ABSTRACT
Rust fungi are obligate plant pathogens, usually restricted to a single host species or genus, although some species such as myrtle rust (Austropuccinia psidii) and some cereal and grass rusts (Puccinia spp.) can infect many members of the families Myrtaceae and Poaceae, respectively. The rust life cycle can involve up to five spore stages, both sexual and asexual. The cycle may be completed on a single host (autoecious rust) or require two, unrelated host plants (heteroecious rust) for completion. About 300 rust species are known in New Zealand, with approximately half comprising native rusts (usually pathogenic on native plants) and half being introduced rusts (usually pathogenic on exotic plants). In this paper, 26 new species of rust fungi discovered in New Zealand are described, comprising one species each of Aecidium and Milesina, two of Pucciniastrum, four of Uromyces and 18 of Puccinia. Puccinia ferocis-forsteri is a new heteroecious species producing uredinia and telia on the endemic Carex forsteri and aecia on endemic Urtica ferox. This is only the third native heteroecious rust fungus reported in New Zealand. In addition, two species are transferred to the genus Milesina, three to Uromyces and seven to Puccinia (including a nom. nov.), either following discovery of the sexual (telial) stage of these rusts or considering the most appropriate sexual genus. A new combination is proposed for Coleosporium based on morphology of its urediniospores and molecular data. Epitypes are designated for eight species, lectotypes are selected for five species and a neotype is designated. Apart from one new species on the introduced Fuchsia magellanica all the rusts are considered native to New Zealand. Where appropriate, DNA was extracted and the 28S region (nuclear large subunit ribosomal DNA [nuLSU]) was amplified with the resulting LSU sequences aligned with sequences from GenBank representing most of the major clades.
Introduction
In the 93 years since Cunningham (Citation1931) published his account of the rust fungi of New Zealand, only 24 new taxa of rust have been described from New Zealand (e.g. Cunningham Citation1945; McNabb Citation1962a, Citation1962b, Citation1966; McKenzie Citation1980, Citation1998, Citation2008; McKenzie and Johnston Citation2004; Padamsee and McKenzie Citation2014). These papers have mostly described species that are considered native to New Zealand, having been discovered on native host plants, and included only a few new species that were obviously introduced. However, many new host/rust pathogen combinations have been published for New Zealand involving both native and exotic host plants, and several introduced rust fungi have been recorded in New Zealand for the first time (e.g. McNabb Citation1966; Laundon Citation1970; Dingley Citation1977; Versluys Citation1977; Latch Citation1980; McKenzie and Latch Citation1980; McKenzie Citation1981; McKenzie et al. Citation2013; Padamsee and McKenzie Citation2019). The sexual (telial) stage has been also found for a few species, which has resulted in the rust being transferred to Puccinia or Uromyces (e.g. McNabb Citation1962a, Citation1966; Cummins Citation1971; McKenzie Citation1991b, Citation2008). The complete life cycle of two heteroecious rusts, Mikronegeria fuchsiae P.E. Crane and R.S. Petersen and Puccinia otagensis (Linds.) McKenzie and Padamsee, has been also elucidated (Crane and Peterson Citation2007; Padamsee and McKenzie Citation2017).
The present paper describes 26 new species of rust fungi from New Zealand, comprising one species each of Milesina and Aecidium, two of Pucciniastrum, four of Uromyces and 18 of Puccinia. In addition, taxonomic transfers are made into Puccinia and Uromyces either following discovery of the sexual stage or through molecular analyses. Uredo puawhananga is transferred to Coleosporium, following confirmation of its taxonomic placement by molecular analysis. Two species of Milesia are recombined in the sexual genus Milesina. Epitypes are designated for eight species and lectotypes are chosen for five species. Apart from one new species on the introduced Fuchsia magellanica it is probable that all the new species are native to New Zealand, with most being endemic to the country.
Materials and methods
The rust specimens were collected throughout New Zealand. All specimens were dried and are preserved as dried vouchers stored in the New Zealand Fungarium (PDD; Thiers Citation2024). Some specimens held in PDD for many years have also been recognised as new species. Microscope slides were prepared with the material mounted in lactophenol for light microscopic examination and for measuring of morphological features. Occasionally hyaline spores were mounted in aniline blue. When available, at least 20 spores were measured for each specimen examined, and the mean size of all spores is given. Spore dimensions are given in the format (a–)b–c(–d), with the smallest approximately 10% of spores (a) and largest 10% of spores (d) given within parentheses; the size of approximately 80% of spores (b–c) are given as a range and lie outside the parentheses. For scanning electron microscopy (SEM), a small amount of dried infected plant material was placed on a sticky carbon tab adhering to a specimen stub. This was teased apart to release the spores and distribute them over the surface of the tab. The sample was then sputter coated with gold for 60 s and viewed by SEM using an accelerating voltage of 10 kV. For the rust fungi examined, the presence of spermatia is indicated by 0, the presence of aeciospores by I, the presence of urediniospores by II, and the presence of teliospores by III. Specimen localities are assigned to New Zealand geographical areas as defined by Crosby et al. (Citation1998) and are based on collections held in PDD. Index Fungorum numbers are provided for new names and when epitypes or lectotypes are designated. When recent collections were available DNA were extracted and the 28S region (nuclear large subunit ribosomal DNA [nuLSU]) was amplified according to the protocol outlined by Padamsee and McKenzie (Citation2014); GenBank numbers are given for LSU sequences (). The resulting LSU sequences were aligned with sequences from GenBank () representing most of the major clades identified by Aime (Citation2006) using MAFFT v. 7 (Katoh and Standley Citation2013). The alignment was analysed using PhyML v. 3.3 (Guindon and Gascuel Citation2003; Guindon et al. Citation2010) as implemented in Geneious Pro 10.2.6 Biomatters (http://www.geneious.com/) to find the best-scoring likelihood tree. The model of evolution specified was GTR+Γ+I. The bootstrap analyses were run with 1000 maximum likelihood bootstrap replicates (MLBS). Mikronegeria fagi and M. fuchsiae were used as outgroups.
Table 1. Species of rust, accession number, hosts, and GenBank numbers of taxa included in the LSU molecular analyses. Holotypes and epitypes in bold. ITS sequences not included in analyses are denoted by +. GenBank records from which information such as the host and/or collection accession number is missing are denoted by *.
Taxonomy
Since January 2013, a fungus can have only one correct name, irrespective of the number of morphs and whether the name is based on sexual or asexual spores [International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) (ICNafp; Turland et al. Citation2018, Article 59)]. Prior to the adoption of ‘one fungus, one name’, five of the species treated in this paper that are known to possess only aecia would have been described in the genus Aecidium, and eight species known to produce only uredinia would have been placed in Uredo. These 13 species are herein described in the genera Puccinia or Uromyces as it is most probable that the New Zealand aecial and uredinial specimens belong within either of these two genera. However, a new aecial species on Ranunculus maculatus is described as an Aecidium. In the molecular analysis this species lay outside of the Puccinia/Uromyces clade. Aime et al. (Citation2018) suggested abandoning the use of the many asexually typified generic names in Pucciniales such as Aecidium, Caeoma, Milesia, Peridermium, Roestelia and Uredo and to use their corresponding sexually typified generic names, and that species known only from an asexual state be placed in the genus most likely to be considered correct. These formal asexually typified generic names are based on type species that are synonyms of species in other genera. Both Aecidium and Uredo are synonyms of sexual genera and new species should not be described in either of these genera. Aecidium is a synonym of Puccinia, but if there is a lack of molecular data or a link between an aecial and telial stage of a rust has not been experimentally shown, then it is impossible to be sure that an aecial specimen belongs in Puccinia (or Tranzschelia or Uromyces, etc.). Uredo is a synonym of Uromyces, although it is also treated as an asexual morph of species in Melampsora, Puccinia, Uromyces and other genera. When DNA can be extracted and sequenced then Uredo species can be often accurately placed into sexual genera. For example, despite telia having not been reported, two Australian rusts that also occur in New Zealand, Uredo dianellae Dietel and U. rhagodiae Cooke and Massee, were recovered in monophyletic clades along with species of Puccinia (van der Merwe et al. Citation2008; McTaggart et al. Citation2016). Hence, they were transferred to Puccinia and the new combinations P. dianellae (Dietel) McTaggart and R.G. Shivas and P. rhagodiae (Cooke and Massee) McTaggart and R.G. Shivas were made (Marin-Felix et al. Citation2017), although it was not type specimens that were sequenced. Although most asexual genera of rust fungi have been reduced to synonymy (Aime et al. Citation2018) some, such as Uredo and Aecidium, contain species that occur in over 50 sexual genera, and it will be a major task to assign these to natural genera (Aime and McTaggart Citation2021).
New species and new combinations
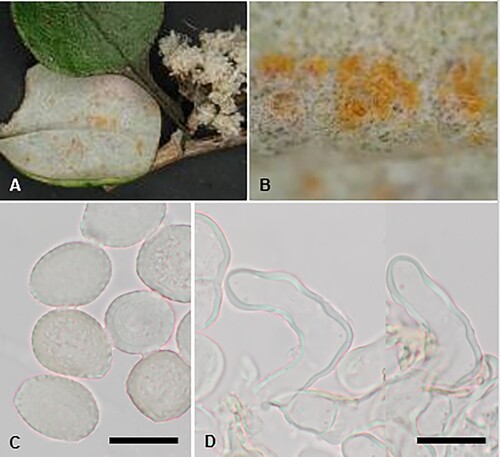
Aecidium ranunculi-maculati McKenzie and Padamsee, sp. nov.
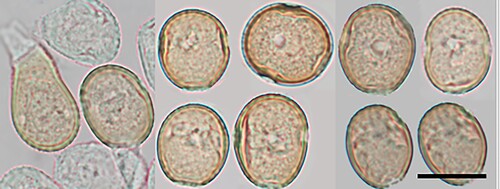
Figure 1. Aecidium ranunculi-maculati on Ranunculus maculatus: A, Aecia. B, Aeciospores (in aniline blue). Scale bar = 20 μm.
IF559594
Etymology. From the host plant Ranunculus maculatus.
Parasitic on Ranunculus maculatus Cockayne and Allan (Ranunculaceae). Spermogonia hypophyllous. Aecia amphigenous but mainly hypophyllous, also on petioles, crowded on leaf, cupulate, ca. 0.3–0.5 mm diameter; peridia tubiform, white or cream, lacerate and revolute at the top. Aeciospores (20.5–)21.5–23.5(–25) × (18–)19.5–21.5(–22.5) μm (mean 22.7 × 20.5 μm), subglobose or broadly ellipsoidal; wall unevenly thickened, 0.75–1(–1.5) μm thick, hyaline, verrucose. Uredinia and telia not known.
Holotype. New Zealand, Central Otago, Dunstan Mountains, Leaning Rock, on Ranunculus maculatus, s.d., P. Heenan (PDD 68337–I).
Distribution. Endemic to New Zealand (Central Otago).
Notes. The host plant is endemic with restricted distribution in the South Island, growing in montane to alpine bogs, and on lake and tarn margins. Another seven rust fungi have been recorded on Ranunculus species in New Zealand, and all produce aecia. Six of these species are considered endemic while Uromyces dactylidis G.H. Otth is especially widespread in the northern hemisphere on R. repens. Aecidium ranunculi-insignis G. Cunn. and A. ranunculi-lyallii G. Cunn. have larger aeciospores than the remaining species, all of which have similar sized aeciospores. In the past, the name A. ranunculacearum DC. was applied to various aecial rust species found on Ranunculus species. Cunningham (Citation1924a) recorded A. ranunculacearum on six endemic species of Ranunculus and on the introduced R. repens. Later, Cunningham (Citation1931) redetermined these rusts to be A. ranunculi-depressi G. Cunn. (on R. brevis), A. ranunculi-insignis (on R. insignis, R. nivicola, R. pachyrrhizus, R. verticillatus), A. ranunculi-lyallii (on R. lyallii) and Uromyces dactylidis (on R. repens). In the molecular analyses A. ranunculi-maculati was recovered in a well-supported clade (91% MLBS) with Pucciniastrum epilobii G.H. Otth that was on a long branch (). However, in a BLAST search of type sequences, the LSU sequence of A. ranunculi-maculati shared 100% sequence identity with 87% query coverage with Puccinia xinyuanensis J.G. Song, Aime and B. Xu, suggesting another gene may be needed to clarify the phylogenetic position of A. ranunculi-maculati.
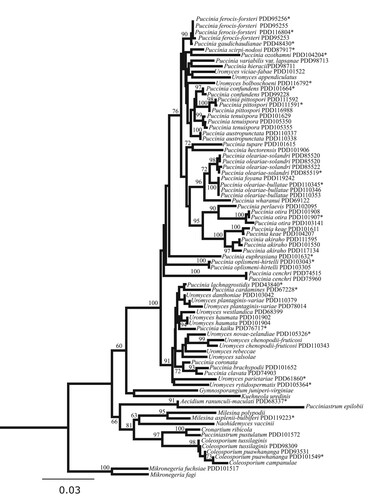
Figure 2. Phylogram obtained from maximum likelihood analysis of nuclear ribosomal DNA internal transcribed spacer region 2 - large subunit sequences (rDNA ITS2-LSU) sequences. The topology was rooted with Mikronegeria fuchsiae and M. fagi. Bootstrap support values (>60%) from a maximum likelihood search with 1000 replicates shown. Epitypes and holotypes are denoted with *.
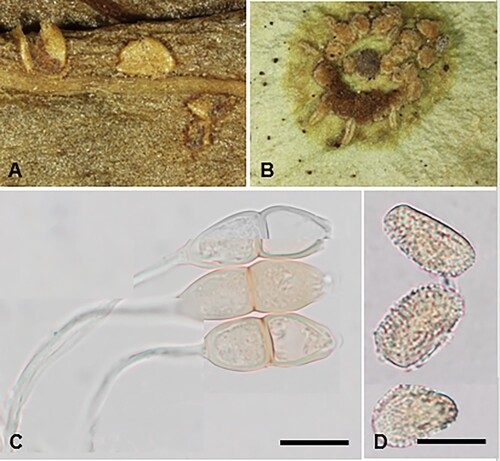
Coleosporium puawhananga (G.T.S. Baylis) McKenzie and Padamsee, comb. nov.
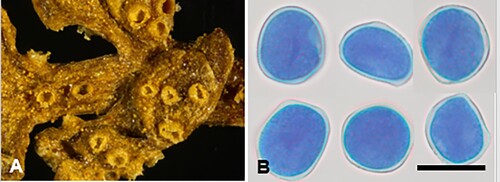
Figure 3. Coleosporium puawhananga on Clematis sp.: A, Uredinia. B, Close-up of uredinia. C, Urediniospores. D, SEM image of urediniospore. Scale bars = 20 μm.
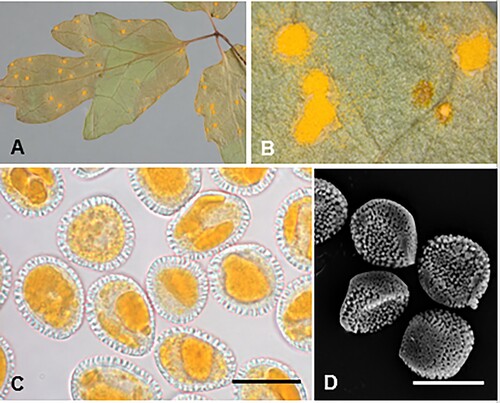
IF557258
≡ Uredo puawhananga G.T.S. Baylis, Transactions of the Royal Society of New Zealand 82: 635, 1954.
Parasitic on leaves of Clematis spp. (Ranunculaceae). Spermogonia not known. Aecia not known. Uredinia hypophyllous, scattered or often in circular groups up to 4 mm diameter, with a pale spot on corresponding upper surface of leaf, chrome orange when fresh, fading through pale yellow to white with age, circular, 0.5–1 mm diameter, pulvinate, becoming pulverulent, long covered by the epidermis. Urediniospores (19.5–)21–27.5(–32) × (14–)16–20(–23) μm (mean 24.5 × 18.1 μm), globose, broadly ellipsoidal or obovoid, sometimes somewhat angular, contents yellow; wall ca. 0.5 μm thick, hyaline, verrucose, germ pores obscure. Telia not known.
Distribution. Endemic to New Zealand (Three Kings Islands, Northland, Auckland, Coromandel, Waikato, Taupo, Hawkes Bay, Wanganui, Wellington, Nelson, Malborough Sounds, Westland, Mid Canterbury, Dunedin, Stewart Island).
Specimens examined. New Zealand, Three Kings Islands, Great Island, on Clematis paniculata J.F. Gmel., 28 November 1997, R.E. Beever (PDD 68371–II). Northland, Waiotemarama Bush Walk, on C. cunninghamii Turcz., 28 January 1988, P.R. Johnston (PDD 48394–II). Auckland, Rangitoto Island, Summit track, on C. paniculata, 14 December 1997, E.H.C. McKenzie, A. McKenzie and J.M. McKenzie (PDD 68342–II); Waitakere Ranges, Matuku, on C. paniculata, 6 December 1997, N.A. Martin (PDD 68343–II). Marlborough Sounds, D'Urville Island, on C. vitalba, 17 November 2005, K. Beach (PDD 83932–II). Westland, Arthur’s Pass, Kelly’s Creek, carpark, on C. paniculata, 9 February 2011, E.H.C. McKenzie, M. Padamsee and M.A. Korver (PDD 101549–II, epitype, designated here, IF901200). Dunedin, Swampy Spur, 370 m, on C. paniculata, 1 March 1954, G.T.S. Baylis (PDD 12908–II, holotype).
Notes. The host plants are all lianes or climbing shrubs. Coleosporium puawhananga occurs throughout the country on several native and introduced Clematis species. Cunningham (Citation1923) first recorded this rust in New Zealand, but he thought the urediniospores were part of the life cycle of Puccinia clavata P. Syd. and Syd. However, Baylis (Citation1954) concluded from inoculation experiments, and from the independent occurrence of telia, that P. clavata is microcyclic (possessing only teliospores) and described Uredo puawhananga for the uredinial rust discovered by Cunningham. Morphologically the urediniospores match those of a Coleosporium and molecular data has confirmed it as a Coleosporium species (98% MLBS) widely separated from P. clavata, which was recovered in a clade (93% MLBS) with Puccinia brachypodii G.H. Otth and P. coronata Corda in the phylogenetic tree (). This result was first shown by Padamsee and McKenzie (Citation2017), but U. puawhananga is only now formally combined with Coleosporium.
The New Zealand rust may eventually be proved to be Co. clematidis Barclay, or to be a formae speciales of Co. tussilaginis (Pers.) Tul. Coleosporium clematidis has been widely recorded on many Clematis spp. in Asia (China, India, Japan, Korea, Nepal, Pakistan, Philippines, Taiwan), South Africa, Russia, East Africa (Kenya, Tanzania, Uganda) and Brazil. Its urediniospores were originally described as measuring 30 × 20 μm (Barclay Citation1890), while Hiratsuka et al. (Citation1992) described them as being 20–36(–44) × 12–22(–28) μm on Clematis spp. in Japan. In general, the New Zealand rust has smaller urediniospores. Hiratsuka et al. (Citation1992) also accepted Co. clematidis-apiifoliae Dietel on Clematis spp. in Japan with urediniospores measuring 16–32(–36) × 12–22 μm. In addition, Sydow and Sydow (Citation1914) described Co. elongatum Syd. and P. Syd. on Clematis in Japan with urediniospores measuring 28–42 × 12–18 μm. Recently, Zhao et al. (Citation2021) described Co. sichuanense on Clematis in China with urediniospores measuring 19–30 × 15–21 μm.
European taxa of Coleosporium are often treated as a single species (Co. tussilaginis) and formae speciales have been designated for the rust on different host genera. For example, Helfer (Citation2013) considered Coleosporium on Senecio spp. in Europe to be Co. tussilaginis f. sp. senecionis-silvatici Boerema and Verh. and noted the urediniospores as being 18.6–37.1 × 11.8–26.4 µm (mean 25.7 × 18.7 µm). Overall, he found that urediniospore size and spore surface ornamentation were inconclusive features for delimitation of taxa in the genus Coleosporium and varied widely within taxa and between different hosts. Coleosporium tussilaginis was discovered in New Zealand in January 1978 (Laundon Citation1978; Sheridan Citation1978) and subsequently it rapidly spread throughout the country, infecting numerous species of Senecio and some other Asteraceae. By contrast, Co. puawhananga was first recorded in New Zealand on Clematis by Cunningham (Citation1923, as Puccinia clavata). It is doubtful that the rust on Clematis was the source of subsequent infections on other host genera such as Senecio, suggesting either separate species or the introduction of a new race or formae speciales into New Zealand.
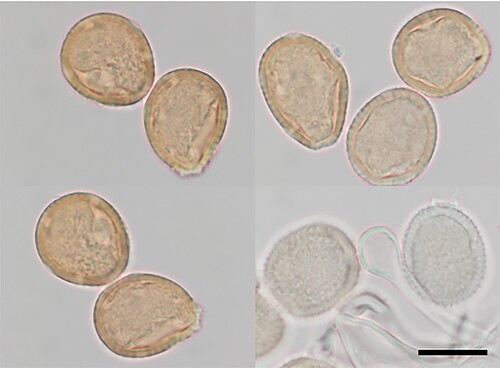
Milesina asplenii-bulbiferi McKenzie and Padamsee sp. nov.
Figure 4. Milesinia asplenii-bulbiferi on Asplenium bulbiferum: A, B, Uredinia (white, arrows; fern sori brown). C, Urediniospores (in aniline blue). Scale bars = 20 μm.
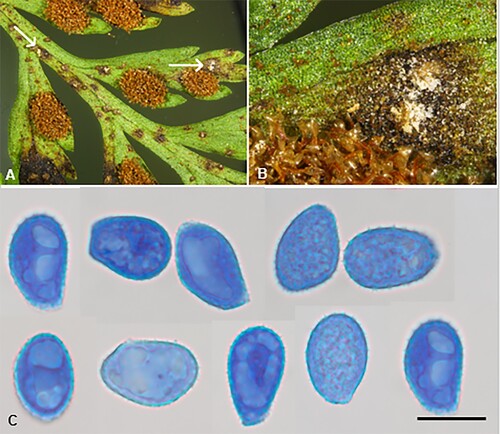
IF559585
Etymology. From Asplenium bulbiferum, the host binomial.
Parasitic on fronds of Asplenium bulbiferum G. Forst. (Aspleniaceae). Spermogonia and aecia not known. Uredinia hypophyllous, inconspicuous, white, elliptical, ca. 0.25 mm long, erumpent, long covered by the epidermis which eventually splits open, surrounded by a rudimentary peridium. Urediniospores (23.5–)25.5–31(–38.5) × (15.5–)17–20(–21.5) µm (mean 28.7 × 18.3 µm), ellipsoidal, oval, obovoid or subglobose, with a flattened base, contents hyaline; wall 0.5–1 µm thick, hyaline, echinulate, germ pores not seen. Telia not known.
Holotype. New Zealand, Stewart Island, Oban, Fuchsia Walk, on fronds of Asplenium bulbiferum, 15 April 2021, E.H.C. McKenzie (PDD 119223–II).
Distribution. Endemic to New Zealand (Stewart Island).
Notes. Asplenium bulbiferum (a reference to the bulbils or vegetative plantlets borne on the fronds, which drop off and reproduce the parent plant) is a widespread coastal to subalpine plant occurring throughout New Zealand. It is a common species in lowland forest, especially in high rainfall areas. A fern identified as ‘Asplenium bulbiferum’ is commonly cultivated in New Zealand and throughout much of the world (Perrie et al. Citation2005). However, this cultivated entity has been recognised as a hybrid between the New Zealand endemic A. bulbiferum and the Norfolk Island endemic A. dimorphum and has been described as Asplenium × lucrosum (Perrie et al. Citation2005).
Although lacking telia this rust is morphologically similar to three other Milesina species found on ferns in New Zealand and is in a highly supported clade (95% MLBS) with M. polypodii (F.B. White) Aime and Rossman (). However, the urediniospores of M. asplenii-bulbiferi are smaller than those of M. polystichi-vestiti (on Polystichum vestitum) but larger than those of M. lindsaeae (on Lindsaea trichomanoides) and M. histiopteridis G. Cunn. (on Histiopteris incisa). Milesina asplenii-incisi (Faull) Hirats. described on Asplenium incisum from Japan and Korea has slightly smaller urediniospores (20–32 × 12–20 µm) and a warted wall (Hiratsuka et al. Citation1992).
As already discussed, Aime et al. (Citation2018) recommended abandoning the use of many asexually typified rust fungus generic names and instead using their corresponding sexually typified generic names. However, Aime and McTaggart (Citation2021) pointed out that the types of Milesina are not congeneric with the type of Milesia. The New Zealand Milesia/Milesina fern rusts appear in a single clade and the two Milesia species previously reported from New Zealand are combined into Milesina.
Milesina lindsaeae (S.D. Baker) McKenzie and Padamsee, comb. nov.
IF901201
≡ Milesia lindsaeae (as lindsayae) S.D. Baker, Transactions of the Royal Society of New Zealand 83: 453, 1956.
Dingley (Citation1969) suggested that Milesia lindsaeae, described from New Zealand, was identical to Uredo lindsaeae Henn. described from Brazil (Hennings Citation1904). The host of the New Zealand rust is Lindsaea trichomanoides, an indigenous terrestrial fern described from New Zealand but also occurring in Australia. Urediniospores of the Brazilian type specimen (18–30 × 15–23 μm) are slightly broader than those of New Zealand specimens (16–31.5 × 12–19 μm, mean 22.4 × 15.6 μm, pers. obs.). Paraphyses were described from Brazil, but they were not seen in the New Zealand material. It is unlikely that this fern rust has a natural distribution that includes New Zealand and Brazil. Therefore, it is considered best to accept the New Zealand rust as a separate species. Uredo lindsaeae is now considered to be a synonym of Calidion lindsaeae (Henn.) Syd. and P. Syd. (Index Fungorum 2023).
Milesina polystichi-vestiti (McKenzie) McKenzie and Padamsee, comb. nov.
IF901202
≡ Milesia polystichi-vestiti McKenzie, Mycoscience 49: 2, 2008.
The host, Polystichum vestitum, is endemic and widespread throughout the New Zealand botanical region. The rust was described from Campbell Island.
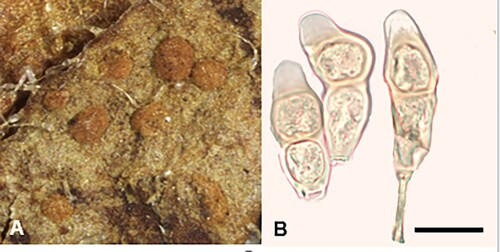
Puccinia abrotanellae McKenzie and Padamsee sp. nov.
Figure 5. Puccinia abrotanellae on Abrotanella pusilla: A, Aecia. B, Aeciospores (in aniline blue). Scale bar = 20 μm.
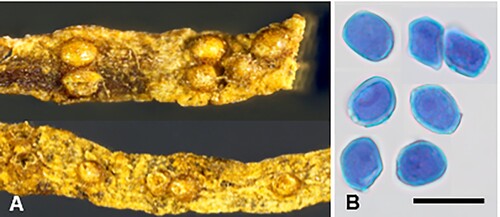
IF559586
Etymology. From the host genus Abrotanella.
Parasitic on Abrotanella pusilla (Hook. f.) Hook. f. (Asteraceae). Spermogonia not known. Aecia mainly hypophyllous, solitary or crowded, yellow, 0.2–0.3 mm diameter, bullate, opening by a small slit or pore. Aeciospores 13–16(–18) × (10–)10.5–13.5 μm (mean 15.0 × 11.8 μm), subglobose to ellipsoidal, often somewhat angular; wall (0.5–)0.75–1 μm thick, hyaline, minutely verrucose, with pore plugs. Uredinia and telia not known.
Holotype. New Zealand, Wellington, Tararua Range, near Oriwa Lake, on Abrotanella pusilla, 4 February 1932, V.D. Zotov (PDD 3264–I).
Distribution. Endemic to New Zealand (Wellington).
Notes. Abrotanella pusilla is a small, almost moss-like endemic herb. It is restricted to the Ruahine and Tararua Ranges in the North Island, growing in montane to subalpine peaty and boggy ground. Due to the age of this specimen, a DNA sequence could not be acquired. Another rust, Aecidium monocystis Berk., occurs on Abrotanella caespitosa, A. pusilla and A. rosulata in New Zealand. However, the aeciospores of Puccinia abrotanellae are much smaller than those of A. monocystis (PDD 110681, PDD 43969), which measure 19.5–26 × 15–21.5 μm (mean 22.9 × 18.6 μm). In fact, G.H. Cunningham has written on the specimen packet of P. (Aecidium) abrotanellae, ‘undescribed, spores 14–18 × 14 far too small for Aec. monocystis Berk.’. The wall of the aeciospores has detachable plugs, like those seen in aeciospores of several other New Zealand rust species.
Puccinia austropunctata McKenzie and Padamsee sp. nov.
Figure 6. Puccinia austropunctata on Galium spp.: A, Aecia. B, Uredinia. C, Aeciospores. D, Urediniospores. E, Figure 87, reproduced from Cunningham (Citation1931). F, Teliospores. Scale bars = 20 μm.
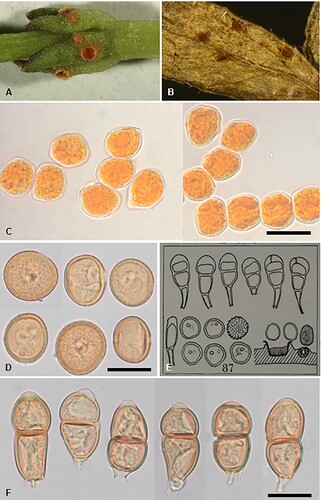
IF901203
Etymology. From Latin austro-, referring to the southern hemisphere origin of this rust, which previously has been confused with Puccinia punctata.
Parasitic on Galium spp. (Rubiaceae). Spermogonia mainly hypophyllous, scattered or in groups, reddish brown. Aecia hypophyllous, scattered or in small groups on pale spots, occasionally on stems, circular, 0.3 mm diameter, appearing disproportionally large on the small leaves, cupulate, orange; peridium short, cream coloured, recurved, slightly lacerate. Aeciospores (15–)17–21(–24) × (13–)14.5–18 μm (mean 19.9 × 16.1 μm), subglobose, ellipsoidal or oval, often angular, contents golden yellow; wall 0.5–1 μm thick, verruculose, hyaline. Uredinia hypophyllous, scattered, cinnamon-brown, circular or oval, up to 0.4–1 mm diameter, occasionally on stems, elliptical up to 1.5 mm long, pulverulent, surrounded by the ruptured epidermis. Urediniospores (19–)21–25(–27) × (15.5–)18–21(–23) μm (mean 22.7 × 19.2 μm), subglobose, obovoid or ellipsoidal, contents pale brown; wall 1–2(–2.5) μm thick, yellowish brown, echinulate, germ pores 2, equatorial or slightly super-equatorial. Telia amphigenous, and on stems, scattered, dark brown to black, circular to oval, up to 1 mm diameter, pulvinate, compact, surrounded by the ruptured epidermis. Teliospores (29–)31–45(–56) × (13.5–)15–20(–21) μm (mean 38.5 × 17.5 μm), 2-celled, ellipsoidal, oblong-ellipsoidal or subclavate, contents pale brown, constricted at septum, straight or curved; wall 0.75–1.5(–2) μm thick at sides, 3–6 μm thick at apex, smooth, greenish yellow, apex rounded or conically rounded, apical pore usually papillate, base rounded or attenuate; pedicels up to 10 × 4–6 μm, thin-walled, hyaline.
Holotype. New Zealand, Otago Lakes, Glenorchy, 365 m, on Galium perpusillum, 15 December 1921, W.D. Reid (PDD 7600–0, I, III).
Distribution. Endemic to New Zealand (Wellington, Buller, Westland, North Canterbury, Mid Canterbury, Mackenzie, Otago Lakes, Central Otago, Dunedin, Southland).
Additional specimens examined. New Zealand, Wellington, Weraroa, on Galium propinquum, 12 October 1922, E.H. Atkinson and G.H. Cunningham (PDD 9514–II, III). Mackenzie, Lake Ohau, on G. perpusillum, s.d., P.B. Heenan (PDD 68338–0, I); Lake Ohau, Raupo Swamp, on G. perpusillum, s.d., P.B. Heenan (PDD 68336–0, I). Otago Lakes, Bold Peak, 640 m, on G. perpusillum, March 1929, J. Scott Thompson and G. Simpson (PDD 3454–0, I, III); Glenorchy, 365 m, on G. perpusillum, 15 December 1921, W.D. Reid (PDD 7600–I, III). Central Otago, Dunstan Mountains, 1065 m, on G. propinquum, 6 February 1921, A.H. Cockayne (PDD 745–II); Mt Barker, Upper Acheron Flat, 580 m, on G. perpusillum, November 1997, P.B. Heenan (PDD 68346–I). Dunedin, Otago Peninsula, yellow-eyed penguin trust lands, on G. propinquum, E.H.C. McKenzie and M. Padamsee (PDD 110338–0, I).
Specimens examined of Puccinia punctata. Austria, Ostalpen, Gunser Gebirge, Burgenland, on Galium verum L., 31 August 1981, J. Hafellner and J. Poelt (PDD 64459–II, III, ex Plantae Graecenses 396). China, Xinjiang, Gongliu, on G. aparine L., 28 August 1986, J.-Y. Zhuang (PDD 66760–II, III, ex HMAS53076). Germany, Brandenburg, Potsdam, Cladow, on G. mollugo L., 10 August 1919, H. Sydow (PDD 42380–II, III, ex Sydow, Mycotheca Germanica 1478).
Notes. The three hosts, Galium perpusillum (Hook. f.) Allan, G. propinquum A. Cunn. and G. trilobum Colenso, are small perennial herbs native to New Zealand; G. propinquum is also native to Australia. They occur throughout most of the country from lowland to montane regions, often in open damp and shady places. There are also several naturalised Galium species in New Zealand, none of which are known to be infected by rust in this country.
Cunningham (Citation1924a, Citation1931) mistakenly identified this rust as Puccinia punctata Link, a species that is widespread on numerous species of Galium in Australia, North, Central and South America, Europe, Africa and Asia. Most specimens held in Fungarium PDD contain aecia and often spermogonia. However, telia are present on three specimens, which were all collected between 1921 and 1929, and probably match material examined by Cunningham. Cunningham’s (Citation1931) illustration of teliospores (E) of this rust appears to show P. punctata, and not the teliospores observed on New Zealand material. In P. punctata the teliospores are often subclavate and rounded at the apex. In P. austropunctata the teliospores are predominantly ellipsoidal or oblong-ellipsoidal. The walls of P. punctata are a darker brown than in P. austropunctata and they are thickened up to 7–16 μm wide at the apex (Gäumann Citation1959; Lindquist Citation1982), whereas in P. austropunctata the apical wall is only 3–6 μm thick. The apical pore of P. austropunctata is usually papillate and quite pale while in P. punctata the apex is usually rounded and rarely papillate. The teliospore pedicel of P. austropunctata is broken very short while in P. punctata it is usually longer. The teliospores of two specimens of P. punctata from Europe and one specimen from China held in Fungarium PDD are slightly longer and broader than those of P. austropunctata (mean 44.4 × 21.5 μm vs 38.5 × 17.5 μm), although the urediniospores of the specimens from overseas average 23.0 × 20.2 μm, which is very similar to the size of P. austropunctata (22.7 × 19.2 μm). Puccinia austropunctata was recovered in a highly supported clade (100% MLBS) and appears to be sister to a clade (with less than 60% MLBS) consisting of P. confundens, P. pittospori and P. tenuispora ().
Puccinia cardamines McKenzie and Padamsee, sp. nov.
IF557265
Etymology. From Cardamine, the host genus.
Parasitic on leaves of Cardamine glara Heenan (Brassicaceae). Spermogonia and aecia not known. Uredinia hypophyllous, scattered, brown, elliptical or circular, ca. 0.3 × 0.25 mm diameter, pulverulent. Urediniospores 23–25.5(–28) × 21–24.5 μm (mean 24.8 × 22.8 μm), subglobose or obovoid, contents pale brown; wall 1.25–1.5 μm thick, pale yellow-brown, moderately echinulate, germ pores 4–5, conspicuous, super-equatorial. Telia not known.
Holotype. New Zealand, Mid Canterbury, Torlesse Range, Ghost Creek, on Cardamine glara, 3 March 1997, P. Heenan (PDD 67228–II).
Distribution. Endemic to New Zealand (Mid Canterbury).
Notes. The host is an endemic perennial, rhizomatous herb, mostly found on scree and alluvial riverbeds in mountainous areas of the North Island (excluding Mt Egmont) and the South Island.
There are no telia on the type specimen. Puccinia cruciferarum subsp. inornata (G. Cunn.) J. Walker, the only other rust recorded on Cardamine spp. in New Zealand, is a microcyclic species, producing only telia. Another microcyclic species, P. drabae F. Rudolphi [ = P. cardamines-cordatae Dietel and Neger], is known on Cardamine cordata from South America (Lindquist Citation1982). Puccinia cardamines was recovered in an unsupported clade sister to a rust on a grass (Lachnagrostis billardieri).
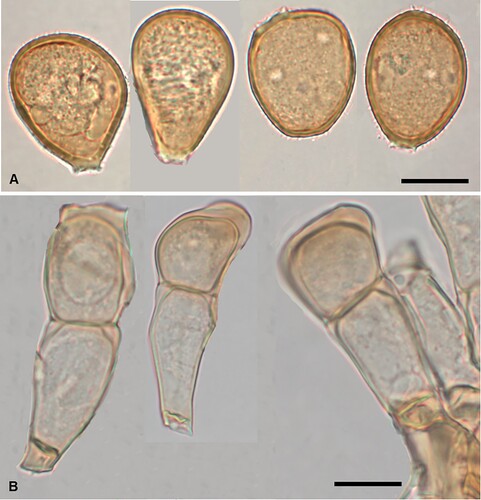
Puccinia cenchricola McKenzie and Padamsee sp. nov.
Figure 8. Puccinia cenchricola on Cenchrus caliculatus: A, Urediniospores. B, Teliospores. Scale bars = 20 μm.
IF557259
Etymology. From Cenchrus, the host genus.
Parasitic on leaves of Cenchrus caliculatus Cav. (Poaceae). Spermogonia and aecia not known. Uredinia amphigenous, but mainly on adaxial surface, reddish brown, linear, up to 1 mm long, long covered by the epidermis which splits longitudinally, pulverulent. Urediniospores (35.5–)37–46(–49.5) × (23.5–)28–33(–34.5) µm (mean 42.5 × 30.2 µm), subglobose, broadly ellipsoidal, ellipsoidal or obovoid, contents pale grey-brown; wall 1–1.5(–2) µm thick, pale yellow-brown, echinulate, germ pores 3(–4), equatorial. Telia amphigenous, dark brown to black, linear, up to 1 mm long, but coalescing into longer streaks, long covered by the epidermis. Teliospores (44–)48–63(–67) × (19–)21.5–26(–36) µm (mean 53.5 × 24.4 µm), 2-celled, subclavate, apex rounded or irregular, constricted at septum, contents pale brown, darker in apical cell; wall 0.5–1.5 µm thick at sides, (1.5–)4–7 µm thick at apex, pale reddish brown, smooth; pedicels up to 10 × 6–9 µm, very pale brown.
Holotype. New Zealand, Kermadec Islands, Raoul Island, Water Line Track, on Cenchrus caliculatus, 17 September 1988, E.H.C. McKenzie (PDD 54650–II, III).
Distribution. Probably indigenous to New Zealand (Kermadec Islands).
Notes. We were unable to obtain an LSU sequence of P. cenchricola, but an internal transcribed spacer region (ITS) sequence (GenBank #PP594476) was obtained; in a BLAST search, P. cenchricola shared only 88.7% sequence identity with P. dichondrae (FN298146).
Specimens examined of Puccinia cenchri var. cenchri. Cook Islands, Aitutaki, on Cenchrus echinatus L., 12 July 2002, E.H.C. McKenzie (PDD 75960–II). Fiji, Viti Levu, Yadua, on C. echinatus, 28 February 1977, E.H.C. McKenzie (PDD 36480–II). French Polynesia, Tubuai, on C. echinatus, 17 September 2001, E.H.C. McKenzie, J. Wright and L. Mu (PDD 74515–II, III). Kiribati, Butaritari, Temanoku, on C. echinatus, 20 January 1976, G.C. Daft (PDD 35333–II). Niue, hotel grounds, on C. echinatus, 13 September 1975, E.H.C. McKenzie (PDD 33943–II). Samoa, Upolu, on C. echinatus, 26 September 1975, E.H.C. McKenzie (PDD 33988–II, III).
Notes. In New Zealand Cenchrus caliculatus is known only from the Kermadec Islands (Edgar and Connor Citation2010). It is also indigenous, but often rare, in other tropical and subtropical Pacific islands.
McKenzie (Citation1992) recorded this rust from the Kermadec Islands as Puccinia sp. Two varieties of another rust, P. cenchri Dietel and Holw., have been described on Cenchrus spp. Puccinia cenchri var. cenchri is widely distributed (perhaps circumglobal in the tropics), while P. cenchri var. africana Cummins is restricted to Africa. These two varieties have very similar spore measurements, differing principally in the number of equatorial germ pores in the urediniospores, with 2(–3) in var. cenchri and 4–5 in var. africana. Puccinia cenchricola has 3(–4) prominent equatorial germ pores.
In the Pacific, Ramachar and Cummins (Citation1965) recorded P. cenchri var. cenchri on Cenchrus caliculatus from the Cook Islands. Later it was recorded from Cook Islands, Fiji, Kiribati, Niue, Samoa, Tonga and Tuvalu on C. echinatus (Dingley et al. Citation1981). Wang and Zhuang (Citation1998) described urediniospores of var. cenchri on C. caliculatus from Taiwan; they gave measurements of 27–37 × 20–31 µm and reported 2–3 equatorial germ pores. Specimens of rust held in Fungarium PDD, on C. echinatus from Pacific Island countries were examined; all had smaller urediniospores (28–41.5 × 21.5–32.5 µm; mean 34.0 × 28.3 µm) than those of P. cenchricola and, where present, smaller teliospores (34–54.5 × 17.5–26.5 µm; mean 44.4 × 20.6 µm); these measurements being similar to those given by Ramachar and Cummins (Citation1965) and Cummins (Citation1971). Combining measurements given by Ramachar and Cummins (Citation1965) and Cummins (Citation1971), the urediniospores of the two varieties of P. cenchri measure 27–43 × 19–31 µm and the teliospores 35–58 × 16–27 µm.
Both the urediniospores and teliospores of P. cenchricola are larger than those of P. cenchri. The mean length and width of the urediniospores of P. cenchricola lie almost entirely outside of the size range for those of P. cenchri, while the mean length and width of the teliospores of P. cenchricola are also larger than those of most P. cenchri spores. Although DNA was extracted from two specimens of P. cenchri from Cook Islands and French Polynesia () it was, unfortunately, not obtained from the P. cenchricola specimen. The sequences of P. cenchri were in a highly supported clade (100% MLBS) and supported as sister (100% MLBS) to a large clade consisting of multiple representatives of Puccinia and Uromyces.
Puccinia confundens McKenzie and Padamsee, sp. nov.
Figure 9. Puccinia confundens on Luzula rufa: A, Urediniospores (PDD 101664, holotype). B, Urediniospores (PDD 99228). Scale bars = 20 μm.
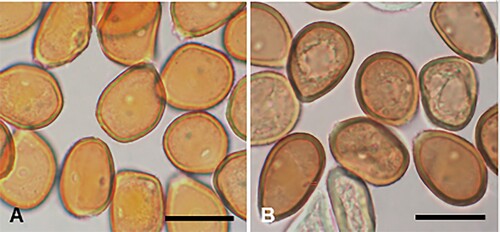
IF901204
Etymology. From the Latin for confusing, referring to the species being confused with Puccinia tenuispora.
Parasitic on Luzula rufa Edgar (Juncaceae). Spermogonia and aecia not known. Uredinia amphigenous, mainly hypophyllous, and on stems, scattered, pale cinnamon, elliptical or circular, 0.25–1 mm, bullate when covered by the epidermis, later pulverulent and covered by a large flap of epidermis. Urediniospores (23–)24–28.5(–31) × (17–)18–21(–23) μm (mean 26.4 × 20.1 μm), subglobose, ellipsoidal or obovoid, contents beige; wall 1–1.5(–2.5) μm thick, pale yellow, echinulate, germ pores 2, equatorial. Telia not known.
Holotype. New Zealand, Mid Canterbury, Craigieburn, Broken River skifield, track to huts, 10 February 2011, on Luzula rufa var. rufa, E.H.C. McKenzie, M. Padamsee and M.A. Korver (PDD 101664–II).
Distribution. Endemic to New Zealand (Mid Canterbury).
Additional specimen examined. New Zealand, Mid Canterbury, Craigieburn Valley skifield, on L. rufa, 4 May 2010, E.H.C. McKenzie and D. Than (PDD 99228–II).
Notes. Morphologically the urediniospores of P. confundens match those of P. tenuispora McAlpine. However, in a molecular analysis they lie in related but separate phylogenetic clades. Two representatives of P. confundens are in a highly supported clade (97% MLBS) and appear to be sister to (<60% MLBS) P. pittospori; P. tenuispora, highly supported (99% MLBS), is sister (also with <65% MLBS) to this clade. Dingley (Citation1977) first recorded rust, which she identified as P. tenuispora, on Luzula rufa in New Zealand. There are four other specimens of rust on L. rufa in Fungarium PDD from Central Otago, Mackenzie and Otago Lakes, but unfortunately they have been subjected to chemical treatment for insects, making it impossible to extract DNA for positive identification.
This species is described here as a Puccinia although, unfortunately, neither of the specimens possesses teliospores. Puccinia confundens differs from P. tenuispora by 11 base pairs in the LSU gene and it is herein introduced as a new species.
Puccinia ferocis-forsteri McKenzie and Padamsee, sp. nov.
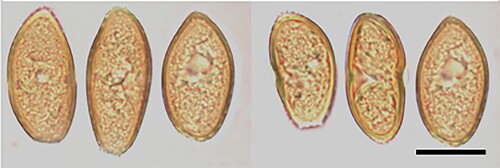
Figure 10. Puccinia ferocis-forsteri on Carex forsteri and Urtica ferox: A, Aecia. B, Teliospores. C, Urediniospores. D, Aeciospores. Scale bars = 20 μm.
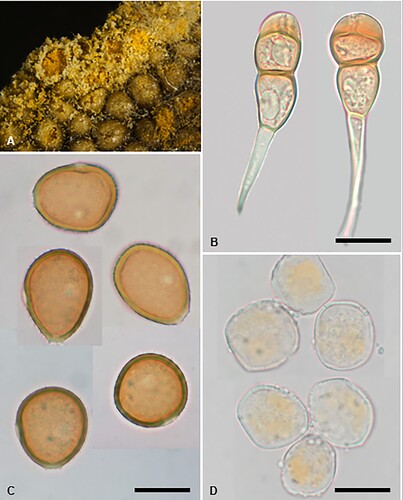
IF559587
Etymology. From the primary host plant, Carex forsteri, and the alternate host, Urtica ferox.
Parasitic on Carex forsteri Wahlenb. (Cyperaceae) and Urtica ferox G. Forst. (Urticaceae). Spermogonia on U. ferox amphigenous, on discoloured spots, honey coloured. Aecia mainly hypophyllous, aggregated on swollen leaf veins, or on swollen and distorted stems up to 20 mm long, yellow, 0.25–0.5 mm wide, leaf blackened on opposite side; peridia white, lacerate and revolute at the top, up to 0.5 mm high. Aeciospores (21–)22.5–27(–29) × (18.5–)19.5–23.5(–24.5) μm (mean 24.7 × 22.1 μm), subglobose, somewhat angular, contents pale yellow; wall 0.75–1(–1.25) μm thick, hyaline, verruculose, with numerous small plugs of varying sizes, usually most pronounced on one area of the spore surface. Uredinia on C. forsteri amphigenous, scattered, rarely coalescing, pale amber to cinnamon, oval, up to 0.5 mm long, restricted in width by leaf veins, pulverulent, surrounded by the ruptured epidermis. Urediniospores (25.5–)27.5–34(–36.5) × (22–)23–26(–28) μm (mean 29.9 × 24.7 μm), subglobose, broadly ellipsoidal or obovoid, contents brown; wall 1.5–2.5 μm thick, tan, echinulate, germ pores 3, often with caps, equatorial. Telia mainly hypophyllous (abaxial), scattered, in lines, dark brown, oval or elliptical, up to 1 mm long, restricted in width by leaf veins, pulvinate, surrounded by the ruptured epidermis. Teliospores (30–)35–49(–59) × (13.5–)15–19.5(–21) μm (mean 42.6 × 17.2 μm), 2-celled, subclavate, contents pale brown, darker towards apex, constricted at septum, straight or slightly curved; wall 1–1.5 μm thick at sides, 6–10.5 μm thick at apex, smooth, tan, apex rounded, pore apical, in lower cell pore immediately below septum, base usually attenuate, basal cell usually narrower, longer and often paler than upper cell; pedicels up to 50 μm long, 4–6 μm wide, thin-walled, hyaline.
Typification. New Zealand, Mid Canterbury, Hoon Hay Reserve, on Carex forsteri, 29 November 2008, J.A. Cooper (PDD 95256–II, III, holotype). New Zealand, Hawkes Bay, Tangoio Forest, on Urtica ferox, 5 December 2019, B.J. Rogan (PDD 116804–I, epitype, designated here, IF559661).
Distribution. Endemic to New Zealand (Hawkes Bay, Mid Canterbury).
Additional specimens examined. New Zealand, Hawkes Bay, Turangakumu, on Carex forsteri, 26 March 1984, E.H.C. McKenzie (PDD 45208–II, III). Mid Canterbury, Hoon Hay Reserve, on Urtica ferox, 23 November 2008, J.A. Cooper (PDD 95253–0, I); Hoon Hay Reserve, on U. ferox, 29 November 2008, J.A. Cooper (PDD 95255–0, I).
Notes. Urtica ferox is an endemic woody shrub that occurs throughout most of New Zealand in coastal to lowland forest margins and scrubland. Carex forsteri is also endemic and is of sporadic occurrence throughout. It occurs in forests in wet areas and often along stream banks from sea level to montane.
The most common and widespread rust on Carex in New Zealand has been referred to as Puccinia caricina DC. (e.g. Dingley Citation1969; McKenzie Citation1998). However, aeciospores of P. ferocis-forsteri (mean 24.7 × 22.1 μm) are larger than those of P. caricina (mean 19.6 × 17.3 μm). In size, they match the measurements given by Zwetko (Citation1993) for P. urticata (Link) F. Kern (18–27 × 16–24 μm). In addition, the aeciospores of P. ferocis-forsteri possess spore plugs, a feature not seen in aeciospores of New Zealand P. caricina, although v. Hofsten and Holm (Citation1968) reported refractive bodies in P. caricina examined by transmission electron microscopy. The plugs in P. ferocis-forsteri are smaller and more numerous than those that occur in aeciospores of several other New Zealand rust fungi (e.g. Puccinia abrotanellae, P. clavata, P. otagensis, P. pulverulenta, Uromyces scaevolae), and do not detach so readily. Savile (Citation1973) classified aeciospores according to the development of wall warts and pore plugs and reported aeciospores on Urtica spp. as type 3. Those of P. ferocis-forsteri fit type 3 spores, whereas those of the other six New Zealand species are type 5.
Zwetko (Citation1993) separated those rusts on Carex, which alternate with Urtica species, into P. urticata, with P. caricina sens. lat. alternating on Ribes species. This separation was accepted by Henderson (Citation2000). In addition, Zwetko (Citation1993) erected 13 host-specific varieties of P. urticata, based on their Carex hosts. More recently, Léveillé-Bourret et al. (Citation2021) sequenced many rust isolates on Cyperaceae and Juncaceae, including a few samples from New Zealand. Among the latter were two collections of P. ferocis-forsteri on Carex forsteri and Urtica ferox, which they labelled as P. urticata agg. 5. These two collections formed a distinct sister clade to all other collections within the P. urticata clade (Léveillé-Bourret et al. Citation2021), and in the current molecular analysis as well as in Padamsee and McKenzie (Citation2017), P. ferocis-forsteri from Carex forsteri was recovered in the same clade to the aecial rust on Urtica ferox (90% MLBS, ). This clade also consists of a representative of a rust on Carex gaudichaudiana (PDD 48430), which shares 99% sequence identity (14 base pairs difference) with P. ferocis-forsteri.
In New Zealand aecia occur on three endemic species of Urtica (U. aspera, U. australis, U. ferox) and on U. incisa, a fully naturalised species. Only the aecia on U. ferox have been connected to a Carex rust through DNA analysis. This rust on U. ferox and on C. forsteri is treated as P. ferocis-forsteri, while the rust on other Urtica spp. and many Carex spp. in New Zealand is regarded as P. urticata sensu lato.
Puccinia gaudichaudianae McKenzie and Padamsee, sp. nov.
Figure 11. Puccinia gaudichaudianae on Carex gaudichaudiana: A, Uredinial paraphyses. B, Urediniospores. Scale bars = 20 μm.
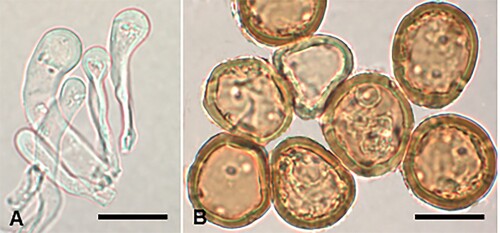
IF557266
Etymology. From the host species, Carex gaudichaudiana.
Parasitic on leaves of Carex gaudichaudiana Kunth (Cyperaceae). Spermogonia and aecia not known. Uredinia hypophyllous (adaxial), scattered, sometimes coalescing, pale brown, elongate, up to 0.5 mm long, restricted in width by leaf veins, surrounded by ruptured epidermis; paraphyses intermixed, capitate or cylindrical, up to 60 μm long × 4–9 μm wide, head 8–15 μm wide, hyaline. Urediniospores 24–33.5 × 22–29 μm (mean 29.1 × 25.8 μm), subglobose or obovoid, contents pale brown; wall 1.5–2.5(–3.5) μm thick, yellow-brown, echinulate, germ pores 6–8, scattered, with small caps. Telia not known.
Holotype. New Zealand, Mid Canterbury, Lake Sarah, on Carex gaudichaudiana, 25 February 1988, E.H.C. McKenzie (PDD 48430–II).
Distribution. Endemic to New Zealand (Mid Canterbury).
Notes. The host is an indigenous sedge found throughout most of New Zealand in boggy ground from sea level to subalpine. It also occurs in Australia and New Guinea. Puccinia gaudichaudianae is characterised by the urediniospores having 6–8 scattered germ pores and by the presence of numerous, hyaline paraphyses. Two other rusts have been recorded on Carex gaudichaudiana. Of these, P. urticata occurs worldwide (including New Zealand) on many Carex species while P. rautahi G. Cunn. is endemic to New Zealand (Cunningham Citation1923, Citation1930, Citation1931). Both these rust species lack paraphyses in the uredinia and their urediniospores have 2–4 equatorial germ pores. In the molecular analysis, P. gaudichaudianae was recovered in a clade with P. ferocis-forsteri, the latter being an endemic heteroecious rust on Carex forsteri and Urtica ferox (); P. gaudichaudianae shares 99% sequence identity with P. ferocis-forsteri. Another locus may be needed to clarify species separation.
Puccinia hebe (G. Cunn.) McKenzie and Padamsee comb. nov.
IF559589
≡ Aecidium hebe G. Cunn., Transactions and Proceedings of the New Zealand Institute 59: 496, 1928.
Cunningham (Citation1928) described Aecidium hebe and gave the following details for the type: on Hebe treadwellii Ckn. et Allan [≡ Veronica treadwellii (Cockayne and Allan) Garn.-Jones], Black Birch Creek, Mt. Cook, Canterbury, 1200 m, January 1928, G.H. Cunningham. In Fungarium PDD there are two specimens (PDD 3401, 3411) with the same collecting details, and hence they must be regarded as syntypes. Consequently, PDD 3411 is selected and designated here as the lectotype (IF559662) of Aecidium hebe (New Zealand, South Canterbury, The Hermitage, Black Birch Creek, on Hebe treadwellii, January 1928, G.H. Cunningham, PDD 3411–I). Although only aecia are known, the opportunity is taken to transfer the species to an appropriate genus, Puccinia.
Puccinia helichrysicola McKenzie and J.A. Cooper, sp. nov.
Figure 12. Puccinia helichrysicola on Helichrysum lanceolatum: A, B, Uredinia. C, Urediniospores. D, Uredinial paraphyses. Scale bars = 20 μm.
IF901393
Etymology. From Helichrysum, the host genus.
Parasitic on leaves of Helichrysum lanceolatum (Buchanan) Kirk (Asteraceae). Spermogonia and aecia not known. Uredinia hypophyllous, scattered or in small groups, golden yellow, oval or elliptical, embedded within leaf tomentum, but becoming exposed and pulverulent, ca. 0.5–0.75 mm diameter, with peripheral paraphyses; paraphyses hyaline, bent, 30–75 × 10–15 μm, sometimes septate, wall 1.0–3.0 μm thick. Urediniospores (21–)22–26(–27.5) × (16–)18–20(–21.5) μm (mean 24 × 19.1 μm), obovoid, subglobose or broadly ellipsoidal, contents yellow; wall (1.0–)1.5–2.0 μm thick, hyaline, echinulate, germ pores obscure. Telia not known.
Holotype. New Zealand, Mid Canterbury, Living Springs, Teddington, on Helichrysum lanceolatum, March 2024, J.A. Cooper (PDD 110711–II).
Distribution. Endemic to New Zealand (Mid Canterbury).
Additional specimen examined. New Zealand, Mid Canterbury, Living Springs, Teddington, on Helichrysum lanceolatum, 8 November 2014, J.A. Cooper (PDD 105891–II).
Notes. The host is an endemic shrub widely distributed throughout New Zealand, especially in coastal and lowland open forest, forest edge, scrub and cliff habitats. Berndt (Citation2009) listed eight species of Puccinia on Helichrysum from South Africa and noted they could not be identified morphologically from a single spore state; P. kalchbrenneri De Toni is the name given to the species complex. However, the uredinia of this species complex do not have paraphyses; rather, they have a peridium that helps form the characteristic bullate to broadly flask-like shape to the uredinia which liberate the spores through an irregular pore or slit at their apex. No rust has been previously recorded on any Helichrysum species in New Zealand. No LSU sequence was obtained for P. helichrysicola; an ITS sequence from PDD 110711 (PP594477) shared 100% sequence identity with P. chunjiei M. Liu, C.J. Li and Hambl. on Triticum aestivum (HQ012446) with only 55% query coverage.
Puccinia kaiku (G. Cunn.) McKenzie and Padamsee, comb. nov.
Figure 13. Puccinia kaiku on Parsonsia heterophylla: A, Caeomata. B, Telia. C, Teliospores. D, Aeciospores. Scale bars = 20 μm.
IF559590
≡ Caeoma kaiku G. Cunn., Transactions and Proceedings of the New Zealand Institute 61: 416, 1930.
Parasitic on leaves of Parsonsia spp. (Apocynaceae). Spermogonia not known. Caeomata hypophyllous, scattered or in circular groups up to 5 mm diameter, chrome-orange, orbicular, becoming elongate, may coalesce, up to 2.5 mm long, 0.5–0.75 mm wide, with small brown spot on corresponding upper surface of leaf, surrounded by a pale green halo, pulverulent, long covered by the epidermis. Aeciospores (19–)21–31(–40) × (14–)16.5–22(–23) μm (mean 25.4 × 18.8 μm), subglobose, ellipsoidal, oblong-ellipsoidal or obovoid, often polygonal, contents golden yellow; wall 1–2 μm thick, hyaline, verrucose. Uredinia not known. Telia hypophyllous, in irregular or circular raised groups up to 3.25 mm diameter, coalescing, surrounded by narrow pale green halo, on upper surface slightly depressed with pale green spot, pulvinate, compact, hard, amber-brown, up to 0.5 mm diameter Teliospores (32–)36–48(–52) × (13–)14.5–16.5(–18) μm, (mean 41.4 × 15.5 μm), 2-celled, fusiform, slightly constricted at septum, apex bluntly acuminate, rarely rounded, pore apical; wall (0.5–)1–1.5(–2) μm thick at sides, (1.5–)2–3(–4) μm thick at apex, cream to pale amber, smooth, contents cream to pale yellow; pedicels up to 90 × 3–6.5 μm, persistent, hyaline.
Typification. New Zealand, Taupo, Waimarino, 460 m, on Parsonsia capsularis × P. heterophylla, February 1930, J.C. Neill (PDD 3471–I, holotype). New Zealand, Hawkes Bay, Elsthorpe Scenic Reserve, on Parsonsia heterophylla A. Cunn., 6 November 2002, R.C. Henderson (PDD 76717–III, epitype designated here, IF559663).
Distribution. Endemic to New Zealand (Bay of Plenty, Taupo, Hawkes Bay, Mid Canterbury).
Additional specimens examined. New Zealand, Bay of Plenty, Whakarewarewa, State Forest area, on Parsonsia capsularis (G. Forst.) DC., 11 June 1945, C.M. Smith (PDD 9782–I). Mid Canterbury, Banks Peninsula, Hinewai Reserve, on P. heterophylla, 22 July 2002, N.A. Martin (PDD 76078–I).
Notes. Both Parsonsia capsularis and P. heterophylla are endemic lianes found in coastal to montane forests, forest margins and shrublands throughout New Zealand. Parsonsia capsularis exists as several varieties; hybridism between the species and the varieties appears to be common. A sequence of the epitype (PDD 76717; GenBank accession number PP594481) was recovered in an unsupported clade sister to two Uromyces species ().
The rust is known from only three aecial collections, and one telial collection. Caeoma kaiku was one of 19 species of rust fungi listed in the New Zealand Threat Classification System (Hitchmough Citation2002) as being ‘data deficient’. Now, with two more recent specimens from 2002, it is suggested that the rust be removed from this category.
Puccinia kokihi McKenzie and Padamsee, nom. nov., stat. nov.
IF901205
= Uredo novae-zelandiae G.F. Laundon, Mycological Papers 91: 16, 1963.
≡ Puccinia tetragoniae var. novae-zelandiae McKenzie, Mycotaxon 41: 307, 1991. [non, Puccinia novozelandica Bubák 1902.]
Etymology. From kokihi, Māori name for the host plant, Tetragonia tetragonioides, New Zealand spinach.
Two other varieties, Puccinia tetragoniae McAlpine var. tetragoniae and P. tetragoniae var. austroafricana Doidge (≡ P. austroafricana (Doidge) Berndt), occur on species of Tetragonia in Australia and South Africa, respectively (Laundon Citation1963; Berndt Citation2009). The Australian rust has larger urediniospores than P. kokihi (29–40 × 27–39 μm vs. 22–32 × 18–25 μm) with thicker walls. The urediniospores of the New Zealand specimens and of P. austroafricana are similar in size, but the teliospores of the South African fungus are smaller (30–50 × 22.5–35 μm vs. a mean of 48 × 33.5 μm, pers. obs.), with a thinner cell wall, especially at the apex. To raise the New Zealand rust to species level it is necessary to propose a new name to prevent the creation of a homonym.
Puccinia lachnagrostidis McKenzie and Padamsee, sp. nov.
Figure 14. Puccinia lachnagrostidis on Lachnagrostis billardierei: Urediniospores and paraphyses. Scale bar = 20 µm.
IF901206
Etymology. From the host genus, Lachnagrostis.
Parasitic on Lachnagrostis billardierei (R. Br.) Trin. (Poaceae). Spermogonia and aecia not known. Uredinia amphigenous, often surrounded by flap of epidermis, scattered, yellowish brown, elliptical or oval, up to 0.5(–0.75) mm long, restricted in width by leaf veins, pulverulent; capitate paraphyses intermixed, hyaline, up to 75 μm long, stalk 5–7 μm wide, head up to 20 μm wide. Urediniospores 29–34(–40) × (22–)24.5–26.5(–28) μm (mean 31.9 × 25.3 μm), obovoid or broadly ellipsoidal, contents pale brown; wall 2–3.25 μm thick, pale brown, echinulate, germ pores 7–8, scattered. Telia not known.
Holotype. New Zealand, Auckland, Kumeu State Forest, on Lachnagrostis billardieri, 3 January 1982, E.H.C. McKenzie (PDD 43840–II).
Distribution. Endemic to New Zealand (Auckland).
Notes. Lachnagrostis billardierei (sand wind grass) is indigenous to New Zealand and is also found naturally in Australia. It occurs throughout most of the country, usually in coastal areas on cliffs, sandy flats and dunes. Puccinia lachnagrostidis is characterised by the presence of capitate paraphyses in the uredinia and relatively thick-walled urediniospores. It is the first rust described from Lachnagrostis although similar rusts with scattered germ pores are known elsewhere on the related genus Deyeuxia, but they lack paraphyses. Two introduced rust species, P. coronata and P. recondita, have been also recorded on Lachnagrostis species in New Zealand (Cunningham Citation1924a; McKenzie Citation1981, Citation1991a). An LSU sequence of P. lachnagrostidis (PDD 43840; GenBank accession number PP594479) was sister to P. cardamines in an unsupported clade ().
Puccinia lagenophoricola McKenzie and Padamsee, sp. nov.
Figure 15. Puccinia lagenophoricola on Lagenophora pumila: A, Telia. B, Teliospores. Scale bar = 20 μm.
IF557260
Etymology. Named for the host genus, Lagenophora.
Parasitic on leaves of Lagenophora pumila (G. Forst.) Cheeseman (Asteraceae). Spermogonia and aecia not known. Uredinia not known. Telia mostly hypophyllous, pulvinate, compact, hard, pale brown, up to 1.5 mm diameter but mainly 0.25–0.5 mm, circular, scattered, sometimes coalescing. Teliospores (30–)33–45(–51) × (11.5–)13–18(–19.5) μm (mean 38.2 × 15.2 μm), ellipsoidal or fusiform, contents very pale brown, palest at apex, 2-celled, slightly constricted at septum, straight or slightly curved, apex bluntly acuminate, rarely rounded, upper cell pore apical, lower cell pore usually immediately below septum, base attenuate; wall 1–2 μm thick at sides, up to 13 μm thick at apex, very pale brown, smooth; pedicels up to 40 × 4.5–6.5 μm, slightly tinted.
Holotype. New Zealand, Dunedin, city, Botanical Gardens, on Lagenophora pumila, 3 February 1924, J.C. Neill and G.H. Cunningham (PDD 1571–III).
Distribution. Endemic to New Zealand (Otago Lakes, Dunedin).
Additional specimen examined. New Zealand, Otago Lakes, Lake Whakatipu, Table Bay, beech forest, 750 m, on Lagenophora pumila, 29 April 1921, W.D. Reid (PDD 350–III).
Notes. The host is a small endemic, rhizomatous herb found throughout the country in lowland to montane grassland, open places and light forest.
Cunningham (Citation1923) identified this rust on Lagenophora pumila as Puccinia lagenophorae. Puccinia lagenophorae is considered a rust of Australasian origin (Wilson et al. Citation1965). It was described from Australia (Cooke Citation1884) and is found throughout New Zealand. However, it is also known from Europe, North and South America, North Africa and Southwest Asia.
Puccinia lagenophoricola differs from P. lagenophorae in the colour, form and shape of the telia, and it lacks mesospores. The telia of P. lagenophoricola are pale brown, pulvinate and compact whereas those of P. lagenophorae are dark brown to black, bullate and long covered by the epidermis. The teliospores of P. lagenophorae are larger, measuring (33.5–)37–52(–57) × (15.5–)18–22(–24.5) μm (mean 43.5 × 20.1 μm), and are darker in colour. Teliospores of P. lagenophoricola are often fusiform whereas those of P. lagenophorae are often clavate or subclavate. Shivas et al. (Citation2023) gave teliospore measurements for P. lagenophorae of 50–65 × 17–27 μm, which is larger again than that of New Zealand material, or of measurements given for P. lagenophorae by Weber et al. (Citation2003). In New Zealand, P. lagenophorae occurs on various genera of Asteraceae including Bellis, Calendula, Emilia, Olearia and Senecio. There are no aecia present on the plants infected with P. lagenophoricola, although a separate plant within the packet of PDD 350 does have aecia; these may be those of P. lagenophorae.
Puccinia liparophylli McKenzie and Padamsee, sp. nov.
Figure 16. Puccinia liparophylli on Liparophyllum gunnii: A, B, Aecia. C, Aeciospores (in aniline blue). Scale bar = 20 μm.
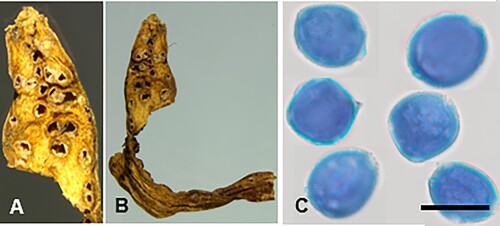
IF559588
Etymology. From the host genus, Liparophyllum.
Parasitic on Liparophyllum gunnii Hook. f. (Menyanthaceae). Spermogonia not known. Aecia amphigenous, scattered, 0.25–0.5 mm diameter, cupulate. Aeciospores (18–)19.5–24.5(–28.5) × (14–)16.5–21(–22.5) μm (mean 23.2 × 18.4 μm), subglobose or ellipsoidal, sometimes angular; wall 1.5–2.5(–3) μm thick, hyaline, verrucose. Uredinia and telia not known.
Holotype. New Zealand, Wellington, Mt Holdsworth, on Liparophyllum gunnii, 19 March 1950, J. Hay (PDD 34547–I).
Distribution. Endemic to New Zealand (Wellington).
Notes. Liparophyllum gunnii (alpine marshwort) is an aquatic, wetland plant indigenous to New Zealand and Tasmania. It grows from about 39°S southwards in montane to subalpine herbfield and boggy places. McAlpine (Citation1906) recorded Aecidium nymphoidis DC. on Limnanthemum indicum [≡ Nymphoides indica], another member of Menyanthaceae, in Queensland, Australia, and suggested that it may be the aecial stage of Puccinia scirpi DC. Soares et al. (Citation2006) also described aeciospores of A. nymphoidis occurring on N. indica, in Brazil. They gave measurements of 15–23 × 20–26 μm, which is similar to the spore size of P. liparophylli, but the walls (1.0–1.5 μm thick) were noticeably thinner. Neither uredinia nor telia have been recorded in Australia, and any connection between the present rust and the rust on Limnanthemum indicum in Australia is unconfirmed.
Puccinia litorosae McKenzie and Padamsee, sp. nov.
IF557267
Etymology. From the host species, Carex litorosa.
Parasitic on leaves of Carex litorosa L.H. Bailey (Cyperaceae). Spermogonia and aecia not known. Uredinia hypophyllous (adaxial), scattered, in brown leaf streaks, brown, elongate, up to 1 mm long, restricted in width by veins, covered by epidermis. Urediniospores 32–44 × 17–22.5 μm (mean 37.7 × 20.0 μm), oval or ellipsoidal, apex rounded or often acute, base often truncate, contents yellow-brown; wall 1–2(–2.5) μm thick, golden yellow, moderately echinulate, germ pores 3, with inconspicuous caps, equatorial. Telia not known.
Holotype. New Zealand, Northland, near Dargaville, Mititai, on Carex litorosa, 24 April 1964, R.F.R. McNabb (PDD 23262–II).
Distribution. Endemic to New Zealand (Northland).
Notes. The host is endemic and occurs in brackish marshes or sandy tidal riverbanks in Northland/Auckland, Wellington, South Island and Stewart Island. Puccinia litorosae has distinctive oval urediniospores, which readily distinguish it from any other rust known on Carex spp. in New Zealand.
Puccinia microseridicola McKenzie and Padamsee, sp. nov.
Figure 18. Puccinia microseridicola on Microseris scapigera. A, Urediniospores. B, Teliospores. Scale bars = 20 μm.
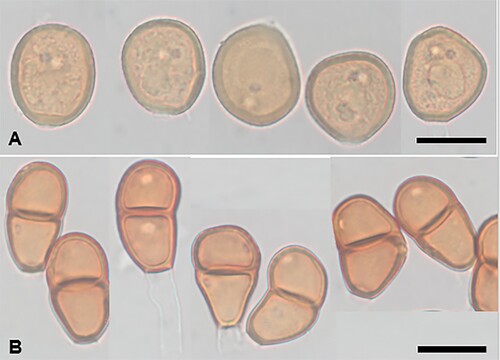
IF559591
Etymology. From the host genus, Microseris.
Parasitic on leaves of Microseris scapigera (Sol. ex A. Cunn.) Sch.Bip. (Asteraceae). Spermogonia and aecia not known. Uredinia mainly hypophyllous, scattered, brown or dark brown, round or oval, 0.1–0.5 mm diameter, surrounded by the ruptured epidermis, pulverulent. Urediniospores (21–)26–31(–32) × (17–)22–26(–29.5) μm (mean 28.0 × 24.0 μm), subglobose, broadly ellipsoidal or ellipsoidal, contents pale brown; wall (1–)1.5–2(–2.5) μm thick, yellow-brown, echinulate, germ pores 2, subapical or slightly lower. Telia mainly hypophyllous, scattered, dark brown, circular or oval, 0.1–0.5 mm diameter, pulvinate, becoming pulverulent. Teliospores (21.5–)27–35(–40) × (15.5–)17–20(–21) μm (mean 31.2 × 18.3 μm), 2-celled, subclavate or ellipsoidal, contents pale brown, constricted at septum; wall 1–2 μm thick at sides, 1.5–2(–2.5) μm thick apex, reddish brown, verrucose especially upper cell, straight or slightly curved, apex rounded, base usually attenuated, pore in upper cell most commonly more or less equatorial but may be towards apex or septum, in lower cell usually more or less equatorial but may be towards septum; pedicels up to 30 × 5–7 μm, hyaline.
Holotype. New Zealand, Mid Canterbury, Craigieburn, on Microseris scapigera, 24 February 1988, E.H.C. McKenzie (PDD 48443–II, III).
Distribution. Endemic to New Zealand (Taupo, Marlborough Sounds, Mid Canterbury).
Additional specimens examined. New Zealand, Taupo, Tongariro National Park, Hauhungatahi, A.P. Druce, on Microseris scapigera, February 1971 (PDD 40281–II, III). Marlborough Sounds, Brothers Islands, on M. scapigera, 17 January 1951, W.H. Dawbin (PDD 40283–II, III); Brothers Islands, on M. scapigera, May 1951, W.H. Dawbin (PDD 40282–II).
Notes. Microseris scapigera occurs throughout New Zealand in grasslands and open places and is probably endemic. Until recently, the ‘native yam daisy’ in Australia was thought to be the same species as M. scapigera from New Zealand. However, Australian representatives of this genus are now known to comprise three undescribed species, endemic to southern Australia (Native Yam Daisy Citation2023).
We were unable to obtain an LSU sequence of P. microseridicola; the ITS sequence of PDD 48443 (GenBank Accession # PP594475) was 97.3% identical to P. aff. hieracii (MW009431) and 90% identical to P. balsamorhizae Peck (JN204182). The distance tree produced by the BLAST search grouped the sequence of P. microseridicola in a clade with Miyagia pseudosphaeria (Mont.) Jørst., P. balsamorrhizae, U. appendiculatus (Pers.) Steud., and P. aff. hieracii.
McAlpine (Citation1895) described Puccinia microseridis McAlpine (as P. microseris) on Microseris forsteri from Australia, but later (McAlpine Citation1906) synonymised this rust with P. hypochaeridis Oudem., pointing out that the host plant had been incorrectly determined and it was Hypochaeris radicata.
McKenzie (Citation1981) recorded rust on M. scapigera in New Zealand as Puccinia hieracii (Röhl.) H. Mart. var. hieracii, which appears to be sister (<60% MLBS) to P. variabilis var. lapsanae (Fuckel) Cummins ().
Puccinia oleariae-bullatae McKenzie and Padamsee, sp. nov.
Figure 19. Puccinia oleariae-bullatae on Olearia bullata: A, Uredinia. B, Urediniospores. Scale bar = 20 μm.
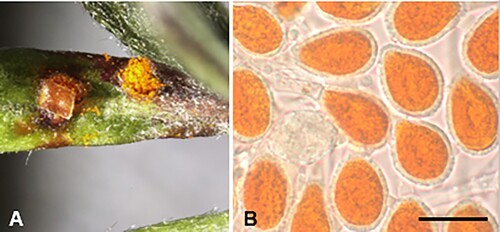
IF557268
Etymology. From Olearia bullata, the host binomial.
Parasitic on leaves of Olearia bullata H.D. Wilson and Garn.-Jones (Asteraceae). Spermogonia and aecia not known. Uredinia epiphyllous, scattered, orange, oval or globose, up to 0.5 mm diameter, pulverulent, epidermis splitting to expose spores, long covered by ruptured epidermis. Urediniospores (20–)21.5–26(–29.5) × (15–)16–18(–20) μm (mean 23.9 × 17.3 μm), ellipsoidal or obovoid, contents golden yellow; wall 1–1.5(–2) μm thick, hyaline, echinulate, germ pores obscure. Telia not known.
Holotype. New Zealand, Otago Lakes, South Mavora Lake, ca. 630 m, on Olearia bullata, 30 November 2016, E.H.C. McKenzie and M. Padamsee (PDD 110345–II).
Distribution. Endemic to New Zealand (Otago Lakes).
Additional specimens examined. New Zealand, Otago Lakes, South Mavora Lake, ca. 630 m, on Olearia bullata, 30 November 2016, E.H.C. McKenzie and M. Padamsee (PDD 110346–II); South Mavora Lake, ca. 630 m, on O.bullata, 30 November 2016, E.H.C. McKenzie and M. Padamsee (PDD 110353–II).
Notes. The host is an endemic divaricating shrub up to 2 m tall, widespread in eastern South Island from North Canterbury to Southland in montane shrubland and scrub.
Seven other species of rust produce urediniospores on Olearia species in New Zealand. Of these, Uredo brownii Syd. and P. Syd. is the only species to produce epiphyllous uredinia. However, the urediniospores of U. brownii are larger (26–38 × 23–34 μm, mean 29.8 × 27.0 μm) than those of Puccinia oleariae-bullatae. The other species all have hypophyllous uredinia and larger urediniospores.
In the molecular analysis P. oleariae-bullatae was in a sister clade to another species on Olearia (). The sequences of P. oleariae-bullatae (uredinia only; GenBank accession #ON622759–622761) are 100% identical to P. foyana (PDD 119242, aecia specimen only) on Ranunculus enysii suggesting that this may be a fourth heteroecious rust from New Zealand, but further data is needed to confirm this observation. The relationship between the aecia and the telia on the type specimen of P. foyana is uncertain; this is also the only specimen in fungarium PDD that has telia.
Puccinia oleariae-solandri McKenzie and Padamsee, sp. nov.
Figure 20. Puccinia oleariae-solandri on Olearia solandri: A, B, Infected tree (Credit: B Doherty, Target Pests). C, Uredinia. D, Teliospores. Scale bar = 20 μm.

IF557262
Etymology. From the host, Olearia solandri.
Parasitic on leaves and stems of Olearia solandri (Hook. f.) Hook. f. (Asteraceae). Spermogonia and aecia not known. Uredinia not known. Telia mainly hypophyllous, also on stems, especially common on young leaves and stems, amber-gold to amber-brown, circular but becoming elliptical, up to 1 × 0.5 mm, pulverulent. Teliospores (32–)34.5–40.5(–44) × (18–)19–22.5(–23) µm (mean 37.5 × 20.6 µm), 2-celled, ellipsoidal, occasionally subclavate, contents golden, constricted at septum, minutely roughened, straight or slightly curved, apex rounded, sometimes truncate, base rounded, sometimes attenuated; wall (1–)1.5–2(–2.5) µm thick at sides, (1.25–)1.5–3 µm thick at apex, golden, pore in upper cell apical, pore in lower cell in bottom ⅓ of the cell, often quite close to the pedicel; pedicels short, deciduous, hyaline.
Holotype. New Zealand, Mid Canterbury, Christchurch, Bromley Road, Old Bromley School Reserve, on Olearia solandri, 23 November 2005, B. Doherty (PDD 85519–III).
Distribution. Probably endemic to New Zealand (Mid Canterbury).
Additional specimens examined. New Zealand, Mid Canterbury, Christchurch, Bromley Road, Old Bromley School Reserve, on Olearia solandri, 23 November 2005, B. Doherty (PDD 85520–III); Christchurch, Bromley Road, Old Bromley School Reserve, on Olearia solandri, 23 November 2005, B. Doherty (PDD 85522–III).
Notes. The host is a small endemic tree up to 4 m tall. It is found mainly in coastal locations throughout the North Island and to latitude 42°S in the South Island. It is popular in Canterbury nurseries and is used there by landscapers as a shelter tree.
In November 2005 rust was found on a tree in Old Bromley School Reserve, Christchurch. It appeared to be causing dieback and to be killing the tree. It was also suspected to be causing witches’ broom type vegetative growth, although other causes such as eriophyid mites (Aceria mayae is recorded on Olearia furfuracea) and phytoplasmas are both contenders for causing these symptoms. The infected tree was cut down, chipped, and buried on site.
Thirteen species of rust fungi, including nine other species of Puccinia, are now known on Olearia species in New Zealand. The Canterbury rust proved to be a new species of Puccinia that is almost certainly endemic to New Zealand. Hopefully, this rust will have survived somewhere in nature! In the molecular analysis P. oleariae-solandri was in a highly supported (98% MLBS) sister clade to P. oleariae-bullatae, which is known to produce only urediniospores on Olearia bullata ().
The teliospores of P. oleariae-solandri are smaller than those of any of the other seven species of Puccinia with telia recorded on Olearia in New Zealand; P. oleariae-bullatae (previous entry) and P. otira (see below) are not known to produce teliospores. In length they are most similar to those of P. tekapo McNabb (mean 49.3 × 27.5 μm) found on Olearia odorata, although they are smaller. Teliospores of P. tekapo also have a long, persistent, thick-walled pedicel while the pedicel on P. oleariae-solandri is deciduous, short and thin-walled. The teliospores of P. osbornii Samuel, described on Olearia rudis from Australia (Samuel Citation1924), measure 30–54 × 18–25 µm, a comparable size to those of P. oleariae-solandri. However, teliospores of P. osbornii are thickened at the apex, whereas those of P. oleariae-solandri are not. Puccinia osbornii also has pedicels that are usually ⅓–⅔ the length of the spore.
In overall appearance the teliospores resemble those of P. atkinsonii G. Cunn. (mean 47.8 × 26.4 μm), but as well as being smaller there is a different placement of germ pores. Both species have an apical germ pore. However, in P. atkinsonii the germ pore in the lower cell is in the upper ⅓ while in P. oleariae-solandri the germ pore in the lower cell is in the lower ⅓ of the cell (often quite close to the pedicel).
Puccinia oplismeni-hirtelli McKenzie and Padamsee, sp. nov.
Figure 21. Puccinia oplismeni-hirtelli on Oplismenus hirtellus subsp. hirtellus: A, B Uredinia. C, Urediniospores. Scale bar = 20 μm.

IF557269
Etymology. From Oplismenus hirtellus, the host binomial.
Parasitic on leaves of Oplismenus hirtellus (L.) P. Beauv. subsp. hirtellus (Poaceae). Spermogonia and aecia not known. Uredinia amphigenous, scattered, yellow-brown, oval, up to 0.5 × 0.25 mm, restricted in width by leaf veins, pulverulent, epidermis splitting to expose spores, long covered by the epidermis. Urediniospores (25–)28–43(–51) × (24–)26–37(–39.5) μm (mean 35.5 × 30.9 μm), ellipsoidal or subglobose, contents golden yellow; wall 1.5–2.5(–3) μm thick, hyaline, coarsely echinulate, germ pores 2–3, equatorial, obscure. Telia not known.
Holotype. New Zealand, Northland, Whangarei, Whau Valley Earth Dam, on Oplismenus hirtellus subsp. hirtellus, 29 January 2013, E.H.C. McKenzie and M. Padamsee (PDD 103043–II).
Distribution. Possibly endemic to New Zealand (Northland).
Additional specimen examined. New Zealand, Northland, Whangarei, Whau Valley Earth Dam, on Oplismenus hirtellus subsp. hirtellus, 15 March 2013, E.H.C. McKenzie, A. McKenzie and A.J. McKenzie (PDD 103305–II).
Notes. The host is an indigenous grass which, in New Zealand, is found in Kermadec Islands (Raoul Island) and North Cape to Auckland, on shaded slopes and in coastal forest. It is also found in tropical America, Asia, Australia and the South Pacific.
Puccinia oplismeni-hirtelli is characterised by its large urediniospores. Several rust fungi have been described from the genus Oplismenus, including species of Puccinia and Uredo. Of these, three species have large urediniospores: P. inclita Arthur (25–40 × 20–28 μm); P. opipara Cummins (31–43 × 22–31 μm); and U. oplismeni-undulatifolii Z.C. Chen (27–35 × 26–37 μm) (Cummins Citation1971; Chen et al. Citation1980). In general, the urediniospores of P. inclita and P. opipara are narrower than those of P. oplismeni-hirtelli while those of U. oplismeni-undulatifolii are shorter. Farr and Rossman (Citation2022) list six species of rust on O. hirtellus, including P. inclita in the Caribbean. Puccinia flaccida Berk. and Broome occurs on O. hirtellus subsp. imbecillis in the Kermadec Islands (McKenzie Citation1992), but this rust has much smaller, brown urediniospores measuring 21–30.5 × 17–24 μm (mean 24.7 × 20.2 μm). In the molecular analysis P. oplismeni-hirtelli formed a distinct clade with 100% BS support ().
Puccinia otira (G. Cunn.) McKenzie and Padamsee, comb. nov.
Figure 22. Puccinia otira on Olearia arborescens. A, Aecia. B, Uredinia. C, Aeciospores. D, Urediniospores. Scale bars = 20 μm.
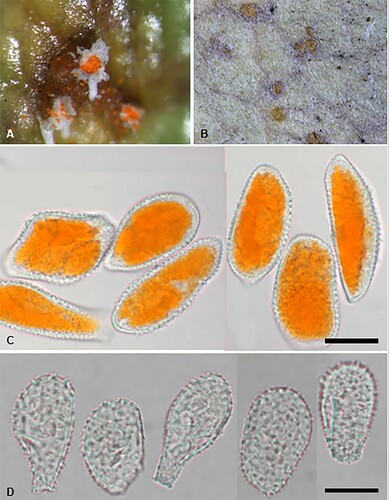
IF557263
≡ Aecidium otira G. Cunn., Transactions and Proceedings of the New Zealand Institute 59: 498, 1928.
Parasitic on Olearia arborescens (G. Forst.) Cockayne and Laing (Asteraceae). Spermogonia mainly epiphyllous, flask-shaped, scattered, immersed, associated with the aecia. Aecia amphigenous, on stems and petioles, most noticeable on upper surface of the leaf, in scattered groups of commonly 3–6, on discoloured spots, 1–2 mm diameter, cupulate, orange, 0.25 mm diameter; peridia white, tubiform, up to 2 mm high, becoming lacerate and revolute. Aeciospores (34–)38–50(–57) × (18.5–)22–30(–32) μm (mean 44.4 × 25.6 μm), oval, ellipsoidal or obovoid, contents bright yellow; wall 1–1.5 μm thick, hyaline, verrucose. Uredinia hypophyllous, scattered or in small groups, circular or elliptical, 0.25–0.5 mm diameter, immersed in leaf tissue, surrounded by split epidermis, orange-yellow, rapidly fading with age, pulverulent. Urediniospores (29–)32–45(–49.5) × (19–)22–27.5 μm (mean 38.7 × 23.9 μm), ellipsoidal, obovoid or pyriform; wall 1–2(–2.5) μm thick, hyaline, coarsely verrucose, germ pores obscure. Telia not known.
Typification. New Zealand, North Canterbury, Arthur's Pass, 900 m, on Olearia arborescens, January 1928, G.H. Cunningham (PDD 38523–I, holotype of Aecidium otira). New Zealand, North Canterbury, Temple Basin track to skifield, on Olearia arborescens, 8 February 2011, E.H.C. McKenzie, M. Padamsee and M. Korver (PDD 101907–II, epitype designated here, IF559664, GenBank Accession #ON622748).
Distribution. Endemic to New Zealand (Taranaki, Taupo, Wellington, Buller, Westland, North Canterbury, Mackenzie, Otago Lakes, Stewart Island).
Additional specimens examined. New Zealand, Taranaki, Mt Egmont, 1200 m, on Olearia arborescens, 20 April 1946, J.M. Dingley (PDD 4519–I). Taupo, National Park, Silica Springs, on O. arborescens, 24 March 1984, E.H.C. McKenzie (PDD 45059–I). Wellington, Tararua Ranges, North of Dundas Hut, on O. arborescens, 10 February 1985, G.W. Ramsay (PDD 47103–I); Tararua Ranges, North of Dundas Hut, on O. arborescens, 11 February 1985, P.R. Johnston (PDD 47106–0, I). North Canterbury, Temple Basin track to skifield, on O. arborescens, 8 February 2011, E.H.C. McKenzie, M. Padamsee and M. Korver (PDD 101908–II, GenBank Accession #ON622747). Otago Lakes, road to Milford Sound, track to Key Summit, on O. arborescens, 14 February 2013, E.H.C. McKenzie and M. Padamsee (PDD 103141–I, GenBank Accession #ON622749).
Notes. The host is a shrub or small tree up to 4 m tall found from latitude 38°S southwards in coastal lowland to montane forest and scrubland. Cunningham (Citation1924b) initially recorded this rust as Puccinia novae-zelandiae G. Cunn. [non, P. novo-zelandica Bubák, 1901] on Olearia arborescens, but later (Cunningham Citation1928) described it as Aecidium otira. Only aecia and spermogonia were known until 2011 when uredinia were found near Arthur’s Pass, which is near the site of the holotype collection of Aecidium otira. A molecular analysis showed that the uredinial and aecial collections belong to the one species (telia have not been found) () and they are sister to P. perlaevis G. Cunn. on Olearia lacunosa. This clade is sister to a highly supported clade (100% MLBS) consisting of P. akiraho G. Cunn. on Olearia avicenniifolia and P. keae G. Cunn. on Olearia species. Puccinia akiraho is a microcyclic species that produces aecia and telia on several species of Olearia, including O. arborescens, while P. keae produces only telia. The aeciospores of P. akiraho are smaller than those of P. otira (mean 35.1 × 20.9 μm vs. 44.4 × 25.6 μm). Puccinia otira appears to be restricted to O. arborescens and probably occurs wherever the host grows, having been found from Taranaki and Taupo southwards to Stewart Island.
A key is provided below to those rust fungi that are known to produce uredinia on Olearia species in New Zealand.
Key to rust fungi that produce urediniospores on Olearia spp. in New Zealand
Urediniospores smooth, 31–57 × 16–26 μm (mean 38.6 × 19.7 μm), fusiform, ellipsoidal or broadly ellipsoidal, often pointed at one or both ends; wall (1–)1.5–2.5(–3) μm thick at sides, (3–)4–6(–8) μm thick at apex Puccinia perlaevis
Urediniospores echinulate or verrucose; wall not thickened at apex
Wall of urediniospore relatively thick (usually more than 3 μm), coarsely and sparsely echinulate 3
Wall of urediniospore thinner (less than 3 μm) 5
Uredinia epiphyllous. Urediniospores 26–38 × 23–34 μm (mean 29.8 × 27.0 μm); wall
3.5–6(–7.5) μm thick Uredo brownii
Uredinia hypophyllous. Mean dimensions of urediniospores more than 40 × 30 μm
Urediniospores 38–56 × 32–45 μm (mean 45.0 × 39.3 μm); wall (1.5–)3–6(–7) μm thick Uredo tupare
Urediniospores 30.5–63 × 25.5–38.5 μm (mean 41.9 × 31.3 μm); wall (1.5–)2.5–4(–5.5) μm thick Uredo oleariae
Uredinia epiphyllous. Urediniospores 20–29.5 × 15–20 μm (mean 23.9 × 17.3 μm), ellipsoidal or obovoid; wall 1–1.5(–2) μm thick, echinulate, germ pores obscure Puccinia oleariae-bullatae
Uredinia hypophyllous. Urediniospores larger
Urediniospores 29–49.5 × 19–27.5 μm (mean 38.7 × 23.9 μm), ellipsoidal, obovoid or pyriform; wall 1–2(–2.5) μm thick, finely echinulate, germ pores obscure Puccinia otira
Urediniospore mean dimensions less than 30 × 22 μm, germ pores equatorial 7
Urediniospores 21–31 × 17–24 μm (mean 26 × 21 μm), obovoid or subglobose; wall 1.25–1.75 μm thick, echinulate, 2 germ pores Puccinia tekapo
Urediniospores 21.5–37 × 15.5–24.5 μm (mean 28.1 × 20.3 μm), subglobose or obovoid; wall (0.5–)1(–1.5) μm thick, verrucose, 4–6 germ pores Puccinia moschata
Puccinia ozothamni McKenzie and Padamsee, sp. nov.
Figure 23. Puccinia ozothamni on Ozothamnus leptophyllus: A, B, Uredinia. C, D, Urediniospores (C, light microscope, D, scanning electron microscope). Scale bars C = 20 μm; D = 10 μm.

IF557270
Etymology. From Ozothamnus, the host genus.
Parasitic on leaves of Ozothamnus leptophyllus (G. Forst.) Breitw. and J.M. Ward (Asteraceae). Spermogonia and aecia not known. Uredinia hypophyllous, scattered, golden, elliptical or subglobose, embedded within leaf tomentum, but becoming exposed and pulverulent, 0.25–0.5 mm long. Urediniospores (20–)23–28(–31.5) × (15–)17–23(–24) μm (mean 26.3 × 20.1 μm), globose, subglobose, obovoid or ellipsoidal, contents yellow; wall 1.0–2.0 μm thick, hyaline, moderately echinulate, germ pores obscure. Telia not known.
Holotype. New Zealand, Auckland, Piha south, Lookout Track, on Ozothamnus leptophyllus, 21 February 2014, N.A. Martin (PDD 104204–II, GenBank Accession #ON622771).
Distribution. Endemic to New Zealand (Auckland).
Additional specimen examined. New Zealand, Auckland, Piha south, Lookout Track, on Ozothamnus leptophyllus, 27 October 2013, N.A. Martin (PDD 103329–II).
Notes. The host is an endemic shrub widely distributed throughout New Zealand from coastal dunes and lowland to subalpine scrubland and grassland. It is a highly polymorphic species.
The demicyclic rust Puccinia lagenophorae, which occurs in New Zealand on various species of Asteraceae, has been recorded on Ozothamnus cordatus in Australia but it lacks urediniospores. In a DNA molecular analysis () P. ozothamni was recovered in a clade (<60% MLBS) with seven other Puccinia and Uromyces species.
Puccinia phormii (G. Cunn.) McKenzie and Padamsee, comb. nov.
IF559593
≡ Uredo phormii G. Cunn., Transactions and Proceedings of the New Zealand Institute 55: 42 [Latin diagnosis p. 54], 1924.
Cunningham (Citation1924a, page 42) described Uredo phormii and gave the following details for the type: on Phormium tenax Forst., Plimmerton (Wellington), 20 m, 16 January 1922, R. Waters, H. Drake and G.H. Cunningham, Herb. Nos. 755, 775. Interestingly, Cunningham (Citation1924a, page 54) gave a description of the rust in Latin, with different collecting details that match those of PDD 757. The description was published before a Latin description became obligatory (1 January 1935) and thus, PDD 775 is selected from the syntypes and designated here as the lectotype (IF559665) of Aecidium phormii. Although only uredinia are known, the opportunity is taken to transfer the species to an appropriate genus, Puccinia.
Puccinia pittospori McKenzie and Padamsee, sp. nov.
Figure 24. Puccinia pittospori on Pittosporum crassifolium: A–C, Uredinia. D, E, Urediniospores. Scale bars = 20 μm.
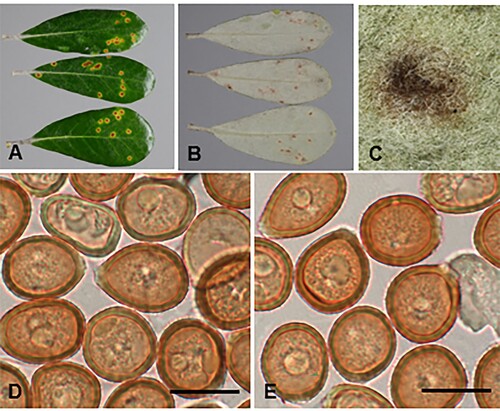
IF557271
Etymology. From Pittosporum, the host genus.
Parasitic on leaves of Pittosporum crassifolium Banks and Sol. ex A. Cunn. (Pittosporaceae). Spermogonia and aecia not known. Uredinia hypophyllous, with a circular or irregular brown spot up to 3 mm diameter and yellow halo on upper surface, embedded in leaf tomentum, often with flap of epidermis, scattered, sparse, dark brown, elliptical or oval, 0.5–1 mm long, pulverulent. Urediniospores 23–28(–36) × (18.5–)19–23(–24) μm (mean 25.8 × 21.0 μm), ellipsoidal, broadly ellipsoidal or subglobose, contents reddish brown; wall 1.5–3 μm thick, reddish brown, moderately echinulate, germ pores 2, equatorial, conspicuous. Telia not known.
Holotype. New Zealand, Auckland, Muriwai Beach, on Pittosporum crassifolium, 7 April 2018, N.A. Martin (PDD 111591–II, GenBank Accession #ON622764).
Distribution. Endemic to New Zealand (Auckland).
Additional specimens examined. New Zealand, Auckland, Eastern Beach, Rogers Park, on Pittosporum crassifolium, 6 April 2018, N.A. Martin (PDD 111592–II, GenBank Accession #OR760478); Eastern Beach, Rogers Park, on P. crassifolium, 13 April 2018, N.A. Martin (PDD 111594–II); Panmure Basin, on P. crassifolium, 17 April 2018, N.A. Martin (PDD 111596–II); Orewa, Arundel Reserve on P. crassifolium, 1 May 2018, N.A. Martin (PDD 116988–II, GenBank Accession #OR760479).
Notes. The host is a shrub or small tree found in forest margins and coastal scrub from North Cape to Poverty Bay. It is widely cultivated.
Farr and Rossman (Citation2022) list four species of rust on Pittosporum spp. Of these, Melampsora euphorbiae (Ficinus and C. Schub.) Castagne, which was recorded on Pittosporum sp. from Azores, is known from New Zealand on Euphorbia spp. However, the present rust is not a species of Melampsora. Nyssopsora citriobati Syd., which was described on Citriobatus spinescens [ = Pittosporum multiflorum] from Queensland (Sydow Citation1938), is known from only the type specimen and produces only teliospores (Loshomboon et al. Citation1990). In those species of Nyssopsora that produce urediniospores, germ pores have not been seen (Loshomboon et al. Citation1990). Two species of Uromyces have been described on Pittosporum abyssinicum; Uromyces kigesianus Cummins from Uganda and U. pittospori Henn. from Eritrea. Uromyces kigesianus produces only teliospores, and although teliospores were described for U. pittospori (Hennings Citation1891), Sydow and Sydow (Citation1908) stated that they were lacking in the type specimen. Hennings (Citation1891) and Sydow and Sydow (Citation1908) described the urediniospores of U. pittospori as being 18–30 × 14–25 μm with a thin wall (1.5–2 μm). These measurements are similar to those recorded for the New Zealand fungus. However, it would seem unlikely that the New Zealand rust is conspecific with an African rust. Uromyces pittospori is not associated with any leaf spotting. In LSU DNA analysis P. pittospori formed a highly supported (99% MLBS) monophyletic clade within the genus Puccinia ().
Puccinia scariosa (Berk.) McKenzie and Padamsee, comb. nov.
IF559595
≡ Uromyces scariosus Berk., in Hooker [as scariosa], Flora Novae-Zelandiae. Part II. Flowerless Plants. The Botany of the Antarctic Voyage II: 195, 1855.
≡ Uredo scariosa (Berk.) G. Cunn. [as scariosus], Transactions and Proceedings of the New Zealand Institute 58: 48, 1927.
= Puccinia geranii-pilosi McAlpine, Rusts of Australia: 179, 1906.
The type specimen of Uromyces scariosus held at Kew consists of four leaves and the isotype (PDD 40803) of only one leaf of Geranium sp. The original description of Uromyces scariosus was based only on urediniospores, and hence Cunningham (Citation1927) proposed the name Uredo scariosa. Later, Cunningham (Citation1931) synonymised Uredo scariosa with Puccinia geranii-pilosi, described from Australia. Following recognition of the 2-celled teliospores and applying the Shenzhen Code (Turland et al. Citation2018), the original oldest name, Uromyces scariosus, is combined into the genus Puccinia.
Puccinia scirpi-nodosi (McAlpine) McKenzie and Padamsee, comb. nov.
IF559666
≡ Uredo scirpi-nodosi McAlpine, Rusts of Australia: 202, 1906.
= Puccinia austrina McKenzie, Mycoscience 49: 4, 2008.
Following the Shenzhen Code (Turland et al. Citation2018) the original, oldest name, Uredo scirpi-nodosi, is combined into the genus Puccinia.
Typification. Australia, Victoria, Mordialloc, on Scirpus nodosus, December 1885, leg. Reader (holotype). New Zealand, Campbell Island, Mt Honey, on Isolepis habra, 7 March 2000, E.H.C. McKenzie (PDD 87917–II, III, holotype of Puccinia austrina, designated here as the epitype of P. scirpi-nodosi, IF901207). The sequence of P. scirpi-nodosi (GenBank accession # PP594478) was sister to that of P. ozothamnus ().
Puccinia tupare (G. Cunn.) McKenzie and Padamsee, comb. nov.
IF901899
≡ Uredo tupare G. Cunn., Transactions and Proceedings of the New Zealand Institute 55: 44 [Latin diagnosis p. 55], 1924.
Cunningham (Citation1924a) described this rust as Uredo tupare on Olearia colensoi Hook. f. (Asteraceae). Thirteen species of rust fungi are known on Olearia species in New Zealand. Seven of these species have been sequenced, and in the molecular analysis lie in four closely related subclades along with Puccinia foyana G. Cunn., P. hectorensis G. Cunn. and P. wharanui (). Of the latter three species, P. foyana produces spermogonia, aecia and telia on Ranunculus enysii Kirk (Ranunculaceae; Cunningham Citation1924a) while P. hectorensis produces aecia and telia on tree daisies (Brachyglottis spp., Asteraceae; Cunningham Citation1931). Puccinia wharanui produces uredinia on Pachystegia insignis (Hook. f.) Cheeseman (syn. Olearia insignis Hook. f.; Cunningham Citation1924a). Puccinia tupare (PDD 101615, GenBank Accession number ON622746) is recovered in a supported clade (72% MLBS) sister to P. hectorensis.
Puccinia wharanui McKenzie and Padamsee, comb.nov.
IF901900
≡ Uredo wharanui G. Cunn., Transactions and Proceedings of the New Zealand Institute 55: 46 [Latin diagnosis p. 55], 1924.
Cunningham (Citation1924a) described Uredo wharanui on Pachystegia insignis, a plant originally described as Olearia insignis. Although teliospores are unknown for this rust, in the phylogenetic analysis it (PDD 69122, GenBank Accession #ON622745) was recovered in a well-supported clade (96% MLBS) sister to the well-supported clade (comprising two other Puccinia species from Olearia, along with P. foyana on Ranunculus (). Morphologically P. wharanui is like several of these species in possessing large urediniospores with a relatively thick wall, obscure germ pores and being prominently echinulate.
Pucciniastrum dingleyae McKenzie and Padamsee, sp. nov.
Figure 25. Pucciniastrum dingleyae on Gaultheria rupestris. A, B, Uredinia. C, Urediniospores. Scale bar = 20 μm.
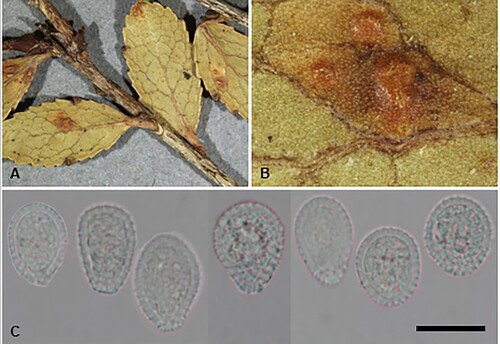
IF557264
Etymology. Named for Joan Marjorie Dingley (14 May 1916–1 January 2008), New Zealand mycologist, collector of the type specimen.
Parasitic on Gaultheria rupestris (L. f.) D. Don (Ericaceae). Spermogonia and aecia not known. Uredinia hypophyllous, scattered or 2–4 pustules grouped on a discoloured spot up to 1 cm in diameter, orbicular or elliptical, up to 1 mm diameter, opening initially through an apical pore that soon splits wider, erumpent, pulverulent. Urediniospores (17–)22–27(–31) × 16–19(–20.5) μm (mean 24.0 × 17.9 μm), subglobose, obovoid or ellipsoidal, sometimes somewhat angular; wall (1.5–)2–2.5(–3) μm thick, hyaline, echinulate, germ pores obscure. Telia not known.
Holotype. New Zealand, North Canterbury, Arthur’s Pass, Avalanche Peak, 915 m, on Gaultheria rupestris, 16 January 1955, J.M. Dingley (PDD 34549–II).
Distribution. Endemic to New Zealand (North Canterbury).
Notes. The host is an endemic shrub up to about 1 m tall. It is restricted to the South Island and is found in montane to lower subalpine habitats, descending to near sea level in the south, including fellfield, shrubland and open places.
At least three species of Pucciniastrum and another rust, Chrysomyxa chiogenis Dietel, have been reported on Gaultheria species, from Canada and India. Of these, P. goeppertianum (J.G. Kühn) Kleb. does not produce urediniospores. The other two Pucciniastrum species produce slightly narrower urediniospores (P. gaultheriae Syd. and P. Syd., 18–32 × 12–18 μm; P. pyrolae (J.F. Gmel.) J. Schröt., 23–42 × 10–19 μm) than those of P. dingleyae, while C. chiogenis has verrucose urediniospores.
Pucciniastrum healyi McKenzie and Padamsee, sp. nov.
Figure 26. Pucciniastrum healyi on Fuchsia magellanica: A, Uredinial paraphyses. B, Urediniospores. Scale bars = 20 μm.
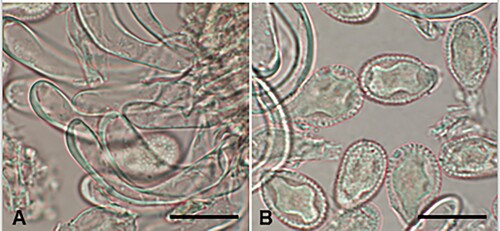
IF559596
Etymology. In recognition of Arthur J. Healy, botanist (1917–2011), who contributed many specimens of rust fungi to Fungarium PDD, including the type of Pucciniastrum healyi.
Parasitic on leaves of Fuchsia magellanica Lam. (Onagraceae). Spermogonia and aecia not known. Uredinia hypophyllous, scattered, circular, ca. 0.5 mm diameter, pulverulent; paraphyses numerous, peripheral, incurved, cylindrical, hyaline or very pale yellow, up to 70 μm long, 10–15(–20) μm wide, wall 0.5–1 μm thick but thickened to 3 μm on outside edge of curve. Urediniospores 22.5–27(–29) × (13.5–)15–17(–20) μm (mean 24.9 × 16.0 μm), obovoid; wall 1–1.5 μm thick, hyaline, moderately echinulate, germ pores obscure. Telia not known.
Holotype. New Zealand, Mid Canterbury, Christchurch, Riccarton, on Fuchsia magellanica, 28 April 1994, A.J. Healy (PDD 63488–II).
Distribution. Introduced to New Zealand (Mid Canterbury).
Notes. Fuchsia magellanica is a widely cultivated, introduced ornamental shrub, which can also be found in the wild.
McKenzie and Dingley (Citation1996) recorded this rust as Uredo fuchsiae (Cooke) Henn., a species that has now been synonymised with Mikronegeria fuchsiae. The urediniospores of Pucciniastrum healyi are similar in size to those of M. fuchsiae (mean 21.1 × 16.3 μm), but M. fuchsiae lacks paraphyses in the uredinia. The urediniospore wall of P. healyi is of uniform thichness, whereas in M. fuchsiae it is often thickened at the apex (up to 7 μm thick) and the spore ornamentation is more pronounced, especially towards the apex. The illegitimate Uredo fuchsiae Arthur and Holw. [≡ Pucciniastrum fuchsiae Hirats. f.] was described on Fuchsia splendens and Lopezia hirsuta in Guatemala (Arthur Citation1918). However, it lacks paraphyses and its urediniospores are smaller (18–24 × 13–16 μm). Pucciniastrum circaeae (Schumach.) Speg., which is widespread on Circaea species in Europe, has been recorded on Fuchsia magellanica and Fuchsia × hybrida in Italy and Chile, respectively (Garibaldi et al. Citation2012; Ferrada et al. Citation2020). In a phylogenetic study this fungus formed a sister clade to P. epilobii (Scholler et al. Citation2022). However, it also lacks paraphyses and has smaller, subglobose or ellipsoidal urediniospores (16–24.5 × 12.5–16.5 μm).
Uromyces bolboschoeni McKenzie and Padamsee, sp. nov.
Figure 27. Uromyces bolboschoeni on Bolboschoenus fluviatilis: A, B, Uredinia and telia. C, Urediniospores. D, Teliospores. Scale bars = 20 μm.
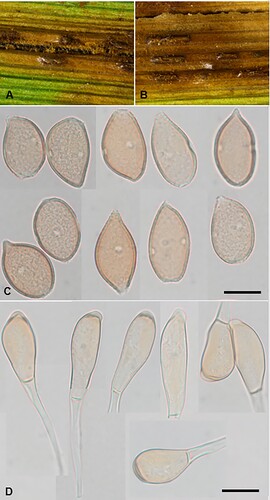
IF557272
Etymology. From the host genus, Bolboschoenus.
Parasitic on leaves of Bolboschoenus fluviatilis (Torr.) Soják (Cyperaceae). Spermogonia and aecia not known. Uredinia on abaxial surface, amber or brown, restricted in width by leaf veins, linear, mainly 0.5–1.5 mm long, but coalescing in streaks up to 5 mm long, covered by epidermis which splits longitudinally, pulverulent. Urediniospores (31–)35–45(–49) × (18.5–)20–26(–27.5) μm (mean 39.0 × 23.0 μm), ellipsoidal, oval or obovoid, base often noticeably flattened at point of attachment, apex rounded or acute, sometimes apiculate, contents pale brown; wall 1–1.5(–2) μm thick, yellow-brown, verrucose, germ pores 3(–4), equatorial. Telia on abaxial surface, restricted in width by leaf veins, linear, mainly 0.5–1.5 mm long, up to 0.6 mm in diameter, scattered, reddish brown. Teliospores (34–)40–54(–58) × (15–)16–18(–20.5) μm (mean 46.3 × 17.2 μm), 1-celled, oblong-ellipsoidal, clavate or fusiform, straight or slightly curved, often with an acute or rounded apical papilla, contents very pale brown; wall 0.5–1 μm thick at sides, 2.5–5 μm thick at apex, pale yellow-brown, smooth; pedicels sometimes detached but usually long and up to 65 × 4–7 μm, tending to taper slightly towards base, hyaline.
Holotype. New Zealand, Auckland, Te Henga, banks of Waiti Stream, near carpark for Lake Wainamu Track, on Bolboschoenus fluviatilis, 9 October 2019, E.H.C. McKenzie and A. McKenzie (PDD 116792–II, III, GenBank Accession #ON622762).
Distribution. Endemic to New Zealand (Auckland).
Additional specimen examined. New Zealand, Auckland, Te Henga, banks of Waiti Stream, near carpark for Lake Wainamu Track, on Bolboschoenus fluviatilis, 17 July 2019, E.H.C. McKenzie and A. McKenzie (PDD 116791–II).
Notes. The host plant grows most commonly in the northern part of the North Island, especially near the coast, and it is usually found on the margin of small freshwater lakes, ponds and streams. It is native to New Zealand, but also occurs in North America, Asia and Australia.
Two rust fungi are known on Bolboschoenus fluviatilis [as Scirpus fluviatilis]; Puccinia obtecta Peck from Australia, based on a specimen held in BPI (BPI 088298; Farr and Rossman Citation2022), and Uromyces scirpi-maritimi Hirats. f. and Yoshin. (Hiratsuka et al. Citation1992). Puccinia obtecta has been widely recorded on species of Schoenoplectus and Schoenoplectiella from North America, West Indies, China and Indonesia. Uromyces scirpi-maritimi is known from Japan and maritime areas of Siberia. Uromyces bolboschoeni has larger urediniospores than described for P. obtecta (19–26 × 14–18 μm, Cummins Citation1940; 25–35 × 13–20 μm, Arthur Citation1934) or for U. scirpi-maritimi (27–40 × 14–20 μm, Hiratsuka et al. Citation1992). It also has broader teliospores than U. scirpi-maritimi (32–68 × 12–16 μm, Hiratsuka et al. Citation1992).
Several other rusts have been recorded on Bolboschoenus maritimus (from Europe or Russia) and on B. medianus (USA, New Zealand) (e.g. Puccinia austrina [ = P. scirpi-nodosi], P. obtectella Cummins, P. scirpi DC., Uromyces lineolatus (Desm.) J. Schröt.), but these all have smaller urediniospores. The urediniospores of U. bolboschoeni are also very distinctive in shape. Urediniospores of U. bolboschoeni usually have three equatorial germ pores whereas those of P. obtectella, which is recorded on B. medianus in New Zealand, have only two equatorial pores. Puccinia obtectella also has much smaller urediniospores (mean 23.4 × 15.8 μm, pers. obs.). Uromyces bolboschoeni was recovered in a clade (<60% MLBS) with seven other Puccinia and Uromyces species ().
Uromyces haumata (G. Cunn.) McKenzie and Padamsee, comb. nov.
IF901191
≡ Uredo haumata G. Cunn., Transactions and Proceedings of the New Zealand Institute 59: 499, 1928.
= Uromyces mcnabbii Cummins, Rust Fungi of Cereals, Grasses and Bamboos: 483, 1971.
Cummins (Citation1971) observed telia on a specimen of Chionochloa rubra collected by R.F.R. McNabb in 1961 and described the rust as Uromyces mcnabbii. However, following the Shenzhen Code (Turland et al. Citation2018), the original, oldest name, Uredo haumata, is combined into the genus Uromyces.
Typification. New Zealand, Canterbury, Mt Cook, Tasman Moraine, 800 m, on Danthonia cunninghamii [probably misdetermination of Chionochloa flavescens], January 1928, G.H. Cunningham [PDD 3395–II, holotype]. New Zealand, Canterbury [Buller], Boyle River, on Danthonia raoulii var. rubra [now, Chionochloa rubra], 23 February 1961, R.F.R. McNabb (PUR F16460, holotype of Uromyces mcnabbii; isotype PDD 19862–II, III).
Notes. Uromyces haumata is recovered in a well-supported (94% MLBS) clade that is sister to a rust on Psychrophila novae-zealandiae ().
Uromyces novae-zelandiae McKenzie and Padamsee, sp. nov.
Figure 28. Uromyces novae-zelandiae on Sarcocornia quinqueflora subsp. quinqueflora: A, C, Uredinia. B, Urediniospores. D, Teliospores. Scale bars = 20 μm.
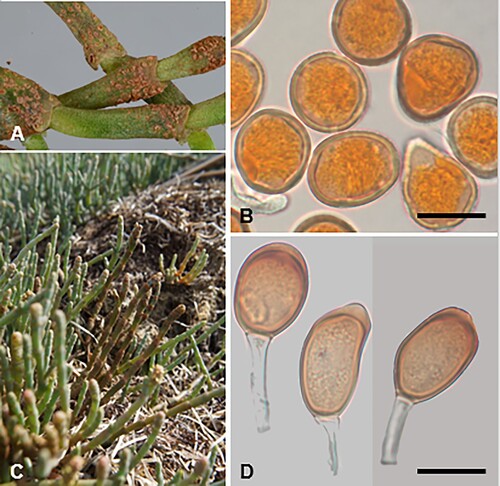
IF557274
= Uredo salicorniae G. Cunn., Transactions and Proceedings of the New Zealand Institute 61: 417, 1930 (provisional name) [non, Uredo salicorniae (DC.) Spreng., 1827.]
Etymology. From New Zealand, the country where the holotype specimen was discovered.
Parasitic on Sarcocornia quinqueflora (Bunge ex Ung.-Sternb.) A.J. Scott subsp. quinqueflora (Chenopodiaceae). Spermogonia and aecia not known. Uredinia amphigenous and on stems and bracts, scattered, amber, elliptical, up to 0.6 × 0.4 mm, bullate, becoming pulverulent, long covered by the epidermis. Urediniospores 25.5–32.5(–34.5) × (20–)21–28(–30) μm (mean 29.7 × 24.6 μm), subglobose or broadly ellipsoidal, contents bright golden yellow; wall 2.25–3 μm thick, pale yellow, moderately echinulate, germ pores 10–12, scattered, with inconspicuous caps; intermixed with numerous, hyaline, cylindrical paraphyses. Telia amphigenous and on stems and bracts, scattered, dark brown, circular or elliptical, up to 0.25 mm diameter, bullate, compact, long covered by the epidermis. Teliospores (22.5–)27–36(–38.5) × 16.5–20(–24) μm (mean 32.3 × 18.4 μm), 1-celled, ellipsoidal, or oval, contents pale tan brown; wall (1–)1.5–2(–2.5) μm thick at sides, 2–4(–5) μm thick at apex, pale tan brown, slightly darker at apex; pedicels up to 45 × 3.5–6.5 μm, thin-walled, hyaline.
Holotype. New Zealand, Dunedin, Otago Peninsula, Okia Flat, on Sarcocornia quinqueflora subsp. quinqueflora, 1 February 2015, M. Padamsee and E.H.C. McKenzie (PDD 105326–II, III, GenBank Accession #ON622755).
Distribution. Endemic to New Zealand (Auckland, Mid Canterbury, Dunedin).
Additional specimens examined. New Zealand, Auckland, Rangitoto Island, on Sarcocornia quinqueflora subsp. quinqueflora, 21 January 1956, S.D. Brook (PDD 15750–II). Mid Canterbury, Lake Ellesmere, coastal salt meadow, on S. quinqueflora subsp. quinqueflora, January 1929, H.H. Allan (PDD 9812–II, ‘holotype’ of Uredo salicorniae).
Notes. The host, which is a succulent subshrub, is indigenous to New Zealand, New Caledonia, and Australia. It is found throughout New Zealand in saltmarsh, and on coastal rocks and cliffs.
The rust was recorded from New Zealand by Cunningham (Citation1930) as Uredo salicorniae. However, Cunningham (Citation1930, 417) stated:
This may be the uredostage of Uromyces salicorniae de Bary, the only rust recorded on this host genus, but as only uredospores are present on the specimens, and as these do not altogether agree with the published descriptions of this species, our form has been provisionally named as new.
Uromyces novae-zelandiae is known from only three collections and is designated ‘nationally critical’ in the New Zealand Threat Classification System (Hitchmough Citation2002). The Dunedin specimen collected in 2015 had both urediniospores and teliospores of a Uromyces. Urediniospores of Uromyces salicorniae (DC.) de Bary described from Europe on various Sarcocornia spp., although of a similar size (22–35 × 18–25 μm) have only four germ pores tending +/− equatorial and narrower walls (about 1.5 μm thick) (Săvulescu Citation1953; Gäumann Citation1959; Wilson and Henderson Citation1966). The teliospores of the two fungi are similar in size. In the molecular analysis U. novae-zelandiae was recovered as sister (<60% MLBS) to a highly supported clade (99% MLBS) consisting of U. chenopodii-fruticosi (DC.) M. Abbasi and Aime (), both species infecting Chenopodiaceae.
Uromyces parietariae McKenzie and Padamsee, sp. nov.
Figure 29. Uromyces parietariae on Parietaria debilis. A, B, Aecia. C, Aeciospores. Scale bar = 20 μm.
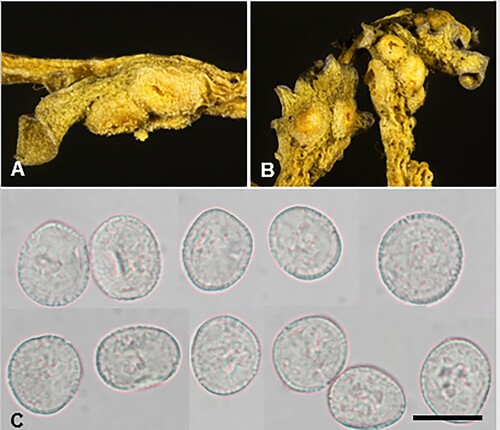
IF557257
Etymology. From the host genus, Parietaria.
Parasitic on leaves of Parietaria debilis G. Forst. (Urticaceae). Spermogonia not known. Aecia on small accessory leaves, not on main leaves, single or 2–5 in a group, bullate, with a slit-like opening, yellow, up to 2 × 1 mm. Aeciospores (22–)22.5–26(–28) × (18–)20–23(–25) μm (mean 24.3 × 21.2 μm), subglobose or ellipsoidal; wall (0.5–)1(–1.5) μm thick, hyaline, verruculose. Uredinia and telia not known.
Holotype. New Zealand, Chatham Islands, Rekohu, Tuku Valley, on Parietaria debilis, 19 November 1992, E.H.C. McKenzie and P.R. Johnston (PDD 61860–I, GenBank Accession #OR760492).
Distribution. Endemic to New Zealand (Chatham Islands).
Notes. This rust was recorded as Aecidium sp. by McKenzie and Johnston (Citation1999). The host plant is indigenous and widespread in New Zealand, occurring in coastal and lowland sites, especially forest margins and open shrubland. It is also indigenous through much of the Southern Hemisphere. Pekel and Azaz (Citation2003) recorded Uromyces tenuicutis McAlpine on Parietaria judaica from Turkey. The telial state of U. tenuicutis occurs on the grass genus Sporobolus, and Cummins (Citation1971) stated that aecia were unknown for this rust. Presumably Pekel and Azaz found aecia on P. judaica, although this was not stated. Members of Urticaceae are aecial hosts for numerous rust fungi. In LSU DNA analyses U. parietariae is sister to (<60% MLBS) a rust on Rytidosperma unarede ().
Uromyces plantaginis-variae (McAlpine) McKenzie and Padamsee, comb. nov.
Figure 30. Uromyces plantaginis-variae on Plantago lanigera (A) and P. triandra (B): A, B, Aecia. C, Aeciospores. D, Teliospores. Scale bars = 20 μm.
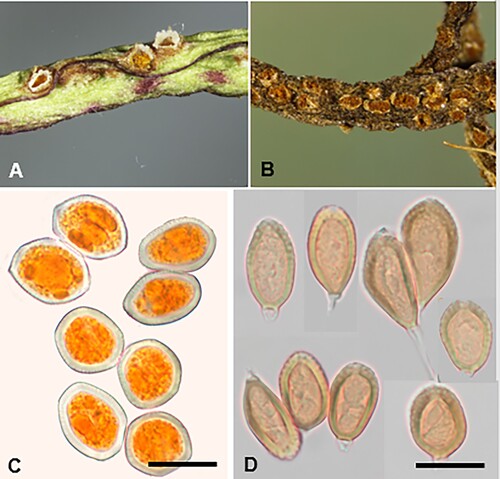
IF557273
≡ Aecidium plantaginis-variae McAlpine, Rusts of Australia: 195, 1906.
Parasitic on leaves of Plantago spp. (Plantaginaceae). Spermogonia amphigenous, numerous. Aecia amphigenous but mainly hypophyllous, cupulate, scattered, sometimes aggregated, orange-yellow, 0.2–0.5 mm diameter; peridia orange, slightly lacerate and revolute at the top. Aeciospores (17–)19–28 × (15–)16–21(–24) μm (mean 23.4 × 18.9 μm), globose, subglobose, ellipsoidal or oblong-ellipsoidal, sometimes somewhat angular or squarish, contents pale yellow; wall 1–2(–2.5) μm thick, hyaline, finely verrucose. Uredinia not known. Telia hypophyllous, surrounded by ruptured epidermis, exposed, scattered, circular or oval, reddish brown, up to 0.6 mm diameter Teliospores (21–)24–30(–32) × (15.5–)17–22(–24) μm (mean 26.5 × 19.7 μm), 1-celled, globose, subglobose, oblong-ellipsoidal, ellipsoidal or broadly ellipsoidal, contents pale yellow; wall 1.5–2 μm thick at sides, 3–6 μm thick at apex, golden yellow, warted but less so towards base; pedicels up to 25 × 2.5–4 μm, thin-walled, collapsing, hyaline.
Typification. Australia, Victoria, Mt Blackwood, on Plantago varia, 27 March 1905, C.C. Brittlebank (VPRI 4387a–0, I, lectotype designated here, IF559668; DAR 33280, isolectotype). New Zealand, Mackenzie, Lake Tekapo, Godley River Valley, on Plantago triandra, 20 December 1958, D. Scott (PDD 18850–I, III, epitype designated here, IF559669).
Distribution. Indigenous to New Zealand (Taranaki, Westland, Mid Canterbury, South Canterbury, Mackenzie, Otago Lakes, Central Otago) and Australia.
Additional specimens examined. New Zealand, Taranaki, Mt Egmont, on Plantago triandra Berggr., February 1929, H.H. Allan (PDD 3436–I). Westland, Landsborough Valley, on P. lanigera Hook. f., 14 February 2003, P. Knightbridge (PDD 78014–I, GenBank Accession #OR760473). Mid Canterbury, Castle Hill, on P. spathulata Hook. f., January 1928, G.H. Cunningham (PDD 3102–I). South Canterbury, Burke’s Pass, on P. spathulata, November 1919, W.D. Reid (PDD 275–I). Mackenzie, Sebastopol Range, on P. triandra, January 1928, G.H. Cunningham (PDD 3106–I, III). Central Otago, Remarkables, head of Wye Creek, 1940m, on P. lanigera, 23 February 2017, D. Lyttle (PDD 110379–I, GenBank Accession #ON622753); Ohau skifield, on P. lanigera, 24 January 2018, D.J. Lyttle (PDD 110628–0, I).
Notes. The host plants are all native to New Zealand; Plantago lanigera also occurs in New Guinea. This rust was first described as Aecidium plantaginis-variae on P. varia from Australia by McAlpine (Citation1906), but he listed syntypes and hence an appropriate lectotype is selected from among his specimens. Recent examination of New Zealand specimens held in Fungarium PDD revealed telia, as well as aecia, on two specimens collected in 1928 and 1958. LSU sequences of PDD 78014 and 110379 (aecial-only specimens) are 99.5% identical and are recovered in a clade (<60% MLBS) sister to U. danthoniae (). McAlpine (Citation1906) gave a similar description for the aecial stage to that reported here. He cited the aeciospores as measuring 22–25(–28) × 16–23 μm. Cunningham (Citation1928) recorded the aecial stage of the rust in New Zealand on Plantago brownii, a synonym of P. triantha, a species found only in the Auckland Islands and in Australia (Tasmania). The specimens on which this record was based have been redetermined as P. triandra.
A microcyclic rust, Uromyces plantaginis Vestergr., described on Plantago tubulosa from Argentina (Sydow and Sydow 1910; Lindquist Citation1982) has evenly warted teliospores that tend to be more globose or subglobose in shape and slightly wider, measuring 24–29 × 20–25 μm.
Uromyces rytidospermatis McKenzie and Padamsee, sp. nov.
Figure 31. Uromyces rytidospermatis on Rytidosperma spp.: A, Urediniospore and teliospores (PDD 105364, holotype). B, urediniospores and teliospores (PDD 19849). C, D, teliospores (PDD 19849). E, SEM image of urediniospore. F, SEM image of teliospore. Scale bars = 20 μm.
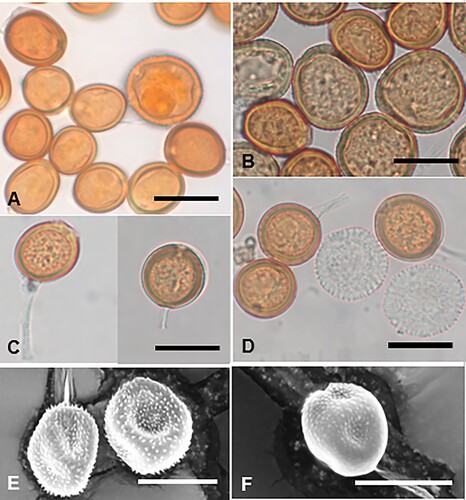
IF901208
Etymology. From Rytidosperma, the host genus.
Parasitic on leaves of Rytidosperma spp. (Poaceae). Spermogonia and aecia not known. Uredinia on adaxial leaf surface, orange-brown, usually 2–3 mm long, pulverulent. Urediniospores (19.5–)20.5–31(–32.5) × (16.5–)19–26.5(–28.5) μm (mean 25.5 × 23.5 μm), globose, subglobose or broadly ellipsoidal, contents pale yellow or orange-yellow; wall 1.5–2.5(–3.5) μm thick, golden yellow, echinulate, germ pores 8–11, scattered, obvious. Telia on adaxial leaf surface, chocolate-brown, compact. Teliospores (18.5–)20–23.5(–24.5) × (16–)17.5–20.5(–21.5) μm (mean 22.5 × 18.9 μm), 1-celled, globose, subglobose or broadly ellipsoidal, contents reddish brown; wall 1.5–2.5(–3.5) μm thick at sides, 2.5–4(–4.5) μm thick at apex, lacking an apical projection, with a small hyaline papilla over the apical germ pore, brown or reddish brown, smooth but verrucose under SEM, especially towards apex; pedicels up to 55 × 2.5–6 μm, hyaline.
Holotype. New Zealand, Northland, near Opua, on Rytidosperma unarede (Raoul) Connor and Edgar, 19 February 2015, E.H.C. McKenzie (PDD 105364–II, III, GenBank Accession #OR760476).
Distribution. Endemic to New Zealand (Northland, Auckland, Bay of Plenty).
Additional specimens examined. New Zealand, Auckland, Warkworth, Dome Valley, on Rytidosperma gracile (Hook. f.) Connor and Edgar, 3 April 1961, R.F.R. McNabb (PDD 19849–II, III). Bay of Plenty, Mt Maunganui, on R. pilosum (R. Br.) Connor and Edgar, 22 December 1978, A.E. Esler and L. Esler (PDD 40285–II, III, ex CHR 354576).
Notes. Rytidosperma is a Southern Hemisphere danthonioid grass genus that was previously referred to in New Zealand as Danthonia and Notodanthonia. The genus is found throughout New Zealand, in grasslands and modified areas, from sea level to montane-alpine areas.
This rust was previously erroneously included within Uromyces danthoniae McAlpine. However, it differs from U. danthoniae in that it has smaller teliospores (mean 22.5 × 18.9 μm vs. mean 31.6 × 21.8 μm), and they are usually globose or subglobose rather than ellipsoidal and lack a pronounced apical thickening. The urediniospores also tend to be smaller than those of U. danthoniae although there is considerable overlap in size. In the molecular analyses U. rytidospermatis and U. danthoniae were not recovered together ().
Uromyces westlandica (G. Cunn.) McKenzie and Padamsee, comb. nov.
Figure 32. Uromyces westlandica on Caltha novae-zelandiae: A, Aecia. B, Telium. C, Aeciospores. D, Teliospores. Scale bars = 20 μm.

IF901209
≡ Aecidium westlandicum G. Cunn., Transactions and Proceedings of the New Zealand Institute 59: 495, 1928.
Parasitic on leaves of Psychrophila novae-zealandiae (Hook. f.) W.A. Weber and P. obtusa (Cheeseman) W.A. Weber (Ranunculaceae). Spermogonia not known. Aecia amphigenous but mainly hypophyllous, with a few aecia on the top part of some petioles, scattered but often concentrated along both sides of the midrib, cupulate, orange fading to yellow, ca. 0.2–0.5 mm diameter; peridia cream, tubiform, lacerate and revolute at the top. Aeciospores (16–)18–24(–27) × (11.5–)13–19(–22) μm (mean 20.6 × 15.5 μm), subglobose, ellipsoidal or oblong-elliptical, sometimes somewhat angular, contents yellow-orange or yellow; wall 0.5–1 μm thick, hyaline, minutely verrucose. Uredinia not known. Telia epiphyllous, scattered, up to 1 mm diameter, immersed, with pale golden yellow spores. Teliospores 20–31 × 16–22 μm (mean 25.2 × 18.8 μm), 1-celled, broadly ellipsoidal, subglobose or obovoid, often with a prominent basal hilum, no pore seen, contents hyaline; wall 2–6(–7.5) μm thick, very pale yellow, smooth; pedicels not observed.
Typification. New Zealand, Mackenzie, Sebastopol Range, Mt Cook, 1300 m, on Caltha novae-zelandiae [≡ Psychrophila novae-zelandiae], January 1928, G.H. Cunningham (PDD 3389–I, holotype). New Zealand, Westland, Mt Rangitaipo, 1050 m, on C. obtusa (≡ P. obtusa), February 1928, G.H. Cunningham (PDD 3388–I, III, epitype designated here, IF901212).
Distribution. Endemic to New Zealand (Wellington, Westland, Mid Canterbury, Mackenzie, Central Otago).
Additional specimens examined. New Zealand, Wellington, Tararua Range, Kareti, on Psychrophila novae-zelandiae, 13 March 1955, R.C. Close (PDD 19021–I). Mid Canterbury, Craigieburn Range, on P. novae-zelandiae, January 1928, H.H. Allan (PDD 3391–I); Porter’s Pass, Fog Hill, on P. obtusa, 31 January 1960, W.R. Philipson (PDD 19164–I). Mackenzie, Sebastopol Range, 1200 m, on P. novae-zelandiae, February 1946, G.H. Cunningham (PDD 4461–I). Central Otago, Dunstan Mountains, 1640 m, on P. obtusa, 9 December 1997, P.B. Heenan (PDD 68399–I, GenBank Accession # PP594480); Dunstan Mountains, bog 1 Km east of Leaning Rock, on P. obtusa, s.d., P.B. Heenan (PDD 68340–I, GenBank Accession #PP594474); Old Man Range, top of Symes Road, on P. obtusa, 6 February 1998, E.H.C. McKenzie, K. Vánky and C. Vánky (PDD 68997–I); Old Man Range, top of Symes Road, on P. obtusa, 6 February 1998, E.H.C. McKenzie, K. Vánky and C. Vánky (PDD 68998–I).
Notes. The hosts are small, endemic, perennial herbs. Psychrophila novae-zelandiae is found southwards from 39°S in montane to subalpine damp grassland and herbfield, while P. obtusa is found in subalpine herbfield from 43 to 45°S.
Petrak (Citation1953) suggested that Aecidium westlandicum may be the aecial stage of Uromyces gaubae Petr., which he described on Caltha introloba (≡ Psychrophila introloba) from New South Wales. According to Cunningham (Citation1928) and J. Walker (pers. comm.) the Australian rust is different. Cunningham (Citation1928, Citation1931) stated that rust infection in New Zealand is systemic, causing etiolation of the leaves and dwarfing of the host plant, a feature not seen with other rusts recorded on the genus Caltha. Certainly, the somewhat larger, darker teliospores described for U. gaubae (27–41 × 16–27 μm) do not match those seen on PDD 3388. However, Petrak (Citation1953) describes the teliospores as sometimes being more or less truncated, and filled with granular plasma, two features that match the current species. But he also described a hyaline, deciduous pedicel up to 70 μm long, which is not seen in U. westlandica. Either way, adoption of ‘one fungus, one name’ (Turland et al. Citation2018), gives priority to Cunningham’s name, Aecidium westlandicum over Petrak’s name (Uromyces gaubae), and hence the new combination is proposed. Aecidium calthae Grev. has also been recorded from Australia (McAlpine Citation1906) on Caltha introloba (≡ P. introloba). However, the aecia were mainly epiphyllous. Uromyces westlandica is recovered as sister to U. haumata (<60% MLBS, ).
Further typification
Phragmidium constrictosporum G.F. Laundon [nom. nov.], Transactions of the British Mycological Society 67: 177, 1976.
≡ Phragmidium constrictum G.F. Laundon [nom. nov.; nom. illeg.], New Zealand Journal of Botany 8: 311, 1970 (non, P. constrictum Bonord., 1869).
≡ Phragmidium acuminatum G. Cunn. [nom. illeg.], Transactions and Proceedings of the New Zealand Institute 61: 403, 1930 (non, P. acuminatum (Fr.) Cooke, 1871).
[Phragmidium potentillae (Pers.) P. Karst., sensu Cunningham, pro parte]
When Cunningham (Citation1930) described Phragmidium acuminatum he cited the type specimen as being on Acaena sanguisorbae var. sericei-nitens, from Table Bay, Wakatipu, 800 m, collected by W.D. Reid. In Fungarium PDD there are two specimens (PDD 770, 1482) both collected in June 1922 that match the given details; each had been labelled ‘part of type collection’. When these specimens were examined, it was found that PDD 1482 is P. acaenae G. Cunn., while PDD 770 is P. constrictosporum. Cunningham (Citation1931) described the urediniospores (of P. acuminatum) as being 18–26 × 15–20 μm (mean 19 × 16 μm), which is larger than found for P. constrictosporum in the present study [15–18.5(–20) × 13–17 μm (mean 16.6 × 15.0 μm)], and matches the size of P. acaenae [(16.5–)18–23(–26.5) × (13–)15–17.5(–19) μm (mean 20.3 × 16.0 μm)]. Thus, PDD 770 is designated here as the lectotype (IF559670) of Phragmidium constrictosporum (New Zealand, Otago Lakes, Table Bay, Wakatipu, 800 m, on Acaena profundeincisa (Bitter) B.H. Macmill., June 1922, W.D. Reid, PDD 770–III, lectotype).
Puccinia euphrasiana G. Cunn., Transactions and Proceedings of the New Zealand Institute 55: 6 [Latin diagnosis p. 12], 1924.
When Cunningham (Citation1924a, page 6) described this species he failed to designate a type specimen, although he did give two numbers (Herb. Nos. 727, 744), but listed three collections. On page 12 he gave a description of the fungus in Latin and under ‘Hab.’ he stated ‘In foliis vivis Euphrasiae cuneatae Forst., York Bay, Wellington, New Zealand, E.H. Atkinson, G.H.C.’. In the New Zealand Fungarium specimen PDD 727 is labelled as the ‘type collection’. However, the collection details do not specifically match those for the collections from York Bay, Wellington, cited by Cunningham (Citation1924a). All the York Bay specimens have sparse infection; however, PDD 727 (Wellington, York Bay, on leaves of Euphrasia cuneata, 7 September 1922, E.H. Atkinson and G.H. Cunningham–II, III) is designated here as the neotype (IF559672) of Puccinia euphrasiana. Cunningham (Citation1931) cited Mt Egmont as a locality for this fungus, but this record is not substantiated by a specimen. However, there is a recent collection from Mt Egmont that is heavily infected with telia although it does not bear any aecia or uredinia; this specimen, which has been sequenced () is designated here as the epitype, IF559671 (New Zealand, Taranaki, Mt Egmont, track to Manganui Lodge, on Euphrasia cuneata, 15 March 2011, E.H.C. McKenzie and M. Padamsee, PDD 101632–III, epitype, GenBank Accession #ON622770).
Puccinia schoenus (G. Cun.) G. Cunn., Transactions and Proceedings of the New Zealand Institute 61: 407, 1930.
≡ Uredo schoenus G. Cunn., Transactions and Proceedings of the New Zealand Institute 59: 499, 1928.
Cunningham (Citation1928) described Uredo schoenus and two years later (Cunningham Citation1930) described Puccinia schoenus, having found a few teliospores associated with the urediniospores. He gave the following details for the type: ‘on Schoenus pauciflorus Hook. f., Canterbury, [Mt] Cook Range, 700 m, January 1928, G.H. Cunningham’. Although Cunningham (Citation1928, Citation1930, Citation1931) listed only one collection of rust from Mt Cook Range, there are five packets in PDD, all with identical collection details on the label, but with two fungarium numbers. Of these five packets, only one appears to have been sorted by Cunningham and the contents placed inside paper, which is labelled in Cunningham’s handwriting as ‘Type’; it is also marked II and III indicating presence of both urediniospores and teliospores. Thus, PDD 3393 is designated here as the lectotype (IF559673) of Puccinia schoenus (New Zealand, Mackenzie, Mt Cook Range, 760 m, on Schoenus pauciflorus, January 1928, G.H. Cunningham, PDD 3393–II, III, lectotype).
Acknowledgements
Nicholas Martin (deceased), former Research Associate, Manaaki Whenua-Landcare Research, is thanked for drawing our attention to two of the new rust species. We thank Tom May, Royal Botanic Gardens, Victoria, for helping to locate specimens of Aecidium plantaginis-variae identified by D. McAlpine, and Jacqueline Edwards and Reannon L. Smith, Agriculture Victoria for selecting an appropriate specimen held in VPRI to designate as lectotype. The staff of the Allan Herbarium, Manaaki Whenua-Landcare Research, Lincoln, are thanked for identification of numerous host plants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aime MC. 2006. Toward resolving family-level relationships in rust fungi (Uredinales). Mycoscience. 47:112–122. doi: 10.1007/s10267-006-0281-0.
- Aime MC, Castlebury LA, Abbasi M, Begerow D, Berndt R, Kirschner R, Marvanová L, Ono Y, Padamsee M, Scholler M, et al. 2018. Competing sexual and asexual generic names in Pucciniomycotina and Ustilaginomycotina (Basidiomycota) and recommendations for use. IMA Fungus. 9:75–89. doi: 10.5598/imafungus.2018.09.01.06.
- Aime MC, McTaggart AR. 2021. A higher-rank classification for rust fungi, with notes on genera. Fungal Systematics and Evolution. 7:21–47. doi: 10.3114/fuse.2021.07.02.
- Anonymous. 2023. State of Victoria (Agriculture Victoria): Victorian Resources Online. Native Yam Daisy | VRO | Agriculture Victoria; [accessed 24 March 2023]. https://vro.agriculture.vic.gov.au/dpi/vro/vrosite.nsf/pages/sip_salt_native_yam.
- Arthur JC. 1918. Uredinales of Guatemala based on collections by E. W. D. Holway. IV. Puccinia on Carduaceae, form-genera, and index. American Journal of Botany. 5:522–550. doi: 10.1002/j.1537-2197.1918.tb05520.x.
- Arthur JC. 1934. Manual of the rusts in United States and Canada. Lafayette, Indiana: Purdue Research Foundation.
- Barclay A. 1890. A descriptive list of the Uredineae occurring in the neighbourhood of Simla (Western Himalayas). Pt. III. Journal of the Asiatic Society of Bengal. Part II. Natural Science. 59(2):75–112.
- Baylis GTS. 1954. Rust fungi on New Zealand Clematis. Transactions of the Royal Society of New Zealand. 82:633–637.
- Berndt R. 2009. New species of rust fungi (Uredinales) from South Africa and new observations on known species. Mycological Progress. 8:99–114. doi: 10.1007/s11557-008-0582-0.
- Chen Z-C, Hu T-L, Koyama T. 1980. A preliminary survey of Uredinales on Formosan Gramineae. Taiwania. 25:152–165.
- Cooke MC. 1884. Some exotic fungi. Grevillea. 13(65):6–7.
- Crane PE, Peterson RS. 2007. Mikronegeria fuchsiae sp. nov., a rust fungus on Fuchsia and Phyllocladus in New Zealand. New Zealand Journal of Botany. 45:707–713. doi: 10.1080/00288250709509745.
- Crosby TK, Dugdale JS, Watt JC. 1998. Area codes for recording specimen localities in the New Zealand subregion. New Zealand Journal of Zoology. 25:175–183. doi: 10.1080/03014223.1998.9518148.
- Cummins GB. 1940. Descriptions of tropical rusts–III. Bulletin of the Torrey Botanical Club. 67:607–613. doi: 10.2307/2481581.
- Cummins GB. 1971. The rust fungi of cereals, grasses and bamboos. New York: Springer. doi: 10.1007/978-3-642-88451-1.
- Cunningham GH. 1923. The Uredinales, or rust-fungi, of New Zealand: part 1–Pucciniaceae, tribe Puccineae (containing descriptions and illustrations of seventy-five species). Transactions and Proceedings of the New Zealand Institute. 54:619–704.
- Cunningham GH. 1924a. The Uredinales, or rust-fungi, of New Zealand: supplement to Part 1; and Part 2. Transactions and Proceedings of the New Zealand Institute. 55:1–58.
- Cunningham GH. 1924b. Second supplement to the Uredinales of New Zealand. Transactions and Proceedings of the New Zealand Institute. 55:392–396.
- Cunningham GH. 1927. Fifth supplement to the Uredinales and Ustilaginales of New Zealand. Transactions and Proceedings of the New Zealand Institute. 58:47–50.
- Cunningham GH. 1928. Sixth supplement to the Uredinales and Ustilaginales of New Zealand. Transactions and Proceedings of the New Zealand Institute. 59:491–505.
- Cunningham GH. 1930. Seventh supplement to the Uredinales and Ustilaginales of New Zealand. Transactions and Proceedings of the New Zealand Institute. 61:402–418.
- Cunningham GH. 1931. The rust fungi of New Zealand. Dunedin: John McIndoe.
- Cunningham GH. 1945. Additions to the rust fungi of New Zealand, I. Transactions of the Royal Society of New Zealand. 75:324–327.
- Dingley JM. 1969. Records of plant diseases in New Zealand. New Zealand Department of Scientific and Industrial Research, Bulletin. 192:1–298.
- Dingley JM. 1977. Additions to the rust fungi of New Zealand–VI. New Zealand Journal of Botany. 15:29–37. doi: 10.1080/0028825X.1977.10429617.
- Dingley JM, Fullerton RA, McKenzie EHC. 1981. Records of fungi, bacteria, algae, and angiosperms pathogenic on plants in Cook Islands, Fiji, Kiribati, Niue, Tonga, Tuvalu, and Western Samoa. UNDP/FAO/SPEC Survey of Agricultural Pests and Diseases 2. Rome: SPEC, UNDP, FAO.
- Edgar E, Connor HE. 2010. Flora of New Zealand. Volume V. Gramineae, 2nd edition. Lincoln: Manaaki Whenua Press.
- Farr DF, Rossman AY. 2022. Fungal databases, U.S. National Fungus Collections. ARS: USDA. https://nt.ars-grin.gov/fungaldatabases/. Last accessed 2 January 2022.
- Ferrada EE, Bohm L, Montenegro O, Llancao M, Romero C, Briceno EX, Morales RA, Diaz GA. 2020. First report of leaf rust of Fuchsia magellanica caused by Pucciniastrum circaeae in Valdivia, Chile. Plant Disease. 104:1548. doi: 10.1094/PDIS-11-19-2356-PDN.
- Garibaldi A, Gilardi G, Ortu G, Gullino ML. 2012. First report of rust caused by Pucciniastrum circaeae on Fuchsia ×hybrida in Italy. Plant Disease. 96:588. doi: 10.1094/PDIS-11-11-0976-PDN.
- Gäumann E. 1959. Die Rostpilze Mitteleuropas. Beiträge zur Kryptogamenflora der Schweiz. 12:1–1407.
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology. 59:307–321. doi: 10.1093/sysbio/syq010.
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology. 52:696–704. doi: 10.1080/10635150390235520.
- Helfer S. 2013. Coleosporium in Europe. Mycotaxon. 124:87–99. doi: 10.5248/124.87.
- Henderson DM. 2000. A checklist of the rust fungi of the British Isles. Kew, Surrey: British Mycological Society.
- Hennings P. 1891 [1892]. Fungi africani. Botanische Jahrbücher für Systematik. Pflanzengeschichte und Pflanzengeographie. 14:337–373.
- Hennings P. 1904. Fungi amazonici I. a cl. Ernesto Ule collecti. Hedwigia. 43:154–186.
- Hiratsuka N, Sato S, Katsuya K, Kakishima M, Hiratsuka Y, Kaneko S, Ono Y, Sato T, Harada Y, Hiratsuka T, Nakayama K. 1992. The rust flora of Japan. Ibariki, Japan: Tsukuba Shuppankai.
- Hitchmough R (comp.). 2002. New Zealand Threat Classification System lists–2002. Threatened Species Occasional Publication 23.
- Hofsten Av, Holm L. 1968. Studies on the fine structure of aeciospores I. Grana Palynologica. 8:235–251. doi: 10.1080/00173136809427469.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 30:772–780. doi: 10.1093/molbev/mst010.
- Latch BJ. 1980. Weeping willow rust in New Zealand. New Zealand Journal of Agricultural Research. 23:535–538. doi: 10.1080/00288233.1980.10417880.
- Laundon GF. 1963. Rust fungi II: on Aceraceae, Actinidiaceae, Adoxaceae and Aizoaceae. Mycological Papers 91.
- Laundon GF. 1970. Additions to the rust fungi of New Zealand–5. New Zealand Journal of Botany. 8:310–319. doi: 10.1080/0028825X.1970.10429131.
- Laundon GF. 1978. A Coleosporium rust new to New Zealand. Plant Disease Reporter. 62:796–797.
- Léveillé-Bourret É, Eggertson Q, Hambleton S, Starr JR. 2021. Cryptic diversity and significant cophylogenetic signal detected by DNA barcoding the rust fungi (Pucciniaceae) of Cyperaceae–Juncaceae. Journal of Systematics and Evolution. 59(4):833–851. doi: 10.1111/jse.12740.
- Lindquist JC. 1982. Royas de la República Argentina y zonas limítrofes. Argentina: Instituto Nacional de Tecnología Agropecuaria.
- Loshomboon P, Kakishima M, Ono Y. 1990. A revision of the genus Nyssopsora (Uredinales). Mycological Research. 94:907–922. doi: 10.1016/S0953-7562(09)81305-1.
- Marin-Felix Y, Groenewald JZ, Cai L, Chen Q, Marincowitz S, Barnes I, Bensch K, Braun U, Camporesi E, Damm U, et al. 2017. Genera of phytopathogenic fungi: GOPHY 1. Studies in Mycology. 86:99–216. doi: 10.1016/j.simyco.2017.04.002.
- McAlpine D. 1895. Australian fungi. Agricultural Gazette of New South Wales. 6:752–758.
- McAlpine D. 1906. The rusts of Australia. Melbourne: Robt. S. Brain, Government Printer.
- McKenzie EHC. 1980. New graminicolous rust fungi. Puccinia rara sp. nov. and P. polypogonobia sp. nov. New Zealand Journal of Botany. 18:335–337. doi: 10.1080/0028825X.1980.10427249.
- McKenzie EHC. 1981. New Zealand rust fungi: additions and corrections. New Zealand Journal of Botany. 19:227–232. doi: 10.1080/0028825X.1981.10425121.
- McKenzie EHC. 1991a. Fungi of the Chatham Islands. Mycotaxon. 41:195–217.
- McKenzie EHC. 1991b. Puccinia tetragoniae var. novae-zelandiae var. nov. and Uredo chathamica sp. nov. from Chatham Islands, New Zealand. Mycotaxon. 41:307–310.
- McKenzie EHC. 1992. Fungi of the Kermadec Islands. Mycotaxon. 45:149–170.
- McKenzie EHC. 1998. Rust fungi of New Zealand–an introduction, and list of recorded species. New Zealand Journal of Botany. 36:233–271. doi: 10.1080/0028825X.1998.9512564.
- McKenzie EHC. 2008. Rust fungi in the subantarctic islands of New Zealand. Mycoscience. 49:1–10. doi: 10.1007/S10267-007-0385-1.
- McKenzie EHC, Dingley JM. 1996. New plant disease records in New Zealand: miscellaneous fungal pathogens III. New Zealand Journal of Botany. 34:263–272. doi: 10.1080/0028825X.1996.10410690.
- McKenzie EHC, Johnston PR. 1999. New records of phytopathogenic fungi in the Chatham Islands, New Zealand. Australasian Plant Pathology. 28:131–138. doi: 10.1071/AP99023.
- McKenzie EHC, Johnston PR. 2004. Puccinia embergeriae sp. nov. on Chatham Islands sow thistle (Embergeria grandifolia) and a note on Miyagia pseudosphaeria on sow thistles (Sonchus spp.) in New Zealand. New Zealand Journal of Botany. 42:657–661. doi: 10.1080/0028825X.2004.9512917.
- McKenzie EHC, Latch GCM. 1980. Occurrence of Uromyces danthoniae and U. macnabbii, and additions to the graminicolous rust fungi of New Zealand. New Zealand Journal of Agricultural Research. 23:393–397. doi: 10.1080/00288233.1980.10425373.
- McKenzie EHC, Padamsee M, Dick M. 2013. First report of rust on Alnus in New Zealand is Melampsoridium betulinum, not M. hiratsukanum. Plant Pathology & Quarantine. 3:59–65. doi: 10.5943/ppq/3/2/1.
- McNabb RFR. 1962a. Additions to the rust fungi of New Zealand–III. Transactions of the Royal Society of New Zealand. Botany. 1:109–116.
- McNabb RFR. 1962b. The graminicolous rust fungi of New Zealand. Transactions of the Royal Society of New Zealand. Botany. 1:235–257.
- McNabb RFR. 1966. Additions to the rust fungi of New Zealand–4. New Zealand Journal of Botany. 4:86–94. doi: 10.1080/0028825X.1966.10443956.
- McTaggart AR, Shivas RG, Doungsa-ard C, Weese TL, Beasley DR, Hall BH, Metcalf DA, Geering ADW. 2016. Identification of rust fungi (Pucciniales) on species of Allium in Australia. Australasian Plant Pathology. 45:581–592. doi: 10.1007/s13313-016-0445-0.
- Padamsee M, McKenzie EHC. 2014. A new species of rust fungus on the New Zealand endemic plant, Myosotidium, in isolated Chatham Islands. Phytotaxa. 174:223–230. doi: 10.11646/phytotaxa.174.4.3.
- Padamsee M, McKenzie EHC. 2017. The intriguing and convoluted life of a heteroecious rust fungus in New Zealand. Plant Pathology. 66:1248–1257. doi: 10.1111/ppa.12672.
- Padamsee M, McKenzie EHC. 2019. Pucciniastrum minimum is the causal agent of rust on blueberries in New Zealand. Australasian Plant Disease Notes. 14(27):3 p. doi: 10.1007/s13314-019-0358-1.
- Pekel FO, Azaz AD. 2003. Parasitic fungi determined on the flora of Akdağ (Olur-Erzurum). Erzincan Eğitim Fakültesi Dergisi. 5(2):75–81.
- Perrie LR, Shepherd LD, Brownsey PJ. 2005. Asplenium ×lucrosum nothosp. nov.: a sterile hybrid widely and erroneously cultivated as ‘‘Asplenium bulbiferum’. Plant Systematics and Evolution. 250:243–257. doi: 10.1007/s00606-004-0239-7.
- Petrak F. 1953. Ein kleiner Beitrage zur Kenntnis der Uredineen Australiens. Sydowia, Annales Mycologici. 7:1–7.
- Ramachar P, Cummins GB. 1965. The species of Puccinia on the Paniceae. Mycopathologia et Mycologia Applicata. 25:7–60. doi: 10.1007/BF02049611.
- Samuel G. 1924. Some new records of fungi for South Australia, III. Transactions and Proceedings of the Royal Society of South Australia. 48:149–161.
- Savile DBO. 1973. Aeciospore types in Puccinia and Uromyces attacking Cyperaceae, Juncaceae and Poaceae. Report of the Tottori Mycological Institute. No. 10:225–241.
- Săvulescu T. 1953. Monografia Uredinalelor din Republica Populară Română II. Bucuresti: Academia Republicii Populare Române.
- Scholler M, Braun U, Buchheit R, Schulte T, Bubner B. 2022. Studies on European rust fungi, Pucciniales: molecular phylogeny, taxonomy, and nomenclature of miscellaneous genera and species in Pucciniastraceae and Coleosporiaceae. Mycological Progress. 21:64 [25 p.]. doi: 10.1007/s11557-022-01810-3.
- Sheridan JE. 1978. Senecio rust caused by Coleosporium senecionis (Pers.) Lev. in the Wellington region. Victoria University of Wellington, Botany Department, Mycology and Plant Pathology Report. 12:7–8. doi: 10.1071/APP9790056.
- Shivas RG, Beasley DR, McTaggart AR. 2023. Rust fungi of Australia; [accessed 16 October 2023]. http://collections.daff.qld.gov.au/web/key/rustfungi/Media/Html/about.html.
- Soares DJ, Ferreira FA, Barreto RW. 2006. First report of the aecial stage of Puccinia scirpi on Nymphoides indica in Brazil, with comments on its worldwide distribution. Australasian Plant Pathology. 35:81–84. doi: 10.1071/AP05091.
- Sydow H. 1938. Neue oder bemerkenswerte australische Micromyceten–III. Annales Mycologici. 36:295–313.
- Sydow H, Sydow P. 1908. Ȕber eine Anzahl aus der Gattung Uromyces auszuschließender resp. unrichtig beschriebener Arten. Annales Mycologici. 6:135–143.
- Sydow H, Sydow P. 1914. Novae fungorum species–XII. Annales Mycologici. 12:195–204.
- Thiers BM. 2024, updated continuously. Index Herbariorum. Available at https://sweetgum.nybg.org/science/ih/.
- Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, editors et al. 2018. International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Glashütten: Koeltz Botanical Books. doi: 10.12705/Code.2018.
- van der Merwe M, Walker J, Ericson L, Burdon JJ. 2008. Coevolution with higher taxonomic host groups within the Puccinia/Uromyces rust lineage obscured by host jumps. Mycological Research. 112:1387–1408. doi: 10.1016/j.mycres.2008.06.027.
- Versluys WS. 1977. New plant disease record in New Zealand. Puccinia oxalidis on Oxalis. New Zealand Journal of Agricultural Research. 20:429–430. doi: 10.1080/00288233.1977.10427355.
- Wang Y-C, Zhuang J-Y, editors. 1998. Flora Fungorum Sinicorum. Vol. 10. Uredinales (I). Beijing: Science Press.
- Weber RWS, Webster J, Engel G. 2003. Phylogenetic analysis of Puccinia distincta and P. lagenophorae, two closely related rust fungi causing epidemics on Asteraceae in Europe. Mycological Research. 107:15–24. doi: 10.1017/S0953756202006937.
- Wilson IM, Walshaw DF, Walker J. 1965. The new groundsel rust in Britain and its relationship to certain Australasian rusts. Transactions of the British Mycological Society. 48:501–511. doi: 10.1016/S0007-1536(65)80025-0.
- Wilson M, Henderson DM. 1966. British rust fungi. University Press: Cambridge.
- Zhao P, Zhang Z-F, Hu D-M, Tsui K-M, Qi X-H, Phurbu D, Gaforov Y, Cai L. 2021. Contribution to rust flora in China I, tremendous diversity from natural reserves and parks. Fungal Diversity. 110:1–58. doi: 10.1007/s13225-021-00482-w.
- Zwetko P. 1993. Rostpilze (Uredinales) auf Carex im Ostslpenraum. Eine neues Artenkonzept. Bibliotheca Mycologica. 153:1–222.