Abstract
The New Zealand fish fauna contains species that are affected not only by river system connectivity, but also by catchment and local-scale changes in landcover, water quality and habitat quality. Consequently, native fish have potential as multi-scale bioindicators of human pressure on stream ecosystems, yet no standardised, repeatable and scientifically defensible methods currently exist for effectively quantifying their abundance or diversity in New Zealand stream reaches. Here we report on the testing of a back-pack electrofishing method, modified from that used by the United States Environmental Protection Agency, on a wide variety of wadeable stream reaches throughout New Zealand. Seventy-three first- to third-order stream reaches were fished with a single pass over 150–345 m length. Time taken to sample a reach using single-pass electrofishing ranged from 1–8 h. Species accumulation curves indicated that, irrespective of location, continuous sampling of 150 stream metres is required to accurately describe reach-scale fish species richness using this approach. Additional species detection beyond 150 m was rare (<10%) with a single additional species detected at only two out of the 17 reaches sampled beyond this distance. A positive relationship was also evident between species detection and area fished, although stream length rather than area appeared to be the better predictor. The method tested provides a standardised and repeatable approach for regional and/or national reporting on the state of New Zealand's freshwater fish communities and trends in richness and abundance over time.
Introduction
With increasing human induced impacts being manifested in freshwater environments globally, the imperative for standardised sampling protocols for consistent monitoring of physical, chemical and biological elements over larger spatial scales is becoming increasingly urgent (Peck et al. Citation2006). For freshwater environments, development of initiatives such as the Environmental Monitoring and Assessment Programme (EMAP) for surface waters by the United States Environmental Protection Agency (US EPA Citation1998), the European Water Framework Directive (WFD2000/60/EC; European Commission, 2000) and the River InVertebrate Prediction and Classification System (RIVPACS; Wright Citation1995) have set precedents for co-ordination and integration of ecosystem information over large areas.
In New Zealand, although integrated approaches to standardised data collection are less advanced, a variety of biotic and abiotic metrics have been developed or tested for use in national State of the Environment (SoE) reporting on aquatic ecosystems (Ward & Pyle Citation1997; Young et al. Citation2004; Gray Citation2009). For example, the Trophic Level Index (TLI) has been developed by Burns et al. (Citation2000) to assess lake water quality, LakeSPI has been developed to assess lake ecological condition (Clayton & Edwards Citation2006), and various macroinvertebrate indices have been applied to represent stream condition (Suren et al. Citation1998; Stark et al. Citation2001; Collier Citation2008, Citation2009). Such indices are frequently used by various agencies (e.g. regional councils, environmental consultants, crown research institutes) not only for SoE monitoring but also for Assessment of Environmental Effects (AEEs), compliance monitoring or to evaluate stream rehabilitation initiatives.
Nationally, freshwater fish form an important and widespread component in aquatic systems and have significant cultural, recreational, conservation and economic value, yet thus far they have been under-utilised as potential indicators for various reasons (but see Joy & Death Citation2003). Many native fish species exhibit diadromous life histories, whereby they often use the ocean at some point in their lifecycle (McDowall Citation1990), and consequently many of the same species occur around New Zealand where habitat and connectivity are suitable (McDowall Citation1993; McDowall & Richardson Citation1983; Jowett & Richardson Citation1996). Because of their high mobility within and between these environments, fish (as opposed to many other freshwater organisms) have the potential to integrate or reflect environmental conditions at multiple spatial scales (i.e. from freshwater to marine environments both locally and nationally).
Despite the value of the ichthyofauna and its widespread coastal distribution, no standardised methods have been developed in New Zealand to enable consistent and effective national reporting on their current status (Gray Citation2009; Joy Citation2009). The New Zealand Freshwater Fish Database (NZFFD, McDowall & Richardson Citation1983) is a national repository for fish survey information collected by different agencies for differing purposes. While this database is a useful tool for assessing and modelling the distribution of fish species throughout New Zealand (e.g. Joy & Death Citation2002, Citation2004; Leathwick et al. Citation2009), the variable methodologies used to collect fish mean that only presence–absence data can be used to assess broad-scale improvements or declines in waterway health (Joy Citation2009).
Recent development of an Index of Biotic Integrity (IBI) for stream fish has led to attempts to use the NZFFD to model expected diversity at a reach-scale (Joy & Death Citation2004). However, the greatest need (at least for national reporting) is for a standardised and quantitative survey methodology for fish communities so that changes not only in diversity, but also measures of relative abundance, can be compared both regionally and nationally (Joy Citation2009). The ability to detect trends in population metrics provides greater sensitivity to change and ultimately improves opportunities for effective management intervention before species disappear (Nicholson & Jennings Citation2004; Jennings Citation2005). Rapid acquisition of quantitative information (and associated metrics) for fish is becoming increasingly critical given that a recent evaluation of historical records in the NZFFD has shown concerning national declines at a coarse presence/absence level of assessment (Joy Citation2009).
In contrast to New Zealand, development and testing of methods for describing the relative abundance and diversity of fish communities has received significant attention overseas. For instance, it has previously been established that fish species richness increases with the number of geomorphic units sampled (Gorman & Karr Citation1978; Angermeier & Schlosser Citation1989) and that effort, stream length, and stream area can all influence species richness and relative abundance at the reach-segment scale (e.g. Lyons Citation1992; Simonson & Lyons Citation1995; Patton et al. Citation2000; Hughes et al. Citation2002; Blocksom et al. Citation2009; Fischer & Paukert Citation2009). Additionally, the level of effort required to effectively describe riverine fish species composition at larger watershed scales has also been investigated (Smith & Jones Citation2005). Information from such studies has been incorporated into methodologies to improve the quality and accuracy of data collected and, because these data are often used for management or conservation decisions, confidence in their accuracy and quality is paramount (Fischer & Paukert Citation2009).
The EMAP protocols for aquatic vertebrates (Peck et al. Citation2006) use 40×the stream wetted width as a standard for setting the stream sampling distance (minimum 150 m). This is based on the likelihood of detecting 90% of the fish (and other aquatic vertebrate) species present using single pass back-pack electrofishing (Patton et al. Citation2000; Cao et al. Citation2001, Citation2002; Reynolds et al. Citation2003). The consistency of data collection across the Western US using the EMAP vertebrate methods has enabled robust assessment of the state of native and exotic fish communities over a wide geographic area (>205,000 km2).
In New Zealand, there is currently no knowledge on the level of effort or sampling distance required to effectively describe reach-scale fish diversity in wadeable streams. Furthermore, fish distribution and diversity is influenced by the wide range of stream types that vary greatly with respect to geology, climate and hydrology (Leathwick et al. Citation2008). The frequently steep topography and a highly variable maritime climate (Mosely & Pearson Citation1997) can cause frequent natural disturbance at the reach-scale in many systems and cause substantial alterations to local habitat conditions. For instance, floods causing major changes to local habitat have been shown to affect fish distributions and abundance and disrupt local movement patterns (Jowett & Richardson Citation1989; David & Closs Citation2002; McEwan Citation2009).
Thus an important consideration for developing a national protocol is to ensure that the range of natural physical (fluvial) processes driving habitat variability (and fish distribution and abundance) within any given reach (Reid et al. Citation2008) is encompassed by the methodology. An assessment of single-pass backpack electrofishing entries in the NZFFD (up to 30 September 2009) indicates that the average stream length sampled by researchers is 45 m (n=4616 records). This distance is less than one third of the distance recommended in the EMAP protocols for characterising reach-scale fish diversity in US streams. While not all data from fish surveys entered into the NZFFD aim to characterise reach-scale diversity (e.g. targeted single species surveys), in many cases it is the primary objective (e.g. data collected for AEEs).
In this investigation, we used a slightly modified version of the EMAP aquatic vertebrates sampling protocol across a variety of New Zealand streams to obtain relative abundance estimates for fish, and to determine what length or area of stream should be sampled before asymptotic species richness occurs. Since New Zealand fish communities are less diverse (38 freshwater species of which approximately one third are diadromous and widely distributed), we predict that the framework on which the EMAP methods are based should be sufficient to characterise fish assemblages here. We anticipate that the results of this project will facilitate the development of standardised reach-scale metrics for long-term assessment of New Zealand's fish communities.
Methods
Preliminary testing of the EMAP methods was undertaken in the Waikato region of New Zealand by Environment Waikato staff in December 2008. Some modifications were made to the EMAP field collection sheets for specific testing of the procedure and to make them more applicable for data collection in New Zealand (for specific details, see David & Hamer Citation2010). Modifications included allowance for New Zealand specific database information (e.g. River Environment Classification—stream segment identification number; Snelder et al. Citation2004), and provisions to enable the evaluation of these methods in greater spatial detail within a reach.
In accordance with EMAP methods, electrofishing machine settings (voltage, pulse width, pulse rate) were standardised based on the conductivity of the water in the reach being sampled. However, different electrofishing machines may use different units of measurement and have different operating capacities. In New Zealand, only one type of backpack electrofishing machine is currently used by different organisations, the ‘Kainga EFM300’ (NIWA Instrument Systems, Christchurch, New Zealand). When using this model, the following slightly modified procedure was applied for the stated conditions: initial voltage setting 1–4 (×100 V) for high conductivity [>300 µS/cm]; 2–5 (×100 V) for medium conductivity [100–300 µS/cm]; 3–6 (×100 V) for low conductivity [<100 µS/cm] waters. Pulse width was set at 2 ms and pulse rate between 60 and 70 Hz. These settings were tested immediately below the selected site. If these settings resulted in all six lights showing on the wand, the voltage was lowered until five lights or less appeared.
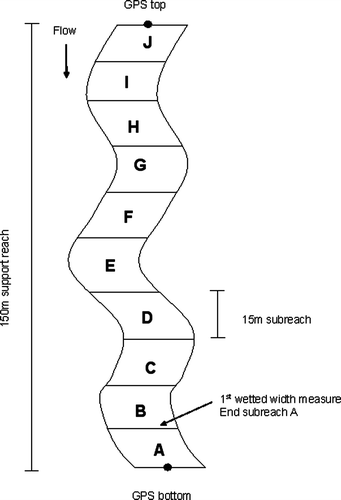
All reaches fished using this machine were undertaken in the same systematic manner, whereby the electrofisher would fish a rectangular ‘lane’ (approximately 3×2 m L×W) in a downstream direction (towards a pole netter) commencing from one bank at the downstream end of the reach. The cathode (‘tail’) was always positioned between the fisher and the pole netter to concentrate the field to the area being fished. Once a ‘lane’ was fished, the team would move one pole net width across the channel and repeat the process until the other side of the channel was reached. The pole netter and electrofishing operator would then move an equivalent ‘lane’ length distance upstream (3 m) to repeat the process until the complete area of the subreach was fished. Stream wetted width was recorded at the end of each subreach () so that area fished and ultimately fish density (fish/100 m2) could be determined for each sampled reach. Fish were then identified, recorded and released at least one pool riffle sequence downstream prior to commencement of the next subreach. To evaluate species distribution and accumulation with stream distance and area sampled, fish species were tallied within each of the 10 equidistant (continuous) subreaches. As per EMAP methods, stop nets were not used.
Fig. 1 General reach layout (150 m). Sampling begins from the bottom end at sub-reach ‘A’ denoted by a Global Positioning System (GPS) coordinate. Once all of sub-reach A is fished (15-m segment), the stream width is measured and fish captured are identified, measured and released downstream. This pattern is repeated until all sub-reaches have been fished.
To ensure that data were collected and recorded in a comparable manner, the modified procedures were demonstrated under field conditions to all parties that later contributed data to this project. Other than testing the utility of the method for evaluating reach-scale fish diversity and relative abundance, there were no other specific hypotheses driving site selection. In effect, sites were chosen opportunistically, with the sampling methodology substituted into various existing research projects and monitoring programmes where possible. These included projects related to topics such as flow allocation and stream rehabilitation, SoE monitoring and reference site benchmarking. Consequently, a range of organisations sampled a range of streams that provided a range of conditions with respect to mean wetted width, conductivity, altitude, distance to the coast, landuse and geographic position ().
Table 1 Range, mean and standard deviation of various parameters measured at 73 sites across New Zealand.
Fish community richness across New Zealand is strongly influenced by stream gradient, altitude and distance inland (Jowett & Richardson Citation1996). In general, high altitude inland sites have fewer species than sites at low elevation that are close to the coast. Thus, to evaluate species accumulation with distance sampled consistently for all sites, the number of fish species detected within each subreach was standardised to a common index value of between 0 and 1 (1 = 100% of the total richness detected for the entire distance sampled). These data were then plotted in 15-m intervals (each 15-m value being the mean index from n=73 sites, ±SD) up to 150 m (the minimum sampling distance for all sites).
All organisations were required to sample 40×the mean wetted width (minimum of 150 m for streams with mean wetted widths <3.75 m), via one pass electrofishing unless time and or operator fatigue precluded site completion (n=3 occasions). Total shock (‘button’) time was recorded to provide an accurate measure of electrofishing effort expended at a site (excluding the time taken to count and process fish).
Results
Between December 2008 and April 2009, 73 first- to third (Strahler)-order streams were sampled by backpack electrofishing by five regional councils (Auckland, Waikato, Horizons, Wellington and Otago), the Department of Conservation (Waikato Conservancy) and two consultancies—National Institute of Water and Atmospheric research (NIWA) and Kessels and Associates (both under contract to Environment Waikato). The streams spanned five regions of New Zealand, four in the North Island (Auckland, Waikato, Manawatu, Wellington), and one in the South Island (Otago) (). Sampled streams varied in mean wetted width from 0.58 to 12.68 m (mean of 10 width measurements/sampled reach) and conductivity (60–640 µS/cm, spot measurement at the time of sampling) (). Streams also covered a broad altitudinal range (<10–350 m asl), were <0.5 km to 170 km inland, and had adjacent landcover ranging from pasture to tall-growing species providing complete canopy cover.
Table 2 Range, mean and standard deviation of fish diversity for each of the five regions sampled.
Of the 73 reaches fished, 17 (23%) were sampled beyond 150 m. The length of stream fished ranged up to 345 m, and fishing area varied over an order of magnitude (121–3151 m2; ). Relative fish abundance derived from single pass electrofishing varied from 0.15 to 248 fish/100 m2 () and reach-scale richness ranged from one to nine species ().
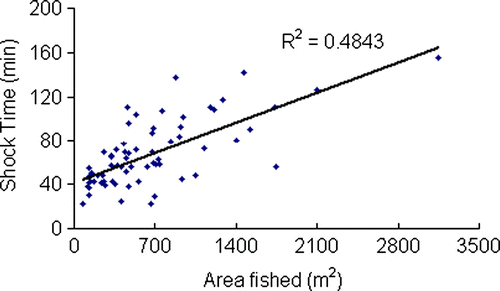
Time to sample a reach ranged from just over an hour up to a maximum of 8 h (). Sampling time was primarily influenced by the total area sampled and the number of fish captured. In effect long, wide reaches combined with high fish densities took the longest to complete. There was a positive linear relationship between the shock time (button) and the area fished indicating that, in general, different operators expended similar effort in proportion to the area sampled using these methods (; R 2=0.48).
Fig. 2 Relationship between shock time and area fished for 73 sites across New Zealand. A positive linear trend indicates that irrespective of operator that effort expended was generally proportional to the area fished.
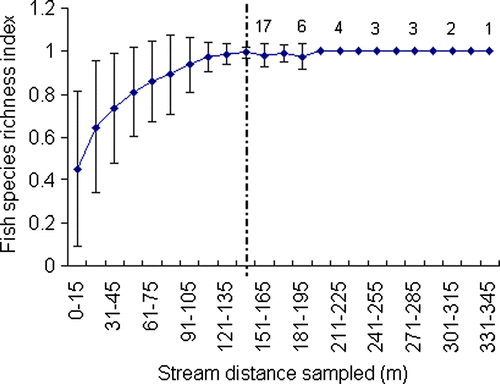
The distance at which maximum species richness was detected varied substantially between different streams. In some streams, full reach-scale species detection was achieved within 15 m, whereas in other streams additional species were still being detected beyond 100 m (). Irrespective of location or stream size, however, the likelihood of detecting new species within any reach declined markedly beyond 120 m. Of the 17 sites that were sampled beyond 150 m, additional species were only recorded at two sites (12%) where a single additional species was detected in each case ().
Fig. 3 Species richness index curve (species accumulation standardised to a common value of between 0 and 1 for every 15 m sampled, 1 = 100% of the richness detected for the total distance sampled). Data points up to 150 m (dashed line) are means derived from n=73 sites across New Zealand. Numbers to the right of the dashed line represent the number of sites where more than 150 m was sampled. Likelihood of detecting previously undetected species becomes lower with increasing stream distance sampled. Error bars are expressed as (±1 SD around the mean).
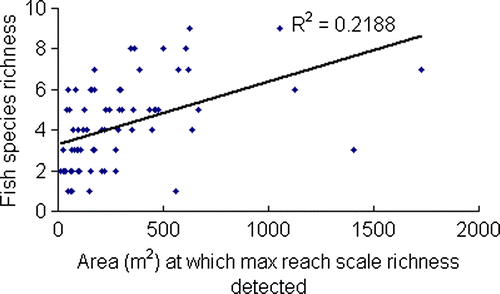
Reach-scale fish community richness for stream area sampled was also investigated (). Although a weak positive relationship was evident (i.e. greater richness tended to be detected if a larger area was sampled R 2=0.22), length fished appeared to be a more accurate predictor of species detection than area sampled.
Discussion
Meaningful estimates of fish species richness in streams can be only be achieved if the length of each stream segment sampled approaches or exceeds the length at which the cumulative species number becomes asymptotic (Lyons Citation1992; Paller Citation1995; Blocksom et al. Citation2009). If the primary goal of the monitoring is to report on reach-scale diversity in wadeable New Zealand streams, our data indicate that single pass electrofishing of at least 150 m is likely to be required to be confident of detecting >90% of the species likely to be present. The asymptotic pattern describing accumulation of species richness for distance sampled was generally consistent across sites irrespective of stream channel width (up to 12.7 m). This pattern suggests a standard minimum reach length is appropriate for fish biomonitoring in New Zealand wadeable streams where the fauna is substantially less diverse than Northern Hemisphere countries (McDowall Citation1990). Our data indicated little or no increase in reach scale richness with sampling lengths greater than this (up to 345 m). In accordance with other studies (e.g. Lyons Citation1992; Angermeier & Smogor Citation1995), our results showed that rates of species accumulation varied among streams. Others have indicated that this variability is a function of probability of individual capture, which is influenced by sampling method, fish size, local physical habitat conditions and number of individuals present in a given area (Bayley & Peterson Citation2001). Consequently, no single method can meet every need, and comparing relative results within reaches over time, rather than between reaches, is likely to be more appropriate and informative.
Some species in New Zealand often respond better to capture after one or more electrofishing passes (e.g. eels and lamprey, particularly juveniles) or are easier to detect with other methods when present but in low densities (e.g. spotlighting of kokopu species, B. David, unpublished data). Although our data for reach-scale richness were close to asymptotic by 150 m, the possibility that not all species may have been detected at every reach cannot be discounted. Nevertheless, with the methodology employed, and the wide range of streams sampled (excluding large braided river systems), additional species detection beyond this distance in other comparable streams is likely to be low.
Because many of New Zealand's native fish species have evolved to occupy and utilise specific habitats (McDowall Citation1990), our reasoning for low species detection beyond 150 m is that the majority of general habitat unit types available to fish at the reach-scale (e.g. pool, riffle, run, backwaters, rapids, woody debris etc.) were more than likely sampled within this distance (authors’ personal observation). A distance of 100–150 m is generally consistent with other standardised biological and physical reach-scale assessments in New Zealand wadeable streams (e.g. Macroinvertebrate sampling protocols; Stark et al. Citation2001; Collier & Kelly Citation2005; Standard Habitat Assessment Protocols; Harding et al. Citation2009). Data held in the NZFFD indicate that 45 m is the average length of stream sampled by single-pass backpack electrofishing with less than 10% of entries indicating a sampling distance of 100 m or more. Consequently we caution that reach-scale community richness (as is often inferred from presence–absence records in the NZFFD) may be underrepresented at many localities (particularly where short distances have been fished), simply because sufficient habitat may not have been sampled.
We consider the additional investment of time to sample a longer reach is justifiable particularly at previously unsampled localities and when assessing long-term changes to fish communities. Knowledge of how variable the species richness and abundance of fish communities are over time (at the reach-scale) is virtually non-existent in New Zealand. An important consideration to obtain these data is to ensure that sample reaches that are of sufficient length to encompass the range and frequency of physical processes shaping local habitat (Reid et al. Citation2008). As with any assessment of the state of biotic communities, a core set of reference sites should be monitored to evaluate natural variability of fish metrics against the variability caused by human induced stressors. Additionally, caution needs to be exercised when calculating fish densities after bed-moving flows, which may disrupt habitat and influence catchability of fish. For SoE sampling we recommend sampling is conducted during warmer seasonal months (early December to late April) when fish are most active and that a stand-down period should be considered following any bed moving flow to allow faunal recovery (e.g. Collier and Kelly Citation2005; David & Hamer Citation2010).
Where possible, information from these methods would be greatly enhanced by the compilation of other biotic and abiotic information collected using other standard procedures such as macro-invertebrate protocols (e.g. Stark et al. Citation2001; Collier Citation2008, Citation2009) and the Stream Habitat Assessment Protocols (SHAP) for wadeable rivers and streams of New Zealand (Harding et al. Citation2009).
Various extensions to the method used here are possible depending on the purpose of the investigation. For instance, better estimates of fish abundance could be examined by undertaking multiple pass depletion within a number of subreaches within a reach. Additionally, if detailed information on recruitment potential of fish is of interest, more detailed information on their size structure could be recorded for specific reaches. As a minimum, the EMAP methods (as followed in this investigation) typically record the maximum and minimum size range for individual species as opposed to measuring all fish. As part of a separate but related study, we are using the same methodological framework and datasheets to investigate and compare the use of spotlighting as a less invasive and more effective method for detecting species known to respond poorly to electrofishing.
Conclusions
Our results suggest that sampling at least 150 m of stream (irrespective of width up to 12.7m) is required to confidently establish reach-scale fish species richness in New Zealand wadeable streams using one pass backpack electrofishing. Reduced sampling effort in terms of length can greatly increase the likelihood of not detecting species that may be present at the reach-scale, and sampling more may result in substantially greater effort for little gain. Based on the use of these methods in New Zealand, each site should take on average about 2–3 h to complete using the minimum sampling requirements but may take longer particularly in wider streams and if high densities of fish are encountered. With sufficient national coverage, use of a consistent and comparable method will enable a more realistic assessment of the state of fish communities across New Zealand. In time, identification of any trends (e.g. local or national recruitment failure/decline) should be possible at relevant spatial and temporal scales, particularly if repeated annual sampling were to occur in conjunction with a core set of national ‘reference’ sites. It is also likely that emerging data will assist in the ongoing development of useful metrics to evaluate current and future impacts on fish in New Zealand wadeable streams.
Acknowledgements
Data were supplied by the Department of Conservation (Waikato Conservancy), National Institute of Water and Atmospheric research (NIWA under contract for Environment Waikato) and five Regional councils (Auckland, Waikato, Horizons, Wellington, Otago). A large number of people contributed to collecting the data contained within this work. In particular, we recognise the efforts of Eric Brown, Peter Hancock, Mike Joy, Wendy Davies, Paul Franklin, Joshua Smith, Louise Mack, Rebecca Lander, Brenda Aldridge, Melany Ginders, Britta Deichmann and Gerry Kessels. Thanks to Clint Prior for numerous formatting and design iterations to the protocol datasheets and to Laura Drummond for extracting and supplying raw NZFFDB information. Two anonymous reviewers provided valuable comments that improved this manuscript.
References
- Angermeier , PL and Smogor , RA . 1995 . Estimating number of species and relative abundances in stream-fish communities: effects of sampling effort and discontinuous spatial distributions . Canadian Journal of Fisheries and Aquatic Sciences , 52 : 936 – 949 .
- Angermeier , PL and Schlosser , IJ . 1989 . Species–area relationships for stream fishes . Ecology , 70 : 1450 – 1462 .
- Bayley , PB and Peterson , JT . 2001 . An approach to estimate probability of presence and richness of fish species . Transactions of the American Fisheries Society , 130 : 620 – 633 .
- Blocksom , K , Emery , E and Thomas , J . 2009 . Sampling effort needed to estimate condition and species richness in the Ohio river, USA . Environmental Monitoring and Assessment , 155 : 157 – 167 .
- Burns , NM , Bryers , G and Bowman , E . 2000 . Protocol for monitoring trophic levels of New Zealand lakes and reservoirs , Wellington : Ministry for the Environment .
- Cao , Y , Larsen , DP and Hughes , RM . 2001 . Evaluating sampling sufficiency in fish assemblage surveys—a similarity-based approach . Canadian Journal of Fisheries and Aquatic Sciences , 58 : 1782 – 1793 .
- Cao , Y , Larsen , DP , Hughes , RM , Angermeier , PL and Patton , TM . 2002 . Sample size affects multivariate comparisons of stream communities . Journal of the North American Benthological Society , 21 : 701 – 714 .
- Clayton JS Edwards T 2006 LakeSPI—a method for monitoring ecological condition of New Zealand lakes Technical Report Version 2 Hamilton NIWA
- Collier , KJ . 2008 . The Average Score Per Metric: an alternative metric aggregation method for assessing stream health . New Zealand Journal of Marine and Freshwater Research , 42 : 367 – 378 .
- Collier , KJ . 2009 . Linking multimetric and multivariate approaches to assess the ecological condition of streams . Environmental Monitoring and Assessment , 157 : 113 – 124 .
- Collier KJ Kelly J 2005 Regional guidelines for ecological assessments of freshwater environments: Macroinvertebrate sampling in wadeable streams Environment Waikato Technical Report 2005/02 Hamilton Environment Waikato
- David , BO and Closs , GP . 2002 . Behaviour of a stream-dwelling fish before, during, and after high discharge events . Transactions of the American Fisheries Society , 131 : 762 – 771 .
- David BO Hamer MP 2010 Regional guidelines for ecological assessments of freshwater environments: Fish monitoring in wadeable streams Environment Waikato Technical Report 2010/09 Hamilton Environment Waikato
- Fischer , JR and Paukert , CP . 2009 . Effects of sampling effort, assemblage similarity, and habitat heterogeneity on estimates of species richness and relative abundance of stream fishes . Canadian Journal of Fisheries and Aquatic Sciences , 66 : 277 – 290 .
- Gorman , OT and Karr , JR . 1978 . Habitat structure and stream fish communities . Ecology , 59 : 3507 – 515 .
- Gray T 2009 Using biological indicators to determine river water quality and ecosystem health for national environmental reporting A scoping study to inform Ministry for the Environment work in 2009 Draft Technical working document, last updated April 2009
- Harding , JS , Clapcott , JE , Quinn , JM , Hayes , JW , Joy , MK , Storey , RG , Greig , HS , Hay , J , James , T , Beech , MA , Ozane , R , Meredith , AS and Boothroyd , IKG . 2009 . Stream habitat assessment protocols for wadeable rivers and streams of New Zealand , Christchurch : University of Canterbury .
- Hughes , RM , Kaufmann , PR , Herlihy , AT , Intelmann , SS , Corbett , SC , Arbogast , MC and Hjort , RC . 2002 . Electrofishing distance needed to estimate fish species richness in raftable Oregon Rivers . North American Journal of Fisheries Management , 12 : 198 – 203 .
- Jennings , S . 2005 . Indicators to support an ecosystem approach to fisheries . Fish and Fisheries , 6 : 212 – 232 .
- Jowett , IG and Richardson , J . 1989 . Effects of a severe flood on in-stream habitat and trout populations in seven New Zealand rivers . New Zealand Journal of Marine and Freshwater Research , 27 : 11 – 17 .
- Jowett , IG and Richardson , J . 1996 . Distribution and abundance of freshwater fish in New Zealand rivers . New Zealand Journal of Marine and Freshwater Research , 30 : 239 – 255 .
- Joy MK 2009 Temporal and land-cover trends in freshwater fish communities in New Zealand's rivers: an analysis of data from the New Zealand Freshwater Fish Database—1970–2007 A report prepared for the Ministry for the Environment Wellington
- Joy , MK and Death , RG . 2002 . Predictive modelling of freshwater fish as a biomonitoring tool in New Zealand . Freshwater Biology , 47 : 2261 – 2275 .
- Joy , MK and Death , RG . 2003 . Assessing biological integrity using freshwater fish and decapod habitat selection functions . Environmental Management , 32 : 747 – 759 .
- Joy , MK and Death , RG . 2004 . Application of the index of biotic integrity methodology to New Zealand freshwater fish communities . Environmental Management , 34 : 415 – 428 .
- Leathwick , JL , Elith , J , Chadderton , WL , Rowe , D and Hastie , T . 2008 . Dispersal, disturbance, and the contrasting biogeographies of New Zealand's diadromous and non-diadromous fish species . Journal of Biogeography , 35 : 1481 – 1497 .
- Leathwick , JL , Elith , J , Rowe , D and Julian , K . 2009 . Robust planning for restoring diadromous fish species in New Zealand's lowland rivers and streams . New Zealand Journal of Marine and Freshwater Research , 43 : 659 – 671 .
- Lyons , J . 1992 . The length of stream to sample with a towed electric fishing unit when fish species richness is estimated . North American Journal of Fisheries Management , 12 : 198 – 203 .
- McDowall , RM . 1990 . New Zealand freshwater fishes—a natural history and guide , Auckland : Heinemann Reed .
- McDowall , RM . 1993 . Implications of diadromy for the structuring and modelling of riverine fish communities in New Zealand . New Zealand Journal of Marine and Freshwater Research , 27 : 453 – 462 .
- McDowall RM Richardson J 1983 The New Zealand freshwater fish survey: guide to input and output New Zealand Ministry of Agriculture and Fisheries, Fisheries Research Division Information Leaflet 12
- McEwan A 2009 Fine scale spatial behaviour of indigenous riverine fish in a small New Zealand stream Unpublished M.Sc. thesis, Massey University
- Mosely PM Pearson CP 1997 Floods and droughts: the New Zealand experience The Caxton Press Christchurch, , New Zealand
- Nicholson , MD and Jennings , S . 2004 . Testing candidate indicators to support ecosystem-based management: the power of monitoring surveys to detect temporal trends in fish community metrics . Journal of Marine Science , 61 : 35 – 42 .
- Paller , MH . 1995 . Relationships amongst number of fish species sampled, reach length surveyed, and sampling effort in South Carolina coastal plain streams . North American Journal of Fisheries Management , 15 : 110 – 120 .
- Patton , TM , Hubert , WA , Rahel , FJ and Gerow , KG. 2000 . Effort needed to estimate species richness in small streams on the great plains in Wyoming . North American Journal of Fisheries Management , 20 : 394 – 398 .
- Peck , DV , Herlihy , AT , Hill , BH , Hughes , RM , Kaufmann , PR , Klemm , DJ , Lazorchak , JM , McCormick , FH , Peterson , SA , Ringold , PL , Magee , T and Cappaert , MR . 2006 . Environmental monitoring and assessment program—surface waters: western pilot study field operations manual for wadeable streams , Washington, DC : US Environmental Protection Agency .
- Reid , HE , Gregory , CE and Brierley , GJ . 2008 . Measures of physical heterogeneity in appraisal of geomorphic river condition for urban streams: twin streams catchment, Auckland, New Zealand . Physical Geography , 29 : 247 – 274 .
- Reynolds , L , Herlihy , AT , Kaufmann , PR , Gregory , SV and Hughes , RM . 2003 . Electrofishing effort requirements for assessing species richness and biotic integrity in western Oregon streams . North American Journal of Fisheries Management , 23 : 450 – 461 .
- Simonson , TD and Lyons , J . 1995 . Comparison of catch per effort removal procedures for sampling stream fish assemblages . North American Journal of Fisheries Management , 15 : 419 – 427 .
- Smith , KL and Jones , ML . 2005 . Watershed level sampling effort requirements for determining riverine fish species composition . Canadian Journal of Fisheries and Aquatic Sciences , 62 : 1580 – 1588 .
- Snelder , TH , Cattaneo , F , Suren , AM and Biggs , BJF . 2004 . Is the River Environment Classification an improved landscape-scale classification in rivers? . Journal of the North American Benthological Society , 23 : 580 – 598 .
- Stark JD Boothroyd IKG Harding JS Maxted JR Scarsbrook MR 2001 Protocols for sampling macroinvertebrates in wadeable streams New Zealand Macroinvertebrate Working Group Report No.1 Prepared for the Ministry for the Environment. Sustainable Management Fund Project No. 5103
- Suren A Snelder T Scarsbrook M 1998 Urban stream habitat assessment method (USHA) NIWA Client Report No. CHC98/60, NIWA Christchurch
- US EPA 1998 Environmental Monitoring and Assessment Program (EMAP): Research plan 1997 EPA/620/R-98/002 Washington, DC US Environmental Protection Agency
- Ward JC Pyle E 1997 Environmental indicators for the sustainable management of freshwater Ministry for the Environment contract report No 2416/1 Wellington
- Wright , JF . 1995 . Development and use of a system for predicting the macroinvertebrate fauna in flowing waters . Australian Journal of Ecology , 20 : 181 – 197 .
- Young , RG , Townsend , CR and Matthaei , C . 2004 . Functional indicators of river ecosystem health—an interim guide for use in New Zealand , Wellington : Ministry for the Environment .