ABSTRACT
Macroalgae of the genus Dictyota produce large amount of diterpenes, which have many ecological and biological functions. We analysed the crude extracts of three Brazilian populations of the brown alga Dictyota ciliolata by hydrogen nuclear magnetic resonance (1H-NMR) spectroscopy and high-resolution gas chromatography coupled to mass spectrometry (HRGC/MS). All of the HRGC/MS profiles showed that D. ciliolata contained prenylated guaiane (Group I), xeniane, and crenulidane diterpenes (Group III) as major constituents. Eleven diterpenes were detected, dictyol B acetate, dictyol B, dictyol C, dictyoxide, isopachydictyol A, pachydictyol A, 4β-acetoxydictyodial A, and four crenulidane diterpenes. Dictyol B acetate was the major product in all of algal populations in Brazil. The diterpene profiles of the Brazilian populations were compared to other ones obtained in populations from the Atlantic and Pacific Oceans. The genomic DNA of a separate Brazilian population was extracted and amplified for psbA, the sequences of which were used to build a haplotype network with other sequences available in Genbank. Ten psbA haplotypes were found, five in the Indian Ocean, four in the Western Pacific Ocean and three in the Atlantic Ocean. The eastern (Western Pacific Ocean) and the western (Indian Ocean) coasts of Australia were the most diverse areas and they contained most of known haplotypes. One haplotype identified via the molecular network was found to be common in all of the oceans and thus is proposed as the ancestral form. The chemical and molecular data are consistent at the recent proposal in which D. ciliolata was classified as an essentially pantropical species, which may prove to be highly beneficial to bioprospection studies as this species is a source of potential bioactive products.
Introduction
Chemotaxonomic markers can be used to separate taxa into several taxonomic ranks and to address taxonomic issues (Zidorn and Stuppner Citation2001; Tauwhare et al. Citation2006; Silva et al. Citation2010; Leite and Castilho Citation2017; Elshamy et al. Citation2018; McDougal et al. Citation2018). Over the past three decades, diterpenes produced by members of the brown algal genera Canistrocarpus De Paula & De Clerck and Dictyota J.V. Lamouroux have been used for this purpose (e.g. Kelecom and Teixeira Citation1986; Teixeira et al. Citation2001; Vallim et al. Citation2005; De-Paula et al. Citation2012) including for the description of Dictyota dolabellana De Paula, Yoneshigue-Valentin & Teixeira (De Paula et al. Citation2007a). An improved understanding of diterpenes and their sources (i.e. the correct determination of species names) is essential for studying their ecological importance, as well for resolving taxonomic difficulties among species and to allow bioprospection (e.g. Lara-Isassi et al. Citation2000; De Paula et al. Citation2011; Fernandes et al. Citation2014; Othmani et al. Citation2014; Lira et al. Citation2016).
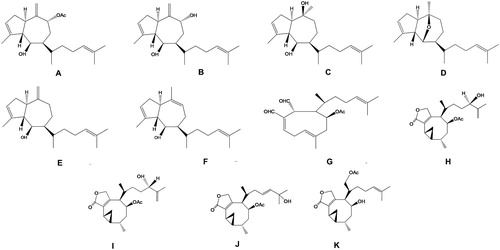
Previous studies have established the hypothetical biosynthetic pathway for Dictyota and Canistrocarpus diterpenes (), the products of which are classified into three categories consisting of Groups I, II, and III (Teixeira and Kelecom Citation1988, Citation1989; Teixeira Citation2010). The natural products synthesised by these algae, including terpenes, have been shown to have many biological activities, and researches have revealed that they play important roles in chemical defence systems and resistance to biological incrustation were established in experimental essays (Barbosa et al. Citation2007; Vallim et al. Citation2007; De Paula et al. Citation2011). All three groups are represented in the southwestern Atlantic coast (, emphasising species from the Brazilian coast).
Figure 1. Hypothetical biosynthetic pathway for the production of Group I, II, and III diterpenes by Dictyota and Canistrocarpus (Teixeira and Kelecom Citation1988, Citation1989; Teixeira Citation2010).
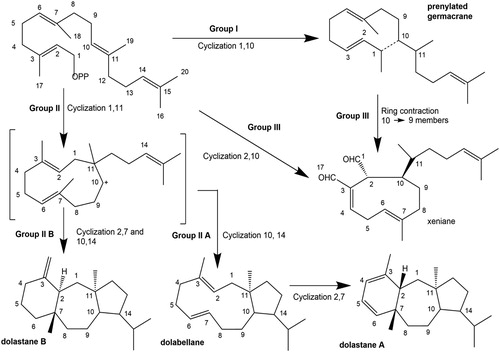
Table 1. Chemotaxonomic groups of diterpenes produced by Canistrocarpus and Dictyota species from Brazila.
The Dictyota ciliolata-crenulata complex is composed of pseudocryptic species with cilio-denticulate margins that are taxonomically challenging and species determination requires the use of combined morphological and molecular phylogenetic data (Tronholm et al. Citation2013; Lozano-Orozco et al. Citation2014, Citation2016). Six species with this feature are known to inhabit the Atlantic Ocean: Dictyota canariensis (Grunow) Tronholm, D. chalchicueyecanensis Lozano-Orozco & Sentíes, D. ciliolata O.G. Sonder ex Kützing, D. dolabellana, D. jamaicensis W.R. Taylor, and D. pleiacantha Tronholm. Of these, only D. pleiacantha does not occur in the Western Atlantic (Guiry and Guiry Citation2018). The natural products of just three of these species have been scrutinised: Dictyota ciliolata from North Carolina (USA), for which dictyol B acetate was determined to be the main product (Cronin et al. Citation1995; Cronin and Hay Citation1996); and D. jamaicensis (De-Paula et al. Citation2008, as D. crenulata J. Agardh) and D. dolabellana (De Paula et al. Citation2007a), both from Brazil, were shown to generate 4β-acetoxydictyodial and 4-hydroxy-7,8-epoxy-2-dolabellane as the main products, respectively. Dictyota ciliolata is unique among these species because of its nearly pantropical distribution, extending well into warm temperate areas, and because it exhibits considerably greater morphological plasticity than the other two species (Tronholm et al. Citation2013).
Dictyota ciliolata comprises four geographically segregated subclades over the extent of its wide range (Tronholm et al. Citation2012). Other than some chemical data obtained from a North Carolinian population, comparisons of the chemical differences among populations of D. ciliolata have not been analysed, in contrast to the molecular data presented by Tronholm et al. (Citation2012, Citation2013). Here, we describe the composition of diterpenes from three populations of D. ciliolata collected along the Brazilian coast and discuss the chemical and genetic data available in the literature concerning other populations of D. ciliolata in the Atlantic, Indian, and Pacific oceans.
Materials and methods
Sampling and identification
For the chemical study, D. ciliolata was sampled from July 1999 to March 2003 at Preta Beach, Grande Island, in the city of Angra dos Reis (23° 00′ 24″ S, 44° 19′ 05″ W), state of Rio de Janeiro; at Itapuã Beach (12° 58′ 16″ S, 38° 30′ 39″ W) in the city of Salvador, state of Bahia; and at the Rocas Atoll (03° 51′ S, 33° 40′ W), state of Rio Grande do Norte state. For the molecular study, samples were collected from Prainha Beach (22° 57′ 58″ S, 42° 1′ 44″ W), in the city of Arraial do Cabo, state of Rio de Janeiro, during August and September 2011. Algae were collected at depths ranging from 2–5 m and screened in the field to remove possible epiphytes, then later dried at room temperature. The specimens selected as vouchers were deposited in the Herbarium of the Universidade do Estado do Rio de Janeiro (HRJ) and at the Herbarium of the Universidade Federal do Estado do Rio de Janeiro (HUNI). Specimens were identified based on morphological guidelines provided in Tronholm et al. (Citation2013).
Chemical analyses
Algal material and extraction
Air-dried D. ciliolata (100 g each, dry weight) from Angra dos Reis, Salvador and Rocas Atoll were extracted with acetone (100%) at room temperature, with. the solvent then allowed to evaporate under reduced pressure to yield crude extracts. Extracts of the Rocas Atoll and Angra dos Reis specimens (1 g) were subjected to silica gel column chromatography and eluted with n-hexane/ethyl acetate to yield 55–60 fractions each. The fractions eluted with n-hexane/ethyl acetate 3:1 yielded three products that were identified via a combination of HRGC/MS and 1H-NMR; dictyol B acetate was determined to be the main product, with isopachydictyol A and pachydictyol A as minor components. These diterpenes were identified through comparisons with available physical and spectroscopic information (Teixeira et al. Citation2001; Cavalcanti et al. Citation2006, Citation2008). All algal extracts were analysed in Brazil with 1H-NMR and HRGC/MS.
1H-NMR analysis
1H-NMR (300 MHz) spectra were recorded on a Varian Unity Plus 300 spectrometer using CDCl3 as solvent and TMS as internal standard. Chemical shifts were reported in δ (ppm) and coupling constants (J) in Hz.
Gas chromatographic analysis (HRGC)
Preliminary HRGC analysis was carried out using an HP 5890 CG equipped with an SE-54 glass capillary column (15 m × 0.25 mm; film thickness 0.25 µm) and an FID detector.
HRGC/MS analysis
An aliquot of extract was diluted with an appropriate volume of ethyl acetate and analysed by HRGC/MS on an HP 6890 series GC system coupled to a HP 5973 mass selective detector in the electron impact mode (70 eV) and equipped with a HP-1 MS capillary column (30 m × 0.25 mm; film thickness 0.25 µm). Injector and detector temperatures were set at 270°C and 290°C, respectively. The temperature programme was maintained at 160°C, then raised at a rate of 4°C/min to 260°C, and finally at a rate of 15°C/min to 290°C for 15 min. Hydrogen was used as the carrier gas, at a flow rate of 1 mL/min. Diluted samples were injected manually in a split mode (1/10 or 1/20). Data were obtained from filtering of raw data (Frd) area percent data. The chemical components were identified based on comparison of their mass spectra with those of known standards and/or literature data, via the co-injection technique in HRGC, and with the Wiley 275 library data of the HRGC/MS system. Silica gel GF254 (Merck) was used for the Analytical thin-layer chromatography (TLC). The 1H-NMR spectra corroborated the HRGC/MS results.
All solvents were HPLC grade. TLC separations were carried out on Merck silica gel 60 F-254 (0.2 mm) percolated aluminium plates. Once developed, the plates were visualised by spraying them with 2% ceric sulfate in sulfuric acid followed by gentle heating. Silica gel 60 (Merck, 70–230 and 230–400 mesh) was used for column chromatography. The 1H-NMR spectra were recorded in deuterated chloroform (CDCl3 100% Aldrich) on a Varian Unity Plus 300 spectrometers using tetramethylsilane (TMS) as an internal standard.
Molecular analysis
The genomic DNA of the specimens from Arraial do Cabo were extracted and purified using the protocol described by Saunders (Citation1993). The amplicons of the plastid-encoded PSII reaction centre D1 gene (psbA) were obtained following the method described by Lopes-Filho et al. (Citation2017) and sequenced by Macrogen Inc. Korea. After editing with Mega 7.0 (Kumar et al. Citation2016), the Brazilian sequences and those retrieved from Genbank (www.ncbi.nlm.nih.gov/genbank) were aligned by ClustalW on the same software. Sequences with missing data were removed from the analysis and the final dataset included 35 sequences with 734 bp (Table S1); a haplotype network was constructed from these sequences by median joining (Bandelt et al. Citation1999) using with Dnasp v.6 (Rozas et al. Citation2017) and Network v.5 (http://www.fluxus-engineering.com/sharenet.htm).
Results
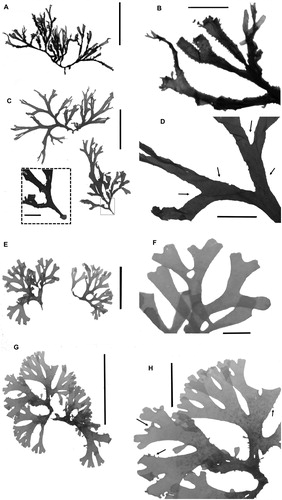
Voucher specimens were up to 10 cm long and were yellow to brown in colour while kept underwater. The margins of the vouchers were variable, ranging in size from conspicuous cilio-teethlike projections (A, B) to very small teeth (C, D), and in number from none (smoothed margin; E, F) to a few (sparsely cilio-dentate margin; G, F). Teeth were curved slighted toward the apex (B) and marginal proliferations were very evident as result of continuous growth of some teeth into adventitious branches or detachable propagules (G). Basal sections were attached to the substrate by rhizoids or by a small, hard discoid holdfast formed by the entanglement of rhizoids, particles, and sediments (C). Branching was dichotomous and the tips were usually rounded, but sometimes tapered. Thallus width varied from 2–4.5 mm at the base, from 1.5–5 mm in the middle section, and from 1–2 mm at the apex; however, all specimens were wider at the dichotomies. The cortex and medullae were monostromatic. Cortical cells were square-like or wider than taller, and measured (length × width) 14.4–36.0 × 12.0–36.0 µm at the base, 14.4–36.0 × 12.0–38.4 µm in the middle section, and 9.6–19.2 × 14.4–21.6 µm at the apex. Medullary cells were taller than wider at the base, square-like in the middle section, and wider than taller at the apex, measuring (length × width) 96.0–136.8 × 60.0–105.6 µm at the base, 67.2–180.0 × 48.0–120.0 µm in the middle section, and 40.8–62.4 × 48.0–74.4 µm at the apex.
Figure 2. Dictyota ciliolata. A, Voucher specimen from Rocas Atoll (HRJ 5516) (scale bar = 10 cm). B, Detailed view of the same specimen showing margins with conspicuous teeth (scale bar = 1 cm). C, Voucher specimen from Preta beach (HUNI 5776) (scale bar = 5 cm). Insert: detailed view of the discoid holdfast (scale bar = 5 mm). D, Detailed view of the same specimen showing margins with very small teeth (black arrows) (scale bar = 1 cm). E, Voucher specimen from Prainha (HUNI 5010) (scale bar = 2 cm). F, Detailed view of the same specimen showing smoothed margins (scale bar = 5 mm). G, Voucher specimen from Prainha (HUNI 5011) (scale bar = 5 cm). H, Detailed view of the same specimen showing sparsely cilio-dentate margins (black arrows) (scale bar = 2 cm).
The three populations of Brazilian D. ciliolata (Angra dos Reis – RJ; Salvador – BA; and the Rocas Atoll – RN) produced 11 diterpenes (), six of which were Group I (prenylated guaiane diterpenes) and five of which were Group III (xeniane and crenulidane diterpenes). Extract from D. ciliolata collected in Angra dos Reis yielded four prenylated guaiane diterpenes, one xeniane, and four crenulidane diterpenes, – possible photooxidation products of xeniane diterpenes (Guella and Pietra Citation1993)-, and trace amounts of other crenulidane diterpenes; Extract from D. ciliolata collected in Salvador yielded three prenylated guaianes and four crenulidane diterpenes; and extract from the Rocas Atoll yielded six prenylated guaianes, one xeniane, and four crenulidane diterpenes. The principal peaks observed in the mass spectra (m/z and relative intensity in parenthesis) of diterpenes from D. ciliolata and the 1H-NMR of dictyol B acetate are shown in Table S2. Spectroscopic data obtained in this study were compared with those of Vázquez et al. (Citation1988).
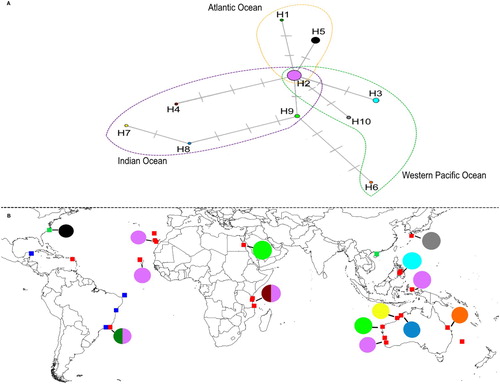
Construction of a haplotype network revealed 10 haplotypes (A), which were plotted in a map containing sites where the chemistry and genetics of D. ciliolata had already been studied (B). Haplotype H2 was found to be the most widespread, and thus the most likely candidate to be the ancestral form. Haplotype diversity was greatest in Australia. Interestingly, three haplotypes were found to occur in the Atlantic Ocean, consisting of H1 (Arraial do Cabo, state of Rio de Janeiro, Brazil), H2 (Arraial do Cabo, state of Rio de Janeiro, Brazil; and the archipelagos of Canary Islands and Cabo Verde) and H5 (state of North Carolina, USA).
Figure 4. Haplotype diversity of psbA sequences taken from D. ciliolata populations. A, Haplotype network of psbA showing the three geographically segregated groups; the haplotype shown in the intersection is the only haplotype found in all three regions. B, Distribution of the 10 haplotypes in the Atlantic and the Indo-West Pacific populations. Squares indicate locations where D. ciliolata had previously been studied. Red squares: DNA sequences available for a given population; blue squares: known diterpenes from a given population; green squares: DNA sequences and known diterpenes available for a given population. Map based on data from Cronin et al. (Citation1995); Cronin and Hay (Citation1996); De Clerck et al. (Citation2006); Tronholm et al. (Citation2010, Citation2012, Citation2013); Caamal-Fuentes et al. (Citation2014b); Cheng et al. (Citation2014); and the present study.
Discussion
Our voucher specimens were in accordance with those presented in Tronholm et al. (Citation2013), the most recent and complete review of the Dictyota ciliolata–crenulata complex. In addition to the products from North Carolina (Cronin et al. Citation1995; Cronin and Hay Citation1996), we also describe here seven other products (). Dictyota ciliolata has many other products – especially prenylated guaiane diterpenes – for which structures could not be elucidated in the present study because only trace amounts were isolated. In the crude extracts, the second most abundant product in the Angra dos Reis and the Rocas Atoll populations was 4β-acetoxydictyodial (G), whereas the Salvador population produced a mixture of crenulidane diterpenes (H–K). The crenulidane diterpenes were identified via a combination of 1H-NMR and HRGC/MS techniques from the Rocas Atoll population. These results suggest that D. ciliolata is among the most important sources in the Atlantic, and also confirms that this species is one of those that produces both Groups I and III diterpenes.
Table 2. Diterpenes produced by Brazilian populations of D. ciliolata.
The haplotype network we constructed had the same general pattern as that observed by Tronholm et al. (Citation2013), that is, a central haplotype that is the most common in the Atlantic and Indo-West Pacific oceans, and from which the other haplotypes derived. However, the haplotype network revealed only three geographically segregated groups instead of four. This is most likely because our study involved slightly fewer sequences than in Tronholm et al. (Citation2013) following the removal of sequences in which there were missing data, which may also explain the absence of Tronholm et al.’s (Citation2013) haplotype ‘Caribbean’; this haplotype therefore could be an artifact of inefficient sequencing processes. Despite the genetic data is limited to the population of Arraial do Cabo, whose diterpenes have not been studied yet, along the coast of Brazil exist at least two haplotypes, H1 (at present, restricted to Brazil) and H2 (which extends from Macaronesia southwards in the Atlantic Ocean).
The closest related species to D. ciliolata is D. coriacea (Holmes) I.K. Wang, Hy. S. Kim & W.J. Lee (Tronholm et al. Citation2012), whose geographical distribution range is the Temperate Northern Pacific realm and includes Korea, Japan, China and Taiwan (Guiry and Guiry Citation2018). It is plausible that the greater haplotype diversity of D. ciliolata in Western Australia might be an indication that this area is a centre of speciation, or, at least, a centre of accumulation of haplotypes, in which new haplotypes have been formed and exported to surrounding areas (Bowen et al. Citation2013), perhaps even as far as the Western Indian and Atlantic oceans. Tronholm et al. (Citation2012) proposed two hypotheses for the dispersal of D. ciliolata into the Atlantic Ocean: (a) Lessepsian migration, which the authors considered less plausible due to the species’ absence from the Mediterranean Sea, despite its occurrence in the Red Sea; (b) by crossing the southern Africa during periods of intermittent operation of the Benguela upwelling system in the Miocene-Pliocene, or, more recently, by means of the occasional occurrence of Agulhas rings that carried the species from the Indian ocean. The haplotypes H1 and H5 suggest ancient events of dispersal into the Atlantic Ocean and colonisation because of their restricted geographical distributions which indicate genetic differentiation driven by regional selective factors over long periods of time. The occurrence of H2 in Brazil reinforces the scepticism of Tronholm et al. (Citation2012) regarding their first hypothesis and strengthens the second one; however, the hypothesis of anthropogenically introduction of this haplotype cannot at present be discarded because its wider geographical distributional range in contrast to the others.
Dictyol B acetate (A) was the main product of all Western Atlantic populations of D. ciliolata (North Carolina, Cronin et al. Citation1995; Cronin and Hay Citation1996; the Yucatan Peninsula, Caamal-Fuentes et al. Citation2014b; and Brazil [34°N to 23°S]) and all populations produced pachydictyol A (F) as well. In addition to other major compounds, dictyol B acetate is known to be a product of D. menstrualis (Hoyt) Schnetter, Hörning & Weber-Peukert (Simas et al. Citation2014) and D. mertensii (C. Martius) Kützing (Freitas et al. Citation2007), albeit as minor components. Because dictyol B acetate is the major component of D. ciliolata, a distinctive 1H-NMR spectrum (as a fingerprint) is generated; thus, dictyol B acetate represent a chemotaxonomic marker for this species, and one that can be effective regardless of geographic variation or haplotypic difference. The dictyodial is exclusive to North Carolina and is closely related to the 4β-acetoxydictyodial (G) skeleton from the Brazilian populations. This is likely to be either an ecological variation, as reported for Canistrocarpus cervicornis (Kützing) De Paula & De Clerck (Oliveira et al. Citation2008; Araujo et al. Citation2018), D. mertensii (Freitas et al. Citation2007) and D. menstrualis (Ortiz-Ramírez et al. Citation2008), or reflects true genetic variation between the North Carolina and the Brazilian populations, as evidenced by the different haplotypes.
The occurrence of D. ciliolata along the Atlantic coast of Africa, previously reported solely in species lists (e.g. John et al. Citation2004), was confirmed by Tronholm et al. (Citation2013) via molecular data collected from populations in Madeira, Canary and Cabo Verde archipelagos. Currently, H2 is the only haplotype known to exist in this region. In regard to natural products, populations of an unidentified Dictyota in the Canary Islands have been shown to generate the crenulidane diterpenes 13β-hydroxy-acetoxycrenulide (H) and the 14-hydroxy-acetoxycrenulide (I) (Norte et al. Citation1990; Zarraga et al. Citation1998), and 4-hydroxycrenulide (J) was reported for a population of another unidentified Dictyota in Senegal (Guella and Pietra Citation1993). Manzo et al. (Citation2009), who studied a Moroccan population of D. ciliolata, reported three xenianes and two prenylated guaianes, including dictyodial (as in the North Carolina population) and dictyol H (a typical diterpene product of D. mertensii, as reported by Freitas et al. Citation2007). However, because the characteristic metabolite product of D. ciliolata (i.e. dictyol B acetate) was not observed in the Canarian, Senegalese, or Moroccan samples, it is possible that none of these populations were D. ciliolata. In the case of the Moroccan population the mixture of different Dictyota species is evident and might be some of those reported to Canary Islands (Guiry and Guiry Citation2018), such as D. cymatophila Tronholm, M. Sanson & Afonso-Carrillo (a sister taxon of D. mertensii that may account for the presence of dictyol H) and one or more dentate species (D. canariensis, D. jamaicensis or D. pleiacantha).
Moreover, diterpene skeletons obtained from a Chinese population (Cheng et al. Citation2014; Zhao et al. Citation2015), that was identified as D. plectens (Allender & Kraft) Kraft, are somewhat comparable to those of Atlantic D. ciliolata populations (i.e. prenylated guaiane, xeniane, and crenulidane diterpenes), despite the fact that they are not the same products as from D. ciliolata. Tronholm et al. (Citation2013) reported that D. ciliolata lineage was comprised of Australian specimens identified as D. plectens, as already noted by previous authors and may explain the morphological resemblance between the two species (Allender and Kraft Citation1983; Tronholm et al. Citation2013). The Chinese population was identified based on a rbcL sequence (Cheng et al. Citation2014) that matched Australian sequences described by De Clerck et al. (Citation2006) and assigned to D. plectens; the lack of psbA sequences from this population precluded its inclusion in any of the 10 haplotypes although it is likely to belong to the Western Pacific Ocean group.
Although D. plectens was known only to its type locality (Kraft Citation2009) prior to the studies carried out in China (Cheng et al. Citation2014; Zhao et al. Citation2015), D. ciliolata and some of its synonyms (Dictyota beccariana Zanardini and Dictyota maxima Zanardini) have been recorded in the literature to the South China Sea (Phang et al. Citation2016). In addition, data reported in the literature and DNA analyses provide further evidence that the Chinese population is D. ciliolata. Differences in the chemical structures between the Chinese population and the Atlantic ones may be attributed to the fact that crude extracts used in Cheng et al. (Citation2014) and Zhao et al. (Citation2015) analyses were obtained with ethanol, which results in polar fractions and more oxidative products (or transformed products). In contrast, the acetone or dichloromethane extracts used in the analyses of Atlantic populations resulted in more nonpolar compounds. Furthermore, the presence of dolabellane and dolastane diterpenes in the extract indicates that the Chinese D. ciliolata population contained at least one other Dictyota species.
As Tronholm et al. (Citation2012) observed, the genetic diversity of D. ciliolata has not been adequately sampled, and as such there are missing haplotypes that have not yet been collected or may possible gone extinct (Tronholm et al. Citation2013). Likewise, the knowledge about the diterpenes produced by this species must be improved. A phylogeographic study combining chemical and molecular tools could not only reveal novel diterpenes but also furnish more data to support new hypotheses about the historical and ecological processes relevant to D. ciliolata populations. Nevertheless, chemical data from Atlantic and Pacific populations of D. ciliolata, in combination with the genetic data from several tropical populations of this species presented by Tronholm et al. (Citation2013), failed to confirm the taxonomic status of D. plectens as a synonym of D. ciliolata. First, there is no chemical information from the type locality of D. plectens (Lord Howe Island) that can be compared to D. ciliolata. Second, Tronholm et al. (Citation2013) used the same Australian specimens from De Clerck et al. (Citation2006), but neither study provided morphological information about the vouchers to allow verification that they matched with the morphological characteristics of D. plectens. Third, the only genetic sequence from the type locality used by Tronholm et al. (Citation2013) was a partial psbA sequences that was much shorter than other D. ciliolata sequences for this gene, and therefore, it was not included in our haplotype network. For these reasons, whether D. ciliolata and D. plectens are conspecific remains undetermined, despite the occurrence of the former on the Lord Howe Island.
The chemical data available for D. ciliolata populations worldwide, but especially from the Western Atlantic populations, provides evidence that this species produces prenylated guaiane (Group I), xeniane, and crenulidane diterpenes (Group III). Although chemical data is lacking for several other dentate species, such as, D. canariensis, D. chalchicueyecanensis and D. pleiacantha, the natural products of D. ciliolata are easily distinguishable from those of D. jamaicensis or D. dolabellana. Despite the highly variable morphology and broad genetic diversity of D. ciliolata (Tronholm et al. Citation2013), this species may be of bioprospecting interest due to the biological activities of the substances it produces (Caamal-Fuentes et al. Citation2014a, Citation2014b; Lira et al. Citation2016; Zubia et al. Citation2017), which could be obtained from many parts of the world due to the species’ nearly pantropical distribution () and apparent chemical similarity among populations.
Table S2: Principal peaks observed in the mass spectra (m/z and relative intensity in parenthesis) of diterpenes from D. ciliolata.
Download MS Word (18.6 KB)Table S1: Sequences of psbA from Dictyota ciliolata used on the haplotype network analysis
Download MS Word (21.4 KB)Acknowledgements
The authors thank Claudia Rezende and Angelo Pinto for the use of high-resolution gas chromatography coupled to mass spectrometry (HRGC) and HRGC/MS equipment, and Sandra Zorat Cordeiro (HUNI) for herbarium assistance. Also, the two anonymous referees for suggestions that improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Joel Campos De-Paula http://orcid.org/0000-0001-5852-7516
Erick Alves Pereira Lopes-Filho http://orcid.org/0000-0001-8085-6084
Fabiano Salgueiro http://orcid.org/0000-0002-0352-0699
Diana Negrão Cavalcanti http://orcid.org/0000-0001-6013-9889
Roberto Campos Villaça http://orcid.org/0000-0002-7329-3516
Valéria Laneuville Teixeira http://orcid.org/0000-0003-4962-6912
Additional information
Funding
References
- Allender BM, Kraft GT. 1983. The marine algae of Lord Howe Island (New South Wales): The Dictyotales and Cutleriales (Phaeophyta). Brunonia. 6:73–130. doi: 10.1071/BRU9830073
- Araujo JM, Tappin MRR, Fortes RR, Lopes-Filho EAP, Salgueiro F, De Paula JC. 2018. Chemodiversity of the brown algae Canistrocarpus cervicornis (Dictyotaceae, Phaeophyceae) in tropical and subtropical populations along the southwestern Atlantic coast of Brazil. J Appl Phycol. 30:611–618. doi: 10.1007/s10811-017-1249-5
- Bandelt H-J, Forster P, Röhl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol. 16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036
- Barbosa JP, Fleury BG, Gama BAP, Teixeira VL, Pereira RC. 2007. Natural products as antifoulants in the Brazilian brown alga Dictyota pfaffii (Phaeophyta, Dictyotales). Biochem Syst Ecol. 35:549–553. doi: 10.1016/j.bse.2007.01.010
- Barbosa JP, Teixeira VL, Pereira RC. 2004. A dolabellane diterpene from the brown alga Dictyota pfaffii as chemical defense against herbivores. Bot Mar. 47:147–151. doi: 10.1515/BOT.2004.015
- Barbosa JP, Teixeira VL, Villaça R, Pereira RC, Abrantes JL, Frugulhetti ICPP. 2003. A dolabellane diterpene from the Brazilian brown alga Dictyota pfaffii. Biochem Syst Ecol. 31:1451–1453. doi: 10.1016/S0305-1978(03)00120-0
- Bowen BW, Rocha LA, Toonen RJ, Karl SA, ToBo Laboratory. 2013. The origins of tropical marine biodiversity. Trends Ecol Evol. 28(6):359–366. doi: 10.1016/j.tree.2013.01.018
- Caamal-Fuentes E, Chale-Dzul J, Moo-Puc R, Freile-Pelegrin Y, Robledo D. 2014a. Bioprospecting of brown seaweed (Ochrophyta) from the Yucatan Peninsula: cytotoxic, antiproliferative, and antiprotozoal activities. J Appl Phycol. 26:1009–1017. doi: 10.1007/s10811-013-0129-x
- Caamal-Fuentes E, Moo-Puc R, Freile-Pelegrin Y, Robledo D. 2014b. Cytotoxic and antiproliferative constituents from Dictyota ciliolata, Padina sanctae-crucis and Turbinaria tricostata. Pharmaceutical Biology. 52(10):1244–1248. doi: 10.3109/13880209.2014.886273
- Cavalcanti DN, Gomes MAV, Pino AC, Rezende CM, Pereira RC, Teixeira VL. 2008. Effects of storage and solvent type in a lipophylic chemical profile of the seaweed Dictyota menstrualis. Braz J Oceanogr. 56:51–57. doi: 10.1590/S1679-87592008000100005
- Cavalcanti DN, Oliveira MAR, De-Paula JC, Barbosa LS, Fogel T, Pinto MA, Paixão ICNP, Teixeira VL. 2011. Variability of a diterpene with potential anti-HIV activity isolated from the Brazilian brown alga Dictyota menstrualis. J Appl Phycol. 23:873–876. doi: 10.1007/s10811-010-9601-z
- Cavalcanti DN, Rezende CM, Pinto AC, Teixeira VL. 2006. Diterpenoid constituents from the brown alga Dictyota menstrualis. Nat Prod Commun. 1:609–611.
- Cheng S, Zhao M, Sun Z, Yuan W, Zhang S, Xiang Z, Cai Y, Dong J, Huang K, Yan P. 2014. Diterpenes from a Chinese collection of the brown alga Dictyota plectens. J Nat Prod. 77:2685–2693. doi: 10.1021/np5006955
- Cronin G, Hay ME. 1996. Chemical defenses, protein content, and susceptibility to herbivory of diploid vs. haploid stages of the isomorphic brown alga Dictyota ciliolata (Phaeophyta). Bot Mar. 39:395–399. doi: 10.1515/botm.1996.39.1-6.395
- Cronin G, Lindquist N, Hay ME, Fenical W. 1995. Effects of storage and extraction procedures on yields of lipophilic metabolites from the brown seaweeds Dictyota ciliolata and D. menstrualis. Mar Ecol Prog Ser. 119:265–273. doi: 10.3354/meps119265
- De Clerck O, Leliaert F, Verbruggen H, Lane CE, De Paula JC, Payo DA, Coppejans E. 2006. A revised classification of the Dictyoteae (Dictyotales, Phaeophyceae) based on rbcL and 26S ribosomal DNA sequence analyses. J Phycol. 42:1271–1288. doi: 10.1111/j.1529-8817.2006.00279.x
- De-Paula JC, Bueno LB, Cavalcanti DN, Yoneshigue-Valentin Y, Teixeira VL. 2008. Diterpenes from the brown alga Dictyota crenulata. Molecules. 13:1253–1262. doi: 10.3390/molecules13061253
- De Paula JC, Bueno LB, Frugulhetti ICPP, Yoneshigue-Valentin Y, Teixeira VL. 2007a. Dictyota dolabellana sp. nov. (Dictyotaceae, Phaeophyceae) based on morphological and chemical data. Bot Mar. 50:288–293.
- De-Paula JC, Cassano V, Yoneshigue-Valentin Y, Teixeira VL. 2007b. Diterpenes from the Brazilian brown alga Dictyota crispata (Dictyotaceae, Phaeophyta). Nat Prod Commun. 2:135–137.
- De-Paula JC, Cavalcanti DN, Yoneshigue-Valentin Y, Teixeira VL. 2012. Diterpenes from marine brown alga Dictyota guineenses. Braz J Pharmacog. 22(4):736–740. doi: 10.1590/S0102-695X2012005000071
- De-Paula JC, Pedrini AG, Pinheiro MD, Pereira RC, Teixeira VL. 2001. Chemical similarity between the brown algae Dictyota cervicornis and D. pardalis (Dictyotales, Phaeophyta). Biochem Syst Ecol. 29:425–427. doi: 10.1016/S0305-1978(00)00066-1
- De Paula JC, Vallim MA, Teixeira VL. 2011. What are and where are the bioactive terpenoids metabolites from Dictyotaceae (Phaeophyceae)? Braz J Pharmacog. 21(2):216–228. doi: 10.1590/S0102-695X2011005000079
- Elshamy AIP, Mohamed TA, Marzouk MM, Hussien TA, Umeyama E, Hegazy MEF, Effert M. 2018. Phytochemical constituents and chemosystematic significance of Pulicaria jaubertii E. Gamal-Eldin (Asteraceae). Phytochem Lett. 24:105–109. doi: 10.1016/j.phytol.2018.01.021
- Fernandes DRP, Oliveira VP, Valentin YY. 2014. Seaweed biotechnology in Brazil: Six decades of studies on natural products and their antibiotic and other biological activities. J Appl Phycol. 26:1923–1937. doi: 10.1007/s10811-014-0287-5
- Freitas OSP, Oliveira AS, De-Paula JC, Pereira RC, Cavalcanti DN, Teixeira VL. 2007. Chemical variation in the diterpenes from the Brazilian brown alga Dictyota mertensii (Dictyotaceae, Phaeophyta). Nat Prod Commun. 2:13–15.
- Guella G, Pietra F. 1993. Photochemical conversion of xenicane into the crenulatane skeleton with diterpenoids of the brown seaweed Dictyota sp. from the coasts of Senegal. J Chem Soc Chem Comm. 20:1539. doi: 10.1039/c39930001539
- Guiry MD, Guiry GM. 2018. AlgaeBase. World-wide Electronic Publication. Galway: National University of Ireland; [searched 2018 February 19]. http://www.algaebase.org.
- John DM, Prud’homme van Reine WF, Lawson GW, Kostermans TB, Price JH. 2004. A taxonomic and geographical catalogue of the seaweeds of the Western Coast of Africa and adjacent islands. Nova Hedwigia. 127:4–139.
- Kelecom A, Teixeira VL. 1986. Diterpenes of marine brown algae of the family Dictyotaceae: their possible role as defense compounds and their use in chemotaxonomy. Sci Total Environ. 58:109–115. doi: 10.1016/0048-9697(86)90081-1
- Kelecom A, Teixeira VL. 1988. Dolastane diterpenes from the marine brown alga Dictyota cervicornis. Phytochemistry. 27:2907–2909. doi: 10.1016/0031-9422(88)80686-1
- Kelecom A, Teixeira VL, Pitombo LF. 1991. Quimiotaxonomia de Dictyotales (Phaeophyta). 6. Da sinonímia entre as algas pardas Dictyota dentata e D. mertensii. Ann. Acad Bras Quim. 40:67–70.
- Kraft GT. 2009. Algae of Australia. Marine benthic algae of Lord Howe Island and the southern Great Barrier Reef, 2. Brown algae. Australian. Canberra and Melbourne: Australian Biological Resources Study and CSIRO Publishing.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870–1874. doi: 10.1093/molbev/msw054
- Lara-Isassi G, Alvarez-Herández S, Collado-Vides L. 2000. Ichtyotoxic activity of extracts from Mexican marine macroalgae. J Appl Phycol. 12:45–52. doi: 10.1023/A:1008103609841
- Leite PM, Castilho RO. 2017. Chemosystematics of Brassicales. Biochem Syst Ecol. 71:205–211. doi: 10.1016/j.bse.2017.02.011
- Lira MLF, Lopes R, Gomes AP, Barcellos G, Verícimo M, Osako K, Ortiz-Ramirez FA, Ramos CJB, Cavalcanti DN, Teixeira VL, Amaral V. 2016. Anti-leishmanial activity of Brazilian green, brown, and red algae. J Appl Phycol. 28:591–598. doi: 10.1007/s10811-015-0538-0
- Lopes-Filho EAP, Salgueiro F, Nascimento SM, Gauna MC, Parodi ER, De Paula JC. 2017. Molecular evidence of the presence of Dictyota dichotoma (Dictyotales: Phaeophyceae) in Argentina based on sequences from mtDNA and cpDNA and a discussion of its possible origin. New Zeal J Bot. 55(3):293–305. doi: 10.1080/0028825X.2017.1326387
- Lozano-Orozco JG, Sentíes A, Díaz-Larrea J, Pedroche FF, De Clerck O. 2014. The occurrence of Dictyota canariensis (Dictyotales, Phaeophyceae) in the Gulf of Mexico. Bot Mar. 57:359–365. doi: 10.1515/bot-2013-0111
- Lozano-Orozco JG, Sentíes A, Pedroche FF, Díaz-Larrea J. 2016. Dictyota chalchicueyecanensis sp. nov. (Dictyotales, Phaeophyceae) en el Golfo de México: Evidencias moleculares y morfológicas. Hidrobiológica. 26(2):225–231.
- Manzo E, Ciavatta ML, Bakkas S, Villani G, Varcamonti M, Zanfardino A, Gavagnin M. 2009. Diterpene content of the alga Dictyota ciliolata from a Moroccan lagoon. Phytochem Lett. 2:211–215. doi: 10.1016/j.phytol.2009.08.003
- McDougal OM, Heenan PB, Perry NB, van Klink JW. 2018. Chemotaxonomy of kōwhai: leaf and seed flavonoids of New Zealand Sophora species. New Zeal J Bot. 56(3):227–236. doi: 10.1080/0028825X.2018.1472107
- Norte M, González AG, Zárraga M, Pérez C, Rodriguez ML, Ruiz-Perez C, Dorta L. 1990. New xenicane diterpenes from the brown algae of Dictyotaceae. Tetrahedron. 46:6125–6132. doi: 10.1016/S0040-4020(01)87934-5
- Oliveira AS, Cavalcanti DN, Bianco EM, De-Paula JC, Pereira RC, Yoneshigue-Valentin Y, Teixeira VL. 2008. Chemical composition of diterpenes from the brown alga Canistrocarpus cervicornis (Dictyotaceae, Phaeophyceae). Nat Prod Commun. 3(9):1469–1472.
- Ortiz-Ramirez FA, Cavalcanti DN, Villaça RC, De-Paula JC, Yoneshigue-Valentin Y, Teixeira VL. 2008. Chemical variation in the diterpenes from the Brazilian brown alga Dictyota menstrualis (Dictyotaceae, Phaeophyceae). Nat Prod Commun. 3:1879–1884.
- Othmani A, Bouzidi N, Viano Y, Alliche Z, Seridi H, Blache Y, Hattab ME, Briand JF, Culioli G. 2014. Anti-microfouling properties of compounds isolated from several Mediterranean Dictyota spp. J Appl Phycol. 26:1573–1584.
- Phang SM, Yeong HY, Ganzon-Fortes ET, Lewmanomont K, Prathep A, Hau LN, Gerung GS, Tan KS. 2016. Marine algae of the South China Sea bordered by Indonesia, Malaysia, Philippines, Singapore, Thailand and Vietnam. Raffles B Zool. 34(Suppl):13–59.
- Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A. 2017. DnaSP 6: DNA sequence polymorphism analysis of large datasets. Mol Biol Evol. 34:3299–3302. doi: 10.1093/molbev/msx248
- Saunders GW. 1993. Gel purification of red algal genomic DNA: an inexpensive and rapid method for the isolation of polymerase chain reaction-friendly DNA. J Phycol. 29:251–254. doi: 10.1111/j.0022-3646.1993.00251.x
- Silva ANO, Oliveira AFM, Santos DYAC, Silva SI. 2010. An approach to chemotaxonomy to the fatty acid content of some Malvaceae species. Biochem Syst Ecol. 38:1035–1038. doi: 10.1016/j.bse.2010.10.006
- Simas DLR, Kaiser CR, Gestinari LM, Duarte HM, Paula JC, Soares AR. 2014. Diterpenes from the brown seaweed Dictyota caribaea (Dictyotaceae, Phaeophyceae): The ecological and taxonomic significance. Biochem Syst Ecol. 52:33–37. doi: 10.1016/j.bse.2013.11.001
- Tauwhare SEK, Newman RH, Scheele S, Kanawa RTE. 2006. Chemotaxonomy of Phormium based on sugar-residue analyses of the leaf exudates. New Zeal J Bot. 44(2):129–133. doi: 10.1080/0028825X.2006.9513013
- Teixeira VL. 2010. A taxonomia química de algas marinhas bentônicas. In: Pedrini AG, editor. Macroalgas: uma introdução à taxonomia. Série Flora Marinha do Brasil, vol. 1, Rio de Janeiro, cap. 6: Technical Books; p. 83–97.
- Teixeira VL, Cavalcanti DN, Pereira RC. 2001. Chemotaxonomic study of the diterpenes from the brown alga Dictyota menstrualis. Biochem Syst Ecol. 29:313–316. doi: 10.1016/S0305-1978(00)00055-7
- Teixeira VL, Kelecom A. 1988. A chemotaxonomic study of diterpenes from marine brown algae of the genus Dictyota. Sci Total Environ. 75(2–3):271–283. doi: 10.1016/0048-9697(88)90040-X
- Teixeira VL, Kelecom A. 1989. Chemotaxonomy of Dictyotales. 2. The Dictyota group. Ínsula. 19(Suppl):249–270.
- Tronholm A, Carrillo JA, Sansón M, Leliaert F, García CF, De Clerck O. 2013. Taxonomy of the Dictyota ciliolata – crenulata complex (Dictyotales, Phaeophyceae). Phycologia. 52(2):171–181. doi: 10.2216/12-005.1
- Tronholm A, Leliaert F, Sansón M, Afonso-Carrillo J, Tyberghein L, Verbruggen H, De Clerck O. 2012. Contrasting geographical distributions as a result of thermal tolerance and long-distance dispersal in two allegedly widespread tropical brown algae. Plos One. 7:E30813. doi: 10.1371/journal.pone.0030813
- Tronholm A, Steen F, Tyberghein L, Leliaert F, Verbruggen H, Siguan MAR, De Clerck O. 2010. Species delimitation, taxonomy and biogeography of Dictyota in Europe (Dictyotales, Phaeophyceae). J Phycol. 46:1301–1321. doi: 10.1111/j.1529-8817.2010.00908.x
- Vallim MA, De-Paula JC, Pereira RC, Teixeira VL. 2005. The diterpenes from Dictyotacean marine brown algae in the Tropical Atlantic American region. Biochem Syst Ecol. 33:1–16. doi: 10.1016/j.bse.2004.06.002
- Vallim MA, Teixeira VL, Pereira RC. 2007. Feeding-deterrent properties of diterpenes of Dictyota mertensii (Phaeophyceae, Dictyotales). Braz J Oceanogr. 55(3):223–229.
- Vázquez JT, Chang M, Nakanishi K, Manta E, Perez C, Martin JD. 1988. Structure of hydroazulenoid diterpenes from a marine alga and their absolute configuration based on circular dichroism. J Org Chem. 53:4797–4800. doi: 10.1021/jo00255a025
- Zarraga M, Arroyo P, Norte M. 1998. New crenulides from brown algae of the Dictyotaceae family. Bol Soc Chil Quim. 43(1):73–79.
- Zhao M, Cheng S, Yuan W, Dong J, Huang K, Sun Z, Yan P. 2015. Further New Xenicanes from a Chinese collection of the brown alga Dictyota plectens. Chem Pharm Bull. 63:1081–1086. doi: 10.1248/cpb.c15-00556
- Zidorn C, Stuppner H. 2001. Chemosystematics of taxa from the Leontodon section Oporinia. Biochem Syst Ecol. 29:827–837. doi: 10.1016/S0305-1978(01)00019-9
- Zubia M, Robledo D, Freile-Pelegrin Y. 2017. Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, Mexico. J Appl Phycol. 19:449–458. doi: 10.1007/s10811-006-9152-5