ABSTRACT
Ecosourcing seed of ‘local genetic stock’ for ecological restoration has been practiced in New Zealand for about 50 years. However, we believe that it has become unnecessarily restrictive. Ecosourcing ensures plants used for restoration are adapted to local conditions and maintains current distributional patterns. It also restricts genetic diversity, confines species to their historic range, and reduces the conservation options for threatened species. For example, New Zealand tree species, the life form most frequently used in restoration plantings, have low population genetic differentiation and high net migration of alleles throughout their range. Therefore, very little is gained through restrictive ecosourcing of tree seed. Furthermore, avoidance of the danger of inbreeding depression and widening the scope for closer environmental matching, argues for larger rather smaller source areas. Climate change, extinctions across multiple trophic levels, habitat loss and fragmentation, spread of invasive species, and novel habitats have completely altered the contemporary biotic landscape. Conservation needs to engage with these changes if it is to protect and restore ecosystems. Restrictive ecosourcing is counter-productive as it limits utilising genotypic, phenotypic and ecotypic diversity, and thus the evolutionary potential of indigenous species and ecosystems. It also reduces opportunities to protect biodiversity when populations are small, and limits response to climate change. A new approach is needed. We recommend that phylogeographic patterns and biogeographic boundaries be used to set nine broad ecosourcing regions and, within these regions, phenotypic adaptation to particular environments be used as a guide to seed selection. This more relaxed approach to ecosourcing will improve restoration outcomes through increasing species and genetic diversity, reducing the detrimental effects of inbreeding and promoting the genetic rescue of populations of threatened species. Examples of adopting an eco-evolutionary approach to ecosourcing are provided for the early-successional coloniser Kunzea ericoides and late-successional conifer species.
Introduction
Conservation in the twenty-first century faces major environmental change in a landscape where indigenous biodiversity is often depleted, fragmented and impacted by introduced species. In New Zealand, the current conservation context includes major shifts in climate, more frequent extreme weather events, extinction of local biota, spread of non-native invasive species and destruction and modification of habitats following land-use intensification and urbanisation (Macinnis-Ng et al. Citation2021). Collectively, these features pose challenges for conservation management if strategies to ensure resilient ecosystems for the preservation of indigenous biodiversity are to be implemented. Globally, conservation is embracing rewilding to restore trophic interactions, landscape corridors to permit species and biome movement, and assisted migration of species and genotypes to match predicted regional climate regime changes (Morse et al. Citation2014; Perring et al. Citation2015; Bonebrake et al. Citation2018; Fremout et al. Citation2021).
In New Zealand, large-scale ecological restoration efforts through active planting of degraded areas, expanding fragments, increasing threatened species populations, and restoring native dominance to key ecosystems are now a conservation priority (Norton et al. Citation2018; Dewes et al. Citation2022). Ambitious attempts to increase indigenous biodiversity, enhance natural landscapes, increase carbon sequestration, and protect soil and water quality are underway, supported by community groups, territorial authorities, the recent One Billion Trees Programme (Te Uru Rakau Forestry New Zealand Citation2018; Case Citation2020) and the Jobs for Nature (RDC Group Citation2021). Other approaches to ecosystem restoration include natural regeneration and enrichment plantings of mature-phase canopy species (Forbes et al. Citation2020).
Internationally, seed collections for ecological restoration plantings are sourced from within ‘seed transfer zones’ to minimise disruption of genetic patterns or loss of local adaptation (Saint Clair Citation2014; Fremout et al. Citation2021; Hancock and Encinas-Viso Citation2021). In New Zealand, when undertaking ecological restoration, ecosourcing of seed with a known wild origin and of ‘local genetic stock’ has been widely advocated and practiced for 50 years and has become a key component of ecological restoration. Eric Godley (Citation1972), in his highly influential article “ … In their natural state”. Does planting achieve its purpose?, suggested that indigenous planting into natural ecosystems be limited to seed sourced from less than 3 miles (5 km) distant to sustain the genetic integrity of the species. Godley was concerned at the then common practice of planting species and varieties into indigenous ecosystems well outside of their natural geographical range and in atypical habitats which he thought would both disrupt their phylogenetic trajectories and often lead to unsuccessful restorations due to poor environmental matches.
At that time in New Zealand, ecological restoration of ‘depleted landscapes’ and ‘lost biotic communities’ as defined by Atkinson (Citation1988) and the recovery of ecosystems that have been degraded, damaged or destroyed (Gann et al. Citation2019) had barely begun, and Godley’s strictures mainly applied to ecosystems ‘in their natural state’. Later, following Godley’s lead, others have expanded the potential range of seed collection by suggesting that Ecological Regions or Districts (McEwen Citation1987) can be used to define seed collection areas (e.g. Wilcox and Ledgard Citation1983; Norton et al. Citation2018) – see ‘Defining ecosourcing regions’ below for further discussion.
Ecosourcing is today widely promoted by New Zealand national agencies (e.g. Department of Conservation; DOC), local authorities (e.g. Regional Councils via Policy Statements) and non-government organisations (e.g. Wetland Trust), supported by codes of practice and operational guidelines (Ferkins Citation2002; Simpson Citation2002; Supplementary Tables S1 and S2). These documents contain frequent references to ‘local genetic stock’ but provide little operational guidance (Erickson and Halford Citation2020). Council-produced guides and DOC (https://www.doc.govt.nz/get-involved/run-a-project/restoration-advice/native-plant-restoration/ecosource-seeds/) promote ecosourcing policies with explicit statements such as: ‘The closer the seed source to the restoration project, the better (in most cases)’ (Supplementary Table S2) (ECan Citation2019). Taranaki Regional Council advocate using locally ecosourced plants to ‘prevent genetic contamination of Taranaki stock and reduce the loss of regional adaptation’ (see Supplementary Table S2). None of the national or regional policy or guidance documents (see Supplementary Tables S1, S2) provide assistance in species selection when taxa become regionally extinct, a frequent occurrence in many intensely developed areas. Locally extinct species are usually translocated from nearby, often being collected exclusively from the nearest remnant in the local geographical area (many times a small and/or isolated remnant) that may have low genetic diversity (Broadhurst et al. Citation2008a). Sometimes the environment of the seed source site is very different to the target restoration site. There is considerable uncertainty about the basis for and application of ecosourcing policies in New Zealand. At Tāne’s Tree Trust national conference in 2009, in response to the question ‘Are current ecosourcing policies and practice scientifically valid?’, 92% of the 87 respondents said ‘No’ or ‘Don’t know’ (Barton et al. Citation2009). In this paper, we aim to provide conservation managers and ecological restoration practitioners ecosourcing guidance. We evaluate the general ecological context in which ecosourcing is proceeding, its principles and goals, discuss when local ecosourcing may limit restoration efforts, and how ecosourcing principles can be modified to ensure better outcomes. We consider knowledge of indigenous plant phylogeography, biogeography and aspects of plant biology that can now be incorporated into ecosourcing and restoration to provide resilient ecosystems and improved conservation outcomes in New Zealand.
Ecological context of ecosourcing and restoration
Forest clearance and remnants
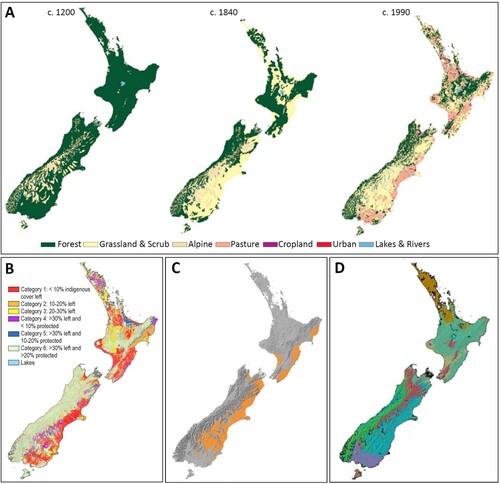
The settlement of New Zealand was accompanied by fire and clearance of forests, particularly in the lowlands (McGlone Citation1983, Citation2001). It is estimated that 90% of land was forested in pre-human times and by 1840, when Europeans settled, the forest cover had reduced to 53% (McGlone Citation2001), and today indigenous forests are reduced to approximately 30% (Ministry for Primary Industries Citation2015; A). The drier lowlands of the eastern North and South Islands have less than 10% of the original indigenous cover remaining and these regions also contain a disproportionately large percentage of New Zealand’s most seriously threatened ecosystems (Walker et al. Citation2006; Holdaway et al. Citation2012; Cieraad et al. Citation2015; B). As the main indigenous tree species lack specific fire adaptations and are mostly slow growing (Perry et al. Citation2014) they have been badly affected. An example of the selective elimination of ecosystems that resulted is provided by the near complete destruction by fire of the formerly dominant dryland conifer forests in eastern North and South Islands (C; Box 1). Remnants of all types of vegetation are under threat from fast-growing, exotic competitors such as species of pine, broom, hakea, willow, etc. Urban spread and intensification of land use has created environments with continuing high extinction rates for native plants, for instance, 0.8% per year in Auckland (Duncan et al. Citation2011). Many of the forest remnants nowadays are too small to be a secure source of local seed and, having usually survived on atypical (poor soils, fire-sheltered) sites, and are an unreliable reference ecosystem for ecological restoration.
Figure 1. A, left, c. 1200 New Zealand forest cover pre-human settlement; middle, c. 1840 post-Māori burning and pre-European settlement; right, c. 1990 post-European settlement (from Weeks et al. Citation2012). B, Threatened Environment Classification (from Cieraad et al. Citation2015). C, Dryland zone shown in orange (from McGlone et al. Citation2017; modified from Walker et al. Citation2009). D, Ten environmental domains (from Overton and Leathwick Citation2001).
Box 1. Case study: late-successional conifers
Extensive deforestation by Polynesian fires of the formerly dominant dryland conifer forests in eastern North and South Islands (Perry et al. Citation2012; McGlone et al. Citation2017; C) affects modern restoration initiatives. In these dry, drought-prone, and frost-prone regions Podocarpus laetus (thin-barked tōtara) and Prumnopitys taxifolia (mataī) were historically abundant and dominant (McGlone et al. Citation2017). Today, dryland conifer forest fragments are few, small, isolated, with a high edge to area ratio and usually an inadequate buffer from impacts of surrounding activities. The conifer remnants are typically indiscriminate, not planned nor representative, and with the total loss of more than 90% of forests from eastern regions there is much missing information, despite palaeoecological reconstruction (McGlone Citation2001; McGlone et al. Citation2017). The deforestation of lowland mature canopy conifer trees results in an absence of seed sources that severely impact natural regeneration (Carswell et al. Citation2007; Forbes et al. Citation2020). Conifer establishment also requires specific conditions of light gaps and canopy openings, and they seldom recruit under closed canopies typical of regenerating broad-leaved angiosperm forests (reviewed by McGlone et al. Citation2017). These factors are unlikely to change in the immediate future.
The current focus of ecological restoration on the planting of new early-successional native forests and a lack of natural seed sources for conifers means they are unlikely to become established in restoration plantings as well as naturally regenerating broadleaf forests without enrichment planting (Tulod et al. Citation2019; Forbes et al. Citation2020). However, the absence of conifers throughout much of lowland New Zealand means when plants are being grown in nurseries for ecological restoration or enrichment plantings seed will usually need to be sourced from populations at locations far distant from the project site. Continuing with the concept of local ecosourcing that excludes conifers due to their local absence is unsatisfactory for the restoration of conifers and return to their pre-human dominance. We consider it as essential that conifers are better represented in ecological restoration initiatives and this can only be achieved by ecosourcing from much larger geographic areas.
Expanding distributional ranges
It may be that less than 25% of indigenous plant species occupy their potential range (Wardle Citation1991, p. 15) and many are thriving in the wild outside of their original ranges. Many native plant species have spread southwards. For example, Metrosideros excelsa (Myrtaceae) and Pittosporum crassifolium (Pittosporaceae) are spreading well south of their original northern North Island limits (Simpson Citation1997). Coprosma autumnalis (Rubiaceae) and Brachyglottis repanda (Asteraceae), are now common in the native forest around Dunedin, and Coprosma repens in the Titi Islands off Stewart Island, all having original range boundaries in the northern to middle South Island. A survey of the Wellington district showed that c. 70 non-local indigenous plants were self-propagating and some, such as the tree Corynocarpus laevigatus (Corynocarpaceae), aggressively spreading (Perrie et al. Citation2013).
The most likely explanation for this southwards spread is that many northern species failed to recapture their potential range at the end of the Pleistocene (Wood et al. Citation2017). Since settlement, disturbed/anthropogenic habitats have facilitated the establishment of native species beyond their natural geographic range but well within their ecological tolerances. These possibly better-adapted but non-local indigenous taxa create conservation dilemmas when they disrupt local plant communities. Under a current ecosourcing philosophy, they are considered aliens and therefore not used.
Macrolearia lyallii (≡ Olearia lyallii; Asteraceae) is a well-studied example of this phenomenon. It has established on the Auckland Islands, well outside its natural range in southern coastal South Island and islands near Foveaux Strait (Godley Citation1965). It was almost certainly accidentally introduced to the Auckland Islands by sealing parties in the early nineteenth century and has succeeded in nutrient-enriched sites similar to those in its native habitat where it is exposed to sea spray and disturbance by nesting sea birds and seals (Wilmshurst et al. Citation2015). Campbell and Rudge (Citation1976) suggested that, as M. lyallii is a non-local indigenous plant, it should be eradicated as it may outcompete the natural population of Metrosideros umbellata (Myrtaceae). Subsequently, it has been shown that M. lyallii on the Auckland Islands is neither ecologically nor biogeographically anomalous and will not threaten M. umbellata (Lee et al. Citation1991; Wilmshurst et al. Citation2015).
Novel ecosystems
New Zealand ecosystems have changed irrevocably during human settlement. Some 50% of indigenous terrestrial avian species were extirpated, and ecosystems fragmented and eliminated through urbanisation, agriculture, and forestry. Novel biotic pressures have been introduced, including grazing, browsing and predatory mammals, wasps and herbivorous insects, nitrogen-fixing shrubs, and exotic forbs and grasses (McWethy et al. Citation2010; Perry et al. Citation2014; Brandt et al. Citation2021). As noted by Perry et al. (Citation2014), anthropogenic fire ‘may have shifted large areas into successional ‘traps’ from which, in the face of recurrent fire, escape from fire-prone low-growing successional communities to more resistant tall forest is difficult.’ The upshot has been the creation of novel ecosystems consisting of exotic and indigenous elements (Hobbs et al. Citation2009; Davis et al. Citation2011) whose long-term composition and trajectory are often unclear. Management of novel ecosystems requires further research into their persistence, values, and restoration thresholds (Santana Citation2022).
These novel ecosystems add a layer of complexity to restoration efforts. Entire taxonomic (e.g. ferns) or life form (e.g. lianas, canopy conifers) indigenous groups are often missing from novel ecosystems. Locating indigenous species which can co-exist or even dominate over the exotic element is a challenge (Thomas et al. Citation2014), and one that probably has to be met by drawing on the indigenous resources over a much wider area than anticipated in current ecosourcing practice.
Environmental shifts
New Zealand faces warmer winters, fewer frosts, and wetter western and drier eastern areas associated with steepened orographic precipitation gradients as well as the possibility of increased disturbances (Hendy et al. Citation2018; Keegan et al. Citation2022). As a special case, urban areas tend to have a very localised combination of higher temperatures (particularly at night), CO2 concentrations and atmospheric nitrogen deposition compared to adjoining regions (Searle et al. Citation2012), providing conditions favourable to the presence of otherwise constrained species. Therefore, irrespective of conservation objectives, plant community compositions over the coming decades will inevitably shift in response to changing environments (McGlone and Walker Citation2011). The goal of climate resilience may require climate-adjusted provenancing, that is sourcing species and genotypes pre-adapted to future environmental states (Prober et al. Citation2015, Citation2019; Harrison et al. Citation2017; Malavasi et al. Citation2018; Carvalho et al. Citation2020; Harrison Citation2021). Resilience, adaptability and ecotypic, phenotypic and genotypic/allelic diversity of species are essential characteristics when ecosourcing plant materials for future climates (Erickson and Halford Citation2020). However, differences in growth, reproductive performance and resistance to herbivory are common between local and more distant provenances (Hancock and Hughes Citation2014). Important considerations are whether the current species have broader environmental ranges than they currently occupy and thus are capable of responding to future change; and the risk that translocated species may prove to be super-competitors and reduce overall indigenous diversity (McGlone and Walker Citation2011).
Inbreeding depression
Flowering plants commonly produce a mix of selfed and outcrossed seed, an important reproductive assurance against cross-pollination failure. Habitat fragmentation, small plant populations that limit mate choice, and the loss of pollinators can increase selfing, resulting in inbreeding depression and the loss of adaptive potential. Pollinator declines, particularly loss of birds, are affecting seed production in indigenous trees and shrubs (Anderson et al. Citation2011; Rodger et al. Citation2021). Inbreeding depression reduces plant performance and induces recruitment failure (Angeloni et al. Citation2011). In New Zealand, the reduction in the fitness of progeny due to pollinator declines (especially among bird-pollinated species) and reduced pollinator service (pollen limitation) and high rates of selfing may be quite widespread (Van Etten et al. Citation2015). Habitat loss and fragmentation in New Zealand lowland forest ecosystems (Perry et al. Citation2014) will exacerbate the selfing syndrome in self-compatible species. Furthermore, low adult densities may increase the chance of crossing with a close relative, thereby increasing the inbreeding coefficient in the seed produced.
Inbreeding depression in New Zealand has been shown to impact seedlings or young plants. Metrosideros excelsa has high rates of geitonogamous selfing leading to lower rates of shoot growth of selfed compared with outcrossed seedlings (Schmidt-Adam et al. Citation2000). Sophora microphylla (Leguminosae) has high selfing rates, pollen limitation and inbreeding depression, producing progeny with poor growth, high mortality and flower failure (Robertson et al. Citation2011; Van Etten et al. Citation2015). In selfed plants, over half of the Sophora seed produced was futile and genetically doomed. In Fuchsia excorticata (Onagraceae), selfed seedlings have low survival and slow growth and seldom reach maturity (Robertson et al. Citation2011). The threatened shrub Olearia adenocarpa (Asteraceae) has self-compatible and self-incompatible genotypes (Heenan et al. Citation2005). Seedlings raised from selfing produced either smaller plants, in comparison to outcrossed plants, or plants that died when young, indicative of inbreeding depression. Hebe amplexicaulis (Plantaginaceae) also exhibits inbreeding depression in selfed progeny (Garnock-Jones and Molloy Citation1982). In our view, to avoid raising plants that may be inbred, seed should preferably be ecosourced from large and healthy populations in natural environments, even if these are some distance from the restoration site.
Restoration and enrichment plantings
Creating appropriate and functional forest and shrubland vegetation successional stages in ecological restoration planting is difficult, especially as the bulk of the planting effort usually occurs over a limited time span. Many plantings in New Zealand are a mix of a small number of early-successional species (e.g. Kunzea ericoides, Myrtaceae; Box 2) produced from a readily available seed source, easily grown in a nursery environment, with a high success rate when planted (satisfying contracted planting success goals), and of rapid growth to achieve canopy closure and weed suppression (Norton et al. Citation2018). Such an approach often leads to insufficient numbers of mid- to late-successional and canopy species and may prevent the development of more diverse and stable communities (Clarkson and Bylsma Citation2016; Norton et al. Citation2018; Forbes et al. Citation2020). With strict local ecosourcing criteria, seed of these later-stage species is often not available in sufficient quantities. Natural dispersal of canopy or emergent species may occur, but only when seed sources are nearby and, for fleshy fruited species, when avian dispersers are abundant (Norton et al. Citation2018; Forbes et al. Citation2020). When natural sources of canopy dominants are constrained, consideration must be given to undertaking later enrichment plantings if full ecosystem restoration is to occur (Norton et al. Citation2018; Tulod et al. Citation2019; Forbes et al. Citation2020).
Box 2. Case study: early-successional Kunzea ericoides
Kunzea ericoides sens. lat. (kānuka) is a widespread early-successional coloniser, with genotypic, phenotypic and ecotypic variability (Heenan et al. Citation2021, Citation2022). Heenan et al. (Citation2021) identified north to south clinal variation and distinguished four broad geographic regions. The genetic study of 1361 SNPs from 49 populations in southern North Island and South Island (Heenan et al. Citation2022) provides data to further investigate the application of an enlarged ecosourcing area and how ecotypes can be applied to selecting seed/plants for restoration projects. Three broad geographic areas of southern North Island, northern South Island and southern South Island can be identified based on genotypic variation of SNPs (Heenan et al. Citation2022, figure 4A; see below). Across each of these regions, and indeed the entire study area of southern North Island and South Island, low population differentiation (FST) and high net migration (Nm) demonstrate elevated levels of gene flow and panmictic genetic structure (Supplementary Table S4).
Genotypic patterns of DNA SNP data in Kunzea are geographic and do not support recognition of multiple Kunzea species (e.g. De Lange Citation2014) with PCA axes 1 and 2 explaining 6.02% of the genetic variation among four species represented by 49 populations (comparable to 7.7% explained for axes 1–3 in DNA microsatellite data; Heenan et al. Citation2021). In contrast, a PCA of environmental variables for the same 49 populations explained 66.0% of the variation on axes 1 and 2 (Heenan et al. Citation2022), suggesting adaptive genetic variation relating to ecotypic differentiation. Reinforcing recognition of ecotypes, utilising genome wide SNP data (Heenan et al. Citation2022) we have done preliminary analyses of adaptive genetic variation (outlier analysis and environmental association analysis) and found evidence that 62 of 1361 SNPs were under selection and correlated with at least one temperature and/or precipitation related variable (Supplementary Table S5). This indicates adaptive genetic variation in kānuka that could identify populations that may be maladapted or pre-adapted under future conditions (Supple et al. Citation2018).
Thus, in Kunzea where there is significant ecotypic differentiation, but minor genetic/allelic variation, seed can be ecosourced from plants from more distant but similar sites. Populations from inland, montane, frost-prone sites and droughty stony soils, for example, can be referred to as a montane-ecotype. Although similar phenotypically and ecotypically, these populations are genetically unrelated and most similar to geographically proximate populations of other forms of kānuka (Heenan et al. Citation2022). Applying a niche matching approach to the ecological restoration of an inland montane site, it would be most appropriate to ecosource seed from other inland montane sites rather than the geographically closest population of another ecotype not suited to the rigours of montane, frost-prone, droughty habitats. In this example, while it is appropriate to expand the geographic context of ecotypic-based ecosourcing to a broader regional context, it does not mandate collecting from the most distant montane-ecotype populations (i.e. central North Island ecosourced seed for planting in Central Otago). In our opinion, where widespread species exhibit phenotypic and/or ecotypic variability, it is more important to ensure the site of the ecosourced plant material is similar to the restoration site rather than focusing on the geographically closest sites.
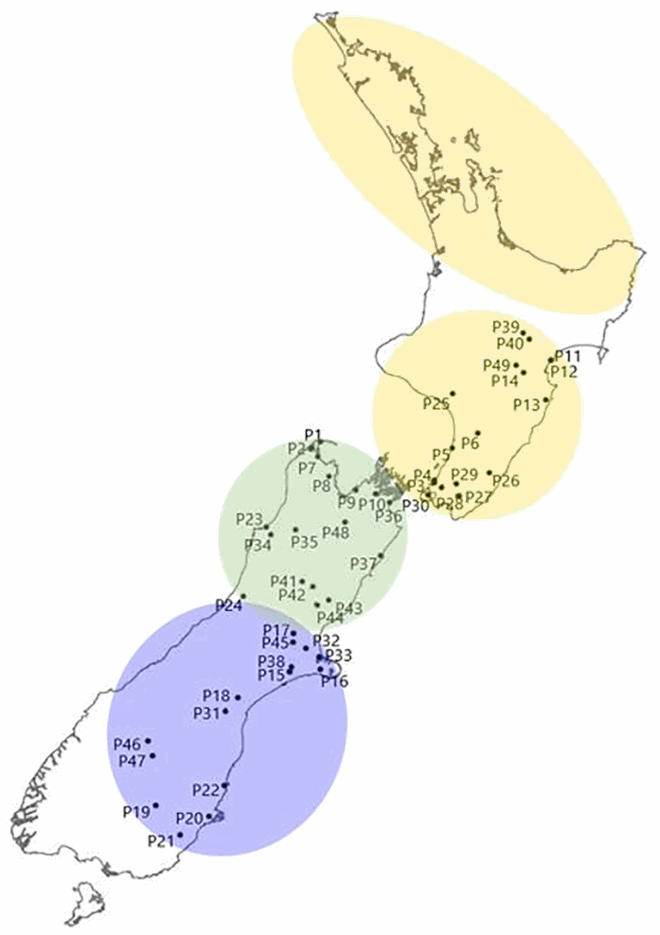
Box 2.1: Four phylogeographic regions for kānuka (Kunzea ericoides) suitable as ecosourcing seed zones based on SNP population genetic data (yellow, green and purple zones) from Heenan et al. (Citation2022) and microsatellite data (orange zone) summarised from Heenan et al. (Citation2021). Individual populations from SNP data have codes from Heenan et al. (Citation2022). SNP population genetic metrics highlight low population differentiation (low FST) and high gene flow (high Nm) (Supplementary Table S4).
Ecosourcing and the conservation goals of restoration
Conservation objectives for dynamic environments
Conservation goals in the twenty-first century are to maintain ecosystem processes, prevent extinctions, and facilitate native species dominance, while reducing threats from weeds, pests and contaminants. A formulation of this goal, Ecological Integrity, has been adopted as the primary conservation objective in New Zealand, is enshrined in Government legislation such as the Environmental Reporting Act 2015, and is now being implemented by governmental organisations. Ecological Integrity as defined in the Act represents a desirable state, namely: ‘ … the full potential of indigenous biotic and abiotic features and natural processes, functioning in sustainable communities, habitats, and landscapes’ (for a full discussion of the concept see Lee et al. (Citation2005) and McGlone et al. (Citation2020)). Note that this formulation reflects the desirability of an ecosystem satisfying Ecological Integrity, but does not mandate any particular past state (McGlone et al. Citation2020). In recent years the Department of Conservation and some Regional Councils have implemented a biodiversity assessment framework based on Ecological Integrity (Wright et al. Citation2020).
The philosophy of ecosourcing as it has developed in New Zealand is predicated on the retention of distributional patterns and ecosystem composition as they were before European settlement. There is much merit in this approach (McGlone Citation2000), not the least because it provides a historical template to co-ordinate conservation restoration activities, but it has limits. It works best at broad landscape scale, as disturbance and competitive interactions ensures a degree of constant disequilibrium across landscapes. Historical data is therefore most useful in showing what was the original suite of species. With regard to vascular plant associations, it is fortunate that there has been so little historical loss of species (only 6 extinctions) and so complete reassembly of original components is technically possible. However, while it may be desirable to have continuity with pre-human ecosystems it is not possible to recreate ecosystems that will function in exactly the same manner as those in the past (McGlone Citation2000). Loss of significant components of the original fauna (plant-browsing moa, bird pollinators and large-bodied insects) and introduction of browsing and grazing mammals, and seed-eating rats, as well as anticipated climate change, means that consideration must also be given to floral elements that can persist in the changed circumstances, even if they were not historically present.
Threatened species management
Crisis conservation often necessitates the translocation of threatened taxa to safer places where the risks are reduced or eliminated. These sites can be outside of the natural distributional range. In New Zealand, threatened animals, mainly birds and reptiles, are translocated to offshore islands and areas of the main islands well outside their historic ranges. Such translocation is rarely done for plants. The discrepancy probably arises because vertebrates account for nearly all (>99%) of the extinctions and most of the ex situ conservation efforts in New Zealand. Under crisis conservation, imminent extinction rightly trumps all other considerations and any mammal-free habitat available has been used.
For nearly all threatened plant species, in situ conservation management is the rule. Small plant populations can exhibit reproductive failure (poor seed set and/or dispersal) such as can occur with self-incompatibility, dioecy and loss of pollinator and dispersal mutualisms. Inbreeding depression can result from pollen limitation and increased selfing (see above), creating genetically depauperate populations. When threatened species have scattered and fragmented populations (at both regional and national scales) and critically low numbers, it is essential that wide geographic ecosourcing and mixing of populations is undertaken to increase allele diversity and expand environmental tolerance. New populations should be established with seed sourced from multiple sites and aggregated to provide increased genotypic and phenotypic variation to facilitate resilient species (e.g. Barnaud and Houliston Citation2010). Outbreeding depression occurring among more distantly related populations being mixed is unlikely at the relatively small geographic scales being proposed herein and within the range of similar genotypic variability.
Defining ecosourcing regions
Ecological regions and provenance trials
The New Zealand Ecological Regions and Districts scheme (McEwen Citation1987) is often suggested as a reliable guide for ecosourcing. For instance, Wilcox and Ledgard (Citation1983), following on from an extensive analysis of provenance performance of Nothofagaceae species recommended it as a ‘ … useful basis for limiting and controlling the transfer of genetic material in Nothofagus.’ Likewise, intensive studies of genotypic variation of Cordyline australis (Asparagaceae) in 28 provenances across its range, led to the same recommendation for local ecosourcing (Harris et al. Citation2006). If widely adopted, this would be a rather restrictive requirement as there are 286 ecological districts and 85 ecological regions. Moreover, the Ecological Region and District scheme is largely subjective, relying for the most part on expert knowledge. The aim was to define ecologically coherent units unified by topography, soils, climate, vegetation cover and cultural history to assist with policy and management, and in particular, establishment of protected natural areas to ensure adequate representation. As the Districts are small (average less than 1000 km2), most plant species resident in them have ranges many times larger, especially trees and shrubs, and we see little evidence in New Zealand of local adaptation at such a fine scale.
The few provenance studies from New Zealand support this concept of a lack of significant fine-scale adaptation. In Wilcox and Ledgard (Citation1983), phenotypic types in Nothofagaceae (Fuscospora and Lophozonia) species typically ranged over very wide areas from the central North Island to the central South Island. Elevational phenotypic gradients were an exception as they showed a strong effect on growth, but of course this does not necessarily imply that a locally sourced plant at a given elevation would perform better than a more distantly sourced plant from a similar elevation. A common garden provenance trial of Podocarpus totara (Podocarpaceae), an important tree in restoration, showed no difference in seedling growth in 28 provenances from Northland to Nelson, but regions from the rest of the South Island had significantly lower growth rates (Bergin and Kimberley Citation1992). Growth form varied widely but showed no pattern. Bergin and Kimberley (Citation1992) also suggested local ecosourcing on the assumption, which was not demonstrated, that there was strong local adaptation. Intensive Cordyline australis (Harris et al. Citation1998, Citation2001, Citation2006) and Kunzea ericoides (Harris Citation1996) provenance studies are important as they were based on specimens grown to adulthood and not just seedlings. These studies revealed largely clinal variation from north to south of growth, cold tolerance and flowering times with local collection site environmental characteristics also important.
Collectively, these studies show there is good evidence for genetically based adaptation within widespread species operating over broad scales, in particular in relation to temperature, but fall short of demonstrating the fine-grained adaptation that would justify an Ecological Regions or District-based approach.
Genetic variation and phylogeography
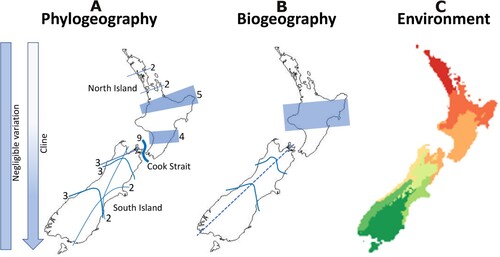
Ecosourcing at small geographic scales in New Zealand assumes extensive fine-scaled genetic variation that remains unconfirmed in any plant taxa. Species with widespread distributions commonly have very broad regional to national patterns of genetic variation (A; Supplementary Figure S2; Supplementary Table S3). Overall, genetic studies of New Zealand trees and shrubs suggest the following: (1) Most species exhibit low levels of population genetic variation as represented by expected heterozygosity (He) and low population differentiation (i.e. high historical gene flow) as shown by low FST, with genotypic variation often being clinal or with a national distribution; (2) Landscape-scale genetic variation and phylogeographic patterns (of individual species) are often consistent with major biogeographic regions and their boundaries (representing multiple species); (3) A few species with widespread distributions and (naturally?) disjunct local populations can have genetically discrete populations. A study of natural and restored populations of Melicytus ramiflorus (Violaceae) near Hamilton, Waikato, showed similar levels of genetic variation in both populations at this local scale (Stevens et al. Citation2015).
Figure 2. Major regions (and their boundaries) identified as potential areas for ecosourcing. A, Phylogeographic regions where two or more species share similar genetic patterns in having a boundary between genotypes (numbers indicate genotypes represented by a general boundary). Lines marking phylogeographic patterns from studies listed in Supplementary Table S3 and shown individually in Supplementary Figure S2. Note the shaded areas represent 4 and 5 species each, respectively, and are therefore generalised boundaries. Other species have negligible or clinal patterns of variation (indicated by left-hand bars). B, Biogeographic regions. The shaded area represents an area where competing hypotheses of the biogeographic boundary occur (see text). Hatched line represents Southern Alps axis. C, Eight environmental domains.
The common patterns of genotypic/allelic variation in New Zealand trees and shrubs are most probably the result of genetic bottlenecks and the loss of genetic diversity in reduced population sizes during Pleistocene glacial episodes, followed by range expansions of particular genotypes (Wood et al. Citation2017). During interglacial cycles, the expansion of forest vegetation occurred over too short a period to enable the accumulation of new mutations through genetic drift, and repeated cycles of glaciation and vegetation contraction would have provided regular genetic bottlenecks further narrowing genotypic variation.
The regions defined by species’ genotypic variation are generally large (A; Supplementary Table S3, and references therein; Supplementary Figure S2), often encompassing 25%–50% of the area of major islands. For example: Helichrysum lanceolatum (Asteraceae) has predominant north to south geographic distribution of genotypic variation as does Kunzea ericoides (see Box 2), although the latter has some differentiated regional groups and concordance with established biogeographic boundaries (e.g. latitude 38°S boundary); Cordyline australis (Asparagaceae) genotypes to the north and south of Lake Taupo are also consistent with the 38°S to 39°S biogeographic boundary (see below); Leptospermum scoparium genetic groups occur over five wide geographic areas in northern North Island, central and southern North Island, East Cape, northern South Island and southern South Island. These examples, and others (Supplementary Table S3, Supplementary Figure S2), demonstrate large-scale geographic zones. Furthermore, for many populations of New Zealand trees expected heterozygosity (He) and genetic differentiation (FST) are low, and much less than the global averages (Supplementary Table S3); this is probably due to the impacts of Pleistocene glacial cycles discussed above.
Not all plant taxa show extended phylogeographic patterns or genotypic change at biogeographic boundaries. Some threatened species may have small and disjunct populations, or assemblages of several disjunct populations, that appear genetically discrete (e.g. Pittosporum obcordatum, Pittosporaceae, Wright et al. Citation2017; Pseudopanax ferox, Araliaceae, Shepherd and Perrie Citation2011). For widespread taxa with predominantly disjunct and discrete populations, a regional focus should be adopted that encompasses an area larger than ‘local genetic stock’ but smaller than a major biogeographic region (see below).
Different genetic markers also provide alternative patterns of genotypic/allelic variation based on their variability. For instance, dominant AFLP, ISSR and RAPD loci have smaller expected heterozygosity (He) values than co-dominant SSR loci (microsatellites) whose values are more-or-less three times larger (Ai et al. Citation2014; Supplementary Table S3). Further, nucleotide sequences with transitions and transversions mutate less than SSR loci with indel mutations (Li et al. Citation2002; Payseur and Cutter Citation2006). cpDNA sequence data are maternally inherited (Birky Citation1995) and often exhibit local introgression and geographical structure which contrasts with nrDNA phylogenetic or taxonomic relationships (Tsitrone et al. Citation2003). These points are exemplified in Fuscospora (Nothofagaceae) with geographic partitioning of chloroplast nucleotide haplotypes that are shared by multiple species but in which SSR genotypes generally represent individual species (Supplementary Table S3). Thus, Fuscospora cliffortioides, F. fusca, F. solandri and F. truncata share two geographically distinct chloroplast haplotypes (Rawlence et al. Citation2021), whereas SSR data provided a greater resolution that separated these four species and provided evidence of hybridisation between F. cliffortioides and F. solandri but did not show phylogeographic patterns (Smissen et al. Citation2014). Sophora provides a similar example with three chloroplast haplotypes generated from genome-wide ddRADseq data distributed geographically (northern North Island, southern North Island and South Island) and each shared among multiple species (Shepherd and Heenan Citation2021). In contrast, SSR data recovered mostly distinct groups representing the species, but little evidence of geographic patterns (Heenan et al. Citation2018). The two closely related lancewood species, Pseudopanax crassifolius and P. ferox provide contrasting patterns with SSR and haplotype data (Shepherd and Perrie Citation2011; Gemmell et al. Citation2022). Pseudopanax ferox has pronounced genetic differentiation of its populations, whereas P. crassifolius has only weakly differentiated genetic clusters. Notably P. ferox, along with Lophozonia menziesii (Nothofagaceae), have unique haplotypes restricted to the southern Wellington region and in both their affinities are with northern South Island populations. For other species Cook Strait is a natural barrier that demarcates patterns of genotypic variation (A; Supplementary Figure S2).
Biogeographic regions
The biogeography of New Zealand at the time of human settlement was the outcome of evolutionary processes through the Cenozoic (65 million years), but the modern pattern of plant distribution largely represents the impact of Pleistocene glaciations, mountain building, volcanic activity, and species responses to complex environmental gradients in a cool-warm temperate oceanic climate over the past 2.6 million years (Wood et al. Citation2017). The majority of extant species (89%) originated after the end of the Miocene Thermal Optimum at about 15.0 Ma and c. 50% have evolved during the late Pliocene-Pleistocene (0–4.99 Ma) (Heenan and McGlone Citation2019). Major New Zealand biogeographic boundaries and smaller regions of endemism have been defined for extant vascular plants by co-occurrence patterns shared by multiple taxa (Cockayne Citation1917; Wardle Citation1963; Burrows Citation1965; McGlone Citation1985; Rogers Citation1989; Heenan et al. Citation2017). These are often overlooked in ecological restoration planning and ecosourcing guidelines. Three major biogeographic boundaries have been identified that relate to trees. First, the central North Island between 38°S and 39°S latitude has several biogeographic lines defined by Wardle (Citation1963, Citation1988), McGlone (Citation1985) and Heenan et al. (Citation2017) which are generally distinguished as the southern limits of northern North Island warm temperate forest trees. Northland and coastlines north of latitude 38°S remained in tall podocarp-broadleaf and beech forest throughout the Pleistocene although there were extensive composition fluctuations in response to glacial cycles (Newnham et al. Citation2013). The location of this boundary is reflected in the nature of the plant community. The lower North Island floristic gap of Rogers (Citation1989), with a northern boundary at approximately 40°S, is not included as it is defined by non-forest species.
Second, in the South Island, the northern and southern boundaries of the southern floristic gap at approximately 42°S and 44°S are significant biogeographic boundaries (Wardle Citation1963, Citation1988; McGlone Citation1985). For these, we follow the boundary positions defined by Heenan et al. (Citation2017), based on spatial analyses of the entire vascular flora. These boundaries combine the Nothofagaceae (beech) gap of previous authors with a newly recognised alpine gap (Heenan et al. Citation2017), both probably demarcated by the repeated impacts of Pleistocene glacial cycles.
Within the major biogeographic regions, smaller areas of endemism have been defined by their distinctive flora and the assemblage of widespread species. Those areas of endemism especially important for tree species are Northland and NW Nelson. Plant disjunctions, such as tree species (e.g. Libocedrus plumosa (Cupressaceae), Phyllocladus trichomanioides (Podocarpaceae), Metrosideros parkinsonii (Myrtaceae)) occurring north of 39°S and disjunct to north-western South Island. Species restricted to the areas of endemism can only be ecosourced within those regions, but for species occurring both within and beyond the area of endemism, options for ecosourcing are less constrained. The northern North Island endemic tree species require comment as these may have different southern limits (see McGlone Citation1985, figure 2; Wardle Citation1991, figure 5.3), making defining a common boundary problematic in the vicinity of 37°S and 39°S, with this region also having a broad phylogeographic boundary (B).
The proposed biogeographic boundaries provide large geographic areas for ecosourcing and can be recognised operationally based on a shared biodiversity, ecological, environmental, and evolutionary history. We identify major boundaries that define large potential seed ecosourcing regions. In North Island to the north of 38°S; between 38°S and 39°S latitude band; and south of 39°S. In South Island boundaries at 42°S and 44°S latitudes, with northern, central, and southern regions. In South Island we also suggest a south-west to north-east line following the axis of the Southern Alps, separating the wetter, humid west coast from drier inner montane basins and east coast. Taking this approach, we provide nine regions that are applicable to ecosourcing.
Ecosourcing parameters in a changing environment
There are two scales of environmental matching that need to be considered in ecosourcing/ecological restoration. First, broad-scale New Zealand-wide environmental assessment to distinguish major ecoregions. This has been undertaken, with 10, 20 and 100 environmental domains identified across New Zealand (Overton and Leathwick Citation2001; Overton et al. Citation2002; Leathwick et al. Citation2003). For environmental regions comparable to the 4–9 areas identified by phytogeography and biogeography (see below), the ten-group domain model (Overton and Leathwick Citation2001), provides the resolution needed (D). Here we include, as an example for how New Zealand can be split into domains for ecosourcing, a new analysis that partitions New Zealand based on both environmental and geographic distance using a Ward-like hierarchical clustering algorithm (C; Supplementary Figure S1; Chavent et al. Citation2018). We selected the mean temperature of the coldest quarter and annual precipitation (McCarthy et al. Citation2021) and implemented the clustering algorithm using the hclustgeo function from the R package ‘ClustGeo’ (Chavent et al. Citation2021). Environmental variables were scaled (mean = 0; SD = 1) before analysis to standardise their units. In hclustgeo, the relative importance between the two distance variables can be adjusted (using the term alpha), before splitting the country into the desired number of domains using the function ‘cutree’ from R 4.1.2 (R Core Team Citation2021). We selected an eight-domain model with alpha = 0.4 since in the South Island this provides separation along the Southern Alps of the high precipitation west and dry east rainfall gradient, and in North Island provides a clear framework for recognising northern North Island and provides some clarity for the central area where there are competing biogeographic boundary hypotheses (). The central North Island environmental domain southern boundary is a new line that does not correspond to the biogeographic lines of Wardle (Citation1963), McGlone (Citation1985), Rogers (Citation1989) and Heenan et al. (Citation2017), and the northern boundary is closest to the 38°S line (McGlone Citation1985). Although this is an eight-domain model, the presence of Cook Strait as an additional boundary separates a domain shared by southern North Island and northern South Island. Therefore, for the purposes of ecosourcing, the environmental domains can be used to distinguish nine regions.
The second scale is the more specific ecotype/niche matching, so that suitable sites for seed ecosourcing can be selected, similar to the conditions at the restoration site. Where anthropogenic changes have shifted the environment envelope outside of the range of many extant local species, new phenotypes from outside the area may be better adapted (Bischoff et al. Citation2006). Ecotype matching identifies the ecosourcing site(s) that best matches the project goals including environmental shifts (e.g. climate change) and novel ecosystems. It remains good practice to ecosource plant material from sites that are close in environmental space to the restoration site (Montalvo and Ellstrand Citation2001).
Mānuka (Leptospermum scoparium; Myrtaceae), although abundant on free-draining soils, is also adapted to partial immersion in water (Cook et al. Citation1980), and therefore provides a good example of ecotype matching. In Canterbury, mānuka from the wetland fringes of Te Waihora/Lake Ellesmere, Yarrs Lagoon (near Lincoln) and Travis Wetland (Christchurch City) occur in permanently wet sites with, for example, the wetland fern Blechnum minus (Blechnaceae). On nearby Banks Peninsula, mānuka often occurs on summer-dry, unstable loess/clay hillslopes in association with a range of broad-leaved trees and shrubs such as Coprosma spp. (Rubiaceae), Kunzea ericoides and Pseudopanax arboreus (Araliaceae). Rather than selecting from the nearest geographical population, ecological restoration projects in wet sites and utilising mānuka should therefore be matched to the most suitable regional populations, such as the wetland ecotypes from Te Waihora, Yarrs Lagoon and Travis Wetland. Another example is provided by Kunzea ericoides (Box 2).
Implementation of an eco-evolutionary ecosourcing paradigm
Establishing biodiversity conservation goals in the twenty-first Century is enormously challenging in the face of increased demands for agricultural land, proliferation of invasive species, climate change, large-scale alterations to abiotic drivers of ecosystem processes and the legacy impacts of ecosystem degradation. Preventing further species loss and restoring habitats and functional indigenous networks remains an operational priority. However, typological approaches based on securing extant community and ecosystem types are, in our view, often unrealistic when rapid biotic/abiotic environmental and climate change are altering habitats and native species assemblages and creating novel niches. We argue for pragmatic guidelines that recognise the uncertainty of predicting biodiversity responses to environmental and climate change over the coming centuries. At multiple scales, ecological integrity can be protected by maintaining systems dominated by native species, where all available occupants are present across the full representation of environmental space (McGlone et al. Citation2020). Ecosourcing as implemented for restoration and rehabilitation programmes is a key operational tool for conservation actions and enhancing ecological integrity and allowing more flexibility in seed sourcing will assist to create more resilient communities.
Ecosourcing regions
The evidence presented herein based on shared plant distributions (e.g. established biogeographic boundaries), individual species phylogeography and population genetics (e.g. low genetic diversity) and biology (e.g. inbreeding depression), supports considerably expanding the size of the geographic area considered for ecosourcing seed and plant material. Seed from small and isolated fragmented remnants, often distant from other remnants, with few pollinators and only a limited number of adults, may result in inbred, unhealthy, doomed plants and consequential cryptic recruitment failure. Seed should preferably be ecosourced from large and healthy populations in natural environments, even if these are some distance from the restoration site. We see little evidence that the ‘local genetic stock’ concept of ecosourcing will make much difference to restoration success, if environmental matching considerations are respected. On the other hand, when the target species has large, diverse local populations and abundant seed available, seed collection from more distant sites is unnecessary because, even though the risk of undesirable consequences is low, as we have demonstrated, there is no benefit in so doing. The one caution is consideration of ecotypic differentiation (see Box 2) and it remains important to ecosource seed from sites similar to the restoration site (Montalvo and Ellstrand Citation2001).
This approach is consistent with international evidence and recommendations that genetic diversity be enriched by ecosourcing seeds from multiple sources to retain the evolutionary potential and adaptive capacity of revegetation plantings and support ecosystem functioning (Broadhurst et al. Citation2008b; Thomas et al. Citation2014; Jordan et al. Citation2019). Ecosourced seeds from a wide geographic area can provide a balance between achieving restored populations that are genetically diverse and maintaining landscape-scale patterns of genetic differentiation (Höfner et al. Citation2022).
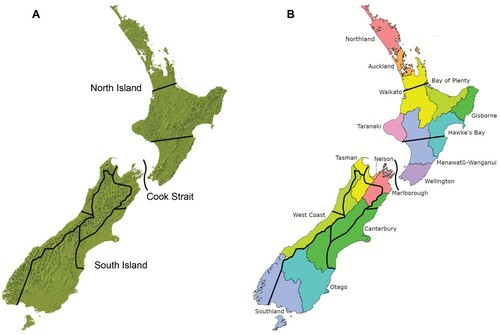
We have integrated national scale phylogeographic patterns of genotypic variation for individual species (or groups of related species), major historical biogeographic boundaries representing the entire vascular flora, and environmental domains to recognise nine regions in New Zealand that can be used as the framework for considering ecosourcing ( and ). These ecosourcing regions broadly correspond with some regional and district council boundaries, which should facilitate ease of implementation (). Some boundaries also coincide with recognised ecological areas (e.g. Canterbury Plains Ecological Region; McEwen Citation1987).
Figure 3. Nine proposed eco-evolutionary regions for ecosourcing. A, Ecosourcing regions overlaid onto New Zealand topographic map. B, Ecosourcing regions overlaid onto Regional Council regions.
The ecosourcing regions provide a general guideline, or rule of thumb, and should not be considered a hard boundary restricting all transfers. The proposed scheme is not to be interpreted as doctrinaire and we encourage common sense and practical application near boundaries – after all our main goal is to encourage ecosourcing from across much larger areas than is currently practiced and not to be overly restrictive. Consideration should always be given to the known range of the species in question. A particular case is that of northern endemic tree species that occur in two biogeographic regions (i.e. north and south of 37°S or 38°S); it is not sensible to apply highly restricted ecosourcing to these species. When a restoration site is near the boundary of an ecosourcing region, this does not preclude sourcing material from across the boundary. For example, if a species largely occurs in one ecosourcing region and is weakly represented in a second, we see little issue in ecosourcing seed from the main region in which it occurs for use elsewhere. We suggest, for example, if suitable ecosourcing populations occur across a boundary and within c. 30–50 km (where in our experience populations tend to have high relatedness) they can be used as a seed source.
Achieving change
Adopting enlarged ecosourcing seed/plant collection zones in New Zealand is consistent with international developments (Saint Clair Citation2014; Fremout et al. Citation2021; Hancock and Encinas-Viso Citation2021). To achieve improved biodiversity outcomes in New Zealand through larger seed-collecting zones, revised policies and management of change are required, led by local, regional, and central government updating Regional Policy Statements, Biodiversity Strategies, and practical guidelines to undertaking ecological restoration plantings and seed ecosourcing. Landscape architects and ecologists creating restoration plans, commercial plant nurseries producing millions of plants annually and ecological restoration companies undertaking plantings, all have key roles to play to operationalise change to increase the resilience of indigenous plant communities. Particular attention needs to be given to engaging with the network of volunteer and community groups that currently collect seed, operate local plant nurseries, and undertake community plantings.
Tangata Whenua (indigenous Māori people of New Zealand) are landowners and managers where much ecological restoration is occurring and we hope that enlarged seed collecting zones will assist kaupapa Māori. Iwi might consider seed collection and restoration for taonga (treasured) species no longer present in their rohe (tribal territory), cultural use associations (e.g. wai, mahinga kai and mahi) where habitats/species have been lost, or in preserving mauri (vital life force), whakapapa (genealogical connections), manaakitanga (respect and care) and kaitiakitanga (guardianship) (e.g. Ratana et al. Citation2019).
Seed ecosourcing and collection guidelines
The general seed collection guidelines suggested below reinforce the enlarged ecosourcing regions that are relevant to New Zealand. Several of these are taken from Erickson and Halford (Citation2020) and include:
Collect seed from 50 or more unrelated parent plants to obtain a representative sampling of genetic diversity in a population.
To minimise the adverse effects of inbreeding, collect seed from unrelated parents, including sourcing and mixing seed from multiple sites. Care should be taken in collecting seed from plants growing closely together as biparental inbreeding may occur through the crossing of related individuals. Genetic and phenotypic diversity and good population representation can be achieved by collecting from plants well-dispersed throughout the collection site.
Collecting seed from larger populations is preferable to small, fragmented populations where inbreeding or past genetic bottlenecks may reduce genetic/allele diversity.
Collection sites should comprise the full range of environmental and climatic conditions within an ecosourcing region, but taking account of ecotypic variation.
Within the designated ecosourcing region collect seed from naturally occurring wild populations and not cultivated plants.
Consider collecting soil to innoculate seedlings with appropriate mycorrhizae.
Record keeping by seed collectors and land managers regarding provenance and species translocations is required for restoration plantings to evaluate outcomes.
Future research to inform ecosourcing regions and ecological restoration
The evidential basis of ecosourcing, phenotypic variation, population genetic structure and phylogeographic patterns of some of the commonly used species in ecological restoration needs to be improved. A good start was made late last century in the form of provenance trials for Podocarpus totara (Bergin and Kimberley Citation1992), Nothofagaceae (Wilcox and Ledgard Citation1983), Cordyline australis (Harris et al. Citation1998) and Kunzea ericoides (Harris Citation1996), but the appetite for such studies appears to have faded as they are time and resource intensive. However, there are opportunities to embed common garden experiments into restoration plantings (Breed et al. Citation2018). Such approaches have been highly successful elsewhere (Breed et al. Citation2018; Bailey et al. Citation2021) and would provide evidence for management of ecological restoration. Experimental restoration sites could initially be established in areas without native vegetation to minimise the risk of unintended gene flow into remnant populations (Laikre et al. Citation2010; Larcombe et al. Citation2016).
Knowledge of adaptive variation can be improved with two complimentary approaches, landscape genomics and common garden experiments (Sork et al. Citation2013). Genetic surveys have become much more feasible as their power has increased and costs decreased. Species which need attention as they are common elements in ecosourcing are Griselinia littoralis (Griseliniaceae), Pittosporum tenuifolium (Pittosporaceae), Plagianthus regius (Malvaceae), Coprosma robusta and a number of small-leaved coprosmas (Rubiaceae), as well as a wider range of conifer species (McGlone et al. Citation2017). These studies should incorporate, where possible, phenotypic, ecotypic and clinal variation. Landscape genomics studies based on these data can identify the genomic signature of adaptive variation by detecting alleles that are under selection and correlated with environmental gradients (Supple et al. Citation2018; Capblancq and Forester Citation2021; See Box 2, Kunzea ericoides case study). However, landscape genomics cannot provide direct information about the fitness of populations under different climate conditions (Sork et al. Citation2013). Replicated common garden experiments are required that span climatic gradients, and use germplasm from across a species range, to allow the detection of differential fitness (genotype by environment interactions) (De Kort et al. Citation2014; Bailey et al. Citation2021). For example, identifying populations that are responding differently to key climatic variables. This information could be used in combination with predictive habitat modelling (e.g. Harrison Citation2021) to design climate-adjusted provenancing strategies within, and where appropriate between, our suggested ecosourcing regions.
Studies are needed regarding the impacts of indigenous New Zealand plants establishing well outside of their natural range (e.g. Lee et al. Citation1991; Wilmshurst et al. Citation2015). Are there desirable or detrimental impacts from indigenous plants such as Corynocarpus laevigatus, Coprosma repens (Rubiaceae), Pittosporum crassifolium, and Metrosideros excelsa on the ecosystems they invade?
Climate change issues must be addressed. An important unknown is the capacity of indigenous species to extend to fill their potential range (Wardle Citation1991, p. 15) or to spread into novel climatic space. For target species, we need to know if regional populations along the main north–south and east–west climate gradients will become maladapted as climates change, and whether genotype movement is needed. Both field and modelling studies are required. If climate resilience does become a restoration goal, gene flow from enrichment plantings into remnant native stands may be desirable (Frankham Citation2015). Natural selection under changing climate can filter gene flow from non-local plantings to improve genetic diversity and the adaptive potential of native populations (Aitken and Whitlock Citation2013; Frankham Citation2015). This is likely to be most beneficial in fragmented landscapes where remnant vegetation has low genetic diversity and natural gene flow has been restricted by anthropogenic barriers (Frankham Citation2015). Outbreeding depression should also be considered when moving and mixing different provenances (Breed et al. Citation2018).
Supplementary Material Table S5
Download MS Word (24.4 KB)Supplementary Material Table S4
Download MS Excel (30.4 KB)Supplementary Material Tables 1-3
Download MS Word (36.9 KB)Supplementary Figure S2
Download JPEG Image (52.3 KB)Supplementary Figure S1
Download PDF (83.2 KB)Acknowledgements
We thank participants at the New Zealand Ecological Society (28 November to 1 December 2022) and New Zealand Plant Conservation Network (4 December to 7 December 2022) conferences for feedback and discussion on seed ecosourcing presentations by Matt Larcombe and Peter Heenan, respectively. Jasmin Mosimann assisted with literature searches. We thank Bruce Clarkson (as referee), an anonymous referee, and the associate editor for their critiques of the draft manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ai B, Kang M, Huang H. 2014. Assessment of genetic diversity in seed plants based on a uniform π criterion. Molecules. 19:20113–20127. DOI:10.3390/molecules191220113.
- Aitken SN, Whitlock MC. 2013. Assisted gene flow to facilitate local adaptation to climate change. Annual Review of Ecology, Evolution, and Systematics. 44:367–388.
- Anderson SH, Kelly D, Ladley JJ, Molloy S, Terry J. 2011. Cascading effects of bird functional extinction reduce pollination and plant density. Science. 331:10681071.
- Angeloni F, Ouborg NJ, Leimu R. 2011. Meta-analysis on the association of population size and life history with inbreeding depression in plants. Biological Conservation. 144:35–43.
- Atkinson IAE. 1988. Presidential-Address—Opportunities for ecological restoration. New Zealand Journal of Ecology. 11:1–12.
- Bailey TG, Harrison PA, Davidson NJ, Weller-Wong A, Tilyard P, Steane DA, Vaillancourt RE, Potts BM. 2021. Embedding genetics experiments in restoration to guide plant choice for a degraded landscape with a changing climate. Wiley Online Library, Report Book No. 1442–7001.
- Barnaud A, Houliston GJ. 2010. Population genetics of the threatened tree daisy Olearia gardneri (Asteraceae), conservation of a critically endangered species. Conservation Genetics. 11:1515–1522.
- Barton I, Gadgil R, Bergin D. 2009. Managing native trees. Towards a national strategy. Proceedings of the Tāne’s Tree Trust 10th Anniversary Conference and Workshops held at The University of Waikato, 18–20 November 2009.
- Bergin D, Kimberley M. 1992. Provenance variation in Podocarpus totara. New Zealand Journal of Ecology. 16:5–13.
- Birky CW. 1995. Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proceedings of the National Academy of Sciences. 92:11331–11338.
- Bischoff AL, Crémieux LC, Smilauerova M, Lawson CS, Mortimer SR, Dolezal J, Lanta V, Edwards AR, Brook AJ, Macel M, et al. 2006. Detecting local adaptation in widespread grassland species – the importance of scale and local plant community. Journal of Ecology. 94:1130–1142.
- Bonebrake TC, Brown CJ, Bell JD, Blanchard JL, Chauvenet A, Champion C, Chen IC, Clark TD, Colwell RK, Danielsen F, Dell AI, et al. 2018. Managing consequences of climate-driven species redistribution requires integration of ecology, conservation and social science. Biological Reviews. 93(1):284–305.
- Brandt AJ, Bellingham PJ, Duncan RP, Etherington TR, Fridley JD, Howell CJ, Hulme PE, Jo I, McGlone MS, Richardson SJ, et al. 2021. Naturalised plants transform the composition and function of the New Zealand flora. Biological Invasions. 23:351–366.
- Breed MF, Harrison PA, Bischoff A, Durruty P, Gellie NJ, Gonzales EK, Havens K, Karmann M, Kilkenny FF, Krauss SL, Lowe AJ. 2018. Priority actions to improve provenance decision-making. BioScience. 68(7):510–516.
- Broadhurst LM, Lowe A, Coates DJ, Cunningham SA, McDonald M, Vesk PA, Yates C. 2008b. Seed supply for broadscale restoration: maximizing evolutionary potential. Evolutionary Applications. 1:587–597. DOI:10.1111/j.1752-4571.2008.00045.x.
- Broadhurst LM, Young AG, Murray BG. 2008a. AFLPs reveal an absence of geographical genetic structure among remnant populations of pohutukawa (Metrosideros excelsa, Myrtaceae). New Zealand Journal of Botany. 46:13–21.
- Burrows CJ. 1965. Some discontinuous distributions of plants within New Zealand and their ecological significance. Part II. Disjunctions between Otago-Southland and Nelson-Marlborough and related distribution patterns. Tuatara. 13:9–29.
- Campbell JD, Rudge MR. 1976. A case for controlling the distribution of Olearia lyallii hook.f. in its type locality, Auckland islands. Proceedings of the New Zealand Ecological Society. 23:491–522.
- Capblancq T, Forester BR. 2021. Redundancy analysis: A Swiss army knife for landscape genomics. Methods in Ecology and Evolution. 12:2298–2309.
- Carswell FE, Richardson SJ, Doherty JE, Allen RB, Wiser SK. 2007. Where do conifers regenerate after selective harvest? A case study from a New Zealand conifer-angiosperm forest. Forest Ecology and Management. 253:138–147. DOI:10.1016/j.foreco.2007.07.011.
- Carvalho CS, Forester BR, Mitre SK, Alves R, Imperatriz-Fonseca VL, Ramos SJ, Resende-Moreira LC, Siqueira JO, Trevelin LC, Caldeira CF, Gastauer M. 2020. Combining genotype, phenotype, and environmental data to delineate site-adjusted provenance strategies for ecological restoration. Molecular Ecology Resources. 21:44–58.
- Case B. 2020. One billion trees: an opportunity for (re)building resilient and multi-functional agricultural landscapes. https://newzealandecology.org/one-billion-trees-opportunity-rebuilding-resilient-and-multi-functional-agricultural-landscapes.
- Chavent M, Kuentz V, Labenne A, Saracco J. 2021. Clustgeo: hierarchical clustering with spatial constraints: R package version 2.1.
- Chavent M, Kuentz-Simonet V, Labenne A, Saracco J. 2018. Clustgeo: an R package for hierarchical clustering with spatial constraints. Computational Statistics. 33(4):1799–1822. DOI:10.1007/s00180-018-0791-1.
- Cieraad E, Walker S, Price R, Barringer J. 2015. An updated assessment of indigenous cover remaining and legal protection in New Zealand’s land environments. New Zealand Journal of Ecology. 39:309–315.
- Clarkson B, Bylsma R. 2016. Restoration planting in urban environments. Indigena (May). 7–10.
- Cockayne L. 1917. A consideration of the terms “species” and “variety” as used in botany, with special reference to the flora of New Zealand. Transactions and Proceedings of the New Zealand Institute. 49:66–79.
- Cook JM, Mark AF, Shore BF. 1980. Responses of Leptospermum scoparium and L. ericoides (Myrtaceae) to waterlogging. New Zealand Journal of Botany. 18:233–246. DOI:10.1080/0028825X.1980.10426922.
- Davis MA, Chew MK, Hobbs RJ, Lugo AE, Ewel JJ, Vermeij GJ, Brown JH, Rosenzweig ML, Gardener MR, Carroll SP, et al. 2011. Don't judge species on their origins. Nature. 474:153–154.
- De Kort H, Vandepitte K, Bruun HH, Closset-Kopp D, Honnay O, Mergeay J. 2014. Landscape genomics and a common garden trial reveal adaptive differentiation to temperature across Europe in the tree species Alnus glutinosa. Molecular Ecology. 23:4709–4721.
- De Lange PJ. 2014. A revision of the New Zealand Kunzea ericoides (Myrtaceae) complex. PhytoKeys. 40:1.
- Dewes D, Burke J, Douglas B, Kincheff S. 2022. Retiring farmland into ngahere. Our Land and Water Science Challenge. 42.
- Duncan RP, Clements SE, Corlett RT, Hahs AK, McCarthy MA, McDonnell MJ, Schwartz MW, Thompson K, Vesk PA, Williams NSG. 2011. Plant traits and extinction in urban areas: a meta-analysis of 11 cities. Global Ecology and Biogeography. 20:509–519.
- ECan. 2019. Guidelines for native plant procurement and ecosourcing. https://www.ecan.govt.nz/your-region/your-environment/our-natural-environment/.
- Erickson VJ, Halford A. 2020. Seed planning, sourcing, and procurement. Restoration Ecology. 28(S3):S216–S224.
- Ferkins C. 2002. Ecosourcing code of practice and ethics. New Zealand: Waitakere City Council.
- Forbes AS, Wallace KJ, Buckley HL, Case BS, Clarkson BD, Norton DA. 2020. Restoring mature-phase forest tree species through enrichment planting in New Zealand’s lowland landscapes. New Zealand Journal of Ecology. 44:1–9.
- Frankham R. 2015. Genetic rescue of small inbred populations: meta-analysis reveals large and consistent benefits of gene flow. Molecular Ecology. 24:2610–2618.
- Fremout T, Thomas E, Bocanegra-Gonzalez KT, Aguirre-Morales CA, Morillo-Paz AT, Atkinson R, Kettle C, González-M R, Alcazar-Caicedo C, Gonzalez MA, Gil-Tobon C, et al. 2021. Dynamic seed zones to guide climate-smart seed sourcing for tropical dry forest restoration in Colombia. Forest Ecology and Management. 490:119–127.
- Gann GD, McDonald T, Walder B, Aronson J, Nelson CR, Jonson J, Hallett JG, Eisenberg C, Guariguata MR, Liu J, et al. 2019. International principles and standards for the practice of ecological restoration. Restoration Ecology. 27(S1):S1–S46.
- Garnock-Jones PJ, Molloy BPJ. 1982. Protandry and inbreeding depression in Hebe amplexicaulis (Scrophulariaceae). New Zealand Journal of Botany. 20:401–403. DOI: 10.1080/0028825X.1982.10428510.
- Gemmell MR, Shepherd LD, Zuccarello GC, Perrie LR. 2022. Phylogeography of the widespread New Zealand tree lancewood/horoeka (Pseudopanax crassifolius; Araliaceae). New Zealand Journal of Botany. DOI:10.1080/0028825X.2022.2037670.
- Godley EJ. 1965. Notes on the vegetation of the Auckland Islands. Proceedings of the New Zealand Ecological Society. 12:57–63.
- Godley EJ. 1972. “ … In their natural state”. Does planting achieve its purpose? Forest and Bird. 185:25–26.
- Hancock N, Encinas-Viso F. 2021. Native seed transfer zones in Australia – How far can seed go? Melbourne: Project Phoenix, Greening Australia.
- Hancock N, Hughes L. 2014. Turning up the heat on the provenance debate: testing the ‘local is best’ paradigm under heatwave conditions. Austral Ecology. 39:600–611.
- Harris W. 1996. Genecological aspects of flowering patterns of populations of Kunzea ericoides and K. sinclairii (Myrtaceae). New Zealand Journal of Botany. 34(3):333–354.
- Harris W, Beever RE, Heenan PB. 1998. Phenotypic variation of leaves and stems of wild stands of Cordyline australis (lomandraceae). New Zealand Journal of Botany. 36(4):593–604.
- Harris W, Beever RE, Parkes S, Smallfield B, Anderson RA, Scheele S. 2006. Genotypic variation of the flowering phenology of Cordyline australis (laxmanniaceae) grown at three locations in New Zealand. New Zealand Journal of Botany. 44(1):23–39.
- Harris W, Beever RE, Smallfield B. 2001. Variation in response to cold damage by populations of Cordyline australis and of some other species of Cordyline (lomandraceae). New Zealand Journal of Botany. 39(1):147–159.
- Harrison PA. 2021. Climate change and the suitability of local and non-local species for ecosystem restoration. Ecological Management & Restoration. 22:S2. DOI:10.1111/emr.12520.
- Harrison PA, Vaillancourt RE, Harris RMB, Potts BM. 2017. Integrating climate change and habitat fragmentation to identify candidate seed sources for ecological restoration. Restoration Ecology. 25:524–531.
- Heenan P, Mitchell C, Houliston G. 2018. Genetic variation and hybridisation among eight species of kōwhai (Sophora: Fabaceae) from New Zealand revealed by microsatellite markers. Genes. 9:11. DOI:10.3390/genes9020111.
- Heenan PB, McGlone MS. 2019. Cenozoic formation and colonisation history of the New Zealand vascular flora based on molecular clock dating of the plastid rbcL gene. New Zealand Journal of Botany. 57:204–226.
- Heenan PB, McGlone MS, Mitchell CM, Houliston GJ. 2021. Genetic variation reveals broad-scale biogeographic patterns and challenges species’ concepts in the Kunzea ericoides (Myrtaceae) complex from New Zealand. New Zealand Journal of Botany. 60. DOI: 10.1080/0028825X.2021.1903946.
- Heenan PB, McGlone MS, Mitchell CM, McCarthy JK, Houliston GJ. 2022. Genotypic variation, phylogeography, unified species concept, and the ‘grey zone’ of taxonomic uncertainty in kānuka: recognition of Kunzea ericoides (A.rich.) Joy thomps. sens. lat. (Myrtaceae). New Zealand Journal of Botany. DOI:10.1080/0028825X.2022.2162427.
- Heenan PB, Millar TR, Smissen RD, McGlone MS, Wilton AD. 2017. Phylogenetic measures of neo- and palaeo-endemism in the indigenous vascular flora of the New Zealand archipelago. Australian Systematic Botany. 30:124–133.
- Heenan PB, Smissen RD, Dawson MI. 2005. Self-incompatibility in the threatened shrub Olearia adenocarpa (Asteraceae). New Zealand Journal of Botany. 43:831–841.
- Hendy J, Kerr S, Halliday A, Owen S, Ausseil AGE, Burton R, Bell K, Deans N, Dickie B, Hale J, et al. 2018. Drought and climate change adaptation: impacts and projections. Deep South National Science Challenge. Motu Note. 31:1–16.
- Hobbs RJ, Higgs E, Harris JA. 2009. Novel ecosystems: implications for conservation and restoration. Trends in Ecology & Evolution. 24:599–605.
- Höfner J, Klein-Raufhake T, Lampei C, Mudrak O, Bucharova A, Durka W. 2022. Populations restored using regional seed are genetically diverse and similar to natural populations in the region. Journal of Applied Ecology. 59:2234–2244. DOI:10.1111/1365-2664.14067.
- Holdaway RJ, Wiser SK, Williams PA. 2012. Status assessment of New Zealand's naturally uncommon ecosystems. Conservation Biology. 26:619–629.
- Jordan R, Breed MF, Prober SM, Miller AD, Hoffmann AA. 2019. How well do revegetation plantings capture genetic diversity? Biology Letters. 5:20190460. DOI:10.1098/rsbl.2019.0460.
- Keegan LJ, White RSA, Macinnis-Ng C. 2022. Current knowledge and potential impacts of climate change on New Zealand’s biological heritage. New Zealand Journal of Ecology. 46:3467.
- Laikre L, Schwartz MK, Waples RS, Ryman N. 2010. Compromising genetic diversity in the wild: unmonitored large-scale release of plants and animals. Trends in Ecology & Evolution. 25:520–529.
- Larcombe MJ, Potts BM, Jones RC, Steane DA, Silva JCE, Vaillancourt RE. 2016. Managing Australia’s eucalypt gene pools: assessing the risk of exotic gene flow. Proceedings of the Royal Society of Victoria. 128:25–39.
- Leathwick JR, Overton JM, McLeod M. 2003. An environmental domain classification of New Zealand and its use as a tool for biodiversity management. Conservation Biology. 17:1612–1623.
- Lee W, McGlone M, Wright E. 2005. Biodiversity inventory and monitoring: a review of national and international systems and a proposed framework for future biodiversity monitoring by the department of conservation. Wellington: Landcare Research; 213 p.
- Lee WG, Wilson JB, Meurk CD, Kennedy PC. 1991. Invasion of the subantarctic Auckland islands, New Zealand, by the asterad tree Olearia lyallii and its interaction with a resident myrtaceous tree Metrosideros umbellata. Journal of Biogeography. 18:493–508.
- Li Y-C, Korol AB, Fahima T, Beiles A, Nevo E. 2002. Microsatellites: genomic distribution, putative functions, and mutational mechanism: a review. Molecular Ecology. 11:2453–2456.
- Macinnis-Ng C, Mcintosh AR, Monks JM, Waipara N, White RSA, Boudjelas S, Clark CD, Clearwater MJ, Curran TJ, Dickinson CJM, et al. 2021. Climate-change impacts exacerbate conservation threats in island systems: New Zealand as a case study. Frontiers in Ecology and the Environment. 19:216–224. DOI:10.1002/fee.2285.
- Malavasi MD, Davis AS, Malavasi UC. 2018. Tree seed sourcing for landscape restoration under climate changes. Ciência Florestal. 28:446–455.
- McCarthy JK, Leathwick JR, Roudier P, Barringer JRF, Etherington TR, Morgan FJ, Odgers NP, Price RH, Wiser SK, Richardson SJ. 2021. New Zealand environmental data stack (NZEnvDS): a standardised collection of spatial layers for environmental modelling and site characterisation. New Zealand Journal of Ecology. 45(2):3440. DOI:10.20417/nzjecol.45.31.
- McEwen WM. ed. 1987. Ecological regions and districts of New Zealand. Wellington: Department of Conservation.
- McGlone M. 2000. The role of history in the creation of a vision of future landscapes. In: Hamblin A, editor. Visions of future landscapes. proceedings of 1999 Australian academy of science Fenner conference on the environment. Canberra: Bureau of Rural Sciences; p. 30–37.
- McGlone M, Walker S. 2011. Potential effects of climate change on New Zealand’s terrestrial biodiversity and policy recommendations for mitigation, adaptation and research. Science for conservation 312. Wellington: Department of Conservation. 77 p.
- McGlone MS. 1983. Polynesian deforestation of New Zealand: a preliminary synthesis. Archaeology in Oceania. 18:11–25.
- McGlone MS. 1985. Plant biogeography and the late cenozoic history of New Zealand. New Zealand Journal of Botany. 23:723–749. DOI:10.1080/0028825X.1985.10434240.
- McGlone MS. 2001. The origin of the indigenous grasslands of southeastern south island in relation to pre-human woody ecosystems. New Zealand Journal of Ecology. 25:1–15.
- McGlone MS, McNutt K, Richardson SJ, Bellingham PJ, Wright EF. 2020. Biodiversity monitoring, ecological integrity, and the design of the New Zealand biodiversity assessment framework. New Zealand Journal of Ecology. 44(2):1–12.
- McGlone MS, Richardson SJ, Burge OR, Perry GLW, Wilmshurst JM. 2017. Palynology and the ecology of the New Zealand conifers. Frontiers in Earth Science. 5:94. DOI:10.3389/feart.2017.00094.
- McWethy DB, Whitlock C, Wilmshurst JM, McGlone MS, Fromont M, Li X, et al. 2010. Rapid landscape transformation in south island, New Zealand, following initial Polynesian settlement. Proceedings of the National Academy of Sciences U.S.A. 107:21343–21348. DOI:10.1073/pnas.1011801107.
- Ministry for Primary Industries. 2015. Sustainable management of New Zealand’s forests: New Zealand’s third country report on the Montreal process criteria and indicators. Wellington: Ministry for Primary Industries.
- Montalvo AM, Ellstrand NC. 2001. Nonlocal transplantation and outbreeding depression in the subshrub Lotus scoparius (Fabaceae). American Journal of Botany. 88(2):258–269.
- Morse NB, Pellissier PA, Cianciola EN, Brereton RL, Sullivan MM, Shonka NK, Wheeler TB, McDowell WH. 2014. Novel ecosystems in the anthropocene: a revision of the novel ecosystem concept for pragmatic applications. Ecology and Society. 19:12.
- Newnham R, McGlone M, Moar N, Wilmshurst J, Vandergoes M. 2013. The vegetation cover of New Zealand at the last glacial maximum. Quaternary Science Reviews. 74:202–214. DOI:10.1016/j.quascirev.2012.08.022.
- Norton DA, Butt J, Bergin DO. 2018. Upscaling restoration of native biodiversity: a New Zealand perspective. Ecological Management & Restoration. 19:26–35.
- Overton JM, Leathwick JR. 2001. Measuring environmental distinctiveness. Science for conservation 174. Wellington: Department of Conservation. 20 p.
- Overton JM, Stephens RTT, Leathwick JR, Lehmann A. 2002. Information pyramids for informed biodiversity conservation. Biodiversity and Conservation. 11:2093–2116.
- Payseur BA, Cutter AD. 2006. Integrating patterns of polymorphism at SNPs and STRs. Trends in Genetics. 22:424–429.
- Perrie L, Russell P, Ogle C, Mitcalfe B, Horne C, Burton E, Rolfe J, Elliot R. 2013. Non-local indigenous New Zealand vascular plants self-propagating in Wellington. Wellington Botanical Society Bulletin. 53:23–47.
- Perring MP, Standish RJ, Price JN, Craig MD, Erickson TE, Ruthrof KX, Whiteley AS, Valentine LE, Hobbs RJ. 2015. Advances in restoration ecology: rising to the challenges of the coming decades. Ecosphere. 6:1–25. DOI:10.1890/ES15-00121.1.
- Perry GLW, Wilmshurst JM, McGlone MS. 2014. Ecology and long-term history of fire in New Zealand. New Zealand Journal of Ecology. 38:157–176.
- Perry GLW, Wilmshurst JM, McGlone MS, Napier A. 2012. Reconstructing spatial vulnerability to forest loss by fire in pre-historic New Zealand. Global Ecology and Biogeography. 21:1029–1041. DOI:10.1111/j.1466-8238.2011.00745.x.
- Prober SM, Byrne M, McLean EH, Steane DA, Potts BM, Vaillancourt RE, Stock WD. 2015. Climate-adjusted provenancing: a strategy for climate-resilient ecological restoration. Frontiers in Ecology and Evolution. 23:65.
- Prober SM, Doerr VAJ, Broadhurst LM, Williams KJ, Dickson F. 2019. Shifting the conservation paradigm: a synthesis of options for renovating nature under climate change. Ecological Monographs. 89:e01333.
- Ratana K, Herangi N, Murray T. 2019. Me pēhea te whakarauora i ngā repo o ngāti maniapoto? How do we go about restoring the wetlands of ngāti maniapoto? New Zealand Journal of Ecology. 43(3):3391.
- Rawlence NJ, Potter BCM, Dussex N, Scarsbrook L, Orlovich DA, Waters JM, McGlone M, Knapp M. 2021. Plio-Pleistocene environmental changes shape present day phylogeography of New Zealand’s southern beeches (Nothofagaceae). New Zealand Journal of Botany. 59:55–71. DOI: 10.1080/0028825X.2020.1791915.
- R Core Team. 2021. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- RDC Group. 2021. Jobs for NATURE PROGRAMME REVIEW. Pp. 1–32.
- Robertson AW, Kelly D, Ladley JJ. 2011. Futile selfing in the tree Fuchsia excorticata (Onagraceae) and Sophora microphylla (Fabaceae): inbreeding depression over 11 years. International Journal of Plant Sciences. 172:191–198.
- Rodger JG, Bennett JM, Razanajatovo M, Knight TM, van Kleunen M, Ashman T-L, Steets JA, Hui C, Arceo-Gómez G, Burd M, et al. 2021. Widespread vulnerability of flowering plant seed production to pollinator declines. Science Advances. 7:eabd3524.
- Rogers GM. 1989. The nature of the lower north island floristic gap. New Zealand Journal of Botany. 27:221–241.
- Saint Clair BS. 2014. The development of forest tree seed zones in the pacific northwest of the United States. In: Bozzano et al. editors. Genetic considerations in ecosystem restoration using native tree species. State of the world’s forest genetic resources – thematic study. Rome: FAO; p. 49–51.
- Santana CG. 2022. The value of and in novel ecosystem(s). Biology & Philosophy. 37(2):1–8.
- Schmidt-Adam G, Young AG, Murray BG. 2000. Low outcrossing rates and shift in pollinators in New Zealand pohutukawa (Metrosideros excelsa: Myrtaceae). American Journal of Botany. 87:1265–1271.
- Searle SY, Turnbull MH, Boelman NT, Schuster WSF, Yakir D, Griffin KL. 2012. Urban environment of New York city promotes growth in northern red oak seedlings. Tree Physiology. 32:389–400.
- Shepherd LD, Heenan PB. 2021. Phylogenomic analyses provide further confirmation of a history of hybridization and introgression between Sophora sect edwardsia (Fabaceae) trees in New Zealand. New Zealand Journal of Botany. 60. DOI: 10.1080/0028825X.2021.1960567.
- Shepherd LD, Perrie LR. 2011. Microsatellite DNA analyses of a highly disjunct New Zealand tree reveal strong differentiation and imply a formerly continuous distribution. Molecular Ecology. 20:1389–1400.
- Simpson P. 1997. Ecological restoration in the Wellington conservancy. Wellington: Department of Conservation.
- Simpson P. 2002. Sustaining biodiversity through the use of local plant provenances. In: Ferkins C, editor. Ecosourcing code of practice and ethics. New Zealand: Waitakere City Council; p. 51–65.
- Smissen RD, Richardson SJ, Morse CW, Heenan PB. 2014. Relationships, gene flow and species boundaries among New Zealand Fuscospora (Nothofagaceae: southern beech). New Zealand Journal of Botany. 52:389–406.
- Sork VL, Aitken SN, Dyer RJ, Eckert AJ, Legendre P, Neale DB. 2013. Putting the landscape into the genomics of trees: approaches for understanding local adaptation and population responses to changing climate. Tree Genetics & Genomes. 9:901–911.
- Stevens MI, Clarke AC, Clarkson FM, Goshorn M, Gemmill CEC. 2015. Are current ecological restoration practices capturing natural levels of genetic diversity? A New Zealand case study using AFLP and ISSR data from mahoe (Melicytus ramiflorus). New Zealand Journal of Ecology. 39:190–197.
- Supple MA, Bragg JG, Broadhurst LM, Nicotra AB, Byrne M, Andrew RL, Widdup A, Aitken NC, Borevitz JO. 2018. Landscape genomic prediction for restoration of a Eucalyptus foundation species under climate change. Elife. 7:e31835.
- Te Uru Rakau Forestry New Zealand. 2018. The One Billion Trees Programme: Our future, our billion trees. Available from www.mpi.govt.nz.
- Thomas E, Jalonen R, Loo J, Boshier D, Gallo L, Cavers S, Bordács S, Smith P, Bozzano M. 2014. Genetic considerations in ecosystem restoration using native tree species. Forest Ecology and Management. 333:66–75. DOI:10.1016/j.foreco.2014.07.015.
- Tsitrone A, Kirkpatrick M, Levin DA. 2003. A model for chloroplast capture. Evolution. 57:1776–1782.
- Tulod AM, Norton DA, Sealey C. 2019. Canopy manipulation as a tool for restoring mature forest conifers under an early-successional angiosperm canopy. Restoration Ecology. 27:31–37.
- Van Etten ML, Tate JA, Anderson SH, Kelly D, Ladley JJ, Merrett MF, Peterson PG, Robertson AW. 2015. The compounding effects of high pollen limitation, selfing rates and inbreeding depression leave a New Zealand tree with few viable offspring. Annals of Botany. 116:833–843. DOI:10.1093/aob/mcv118.
- Walker S, King N, Monks A, Williams S, Burrows L, Cieraad E, Meurk C, Overton JM, Price R, Smale M. 2009. Secondary woody vegetation patterns in New Zealand’s south island dryland zone. New Zealand Journal of Botany. 47:367–393. DOI:10.1080/0028825x.2009.9672713.
- Walker S, Price R, Rutledge D, Stephens RTT, Lee WG. 2006. Recent loss of indigenous vegetation. New Zealand Journal of Ecology. 30:169–177.
- Wardle P. 1963. Evolution and distribution of the New Zealand flora as affected by quaternary climates. New Zealand Journal of Botany. 1:3–17.
- Wardle P. 1988. Effects of glacial climates on floristic distribution in New Zealand 1. A review of the evidence. New Zealand Journal of Botany. 26:541–555.
- Wardle P. 1991. Vegetation of New Zealand. Cambridge: Cambridge University Press.
- Weeks ES, Overton JM, Walker S. 2012. Estimating patterns of vulnerability in a changing landscape: a case study of New Zealand's indigenous grasslands. Environmental Conservation. 40:84–95. DOI:10.1017/S0376892912000343.
- Wilcox MD, Ledgard NJ. 1983. Provenance variation in the New Zealand species of Nothofagus. New Zealand Journal of Ecology. 6:19–21.
- Wilmshurst JM, McGlone MS, Turney CSM. 2015. Long-term ecology resolves the timing, region of origin and process of establishment for a disputed alien tree. AoB PLANTS. 7:plv104. DOI:10.1093/aobpla/plv10.
- Wood J, Wilmshurst J, Newnham R, McGlone M. 2017. Evolution and ecological change during the New Zealand quaternary. In: Shulmeister J, editor. Landscape and quaternary environmental change in New Zealand, Atlantis advances in quaternary science. Vol. 3. p. 235–291.
- Wright EF, Bellingham PJ, Richardson SJ, McKay M, MacLeod CJ, McGlone MS. 2020. How to get a national biodiversity monitoring programme off the ground: lessons from New Zealand. Parks. 26:67–78.
- Wright SA, Hutchison M, Hale ML, Gemmill CEC, De Lange PJ, Pelser PB. 2017. A preliminary conservation genetic study of Pittosporum obcordatum (Pittosporaceae), an endemic New Zealand species with a disjunct distribution. New Zealand Journal of Botany. 55:424–438. DOI: 10.1080/0028825X.2017.1363789.
