ABSTRACT
The island syndrome is defined as a suite of predictable and consistent differences between island and mainland organisms. In seed plants, much of what we know about the island syndrome comes from work in the Southwest Pacific, which is comprised of the three main islands of New Zealand and ten surrounding archipelagos. These surrounding archipelagos are all remote and relatively small. They were never connected to another landmass and their floras are derived by overwater dispersal, mostly from the main islands of New Zealand, making them an ideal system for the study of island evolution. In this review, we summarise work on the island syndrome conducted on New Zealand’s outlying islands, establish whether these findings are in line with global patterns of island biology or whether they are unique to this region, and propose directions for future research.
Introduction
Geographic isolation can have a profound effect on insular floras. Isolation from the mainland causes dispersal limitation, favouring species with effective long-distance dispersal and subsequent establishment. Once on an island, plants are met with peculiar ecological conditions resulting from unique climates and an impoverished set of pollinators, dispersers, competitors, and predators, making in situ island evolution predictable. Island plants often differ from their mainland relatives, being self-compatible, lacking most defensive adaptations, possessing unusually large seeds and leaves and unspecialised, inconspicuous flowers (Darwin Citation1859; Wallace Citation1878; Baker Citation1967; Carlquist Citation1974; Givnish Citation1998; Whittaker et al. Citation2023). This suite of predictable and consistent differences is known as the ‘island syndrome’ (Burns Citation2019).
The New Zealand region comprises three main islands (North, South and Stewart Island, termed the ‘New Zealand mainland’ hereafter) and ten surrounding archipelagos (termed ‘New Zealand’s outlying islands’ hereafter) (). The flora of the New Zealand mainland derives from a combination of long-distance dispersal and vicariance (McGlone et al. Citation2001), and has been shaped by its long history of isolation, elevational range, rainfall gradients, climatic history, and diversified series of volcanic, metamorphic, and sedimentary soils (Carlquist Citation1974).
Figure 1. Map of the New Zealand region, comprising three main islands (i.e. the New Zealand mainland) and ten surrounding archipelagos (i.e. New Zealand’s outlying islands).
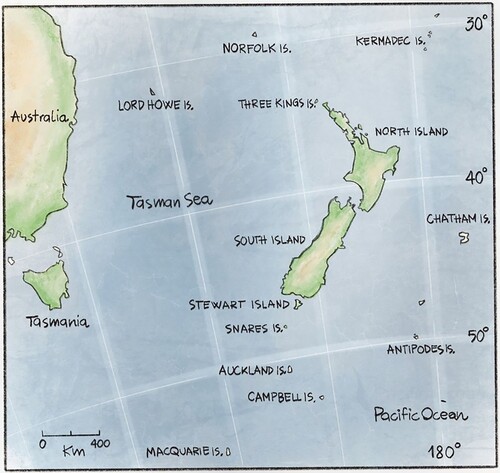
All of New Zealand’s outlying islands are relatively young geologically and were never connected to another landmass. Norfolk island is a remnant of a volcano active in the late Pliocene. The subaerial portion of the Norfolk volcano was constructed during several volcanic episodes from about 3.05 m. y. to about 2.3 m.y. ago (Jones and McDougall Citation1973). The Kermadec islands are the emergent part of a linear group of volcanic mountains. The Islands themselves are of Pleistocene and Holocene age (Brook Citation1998; Burns Citation2022). Lord Howe Island comprises remnants of the subaerial portion of a Late Miocene shield volcano and is approximately 7 m. y. old (Woodroffe et al. Citation1995). The origin of the Three Kings archipelago is unknown, but the group is unlikely to have been connected to the New Zealand mainland during the last few million years. The area is currently being uplifted (Hayward and Moore Citation1987). The Chatham Islands were uplifted above the surface of the sea approximately 4 m. y. ago (Burns Citation2022). Antipodes, Auckland, Campbell and Snares islands represent the only sub-aerial exposures of the Campbell Plateau (Scott and Turnbull Citation2019). Antipodes island is less than 0.5 m. y. old (Scott et al. Citation2013). Auckland islands was formed by two volcanoes during the Miocene and is at least 12 m. y. old (Adams Citation1983). Campbell island is the remains of a shield volcano which reached sea level during the Miocene between 12 and 6 m. y. ago (Scott and Turnbull Citation2019). Although glaciation may have played a role in shaping their floras, recent evidence suggests that during the last glacial maximum (∼ 21 ka) a combination of high seasonality and low precipitation left these islands incapable of sustaining significant glaciation (Rainsley et al. Citation2019). Finally, Macquarie island was formed by oceanic crust uplift, reaching above sea-level during the late Pleistocene (Varne et al. Citation2000).
The flora of New Zealand’s outlying islands derives from overwater dispersal. Most specifically, the floras of Antipodes, Auckland, Campbell, Chatham, Macquarie, Snares and Three Kings Islands are derived by overwater dispersal largely from New Zealand. On the other hand, Kermadec, Lord Howe and Norfolk Island floras are derived from both New Zealand floras and other surrounding landmasses such as Australia and New Caledonia (Oliver Citation1948; Godley Citation1969; Taylor Citation1971; Johnson and Campbell Citation1975; Meurk Citation1977; Sykes Citation1977; Wright Citation1983; Godley Citation1989; Green Citation1994; Meurk et al. Citation1994; Sykes and West Citation1996; De Lange et al. Citation2011; De Lange Citation2014; De Salas and Baker Citation2015).
Work on New Zealand’s outlying islands is significant for several reasons. First, because they were never connected to another landmass, and because most have a clear mainland source pool, they collectively comprise an excellent system for the study of insular assembly rules i.e. processes of immigrant selection/biased colonisation and ecological filtering on establishment. Secondly, their remoteness and small size make them ideal for the study of evolution in extreme isolation, facilitating the evolution of patterns that may be difficult to appreciate on bigger, less isolated islands (Whittaker et al. Citation2023). Thirdly, these archipelagos are distributed across 25 degrees of latitude and therefore encompass a massive range of environmental conditions (Ciarle and Burns Citation2023). Lastly, because their floras are derived largely from the New Zealand mainland, they can help us better understand the ecology and evolution of the New Zealand flora in general.
Here, we review work on the island syndrome in seed plants conducted on New Zealand’s outlying islands. We systematically review previous work on the biogeography and evolution of their floras to: (1) summarise the components of the island syndrome of New Zealand’s outlying islands, (2) determine whether these findings are consistent with global evidence for the island syndrome, and (3) propose directions for future research.
Quantitative analysis
Data collection and categorisation
We selected plant traits following Burns (Citation2019) and Whittaker et al. (Citation2023) and ran distinct searches on Google Scholar using different keywords (supplementary material S1), sorting the results by relevance. For each search, we inspected the first 10 pages of results (n = 100 studies) and considered all studies in English discussing plant trait evolution and/or assembly rules on New Zealand’s outlying islands. The selected studies were then manually screened and classified based on their islands of interest and the plant trait investigated.
The distribution of plant traits on islands can be investigated in two ways: (1) at the community level, the insular assembly rule method (sensu Whittaker et al. Citation2023) compares the island species pool to the mainland source pool, searching for differences in trait distribution, which can arise from dispersal filters, in situ evolution, or both; (2) at the species level, the pairwise comparison contrasts the traits of island species to those of their closest relative(s) on the mainland, which helps narrow down the mechanisms causing trait differences to in situ evolution alone. The first method cannot disentangle the relative roles of ecological and evolutionary processes but can detect processes of immigrant selection/biased colonisation and/or ecological filtering on establishment (Lloyd Citation1985). The second method can isolate and detect evolutionary processes but assumes that traits of island species are always derived. It is not always clear whether the species chosen for the comparison are representative of the island species pool, meaning the results cannot necessarity be extended to the whole island flora. It is also worth noting that based on colonisation time and island age, endemic species on different archipelagos have had different amounts of time for in situ evolution. Furthermore, the general dynamic model of island biogeography suggests that opportunities for morphological evolution and speciation can depend on the age and geological history of the island (Whittaker et al. Citation2008).
Pairwise comparison of plant traits was pioneered in the Southwest Pacific and most studies conducted in this region follow this methodology (Burns Citation2019). Unless otherwise stated, all studies discussed in the next section (see ‘Components of the island syndrome’) follow the pairwise comparison method.
Results
We found 19 studies investigating the island syndrome of New Zealand’s outlying islands (supplementary material S1). Of these, four failed to find differences between island and mainland plants (Kavanagh and Burns Citation2015; Lord Citation2015; Biddick et al. Citation2019a; Biddick Citation2023). Overall, out of 21 traits globally considered possible components of the plant island syndrome, 16 were investigated on New Zealand’s outlying islands. Only 4 components are currently well supported, while evidence for 9 of them is tentative. Limited evidence from 3 components suggest that they may not be part of the island syndrome (). These components can be grouped under 4 traits, namely growth habit, leaves & stems, flowers and seeds (). In the next section, each component will be discussed individually.
Table 1. Components of the island syndrome known in seed plants, divided by trait. For each component, we indicate the observed pattern, how well supported it is on New Zealand’s outlying islands and globally, and provide a summary of the topic and leading references. Green tick = Reasonably well supported component. Yellow tilde = Equivocal/insufficient support. Possible component of the island syndrome, requiring further investigation. Red cross = Component so far rejected as part of the plant island syndrome. Question mark = Component not yet investigated on New Zealand’s outlying islands or outside of New Zealand.
Components of the island syndrome
Growth habit
Stature
The island rule in body size predicts that, after island colonisation, small organisms evolve to become larger while large organisms evolve to become smaller. This rule was initially proposed for mammals (Van Valen Citation1973) and later expanded to vertebrates (Lomolino et al. Citation2012). Despite decades of work, its generality in explaining evolutionary trajectories on islands is still fiercely debated (Faurby and Svenning Citation2016; Benítez-López et al. Citation2021).
Using an extensive dataset of 175 island/mainland pairs, Biddick et al. (Citation2019b) found support for the island rule in plant stature on all New Zealand’s outlying islands (Burns et al. Citation2012; Biddick et al. Citation2019b). Three other studies found evidence for gigantism in Azorella (up to 100 cm tall) on all subantarctic islands (Mitchell et al. Citation1999), gigantism in Leptinella featherstonii (up to 150 cm tall) on Chatham islands (Lloyd Citation1981) and dwarfism in Alyxia ruscifolia (up to 200 cm tall) on Lord Howe Island (Burns Citation2016a). One study tested the island rule while accounting for different growth forms on the Chatham islands. Herbs and woody species showed evidence of gigantism, while graminoids showed evidence of dwarfism (Cox and Burns Citation2017).
The island rule in body size was extended to plants only recently (Burns Citation2019), and while established in the New Zealand region, not enough studies have been conducted worldwide to consider this pattern an established component of the plant island syndrome. Moreover, the evolutionary processes behind this pattern are still unclear, although it has been suggested that plant stature may evolve indirectly on islands because of genetic drift or as a by-product of selection acting on correlated traits (Burns et al. Citation2012; Biddick et al. Citation2019b).
Moving forward, it would be beneficial to address the following questions: is the island body size rule a consistent pattern in plants worldwide? Do different growth forms respond differently to insularity, with herbaceous species increasing in size and woody species decreasing in size? Given that herbaceous plant stature can vary considerably throughout the year (e.g. if peduncle and inflorescences are present or absent), is height the best proxy of plant body size?
Divaricate branching
Compositional differences between islands and continents are thought to drastically alter the defensive adaptations of plant taxa following island colonisation. This loss (and gain) of defence hypothesis predicts the loss of non-bird-specific defences and the gain of bird-specific defences (Ciarle et al. Citation2024). Divaricate branching is characterised by longer outer branches with well-spaced internodes and fewer, small leaves (Greenwood and Atkinson Citation1977). Divaricate species also possess high tensile stem strengths and produce right-angled branches (Pollock et al. Citation2007). Divaricate branching is thought to be an anti-browsing defence prevalent on islands lacking browsing mammals but browsed by giant ratites and reptiles, and subsequently lost when these browsing pressures cease (Greenwood and Atkinson Citation1977; McGlone and Webb Citation1981; Bond and Silander Citation2007; Lusk et al. Citation2016).
While the New Zealand mainland housed giant ratites, these species never reached any outlying island (Howard et al. Citation2022). Accordingly, evidence for loss of divaricate branching was found in 12 Chatham Island species (Kavanagh Citation2015; Burns Citation2016b). When compared to their mainland relatives, island plants consistently produced bigger leaves and smaller-angled branches (Kavanagh Citation2015). Similarly, using 22 island/mainland pairs, Howard et al. (Citation2022) showed how divaricate branching was consistently lost on the Kermadec and Three Kings islands and New Zealand subantarctic archipelagos.
The loss (and gain) of defensive adaptations is globally supported (but see Meredith et al. Citation2019; Monroy and García-Verdugo Citation2019; Moreira et al. Citation2021), as the emerging global pattern seems to be one in which many island species lost defences that evolved in mammal-dominated ecosystems and gained new defences against bird-dominated islands (Ciarle et al. Citation2024). However, whether the loss of divaricate branching represents a general trend in island evolution, or an idiosyncrasy of New Zealand’s outlying islands remains to be established.
Evidence suggests that the divaricate architecture evolved in the New Zealand mainland mostly in the last 5 million years, when Plio-Pleistocene climatic constraints prevented plants from quickly growing out of the reach of browsers (Lusk et al. Citation2016; Maurin et al. Citation2022). Is it therefore plausible that divarication evolved in the mainland lineage after splitting from the island lineage, meaning their common ancestor was not-divaricate. Before testing the loss of divarication hypothesis globally, it would be worth testing it further in the New Zealand region, as previous studies always assumed the divaricate state of the common ancestor.
Spinescence encompasses all types of sharp projections from plant surfaces and can be divided into three classes. Spines are modified leaves or stipules; thorns are modified twigs or branches; prickles are derived from epidermal tissue (Cornelissen et al. Citation2003). Although spines, thorns and prickles can have multiple functions (Stanton et al. Citation2005; Forster and Bonser Citation2009), most forms of spinescence play a defensive role and deter herbivores (Burns Citation2019). The loss (and gain) of defence hypothesis predicts that leaf spinescence should be lost on islands.
Comparisons between Lord Howe Island and Australia showed a marked reduction in leaf spines in island populations relative to mainland populations in several plant species (Burns Citation2016b). Similarly, analyses of Aciphylla dieffenbachia, Melicytus chathamicus and Pseudopanax chathamica on the Chatham islands indicate that reduced leaf spinescence may be a hallmark of Chatham endemics (Burns Citation2016b).
Much like divarication, leaf spinescence appears to be prevalent on islands browsed in the past by large birds and reptiles and lost following predatorial release (Ciarle et al. Citation2024). Support for the loss of spinescence on islands comes from New Zealand, the Channel islands (Salladay and Ramirez Citation2018), and Madagascar (Grubb Citation2003), with one study failing to find support for it in Australia (Meredith et al. Citation2019).
Sexual dimorphism in secondary sex characters of gender dimorphic species, including any aspect of morphology and physiology, appears to be common in dioecious Angiosperms (Lloyd and Webb Citation1977; Barrett and Hough Citation2013). The niche variation hypothesis predicts that insular populations should exhibit increased sexual size dimorphism to minimise intraspecific competition (Meiri et al. Citation2005).
Using 14 island/mainland species pairs from Kermadec, Three Kings, Chatham and Auckland Islands, Kavanagh and Burns (Citation2015) tested for differences in sexual dimorphism between islands and the mainland, but failed to find any significant difference. The authors conclude that the niche variation hypothesis may simply not apply to dioecious plants. Alternatively, environmental factors, differences in the amounts of resources allocated to reproduction between the sexes and selective pressures acting on traits correlated to sexual characters may lead to unpredictable patterns in sexual size dimorphism (Kavanagh and Burns Citation2015).
Despite the high incidence of dioecious plants typical of many islands (Baker and Cox Citation1984; Schlessman et al. Citation2014; McGlone and Richardson Citation2023), insular patterns of sexual dimorphism are still poorly understood, with the only available study suggesting that this is not a feature of island floras. Testing the niche variation hypothesis on a global scale could help understand not only island evolution, but the reproductive biology of flowering plants in general.
Three possible “growth habit” components of the plant island syndrome have never been investigated on New Zealand outlying islands: insular woodiness, polyploidy and mycorrhiza. Secondary insular woodiness (i.e. the evolutionary transition from herbaceous toward woody habit after island colonisation) is a key factor in the adaptive radiations of many island plants (Zizka et al. Citation2022). Insular woodiness appears to be rare on New Zealand’s outlying islands, with Lordhowea insularis being a notable exception (Conn Citation1996). Conversely, New Zealand subantarctic islands are known for their megaherbs, flowering plants that evolved independently toward gigantism while retaining their herbaceous habit (Mitchell et al. Citation1999; Wagstaff and Tate Citation2011) (A). Instead of focusing on insular woodiness per se, we believe future research would benefit from investigating how plant growth form relates to insular gigantism.
Figure 2. Six endemic plant species from New Zealand’s outlying islands. Each species represents a possible avenue for future work. A, Anisotome latifolia, a megaherb endemic to Auckland and Campbell islands. Gigantism associated with the retention of the herbaceous habit is a repeated and puzzling pattern of New Zealand’s subantarctic islands. B, Leptecophylla robusta, endemic to the Chatham islands. Leptecophylla juniperina, its closest mainland ancestor, has smaller leaves. Despite leaf area following an island-rule-like pattern on New Zealand’s outlying islands, leaf gigantism is common on the Chatham islands. Different island systems may therefore favour different evolutionary trajectories. C, Plantago aucklandica, endemic to Auckland islands. This wind-pollinated plant produces flowers that are larger than its closest mainland relative, but whether this represents a common island pattern remains unclear. D, Abrotanella spathulata on Auckland and Campbell islands evolved vividly coloured flowers in a pollinator-poor environment. The in situ evolution of conspicuous flower may be a repeated pattern on New Zealand’s subantartic islands. E, Corokia macrocarpa, endemic to the Chatham islands. Seed gigantism is pronounced in many fleshy-fruited island plants, challenging the loss of dispersibility hypothesis. F, Alixya ruscifolia from Lord Howe island. Island populations evolved narrower, more oblong fruits. Additionally, stature, leaf area and seed size, which typically correlate on the mainland, seem to follow independent evolutionary trajectories on islands. The reason though remains unclear.

Polyploidy (i.e. possessing multiple pairs of homologous chromosomes following events of genome duplication) has played a key role in plant speciation and diversification (Soltis et al. Citation2004; Thompson and Merg Citation2008). Results from four archipelagos including the New Zealand mainland suggest that polyploidy may favour insular establishment and subsequent diversification (Meudt et al. Citation2021). Focusing on 270 species from the grass subfamily Danthonioideae (with 61 species present on the New Zealand mainland), Linder and Barker (Citation2014) found that polyploidy facilitates establishment following long-distance dispersal. As the New Zealand mainland is rich in polyploid species (Murray et al. Citation2011), it would be interesting to see whether ploidy played a role in the colonisation of New Zealand outlying islands.
Finally, evidence from a single, global study that included the New Zealand region suggests that symbiotic mycorrhiza may hinder insular establishment but favour diversification (Delavaux et al. Citation2021).
Leaves & stems
Leaf size
The modular nature of plants (a module being defined as a cluster of traits which are highly correlated, but show weak correlation to other such trait clusters; Dellinger et al. Citation2019) led to the island rule in body size being extended to multiple plant traits (Burns Citation2019).
Using a dataset of 175 island/mainland pairs, Biddick et al. (Citation2019b) found support for a pattern analogous to the island rule in leaf area. However, most other studies on the subject, albeit with much smaller datasets, found consistent evidence for leaf gigantism (Lloyd Citation1981; Burns et al. Citation2012; Cox and Burns Citation2017; Biddick et al. Citation2019a). In a study on Alyxia ruscifolia, Burns (Citation2016a) was the only one to find no size changes in leaf area.
Although established on New Zealand’s outlying islands, this pattern requires further investigation, as current evidence comes from a limited number of archipelagos. Also, leaf gigantism appears to be a feature of many plant lineages (B), suggesting that different island systems might favour different evolutionary trajectories.
The modularity of plants makes it so that different modules may follow the same pattern because of different underlying mechanisms. While leaf area may follow an island-rule-like pattern, it may do so because of selective pressures (or random processes) unrelated to body size or other modules. Future research would benefit from attempts to shed light on these mechanisms, as currently leaf area has been proposed to vary in response to altered predation pressures (Biddick et al. Citation2019b; Ciarle et al. Citation2024), evolutionary drift (Biddick and Burns Citation2018) or as a by-product of selection acting on a developmentally correlated trait (Burns et al. Citation2012).
Leaf heteroblasty can be defined as a ‘rather sudden and substantial change in the form of individual metamers or plant habit during ontogeny’ (Zotz et al. Citation2011). Heteroblastic woody plants either produce smaller, more elongated, or more lobed leaves as juveniles than as adults (Burns Citation2019). Like divaricate branching, leaf heteroblasty is thought to be a form of defence common in archipelagos browsed by giant ratites and subsequently lost following predatorial release (Heenan Citation1997; Burns and Dawson Citation2006).
Evidence for the loss of leaf heteroblasty was found in three Chatham Island species (Muehlenbeckia australis, Plagianthus, regius, and Pseudopanax chathamicus) (Burns and Dawson Citation2009). However, the same study found two endemic tree species (Dracophyllum arboretum and Coprosma chathamica) to be clearly heteroblastic. Contrary to what is observed in most heteroblastic species, these two species produced considerably larger leaves as juveniles (Burns and Dawson Citation2006; Burns and Dawson Citation2009). Loss of leaf heteroblasty was also found in a study comparing several species between Lord Howe Island and mainland Australia (Burns Citation2016b).
Evidence from New Zealand, Madagascar (Bond and Silander Citation2007), the Mascarene islands (Eskildsen et al. Citation2004) and Hawaii (Givnish et al. Citation1994) suggests that heteroblasty evolved as a form of defence on archipelagos featuring giant birds/reptiles. Not enough studies have been conducted to determine whether its subsequent loss on more isolated archipelagos is a general trend of island evolution.
We encourage two types of future research. First, a common pattern seems to emerge for heteroblasty and spinescence, where these forms of defence evolve on big continental fragments and land-bridge islands and are then lost on smaller, more isolated oceanic islands. If proven, this hypothesis would provide a unifying framework for what are now considered opposite patterns. Secondly, why certain species would evolve bigger leaves as juveniles after island colonisation remains unclear. Burns and Dawson (Citation2009) suggest that possessing smaller adult leaves may be a biomechanical adaptation to generate less stress on flowering stalks in high winds. It would be worth testing this hypothesis, and examining whether this pattern can be extended on more species on multiple archipelagos.
Leaf domatia are distinctive structures on leaves and shoots that house invertebrate mites. In New Zealand, domatia provide refuge and shelter to the mites, which in turn eat herbivorous arthropods and fungal pathogens (Agrawal Citation1997; Romero and Benson Citation2005). Therefore, leaf domatia can be an indirect form of plant defence, and may be lost on isolated islands.
A single study tested for the loss of domatia in the New Zealand region, albeit not on the outlying oceanic islands. Using 6 species distributed across 60 less isolated islands off the coast of New Zealand, Biddick (Citation2023) found no support for loss of defence on islands, as changes in domatia production were linked to leaf area, not insularity. This trait has yet to receive global attention. It is possible that insects and fungi, predated by mites, which readily disperse to islands, create predation pressures similar to those on the mainland. However, it should be noted that the study mostly considered land-bridge islands housing young floras. The pattern may change drastically on oceanic islands (Biddick Citation2023).
Most work on the loss of defence hypothesis in island plants focuses on vertebrates (Ciarle et al. Citation2024). Little is known about how invertebrate defences vary on islands (but see Moreira et al. Citation2021). Future research would benefit from testing the loss of domatia on the oceanic archipelagos surrounding New Zealand and investigating the potential loss of other forms of defence against invertebrates (e.g. trichomes, deceptive colouration, chemical defences) (War et al. Citation2012; Farmer Citation2014).
Stem size
Burns et al. (Citation2012) tested for an island-rule-like pattern in stem size (measured as the diameter of the stem immediately adjacent to the point of petiole attachment) using 14 island/mainland pairs from 6 archipelagos and found a consistent trend toward gigantism. Plasticity was taken into account by sampling all plant species from the same garden. Testing for changes in leaf-stem allometry on islands, Biddick et al. (Citation2019a) found that stem size varied with latitude, not insularity. The trait has never been investigated outside of the New Zealand region. From our current understanding, this trait seems to be largely correlative, and would be informative only when analysed alongside other traits (e.g. leaf size) (Burns et al. Citation2012).
One possible leaf component of the island syndrome has never been investigated in the New Zealand region: the in situ evolution of tufted-leaved growth (i.e. plants with leaves only in rosettes at branch tips), a rare feature of some island plants (Moreira-Munoz et al. Citation2013). The selective agents behind this trait remain unclear, but tufted-leaved growth is especially common in the dry coastal zones of the Canary Islands (Whittaker et al. Citation2023), and may be an uncommon feature of New Zealand’s subtropical and cool-temperate islands.
Flowers
Baker’s rule/self-compatibility
Following long-distance dispersal and colonisation, mate limitation can favour the establishment of species capable of self-fertilisation (Baker Citation1967; Pannell et al. Citation2015). This phenomenon, termed Baker’s rule, suggests that self-compatible and dimorphic species (i.e. individuals possessing either male or female function) should be over- and underrepresented respectively among early island colonisers.
The New Zealand flora has long been known for its abundance of gender dimorphic species (Carlquist Citation1974; Webb et al. Citation1999; McGlone and Richardson Citation2023). Although the breeding systems of several New Zealand outlying island plants have been studied extensively (Lloyd and Horning Citation1979; Powlesland Citation1984; Webb and Pearson Citation1993; Bergstrom et al. Citation1997; Schmidt-Adam et al. Citation2000; Lord Citation2012), only a single study tested whether self-compatible and dimorphic species are over- and underrepresented respectively on New Zealand’s outlying islands. Using 71 species, Lord (Citation2015) showed how the proportion of species with self-compatible flowers is higher on subantarctic islands compared to the New Zealand mainland. Conversely, the proportion of dimorphic species did not differ between islands and the mainland.
Globally, when limited to the initial stages of colonisation, Baker’s rule has been supported as an assembly rule of island floras (Pannell et al. Citation2015; Grossenbacher et al. Citation2017; Razanajatovo et al. Citation2019; Whittaker et al. Citation2023). Lord’s analysis (Citation2015) tested for possible island assembly rules, but the aim of the study was not to test Baker’s rule. Consequently, it made no distinction between early colonisers and ancient endemics and it did not investigate whether gender dimorphism arose in situ or was already present in the island lineages at colonisation. Although an assembly rule seems to exist for self-compatible species, Baker’s rule needs to be tested further in the New Zealand region.
Selfing avoidance
After the initial stages of colonisation (i.e. beyond the scope of Baker’s rule) selection may favour self-incompatible species to avoid inbreeding depression. In the New Zealand region, the the only work on the subject comes from Chatham Island, where Gentianella lineata and Gentianella chathamica lost the dichogamous system (i.e. receptivity of the stigma and shedding of the pollen occurring at different times) of their mainland ancestor and became self-compatible. The two species then evolved to be approach herkogamous (i.e. stigma and anthers being spatially separated, so that pollinators come in contact with the stigma before removing the pollen) (Webb and Pearson Citation1993). While both breeding systems limit selfing, herkogamy has the advantage over dichogamy of requiring a single insect visit to pick up and deposit pollen, a crucial advantage in environments with impoverished pollinator services (Webb and Pearson Citation1993).
Globally, transitions toward herkogamy have not been investigated. Conversely, transitions toward dioecy are globally supported (Sakai et al. Citation1995; Francisco-Ortega et al. Citation2000; Burns Citation2019), but were never explored on New Zealand’s outlying islands. Additionally, selfing-avoiding mechanisms such as stylar polymorphism (e.g. distyly and tristyly) have been proposed to hinder island establishment (Watanabe and Sugawara Citation2015), but support for this pattern remains tentative.
Anemophily
Despite the great variety of pollination syndromes (i.e. suites of floral traits appearing in connection with specific functional pollinator groups) present across Angiosperms (Dellinger Citation2020), only a subset of this diversity seems to dominate insular systems. It has been hypothesised that anemophilous flowers and flowers relying on multiple, unspecialised pollinators would be overrepresented on islands as they are less reliable on specific pollination syndromes (Carlquist Citation1974; Whittaker et al. Citation2023).
Although numerous studies focused on the pollination syndromes of plants on New Zealand’s mainland and subantarctic islands (Anderson Citation2003; Kelly et al. Citation2004; Newstrom and Robertson Citation2005; Lord Citation2012; Lord et al. Citation2013; Burns Citation2019; Buxton et al. Citation2019), a single study tested for an assembly rule in anemophilous species. Using a dataset comprising 321 species, Lord (Citation2015) found no difference in the incidence of anemophilous species between New Zealand subantarctic islands and the New Zealand mainland.
Globally, anemophily does not seem to be overrepresented on islands (Whittaker et al. Citation2023). Instead, switching from insect pollination toward wind or vertebrate pollination, which is a reasonably well-supported evolutionary component of the plant island syndrome, has never been investigated in the New Zealand region.
Size
Size coupling is an important aspect of pollination mutualisms, as smaller flowers tend to be pollinated by smaller pollinators and larger flowers by larger pollinators (Kuriya et al. Citation2015; Biddick and Burns Citation2018). Consequently, the reduced size-range of island pollinators may reduce the flower-size diversity of zoophilous island flowers, causing flower size to follow a pattern analogous to the island rule in body size.
Using 11 island/mainland pairs of zoophilous plants from New Zealand’s outlying islands, Burns (Citation2019) found evidence for an island-rule-like pattern in flower perianth size. Globally, a single study tested for a general trend in the in situ evolution of flower size, but failed to find any significant pattern (Hetherington-Rauth and Johnson Citation2020). As most peculiarities of island flowers are regarded as products of biased colonisation (Bernardello et al. Citation2001; Newstrom and Robertson Citation2005; Hetherington-Rauth and Johnson Citation2020), confirming the island rule in flower size would provide the first evidence of a generalised in situ evolutionary trajectory of flowers on islands (C).
Attractiveness
Because islands typically provide impoverished pollinator services (Schueller Citation2007; Yamada et al. Citation2014; Grossenbacher et al. Citation2017), island flowers are often predicted to be inconspicuous, dull-coloured and actinomorphic (Carlquist Citation1974; Barrett Citation1996; Whittaker et al. Citation2023).
Even though various studies noted the conspicuous flower displays in a number of unrelated genera on New Zealand’s southern outlying islands (Wardle Citation1978; Godley Citation1982; Lloyd Citation1985; Lord et al. Citation2013), the only study to compare island and mainland species comes from Auckland and Campbell Island, where species of the genus Abrotanella, present on the mainland with white, inconspicuous flowers evolved purple, vividly coloured capitula (Swenson and Bremer, Citation1997) (D).
The unusual in situ evolution of vividly coloured flowers may reflect evolution in an environment where every possibility of attracting insect pollinators needs to be exploited. Under this hypothesis, the phenomenon should be accentuated in dioecious entomophilous species (Godley Citation1979). Resources allocated to flowers need to increase reproductive success, which is pollinator-driven. However, flowers are also subjected to non-pollinator selective pressures such environmental stresses, which determine flower maintenance costs. If reproductive success increases at a higher rate than maintenance costs, limiting pollinators will increase selective pressures toward pollinator attraction. Only when pollinators become too scarce and/or mating partners are limited, selection will favour individuals that rely on abiotic vectors/self-fertilisation (Maciel et al. Citation2020; Roddy et al. Citation2021). Consequently, depending on the biotic and abiotic factors of islands, pollinator paucity could result in showy flowers.
Globally, this component remains an understudied aspect of the island syndrome. Future research would benefit from testing changes in attractiveness in island taxa pollinated by organisms that responds to the same stimuli (e.g. colour, scent) as the mainland relative(s). As switching from insect pollination toward wind or vertebrate pollination appears to be a feature of island floras, it would also be interesting to quantify changes in resources allocated to attractiveness when the floral characters required to attract pollinators change abruptly.
Finally, generalised floral morphologies (i.e. morphologies allowing pollination by multiple biotic vectors) appear to facilitate island establishment. Supported by several island systems (Barrett Citation1996; Crawford et al. Citation2011; Whittaker et al. Citation2023), this pattern has never been investigated on New Zealand’s outlying islands.
Seeds
Seed size
The loss of dispersal ability hypothesis predicts that wind-dispersed island plants should evolve bigger seeds to avoid being blown out at sea (Darwin Citation1859; Burns Citation2019). Alternatively, the density-compensation hypothesis predicts that seeds of all species should increase in size as a way to increase the competitive ability of offspring to cope with the increased population density typical of many islands (Burns Citation2019).
Testing for an island-rule-like pattern in seed size, Biddick et al. Citation2019b found that island seeds were predominantly larger than mainland seeds. This pattern was confirmed by other studies, all of which found robust support for gigantism in seed size (Kavanagh and Burns Citation2014; Burns Citation2016a; Cox and Burns Citation2017). Using 36 island/mainland pairs, Kavanagh and Burns (Citation2014) also accounted for fruit type, finding that fleshy-fruited taxa tend to show greater increases in seed size than dry-fruited taxa. Using 14 island/mainland pairs, Burns et al. (Citation2012) was the only study to find no evolutionary pattern in seed size.
Globally, seed gigantism in dry-fruited taxa is a reasonably well supported component of the island syndrome, although the mechanisms behind it remain unclear (Carlquist Citation1974; Fresnillo and Ehlers Citation2008; Knope et al. Citation2020). Whether the same pattern applies to fleshy-fruited species, as suggested from New Zealand studies (Kavanagh and Burns Citation2014) remains unclear (Burns Citation2019) (E).
Kavanagh and Burns (Citation2014) proposed that the accentuated increase in seed size in fleshy-fruited species may result from size coupling between fruits and frugivores, where putative selective pressures for increased seed size may not result in loss of dispersibility. We suggest future research to test for the loss of dispersibility in fleshy-fruited plants, as this would provide the first evidence for/against loss of dispersibility in endozoochorous species. We also propose that future studies test whether seed size acts as a constraint for the colonisation of New Zealand outlying islands, as this globally well-supported pattern (Whittaker et al. Citation2023) has never been tested in the New Zealand region.
Seed shape
As seed size increases, seed shape of fleshy-fruited species may change to facilitate ingestion and dispersal by island birds (Burns Citation2019). In a study on Alyxia ruscifolia on Lord Howe Island, Burns (Citation2016a) found that seed shape differed between island and mainland plants. Seeds produced by island plants were less circular and more oblong than seeds produced by mainland plants (F).
This trait has yet to receive global attention. The oblong shape of island seeds may represent an adaptation to facilitate dispersal. Seed gigantism is common in various island floras, and the upper size limit of fruits that can be swallowed whole by birds is set by their smallest transverse dimension. Therefore, as seed size increases, plants may be able to retain small-gaped birds as seed dispersers by producing less circular, more oblong fruits (Lord Citation2004).
We encourage future studies to test this hypothesis outside the New Zealand region, comparing dry- and fleshy-fruited lineages. Also, as past studies have mostly focused on seed mass and trade-offs between seed size and number (Moles and Westoby Citation2006), we encourage future work to view the seed economics spectrum in its entirety, investigating how the ecological and evolutionary dynamics of islands influence seed morphology (e.g. seed size, coat thickness, shape, surface), physiology (e.g. germination timing, light and temperature requirements) and chemistry (e.g. chemical defences) (Saatkamp et al. Citation2019).
Serotiny
Wildfires can occasionally occur in even the coldest and wettest environments on Earth (see Burns Citation2019). The evolutionary history of plants has therefore been tightly intertwined with wildfires (Keeley et al. Citation2011; Lamont and He Citation2017), and plants have evolved several traits to cope with fire, such as serotiny (i.e. retaining seeds in woody cones/fruits and releasing them after fires) (Lamont et al. Citation2020), thick bark (Pausas Citation2015) and lignotubers (i.e. woody growth containing food reserves and buds) (Pausas et al. Citation2018). Island environments are usually less prone to wildfires than mainland environments (Perry et al. Citation2014). Consequently, plants are expected to lose fire-adapted traits after island colonisation.
Australia is one of the most fire-prone places on earth (Bradstock et al. Citation2012), making the islands lying between Australia and New Zealand ideal for testing the loss of fire-adapted traits hypothesis. Burns (Citation2019) compared the degree of serotiny between Melaleuca howeana on Lord Howe Island and Melaleuca ericifolia on the Australian mainland and found clear evidence for loss of serotiny. While this can be viewed as a loss of fire-adapted traits, other ecological factors could not be ruled out (Hamilton-Brown et al. Citation2009; Burns Citation2019). Globally, the handful of available studies provide limited support for the loss of fire adaptations as a component of the island syndrome (Climent et al. Citation2004; Stephens and Libby Citation2006; Garcillán Citation2010; Briand et al. Citation2015).
Synthesis and future directions
Most studies on the island syndrome in plants on New Zealand’s outlying islands (and worldwide) focus on a single trait. Yet, the island syndrome is by definition a suite of multiple traits. To understand whether a plant will successfully colonize/establish on an island and the directions in which it will evolve once established, a multi-trait approach is needed. This is especially relevant considering that many plant traits are known to covary allometrically (Corner Citation1949; Harvey and Pagel Citation1991; Niklas Citation1994; Minelli Citation2018; E-Vojtkó et al. Citation2022) and that various components of the island syndrome in the New Zealand region are thought to be largely correlative (Burns Citation2019).
We believe future research would benefit from formalising the evolutionary component of the plant island syndrome as a multidimensional space, with each dimension being a trait component. By defining the position in trait space of island endemics, relative to their closest mainland relatives and their common ancestors, this approach would facilitate (I) Quantifying and comparing the degree of morphological evolution in island and mainland lineages; (II) Testing for convergent evolution (i.e. an island syndrome) in island endemics; (III) Testing for the possible existence of multiple island syndromes (i.e. multiple focal points in trait space) (Stayton Citation2015; Castiglione et al. Citation2019; Gómez et al. Citation2022).
Additionally, as suggested by traits such as divarication and spinescence, the island syndrome may not be monotonic, and its direction may vary considerably depending on the characteristics of islands. It could be beneficial to group islands based on their abiotic and biotic factors (e.g. island area, isolation, elevation, bird species diversity) and test for modularity of trait space, i.e. whether species are clustered in the trait space based on island types (Gómez et al. Citation2022), and the existence of different focal points in trait space for different island types. Both approaches require an extensive dataset including a significant portion of the traits discussed in this review and a well-resolved phylogeny of the target species.
In conclusion, New Zealand’s outlying islands have played a pivotal role in our understanding of the plant island syndrome and its components. However, only a fraction of these are currently well-supported. Overall, while the further study of individual traits would be valuable, it would be most beneficial to adopt a multi-trait approach, trying to address the plant island syndrome in its entirety.
Supplementary material
Download MS Excel (14.3 KB)Acknowledgements
We thank Alessandra Franco for drawing . D was taken from the iNaturalist profile of Christopher Stephens.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The list of studies consulted to make this review is available in the supplementary material of this article.
References
- Adams CJ. 1983. Age of the volcanoes and granite basement of the Auckland Islands, Southwest Pacific. New Zealand Journal of Geology and Geophysics. 26(3):227–237. doi: 10.1080/00288306.1983.10422237.
- Agrawal A. 1997. Do leaf domatia mediate a plant–mite mutualism? An experimental test of the effects on predators and herbivores. Ecological Entomology. 22(4):371–376. doi: 10.1046/j.1365-2311.1997.00088.x.
- Anderson SH. 2003. The relative importance of birds and insects as pollinators of the New Zealand flora. New Zealand Journal of Ecology. 27(2):83–94.
- Baker HG. 1967. Support for Baker's Law-as a rule. Evolution. 21(4):853–856. doi: 10.2307/2406780.
- Baker HG, Cox PA. 1984. Further thoughts on dioecism and islands. Annals of the Missouri Botanical Garden. 71(1):244–253. doi: 10.2307/2399068.
- Barrett SC, Hough J. 2013. Sexual dimorphism in flowering plants. Journal of Experimental Botany. 64(1):67–82. doi: 10.1093/jxb/ers308.
- Barrett SCH. 1996. The reproductive biology and genetics of island plants. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 351(1341):725–733. doi: 10.1098/rstb.1996.0067.
- Benítez-López A, Santini L, Gallego-Zamorano J, Milá B, Walkden P, Huijbregts MA, Tobias JA. 2021. The island rule explains consistent patterns of body size evolution in terrestrial vertebrates. Nature Ecology & Evolution. 5(6):768–786. doi: 10.1038/s41559-021-01426-y.
- Bergstrom DM, Selkirk PM, Keenan HM, Wilson ME. 1997. Reproductive behaviour of ten flowering plant species on subantarctic Macquarie Island. Opera Botanica. 132:109–120.
- Bernardello G, Anderson GJ, Stuessy TF, Crawford DJ. 2001. A survey of floral traits, breeding systems, floral visitors, and pollination systems of the angiosperms of the Juan Fernández Islands (Chile). The Botanical Review. 67:255–308. doi: 10.1007/BF02858097.
- Biddick M. 2023. Are all forms of defence lost on islands? Persistence of a defensive mutualism in six island colonists from New Zealand. Biology Letters. 19(4):20220425. doi: 10.1098/rsbl.2022.0425.
- Biddick M, Burns KC. 2018. Phenotypic trait matching predicts the topology of an insular plant-bird pollination network. Integrative Zoology. 13(3):339–347. doi: 10.1111/1749-4877.12319.
- Biddick M, Hendriks A, Burns KC. 2019b. Plants obey (and disobey) the island rule. Proceedings of the National Academy of Sciences. 116(36):17632–17634. doi: 10.1073/pnas.1907424116.
- Biddick M, Hutton I, Burns KC. 2019a. Independent evolution of allometric traits: a test of the allometric constraint hypothesis in island vines. Biological Journal of the Linnean Society. 126(1):203–211. doi: 10.1093/biolinnean/bly158.
- Bond WJ, Silander JA. 2007. Springs and wire plants: anachronistic defences against Madagascar's extinct elephant birds. Proceedings of the Royal Society B: Biological Sciences. 274(1621):1985–1992. doi: 10.1098/rspb.2007.0414.
- Bradstock RA, Williams RJ, Gill AM. 2012. Flammable Australia: fire regimes, biodiversity and ecosystems in a changing world. Melbourne: CSIRO publishing.
- Briand CH, Schwilk DW, Gauthier S, Bergeron Y. 2015. Does fire regime influence life history traits of jack pine in the southern boreal forest of Québec, Canada? Plant Ecology. 216:157–164. doi: 10.1007/s11258-014-0424-x.
- Brook FJ. 1998. Stratigraphy and paleontology of pleistocene submarine volcanic-sedimentary sequences at the northern Kermadec Islands. Journal of the Royal Society of New Zealand. 28(2):235–257. doi: 10.1080/03014223.1998.9517561.
- Burns KC. 2016a. Size changes in island plants: independent trait evolution in Alyxia ruscifolia (Apocynaceae) on Lord Howe Island. Biological Journal of the Linnean Society. 119(4):847–855. doi: 10.1111/bij.12851.
- Burns KC. 2016b. Spinescence in the New Zealand flora: parallels with Australia. New Zealand Journal of Botany. 54(2):273–289. doi: 10.1080/0028825X.2015.1130727.
- Burns KC. 2019. Evolution in isolation: the search for an island syndrome in plants. Cambridge (UK): Cambridge University Press.
- Burns KC. 2022. The paradox of island evolution. Journal of Biogeography. 49(2):248–253. doi: 10.1111/jbi.14303.
- Burns KC, Dawson JW. 2006. A morphological comparison of leaf heteroblasty between New Caledonia and New Zealand. New Zealand Journal of Botany. 44(4):387–396. doi: 10.1080/0028825X.2006.9513030.
- Burns KC, Dawson JW. 2009. Heteroblasty on Chatham Island: a comparison with New Zealand and New Caledonia. New Zealand Journal of Ecology. 33(2):156–163.
- Burns KC, Herold N, Wallace B. 2012. Evolutionary size changes in plants of the south-west Pacific. Global Ecology and Biogeography. 21(8):819–828. doi: 10.1111/j.1466-8238.2011.00730.x.
- Buxton MN, Anderson BJ, Hoare RJ, Lord JM. 2019. Are moths the missing pollinators in subantarctic New Zealand? Polar Research. 38:3545. doi: 10.33265/polar.v38.3545.
- Carlquist S. 1974. Island biology. New York (NY): Columbia University Press.
- Castiglione S, Serio C, Tamagnini D, Melchionna M, Mondanaro A, Di Febbraro M, Profico A, Piras P, Barattolo F, Raia P. 2019. A new, fast method to search for morphological convergence with shape data. PLoS One. 14(12):e0226949. doi: 10.1371/journal.pone.0226949.
- Ciarle R, Burns KC. 2023. Island biogeography of birds in the south west Pacific: direct and indirect effects of physical geography and co-occurring vegetation. Journal of Biogeography.
- Ciarle R, Burns KC, Mologni F. 2024. The loss (and gain) of defensive adaptations in island plants and animals: a comparative review. In: Moreira X, Abdala-Roberts L, editors. Ecology and evolution of plant-herbivore interactions on islands, vol. 249. London: Springer Nature; p. 69–93.
- Climent J, Tapias R, Pardos JA, Gil L. 2004. Fire adaptations in the Canary Islands pine (Pinus canariensis). Plant Ecology. 171:185–196. doi: 10.1023/B:VEGE.0000029374.64778.68.
- Conn B. 1996. Flora of Australia volume 49: Oceanic islands 1. Melbourne: CSIRO Publishing.
- Cornelissen JH, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, et al. 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany. 51(4):335–380. doi: 10.1071/BT02124.
- Corner EJH. 1949. The durian theory or the origin of the modern tree. Annals of Botany. 13(52):367–414. doi: 10.1093/oxfordjournals.aob.a083225.
- Cox BTM, Burns KC. 2017. Convergent evolution of gigantism in the flora of an isolated archipelago. Evolutionary Ecology. 31:741–752. doi: 10.1007/s10682-017-9909-0.
- Crawford DJ, Anderson GJ, Bernardello G. 2011. The reproductive biology of island plants. In: Bramwell D, Caujapé-Castells J, editors. The biology of island floras. Cambridge, England: Cambridge University Press; p. 11–36.
- Darwin C. 1859. On the origins of species by means of natural selection. London: Murray.
- De Lange PJ. 2014. The flora of Egeria Rock, Northern Kermadec Island Group. Wellington Botanical Society Bulletin. 55:41–49.
- De Lange PJ, Heenan PB, Rolfe JR. 2011. Checklist of vascular plants recorded from Chatham Islands. Wellington: Department of Conservation, Wellington Hawke's Bay Conservancy.
- Delavaux CS, Weigelt P, Dawson W, Essl F, van Kleunen M, König C, Pergl J, Pyšek P, Stein A, Winter M, et al. 2021. Mycorrhizal types influence island biogeography of plants. Communications Biology. 4(1):1128. doi: 10.1038/s42003-021-02649-2.
- Dellinger AS. 2020. Pollination syndromes in the 21st century: where do we stand and where may we go? New Phytologist. 228(4):1193–1213. doi: 10.1111/nph.16793.
- Dellinger AS, Artuso S, Pamperl S, Michelangeli FA, Penneys DS, Fernández-Fernández DM, Alvear M, Almeda F, Armbruster WS, Staedler Y, Schönenberger J. 2019. Modularity increases rate of floral evolution and adaptive success for functionally specialized pollination systems. Communications Biology. 2(1):453. doi: 10.1038/s42003-019-0697-7.
- De Salas MF, Baker ML. 2015. A census of the vascular plants of Tasmania, including Macquarie Island. Tasmanian Herbarium, Tasmanian Museum and Art Gallery.
- Eskildsen LI, Olesen JM, Jones CG. 2004. Feeding response of the Aldabra giant tortoise (Geochelone gigantea) to island plants showing heterophylly. Journal of Biogeography. 31(11):1785–1790. doi: 10.1111/j.1365-2699.2004.01092.x.
- E-Vojtkó A, Junker RR, de Bello F, Götzenberger L. 2022. Floral and reproductive traits are an independent dimension within the plant economic spectrum of temperate central Europe. New Phytologist. 236(5):1964–1975. doi: 10.1111/nph.18386.
- Farmer EE. 2014. Leaf defence. Oxford: Oxford University Press.
- Faurby S, Svenning JC. 2016. Resurrection of the island rule: human-driven extinctions have obscured a basic evolutionary pattern. The American Naturalist. 187(6):812–820. doi: 10.1086/686268.
- Forster MA, Bonser SP. 2009. Heteroblastic development and the optimal partitioning of traits among contrasting environments in Acacia implexa. Annals of Botany. 103(1):95–105. doi: 10.1093/aob/mcn210.
- Francisco-Ortega J, Santos-Guerra A, Kim SC, Crawford DJ. 2000. Plant genetic diversity in the Canary Islands: a conservation perspective. American Journal of Botany. 87(7):909–919. doi: 10.2307/2656988.
- Fresnillo B, Ehlers BK. 2008. Variation in dispersability among mainland and island populations of three wind dispersed plant species. Plant Systematics and Evolution. 270:243–255. doi: 10.1007/s00606-007-0615-1.
- Garcillán PP. 2010. Seed release without fire in Callitropsis guadalupensis, a serotinous cypress of a Mediterranean-climate oceanic island. Journal of Arid Environments. 74(4):512–515. doi: 10.1016/j.jaridenv.2009.09.017.
- Givnish TJ. 1998. Adaptive plant evolution on islands: classical patterns, molecular data, new insights. In: Grant PR, editor. Evolution on islands. Oxford: Oxford University Press; p. 281–304.
- Givnish TJ, Sytsma KJ, Smith JF, Hahn WJ. 1994. Thorn-like prickles and heterophylly in cyanea: adaptations to extinct avian browsers on Hawaii? Proceedings of the National Academy of Sciences. 91(7):2810–2814. doi: 10.1073/pnas.91.7.2810.
- Godley EJ. 1969. Additions and corrections to the flora of the Auckland and Campbell Islands. New Zealand Journal of Botany. 7(4):336–348. doi: 10.1080/0028825X.1969.10428849.
- Godley EJ. 1979. Flower biology in New Zealand. New Zealand Journal of Botany. 17(4):441–466. doi: 10.1080/0028825X.1979.10432564.
- Godley EJ. 1982. Breeding systems in New Zealand plants 6. Gentiana Antarctica and G. Antipoda. New Zealand Journal of Botany. 20(4):405–420.
- Godley EJ. 1989. The flora of Antipodes Island. New Zealand Journal of Botany. 27(4):531–564. doi: 10.1080/0028825X.1989.10414138.
- Gómez JM, González-Megías A, Narbona E, Navarro L, Perfectti F, Armas C. 2022. Phenotypic plasticity guides Moricandia arvensis divergence and convergence across the Brassicaceae floral morphospace. New Phytologist. 233(3):1479–1493. doi: 10.1111/nph.17807.
- Green PS. 1994. Flora of Australia, volume 49, Oceanic islands 1. Canberra: Australian Government Printing Service.
- Greenwood RM, Atkinson IAE. 1977. Evolution of divaricating plants in New Zealand in relation to moa browsing. New Zealand Ecological Society. 24:21–33.
- Grossenbacher DL, Brandvain Y, Auld JR, Burd M, Cheptou PO, Conner JK, Grant AG, Hovick SM, Pannell JM, Pauw A, et al. 2017. Self-compatibility is over-represented on islands. New Phytologist. 215(1):469–478. doi: 10.1111/nph.14534.
- Grubb PJ. 2003. Interpreting some outstanding features of the flora and vegetation of Madagascar. Perspectives in Plant Ecology, Evolution and Systematics. 6(1-2):125–146. doi: 10.1078/1433-8319-00046.
- Hamilton-Brown S, Boon PI, Raulings E, Morris K, Robinson R. 2009. Aerial seed storage in Melaleuca ericifolia Sm. (Swamp Paperbark): environmental triggers for seed release. Hydrobiologia. 620:121–133.
- Harvey PH, Pagel MD. 1991. The comparative method in evolutionary biology, vol. 239. Oxford: Oxford University Press.
- Hayward BW, Moore PR. 1987. Geology of the Three Kings Islands, northern New Zealand. Records of the Auckland Institute and Museum. 24:215–232.
- Heenan PB. 1997. Heteroblasty in Carmichaelia, Chordospartium, Corallospartium, and Notospartium (Fabaceae—Galegeae) from New Zealand. New Zealand Journal of Botany. 35(2):243–249. doi: 10.1080/0028825X.1997.10414160.
- Hetherington-Rauth MC, Johnson MT. 2020. Floral trait evolution of angiosperms on Pacific islands. The American Naturalist. 196(1):87–100. doi: 10.1086/709018.
- Howard J, Cameron E, Bellve A, Baba Y, Wright S. 2022. New Zealand divaricate plant species: tensile strength and remote island occurrence. Austral Ecology. 47(5):1091–1100. doi: 10.1111/aec.13198.
- Johnson PN, Campbell DJ. 1975. Vascular plants of the Auckland Islands. New Zealand Journal of Botany. 13(4):665–720. doi: 10.1080/0028825X.1975.10430354.
- Jones JG, McDougall I. 1973. Geological history of Norfolk and Philip Islands, southwest Pacific Ocean. Journal of the Geological Society of Australia. 20(3):239–254. doi: 10.1080/14400957308527916.
- Kavanagh PH. 2015. Herbivory and the evolution of divaricate plants: structural defences lost on an outlying island. Austral Ecology. 40(2):206–211. doi: 10.1111/aec.12196.
- Kavanagh PH, Burns KC. 2014. The repeated evolution of large seeds on islands. Proceedings of the Royal Society B: Biological Sciences. 281(1786):20140675. doi: 10.1098/rspb.2014.0675.
- Kavanagh PH, Burns KC. 2015. Sexual size dimorphism in island plants: the niche variation hypothesis and insular size changes. Oikos. 124(6):717–723. doi: 10.1111/oik.01753.
- Keeley JE, Pausas JG, Rundel PW, Bond WJ, Bradstock RA. 2011. Fire as an evolutionary pressure shaping plant traits. Trends in Plant Science. 16(8):406–411. doi: 10.1016/j.tplants.2011.04.002.
- Kelly D, Ladley JJ, Robertson AW. 2004. Is dispersal easier than pollination? Two tests in New Zealand Loranthaceae. New Zealand Journal of Botany. 42(1):89–103. doi: 10.1080/0028825X.2004.9512892.
- Knope ML, Funk VA, Johnson MA, Wagner WL, Datlof EM, Johnson G, Crawford DJ, Bonifacino JM, Morden CW, Lorence DH, et al. 2020. Dispersal and adaptive radiation of Bidens (Compositae) across the remote archipelagoes of Polynesia. Journal of Systematics and Evolution. 58(6):805–822. doi: 10.1111/jse.12704.
- Kuriya S, Hattori M, Nagano Y, Itino T. 2015. Altitudinal flower size variation correlates with local pollinator size in a bumblebee-pollinated herb, Prunella vulgaris (Lamiaceae). Journal of Evolutionary Biology. 28(10):1761–1769. doi: 10.1111/jeb.12693.
- Lamont BB, He T. 2017. Fire-proneness as a prerequisite for the evolution of fire-adapted traits. Trends in Plant Science. 22(4):278–288. doi: 10.1016/j.tplants.2016.11.004.
- Lamont BB, Pausas JG, He T, Witkowski ET, Hanley ME. 2020. Fire as a selective agent for both serotiny and nonserotiny over space and time. Critical Reviews in Plant Sciences. 39(2):140–172. doi: 10.1080/07352689.2020.1768465.
- Linder HP, Barker NP. 2014. Does polyploidy facilitate long-distance dispersal? Annals of Botany. 113(7):1175–1183. doi: 10.1093/aob/mcu047.
- Lloyd DG. 1981. Evolution of prostrate and erect habits in Cotula section Leptinella and other New Zealand plant groups. New Zealand Journal of Botany. 19(3):247–253. doi: 10.1080/0028825X.1981.10426376.
- Lloyd DG. 1985. Progress in understanding the natural history of New Zealand plants. New Zealand Journal of Botany. 23(4):707–722. doi: 10.1080/0028825X.1985.10434239.
- Lloyd DG, Horning DS. 1979. Distribution of sex in Coprosma pumila on Macquarie Island, Australia. New Zealand Journal of Botany. 17(1):5–7. doi: 10.1080/0028825X.1979.10425155.
- Lloyd DG, Webb CJ. 1977. Secondary sex characters in plants. The Botanical Review. 43(2):177–216. doi: 10.1007/BF02860717.
- Lomolino MV, Sax DF, Palombo MR, van der Geer AA. 2012. Of mice and mammoths: evaluations of causal explanations for body size evolution in insular mammals. Journal of Biogeography. 39(5):842–854. doi: 10.1111/j.1365-2699.2011.02656.x.
- Lord JM. 2004. Frugivore gape size and the evolution of fruit size and shape in southern hemisphere floras. Austral Ecology. 29(4):430–436. doi: 10.1111/j.1442-9993.2004.01382.x.
- Lord JM. 2012. Hermaphroditism and dichogamy in Stilbocarpa polaris (Araliaceae) on Campbell Island. New Zealand Journal of Botany. 50(1):89–93. doi: 10.1080/0028825X.2011.638645.
- Lord JM. 2015. Patterns in floral traits and plant breeding systems on Southern Ocean islands. AoB PLANTS. 7:plv095.
- Lord JM, Huggins L, Little LM, Tomlinson VR. 2013. Floral biology and flower visitors on subantarctic Campbell Island. New Zealand Journal of Botany. 51(3):168–180. doi: 10.1080/0028825X.2013.801867.
- Lusk CH, McGlone MS, Overton JM. 2016. Climate predicts the proportion of divaricate plant species in New Zealand arborescent assemblages. Journal of Biogeography. 43(9):1881–1892. doi: 10.1111/jbi.12814.
- Maciel AA, Cardoso JCF, Oliveira PE. 2020. On the low reproductive success of two Cyrtopodium species (Orchidaceae: Cyrtopodiinae): the relative roles of biotic and abiotic pollination. Plant Species Biology. 35(1):49–58. doi: 10.1111/1442-1984.12260.
- Maurin KJ, Smissen RD, Lusk CH. 2022. A dated phylogeny shows plio-pleistocene climates spurred evolution of antibrowsing defences in the New Zealand flora. New Phytologist. 233(1):546–554. doi: 10.1111/nph.17766.
- McGlone MS, Duncan RP, Heenan PB. 2001. Endemism, species selection and the origin and distribution of the vascular plant flora of New Zealand. Journal of Biogeography. 28(2):199–216. doi: 10.1046/j.1365-2699.2001.00525.x.
- McGlone MS, Richardson SJ. 2023. Sexual systems in the New Zealand angiosperm flora. New Zealand Journal of Botany. 61(4):201–231. doi: 10.1080/0028825X.2022.2118613.
- McGlone MS, Webb CJ. 1981. Selective forces influencing the evolution of divaricating plants. New Zealand Journal of Ecology. 4:20–28.
- Meiri S, Dayan T, Simberloff D. 2005. Variability and sexual size dimorphism in carnivores: testing the niche variation hypothesis. Ecology. 86(6):1432–1440. doi: 10.1890/04-1503.
- Meredith FL, Tindall ML, Hemmings FA, Moles AT. 2019. Prickly pairs: the proportion of spinescent species does not differ between islands and mainlands. Journal of Plant Ecology. 12(6):941–948. doi: 10.1093/jpe/rtz031.
- Meudt HM, Albach DC, Tanentzap AJ, Igea J, Newmarch SC, Brandt AJ, Lee WG, Tate JA. 2021. Polyploidy on islands: its emergence and importance for diversification. Frontiers in Plant Science. 12:637214. doi: 10.3389/fpls.2021.637214.
- Meurk CD. 1977. Alien plants in Campbell Island's changing vegetation. Mauri Ora. 5:93–118.
- Meurk CD, Foggo MN, Wilson JB. 1994. The vegetation of subantarctic Campbell Island. New Zealand Journal of Ecology. 18(2):123–168.
- Minelli A. 2018. Plant evolutionary developmental biology: the evolvability of the phenotype. Cambridge (UK): Cambridge University Press.
- Mitchell AD, Meurk CD, Wagstaff SJ. 1999. Evolution of Stilbocarpa, a megaherb from New Zealand's sub-antarctic islands. New Zealand Journal of Botany. 37(2):205–211. doi: 10.1080/0028825X.1999.9512628.
- Moles AT, Westoby M. 2006. Seed size and plant strategy across the whole life cycle. Oikos. 113(1):91–105. doi: 10.1111/j.0030-1299.2006.14194.x.
- Monroy P, García-Verdugo C. 2019. Testing the hypothesis of loss of defenses on islands across a wide latitudinal gradient of Periploca laevigata populations. American Journal of Botany. 106(2):303–312. doi: 10.1002/ajb2.1232.
- Moreira X, Castagneyrol B, García-Verdugo C, Abdala-Roberts L. 2021. A meta-analysis of insularity effects on herbivory and plant defences. Journal of Biogeography. 48(2):386–393. doi: 10.1111/jbi.14003.
- Moreira-Munoz A, Francioli SE, Hobohm C, da Silva Menezes de Sequeira MP. 2013. Endemism on Islands–case studies. In: Hobohm C, editor. Endemism in vascular plants. Dordrecht: Springer Netherlands; p. 165–204.
- Murray BG, de Lange PJ, Bramwell D, Caujape-Castells J. 2011. Chromosomes and evolution in New Zealand endemic angiosperms and gymnosperms. In: Bramwell D, Caujapé-Castells J, editors. Biology of island floras. Cambridge (UK): Cambridge University Press; p. 265–283.
- Newstrom L, Robertson A. 2005. Progress in understanding pollination systems in New Zealand. New Zealand Journal of Botany. 43(1):1–59. doi: 10.1080/0028825X.2005.9512943.
- Niklas KJ. 1994. Plant allometry: the scaling of form and process. University of Chicago Press.
- Oliver WRB. 1948. The flora of the Three Kings Islands. Records of the Auckland Institute and Museum. 3(4/5):211–238.
- Pannell JR, Auld JR, Brandvain Y, Burd M, Busch JW, Cheptou PO, Conner JK, Goldberg EE, Grant A, Grossenbacher DL, et al. 2015. The scope of Baker's Law. New Phytologist. 208(3):656–667. doi: 10.1111/nph.13539.
- Pausas JG. 2015. Bark thickness and fire regime. Functional Ecology. 29(3):315–327. doi: 10.1111/1365-2435.12372.
- Pausas JG, Lamont BB, Paula S, Appezzato-da-Glória B, Fidelis A. 2018. Unearthing belowground bud banks in fire-prone ecosystems. New Phytologist. 217(4):1435–1448. doi: 10.1111/nph.14982.
- Perry GL, Wilmshurst JM, McGlone MS. 2014. Ecology and long-term history of fire in New Zealand. New Zealand Journal of Ecology. 38(2):157–176.
- Pollock ML, Lee WG, Walker S, Forrester G. 2007. Ratite and ungulate preferences for woody New Zealand plants: influence of chemical and physical traits. New Zealand Journal of Ecology. 31(1):68–78.
- Powlesland MH. 1984. Reproductive biology of three species of Melicytus (Violaceae) in New Zealand. New Zealand Journal of Botany. 22(1):81–94. doi: 10.1080/0028825X.1984.10425235.
- Rainsley E, Turney CS, Golledge NR, Wilmshurst JM, McGlone MS, Hogg AG, Bo L, Thomas ZA, Roberts R, Jones RT, et al. 2019. Pleistocene glacial history of the New Zealand subantarctic islands. Climate of the Past. 15(2):423–448. doi: 10.5194/cp-15-423-2019.
- Razanajatovo M, van Kleunen M, Kreft H, Dawson W, Essl F, Pergl J, Pyšek P, Winter M, Weigelt P. 2019. Autofertility and self-compatibility moderately benefit island colonization of plants. Global Ecology and Biogeography. 28(3):341–352. doi: 10.1111/geb.12854.
- Roddy AB, Martínez-Perez C, Teixido AL, Cornelissen TG, Olson ME, Oliveira RS, Silveira FA. 2021. Towards the flower economics spectrum. New Phytologist. 229(2):665–672. doi: 10.1111/nph.16823.
- Romero GQ, Benson WW. 2005. Biotic interactions of mites, plants and leaf domatia. Current Opinion in Plant Biology. 8(4):436–440. doi: 10.1016/j.pbi.2005.05.006.
- Saatkamp A, Cochrane A, Commander L, Guja LK, Jimenez-Alfaro B, Larson J, Nicotra A, Poschlod P, Silveira FAO, Cross AT, et al. 2019. A research agenda for seed-trait functional ecology. New Phytologist. 221(4):1764–1775. doi: 10.1111/nph.15502.
- Sakai AK, Wagner WL, Ferguson DM, Herbst DR. 1995. Origins of dioecy in the Hawaiian flora. Ecology. 76(8):2517–2529. doi: 10.2307/2265825.
- Salladay RA, Ramirez AR. 2018. Reduced defenses and increased herbivore preference of island chaparral shrubs compared to mainland relatives. Western North American Naturalist. 78(4):768–776. doi: 10.3398/064.078.0416.
- Schlessman MA, Vary LB, Munzinger J, Lowry PP. 2014. Incidence, correlates, and origins of dioecy in the island flora of New Caledonia. International Journal of Plant Sciences. 175(3):271–286. doi: 10.1086/674452.
- Schmidt-Adam G, Young AG, Murray BG. 2000. Low outcrossing rates and shift in pollinators in New Zealand pohutukawa (Metrosideros excelsa; Myrtaceae). American Journal of Botany. 87(9):1265–1271. doi: 10.2307/2656719.
- Schueller SK. 2007. Island–mainland difference in Nicotiana glauca (Solanaceae) corolla length: a product of pollinator-mediated selection? Evolutionary Ecology. 21:81–98. doi: 10.1007/s10682-006-9125-9.
- Scott JM, Turnbull IM. 2019. Geology of New Zealand’s sub-Antarctic islands. New Zealand Journal of Geology and Geophysics. 62(3):291–317. doi: 10.1080/00288306.2019.1600557.
- Scott JM, Turnbull IM, Auer A, Palin JM. 2013. The sub-Antarctic Antipodes Volcano: a <0.5 Ma HIMU-like Surtseyan volcanic outpost on the edge of the Campbell Plateau, New Zealand. New Zealand Journal of Geology and Geophysics. 56(3):134–153. doi: 10.1080/00288306.2013.802246.
- Soltis DE, Soltis PS, Tate JA. 2004. Advances in the study of polyploidy since plant speciation. New Phytologist. 161(1):173–191. doi: 10.1046/j.1469-8137.2003.00948.x.
- Stanton ML, Palmer TM, Young TP. 2005. Ecological barriers to early colony establishment in three coexisting acacia-ant species in Kenya. Insectes Sociaux. 52:393–401. doi: 10.1007/s00040-005-0826-9.
- Stayton CT. 2015. The definition, recognition, and interpretation of convergent evolution, and two new measures for quantifying and assessing the significance of convergence. Evolution. 69(8):2140–2153. doi: 10.1111/evo.12729.
- Stephens SL, Libby WJ. 2006. Anthropogenic fire and bark thickness in coastal and island pine populations from Alta and Baja California. Journal of Biogeography. 33(4):648–652. doi: 10.1111/j.1365-2699.2005.01387.x.
- Swenson U, Bremer K. 1997. Patterns of floral evolution of four Asteraceae genera (Senecioneae. Blennospermatinae) and the origin of white flowers in New Zealand. Systematic Biology. 46(3):407–425.
- Sykes WR. 1977. Kermadec Islands flora: an annotated check list. Lincoln, New Zealand: Manaaki Whenua Press.
- Sykes WR, West CJ. 1996. New records and other information on the vascular flora of the Kermadec Islands. New Zealand Journal of Botany. 34(4):447–462. doi: 10.1080/0028825X.1996.10410126.
- Taylor RH. 1971. Influence of man on vegetation and wildlife of Enderby and Rose Islands, Auckland Islands. New Zealand Journal of Botany. 9(2):225–268. doi: 10.1080/0028825X.1971.10429139.
- Thompson JN, Merg KF. 2008. Evolution of polyploidy and the diversification of plant–pollinator interactions. Ecology. 89(8):2197–2206. doi: 10.1890/07-1432.1.
- Van Valen L. 1973. Patterns and the balance of nature. Evolutionary Theory. 1:31–49.
- Varne R, Brown AV, Falloon T, Dilek Y. 2000. Macquarie Island: its geology, structural history, and the timing and tectonic setting of its N-MORB to E-MORB magmatism. Special Papers-Geological Society of America. 349:301–320.
- Wagstaff SJ, Tate JA. 2011. Phylogeny and character evolution in the New Zealand endemic genus Plagianthus (Malveae, Malvaceae). Systematic Botany. 36(2):405–418. doi: 10.1600/036364411X569589.
- Wallace AR. 1878. Tropical nature, and other essays. London: Macmillan and Company.
- War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC. 2012. Mechanisms of plant defense against insect herbivores. Plant Signaling & Behavior. 7(10):1306–1320. doi: 10.4161/psb.21663.
- Wardle P. 1978. Origin of the New Zealand mountain flora, with special reference to trans-Tasman relationships. New Zealand Journal of Botany. 16(4):535–550. doi: 10.1080/0028825X.1978.10426881.
- Watanabe K, Sugawara T. 2015. Is heterostyly rare on oceanic islands? AoB Plants. 7:plv087. doi: 10.1093/aobpla/plv087.
- Webb CJ, Lloyd DG, Delph LF. 1999. Gender dimorphism in indigenous New Zealand seed plants. New Zealand Journal of Botany. 37(1):119–130. doi: 10.1080/0028825X.1999.9512618.
- Webb CJ, Pearson PE. 1993. The evolution of approach herkogamy from protandry in New Zealand Gentiana (Gentianaceae). Plant Systematics and Evolution. 186:187–191. doi: 10.1007/BF00940797.
- Whittaker RJ, Fernández-Palacios JM, Matthews TJ. 2023. Island biogeography: geo-environmental dynamics, ecology, evolution, human impact, and conservation. Oxford: Oxford University Press.
- Whittaker RJ, Triantis KA, Ladle RJ. 2008. A general dynamic theory of oceanic island biogeography. Journal of Biogeography. 35(6):977–994. doi: 10.1111/j.1365-2699.2008.01892.x.
- Woodroffe CD, Murray-Wallace CV, Bryant EA, Brooke B, Heijnis H, Price DM. 1995. Late quaternary sea-level highstands in the Tasman Sea: evidence from Lord Howe Island. Marine Geology. 125(1-2):61–72. doi: 10.1016/0025-3227(95)00028-W.
- Wright AE. 1983. Conservation status of the Three Kings Islands endemic flora in 1982. Records of the Auckland Institute and Museum. 20:175–184.
- Yamada T, Kodama K, Maki M. 2014. Floral morphology and pollinator fauna characteristics of island and mainland populations of Ligustrum ovalifolium (Oleaceae). Botanical Journal of the Linnean Society. 174(3):489–501. doi: 10.1111/boj.12092.
- Zizka A, Onstein RE, Rozzi R, Weigelt P, Kreft H, Steinbauer MJ, Bruelheide H, Lens F. 2022. The evolution of insular woodiness. Proceedings of the National Academy of Sciences. 119(37):e2208629119. doi: 10.1073/pnas.2208629119.
- Zotz G, Wilhelm K, Becker A. 2011. Heteroblasty—a review. The Botanical Review. 77:109–151. doi: 10.1007/s12229-010-9062-8.
