Abstract
A re-evaluation of the threat status of New Zealand's marine invertebrates was undertaken in 2009, following earlier review of New Zealand's Threat Classification System and subsequent refinement of the national criteria for classifying threat of extinction to New Zealand's flora and fauna. Sufficient information was available to enable 295 marine invertebrate taxa to be fully evaluated and assigned to a national threat category. The 10 taxa at most risk of extinction (‘nationally critical’) were the giant seep clam Calyptogena sp., the primitive acorn barnacle Chionelasmus crosnieri, O'Shea's vent barnacle Volcanolepas osheai, the stalked barnacle Ibla idiotica, the four-blotched umbrella octopus Cirroctopus hochbergi, the roughy umbrella octopus Opisthoteuthis chathamensis, the giant squid Idioteuthis cordiformis, the large-egged polychaete Boccardiella magniovata and two gravel maggots, Smeagol climoi and Smeagol manneringi. The key threatening processes identified for marine invertebrates were fishing and land-use associated impacts such as sedimentation. We identified no taxa that had improved in threat status as a result of past or ongoing conservation management action, nor any taxa that had worsened in threat status because of known changes in their distribution, abundance or rate of population decline. We evaluated a small fraction of New Zealand's marine invertebrate fauna for their threat status. Many taxa remain ‘data deficient’ or unlisted. In addition to the most threatened taxa, we recommend these taxa and their habitats as priorities for further survey and monitoring.
Introduction
Most marine species are thought to be more resilient to extinction than terrestrial species because of their large effective population sizes, often over broad ranges (Carlton et al. Citation1991, McKinney Citation1998). However, marine species with particular characteristics, such as slow growth rate, low adult mobility and small geographic range, are vulnerable to extirpation and extinction, with several examples of recent extinctions and near-extinctions (Roberts & Hawkins Citation1999). The first documented extinction of a marine invertebrate was of the eelgrass limpet, Lottia alveus, which became extinct following a disease outbreak that wiped out its eelgrass habitat (Carlton et al. Citation1991). A number of other marine invertebrates are thought to have become extinct in recent history (Carlton Citation1993; Carlton et al. Citation1999).
A species’ risk of extinction can be a critical consideration in its management, not only at a species level, but at a habitat and ecosystem level. Listing a species by its level of threat of extinction can help highlight where management action and associated resources need to be focussed (Nielsen & Kenchington Citation2001; Joseph et al. Citation2008) and inform consideration of decisions such as habitat protection and resource utilisation (Roberts et al. Citation2003a, Citation2003b). Ongoing assessments of changes in species’ threatened status can also provide a way of measuring the effectiveness of conservation management. However, as any conservation management action may have substantial impacts on economic activities (particularly in the marine environment), accurate identification of species at risk of extinction is an important issue (Powles et al. Citation2000).
The IUCN Red List of Threatened Species (IUCN Citation2010) identifies and documents those species most in need of conservation attention if global extinction rates are to be reduced, and provides a global index of the state of change of biodiversity. In 2002, to complement the world view provided by the Red List, New Zealand developed a Threat Classification System focussed at the national level (Molloy et al. Citation2002). This system provided a process and criteria for assessing the threat status of New Zealand's flora and fauna and provided a more sensitive classification for taxa with naturally restricted distributions and small numbers as a result of insular rarity. Hitchmough (Citation2002) presented the results of applying that system to a range of taxa. An update of the list was undertaken in 2005 (Hitchmough et al. Citation2007), which documented changes in the threat status of species and added new species to the list.
Internationally, marine species have received less attention than their terrestrial counterparts, both in terms of assessments of their threat status and associated management responses. Just 5% of the species listed on the IUCN Red List are marine species and of these, few are invertebrate taxa (IUCN Citation2010). There have been few attempts to collate information on the conservation of marine invertebrates for particular regions (but see Ponder et al. Citation2002). However, their importance for fisheries, tourism, ecosystem services and as the major component of biodiversity in the marine environment highlights the need for appropriate conservation management.
Although all marine mammals, most seabirds and two marine fish are fully protected in New Zealand waters, the only protected marine invertebrates are black corals (all antipatharian species) and all species of ‘red coral’ (Stylasteridae), which are protected under the Wildlife Act 1953. Despite their legal protection, bycatch of these species does occur across some regions, primarily as a result of bottom trawling and dredging (Probert et al. Citation1997; Clark & O'Driscoll Citation2003; Consalvey et al. Citation2006). Further, some localised coral populations are vulnerable to other damage associated with human activities, such as scuba diving (Miller et al. Citation2004). There is also some confusion over what species comprise the legally-protected ‘red corals’ (Consalvey et al. Citation2006). Many other marine invertebrates are at risk from human activities including pollution, habitat loss or modification, collection, disturbance and fisheries bycatch. Marine invertebrates also support important recreational, commercial and customary fisheries in New Zealand and in 2007, four of the 10 marine species with the highest export dollar value were invertebrates—arrow squid, paua (abalone), green-lipped mussel and rock lobster (Ministry of Fisheries Citation2009). Some areas that support particularly sensitive, at risk or ecologically important marine invertebrate communities have received protection from fishing and other threats in New Zealand (Anon Citation2001; Grange et al. Citation2003).
For some taxa, it is possible confidently to list and assess the risk of extinction of all species known to exist in New Zealand (e.g. marine mammals, terrestrial birds), but the task is large for many groups, including the marine invertebrates. For example, over 3000 marine mollusc species and subspecies are known from New Zealand waters, of which more than a third remain undescribed (Spencer et al. Citation2009) and the threat list for marine invertebrates completed in 2005 was known to be incomplete (Hitchmough et al. Citation2007). In 2007, a review of New Zealand's Threat Classification System (Molloy et al. Citation2002) was undertaken, which resulted in a new manual for classifying New Zealand's flora and fauna according to their threat of extinction (Townsend et al. Citation2008). As part of the implementation of this revised system, we re-evaluated the threat status of New Zealand marine invertebrates in 2009. This paper reports the results of these assessments.
Methods
Our starting list for re-evaluation of the conservation status of New Zealand marine invertebrates was the result of the previous listing process (Hitchmough et al. Citation2007), which included 285 taxa from a range of phyla. A call for submissions on the list was made via the Department of Conservation website (http://www.doc.govt.nz/) in December 2008 and via contact with the New Zealand Marine Sciences Society. Submissions closed on 22 March 2009.
In May 2009, a range of experts on New Zealand marine invertebrates was contacted by the Department of Conservation and invited to be part of an expert panel to be convened to undertake the re-evaluation process. The role of the expert panel members was to provide knowledge on their particular field of expertise at the threat classification list meeting, to answer queries on listing decisions reached, and to consult with peers to bring as much information as possible to the meetings (Townsend et al. Citation2008).
A one-day workshop was held in June 2009, and taxa were placed into risk categories based on the criteria provided by Townsend et al. (Citation2008), submissions received, advice from invited panel members that were unable to attend the meeting, panel knowledge and referral to recent publications relating to taxonomic and population status information (e.g. Tracey et al. Citation2005; Consalvey et al. Citation2006; Gordon Citation2009). Where there was doubt, we referred our provisional assessments to the relevant experts subsequent to the workshop.
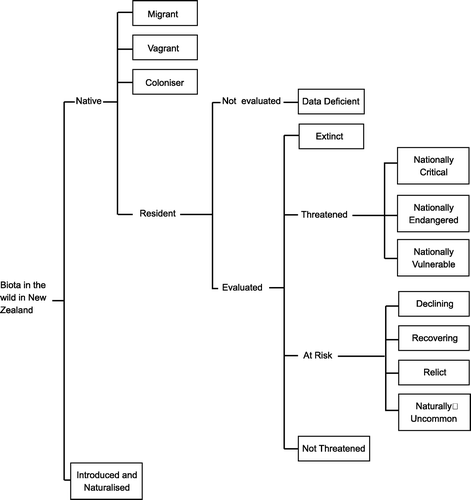
The categories used in our evaluation () are as defined in Townsend et al. (Citation2008) and are specific to the New Zealand region:
-
Extinct;
-
Threatened [including Nationally Critical (NC), Nationally Endangered (NE) and Nationally Vulnerable (NV)];
-
At Risk [including Declining (Dec), Recovering (Rec), Relict (Rel) and Naturally Uncommon (NU)];
-
Not Threatened (NT);
-
Non-resident Native [including Coloniser (Col), Migrant, and Vagrant)];
-
Introduced and Naturalised (self-sustaining populations exist in the wild);
-
Data Deficient.
Fig. 1 The structure of the New Zealand Threat Classification System (Townsend et al. Citation2008), reproduced with permission of the Department of Conservation.
Results
A total of 311 (2.7%) of the 11544 known New Zealand marine invertebrate species (Gordon Citation2009) were considered during the threat classification process, including four annelids, 21 arthropods, one brachiopod, seven bryozoans, 39 cnidarians, four echinoderms, one sponge and 234 molluscs ( and ; complete list in Appendix 1). Of these, 12 taxa were considered ‘data deficient’ and were not evaluated for their threat status. Four species [three limpets: Micropilina sp. C (NMNZ M.171275), Actinoleuca campbelli bountyensis Powell, 1956, and Notoacmea scapha (Suter, 1907); and one shrimp Chorocaris sp. (NIWA specimen, coll. 2001)] were removed from the revised list because of recent taxonomic revisions. The remaining 295 taxa were evaluated and assigned to the relevant threat category. The vast majority of taxa evaluated were endemic to New Zealand waters and included a number of endemic genera. In accordance with recent taxonomic revisions, 18 taxa (two crabs, two barnacles and 14 molluscs) were renamed in the list. Of the 295 taxa we evaluated, plus the 12 ‘data deficient’ taxa, 91 remained taxonomically indeterminate.
Table 1 Number of taxa evaluated and assigned to threat categories defined by Townsend et al. (Citation2008).
Table 2 Number of marine invertebrate taxa evaluated and assigned to threat categories, or listed as data deficient, as a percentage of the total known New Zealand species diversity in the coastal and marine environment (from Gordon Citation2009).
Some 26 taxa were added to the previous list, including 12 isidids (bamboo corals), nine paragorgiids (bubblegum corals), one coralliid (precious coral), three vent shrimps (Alvinocaris alexander, Lebbeus wera and Nautilocaris saintlaurentae) and the king crab, Paralomis hirtella. For several cnidarian taxa, listings were made at the genus level (and therefore may include more than one species) to reflect difficulties in identification and the large number of undescribed but apparently endemic and in some cases, threatened species. For example, red coral, Errina novazealandica was changed to Errina spp. to reflect difficulties in identification of these species. Several deepwater corals were also listed at the genus level, including the precious corals, Corallium, and many of the bamboo corals.
Threatened taxa
We placed 33 taxa in the ‘threatened’ category (). In 2004, there were 11 ‘nationally critical’ marine invertebrates, based on the previous classification system. Under the new criteria, 10 taxa were listed as being at most risk of extinction. This list included seven species previously listed as ‘nationally critical’, plus three additional taxa: the squid Idioteuthis cordiformis, and two gravel maggots, Smeagol climoi and Smeagol manneringi. The status of four taxa previously listed in 2004 as ‘nationally critical’ was changed (the octopus Opisthoteuthis mero was relisted as ‘nationally vulnerable’; the echinoid Porterpygus kieri was relisted as ‘data deficient’; the polychaete Spio aequalis was relisted as ‘nationally endangered’; and the seadaisy Xyloplax medusiformis was relisted as ‘data deficient’).
Two taxa were listed as ‘nationally endangered’—the polychaete Spio aequalis (previously listed as ‘nationally critical’) and the brachiopod Pumilus antiquatus. A total of 21 taxa were listed as ‘nationally vulnerable’ and all were assigned to this category because of their patterns of decline as a result of existing threats. Most of the taxa in this category were deepwater corals.
The following 10 taxa have been listed as ‘nationally critical’ and are the marine invertebrates known to be at most risk of extinction in New Zealand waters:
Giant seep clam, Calyptogena spp.
The genus Calyptogena comprises highly specialised bivalves that live in symbiosis with sulphur-oxidising bacteria in habitats such as hydrothermal vents (Krylova & Sahling Citation2006). Calyptogena spp. have been found in methane seeps from Cape Palliser to Castlepoint offshore of the southeast North Island coast. The small spatial area of these species’ highly specialised habitat placed them in the ‘nationally critical’ category.
Primitive acorn barnacle, Chionelasmus crosnieri (Buckeridge, Citation1998)
Chionelasmus crosnieri was formerly listed as C. darwini, but has been relisted as C. crosnieri, in accordance with Buckeridge's (Citation1998) revision. In New Zealand, this species is known only from an area at around 500 m depth, on the Kermadec Ridge (Foster Citation1981), and is one of the most primitive living acorn barnacles. The small area of its known habitat placed it in the ‘nationally critical’ category.
Four-blotched umbrella octopus, Cirroctopus hochbergi O'Shea, 2000
Recorded only from New Zealand, C. hochbergi has been captured from several locations at depths between 700 and 1350 m and in association with seamounts, cold seep and vent habitats (O'Shea Citation1999). Its probable small population size and ongoing pattern of decline because of fishing impacts placed this species in the ‘nationally critical’ category.
Stalked barnacle, Ibla idiotica Batham, Citation1945
Although historically found at several sites on the Otago Peninsula, the small—the female is 2.0–3.5 mm and the male 0.4 mm in maximum dimension (Batham Citation1945)—stalked barnacle I. idiotica appears to have vanished from the intertidal and may now be restricted to a few subtidal pockets. This pattern of decline placed this species in the ‘nationally critical’ category.
Giant squid, Idioteuthis cordiformis (Chun, 1908)
The giant, or whip-lash squid, I. cordiformis, is known from several seamounts in the New Zealand region, including on the Chatham Rise and in the Bay of Plenty. Its ongoing or predicted decline because of fishing impacts placed this species in the ‘nationally critical’ category.
Roughy umbrella octopus, Opisthoteuthis chathamensis O'Shea, 2000
Recorded only from New Zealand, this octopus species has been captured from soft sediment habitat at depths between 900 and 1438 m off East Cape and the Chatham Rise (O'Shea Citation1999). Taken as bycatch in the deepwater trawl fishery, this species has not been recorded since 1999. The apparent pattern of decline in this species placed it in the ‘nationally critical’ category.
Gravel maggot, Smeagol climoi Tillier & Ponder, 1993
Previously listed as ‘range restricted’ under the 2002 criteria (Molloy et al. Citation2002), the pulmonate gastropod S. climoi has been recorded only on the gravel beaches of Wellington's South Coast (Tillier & Ponder Citation1992). All five species of Smeagol are restricted to the upper littoral of very small areas of gravel or cobble beaches in New Zealand and southeastern Australia, with each species having a very small geographic distribution (Ponder et al. Citation2002). S. climoi's highly restricted range placed it in the ‘nationally critical’ category.
Gravel maggot, Smeagol manneringi Climo, 1981
As with S. climoi, S. manneringi was also listed as ‘range restricted’ under the 2002 criteria. This species is found only on Kaikoura gravel beaches, and this highly restricted range placed it in the ‘nationally critical’ category.
O'Shea's vent barnacle, Volcanolepas osheai (Buckeridge, Citation2000)
Known only from the Brothers Caldera, northeast of the North Island, at depths between 1200 and 1700 m, this stalked barnacle species is the only hydrothermal vent-associated barnacle known from New Zealand waters (Buckeridge Citation2000). It is one of two species in the genus Volcanolepas (Southward & Jones Citation2003). Its apparent highly restricted distribution and single population placed this species in the ‘nationally critical’ category.
Large-egged polychaete, Boccardiella magniovata (Read, 1975)
An intertidal estuarine species, B. magniovata has been recorded from several locations but is nowhere abundant. Sites where this species has been found previously are being increasingly modified through urbanisation and a search in 2002 of its type locality revealed no individuals of this species (G. Read, personal communication). The few populations, apparent small population sizes and pattern of decline because of anthropogenic threats placed this endemic species in the ‘nationally critical’ category.
At risk taxa
A total of 251 taxa were placed in the ‘at risk’ category, with most (243) being listed as ‘naturally uncommon’ (). These are taxa whose distributions are naturally confined to specific substrates, habitats or geographic areas, or taxa that occur within naturally small and widely scattered populations. This includes a large number of species with distributions restricted to particular islands (e.g. Calliostoma spp.), and species associated with particular habitats, such as seamounts. The remaining eight taxa were classified as ‘declining’, within the ‘at risk’ category.
Other categories
Some 12 taxa were listed as being ‘data deficient’, where information relating to them was so poor that an assessment of threat status could not be made (Townsend et al. Citation2008). This included two annelids, one arthropod, two cnidarians, two echinoderms and five molluscs.
A further 11 taxa (three bryozoans and eight molluscs) were evaluated but did not fit any of the other categories and were listed as ‘not threatened’.
Discussion
Some 33 ‘threatened’ and 251 ‘at risk’ marine invertebrates were identified through our threat classification process, which involved the assessment of 295 taxa. It is known that marine taxa generally have much smaller percentages of threatened species, but also many more undescribed and unrecorded species than do terrestrial or freshwater plants or vertebrates (McKinney Citation1999; Regnier et al. Citation2009). This is certainly the case for New Zealand marine invertebrates, where just a small fraction of the fauna has been surveyed and described to date (Gordon Citation2009). Unlike New Zealand birds and terrestrial plants, where all taxa can be evaluated for their threat status (Miskelly et al. Citation2008; de Lange et al. Citation2009), this is currently an unachievable task for marine invertebrates, where many of the taxa remain unknown and undescribed.
There are, therefore, several sources of bias in relation to the list of threatened marine invertebrates presented here. While some phyla and geographic areas are relatively well studied in New Zealand waters, there are substantial gaps in our knowledge, which prevent us from not only knowing more about species’ distribution and abundance, but about their existence and identity. A huge number of marine species in New Zealand remain undiscovered and undescribed, and many habitats, such as those in depths beyond the continental shelf, remain largely unsurveyed. In addition, available taxonomic and ecological expertise is inconsistent among marine phyla and habitats, resulting in some taxa receiving more attention than others. Nearly a third of the marine invertebrate taxa we evaluated remain taxonomically indeterminate. Taxonomic resolution is seen as vital for furthering conservation management of these species (de Lange et al. Citation2009).
Much of the data available on marine species distribution and abundance has been derived from fisheries surveys and museum collections. Although such data can be very useful for assessing biodiversity (e.g. Ponder et al. Citation2000; Beaumont et al. Citation2008), the geographic distribution of sampling effort and the sampling methodology employed often prevents reliable description or even estimation of a species’ actual distribution and abundance. Our evaluations have been based on the best available information, which is incomplete for many taxa.
Edgar et al. (Citation2005) suggested that population declines for marine species at risk of extinction will go largely unnoticed, because of the ‘hidden’ nature of their environment and the lack of quantitative data on species distribution and abundance. Priorities for the collection of demographic data should therefore be not only on the species at most risk of extinction, but also on the ‘data deficient’ taxa (McKinney Citation1999; Townsend et al. Citation2008). It is likely that the vast majority of marine invertebrate species not evaluated here (which can be a large percentage of the known diversity; ) would be listed as ‘data deficient’, but this would highlight particular taxa and geographical areas where survey effort should be directed. We also consider that there is a strong likelihood that many marine invertebrates listed as ‘data deficient’ would be relisted as ‘threatened’ or ‘at risk’ if sufficient data were available to allow their evaluation.
A range of marine habitats are under ongoing risk of loss or degradation, through human activities such as reclamation, destructive fishing methods and sedimentation. It may therefore also be important to survey and monitor species associated with habitats known to be particularly vulnerable, as the loss of some habitats may result in the loss of associated fauna, including marine invertebrates. Seagrasses and seamounts are examples of vulnerable habitats that may support threatened dependent marine invertebrate species (O'Hara Citation2002; Hughes et al. Citation2009).
The threat status of several species appeared to have improved since the last listing process. These apparent improvements were related to changes in the evaluation criteria or to changes in knowledge of a taxon. For example, two species, the octopus Opisthoteuthis mero and polychaete Spio aequalis, ‘improved’ since the 2004 listing process (Hitchmough et al. Citation2007). Previously listed as ‘nationally critical’ under the old criteria (Molloy et al. Citation2002), O. mero was relisted as ‘nationally vulnerable’ and S. aequalis was relisted as ‘nationally endangered’. The change in classification of the former was related to the change in the classification criteria, and the change of the latter was related to the discovery of several new populations of that species.
Although management action such as the implementation of marine protected areas and benthic protected areas has been undertaken since the last marine invertebrate threat listing process (e.g. Ministry of Fisheries Citation2007), we know of no instance where any recovery or slowing in the rate of decline of a taxon as a whole has been documented in response to management. However, a lack of monitoring may explain this lack of documentation in some areas (deepwater habitats for example), and it is also likely that individual populations of some taxa have responded to management through the removal of threatening processes such as fishing (Clark & O'Driscoll Citation2003).
We could identify no taxon that was ‘recovering’ following a decline in population abundance, or that could be considered a ‘relict’ (Townsend et al. Citation2008). The particular characteristics of marine invertebrate species (e.g. dispersal mechanisms, body size) and lack of completeness of the list, also excluded the ‘migrant’, ‘vagrant’, ‘coloniser’ and ‘introduced and naturalised’ categories.
Two species formerly listed as ‘nationally critical’ (each with the qualifier ‘data poor’) were relisted as ‘data deficient’ under our evaluation process. We considered that there were too little data available (e.g. on population size or distribution) for the echinoid Porterpygus kieri and the seadaisy Xyloplax medusiformis to enable an adequate assessment of their threat status. As ‘data deficient’ species, these species remain priorities for future collection of population information.
A range of known threatening processes continue to act upon many of the marine invertebrates listed during our evaluation process, and are consistent with the threats that continue to be identified worldwide. The activities we noted as being key threatening processes for the majority of taxa thought to be in decline were the impacts of fishing (including bycatch or habitat loss), and land use/coastal development-associated impacts such as sedimentation. Some species were also noted to be at risk from shell collectors and traders, but such threats are usually considered much less significant than either fishing or coastal development, which can affect the survival of even some relatively common taxa (Ponder & Grayson Citation1998; Morrison et al. Citation2009). Management of these effects may result in the improvement in the threat status of some species we have listed, but ongoing monitoring would be required to assess fully the magnitude of any such improvement.
Marine invertebrates have been suggested to be vulnerable to the effects of climate change and associated effects such as sea level rise, climate warming and acidification (Harvell et al. Citation2002; Orr et al. Citation2005; Przeslawski et al. Citation2008). A number of taxa we have listed as being threatened or at risk, such as the deepwater corals and other calcified taxa, have been suggested to be particularly vulnerable to the effects of ocean acidification (Turley et al. Citation2007; Smith Citation2009). Evaluation of these species’ population status provides a baseline for the long-term assessment of the potential impacts of such environmental change.
Encouragingly, no taxa listed in 2004 (Hitchmough et al. Citation2007) were relisted here in a more threatened category as a result of an actual change in the distribution and abundance of the taxon, or an increase in the rate of decline in abundance. The vast majority of taxa that appeared to worsen in their threat status were actually relisted in a more threatened category as a result of the change in criteria between 2002 (Molloy et al. Citation2002) and 2008 (Townsend et al. Citation2008), or an increase in knowledge of the taxa. The marine slugs, or gravel maggots S. climoi and S. manneringi are two examples of ‘nationally critical’ species, with highly restricted distributions. Smeagol hilaris has recently been listed as a critically endangered species in New South Wales, Australia, for the same reasons as the two conspecifics listed here (Fisheries Scientific Committee Citation2009). The list of threatened New Zealand marine invertebrates includes a large number of narrow-range endemics, which are known to be at particular risk of extinction as a result of their vulnerability to small-scale threatening processes such as stormwater discharges, pollution or urbanisation (Ponder et al. Citation2002). Often, legal protection of their geographic range through the establishment of a marine protected area, or other such management action, may do little to protect such species. Smeagol climoi and S. manneringi were previously listed as ‘range restricted’ (Hitchmough et al. Citation2007) but revision of the classification criteria (Townsend et al. Citation2008) has ensured that such narrow-range endemic species with no predicted pattern of decline or history of human influence are highlighted as being at the highest risk of extinction.
The results of this threat listing process provide guidance for marine conservation management in New Zealand and also highlight key areas where further monitoring and research is required. As found in several international threat listing processes, a general lack of knowledge of population distribution and abundance, as well as life history characteristics, is an important issue to address to allow the threat classification of marine invertebrates (Gardenfors Citation2001; Miller et al. Citation2007). Additionally, the management of key threatening processes and the responses of marine invertebrate populations to such management are important areas of future research. As noted by Ponder et al. (Citation2002) for Australia, our lack of knowledge in these areas may have serious consequences for marine ecosystems. Although international studies have reported difficulty in applying some threat classification criteria to marine species (Miller et al. Citation2007), we have shown that the New Zealand criteria can be successfully applied to marine species, and may be suited to other countries with similar requirements, geography and ecological characteristics (Townsend et al. Citation2008).
Acknowledgements
We thank the following for their valuable input into the relisting process: Geoff Read, Di Tracey and Michelle Kelly. We also thank our two reviewers for their constructive comments on the manuscript. was reproduced with permission of the Department of Conservation.
References
- Anon 2001 Seamount closures Seafood New Zealand June 2001 21
- Batham , EJ . 1945 . Description of female, male and larval forms of a tiny stalked barnacle, Ibla idiotica n. sp . Transactions of the Royal Society of New Zealand , 75 : 347 – 356 .
- Beaumont J Oliver M MacDiarmid A 2008 Mapping the values of New Zealand's coastal waters 1 Environmental values Biosecurity New Zealand. Biosecurity New Zealand technical paper no. 2008/16 Wellington
- Buckeridge , JS . 1998 . A new coral inhabiting barnacle, Chionelasmus crosnieri sp. nov. (Cirripedia: Balanomorpha) from New Caledonia, Southwest Pacific . Zoosystema , 20 : 167 – 176 .
- Buckeridge , JS . 2000 . Neolepas osheai sp. nov., a new deep-sea vent barnacle (Cirripedia: Pedunculata) from the Brothers Caldera, south-west Pacific Ocean . New Zealand Journal of Marine and Freshwater Research , 34 : 409 – 418 .
- Carlton , JT . 1993 . Neoextinctions of marine invertebrates . American Zoologist , 33 : 499 – 509 .
- Carlton , JT , Geller , JB , Reaka-Kudla , ML and Norse , EA . 1999 . Historical extinctions in the sea . Annual Review of Ecology and Systematics , 30 : 515 – 538 .
- Carlton , JT , Vermeij , GJ , Lindberg , DR , Carlton , DA and Dudley , EC . 1991 . The first historical extinction of a marine invertebrate in an ocean basin: The demise of the eelgrass limpet Lottia alveus . Biological Bulletin , 180 : 72 – 80 .
- Clark , M and O'Driscoll , R . 2003 . Deepwater fisheries and aspects of their impact on seamount habitat in New Zealand . Journal of Northwest Atlantic Fishery Science , 31 : 441 – 458 .
- Consalvey M Mackay K Tracey D 2006 Information review for protected deep-sea coral species in the New Zealand region NIWA Client Report WLG2006–85, prepared for the Department of Conservation Wellington National Institute of Water and Atmospheric Research
- de Lange , PJ , Norton , DA , Courtney , SP , Heenan , PB , Barkla , JW and Cameron , EK . 2009 . Threatened and uncommon plants of New Zealand (2008 revision) . New Zealand Journal of Botany , 47 : 61 – 96 .
- Edgar , GJ , Samson , CR and Barrett , NS . 2005 . Species extinction in the marine environment: Tasmania as a regional example of overlooked losses in biodiversity . Conservation Biology , 19 : 1294 – 1300 .
- Fisheries Scientific Committee 2009 Proposed determination: Smeagol hilaris, a marine slug, as a critically endangered species Port Stephens, NSW Port Stephens Fisheries Centre
- Foster , BA . 1981 . Cirripedes from ocean ridges north of New Zealand . New Zealand Journal of Zoology , 8 : 349 – 367 .
- Gardenfors , U . 2001 . Classifying threatened species at national versus global levels . Trends in Ecology & Evolution , 16 : 511 – 516 .
- Gordon DP . 2009 New Zealand Inventory of Biodiversity Volume One: Kingdom Animalia, Radiata, Lophotrochozoa, Deuterostomia Christchurch Canterbury University Press
- Grange KR Tovey A Hill AF 2003 The spatial extent and nature of the bryozoan communities at Separation Point, Tasman Bay Marine Biodiversity Biosecurity Report No. 4 Wellington Ministry of Fisheries
- Harvell , CD , Mitchell , CE , Ward , JR , Altizer , S , Dobson , AP , Ostfeld , RS and Samuel , MD . 2002 . Climate warming and disease risks for terrestrial and marine biota . Science , 296 : 2158 – 2162 .
- Hitchmough , R . 2002 . New Zealand threat classification system lists , Wellington : Department of Conservation .
- Hitchmough , R , Bull , L and Cromarty , P . 2007 . New Zealand threat classification system lists 2005 , Wellington : Department of Conservation .
- Hughes , AR , Williams , SL , Duarte , CM , Heck , KL and Waycott , M . 2009 . Associations of concern: declining seagrasses and threatened dependent species . Frontiers in Ecology , 7 : 242 – 246 .
- IUCN 2010 IUCN Red List of Threatened Species, version 2010.1 http://www.iucnredlist.org (accessed 28 April 2010)
- Joseph , LN , Maloney , RF and Possingham , HP . 2008 . Optimal allocation of resources among threatened species: a project prioritisation protocol . Conservation Biology , 23 : 328 – 338 .
- Krylova , EM and Sahling , H . 2006 . Recent bivalve molluscs of the genus Calyptogena (Vesicomyidae) . Journal of Molluscan Studies , 72 : 359 – 395 .
- McKinney , ML . 1998 . Is marine biodiversity at less risk? Evidence and implications . Diversity and Distributions , 4 : 3 – 8 .
- McKinney , ML . 1999 . High rates of extinction and threat in poorly studied taxa . Conservation Biology , 13 : 1273 – 1281 .
- Miller , KJ , Mundy , CN and Chadderton , WL . 2004 . Ecological and genetic evidence of the vulnerability of shallow-water populations of the stylasterid hydrocoral Errina novaezelandiae in New Zealand's fiords . Aquatic Conservation: Freshwater and Marine Ecosystems , 14 : 75 – 94 .
- Miller , RM , Rodriguez , JP , Aniskowicz-Fowler , T , Bambaradeniya , C , Boles , R , Eaton , MA , Gardenfors , U , Keller , V , Molur , S , Walker , S and Pollock , C . 2007 . National threatened species listing based on IUCN criteria and regional guidelines: current status and future perspectives . Conservation Biology , 21 : 684 – 696 .
- Ministry of Fisheries 2007 Habitat protection and research http://www.fish.govt.nz/en-nz/Environmental/Seabed+Protection+and+Research/default.htm
- Ministry of Fisheries 2009 Commercial quota value http://www.fish.govt.nz/en-nz/SOF/ValueIndicator.htm
- Miskelly , CM , Dowding , JE , Elliot , GP , Hitchmough , RA , Powlesland , RG , Robertson , HA , Sagar , PM , Scofield , RP and Taylor , GA . 2008 . Conservation status of New Zealand birds, 2008 . Notornis , 55 : 117 – 135 .
- Molloy J Bell B Clout M de Lange P Gibbs G Given D Norton DA Smith N Stephens T 2002 Classifying species according to threat of extinction A system for New Zealand Threatened Species Occasional Publication 22 Wellington, Department of Conservation
- Morrison MA Lowe ML Parsons DM Usmar NR McLeod IM 2009 A review of land-based effects on coastal fisheries and supporting biodiversity in New Zealand New Zealand Aquatic Environment and Biodiversity Report No. 37 Wellington, Ministry of Fisheries
- Nielsen , EE and Kenchington , E . 2001 . A new approach to prioritising marine fish and shellfish populations for conservation . Fish and Fisheries , 2 : 328 – 343 .
- O'Hara , T . 2002 . Endemism, rarity and vulnerability of marine species along a temperate coastline . Invertebrate Systematics , 16 : 671 – 684 .
- O'Shea S 1999 The marine fauna of New Zealand: Octopoda (Mollusca: Cephalopoda) NIWA Biodiversity Memoir 112 National Institute of Water and Atmospheric Research Wellington
- Orr , JC , Fabry , VJ , Aumont , O , Bopp , L , Doney , SC , Feely , RA , Gnanadesikan , A , Gruber , N , Ishida , A , Joos , F , Key , RM , Lindsay , K , Maier-Reimer , E , Matear , R , Monfray , P , Mouchet , A , Najjar , RG , Plattner , G , Rodgers , KB , Sabine , CL , Sarmiento , JL , Schlitzer , R , Slater , RD , Totterdell , IJ , Weirig , M , Yamanaka , Y and Yool , A . 2005 . Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms . Nature , 437 : 681 – 686 .
- Ponder , WF , Carter , GA , Flemons , P and Chapman , RR . 2000 . Evaluation of museum collection data for use in biodiversity assessment . Conservation Biology , 15 : 648 – 657 .
- Ponder WF Grayson JE 1998 The Australian marine molluscs considered to be potentially vulnerable to the shell trade Report for Environment Australia Sydney Australian Museum
- Ponder WF Hutchings P Chapman R 2002 Overview of the conservation of Australian marine invertebrates Report for Environment Australia Sydney Australian Museum
- Powles , H , Bradford , MJ , Bradford , RG , Doubleday , WG , Innes , S and Levings , CD . 2000 . Assessing and protecting endangered marine species . ICES Journal of Marine Science , 57 : 669 – 676 .
- Probert , PK , McKnight , DG and Grove , SL . 1997 . Benthic invertebrate bycatch from a deep-water trawl fishery. Chatham Rise, New Zealand . Aquatic Conservation: Freshwater and Marine Ecosystems , 7 : 27 – 40 .
- Przeslawski , R , Ahyong , ST , Byrne , M , Wörheide , G and Hutchings , P . 2008 . Beyond corals and fish: the effects of climate change on noncoral benthic invertebrates of tropical reefs . Global Change Biology , 14 : 2773 – 2795 .
- Regnier , C , Fontane , B and Bouchet , P . 2009 . Not knowing, not recording, not listing: numerous unnoticed mollusk extinctions . Conservation Biology , 23 ( 5 ) : 1214 – 1221 .
- Roberts , CM , Andelman , S , Branch , G , Bustamante , RH , Castilla , JC , Dugan , J , Halpern , BS , Lafferty , KD , Leslie , H , Lubchenco , J , McArdle , D , Possingham , HP , Ruckelshaus , M and Warner , RR . 2003a . Ecological criteria for evaluating candidate sites for marine reserves . Ecological Applications , 13 : S199 – S214 .
- Roberts , CM , Branch , G , Bustamante , RH , Castilla , JC , Dugan , J , Halpern , BS , Lafferty , KD , Leslie , H , Lubchenco , J , McArdle , D , Ruckelshaus , M and Warner , RR . 2003b . Application of ecological criteria in selecting marine reserves and developing reserve networks . Ecological Applications , 13 : S215 – S228 .
- Roberts , CM and Hawkins , JP . 1999 . Extinction risk in the sea . Trends in Ecology and Evolution , 14 : 241 – 246 .
- Smith , AM . 2009 . Bryozoans as southern sentinels of ocean acidification: a major role for a minor phylum . Marine and Freshwater Research , 60 : 475 – 482 .
- Southward , AJ and Jones , DS . 2003 . A revision of stalked barnacles (Cirripedia: Thoracica: Scalpellomorpha: Eolepadidae: Neolepadinae) associated with hydrothermalism, including a description of a new genus and species from a volcanic seamount off Papua New Guinea . Marine Biodiversity , 32 : 77 – 93 .
- Spencer , HG , Marshall , BA and Willan , RC . 2009 . “ Checklist of New Zealand living mollusca ” . In New Zealand Inventory of Biodiversity 1 , Edited by: Gordon , DP . Kingdom Animalia : Radiata, Lophotrochozoa, Deuterostomia. Christchurch, Canterbury University Press .
- Tillier , S and Ponder , WF . 1992 . New species of Smeagol from Australia and New Zealand, with a discussion of the affinities of the genus (Gastropoda: Pulmonata) . Journal of Molluscan Studies , 58 : 135 – 155 .
- Townsend AJ de Lange PJ Duffy CAJ Miskelly CM Molloy J Norton DA 2008 New Zealand threat classification system manual Wellington Department of Conservation
- Tracey DM Anderson OF Clark MR Oliver MD 2005 A guide to common deepsea invertebrates in New Zealand waters New Zealand Aquatic Environment and Biodiversity Report No. 1 Wellington Ministry of Fisheries
- Turley , CM , Roberts , JM and Guinotte , JM . 2007 . Corals in deep-water: will the unseen hand of ocean acidification destroy cold-water ecosystems . Coral Reefs , 26 : 445 – 448 .
Appendix 1: Threat rankings for marine invertebrates.
The following is a list of all marine invertebrate taxa we assessed according to Townsend et al. (Citation2008). Taxa are grouped by threat category, then alphabetically by scientific name. * denotes an addition to this list (c.f. Hitchmough et al. Citation2007). Townsend et al. (Citation2008) provided further detail regarding the qualifiers, which are abbreviated as: CD, Conservation Dependent; DP, Data Poor; De, Designated; EF, Extreme Fluctuations; EW, Extinct in the Wild; Inc, Increasing; IE, Island Endemic; OL, One Location; PD, Partial Decline; RF, Recruitment Failure; RR, Range Restricted; SO, Secure Overseas; Sp, Sparse; St, Stable; TO, Threatened Overseas.
Threatened
Nationally critical
Criteria for nationally critical: A, very small population (natural or unnatural); B, small population (natural or unnatural) with a high ongoing or predicted decline; C, population (irrespective or size or number of sub-populations) with a very high ongoing or predicted decline (>70%).
Nationally endangered
Criteria for nationally endangered: A, small population (natural or unnatural) that has a low to high ongoing or predicted decline; B, small stable population (unnatural); C, moderate population and high ongoing or predicted decline.
Nationally vulnerable
Criteria for nationally vulnerable: A, small, increasing population (unnatural); B, moderate, stable population (unnatural); C, moderate population, with population trend that is declining; D, moderate to large population and moderate to high ongoing or predicted decline; E, large population and high ongoing or predicted decline.
At risk
Declining
Criteria for declining: A, moderate to large population and low ongoing or predicted decline; B, large population and low to moderate ongoing or predicted decline; C, very large population and low to high ongoing or predicted decline.
Recovering
Criteria for recovering: A, moderate population; B, moderate to large population.
No taxa listed in this category.
Relict
No taxa listed in this category.
Naturally uncommon
Other categories
Introduced and naturalised
No taxa listed in this category.
Migrant
No taxa listed in this category.
Vagrant
No taxa listed in this category.
Coloniser
No taxa listed in this category.
Data deficient
Extinct
No taxa listed in this category.