Abstract
Growth and reproductive characteristics of gold striped amberjack Seriola lalandi collected in the waters off western Kyushu from April 2003 to March 2008 were determined based on vertebral centrum readings and gonad histology, respectively. Translucent and opaque zones on vertebral centrum were identified, and the number of opaque rings counted. Von Bertalanffy growth parameters did not significantly differ between males and females (F=0.28, P>0.05), and transformed value for the von Bertalanffy growth parameter FL ∞ and K were 1108 and 0.3091, respectively. The spawning period was evaluated to be from April to June for S. lalandi, based on the results from monthly changes in the gonadosomatic index and histological observations. Based on the relationships between the fork length and the developmental stage of testes and ovaries, the smallest fork length of male and female with mature gonads was 624 and 662 mm, respectively. The first maturation age was estimated to be 2 years old for both sexes.
Introduction
Gold striped amberjack Seriola lalandi is widely distributed in temperate and subtropical waters of the world. In Japan, this species is distributed in the Pacific Ocean off the south coast of Honshu and throughout the Sea of Japan to the East China Sea, and is caught as commercially important species and grouped in fisheries landing statistics with yellowtail Seriola quinqueradiata, greater amberjack Seriola dumerili and almaco jack Seriola rivoliana. The main methods of fishing for the genus Seriola in the East China Sea is by set nets and purse seines, and the annual catch has ranged from 7000 to 16,000 tons since 1970. The catch of the gold striped amberjack is less than that of the yellowtail; however, as most of the catch has been previously grouped and landings treated with other Seriola spp. without identification, accurate catch statistics for this species are not available. In New South Wales (NSW), Australia, annual catches have fluctuated from 400 to 600 metric tonnes from 1990 to 2000 (Gillanders et al. Citation1999b), although this level has fallen dramatically in recent years (Stewart et al. Citation2004). This species is important commercially in many areas of the world.
As gold striped amberjack have been managed as a part of the resources of the genus Seriola by the government of Japan, it is necessary to clarify how the biological characteristics of each species in the genus Seriola differ. Many studies on the biological characteristics of yellowtail, S. quinqueradiata, have been reported in Japan. Age was determined using various hard tissues (Mitani Citation1955, Citation1958; Mitani & Sato Citation1959), and distribution and migration of yellowtail, ranging from Hokkaido to the continental shelf margin, have been investigated (Mitani Citation1960; Watanabe Citation1979; Murayama Citation1992). The reproductive characteristics of yellowtail have been reported for the spawning season, the maturity process of oocytes and the spawning ground (Mitani Citation1959; Murayama Citation1992; Uehara et al. Citation1998). However, there are no studies of biological (growth and reproduction) characteristics of the gold striped amberjack, S. lalandi, around Japan. Consequently, the aims of the present study are to determine the age, growth and reproductive characteristics of the gold striped amberjack in the East China Sea. Then, we compared the biological characteristics of S. lalandi in the East China Sea, Japan, with that of S. lalandi in NSW, Australia and that of S. lalandi in Northern New Zealand.
Materials and methods
Collection of samples and general biological data
A total of 164 specimens of gold striped amberjack obtained from April 2003 to March 2008 in the waters off western Kyushu were analysed (). Specimens were caught by large-sized purse seines and set nets mainly in the coastal waters off the Goto Islands.
Fig. 1 The location of Seriola lalandi commercially caught in the waters off western Kyushu. The size of circle indicates the number of fish caught at each site in the present study. The total number of collected specimens of gold striped amberjack was 164.
Specimens were measured to the nearest millimetre in total length and fork length (FL) and to the nearest gram of body weight (BW). The gonad weight (GW) was measured to the nearest 0.1 g after determining the sex, and small pieces of the gonad were fixed in 10% formalin for histological observations. The vertebral centra were removed and frozen for later analysis. The gonadosomatic index (GSI) was calculated as the ratio of GW/(BW-GW).
Age determination
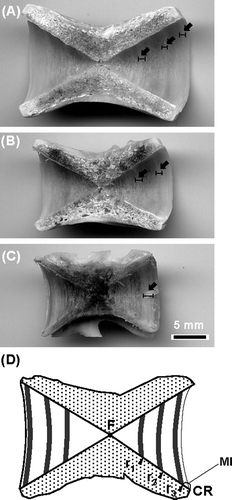
The 17th vertebra was used for determining the age, or if the 17th vertebra was damaged, then the 16th or 18th vertebra was used. The vertebra was boiled in water for several minutes, cleaned, sectioned along the longitudinal axis, bleached with 10% solution of H2O2 and dried for 1 day. Each centrum was examined by three readers, and ring marks on the posterior face of the centrum were counted and directly measured using calipers. Broad translucent zones alternated with narrow opaque zones on the centrum surface (76; A–C). The opaque zones that encircled the centrum were considered annual rings and were counted following the method of Gillanders et al. (Citation1999a) and Mitani (Citation1958). The distance from the focus (F) along a straight line to the centrum margin was denoted the centrum radius (CR; D), and the distance from F to the outer edge of each opaque zones was the ring radius (rn). To define the period of the formation of the ring marks (opaque zone), we examined monthly changes in the frequency of individuals with opaque zones on the outer margin of the centrum and the marginal increment (MI). The MI was determined according to the equation:
Fig. 2 Photograph of centrum of Seriola lalandi with three ring marks (A, FL = 758 mm, female), two ring marks (B, FL = 710 mm, female) and one ring mark (C, FL = 586 mm, male), and a schematic figure (D) of the measurement region on the centrum shown in (A). Black arrows and grey areas in (D) indicate ring marks and the opaque zone, respectively. F, focus; CR, centrum radius; rn, ring radius of the boundary from an opaque zone to a translucent zone; MI, marginal increment.
where CR represents the centrum radius (mm) and rn the ring radius (mm) of the nth outer opaque zone of the centrum.
Age was determined for each fish on the basis of the number of ring marks (annuli) on the centrum, assuming a hatch date of 1 May, which approximately corresponded to the middle of the spawning period (see Results). For some specimens sampled from May to June, which did not form a new translucent zone on the outer margin, the age was calculated as the sum of the number of ring marks plus 1 year. In order to describe the growth of males and females, von Bertalanffy growth equations were fitted to the observed FL values at age t by using non-linear least-square regression for both sexes. The growth equation is:
Histological observations
The fixed gonads were dehydrated and embedded in paraffin, and sections (thickness; 4 µm) were obtained and stained by the Mayer's haematoxylin and eosin method. The stained sections were observed under an optical microscope and the most advanced testes and oocyte stages were recorded. The developmental stages of testes and ovaries were classified as five and six stages of maturity, respectively, based on the development of the most advanced testes and oocytes and their histological characteristics ( and ).
Fig. 3 Photomicrographs of testes at different developmental stages in Seriola lalandi: (A) spermatogonia proliferation stage; (B) early spermatogenesis stage; (C) late spermatogenesis stage; (D) functional maturation stage; (E) post-spawning stage. sg, spermatogonial; st, spermatid; sz, spermatozoon. Scale bar equals 100 µm.
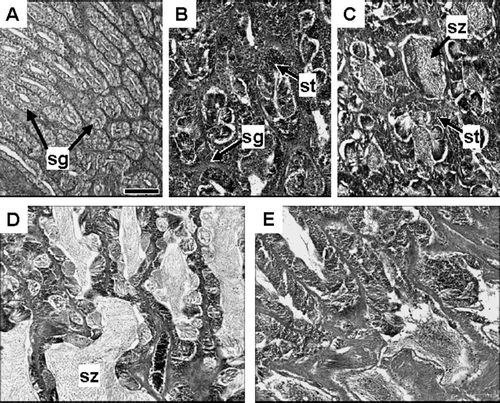
Fig. 4 Photomicrographs of ovaries at different developmental stages in Seriola lalandi: (A) immature stage; (B) developing stage; (C) vitellogenic stage; (D-E) mature stage; (F) spawning stage; (G) resting stage. at, atretic oocyte; ey, early yolk oocyte; gvm, germinal vesicle migration oocyte; hy, hydration oocyte; ly, late yolk oocyte; my, mid-yolk oocyte; pn, perinucleolus oocyte; pof, postovulatory follicle. Scale bar equals 100 µm.
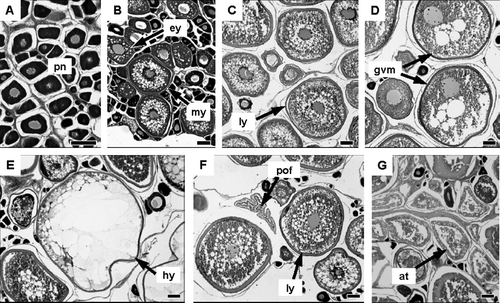
The five stages of testes were as follows:
-
The spermatogonial proliferation stage (Sp; A)—only spermatogonia are abundant in the seminal lobule.
-
The early spermatogenesis stage (Es; B)—spermatogonia and spermatids are organized in the seminal lobules.
-
The late spermatogenesis stage (Ls; C)—spermatogenesis proceeds in the testis. Spermatids of the seminal lobules increase and spermatozoa are found in the lumina of the seminal lobules.
-
The functional maturation stage (Fm; D)—spermatozoa are abundant in the lumina of the seminal lobules and main sperm duct. Spermatogonial division and further spermatogenesis proceeds in the seminal lobules.
-
The post-spawning stage (Ps; E)—spermatogonia are found in the seminal lobules, although spermatozoa occur in the lumina of the seminal lobules.
The six stages of oocytes were as follows:
-
The immature stage (Im; A)—only previtellogenic oocytes are present, including those in the perinucleolus and yolk vesicle stages.
-
The developing stage (D; B)—the most advanced oocytes are at the early yolk or mid-yolk stages.
-
The vitellogenic stage (Vi; C)—the most advanced oocytes are at the late yolk stage, which marks the end of vitellogenesis.
-
The mature stage (M; D, E), the most advanced oocytes are at the germinal vesicle 127 migration or hydration stages. The degenerated old postovulatory follicles appear in some ovaries at the germinal vesicle migration.
-
The spawning stage (Sp; F)—yolked oocytes and newly postovulatory follicles are present. Most postovulatory follicles disappear from the ovaries before the developing oocytes attain the germinal vesicle migration stage.
-
The resting stage (Re; G)—all yolked oocytes are degenerating (atretic stage) and non-yolked oocytes are present.
Sexual maturity
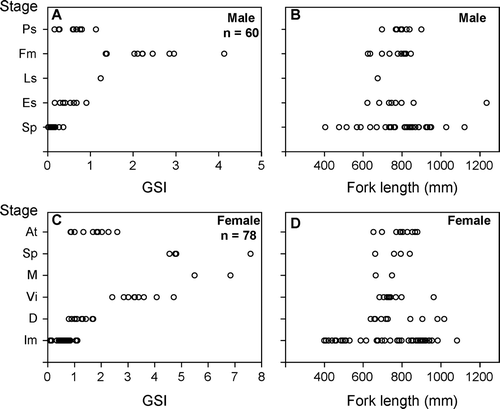
Estimates of size at maturity for 60 males and 78 females were based on the relationship between gonad developmental stages and fork length. Sexually mature males were defined as individuals with testes at the Fm stage. Sexually mature females were defined as individuals with ovaries with Vi, M or Sp stage oocytes.
Results
Growth
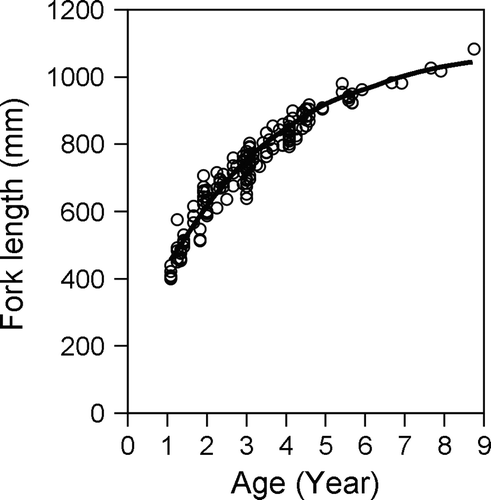
A total of 164 specimens (73 males ranging in size from 404 to 1121 mm FL, 91 females ranging in size from 399 to 1235 mm FL) of gold striped amberjack were sampled, and the ages of 162 specimens could be identified. The largest specimens (1121 and 1235 mm FL) were excluded from the analysis because it was too difficult to read the ring marks. For the other specimens, the estimated maximum ages for male and female were 7 and 8 years old, respectively. One clear ring mark was observed on vertebrae of 29 individuals from 408 to 615 mm FL and validated by three independent readers (C), and individuals more than 630 mm FL had the first ring mark at the same position as the 1-year-old fish (A,B). All specimens examined from July to December had a translucent zone on the outer margin of the centrum (A), and the frequency with an opaque zone was high from January to June. Some specimens examined from April to June had only minimal translucent zones (B), and the MI values gradually increased from July to January.
Fig. 5 Monthly changes in (A) frequency of appearance of an opaque zone on the outer margin of the centrum and in the (B) marginal increments (MI) of centrum in Seriola lalandi. Open and closed circles indicate the specimens with translucent and with opaque zones on the outer margin, respectively. Number of fish examined each month is indicated.
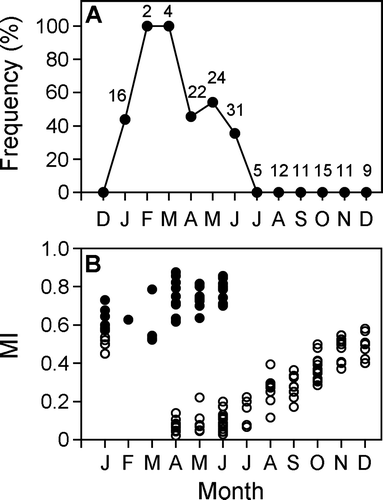
The hatch date of all the individuals was assumed to be 1 May for gold striped amberjack (see below), and the age of each individual was decided as the monthly age using the results of age determination. The relationship between age and FL of gold striped amberjack is shown in . As there were no significant differences in the growth between males and females (F=0.28, P>0.05), all the individuals were pooled for the estimation of the growth model. Growth model of gold striped amberjack was estimated as follows:
Annual reproductive cycle
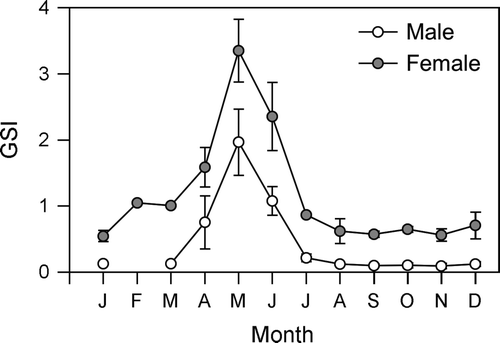
The mean GSI value of the gold striped amberjack was high from April to June and peaked in May for both sexes (A,B). The maximum values of the mean GSI in males and females in May were 2.0 and 3.3, respectively. The mean GSI values decreased rapidly in July and were less than 1.0 from July to March.
Fig. 7 Monthly changes in the gonadosomatic index (GSI) of male (n=60) and female (n=78) Seriola lalandi. Vertical bars denote the standard error.
The gonads of all males collected from August to March were at the Sp stage (A).
Fig. 8 Monthly changes in the frequency of occurrence of various maturity stages of (A) testes and (B) ovaries in Seriola lalandi. For males, Sp, spermatogonial proliferation stage; Es, early spermatogenesis stage; Ls, late spermatogenesis stage; Fm, functional maturation stage; Ps, post-spawning stage, and for females, Im, immature stage; D, developing stage; Vi, vitellogenic stage; M, mature stage; Sp, spawning stage; Re, resting stage. The numbers over each column represent the number of fish examined.
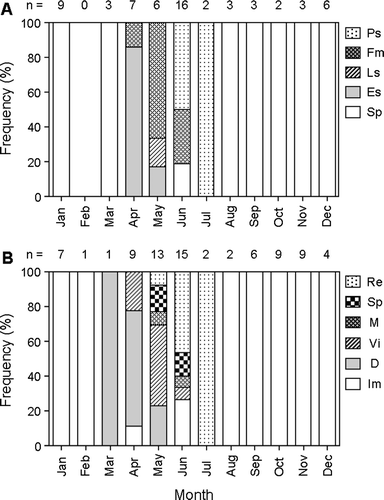
Individuals at the Fm stage appeared from April, and c. 70% of specimens in May and c. 30 % in June had abundant spermatozoa in the lumina of the seminal lobules and main sperm duct. Specimens in the Ps stage appeared from June, and only Ps stage specimens (n=2) were observed in July.
No maturing (Vi, M and Sp stages) females of the gold striped amberjack were collected from July to March (B). Maturing females appeared from April, and c. 70% of specimens in May exhibited the maturing condition, although specimens with hydrated oocytes were not observed. Half of the specimens collected in June had atretic oocytes, but females with ovaries in M and Sp stages were also observed.
Size at maturation
GSI values of male individuals were less than 0.9 in immature (Sp and Es) stages, 1.2 in the Ls stage, and from 1.4 to 4.1 in the Fm stage (A). Individuals with testes at the Fm stage were larger than 624 mm FL (B). GSI values of female individuals were less than 1.1 in the immature (Im) stage, and the values for the oocytes ranged from 0.8 to 1.7 in the developing (D) stage (C). GSI values for females in the Vi, M and Sp stages ranged from 2.4 to 7.6, and most of these individuals exceeded 3.0. The minimum fork length of females in the mature (from Vi to Sp) stages was 662 mm FL (D).
Fig. 9 Relationships between (A) the five maturation stages of testes and gonadosomatic index (GSI); (B) the five maturation stages of testes and fork length; (C) the six maturation stages of ovaries and GSI; (D) the six maturation stages of ovaries and fork length of Seriola lalandi. Refer to and 4 for each testis and ovarian stage, respectively. n, number of fish examined.
Discussion
There have been no studies on age, growth and reproduction of this species in the northern hemisphere. In the southern hemisphere, the growth pattern of S. lalandi in NSW, Australia, was reported by Gillanders et al. (Citation1999a) using several structures (scales, otoliths and vertebrae) and length frequency data. They reported that the otoliths, scales and vertebrae can be used for age determination, although it is necessary to determine the position of the first zone and to validate estimates for all age classes. In the present study, a clear ring mark was observed on vertebrae and validated by three independent readers, and the first ring mark on vertebrae of larger fish was clearly observed at the same position as that of individuals with one ring mark only. Moreover, there was a clear periodicity in the formation of ring marks based on monthly changes in MI and the frequency of appearance of an opaque zone on the outer margin. Therefore, it is considered that the vertebrae can be used for age determination in S. lalandi as shown by Gillanders et al. (Citation1999a).
Gillanders et al. (Citation1999a) reported that average fork lengths were about 485 mm FL at age 1 and 788 mm FL at age 5, and the maximum age of fish was 11 years old (approximately 900 mm FL) based on age determination using vertebrae. In the present study, the maximum age estimated was estimated at 8 years old (1083 mm FL), and the predicted fork lengths at age 1 and 5 were 430 and 911 mm FL, respectively. The sizes of S. lalandi in NSW and East China Sea were approximately equal at age 1, but the size in the East China Sea was larger than that in NSW at age 5.
There are no reports regarding gold striped amberjack maturation in Japan using histological techniques, but size and age at maturity, and seasonal changes in gonad activity have been reported for S. lalandi using histological techniques from NSW (Gillanders et al. Citation1999b) and from Northern New Zealand (NNZ) (McGregor Citation1995; Poortenaar et al. Citation2001). The peak gonad activity of S. lalandi from NSW was in December suggesting an early summer spawning period, and the smallest mature male and female were 360 mm FL (0 years old) and 698 mm FL (3 years old), respectively (Gillanders et al. Citation1999b). GSI of S. lalandi from NNZ was high from November 1998 to January 1999, and from October 1999 to January 2000, suggesting spawning during the spring and summer period (Poortenaar et al. Citation2001). Poortenaar et al. (Citation2001) reported that the smallest mature males and females S. lalandi in NNZ were 750 mm FL (4 years old) and 775 mm FL (4 years old), respectively. McGregor (Citation1995) reported that NNZ population of S. lalandi mature between 580 and 670 mm FL. In the present study, the spawning period of this species is estimated to occur in late spring/early summer (April to June; peak May) based on seasonal changes in the GSI and in maturation stage. Individuals under 500 mm FL (1 year old) had immature testes and oocytes. The minimum size of males and females with mature gonads is approximately 620 mm and 660 mm FL, respectively, and the estimated age at maturity is 2 years old for both sexes. Differences in size and age of sexual maturity among waters or year class would be attributed to different environmental condition (water temperatures, food condition, etc), or behavioural and physiological differences among populations.
In a previous study for S. quinqueradiata, it was reported that they stay around same sea area from 1 to 2 years old, then migrate over a wider range after 3 years old (Mitani Citation1960; Murayama Citation1992), and the growth rates were different in the habitat attributed to water temperature (Murayama Citation1992). Although migration of S. lalandi is not clarified, it is considered that S. lalandi make a similar migration to the S. quinqueradiata, and differences in the growth rates will occur between water regions. Seasonal changes in GSI and maturation stage of female giant yellowtail showed that the spawning season of this species was from April to June, which is slightly late compared with the Japanese amberjack (from March to May). Maturity age of both S. quinqueradiata and S. lalandi was 2 years old.
The growth and reproductive parameter is different in habitation area or year class. It is not clear whether these differences in growth (estimated sizes for > 1-year-old fish) and reproduction (age and size of first spawning) occur because of the geographic differences in the distribution (i.e. respective latitudes and physical conditions) or are genetic differences. In the present study, the biological characteristics (growth and reproduction) of gold striped amberjack in the East China Sea were clarified. Therefore, it will be possible to evaluate S. quinqueradiata as a separate resource to manage the genus Seriola precisely, and to make a comparative study with the species from NSW and NNZ. In the future, it will be necessary to investigate the growth and reproduction of gold striped amberjack over a wide range in Japan, to clarify any differences in growth and reproduction among the areas of Japan.
Acknowledgements
The authors thank Dr Yamamoto of the Tsukuba Office, Agriculture, Forestry and Fisheries Research Council Secretariat Ministry of Agriculture, Forestry and Fisheries for advice on the method of age determination using vertebral centrum.
References
- Gillanders , BM , Ferrell , DJ and Andrew , NL . 1999a . Aging methods for yellowtail kingfish, Seriola lalandi V., and results from age- and size-based growth models . Fishery Bulletin , 97 : 812 – 827 .
- Gillanders , BM , Ferrell , DJ and Andrew , NL . 1999b . Size at maturity and seasonal changes in gonad activity of yellowtail kingfish (Seriola lalandi; Carangidae) in New South Wales, Australia . New Zealand Journal of Marine and Freshwater Research , 33 : 457 – 468 .
- McGregor , G.A. 1995 . Is the northern region the kingfish capital of Pacific? Part 1: the fish. Seafood New Zealand : 28 30 .
- Mitani , F . 1955 . Studies on the fishing conditions of some useful fishes in the western region of Wakasa Bay—II. Relation between the scale of the yellowtail, Seriola quinqueradiata (T. & S.), and fork length and age . Bulletin of the Japanese Society of Scientific Fisheries , 21 : 463 – 466 .
- Mitani , F . 1958 . Studies on the growth and age of the yellowtail, Seriola quinqueradiata (T. & S.), found in Japan and the adjacent region—I. A fundamental consideration on age estimation by vertebrae . Bulletin of the Japanese Society of Scientific Fisheries , 24 : 626 – 631 .
- Mitani , F . 1959 . On the spawning shoals of the yellowtail, Seriola quinqueradiata (T. & S.), migrating to the Danjo Islands in the East China Sea . Bulletin of the Japanese Society of Scientific Fisheries , 24 : 708 – 713 .
- Mitani , F . 1960 . Fishery biology study of yellowtail, Seriola quinqueradiata T. & S., inhabiting in the waters around Japan . Memoirs of the Faculty of Agriculture of Kinki University , 1 : 81 – 300 .
- Mitani , F and Sato , T . 1959 . Studies on the growth and age of the yellowtail, Seriola quinqueradiata (T. & S.), found in Japan and the adjacent region—II. Estimation of age and growth from the opercular bone . Bulletin of the Japanese Society of Scientific Fisheries , 24 : 803 – 808 .
- Murayama , T . 1992 . Studies on the resources ecology of the yellowtail, Seriola quinqueradiata (T. & S.), in the Japan Sea Coast . Bulletin of Shimane Prefecture Fisheries Experimental Station , 7 : 1 – 64 .
- Poortenaar , CW , Hooker , SH and Sharp , N . 2001 . Assessment of yellowtail kingfish (Seriola lalandi lalandi) reproductive physiology, as a basis for aquaculture development . Aquaculture , 201 : 271 – 286 .
- Stewart , J , Ferrell , DJ and Van Der Walt , B . 2004 . Sizes and ages in commercial landing with estimates of growth, mortality and yield per recruit of yellowtail kingfish (Seriola lalandi) from New South Wales, Australia . Marine and Freshwater Research , 55 : 489 – 497 .
- Uehara , S , Mitani , T and Ishida , M . 1998 . Fishing and spawning ground of yellowtail, Seriola quinqueradiata, in the East China Sea . Fisheries Biology Oceanography of South-western waters Japan , 14 : 55 – 62 .
- Watanabe , K . 1979 . Distribution and migration of yellowtail in the Tsushima Warm Current waters based on recapture of fish tagged in spring and summer . Bulletin of Seikai Regional Fisheries Research Laboratory , 30 : 131 – 164 .