Abstract
Shells of commercially valued bivalves in New Zealand, Crassostrea gigas, Perna canaliculus and Pecten novaezelandiae, are damaged by blister-causing Polydora polychaete species known to be close in morphology to the widely recorded oyster pest Polydora websteri Hartman. Recent New Zealand occurrences are here confirmed to relate to two species, P. websteri, and a second similar species, Polydora haswelli Blake & Kudenov, a new record for New Zealand, previously known only from Australia; the two species are described and compared. The worms have limited distributions, with P. websteri confirmed only for Pacific oysters (C. gigas) in northern New Zealand, although prior reports indicate it may also occur on scallops and have reached the northern South Island. Polydora haswelli has been found only in northern New Zealand, occurring on subtidal mussels and scallops and native oysters (Perna canaliculus, Pecten novaezelandiae, Ostrea chilensis), as well as co-existing with intertidal P. websteri on Pacific oysters. The worms are not present in Foveaux Strait O. chilensis beds, a major source of past oyster exports to Australia. The history of mud-blister worm outbreaks in Australasia is examined. While trans-Tasman exports of live oysters from New Zealand were commonplace during the nineteenth century, there is no evidence that mud-blister worms were present in New Zealand then. The earliest reports only date from the early 1970s and only from northern New Zealand, whereas a century earlier in the 1870s at least one of these pest worms had become widespread along eastern Australian coasts.
Introduction
Polydora and related genera, notably including Boccardia, comprise the Polydora-group or the ‘polydorids’, a speciose group of small spionid worms of similar morphology, many of which have the ability to live on and in mollusc shells, and all having rigid spine-like chaetae on chaetiger 5. The shell-damaging polychaetes present in the shells of cultured edible oysters worldwide are mostly species of Polydora. Activities of certain of these worms, commonly known to farmers as mudworms or mud-blister worms, can distort shell shape, foul oyster flesh and weaken the integrity of the host oyster shell. One scenario for the process is that juvenile worms settle in shell lip crevices, and the growing worm extends its activity into the peripheral extrapallial space, establishing a cover of mucus and debris over an enlarging U-shaped run on the inner surface of the shell valve. The worm and the accumulated particles become encased in a thin flexible layer of dark shell secretion (except for the opening at the shell edge where the worm feeds), forming a mud-blister, the wall of which thickens and hardens over time with increasing biomineralisation. In this type of blistering, one blister may overlay another, and blisters can even be removed leaving no trace of a boring on the shell valve surface (Whitelegge Citation1890, personal observation). Blister-dweller worms can enter the extrapallial spaces between living mollusc and shell also via direct external surface borings, either from near the shell edge, or inside the pallial line (mantle edge), in this instance penetrating the shell valve to establish in the otherwise sealed-off central extrapallial space. The blisters formed in the latter process can be very large and damaging.
There is a large literature on polydorid shell-dwellers as apparent problems for shellfish, both wild stock and farmed (e.g. Lauckner Citation1983) although in many publications evidence is often lacking on the process by which the host mollusc has been ‘attacked’ or otherwise disadvantaged by the presence of superficial shell worm burrows following along the plane of the shell, and misidentifications of the actual species involved are probably commonplace. Spionid shell borers have an ancient lineage, dating back about 400 million years to the Devonian (Cameron Citation1969). Only a few species induce conspicuous blistering, whereas many can have no direct impact, never through-penetrating a shell valve to reach the extrapallial space. For example, several shell-specialist polydorids in New Zealand appear to co-exist with their hosts as relatively harmless external shell borers at densities insufficient to compromise shell strength (personal observation). Certain Polydora-group species mostly living in sediment or rock crevices also can occur on shell as surface fouling or as crevice-dwelling non-borers (Sato-Okoshi & Okoshi Citation1997; Sato-Okoshi Citation1999, personal observation).
Most New Zealand aquaculture of oysters takes place in northern sheltered harbours, farming the introduced Pacific oyster, Crassostrea gigas, using intertidal racks (Holland & Jeffs Citation2000). Oyster-associated shell-blistering worms were not a concern for New Zealand oyster growers until around 1970 (see Discussion), and then recognition of Polydora websteri Hartman as the main species involved was not explicit in scientific reports until much later (Handley Citation1995). The additional presence in oysters and other shellfish of the similar P. haswelli Blake & Kudenov was not known until comparatively recently (Read & Handley Citation2004). Polydora websteri was first described from Connecticut, USA (Hartman Citation1943) and P. haswelli from southeastern Australia (Blake & Kudenov Citation1978).
Polydora websteri and P. haswelli were placed in a large ‘ciliata/websteri’ Polydora subgroup of similar species by Blake (Citation1996). This is an informal grouping, mostly of shell-dwellers, all with a lateral subdistal flange or ‘tooth’ on falcate spines of chaetiger 5. New Zealand specimens of these two species are described in detail here, compared to facilitate recognition by aquaculture biologists, and their taxonomic history, distribution, similarities to other species, and probable sequence of incidence in Australasian oyster farming areas are discussed.
Methods
Worms on C. gigas were extracted from shell-blistered oysters collected from several North Island, New Zealand, oyster farms. Worms on Pecten novaezelandiae were extracted from shell-blistered wild scallops collected by divers off Kawau Island and in Whangarei Harbour, and dredged in the Firth of Thames. Worms on Perna canaliculus mostly came from farmed long-line mussels from the Coromandel area. Material examined alive included Kawau and Coromandel Polydora haswelli and Clevedon and Houhora P. websteri, all extracted by shell dissection under stereo microscopy. Material retained for preservation is held in the Collection of the National Institute of Water and Atmospheric Research, Wellington (NIWA), stations in the series Ynnnnn, registrations as NIWA nnnnn. Paratype material of Australian P. haswelli and Australian specimens of P. websteri determined by Blake & Kudenov (Citation1978) held at Museum Victoria, Melbourne, Australia (MV), were borrowed and examined. SEM images for P. haswelli and P. websteri are from New Zealand worms from stations Y10170 and Y10329 respectively. Specimens were Osmium post-fixed from formalin fixed, platinum coated and examined in a JEOL 5300LV at 20 kV.
Systematic description
Genus Polydora Bosc, 1802
Diagnosis: Polydora-group spionid with prostomium anteriorly entire or incised, extending posteriorly as caruncle; eyes usually present. Chaetiger 1 without notochaetae. Chaetiger 5 greatly modified, with major spines of one type in a curved row, usually accompanied by slender companion chaetae. Posterior notopodial spines present or absent. Neuropodial hooded hooks bidentate with conspicuous angle between teeth, with constriction and manubrium on shaft and beginning on chaetigers 7–14. Branchiae beginning posterior to chaetiger 5. Pygidium usually saucer-like or disc-like. Anterior part of digestive tract usually without gizzard-like structure. (Adapted after Blake Citation1996.)
Polydora haswelli Blake & Kudenov Citation1978 (A–G, A, C, E, , A–C)
Polydora haswelli Blake & Kudenov Citation1978: 259–260, fig. 44a–f [type locality Sydney Harbour, New South Wales, Australia].
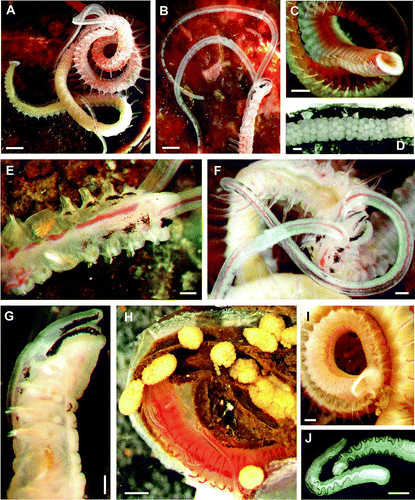
Fig. 1 (A–G) Living Polydora haswelli: (A) whole body; (B) anterior lateral right body and palps; (C) posterior and pygidium; (D) egg capsule string; (E) anterior dorsal body; (F) palps and dorsal head; (G) head in right lateral view. (H–I) Living Polydora websteri: (H) anterior body and palps, with individual egg capsules; (I) posterior and pygidium in lateral view. (J) Preserved Polydora websteri palps groove-edge pigmentation. Scale bars: A, B, H, 500 µm; C–F, I–J, 200 µm; G, 100 µm.
Diagnosis: Polydora species with chaetiger 5 bearing narrow-flanged falcate spines with flange subdistal, and hastate companion chaetae, large white glandularly thickened pygidial disk, black-banded palps, without palp groove pigment, and with dorsal black pigmentation on lateral prostomium and first few segments.
Material examined
MV F42883 (former NMV G2883), 5 paratypes. Original label: ‘Parallel with beach between navigation light & Parsons Peak, (48mi) Nth of Chinamans Beach, NSW, 20–35 feet, sandy bottom, sparse weeds, shells, starfish. Coll. P. Hutchings. 08.05.1971’. Det. & pub. Blake & Kudenov Citation1978: 259.
MV F43058 (former NMV G3058), 1 specimen. Original label: ‘Camden, Haven, Kennedy. Oyster lease in mud blisters 20.4.77. Coll. M. Skeel’. New label ‘Loc. 31° 40’, 152° 48’, Camden Haven NSW. Coll. M. Stebbe [error for Skeel] 20.04.1977. Det Blake & Kudenov Citation1978’. Pub. Blake & Kudenov Citation1978: 259.
Y10118, NIWA 48055, 10 specimens, 15 February 2002, Offshore West of Kawau Island, 36° 25.2’ S, 174° 52.8’ E, 17 m, coll. Ian Paterson, on P. novaezelandiae.
Y10120, NIWA 48056, 2 specimens, 9 October 1998, Mahurangi Harbour, 36° 26.4’ S, 174° 42.6’ E, 0 m, coll. Sean Handley, on C. gigas shell.
Y10123, NIWA 48057, 1 specimen, 19 October 1998, Coromandel Harbour, 36° 48’ S, 175° 25.8’ E, 3 m, coll. Sean Handley, on C. gigas shell.
Y10124, NIWA 48058, 2 specimens, 20 October 1998, near Clevedon, South Auckland, 36° 56.2’ S, 175° 6.8’ E, 0 m, coll. Sean Handley, on C. gigas shell.
Y10125, 5 specimen lots, 14 October 1999, off Waimangu Point, Firth of Thames, 36° 59.4’ S, 175° 16.8’ E, 10 m, coll. B. Brownell, extracted Sean Handley, all on P. novaezelandiae shell: NIWA 48059, 5 specimens; NIWA 48060, 7 specimens; NIWA 48061, 8 specimens; NIWA 48062, 2 specimens; NIWA 48063, 2 specimens.
Y10128, NIWA 48064, 1 specimen, 2 August 1996, Mahurangi Harbour, 36° 26.4’ S, 174° 42.6’ E, 0 m, coll. Sean Handley, on C. gigas shell.
Y10148, NIWA 48065, 2 specimens, 14 August 2003, Wilson Bay, Coromandel, 36° 52.8’ S, 175° 24.6’ E, 5 m, commercial-supplied, on longline cultivated P. canaliculus.
Y10154, NIWA 48066, 3 specimens, NIWA 48067, 2 specimens, NIWA 48068, 6 blistered valves, 1 September 2003, Wilson Bay, Coromandel, 36° 52.8’ S, 175° 24.6’ E, 5 m, commercial-supplied, on longline cultivated P. canaliculus.
Y10170, NIWA 48069, 6 specimen, 16 March 2004, Parua Bay, Whangarei Harbour, 35° 47.676’ S, 174° 27.096’ E, 4 m, coll. Ian Cameron, on P. novaezelandiae shell,
Y10330, NIWA 48070, 9 specimen, 1 August 2004, Coromandel Harbour area, commercial-supplied, on longline cultivated P. canaliculus.
Description
Dimensions: Up to 28 mm long and 1 mm wide. Segment total about 150 in adults. Palps extend to about chaetiger 20 in life (A–B).
Morphological characters: Prostomium tip weakly notched/bilobed. Occipital antenna absent. Caruncle extending to anterior chaetiger 4 (A). Eyes present, two pairs.
Fig. 2 (A) Polydora haswelli dorsal anterior SEM, palps regenerating, obscured prostomial tip bilobed as in Polydora websteri. (B) Polydora websteri dorsal anterior SEM. Arrows in A–B mark chaetiger 3 posterior boundaries. (C–D) Chaetiger 5 spines under light microscopy: (C) P. haswelli left side ventral view; (D) P. websteri in right side dorsal view. (E–F) SEM detail of pygidial fan inner surfaces: (E) P. haswelli fan; (F) P. websteri fan. Scale bars: A–B, 100 µm; C–D, 10 µm; E–F, 20 µm.
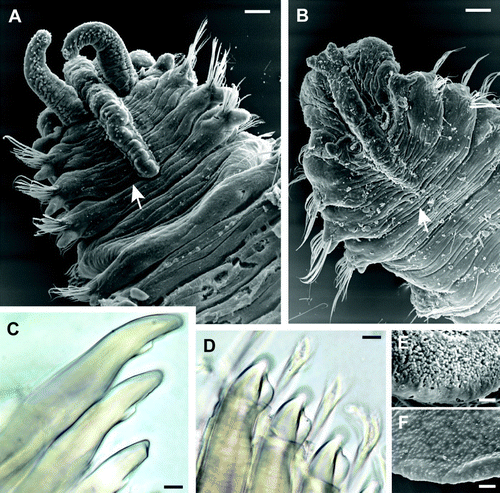
Branchiae from chaetiger 7 and on following chaetigers until almost end of body; longest branchiae on chaetiger 10. Anterior gut gizzard-like structure absent. Pygidium large, distinctly wider than last chaetigers, a plump glandular shallow disc with dorsal notch/gap (C), densely glandular surface apparent (E).
Chaetiger one notopodial lobe present, notochaetae absent. Chaetiger 5 elongated relative to adjacent segments (E); thickened chaetae and companion chaetae arranged in a shallow U with rows closely adjacent, companion chaetae slightly more ventral (A–B). Thickened chaetae falcate spines with a narrow subterminal flange on posterior edges (C), forming asymmetric scoop (A), with flange delineated from shaft by slight groove, more apparent in newly emergent chaetae (C), in which flange sometimes appears subdivided into two parts (B). Companion chaetae hastate, with flattened broad blades with fine frayed tips, and closely adjoin the falcate spines (B), the blade often appearing to wrap over the dorsal ‘scoop’ side of the spine (A). Up to seven falcate spines emergent in large worms. Superior notochaetae and inferior neurochaetal fascicles of chaetiger 5 present. Hooded hooks begin chaetiger 7; bifid, shaft constriction (manubrium) present, main fang more or less oriented at right angles to the shaft with wide angle to apical tooth. Alternating capillaries adjacent hooded hooks absent; neurochaetal inferior chaetae below hooded hooks absent. Posterior notochaetal spines absent.
Fig. 3 Polydora haswelli chaetiger 5 spine SEM: (A) left side lateral view; (B) right side ventro-lateral view. Scale bars: 10 µm.
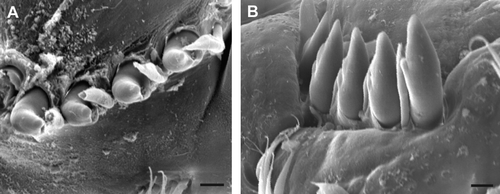
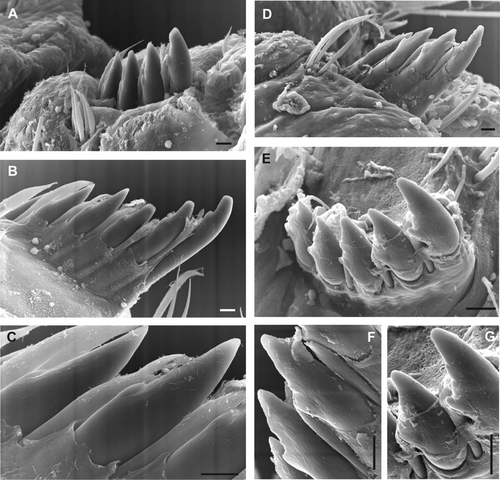
Fig. 4 Chaetiger 5 spine SEM, right side ventral views. (A–C) Polydora haswelli (2 specimens, B–C same specimen). (D–G) Polydora websteri (2 specimens, D & F, E & G same specimens). Chipping and grinding wear of the subdistal shelf is more pronounced for the wider shelf of the P. websteri spines where the lower contact edge has become irregular (image F & G). Scale bars: 10 µm.
Colouration in life: Fine bands of black on palps (A–B, F), usually as paired groups of small adjacent pigment spots on either side of deep palp groove, with about 12 pairs, starting a third of the way from palp insertion, always terminal pair at the tip. Palps may have pale dusky background colour over distal two-thirds (not white pigment as such); palp groove edges without pigment line. Body surface pigment (A–B, E–G) includes prostomium pigment as short subdistal black stripe either side; also black patch either side of peristomium, just in front of palp insertion; thin black line laterally on lips of mouth, pairs of irregular black patches on dorsal surface of first four segments. In heavily pigmented worms, black pigment extensive enough to be nearly continuous across the dorsum and to occur also on chaetigers 2–3 as small ventro-lateral patches. Pygidium glandular area distinctly white. Worm body otherwise without surface pigment and worms otherwise pale and semi-transparent anteriorly (E, G) but worms may appear distinctly greenish from midbody.
Methyl Green staining: Body stains extensively with a distinct granular result (apparently outlining individual cells), and additional uniform diffuse staining lightly over most of the surface. Granular staining in anterior branchiae often along full length of each edge. Granular and diffuse staining on ventral peristomium, and some granular staining on the ventral surface of chaetiger 1–4. Little staining on chaetiger 5. A triangular unstained dorsal area present lateral to anterior caruncle.
Reproduction: Egg masses with connected capsules (D), without nurse eggs, with planktotrophic early-released larvae. No other information known.
Habitat: Intertidal marine, or inshore marine embayment, or marine shelf. Known from bivalve mollusc shell, in debris packed shell blisters; shell penetration by external borings, not limited to shell margins. Recorded in New Zealand from living shells of commercial shellfish C. gigas, Pecten novaezelandiae and Perna canaliculus. In addition in the Auckland–Northland region P. haswelli has been noticed on Ostrea chilensis, the other main commercial oyster, as well as on endemic Austrovenus stutchburyi and Atrina zelandica (I. Paterson 2003, personal communication). However, O. chilensis beds extensively harvested by dredging in Foveaux Strait, southern New Zealand did not have P. haswelli, at least up to 2002. Many freshly opened O. chilensis shells obtained from Foveaux Strait were examined by the author during annual oyster surveys 2000–2002. No blistering Polydora-group species were detected, although crevice dweller species and surface borers such as Boccardia knoxi (Rainer) and B. acus (Rainer) were present, and no industry reports of shell blistering damage are known. Occurrences in empty/dead shell valves never reported. Tube lining present as packing within blister cavity. Tube chimney present as glued debris/sand-grains.
Distribution: Global distribution includes New Zealand mainland and southeast coast of Australia. Within New Zealand, confirmed records are known for the Hauraki Gulf, Whangarei Harbour, Firth of Thames and Coromandel Peninsula inshore waters. The occurrence of P. haswelli further southwards in New Zealand is not yet confirmed.
Remarks
The original description (Blake & Kudenov Citation1978: 259) contains the text ‘occipital tentacle’ as the second subclause of a sentence, but not followed by the word ‘present’. An occipital tentacle is not represented in the original figure, or present in any specimens I have examined (A); there is no occipital tentacle in P. haswelli and the mention is probably a lapsus.
The P. haswelli paratype specimens of MV F42883 are mostly in poor condition. However, one specimen has the characteristic dorsal pigmentation of the species, and the MV F43058 specimen is in good condition and closely similar to New Zealand worms. No palps remain, but Blake & Kudenov's ‘additional pigment on palps’ in P. haswelli was present as pigment spots (James Blake 2003, personal communication). Chaetiger 5 spines in both Australian specimen lots of P. haswelli were closely examined. The combination of both a tooth and a flange according to the type description and figures of P. haswelli is not present. Rather, as in the New Zealand worms, there is a narrow ridge structure on one side, always a flange rather than a tooth, since it is never separated from the shaft. During the life cycle of the emergent spines, the ridge may appear to be in two parts, and the wear process may render its outer edge tooth-like, especially under light microscopy. SEM views show the flange descends onto the trailing posterior ventral side if the spines are in situ with the concave side of the falcate tip uppermost ( and A–C). Thus spines of the left and right side fascicles are enantiomorphs (mirror-image asymmetry).
Polydora haswelli has had only two further apparent taxonomic records in Australasia until now; these not formally included or excluded from synonymy here as the records are brief and the specimens have not been re-examined. Hutchings & Murray (Citation1984) recorded P. haswelli from Botany Bay, New South Wales, Australia. This record did not extend the known distribution much beyond the type locality, but the habitat information as given (sand of seagrass beds) is difficult to reconcile to the specialist shell-boring life of P. haswelli. However, some of the Blake & Kudenov (Citation1978) collection data similarly did not indicate an association with shellfish (only one lot was from oyster mud blisters), and the authors did not comment on the ecology of P. haswelli, although Blake (Citation1996: 172) later indicated it was a shell borer. A second apparent Australian P. haswelli record (Sato-Okoshi et al. Citation2008), from Albany, south-western Australia, in Saccostrea glomerata (as S. commercialis), appears likely to be a misidentification of P. websteri, judging from the information presented. See Remarks for P. websteri for further comparison between the two species.
Radashevsky et al. (Citation2006) reported an oyster-shell borer Polydora from Espírito Santo Bay and other sites in Brazil as P. cf. haswelli, and included in it specimens previously reported as P. websteri by Bolívar & Lana (Citation1987). Also Polydora neocaeca Williams & Radashevsky, a species described from Rhode Island, Atlantic coast North America, is remarkably similar to P. haswelli, including spine morphology (apparent in the A side view in situ SEM of Williams & Radashevsky Citation1999) and in anterior dorsal pigmentation (Williams & Radashevsky Citation1999: and ), and in the presence of palp banding. Polydora neocaeca is a species found on both bivalves and gastropods, occurring in shells alongside P. websteri (Williams & Radashevsky Citation1999: 122). There are as yet no confirmed reports of P. neocaeca in oysters but according to Radashevsky & Williams (Citation1998: 213), very similar worms had been found in ‘oysters’ (species unstated) from off Virginia. Read (Citation2000) had earlier raised the possibility that P. neocaeca was a modern introduction to the US eastern coast, especially since he did not concur that it conformed to the 1879 description of P. caeca Webster. Williams & Radashevsky (Citation1999) did not initially compare P. neocaeca to P. haswelli, but after the similarity became apparent, Radashevsky et al. (Citation2006) were uncertain if P. neocaeca differed from the senior name P. haswelli. Radashevsky et al. (Citation2006) identified their Brazilian species as P. cf. haswelli rather than P. cf. neocaeca, apparently because the sperm were slightly larger and only tetrad spermatid groups were observed rather than the octads of P. neocaeca (sperm characteristics are as yet unknown in P. haswelli). However, chaetiger 5 spine morphology, based only on original descriptions, suggests that the Brazilian taxon is more similar to P. neocaeca. Pigmentation and sperm development of P. cf. haswelli were not illustrated. As concluded by Radashevsky et al (Citation2006: 31) inclusion of genetic information is needed to evaluate fully putative records of P. haswelli versus P. neocaeca. A wider molecular study of all known Polydora close to P. haswelli should clarify its phylogeography and aid decisions on the management of the New Zealand worm from a biosecurity viewpoint, but all indications are that it is only a very recent pest of aquaculture here.
Polydora websteri Hartman in Loosanoff & Engle 1943 (H–J, B, D, F, D–G)
Polydora websteri Hartman in Loosanoff and Engle 1943: 70–72, a–g [locality Milford River mouth, Long Island Sound, Connecticut, USA].—Radashevsky, 1999: 110–112, a–f, [redescription of proposed lectotype].— ICZN, Citation2001: 152 [Ruling, P. websteri a new nominal species, etc.].
Diagnosis: Polydora species with chaetiger 5 bearing broad-flanged spines, with flange almost terminal, and hastate companion chaetae, large thin pygidial disk, palps with palp groove black pigment, without banding, body lacking dorsal black pigmentation.
Material examined
MV F43057 (former NMV G3057), 2 specimens and fragments, original label missing. New label: ‘36° 3’, 150° 7’, Tuross Lake NSW. coll. J. Gilartine [spelling uncertain]. 18 May 1975, det. Blake & Kudenov, Citation1978’. (pub. 1978: 258 as coll. M. Skeel, April 1977, from Crassostrea glomerata as C. commercialis).
Y10117, NIWA 48054, 13 specimens, 23 February 2001, Pakihi Marine Farms, Clevedon, South Auckland, 36° 56.2’ S, 175° 6.8’ E., 0 m, coll. Sean Handley, on C. gigas on oyster rails.
Y10121, NIWA 48072, 10 specimens, 12 October 1998, Parengarenga Harbour, 34° 31.2’ S, 172° 55.8’ E, 0 m, coll. Sean Handley, on C. gigas shell.
Y10122, NIWA 48073, 9 specimens, 13 October 1998, Houhora Harbour, 34° 47.4’ S, 173° 7.2’ E, 0 m, coll. Sean Handley, on C. gigas shell.
Y10126, NIWA 48074, 4 specimens, 1 January 1992, Mahurangi Harbour, 36° 26.4’ S, 174° 42.6’ E, 0 m, coll. Sean Handley, on C. gigas shell.
Y10127, NIWA 48075, 1 specimen, 1 January 1997, Mahurangi Harbour, 36° 26.4’ S, 174° 42.6’ E, 0 m, coll. Sean Handley, on C. gigas shell.
Y10128, NIWA 48076, 3 specimens, 2 August 1996, Mahurangi Harbour, 36° 26.4’ S, 174° 42.6’ E, 0 m, coll. Sean Handley, on C. gigas shell.
Y10145, NIWA 48077, 30 specimens, February 1991, Mahurangi Harbour, 36° 27.6’ S, 174° 42’ E, 0 m, coll. Sean Handley, on C. gigas shell, voucher set of 10 slides, data published Handley & Bergquist (Citation1997).
Y10329, NIWA 48078, 38 specimens, NIWA 48079, 71 specimens, 27 July 2004, Houhora Harbour, 34° 47.4’ S, 173° 7.2’, 0 m, coll. Sanford Ltd, on C. gigas shell.
Description
Dimensions: Up to 24 mm long and 1.2 mm wide. Segment total about 110 in adults. Palps extend to about chaetiger 20 in life (H).
Morphological characters: Prostomium tip weakly bilobed/notched; occipital antenna absent; caruncle extends to mid-chaetiger 3 (B). Eyes present, two pairs.
Branchiae from chaetiger 7 and on following chaetigers until almost end of body; longest branchiae on chaetiger 9. Anterior gut gizzard-like structure absent. Pygidium large, distinctly wider than last chaetigers, a flattened shallow disc with dorsal notch/gap (I) with weakly glandular surface (F).
Chaetiger 1 notopodial lobe present, notochaetae absent. Chaetiger 5 elongated relative to adjacent segments; thickened chaetae and companion chaetae arranged in a shallow U with rows closely adjacent, companion chaetae slightly more ventral (D–G). Thickened chaetae asymmetric falcate spines with broad subterminal lateral flange near continuous with shaft (D, E). Companion chaetae hastate, with flattened broad blades and frayed tips; chaetae closely adjoining the falcate spines and may wrap dorsally over spine (D–E). Up to seven falcate spines emergent in large worms. Superior notochaetae and inferior neurochaetal fascicles of chaetiger 5 present. Hooded hooks begin chaetiger 7; bifid, shaft constriction (manubrium) present, main fang more or less oriented at right angles to the shaft with wide angle to apical tooth. Alternating capillaries adjacent hooded hooks absent; neurochaetal inferior chaetae below hooded hooks absent. Posterior notochaetal spines absent.
Colouration in life: Dorsal pigmentation pattern absent. Palp pigmentation a black line on groove edge (H, J). No surface pigment other than on palps (H). Pygidium semi-transparent, white, but not noticeably pigmented except faintly at centre. In overall view, body appears somewhat orange-red in life from gut and surface blood vessels.
Methyl Green staining: Body stains extensively with a distinct granular (apparently outlining individual cells) result. Little diffuse staining occurs. Granular staining in anterior branchiae at lateral bases only. Little staining on ventral peristomium or on chaetiger 5, but distinct granular staining on the ventral surface of chaetigers 1–4. A triangular unstained dorsal area present lateral to anterior caruncle.
Reproduction: Egg masses with separate capsules, not fused into a string, without nurse eggs, with planktotrophic early released larvae (see Remarks).
Habitat: Intertidal marine, or inshore marine embayment. Known from bivalve mollusc shells, in debris-packed blisters on valve interior surface; colonisation by shell lip ingress, or less commonly by external through-shell borings. Recorded from living shells of New Zealand commercial shellfish C. gigas and P. novaezelandiae (latter fide Handley), but not Perna canaliculus. Like P. haswelli, P. websteri has not been found on subtidal O. chilensis from the main commercial harvest area of Foveaux Strait, although many freshly opened shells have been dissected by the author. However, it has been found on Auckland region O. chilensis (I. Paterson 2003, personal communication). Occurrences in empty/dead shell also never reported. Tube lining present as ‘mud’ packing within blister. Tube chimney present as glued debris/sand-grains.
Distribution: Known to be anthropochoric/invasive. Global distribution includes New Zealand mainland, Australia, within south east coast and Tasmanian zone, North America Pacific coast (to Panamanian Isthmus), Atlantic coast and Gulf of Mexico. Polydora websteri appears to be common where feral C. gigas has large intertidal populations in northern New Zealand, north of 37° S. It may be rare in cooler waters further south although Handley (Citation1995, Citation1997a, Citation1997b) found small numbers in P. novaezelandiae, and in subtidally cultured C. gigas in northern South Island's Marlborough Sounds at 41° S.
Remarks
Following application to the ICZN by Radashevsky & Williams (Citation1998), the name Polydora websteri has been conserved as the valid name for Hartman's species from Long Island Sound, Connecticut, and a lectotype, as proposed and described by Radashevsky (Citation1999) using Hartman's original P. websteri material, has been designated (ICZN Citation2001). Radashevsky & Williams' application (discussed by Petersen Citation2000; Read Citation2000) established to the satisfaction of the Commission that P. websteri, Hartman's replacement name for P. caeca Webster, a junior homonym, was not based on Webster's original specimen (Webster Citation1879), and that Hartman was mistaken that P. websteri was the same taxon as represented by the unavailable name P. caeca. As mentioned earlier, Williams & Radashevsky (Citation1999) also described a new shell-boring species, P. neocaeca from Rhode Island, which they propose was Webster's original species. However, despite the suggestive choice of name it cannot be a replacement name for Webster's permanently invalid species name (P. neocaeca nomenclatural status clarified in Radashevsky & Williams Citation2000).
The Australian lot of P. websteri (MV F43057) contains one anterior end in good condition and matching New Zealand specimens, and one indeterminable Polydora anterior end doubtfully belonging to this species.
The similar gross morphology of P. websteri and P. haswelli renders separation during identification difficult if material is poor. The only striking differences are in pigmentation. In P. haswelli palp pigment is distinct as black transverse bands or spots, whereas in P. websteri there are no bands but there is a very narrow black line along the palp groove edge (see Discussion). Notably the anterior-most segments in P. haswelli have considerable black pigmentation dorsally (described above, and see fig. 44 in Blake & Kudenov Citation1978), including a conspicuous stripe alongside the prostomium. This pigmentation is absent in P. websteri. The pygidial fan of P. haswelli is distinctly white and glandular, whereas that of P. websteri is neither (EF). These colouration differences are usually sufficient for accurate discrimination of respective species, including preserved material if of good quality. In addition, spines of chaetiger 5 ‘upper’ row in P. haswelli are falcate with a delicate long narrow subterminal flange (sometimes appearing in two parts—the ‘tooth and flange’ of Blake & Kudenov), and in P. websteri falcate with a broad, short, almost-terminal lateral flange. This difference is readily apparent when viewing whole mounts of chaetiger 5 from either dorsal or ventral aspect. Radashevsky (Citation1999) reported the caruncle of the P. websteri type series only extended to the posterior of chaetiger 2, and in the original description of P. haswelli the caruncle is described as extending to mid-chaetiger 3. Here I report caruncles maximally to anterior chaetiger 4 in P. haswelli and to mid-chaetiger 3 in P. websteri (A–B). This is a size-variable character as noted by Williams & Radashevsky (Citation1999) for the related species P. neocaeca where the caruncle length ranged from post chaetiger 2 to mid-chaetiger 4. It is thus an indicative rather than invariant character state.
It is interesting that differences in Methyl Green staining were apparent between P. haswelli and P. websteri. While there was a similar distribution of staining cells in both species, P. websteri had less extensive granular staining on branchiae, and on the ventral peristomium, but a more pronounced area of granular staining on the ventral surface of chaetigers 1–4. Polydora haswelli took up stain more widely as a diffuse stain than P. websteri; thus P. websteri, with less background stain present, paradoxically showed a clearer overall patterning of granular staining. Use of Methyl Green is unlikely to be necessary to distinguish between good material of the two, but detection of the line of palp groove edge black pigment (if faint) in P. websteri may be easier after staining, as a result of the increased contrast.
Uncertainty over accuracy of P. websteri identifications in past reports makes problematic comparisons of reproductive modes and egg capsule forms stated in the literature. There are reports, however, from the Atlantic coast of the United States of planktotrophic reproduction (Hopkins Citation1958, Louisiana), and of similar distinct capsules with planktotrophic larvae (Blake Citation1969, Maine).
Discussion
The identity of P. websteri and P. haswelli
Blister-causing worms in shellfish were for some time mostly reported as Polydora ciliata (Johnston, Citation1838), a species first described from Berwick Bay, northern England, but there inhabiting rock crevices not shellfish (Johnston 1838). Later on, as an increasing diversity of Polydora species became apparent, Polydora hoplura Claparède, 1869 was more often reported in European oysters, and usually P. websteri was reported in North American oysters since the 1940s. Polydora websteri rapidly overtook the popularity of ‘P. ciliata’ as the species name most reported from harvested shellfish, although more than a decade after its discovery, scallop shell blistering was still attributed to P. ciliata at a Massachusetts site (Turner & Hanks Citation1959).
The original (Hartman Citation1943) description of P. websteri was based on specimens obtained from ‘vesicles’ on empty shells of the North American oyster, Crassostrea virginica. Interestingly, a number of subsequent reports of ‘P. websteri’ are of worms boring externally in the shell rather than blister-inducing penetrations into the extrapallial spaces, and there are also reported variations in pigmentation. It is probable that more than one species has been included under the name, with closely similar species such as P. neocaeca responsible for some of the reports. As yet, it is not possible to know how common P. neocaeca is on the Atlantic coast of the USA. Subsequent records of P. websteri are mostly from the eastern seaboard of North America and from Gulf of Mexico, but there are a few from the western coast and the Pacific Ocean. These include California and Oregon (Hartman Citation1961), the Gulf of California (Blake Citation1981), Japan (Sato-Okoshi Citation1999), and Australia (Blake & Kudenov Citation1978). This species has become the most widely reported blister-dweller in bivalves.
The surface pigmentation present on P. websteri compared to its close relatives is important because different pigmentation patterns between worm populations almost certainly indicate genetic differences, and in practice facilitate live identifications (a factor worth noting is that dark pigmentation in Polydora-group live worms may fade considerably while under strong lighting of microscopy; personal observation). Absence of pigmentation patterns in certain individuals of an otherwise pigmented population is not necessarily significant; Manchenko & Radashevsky (Citation2002) reported individuals electrophoretically defined as their new species Dipolydora melanopalpa that did not have the characteristic pigment pattern of the species. Williams & Radashevsky (Citation1999) described P. neocaeca as distinct from P. websteri, particularly on the basis that palps were distinctively banded with pigment. Previously, Blake & Kudenov (Citation1978) had described P. haswelli from Australia as distinct from their simultaneously published first Australian records of P. websteri, probably partly because head pigmentation patterns were present. Live P. websteri from the type locality seemingly lacked pigmentation patterns (Hartman Citation1943, but palps not described), as apparently do the preserved type specimens now (Radashevsky Citation1999, one unpigmented palp found). Hartman (Citation1951: 82) later reported that in Gulf of Mexico P. websteri from shell blisters ‘palpi have dusky or purple markings’, which is ambiguous and could indicate either lines or bands, but she subsequently commented that the P. websteri-type specimens have palp pigment present as ‘a pair of fine black lines along the edges of the longitudinal grooves’ (Hartman Citation1961: 100). This crucial observation, describing pigmentation identical to the New Zealand P. websteri, appears to have been overlooked in previous discussions of the species (Radashevsky & Williams Citation1998; Radashevsky Citation1999; Williams & Radashevsky Citation1999). It was also reported by Haigler (Citation1969), who used presence of fine longitudinal lines of black pigment on the palps to distinguish P. websteri from another Polydora species. However, Blake (Citation1971: 6) reported P. websteri, in which some specimens had ‘dense anterior and posterior black pigment’, but did not mention palp pigment. This description was of specimens mostly from the eastern coasts, but also included one lot from California. Body pigmentation was present in Japanese populations reported by Sato-Okoshi (Citation1999), but varied with location, and worms from one site had none. These two reports of anterior body pigment are at odds with the P. websteri description and could represent one of several other similar species.
Detectable morphological differences between genetically distinct Polydora-group species can be subtle to non-existent (Rice et al. Citation2008). Reports of the ‘tooth and flange’ falcate spines widely present in Polydora-group species may be particularly difficult to interpret. Caution is necessary, as the appearance of these spines is very dependent on age and wear, as noted by Blake & Maciolek (Citation1987: 12) for very similar spines in Polydora cornuta Bosc, and as apparent in the spines figures for Polydora woodwicki in Blake & Kudenov (Citation1978). The other variables are orientation when viewed (aspect rarely stated) and viewing technique, which is usually standard light microscopy of whole body slide-mounts or excised chaetal groups. Blake & Kudenov (Citation1978) stated P. haswelli differed from P. websteri because chaetiger 5 spine ornamentation included a distinct lateral tooth as well as a flange, although in contradiction, Kudenov (Citation1982: 573) later commented that two flanges are present in P. haswelli, and a flange and tooth in P. websteri and P. cornuta (then as P. ligni). He also reported observations of W.J. Light that in P. websteri the flange and tooth were not connected in many specimens (California region). However, in authoritative re-descriptions there is no ‘tooth’ on P. websteri spines, only a flange (fide Radashevsky Citation1999), and no ‘flange’ on P. cornuta spines, only a small tooth (fide Blake & Maciolek Citation1987). Nevertheless, these authors are describing variations in the same structure—a subdistal asymmetric flange, of different length, thickness and outline shape between species, and which may thus appear under light microscopy to be so distinctly developed as to be tooth-like, or can be worn into an apparent tooth-and-flange outline.
There is no doubt that P. websteri and P. haswelli are distinct species, while the situation for P. haswelli and P. neocaeca is unresolved and intriguing. There are also other comparatively little known species with apparent close similarly to P. websteri and P. haswelli, such as Polydora triglanda in Radashevsky & Hsieh (Citation2000), also described from C. gigas. As noted earlier, molecular studies within the ciliata/websteri group would be helpful to clarify relationships of species close to P. websteri.
History of oyster pest worms in Australasia
The sequence of events concerning records of oyster pest worms in the Australasian region may be important for deciding whether the species P. websteri and P. haswelli as known now are endemic or alien to either or both New Zealand and Australia.
Concerns over mud-blister worms in New Zealand arise in the literature during the 1970s and follow the accidental arrival and population expansion of the alien oyster C. gigas in Northland around 1970 (Dinamani Citation1971). There is no evidence the events are associated apart from a coincidence in timing. At around that time regional systematic studies of Polydora-group shell-dwelling species from much further south by Rainer (Citation1973, Otago) and Read (Citation1975, Wellington) did not detect P. websteri, and it is particularly significant that Rainer did not find it, since he examined many O. chilensis. Similarly, there have been no observations of any mudworm presence in the Foveaux Strait O. chilensis (‘dredge oyster’) populations (Stead Citation1971; this paper). Earlier publications indicate Polydora problems had not yet been encountered in the fledgling northern oyster farming industry by 1966 (Rainer Citation1964; Elliott Citation1966). However, probably by 1971 (Curtin Citation1971), and definitely by 1982 (Curtin Citation1982, Citation1986), worm infestations were a significant concern in advice given to Northland farmers. Dinamani (Citation1986: records from 1979 onwards) gave the first tentative suggestion that the New Zealand C. gigas ‘mudworm’ might be P. websteri. In 1993–94, popular articles (Handley Citation1993, Citation1994) correctly identified peripheral extrapallial space blistering by P. websteri (‘larval blisters’ in the article), and in addition, Handley's categories of blisters away from edges as P. websteri ‘adult blisters’ and ‘internal new mudblisters’ (Handley & Bergquist Citation1997) could have included external borings of other species including P. haswelli. Finally, over 25 years after C. gigas arrived in New Zealand, P. websteri was recorded for the first time in the scientific literature (Handley Citation1995). Handley reported finding P. websteri in C. gigas shells from Mahurangi Harbour, Northland (Handley & Bergquist Citation1997: 1991 data), and from Admiralty Bay, Marlborough Sounds in C. gigas and P. novaezelandiae (Handley Citation1995: oysters, 1993–94 data; Handley Citation1997a: scallops, collected 1992). The Mahurangi Harbour P. websteri might have included some P. haswelli, since its presence was not recognised at that time and it now occurs there. However, Handley's voucher set of Polydora collected from Mahurangi in 1991 contains only P. websteri and a few P. hoplura. These are the earliest verified P. websteri specimens known from New Zealand. Handley (Citation2002: 1999 data) subsequently reported only P. hoplura from blistered C. gigas from Houhora Harbour oyster farms.
There is no verified history of severe pest Polydora records in New Zealand scallops before 1998–99. During that period, Nesbit (Citation1999) discovered extensive blister infestation by a Polydora species in scallops harvested around Kawau Island. This was probably P. haswelli, as it was present there in scallops in 2002, and instances of P. haswelli on scallops in the nearby Firth of Thames were noticed by shellfishers in 1999 (this paper). Bull (Citation1976) found only a low percentage infestation of scallops by only P. hoplura at the time of his studies in the South Island Marlborough Sounds (identifications by Read). The earliest verified records of P. haswelli in farmed oysters (C. gigas) and mussels (P. canaliculus) in New Zealand are 1996 and 2003 respectively (this paper).
In contrast to events in New Zealand, the history of reports of Polydora-group blister-causing worms in mainland Australian oysters began in the nineteenth century, more than 80 years before C. gigas arrived in the affected New South Wales (NSW) area—this about 1967 fide Medcof & Wolf (Citation1975), whereas imports of C. gigas to other areas of Australia began 20 years earlier in 1947 (Thomson Citation1952). While C. gigas may be more heavily colonised (‘attacked’) by P. websteri than are native oysters (Lauckner Citation1983: 808), perhaps contrary to expectation, problem outbreaks by Polydora species do not appear to have coincided with the many intentional introductions around the world of Japanese origin C. gigas, although several other unwanted organisms have travelled with this oyster (Mann Citation1978).
Haswell (Citation1885) and Whitelegge (1890) described worm damage in native rock oysters from Hunter River native oyster beds at Newcastle, NSW, attributed at the time to P. ciliata. The worm infestations were a new problem for the infant NSW aquaculture industry and, as the pests appeared suddenly, rather than just becoming more obvious when oysters were cultivated, there are grounds to suspect an accidental introduction. Roughley (Citation1939) states that it was about 1870 that mudworm-infected oysters were found to be ‘dying in large numbers’ in the Hunter River and that ‘until that time there appears to have been no reference to such an infection, and there is no satisfactory evidence as the cause of the outbreak’. Roughley (Citation1929 (1925): 22) records that ‘as the ravages of the worm spread from river to river, dredge [shallow subtidal] beds were abandoned’ in favour of intertidal beds (although both P. websteri and P. haswelli tolerate some air exposure, with the latter in New Zealand favouring the subtidal more than P. websteri).
The scenario of gradually spreading aliens has further credibility in that the shell-blister ‘worm disease’ was not recorded until 1888 in the oyster beds of George's River, Sydney, to the south (Roughley Citation1922: 17), although this seems a long lag time from 1870 (but not from the 1885 outbreak). Additionally, a review by Nell (Citation1993) reports that in 1895, not long after the 1888 Sydney event, ‘disaster struck’ to the north in Queensland where it is claimed a Polydora species killed large numbers of oysters. These reports of Polydora-related deaths are interesting in that, irrespective of how unpleasant the content of blisters broken during processing may look and smell to oyster farmers and to consumers, in the sea the worm is sealed off from contact with the mollusc flesh. Healthy bivalves normally tolerate Polydora blistering (Handley & Bergquist Citation1997; personal observation). Other more certain causes of oyster deaths include metazoan predators (Stead Citation1907), but especially important are outbreaks of a variety of diseases including Bonamiosis, caused by microorganisms as yet undiscovered in the nineteenth century, some of which cause large-scale mortalities and ‘extreme commercial losses’ (Lauckner Citation1983). It may be pertinent that the same highly injurious haplosporidian parasite Bonamia exitiosa Hine et al., occurs in both Australian O. angasi and New Zealand O. chilensis flat oysters (Hine & Jones Citation1994; Corbeil et al. Citation2006). Nevertheless, Ogburn et al (Citation2007) recently made the remarkable claim that ‘permanent extinction of natural oyster populations below the neap tide level in [eastern Australian] estuaries occurred rapidly once mudworm appeared’, and that the cause was ‘the introduction of [un-named species of] mudworm in oysters translocated from New Zealand’. Nell (Citation1993, Citation2001) mentions earlier suggestions that the outbreak in NSW was related to importation of rock oyster (Saccostrea glomerata as C. commercialis) spat from New Zealand c. 1888 (region unstated, but S. glomerata populations are in northern North Island).
Historical reviews repeatedly mention imports into Australia of New Zealand oysters, namely ‘[In the 1870s] additional oyster [Foveaux Strait O. chilensis] supplies to supplement the Sydney and Melbourne markets were imported from the South Island of New Zealand during the winter months … ’ (Smith Citation1982), and see also (Smith Citation1985: 38). However, apart from the non-fatal nature of parasitism by ‘mudworm’, a ‘lethal alien worm from New Zealand’ scenario founders on the unproven existence of P. websteri or P. haswelli in any oyster species in New Zealand in the 1870s. Polydora hoplura, which does currently occur through most of the country and can blister oysters, would be a candidate for a translocated species, but only if it was in New Zealand by then. Its provenance and time of arrival in New Zealand is unknown, but it is thought to be an alien (Cranfield et al. Citation1998), was widespread in the Wellington region by the 1970s (Read Citation1975), but was not found in Otago then by Rainer (Citation1973). In any case, P. hoplura is unlikely to be the Hunter River species examined by Whitelegge (1890) and Haswell (Citation1885) for reasons suggested below.
Whitelegge (1890) made accurate and detailed observations on the blistering rather than boring habit of the ‘P. ciliata’ worms from Hunter River, but his identification was treated sceptically by McIntosh (Citation1902: 306). European ‘P. ciliata’ did not produce shell blisters and McIntosh suspected this was instead an occurrence of P. hoplura, which he considered ‘a southern [hemisphere] species, as shown by Dr Carazzi … ’. Here McIntosh appears to be relying on Carazzi's (Citation1893: 38) comment that he saw P. hoplura in oyster shells sent to him from Sydney. Elsewhere in Carazzi's text (table of distributions, p. 36) he reported P. hoplura from the Mediterranean, Madeira, US Atlantic coast, and a place called ‘Pacifico (Double Island)’, possibly a reference to a NSW location at 30° 52’ S, 152° 59’ E, although there are at least 10 islands with that name on the Australian coast. Polydora hoplura occurs in Australian Crassostrea spp. oysters (e.g. Blake & Kudenov Citation1978: 264), but it is a distinctive species (or species-group) possessing conspicuous posterior notochaetal spines, and unlikely to be mistaken for P. ciliata or P. websteri. Whitelegge most probably had found P. websteri in the Hunter River oysters, as Haswell did not long later (below).
Oyster blistering worms subsequently received little attention in Australian scientific literature up until the last quarter of the twentieth century, although they periodically manifested in densities sufficient to be a nuisance for growers (Smith Citation1985). Skeel (Citation1979) applied the names P. websteri, P. hoplura and the newly named P. haswelli in a brief formal report after they were authoritatively recorded from Australia (Blake & Kudenov Citation1978), and after previous popular articles (Skeel Citation1975, Citation1977, Citation1978). Polydora haswelli was described based on 28 specimens collected in NSW, Australia, at five locations, host shellfish unstated except one from off oysters (Blake & Kudenov Citation1978). In the same paper, seven P. websteri were recorded from two other sites, both from off oysters, including the three Australian Museum P. websteri dated 1885 and thus likely to be from Haswell's collection of ‘P. ciliata’ oyster blister worms from Newcastle (fide Blake & Kudenov Citation1978: 259). A further mud-blister species, P. latispinosa Blake & Kudenov, was described, reported from scallops and oysters. There have been no subsequent reports of P. latispinosa, which is similar to P. websteri and P. haswelli, but differs in possessing posterior notochaetal spines and an occipital tentacle.
Thus in summary it is likely that one or both of the oyster-blistering P. websteri and P. haswelli pair either first appeared or first greatly increased in density on the Australian eastern coast by about 1870. Possibly the two have been confounded during some of the time since, but there is no information on this, or on whether P. haswelli is an Australian native. Polydora websteri did not become obvious in New Zealand oyster-farming regions until a century later, and notably with a limited distribution only in the north, with P haswelli only becoming apparent more recently, also in the north, though perhaps confounded with P. websteri for a time. The evidence points to P. websteri and P. haswelli as adventive alien arrivals in New Zealand after (at earliest) the mid-1960s, most plausibly via the intermediary of Australian waters, since at least P. websteri has long been present there.
Acknowledgements
I was much helped by provision of specimens and information from Ian Paterson, Bill Brownell, and from several NIWA staff, especially Sean Handley, who also arranged for supplies of live farmed shellfish. I also thank Chris Rowley, Museum Victoria, for arranging loan of Australian Polydora material, and David Flynn (Victoria University of Wellington), who took the SEM images. Helpful comments on versions of the manuscript were provided by Shane Ahyong, Chris Glasby, Sean Handley, Ian Paterson, Vasily Radashevsky and anonymous referees. This work in part funded by FRST contract C01X0219 to NIWA.
References
- Blake , JA . 1969 . Reproduction and larval development of Polydora from northern New England (Polychaeta: Spionidae) . Ophelia , 7 : 1 – 63 .
- Blake , JA . 1971 . Revision of the genus Polydora from the east coast of North America (Polychaeta: Spionidae) . Smithsonian Contributions to Zoology , 75 : 1 – 32 .
- Blake , JA and Kudenov , JD . 1978 . The Spionidae (Polychaeta) from southeastern Australia and adjacent areas with a revision of the genera . Memoirs of the National Museum of Victoria , 39 : 171 – 280 .
- Blake , JA . 1981 . Polydora and Boccardia species (Polychaeta: Spionidae) from western Mexico, chiefly from calcareous habitats . Proceedings of the Biological Society of Washington , 93 : 947 – 962 .
- Blake , JA and Maciolek , NJ . 1987 . A redescription of Polydora cornuta Bosc (Polychaeta: Spionidae) and designation of a neotype . Bulletin of the Biological Society of Washington , 7 : 11 – 15 .
- Blake JA 1996 . Family Spionidae Grube, 1850. Including a review of the genera and species from California and a revision of the genus Polydora Bosc, 1802 . In: Blake JA , Hilbig B , Scott PH Taxonomic atlas of the benthic fauna of the Santa Maria Basin and Western Santa Barbara Channel . 6 . The Annelida Part 3. Polychaeta: Orbiniidae to Cossuridae. 1st ed . Santa Barbara , Santa Barbara Museum of Natural History 81 223 .
- Bolívar , GA and Lana , PC . 1987 . Spionidae (Annelida: Polychaeta) Do Litoral Do Estado Do Paraná . Nertica Pontal do Sul PR , 2 : 107 – 148 .
- Bull MF 1976 . Aspects of the biology of the New Zealand scallop, Pecten novaezelandiae Reeve 1853, in the Marlborough Sounds . Unpublished Ph.D. thesis , Victoria University of Wellington .
- Cameron , B . 1969 . Paleozoic shell-boring annelids and their trace fossils . American Zoologist , 9 : 689 – 703 .
- Carazzi D 1893 . Revisione del genere Polydora Bosc e cenni su due specie che vivono sulle ostriche . Mittheilungen aus der Zoologischen Station zu Neapel 11 : 4 45 , Pl. 2 .
- Corbeil , S , Arzul , I , Robert , M , Berthe , FCJ , Besnard-Cochennec , N and Crane , MSJ . 2006 . Molecular characterisation of an Australian isolate of Bonamia exitiosa . Diseases of Aquatic Organisms , 71 : 81 – 85 .
- Cranfield , HJ , Gordon , DP , Willan , RC , Marshall , BA , Battershill , CN , Francis , MP , Nelson , WA , Glasby , CJ and Read , GB . 1998 . Adventive marine species in New Zealand . NIWA Technical Report , 34 : 5 – 48 .
- Curtin , L . 1971 . Oyster farming in New Zealand . Fisheries Technical Report , 72 : 1 – 99 .
- Curtin , L . 1982 . Longlines for improving oyster condition . Catch , 9 : 15
- Curtin , L . 1986 . Mudworm menace in oyster farms . Catch , 13 : 10 – 11 .
- Dinamani , P . 1971 . Occurrence of the Japanese oyster Crassostrea gigas (Thunberg), in Northland, New Zealand . New Zealand Journal of Marine and Freshwater Research , 5 : 352 – 357 .
- Dinamani P 1986 . Potential disease-causing organisms associated with mantle cavity of Pacific oyster Crassostrea gigas in northern New Zealand . Diseases of Aquatic Organisms 2 : 55 63 .
- Elliott , H . 1966 . Some aspects of the Crassostrea species in Australian and New Zealand waters, with special reference to the New Zealand rock oyster, C. glomerata . Fisheries Technical Report , 11 : 1 – 75 .
- Haigler , SA . 1969 . Boring mechanism of Polydora websteri inhabiting Crassostrea virginica . American Zoologist , 9 : 821 – 828 .
- Handley , SJ . 1993 . Mudworm infestations and oyster production . Aquaculture Update , 8 : 6 – 7 .
- Handley , SJ . 1994 . Waiter … there's a worm in my oyster—the problem of mudworm in shellfish . Seafood New Zealand , 2 : 51 – 53 .
- Handley , SJ . 1995 . Spionid polychaetes in Pacific Oysters, Crassostrea gigas (Thunberg) from Admiralty Bay, Marlborough Sounds, New Zealand . New Zealand Journal of Marine and Freshwater Research , 29 : 305 – 309 .
- Handley , SJ and Bergquist , PR . 1997 . Spionid polychaete infestations of intertidal Pacific oysters Crassostrea gigas (Thunberg), Mahurangi harbour, northern New Zealand . Aquaculture , 153 : 191 – 205 .
- Handley SJ 1997a . Spionid polychaete worm infestations of the Pacific oyster Crassostrea gigas in New Zealand . Unpublished Ph.D. thesis , University of Auckland .
- Handley , SJ . 1997b . Optimizing subtidal oyster production, Marlborough Sounds, New Zealand: Spionid polychaete infestations, water depth, and spat stunting . Journal of Shellfish Research , 16 : 143 – 150 .
- Handley , SJ . 2002 . Optimizing intertidal Pacific oyster [Crassostrea gigas] (Thunberg) culture, Houhora Harbour, northern New Zealand . Aquaculture Research , 33 : 1019 – 1030 .
- Hartman O 1943 . Polydora websteri Hartman. In: Loosanoff & Engle. Polydora in oysters suspended in the water . Biological Bulletin 85 : 69 78 .
- Hartman , O . 1951 . The littoral marine annelids of the Gulf of Mexico . Publications of the Institute of Marine Science , 2 : 7 – 124 .
- Hartman O 1961 . Polychaetous annelids from California . Allan Hancock Pacific Expeditions 25 : 1 226 , pl. 1–34 .
- Haswell , WA . 1885 . On a destructive parasite of the rock oyster . Proceedings of the Linnean Society of New South Wales , 10 : 272 – 275 .
- Hine , PM and Jones , JB . 1994 . Bonamia and other aquatic parasites of importance to New Zealand . New Zealand Journal of Zoology , 21 : 49 – 56 .
- Holland , RC and Jeffs , AG . 2000 . The status of aquaculture in New Zealand . World Aquaculture , 31 : 24 – 27 .
- Hopkins , SH . 1958 . The planktonic larvae of Polydora websteri Hartman (Annelida, Polychaeta) and their settling on oysters . Bulletin of Marine Science Gulf and Caribbean , 8 : 267 – 277 .
- Hutchings , PA and Murray , A . 1984 . Taxonomy of polychaetes from the Hawkesbury River and the southern estuaries of New South Wales, Australia . Records of the Australian Museum Supplement , 3 : 1 – 118 .
- ICZN 2001 . Opinion 1974. Polydora websteri Hartman in Loosanoff & Engle, 1943 (Annelida, Polychaeta): specific name conserved by a ruling that it is not to be treated as a replacement for P. caeca Webster, 1879, and a lectotype designated for P. websteri . Bulletin of Zoological Nomenclature 58 : 152 153 .
- Johnston G 1838 . Miscellanea Zoologica Ariciadae . Magazine of Zoology and Botany, Edinburgh 2 : 63 73 , plates 2–3 .
- Kudenov , JD . 1982 . Redescription of the major spines of Polydora ligni Webster (Polychaeta: Spionidae) . Proceedings of the Biological Society of Washington , 95 : 571 – 574 .
- Lauckner , G . 1983 . “ Diseases of Mollusca: Bivalvia ” . In Diseases of marine animals , Edited by: Kinne , O . 477 – 961 . Hamburg : Biologische Anstalt Helgoland .
- Manchenko , GP and Radashevsky , VI . 2002 . Genetic differences between two sibling sympatric Dipolydora species (Polychaeta: Spionidae) from the Sea of Japan, and a new species description . Journal of the Marine Biological Association of the United Kingdom , 82 : 193 – 200 .
- Mann R 1978 . Exotic species in mariculture [Symposium on exotic species in mariculture: case histories of the Japanese oyster, Crassostrea gigas (Thunberg), with implications for other fisheries] . Cambridge, MA : MIT Press .
- McIntosh , WC . 1902 . Notes from the Gatty Marine Laboratory, St. Andrews. No. 22. 1. On abnormal coloration in Pleuronectids. 2. On the British Syllidae. 3. On the Syllidae of the ‘Porcupine’ Expeditions. 4. On Syllidae collected in Norway by Canon Norman. 5. On the boring of Polydora in Australian oysters . Annals and Magazine of Natural History , 9 : 291 – 308 .
- Medcof , JC and Wolf , PH . 1975 . Spread of Pacific oyster worries NSW culturists . Australian Fisheries , 34 : 32 – 38 .
- Nell , JA . 1993 . Farming the Sydney rock oyster (Saccostrea commercialis) in Australia . Reviews in Fisheries Science , 1 : 97 – 120 .
- Nell , JA . 2001 . The History of Oyster Farming in Australia . Marine Fisheries Review [NOAA] , 63 : 14 – 25 .
- Nesbit GJ 1999 . Reseeding and hatchery potential of Pecten novaezelandiae and effects of recreational harvesting . Unpublished M.Sc. thesis , University of Auckland .
- Ogburn , DM , White , I and Mcphee , DP . 2007 . The disappearance of oyster reefs from eastern Australian estuaries—impact of colonial settlement or mudworm invasion? . Coastal Management , 35 : 271 – 287 .
- Petersen , ME . 2000 . Comments on the proposed conservation of Polydora websteri Hartman in Loosanoff & Engle, 1943 (Annelida, Polychaeta) by a ruling that it is not to be treated as a replacement name for P. caeca Webster, 1879, and designation of a lectotype for P. websteri (Case 3080; see BZN 55: 212–216) . Bulletin of Zoological Nomenclature , 57 : 45
- Radashevsky , VI and Williams , JD . 1998 . Polydora websteri Hartman in Loosanoff and Engle, 1943 (Annelida, Polychaeta): Proposed conservation of the specific name by a ruling that it is not to be treated as a replacement for P. caeca Webster, 1879, and designation of a lectotype for P. websteri . Bulletin of Zoological Nomenclature , 55 : 212 – 216 .
- Radashevsky , VI . 1999 . Description of the proposed lectotype for Polydora websteri Hartman in Loosanoff & Engle, 1943 . Ophelia , 51 : 107 – 113 .
- Radashevsky , VI and Hsieh , HL . 2000 . Polydora (Polychaeta: Spionidae) species from Taiwan . Zoological Studies , 39 : 203 – 217 .
- Radashevsky , VI and Williams , JD . 2000 . Comment on the proposed conservation of Polydora websteri Hartman in Loosanoff & Engle, 1943 (Annelida, Polychaeta) by a ruling that it is not to be treated as a replacement name for P. caecaWebster, 1879, and designation of a lectotype for P. websteri (Case 3080; see BZN 55: 212–216) . Bulletin of Zoological Nomenclature , 57 : 110 – 111 .
- Radashevsky , VI , Lana , PC and Nalesso , RC . 2006 . Morphology and biology of Polydora species (Polychaeta: Spionidae) boring into oyster shells in South America, with the description of a new species . Zootaxa , 1353 : 1 – 37 .
- Rainer SF 1964 . The ecology of the Auckland rock oyster, Crassostrea glomerata . Unpublished M.Sc. thesis , University of Auckland .
- Rainer , SF . 1973 . Polydora and related genera (Polychaeta: Spionidae) from Otago waters . Journal of the Royal Society of New Zealand , 3 : 545 – 564 .
- Read , GB . 1975 . Systematics and biology of polydorid species (Polychaeta: Spionidae) from Wellington Harbour . Journal of the Royal Society of New Zealand , 5 : 395 – 419 .
- Read , GB . 2000 . Comments on the proposed conservation of Polydora websteri Hartman in Loosanoff & Engle, 1943 (Annelida, Polychaeta) by a ruling that it is not to be treated as a replacement name for P. caeca Webster, 1879, and designation of a lectotype for P. websteri (Case 3080; see BZN 55: 212–216) . Bulletin of Zoological Nomenclature , 57 : 43 – 45 .
- Read , GB and Handley , SJ . 2004 . New alien mudworm now becoming a pest in longline mussels . Water & Atmosphere , 12 : 30 – 31 .
- Rice , SA , Karl , S and Rice , KA . 2008 . The Polydora cornuta complex (Annelida: Polychaeta) contains populations that are reproductively isolated and genetically distinct . Invertebrate Biology , 127 : 45 – 64 .
- Roughley , TC . 1922 . Oyster culture on the George's River, New South Wales . Technical Education Series, Technological Museum, Sydney , 25 : 1 – 69 .
- Roughley TC 1929 (1925) The story of the oyster. Its history, growth, cultivation and pests in New South Wales . Sydney , A. J. Kent, Govt Printer .
- Roughley , TC . 1939 . A review of the scientific investigation of the fisheries of New South Wales [Presidential address to Linnean Society] . Proceedings of the Linnean Society of New South Wales , 64 : vi – xxvii .
- Sato-Okoshi , W and Okoshi , K . 1997 . Survey of the genera Polydora, Boccardiella and Boccardia (Polychaeta, Spionidae) in Barkley Sound (Vancouver Island, Canada), with special reference to boring activity . Bulletin of Marine Science , 60 : 482 – 493 .
- Sato-Okoshi , W . 1999 . Polydorid species (Polychaeta: Spionidae) in Japan, with descriptions of morphology, ecology and burrow structure. 1. Boring species . Journal of the Marine Biological Association of the United Kingdom , 79 : 831 – 848 .
- Sato-Okoshi , W , Okoshi , K and Shaw , J . 2008 . Polydorid species (Polychaeta: Spionidae) in south-western Australian waters with special reference to Polydora uncinata and Boccardia knoxi . Journal of the Marine Biological Association of the UK , 88 : 491 – 501 .
- Skeel , ME . 1975 . Vice of the mudworm . Australian Fisheries , 34 : 24
- Skeel , ME . 1977 . Further investigations on mudworms in oysters . Australian Fisheries , 36 : 22 – 23 .
- Skeel , ME . 1978 . Water clean up helps keep down pests . Australian Fisheries , 37 : 12 – 13 .
- Skeel , ME . 1979 . “ Shell boring worms (Spionidae: Polychaeta) infecting cultivated bivalve molluscs in Australia ” . In Proceedings of the World Mariculture Society , Edited by: Avault , JW . 529 – 533 . Baton Rouge : Louisiana State University .
- Smith , GS . 1982 . What happened to the Queensland oyster industry? A lesson from history . Australian Fisheries , 41 : 42 – 45 .
- Smith GS 1985 . The Queensland oyster fishery: an illustrated history . Queensland Department of Primary Industries Information series: QI85007 i–ix 1 109 .
- Stead DG 1907 . Preliminary note on the wafer (Leptoplana australis): a species of dendrocoelous turbellarian worm, destructive to oysters , Department of Fisheries , New South Wales .
- Stead , DH . 1971 . Observations on the biology and ecology of the Foveaux Strait dredge oyster (Ostrea lutaria Hutton) . Fisheries technical report , 68 : 1 – 49 .
- Thomson , JM . 1952 . The acclimatization and growth of the Pacific oyster (Gryphaea gigas) in Australia . Australian Journal of Marine and Freshwater Research , 3 : 64 – 73 .
- Turner , HJ Jr and Hanks , JE . 1959 . Infestation of Pecten irradians by Polydora . Nautilus , 72 : 109 – 111 .
- Webster , HE . 1879 . The Annelida Chaetopoda of the Virginian coast . Transactions of the Albany Institute, New York , 9 : 202 – 269 .
- Whitelegge , T . 1890 . Report on the worm disease affecting the oysters on the coast of New South Wales . Records of the Australian Museum, Sydney , 1 : 41 – 54 .
- Williams , JD and Radashevsky , VI . 1999 . Morphology, ecology, and reproduction of a new Polydora species from the east coast of North America . Ophelia , 51 : 115 – 127 .