ABSTRACT
A fossil cycad pinna fragment with dichotomising and anastomosing venation and cuticular preservation from the middle Miocene Hindon Maar deposits, Otago, New Zealand, is assigned to the extinct genus Pterostoma (Cycadales: cf. Zamiaceae) as a new species: P. neehoffii. The leaf features of the Hindon Maar fossil differ from previously reported Pterostoma-like macrofossils and dispersed cuticles from New Zealand and Australia in having the combination of narrow guard cell flanges, poorly developed epidermal cuticular ridges and apparently lacking trichomes, suggesting that there were at least three cycad species present in the Neogene of New Zealand. The fossil is also placed into a broader overview of the macrofossil and pollen record for cycads in New Zealand and their possible paleoenvironments.
Introduction
Cycads (Class Equisetopsida: Subclass Cycadidae sensu Chase and Reveal (Citation2009) and Mabberley (Citation2017)) are an ancient, worldwide group of gymnosperms with rigid, large, pinnate leaves arising from woody trunks, making them superficially resemble palms. There are currently 355 living species and ten genera in two families (Lindstrom Citation2009; Christenhusz et al. Citation2011; Calonje et al. Citation2019), but they have a long, much more diverse fossil history extending back to the Carboniferous: Pennsylvanian (Pott et al. Citation2009; Taylor et al. Citation2009; Hill and Stevenson Citation2012). Although there are no extant cycads in New Zealand, macrofossil impressions of several taxa have been reported from the Jurassic Curio Bay fossil forest (Arber Citation1917; Thorn Citation2001; Pole Citation2009) and the early Miocene of Otago and Southland (Pole Citation1992, Citation2007). Dispersed cuticles of Pterostoma R.S.Hill (cf. Zamiaceae) are known from the Late Cretaceous (Pole and Douglas Citation1999) to the Miocene of southern South Island (Hill and Pole Citation1994; Pole et al. Citation2003; Pole Citation2007), with Macrozamia Miq. (Zamiaceae) cuticle also present in the Late Cretaceous (Pole and Douglas Citation1999). An unidentified cycad genus described by Pole (Citation2007) from Southland was possibly also present in the Early Pleistocene of Waikato, North Island (Pole Citation2019). Other reports of cycad-like impression fossils by von Ettingshausen (Citation1887, Citation1891) have generally been regarded as uncertain and requiring more study, with Pole (Citation2012) recognising only three named fossil cycad macrofossil taxa for New Zealand.
The extinct genus Pterostoma was first described from the early Eocene of Tasmania (Hill Citation1980), but is now known in Australia from the mid-Cretaceous of Queensland (Pole and Douglas Citation1999) and the Miocene of Victoria (Pole Citation2007). Pterostoma is also recorded from the Late Cretaceous and Eocene to Miocene of New Zealand (Pole Citation1992, Citation2007; Hill and Pole Citation1994; Pole and Douglas Citation1999; Pole et al. Citation2003). The possible cycad frond impressions illustrated by Pole (Citation1992) from Manuherikia Group sediments of Otago are now regarded as a Pterostoma (Pole et al. Citation2003; Pole Citation2007) and the coeval dispersed cuticle taxon (P. douglasii R.S.Hill & Pole) is common and widespread in the St Bathans Member and Southland Gore Lignite Measures (Pole Citation2007).
However, despite the presence of impressions and dispersed cuticle samples, a cycad frond piece with cuticular preservation has not been described and illustrated from New Zealand. Accordingly, the discovery of such a Pterostoma-like specimen at the middle Miocene-aged Hindon Maar Complex allows for direct comparison with previously reported fossils from New Zealand and Australia, helping to inform better our understanding of diversity and paleoecology of Pterostoma in the Neogene of New Zealand.
Materials and methods
Geological setting
The Hindon Maar Complex (c. 30 km W of Dunedin, ) comprises four partly-eroded, maar-diatreme volcanoes, with three craters filled by biogenic and highly fossiliferous lacustrine sediments. The maars were identified from sub-circular magnetic anomalies and are infilled predominantly by gyttja (organic-rich lacustrine mud), spiculite and laminated diatomite sediments (Kaulfuss et al. Citation2018). The preliminary age determined for Hindon Maar was earliest Miocene (23–16 Ma), based on palynology (D.C. Mildenhall pers. comm. in Youngson Citation1993), but a new 40Ar/39Ar radiometric age of 14.60 ± 0.09 Ma from associated volcanics, suggests that the complex is of mid-Miocene age (Kaulfuss et al. Citation2018).
Figure 1. Map of Cenozoic New Zealand localities with recorded leaf fragments (red) of cycad fossils with anastomosing venation (Pole Citation1992, Citation2007; this study) and Late Cretaceous to Cenozoic cycad pollen reports from FRED, Raine et al (Citation2011), or as unpublished reports (D.C. Mildenhall, 2017, pers. comm.): Cretaceous to Paleocene (green), Eocene (blue), Oligocene (yellow) and Miocene (pale purple). See Appendix 1 for pollen locality details.
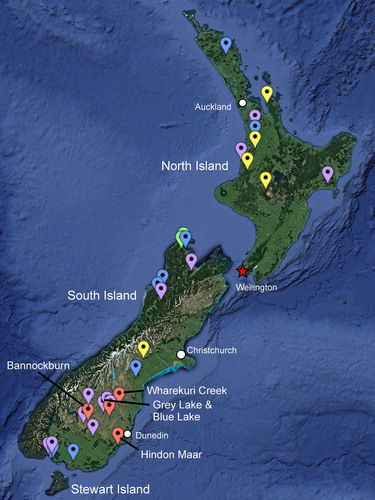
Excavations in two pits ca.100 m apart, near the centre of one maar crater have yielded abundant and diverse animal and plant fossils with excellent preservation, similar to that seen at the nearby, earliest Miocene Foulden Maar, but with a very different taxonomic composition (Lee et al. Citation2016a, Citation2016b; Kaulfuss et al. Citation2018). The most common plant fossils at Hindon Maar are angiosperm leaves, seeds and fruits, mainly Nothofagus Blume, but also Ripogonum J.R.Forst. & G.Forst., Araliaceae leaves and flowers with in situ pollen, podocarp leaves and cones, a cycad, and fern fronds. Together, these fossils are indicative of a Nothofagus–podocarp forest growing under humid, mesothermal conditions around the maar. This is in marked contrast with the Lauraceae-dominated evergreen mesothermal rainforest that surrounded the Foulden Maar site.
Articulated fish fossils are abundant at Hindon Maar, some with soft parts (e.g. skin and eyes) preserved and include a species of eel (Anguilla de Garsault, Citation1764: Anguillidae) and the larval to adult stages of one or more species of Galaxias Cuvier, Citation1816 (Galaxiidae). The fossil assemblage also includes the first pre-Quaternary bird feathers from New Zealand and fish- and bird-derived coprolites. Insects are the most abundant animal fossils in terms of both numbers and diversity, with at least six orders and 25 families present and weevils particularly diverse (Kaulfuss et al. Citation2018). Many of the plant fossils from the site exhibit insect-mediated damage, such as diverse feeding traces, galls and mines (Möller et al. Citation2017).
Specimen preparation
The compressed cycad pinna fragment is preserved on a bedding plane in laminated carbonaceous sediment and was exposed by manual splitting with a knife. Fine needles and wet paintbrushes were used to free the sample and remove surface debris. Fossil cuticle preparations were made using standard methods (e.g. Bannister et al. Citation2012) by warming in 6% H2O2, staining with 0.1% crystal violet and mounting in thymol-glycerine jelly. Slides were photographed under a Leica DM1000 LED microscope (Leica Microsystems GmbH, Wetzlar, Germany) with LAS software (Leica Microsystems Citation2015). Cuticles were also examined by SEM in a JEOL6700F Field Emission Scanning Electron Microscope (JEOL Ltd, Tokyo, Japan). Measurements were averaged from 20 cells unless otherwise indicated.
Reference leaves of extant cycads cultivated at the Adelaide Botanic Gardens and leaf and cuticle collections at The University of Adelaide (ADU) were used for comparison with the fossil, with cuticles prepared in a similar way to the fossils. The fossil and extant specimens were photographed using a Sony D80 DSLR camera (Sony Corporation, Tokyo, Japan) and the cuticles with a Leica microscope as above. All fossil specimens are held at the Geology Museum (OU), University of Otago, Dunedin, New Zealand.
Systematics
Cycadales Pers. ex Bercht. & J.Presl
cf. Zamiaceae Horianow
Pterostoma R.S.Hill
Pterostoma neehoffii Conran, Bannister, U.Kaulf. & D.E.Lee sp. nov.
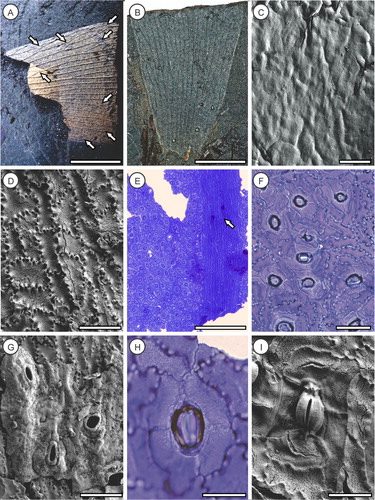
Holotype: OU35425a (A, C–I); paratype: counterpart OU35425b (B).
Figure 2. Pterostoma neehoffii sp. nov., all cuticle images derived from OU35425a. A, Holotype (OU35425a), showing anastomosing veins (arrows); B, Paratype counterpart (OU35425b); C, SEM of adaxial epidermis showing smooth outer surface without obvious cellular detail or hairs; D, SEM of adaxial epidermis inner surface showing strongly sinuous anticlinal walls with buttressing; E, TLM of abaxial surface showing intercostal stomatal band, elongated costal cells and two crystalliferous cells along the vein (arrow); F, TLM of abaxial intercostal cells showing randomly oriented stomata and buttressing of sinuous anticlinal walls; G, SEM of abaxial intercostal cells showing sparse cuticular ridges, sunken stomata and prominent stomal rings; H, TLM of sunken stomate and stomatal ring (external view); I, SEM of stomate (internal view) showing guard cell flanges and thin-walled subsidiary cells. Scale bars A, B = 20 mm, C = 100 µm, D, F, G = 50 µm, E = 100 µm, H, I = 25 µm.
Repository: Geology Department (OU), University of Otago, Dunedin.
Type Locality: Pine Tree Pit within the Hindon Maar Complex (I44/f0392 in the New Zealand Fossil Record File), Waipiata Volcanic Field, c. 30 km W of Dunedin, Otago, southern New Zealand (45.76144°S 170.26466°E (NZGD49); ).
Age and stratigraphy: Middle Miocene (14.6 Ma) lacustrine sediments.
Etymology. Named for Mr Clem Neehoff (1927–2017), who encouraged our fossil explorations of the maar complex on the family farm.
Diagnosis. Veins simple or dichotomously branching with frequent anastomoses, uniformly thickened. Adaxial intercostal epidermal cells irregularly rectangular, oriented randomly; costal cells longer and narrower, oriented along the leaf axis; anticlinal walls sinuous, buttressed internally, periclinal walls slightly domed, outer surface smooth, inner very finely granulose. Abaxial intercostal epidermal cells irregularly rectangular, oriented randomly; costal cells longer and narrower, oriented along the leaf axis; anticlinal walls sinuous, buttressed internally, periclinal walls ridged and corrugated, outer surface smooth, inner very finely granulose. Lamina hypostomatic, stomata intercostal, orientated randomly, with a prominent raised ring. Subsidiary cells irregularly elongate, 4–7 stomate−1; anticlinal walls curved, periclinal walls ridged and corrugated, outer surface smooth, inner very finely granulose.
Description. Pinna fragment, at least 60 mm long and 40 mm wide (A, B) missing basal attachment and apex, but both margins preserved; lower margins entire. Veins simple or dichotomously branching, exhibiting frequent anastomoses, increasing towards the pinna margins and apex (A); c. 5 veins cm−1 across pinna and at least 20 veins pinna−1; uniformly thickened, without anastomosing secondary veins. Adaxial intercostal epidermal cells irregularly rectangular, oriented randomly (D); cells 49–67 µm long (51.2 ± 7.2 µm, average ± SD), 27–43 µm wide (33.2 ± 3.8 µm); costal cells not obviously differentiated; anticlinal walls sinuous, buttressed internally (D), periclinal walls slightly domed, outer surface smooth (C), inner very finely granulose (D); trichome bases not observed. Abaxial intercostal epidermal cells irregularly rectangular, oriented randomly (F); cells 44–73 µm long (55.9 ± 8.2 µm), 22–58 µm wide (41.3 ± 8.6 µm); costal cells longer and narrower, oriented along the leaf axis (E); cells 51–111 µm long (75.1 ± 16.5 µm), 18–44 µm wide (27.0 ± 5.9 µm); anticlinal walls sinuous, buttressed internally (F), periclinal walls ridged and somewhat corrugated, outer surface smooth (G), inner very finely granulose (I); crystalliferous cells uncommon (E); trichome bases not observed. Lamina hypostomatic, stomata intercostal, c. 29 mm−2, orientated randomly, with a raised thickened ring externally (F–H), relatively narrow internal guard cell lamellae and depressed polar ends (I); stomata 30–44 µm long (36.7 ± 4.1 µm), 17–35 µm wide (26.7 ± 5.8 µm). Subsidiary cells irregularly elongate, 4–7 stomate−1 (5.6 ± 0.8); anticlinal walls thin, rounded to curved, periclinal walls slightly ridged and corrugated (F), outer surface smooth, inner very finely granulose (I); cells 27–78 µm long (44.9 ± 12.2 µm), 9–36 µm wide (17.8 ± 7.1 µm).
Discussion
Affinities of the fossil
Anastomosing venation is rare in living cycads, although simple anastomoses occasionally occur in Stangeria and Dioon. Encephalartos and Macrozamia also show apical and lateral anastomosing in the spinescent tips, but always only very near the apex (Brashier Citation1968; Greguss Citation1968; Erdei and Manchester Citation2015). In contrast, anastomosing venation is present in fossil species of Ctenis Lindl. & Hutton from the Jurassic to Eocene of the Northern Hemisphere (Harris Citation1964; Pant Citation1987) and Mesodescolea S.Archang. emend. S.Archang. & Petriella (cf. Stangeriaceae) from the Lower Cretaceous of Argentina (Artabe and Archangelsky Citation1992) Leptocycas gracilis Delev. & R.C.Hope from the Upper Triassic of North Carolina (Delevoryas and Hope Citation1971) and some species of the widespread Mesozoic genus Pseudoctenis Seward also display occasional anastomosing veins (Pant Citation1987; Artabe and Stevenson Citation1999), although the latter genus is normally distinguished from Ctenis in lacking anastomoses (Pott et al. Citation2007).
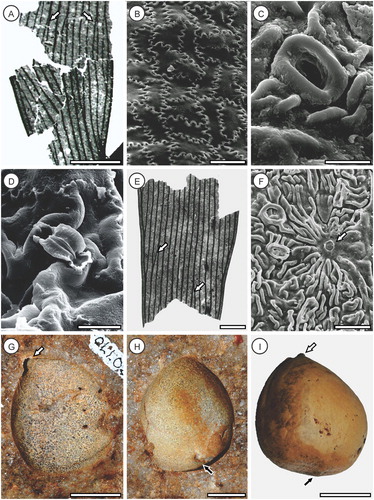
Hill (Citation1980) described a new cycad genus, Pterostoma, with two species, P. zamioides R.S.Hill and P. anastomosans R.S.Hill, from the middle Eocene of eastern Australia, with a third species (P. hirsutus R.S.Hill & Pole) described from dispersed cuticle from the Early Eocene of Tasmania (Hill and Pole Citation1994). The pinnae of these species are dominated by veins that are either simple or dichotomously branched, occasionally anastomosing (A, E) and, in P. anastomosans, with a network of finer veins in between (indicated by impressions in the preserved cuticle). All the Eocene Australian species have trichome bases (F), conspicuously sinuous adaxial epidermal cells (B), abaxially ridged epidermal cells (C, F), sunken stomata below a prominent ring (C, F) and a cuticular ledge between the guard cells and haplocheilic subsidiary cells (D). These features are also seen in the dispersed cuticle taxon P. douglasii R.S.Hill & Pole from the Miocene of New Zealand (Hill and Pole Citation1994).
Figure 3. Comparative Eocene Australian Pterostoma fossils described by Hill (Citation1980). A–D, Pterostoma zamioides from Anglesea (P150055 and AN 2284), Victoria, E, F, P. anastomans from Nerriga, New South Wales (N0157); all samples held at the Melbourne Museum. A, Pinna fragment (AN 2284) showing anastomosing veins (arrows); B, SEM of P150055 adaxial surface showing strongly sinuous anticlinal cell walls; C, SEM of P150055 stomatal ring and cuticular ridges; D, SEM of P150055 stomate (interior view) showing guard cell flanges; E, Pinna fragment of distal pinna fragment of N0157 showing anastomosing veins (arrows); F, SEM of N0157 intercostal cells showing cuticular ridges, a trichome base (arrow) and sunken stomata with prominent rings; G, Cycad-like ?Avicennia diaspore (OU 30770) described from Landslip Hill by Campbell (Citation2002) showing short apical beak (arrow); H, Cycad-like ?Avicennia diaspore (OU 30771) described from Landslip Hill by Campbell (Citation2002) showing basal attachment scar (arrow); I, Cycas revoluta Thunb. seed showing short apical beak (white arrow) and basal attachment (hilum) scar (black arrow). Scale bars A, E = 2 mm B, F = 50 µm, C, D = 20 µm, G–I = 10 mm; photos A–F courtesy of Bob Hill.
Although Pant (Citation1987) synonymised Pterostoma under Macrozamia Miq. (Zamiaceae), the latter genus has pinna bases that narrow towards the point of attachment (Johnson and Wilson Citation1990b), often with a pale, ∼petiole-like basal gland (Hill and Stevenson Citation2012) and Carpenter (Citation1991) clearly defined the cuticular criteria by which Pterostoma can be distinguished from other cycads. The separation of these two genera was supported further by Pole and Douglas (Citation1999) and Hill and Pole (Citation1994), with the description of additional Cenozoic dispersed cuticle Pterostoma species from Australia and New Zealand. In particular, cuticle of P. douglasii is widespread in Manuherikia Group sediments (Pole et al. Citation2003), with Pole’s (Citation1992) cf. cycad pinna fragment impressions bearing anastomosing venation () now considered to be conspecific with P. douglasii (Pole Citation2007). A second cycad leaf fragment with distinctive cuticle was also recovered from Manuherikia Group sediments by Pole (Citation2007) and may represent a new genus which then survived to the early Pleistocene-aged sediments at Mokau, North Island (Pole Citation2019).
Figure 4. Photographs of specimens reported by Pole (Citation1992) as possible cycads from Bannockburn. A, OU13684, showing broad attachment of two pinnae to the rachis (arrow); B, C, details of same showing anastomosing veins; D, OU13672, possibly two pinna side by side; E, detail of same showing anastomosing veins; F, OU12583, fragment of pinna showing anastomosing veins; G, OU13680, proximal pinna fragment; H, OU13606, fragment of pinna showing anastomosing veins; I, OU13075, bases of two pinnae showing very broad attachment to the rachis (arrow). See Pole (Citation1992) for detailed drawings of the venation. Scale bars A–F, H, I = 10 mm, G = 5 mm.

Comparison of P. neehoffii with previously named Pterostoma taxa, as well as Pole’s (Citation2007) ?Zamiaceae gen. et sp. indet. taxon from the Grey Lake and Blue Lake sites near St Bathans in Central Otago, shows that the new species described here differs in a combination of characters from these other taxa (). Although the two Manuherikia Group taxa were found in sediments of similar age, they both differ from P. neehoffii by having distinctive trichome bases and broad, inward-curving (‘Batman logo’ sensu Pole Citation2007) guard cell lamellae; the basis for the name Pterostoma (Greek: winged mouth), as well as by having deeply depressed polar cells with prominent flanges. Similarly, although cuticle was not preserved in the cf. cycad fossils from Central and North Otago described by Pole (Citation1992), his (B) drawing of OU13684 was comparable to Hill’s (Citation1980; Fig. 17) illustration of P. zamioides, showing the broad attachment of the apical pinnae, which is also very clear in OU13075 (I). The Australian Pterostoma species have fewer vein anastomoses (A, E) and all possess trichome bases, have well-developed cuticular sculpturing (C, F), and stomata with prominent inward-curving guard cell lamellae and depressed polar ends (D). The proportionately much narrower guard cell lamellae of P. neehoffii are somewhat similar to those of the Early Eocene Tasmanian P. hirsutus, but the latter has very numerous trichome bases.
Table 1. Distinguishing characteristics of Pterostoma species and the Miocene New Zealand taxon labelled ?Zamiaceae gen. et sp. indet. by Pole (Citation2007).
Overall P. neehoffii is most similar to P. douglasii, especially if the cf cycad pinna segment impressions described by Pole (Citation1992) belong to the latter, but still differs markedly in the development of the stomatal guard cell lamellae, as well as by having deeply depressed polar cells with prominent flanges; both features absent in P. neehoffii. The apparent absence of hairs in P. neehoffii is another difference, but this may just be a sampling issue. Nevertheless, as dispersed cuticle of P. douglasii is defined in part by the presence of hairs, it suggests that the latter is more hirsute.
Pterostoma shares some morphological similarities with Dioonopsis nipponica Horiuchi & Kimura, a zamioid cycad from the Paleogene of Japan and North America (Horiuchi and Kimura Citation1987; Erdei et al. Citation2012). In particular, Hill and Pole (Citation1994) found that the fronds of Dioonopsis resembled Pterostoma in sharing major vein anastomoses and some aspects of cuticle morphology, but noted that Dioonopsis differed in possessing apically spinose fronds, unicellular trichome bases, much finer cuticular ridges and internally buttressed epidermal cells. Nevertheless, they agreed that both genera were probably related and show affinities to Zamiaceae, although they differ from many of the extant genera in venation and some stomatal features (Erdei et al. Citation2018).
The prominent Florin-like stomatal rings seen in Pterostoma fossils are also seen in extant Dioon species (Greguss Citation1968; Vovides and Galicia Citation2016), further supporting a relationship with Zamiaceae, where sunken stomata with over-arching ridges from the subsidiary cells are widespread (Pant and Nautiyal Citation1963; Greguss Citation1968), possibly (together with prominent cuticular sculpturing) reflecting exaptation to aridity (Barone Lumaga et al. Citation2013, Citation2015). However, Dioon is distant from a Macrozamia–Encephalartos clade and basal within Zamiaceae (Nagalingum et al. Citation2011; Condamine et al. Citation2015), apparently diverging in the earliest Cretaceous. Condamine et al. (Citation2015) found that the Encephalarteae (Macrozamia and Encephalartos Lehm. + Lepidozamia Regel) probably arose in the Late Cretaceous, although they cautioned that branching process prior choice can impact Bayesian divergence time estimates significantly, with Nagalingum et al. (Citation2011) finding that most extant cycad clades display Miocene–Pliocene crown radiations, apparently coinciding with increased aridity and seasonality (Barone Lumaga et al. Citation2013).
Mesodescolea can be excluded as a likely relative, based on the deep lobing of its pinnae and very different cuticles (Artabe and Archangelsky Citation1992). Similarly, Leptocycas Delev. & R.C.Hope can probably be discounted, based on pinna morphology and cuticular anatomy (Zhang et al. Citation2010). In contrast, Ctenis fossils possess pinnate fronds, pinnae with broad, often decurrent attachments and frequently anastomosing venation (Artabe and Stevenson Citation1999). Erdei and Manchester (Citation2015) regarded Eocene North American material of Ctenis to be very similar to coeval Dioonopsis fossils, as well as the Miocene New Zealand Pterostoma-like impressions described by Pole (Citation1992). Pterostoma also shares randomly-oriented stomata with Ctenis and Dioonopsis (Erdei et al. Citation2018). Similarly, Pseudoctenis shares numerous morphological and epidermal features with these three genera (Artabe and Stevenson Citation1999) and the precise relationships between these four genera require further study.
Fossil cycad pollen from New Zealand
Raine et al. (Citation2011) list four cycad palynomorphs from New Zealand: the Mesozoic Cycadopites follicularis L.R.Wilson & R.M.Webster; C. granulatus (Jersey) Jersey and Cycadopites sp. A (de Jersey and Raine Citation1990), all from the Triassic; and Cycadopites sp. B (Pocknall Citation1985) from the Oligocene. In addition, there are numerous records of cycad pollen (as Cycadopites Wodehouse or Ginkgocycadophytus Samojlovitch) recorded in FRED (GNS Science Citation2019) from Cretaceous and Cenozoic localities across New Zealand, including some from the Miocene of Central Otago ( and Appendix 1). In addition, there are unpublished records of Cycadopites pollen from at least three sites in the Waikoikoi Stream and Pomahaka River sections in South Otago (D.C. Mildenhall 2017, pers. comm.). However, most of these records are either single pollen grains, or specimens observed outside abundance counts and some may represent redeposited Mesozoic pollen (J.I. Raine and D.C. Mildenhall 2019, pers. comm.). Similarly, although Pocknall (Citation1985) reported relatively abundant Cycadopites pollen in some samples from the Waikato Coal Measures dated to the late Oligocene Upper Nothofagidites matauraensis and Rhoipites waimumuensis Zones of Pocknall and Mildenhall (Citation1984), he noted that it was uncommon elsewhere in New Zealand after the Eocene. Because cycad-like monosulcate pollen grains are also seen in Ginkgo L., bennettites and some angiosperms (e.g. Vajda et al. Citation2013), caution must be used in assigning all of these pollen records to cycads; however, Miocene dispersed cycad cuticle is relatively common (Pole Citation2007), Ginkgo and bennetites are not known from Cenozoic New Zealand sites (Raine et al. Citation2011), and the palynomorphs mapped here are quite different from the various monosulcate angiosperm pollens known from Cenozoic New Zealand (J.I. Raine and D.C. Mildenhall 2019, pers. comm.).
The apparent disconnect between the low reported abundance of fossil cycad pollen (GNS Science Citation2019) and prevalence of dispersed cuticle cycad fossils in the Neogene of New Zealand (Pole Citation2007) can perhaps be explained by pollination in modern cycads, where the majority of species are insect pollinated and show long-term coevolution with beetles (Cai et al. Citation2018) and thrips (Peñalver et al. Citation2012; Brookes et al. Citation2015). As a result, cycad pollen undergoes aggregation at release and shows very poor aerodynamics and aerial dispersal abilities, with most airborne grains only moving a few metres from the parent cone (Walter and Hall Citation2011; Hall and Walter Citation2018). Although no definitive fossil pollinator associations have yet been identified for New Zealand, fossil thrips and diverse Curculionidae (weevils) are present at Hindon Maar (Kaulfuss et al. Citation2018) and some of these may have acted as pollinators for P. neehoffii.
Possible fossil cycad seeds from the Landslip Hill silcretes
The Landslip Hill fossil site near Gore, Southland consists of Miocene-aged silica-cemented quartzose sandstones and conglomerates from the Upper Gore Lignite Measures (Wood Citation1956), representing a probable riparian point-bar deposit (Lindqvist Citation1990; Lee et al. Citation2003). The silcrete contains abundant mineralised conifer and angiosperm leaf impressions, wood, some with cellular detail preserved (Vanner Citation2019), at least one fern (Conran et al. Citation2017), and fruit and seeds representing diverse angiosperms (Holden Citation1983; Campbell and Holden Citation1984; Campbell Citation2002; Jackson Citation2015). Campbell (Citation2002) also considered that the presence of taxa identified as possible Avicennia L. (Acanthaceae), Casuarinaceae, Corynocarpus J.R.Forst. & G.Forst.(Corynocarpaceae), Planchonella Pierre (Sapotaceae, as Pouteria Aubl.) and Pomaderris Labill. (Rhamnaceae) were indicative of a warm temperate lowland environment.
Campbell (Citation2002) described moulds of two cavities with oval-ellipsoidal steinkerns (OU30770 and OU30771) recovered from Landslip Hill as ?Avicennia capsules, noting that they were somewhat compressed, smooth and of a tough, leathery appearance and possessed a short, stout basal stalk and an indistinct peripheral suture. However, re-examination of the original specimens (G, H) suggests that they are more radiosymmetric than compressed and closely resemble some cycad seeds.
The short-lived, cryptoviviparous, water-dispersed fruits of Avicennia are laterally compressed, ellipsoid to ovoid capsules, often with a persistent stylar beak, a very thin (<0.5 mm thick), pubescent pericarp with a bilateral, slightly indented suture line and the bract, bracteoles and calyx are usually persistent on the pericarp (Duke Citation1991). In contrast, the often similar-sized seeds of cycads are characterised by having an ephemeral, fleshy sarcotesta overlying a smooth, thick, shell-like sclerotesta. Once released from the megasporophyll, the fleshy seeds are mainly animal dispersed (Hall and Walter Citation2013) and once the sarcotesta has been consumed or decomposes, the thick sclerotesta protects the still-developing embryo for several months prior to germination (Dehgan and Yuen Citation1983). As with living Avicennia fruits, cycad seeds are ellipsoid to ovoid and may also have a short beak at the micropylar end and a well-developed hilum scar at the base (I).
Unfortunately, due to the relatively coarse nature of the silcrete matrix that formed these steinkerns, it is not possible to obtain sufficient anatomical information of the outer wall of these diaspores to determine if they represent angiosperm fruits (e.g. Avicennia, palms, laurels, or other large-seeded taxa with smooth fruits, seeds or endocarps) or cycad sclerotestas and the precise affinities of these steinkerns requires further study.
Paleoecology of Pterostoma in the Oligo–Miocene of New Zealand
Modern cycads occupy a wide range of habitats from rainforests to arid lands in tropical or subtropical climates; most species growing in regions of predominantly summer rainfall on well-drained, often nutrient-poor soils (Hill and Stevenson Citation2012), often in areas subject to fire (Johnson and Wilson Citation1990a).
The fossil Pterostoma species described by Hill (Citation1980), although associated with highly diverse mesothermal rainforests, nevertheless displayed clean abscission of the fronds and this was interpreted as possible evidence for winter (or dry season) deciduousness at the prevailing high paleolatitudes where fossils of the genus have been found (Hill and Pole Citation1994). Facultative deciduousness is uncommon in cycads, but does occur in some modern Cycas L. (Cycadaceae) species in response to seasonal water stress, e.g. C. armstrongii Miq. (Ornduff Citation1992), C. circinalis L. (Raju and Rao Citation2011) and C. siamensis Miq. (Rundel et al. Citation2002). It has also been reported for the arenicolous dryland African Encephalartos poggei Asch. and related species, as well as the Australian dry sclerophyll forest understorey species Bowenia serrulata (W.Bull) Chamb. (Rundel et al. Citation2002); both genera in Zamiaceae sens. lat. (Salas-Leiva et al. Citation2013; Condamine et al. Citation2015). Cold-induced deciduousness also occurs in C. panzhihuaensis L.Zhou & S.Y.Yang from Sichaun and Yunnan in China below −7°C (LLIFLE Citation2005).
If Pterostoma was in fact deciduous this may have been due to a combination of water availability and high paleolatitude long-winter effects; however, caution is needed, as at least six of the 10 extant cycad genera have at least some species with non-persistent leaf bases, abscising at senescence (Hill and Stevenson Citation2012). Erdei et al. (Citation2012) discussed a possible stress-related deciduous habit for Dioonopsis, as did Hermsen et al. (Citation2009) for the Triassic Antarctic genus Antarcticycas Smoot, T.N.Taylor & Delev., but the evidence in both genera is considered inconclusive.
The late Oligocene forests in the Karamu region in the Waikato Coal Measures where cycad pollen was abundant were dominated by Nothofagus Blume subgenus Brassospora Philipson & M.N.Philipson beeches with emergent gymnosperms (Pocknall Citation1985) growing under a temperate climate, with cycads as part of a diverse understorey, rather similar in composition to the mesothermal modern ‘dry rainforests’ of northern New South Wales (Bowman Citation2000). Similarly, the Oligo–Miocene cycad-bearing Bannockburn Inlet and Wharekuri Creek sites consisted of diverse mesothermal mixed rainforest (e.g. Lauraceae and Arecaceae) and sclerophyllous (e.g. Casuarinaceae) macrofossils (Pole Citation1993, Citation2007, Citation2014; Pole et al. Citation2003). The paleoecology and paleoclimate of the region have been summarised recently, with paleotemperature estimates ranging from temperate to marginally subtropical with floristic evidence for seasonality, wet winters and dry summers, with fire thought to have been relatively common and ecologically important (Pole Citation2014; Reichgelt et al. Citation2015, Citation2018).
It is therefore possible that the relative abundance of cycad cuticles and widespread (albeit rare) pollen records in the early to middle Miocene reflects this increased seasonality leading to drier, potentially more flammable forests; conditions under which many extant cycads now thrive. Nevertheless, the falling temperatures during the Quaternary were probably the main reason for the eventual extinction of Pterostoma in New Zealand.
Acknowledgements
The authors thank the Neehoff family for kindly allowing access to the Hindon Maar Complex on numerous occasions and the Department of Geology, University of Otago and the School of Biological Sciences, The University of Adelaide are thanked for provision of resources to undertake this research. The Department of Botany, University of Otago is thanked for providing access to photomicrographic equipment, as is Liz Girvan, Otago Centre for Electron Microscopy, University of Otago for help with the SEM. Ian Raine and Dallas Mildenhall are thanked for information on New Zealand fossil cycad pollen records. Bob Hill is thanked for photographs of Australian Pterostoma and discussions about fossil and living cycad morphology and both he and Bill Lee provided helpful comments on drafts of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arber EAN. 1917. The earlier Mesozoic floras of New Zealand. New Zealand Geological Survey Palaeontological Bulletin. 6:1–80.
- Artabe AE, Archangelsky S. 1992. Las Cycadales Mesodescolea Archangelsky emend. Archangelsky y Petriella 1971 (Cretácico) y Stangeria Moore (Actual). Ameghiniana. 29(2):115–123.
- Artabe AE, Stevenson DW. 1999. Fossil Cycadales of Argentina. The Botanical Review. 65(3):219–238. doi: 10.1007/BF02857630
- Bannister JM, Lee DE, Conran JG. 2012. Lauraceae from rainforest surrounding an early Miocene maar lake, Otago, southern New Zealand. Review of Palaeobotany and Palynology. 178(1):13–34. doi: 10.1016/j.revpalbo.2012.03.015
- Barone Lumaga MR, Coiro M, Erdei B, Mickle J. 2013. Cuticle micromorphology in cycads: what is the role of ecological pressures? In: Conference Organising Committee, editor. Botany 2013 celebrating diversity!; Jul 27–31; New Orleans. New Orleans: Botanical Society of America; p. Abstract 313; [accessed 2014 Jan 10]. http://www.2013.botanyconference.org/engine/search/index.php?func=detail&aid=313.
- Barone Lumaga MR, Coiro M, Truernit E, Erdei B, De Luca P. 2015. Epidermal micromorphology in Dioon: did volcanism constrain Dioon evolution? Botanical Journal of the Linnean Society. 179(2):236–254. doi: 10.1111/boj.12326
- Bowman DMJS. 2000. Australian rainforests: islands of green in a land of fire. Cambridge (UK): Cambridge University Press. p. 345.
- Brashier CK. 1968. Vascularization of cycad leaflets. Phytomorphology. 18:35–43.
- Brookes DR, Hereward JP, Terry LI, Walter GH. 2015. Evolutionary dynamics of a cycad obligate pollination mutualism – pattern and process in extant Macrozamia cycads and their specialist thrips pollinators. Molecular Phylogenetics and Evolution. 93:83–93. doi: 10.1016/j.ympev.2015.07.003
- Cai C, Escalona HE, Li L, Yin Z, Huang D, Engel MS. 2018. Beetle pollination of cycads in the Mesozoic. Current Biology. 28(17):2806–2812. doi: 10.1016/j.cub.2018.06.036
- Calonje M, Stevenson DW, Osborne R. 2019. The world list of cycads, online edition [internet] 2013–2019; [accessed 2019 Jun 24]. http://www.cycadlist.org.
- Campbell JD. 2002. Angiosperm fruit and leaf fossils from Miocene silcrete, Landslip Hill, northern Southland, New Zealand. Journal of the Royal Society of New Zealand. 32(1):149–154. doi: 10.1080/03014223.2002.9517687
- Campbell JD, Holden AM. 1984. Miocene casuarinacean fossils from Southland and Central Otago, New Zealand. New Zealand Journal of Botany. 22(1):159–167. doi: 10.1080/0028825X.1984.10425242
- Carpenter RJ. 1991. Macrozamia from the early Tertiary of Tasmania and a study of the cuticles of extant species. Australian Systematic Botany. 4(2):433–444. doi: 10.1071/SB9910433
- Chase MW, Reveal JL. 2009. A phylogenetic classification of the land plants to accompany APG III. Botanical Journal of the Linnean Society. 161(2):122–127. doi: 10.1111/j.1095-8339.2009.01002.x
- Christenhusz MJM, Reveal J, Farjon A, Gardner MF, Mill RR, Chase MW. 2011. A new classification and linear sequence of extant gymnosperms. Phytotaxa. 19:55–70. doi: 10.11646/phytotaxa.19.1.3
- Condamine FL, Nagalingum NS, Marshall CR, Morlon H. 2015. Origin and diversification of living cycads: a cautionary tale on the impact of the branching process prior in Bayesian molecular dating. BMC Evolutionary Biology. 15(1):65. doi: 10.1186/s12862-015-0347-8
- Conran JG, Jackson JA, Lee DE, Kennedy EM. 2017. Gleichenia-like Korallipteris alineae sp. nov. macrofossils (Polypodiophyta) from the Miocene Landslip Hill silcrete, New Zealand. New Zealand Journal of Botany. 55(3):258–275. doi: 10.1080/0028825X.2017.1317278
- Cuvier G. 1816. Le règne animal distribué d’après son organisation, pour servir de base à l’histoire naturelle des animaux et d’introduction à l’anatomie comparée. Les reptiles, les poissons, les mollusques et les annélides. 1st ed. Vol. 2. Paris: Déterville. p. 532.
- de Garsault FAP. 1764. Les figures des plantes et animaux d’usage en medecine, décrits dans la Matiere Medicale de Géoffroy medecin. Vol. 5, plates 644–729. Paris: Desprez. p. 177.
- Dehgan B, Yuen CKKH. 1983. Seed morphology in relation to dispersal, evolution, and propagation of Cycas L. Botanical Gazette. 144(3):412–418. doi: 10.1086/337391
- de Jersey NJ, Raine JI. 1990. Triassic and earliest Jurassic miospores from the Murihiku Supergroup, New Zealand. New Zealand Geological Survey Paleontological Bulletin. 62:1–164.
- Delevoryas T, Hope RC. 1971. A new Triassic cycad and its phyletic implications. Postilla. 150:1–21.
- Duke NC. 1991. A systematic revision of the mangrove genus Avicennia (Avicenniaceae) in Australasia. Australian Systematic Botany. 4(2):299–324. doi: 10.1071/SB9910299
- Erdei B, Calonje M, Hendy A, Espinoza N. 2018. A review of the Cenozoic fossil record of the genus Zamia L.(Zamiaceae, Cycadales) – with recognition of a new species from the late Eocene of Panama – evolution and biogeographic inferences. Bulletin of Geosciences. 93(2):185–204. doi: 10.3140/bull.geosci.1671
- Erdei B, Manchester SR. 2015. Ctenis clarnoensis sp. n., an unusual cycadalean foliage from the Eocene Clarno Formation, Oregon. International Journal of Plant Sciences. 176(1):31–43. doi: 10.1086/678467
- Erdei B, Manchester SR, Kvaček Z. 2012. Dioonopsis Horiuchi et Kimura leaves from the Eocene of western North America: a cycad shared with the Paleogene of Japan. International Journal of Plant Sciences. 173(1):81–95. doi: 10.1086/662654
- GNS Science. 2019. FRED: the fossil record electronic database; [updated 2019 Mar 11]. Lower Hutt, N.Z., Geoscience Society of New Zealand and GNS Science; [accessed 2018 Jun 21]. http://www.fred.org.nz/index.jsp.
- Greguss P. 1968. Xylotomy of living cycads: with a description of their leaves and epidermis. Budapest: Akadémiai Kiadó. p. 260.
- Hall JA, Walter GH. 2013. Seed dispersal of the Australian cycad Macrozamia miquelii (Zamiaceae): are cycads megafauna-dispersed “grove forming” plants? American Journal of Botany. 100(6):1127–1136. doi: 10.3732/ajb.1200115
- Hall JA, Walter GH. 2018. Pollination of the Australian cycad Cycas ophiolitica (Cycadaceae): the limited role of wind pollination in a cycad with beetle pollinator mutualists, and its ecological significance. Journal of Tropical Ecology. 34(2):121–134. doi: 10.1017/S0266467418000111
- Harris TM. 1964. The Yorkshire Jurassic Flora. II. Caytoniales, Cycadales & Pteridosperms. London: British Museum (Natural History). p. 191.
- Hermsen EJ, Taylor EL, Taylor TN. 2009. Morphology and ecology of the Antarcticycas plant. Review of Palaeobotany and Palynology. 153(1–2):108–123. doi: 10.1016/j.revpalbo.2008.07.005
- Hill RS. 1980. Three new Eocene cycads from Eastern Australia. Australian Journal of Botany. 28(1):105–122. doi: 10.1071/BT9800105
- Hill RS, Pole MS. 1994. Two new species of Pterostoma R.S.Hill from Cenozoic sediments in Australasia. Review of Palaeobotany and Palynology. 80(1–2):123–130. doi: 10.1016/0034-6667(94)90097-3
- Hill KD, Stevenson DW. 2012. The cycad pages; [accessed 2019 Mar 12]. http://plantnet.rbgsyd.nsw.gov.au/PlantNet/cycad/index.html.
- Holden AM. 1983. Studies in New Zealand Oligocene and Miocene plant macrofossils [unpublished PhD thesis]. Wellington (NZ): Victoria University. p. 369.
- Horiuchi J, Kimura T. 1987. Dioonopsis nipponica gen. et. sp. nov., a new cycad from the Palaeogene of Japan. Review of Palaeobotany and Palynology. 51(1–3):213–225. doi: 10.1016/0034-6667(87)90031-5
- Jackson JA. 2015. A study of plant fossils from Landslip Hill silcrete [unpublished MSc thesis]. Dunedin: University of Otago. p. 149.
- Johnson LAS, Wilson KL. 1990a. General traits of the Cycadales. In: Kramer KU, Green PS, editors. The families and genera of vascular plants, edited by K. Kubitzki. Volume 1. Pteridophytes and gymnosperms. Berlin: Springer Verlag; p. 363–368.
- Johnson LAS, Wilson KL. 1990b. Zamiaceae. In: Kramer KU, Green PS, editors. The families and genera of vascular plants, edited by K. Kubitzki. Volume 1. Pteridophytes and gymnosperms. Berlin: Springer Verlag; p. 371–377.
- Kaulfuss U, Lee DE, Wartho J-A, Bowie E, Lindqvist JK, Conran JG, Bannister JM, Mildenhall DC, Kennedy EM, Gorman AR. 2018. Geology and palaeontology of the Hindon Maar Complex: a Miocene terrestrial fossil Lagerstätte in southern New Zealand. Palaeogeography, Palaeoclimatology, Palaeoecology. 500:52–68. doi: 10.1016/j.palaeo.2018.03.022
- Lee DE, Kaulfuss U, Bannister JM, Conran JG. 2016a. Biodiversity and palaeoecology of Hindon and Foulden Maars: two early Miocene Konservat-Lagerstätten from New Zealand. In: Laurie JR, Kruse PD, Garcia-Bellido DC, Holmes JD, editors. Geological society of Australia abstracts number 117, Palaeo Down Under 2; Jul 11–15. Adelaide: Geological Society of Australia Inc; p. 40.
- Lee DE, Kaulfuss U, Conran JG, Bannister JM, Lindqvist JK. 2016b. Biodiversity and palaeoecology of Foulden Maar: an early Miocene Konservat-Lagerstätte deposit in southern New Zealand. Alcheringa. 40(4):525–541. doi: 10.1080/03115518.2016.1206321
- Lee DE, Lindqvist JK, Douglas B, Bannister JM, Cieraad E. 2003. Field trip 9. Paleobotany and sedimentology of Late Cretaceous – Miocene nonmarine sequences in Otago and Southland. In: Cox S, Smith Lyttle B, editors. Geological Society of New Zealand Inc 2003 annual conference; 1–4 Dec; University of Dunedin. Otago: Geological Society of New Zealand, Miscellaneous Publication; p. FT2–FT48.
- Leica Microsystems. 2015. Leica application suite (LAS), version 4.1. Wetzlar: Leica Microsystems.
- Lindqvist JK. 1990. Deposition and diagenesis of Landslip Hill silcrete, Gore Lignite Measures (Miocene), New Zealand. New Zealand Journal of Geology and Geophysics. 33(1):137–150. doi: 10.1080/00288306.1990.10427579
- Lindstrom AJ. 2009. Typification of some species names in Zamia L. (Zamiaceae), with an assessment of the status of Chigua D. Stev. Taxon. 58(1):265–270. doi: 10.1002/tax.581025
- LLIFLE. 2005. Cycas panzhihuaensis. Text available under a CC-BY-SA creative commons attribution license. www.llifle.com 14 Nov. 2005; [accessed 2019 Mar 14]. www.llifle.com/Encyclopedia/PALMS_AND_CYCADS/Family/Cycadaceae/31950/Cycas_panzhihuaensis.
- Mabberley DJ. 2017. Mabberley’s plant-book: a portable dictionary of plants, their classification and uses. 4th ed. Cambridge (UK): Cambridge University Press. p. 1120.
- Möller AL, Kaulfuss U, Lee DE, Wappler T. 2017. High richness of insect herbivory from the early Miocene Hindon Maar crater, Otago, New Zealand. PeerJ. 5:e2985. doi: 10.7717/peerj.2985
- Nagalingum NS, Marshall CR, Quental TB, Rai HS, Little DP, Mathews S. 2011. Recent synchronous radiation of a living fossil. Science. 334(6057):796–799. doi: 10.1126/science.1209926
- Ornduff R. 1992. Features of coning and foliar phenology, size classes, and insect associates of Cycas armstrongii (Cycadaceae) in the Northern Territory, Australia. Bulletin of the Torrey Botanical Club. 119(1):39–43. doi: 10.2307/2996918
- Pant DD. 1987. The fossil history and phylogeny of the Cycadales. Geophytology. 17(2):125–162.
- Pant DD, Nautiyal DD. 1963. Cuticle and epidermis of recent Cycadales. Leaves, sporangia and seeds. Senckenbergiana Biologia. 44:257–347.
- Peñalver E, Labandeira CC, Barrón E, Delclòs X, Nel P, Nel A, Tafforeau P, Soriano C. 2012. Thrips pollination of Mesozoic gymnosperms. Proceedings of the National Academy of Sciences. 109(22):8623–8628. doi: 10.1073/pnas.1120499109
- Pocknall DT. 1985. Palynology of Waikato Coal Measures (late Eocene—late Oligocene) from the Raglan area, North Island, New Zealand. New Zealand Journal of Geology and Geophysics. 28(2):329–349. doi: 10.1080/00288306.1985.10422231
- Pocknall DT, Mildenhall DC. 1984. Late Oligocene – early Miocene spores and pollen from Southland, New Zealand. New Zealand Geological Survey Paleontological Bulletin. 51:1–66.
- Pole MS. 1992. Early Miocene flora of the Manuherikia Group, New Zealand. 3. Possible cycad. Journal of the Royal Society of New Zealand. 22(4):303–306. doi: 10.1080/03036758.1992.10420823
- Pole MS. 1993. Early Miocene flora of the Manuherikia Group, New Zealand. 10. Paleoecology and stratigraphy. Journal of the Royal Society of New Zealand. 23(4):393–426. doi: 10.1080/03036758.1993.10721232
- Pole MS. 2007. Conifer and cycad distribution in the Miocene of southern New Zealand. Australian Journal of Botany. 55(2):143–164. doi: 10.1071/BT06056
- Pole MS. 2009. Vegetation and climate of the New Zealand Jurassic. GFF. 131(1–2):105–111. doi: 10.1080/11035890902808948
- Pole MS. 2012. Plant macrofossils. In: Gordon DP, editor. New Zealand inventory of biodiversity, volume 3: Kingdoms Bacteria, Protozoa, Chromista, Plantae, Fungi. Christchurch (NZ): Canterbury University Press; p. 460–475.
- Pole MS. 2014. The Miocene climate in New Zealand: estimates from paleobotanical data. Palaeontologia Electronica. 17(2):79. Article 17.2.27A.
- Pole MS. 2019. A survey of Pliocene to mid-quaternary leaf cuticle from the North Island, New Zealand. Palaeontologia Electronica. 22(1):1–32. 12A. doi: 10.26879/862
- Pole MS, Douglas B. 1999. Plant macrofossils of the Upper Cretaceous Kaitangata coalfield, New Zealand. Australian Systematic Botany. 12(3):331–364. doi: 10.1071/SB98003
- Pole MS, Douglas B, Mason G. 2003. The terrestrial Miocene biota of southern New Zealand. Journal of the Royal Society of New Zealand. 33(1):415–426. doi: 10.1080/03014223.2003.9517737
- Pott C, Kerp H, Krings M. 2007. Pseudoctenis cornelii nov. spec. (cycadalean foliage) from the Carnian (Upper Triassic) of Lunz, Lower Austria. Annalen des Naturhistorischen Museums in Wien. Serie A für Mineralogie und Petrographie, Geologie und Paläontologie, Anthropologie und Prähistorie. 109:1–17.
- Pott C, McLoughlin S, Lindström A. 2009. Late Palaeozoic foliage from China displays affinities to Cycadales rather than to Bennettitales necessitating a re-evaluation of the Palaeozoic Pterophyllum species. Acta Palaeontologica Polonica. 55(1):157–168. doi: 10.4202/app.2009.0070
- Raine JI, Mildenhall DC, Kennedy EM. 2011. New Zealand fossil spores and pollen: an illustrated catalogue. 4th ed. (GNS science miscellaneous series no. 4); [updated 2013 May 5; accessed 2018 Dec 10]: http://www.gns.cri.nz/what/earthhist/fossils/spore_pollen/catalog/index.htm, 853 html pages; issued also in CD version.
- Raju A, Rao N. 2011. Taxonomic aspects and coning ecology of Cycas circinalis L. (Cycadales: Cycadaceae), a threatened species of India. Journal of Threatened Taxa. 3(1):1425–1431. doi: 10.11609/JoTT.o2372.1425-31
- Reichgelt T, Kennedy EM, Conran JG, Lee WG, Lee DE. 2018. The presence of moisture deficits in Miocene New Zealand. Global and Planetary Change. 172:268–277. doi: 10.1016/j.gloplacha.2018.10.013
- Reichgelt T, Kennedy EM, Conran JG, Mildenhall DC, Lee DE. 2015. The early Miocene paleolake Manuherikia: vegetation heterogeneity and warm-temperate to subtropical climate in southern New Zealand. Journal of Paleolimnology. 53(4):349–365. doi: 10.1007/s10933-015-9827-5
- Rundel PW, Patterson MT, Boonpragob K, Esler KJ. 2002. Demography and ecophysiology of Cycas siamensis in a deciduous dipterocarp forest of northeast Thailand. Natural History Bulletin – Siam Society. 50(1):15–24.
- Salas-Leiva DE, Meerow AW, Calonje M, Griffith MP, Francisco-Ortega J, Nakamura K, Stevenson DW, Lewis CE, Namoff S. 2013. Phylogeny of the cycads based on multiple single-copy nuclear genes: congruence of concatenated parsimony, likelihood and species tree inference methods. Annals of Botany. 112(7):1263–1278. doi: 10.1093/aob/mct192
- Taylor TN, Taylor EL, Krings M. 2009. Paleobotany: The biology and evolution of fossil plants. 2nd ed. Burlington (MA): Academic Press; 1230 p.
- Thorn V. 2001. Vegetation communities of a high palaeolatitude Middle Jurassic forest in New Zealand. Palaeogeography, Palaeoclimatology, Palaeoecology. 168(3–4):273–289. doi: 10.1016/S0031-0182(01)00203-6
- Vajda V, Lyson TR, Bercovici A, Doman JH, Pearson DA. 2013. A snapshot into the terrestrial ecosystem of an exceptionally well-preserved dinosaur (Hadrosauridae) from the Upper Cretaceous of North Dakota, USA. Cretaceous Research. 46:114–122. doi: 10.1016/j.cretres.2013.08.010
- Vanner MR. 2019. Miocene Casuarinaceae wood from Landslip Hill, Southland, New Zealand. IAWA Journal. doi:10.1163/22941932-40190244.
- von Ettingshausen CB. 1887. Beiträge zur Kenntniss der fossilen Flora Neuseelands. Denkschriften der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Classe. 53(1):143–194.
- von Ettingshausen CB. 1891. Contributions to the knowledge of the fossil flora of New Zealand. Transactions of the New Zealand Institute. 23:237–249.
- Vovides AP, Galicia S. 2016. G-fibers and Florin ring-like structures in Dioon (Zamiaceae). Botanical Sciences. 94(2):263–268. doi: 10.17129/botsci.274
- Walter GH, Hall JA. 2011. Does pollen aerodynamics correlate with pollination vector? Pollen settling velocity as a test for wind versus insect pollination among cycads (Gymnospermae: Cycadaceae: Zamiaceae). Biological Journal of the Linnean Society. 104(1):75–92. doi: 10.1111/j.1095-8312.2011.01695.x
- Wood BL. 1956. The geology of the gore subdivision (New Zealand geological survey bulletin no. 53). Wellington (NZ): DSIR. p. 128.
- Youngson JH. 1993. Mineralized vein systems and Miocene maar crater sediments at Hindon, East Otago, New Zealand [unpublished MSc thesis]. University of Otago. p. 258.
- Zhang J, Yao J-X, Chen J-R, Li C-S. 2010. A new species of Leptocycas (Zamiaceae) from the Upper Triassic sediments of Liaoning Province, China. Journal of Systematics and Evolution. 48(4):286–301. doi: 10.1111/j.1759-6831.2010.00079.x
