Fagus sylvatica L.). Plant Biology. 11(3):425–433. doi: 10.1111/j.1438-8677.2008.00111.x
- Houliston GJ, Goeke DF, Smith LA, Fowler SV. 2015. The genetic variation in giant buttercup in New Zealand pastures. New Zealand Plant Protection. 68:112–117.
- Hӧrandl E, Emadzade K. 2012. Evolutionary classification: a case study on the diverse plant genus Ranunculus L. (Ranunculaceae). Perspectives in Plant Ecology, Evolution and Systematics. 14:310–324. doi: 10.1016/j.ppees.2012.04.001
- Johnson JR, Thomson RC, Micheletti SJ, Shaffer HB. 2011. The origin of tiger salamander (Ambystoma tigrinum) populations in California, Oregon, and Nevada: introductions or relicts? Conservation Genetics. 12(2):355–370. doi: 10.1007/s10592-010-0144-2
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution. 33(7):1870–1874. doi: 10.1093/molbev/msw054
- Lusk CS, Bourdôt GW, Harrington KC, Hurrell GA. 2011. Pasture tolerance and efficacy of three herbicides used against giant buttercup (Ranunculus acris subsp. acris L.). New Zealand Plant Protection. 64:86–92.
- Paun O, Lehnebach C, Johansson JT, Lockhart P, Hörandl E. 2005. Phylogenetic relationships and biogeography of Ranunculus and allied genera (Ranunculaceae) in the Mediterranean region and in the European alpine system. Taxon. 54:911–930. doi: 10.2307/25065478
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. Mrbayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 61:539–542. doi: 10.1093/sysbio/sys029
- Shaw J, Lickey EB, Schilling EE, Small RL. 2007. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. American Journal of Botany. 94:275–288. doi: 10.3732/ajb.94.3.275
- Sorokin H. 1927a. The chromosomes of Ranunculus acris. The American Naturalist. 61:571–574. doi: 10.1086/280180
- Sorokin H. 1927b. Cytological and morphological investigations of gynodimorphic and normal forms of Ranunculus acris L. Genetics. 12:59–83.
- Sutton G, Paterson I, Paynter Q. 2017. Genetic matching of invasive populations of the African tulip tree, Spathodea campanulata Beauv.(Bignoniaceae), to their native distribution: maximising the likelihood of selecting host-compatible biological control agents. Biological Control. 114:167–175. doi: 10.1016/j.biocontrol.2017.08.015
