Abstract
We analyse short-term individual and group behavioural reactions and long-term individual responses of bottlenose dolphins (Tursiops truncatus) in New Zealand to remote skin biopsy sampling. The biopsy system employed uses a small stainless steel tip (5-mm diameter, 9-mm length) mounted on a lightweight polycarbonate projectile, fired from a modified veterinary rifle with adjustable pressure. Individual and group behavioural reactions were scored on a 5-point scale from 0 (no reaction) to 5 (strenuous reaction). A total of 187 individual and 145 group behavioural reactions were recorded from the Bay of Islands subpopulation, while 38 individual and 39 group behavioural reactions were recorded from the Doubtful Sound subpopulation. Short-term reactions (<1 min duration) were ranked from low or mild (levels 1–2), as evidenced by startle responses, to moderate (level 3), as evidenced by multiple leaps of the sampled individual. Most attempts (99%) resulted in only mild reactions with only two attempts resulting in moderate reactions. There were no strenuous reactions by individuals or groups and no significant differences in behavioural reactions between the two subpopulations. Analyses of resighting rates and capture probabilities based on individual identification records for 40 of the biopsied dolphins showed no evidence of long-term avoidance responses. Wound healing was documented in for 10 dolphins across periods of 3 days to 7 months. Results reported here agree with previous findings showing that remote biopsy sampling causes only short-term reactions in both the targeted individual and its group.
Introduction
The collection of small tissue samples from live cetaceans is essential to addressing questions in population genetics (Baker et al. Citation1990; Tezanos-Pinto et al. Citation2009), phylogenetics (Caballero et al. Citation2008), taxonomy (Rosenbaum et al. Citation2002; Caballero et al. Citation2007), foraging ecology (de Stephanis et al. Citation2008; Valenzuela et al. Citation2009), social structure (Duffield & Wells Citation1991; Krützen et al. Citation2005), toxicology (Fossi et al. Citation2000), reproductive success (Rosenbaum et al. Citation2002), population dynamics (Oremus et al. Citation2007) and aging of individuals (Herman et al. Citation2009). Methods used for the collection of samples from wild cetaceans include ‘skin swabbing’ the animals using a velcro pad (Harlin et al. Citation1999), collection of sloughed skin (Amos & Hoelzel Citation1990), faeces from the water column (Parsons et al. Citation1999), use of a biopsy dart mounted on a pole (Bilgmann et al. Citation2008), the deployment of a remote biopsy dart using a crossbow (Lambertsen Citation1987) or a modified veterinary capture rifle (Krützen et al. Citation2002). Recently, DNA has been successfully extracted from the blow of wild whales and dolphins kept in captivity (Frère et al. Citation2010); however, the efficiency of this method has yet to be adequately tested in wild small cetacean populations.
Skin swabbing and the collection of faeces are considered ‘non-invasive’ as they do not penetrate the epidermis. Most sampling methods suffer from some form of limitation; with skin swabbing and faecal collection there are limits to the quantity or quality of the sample obtained (Taberlet & Waits Citation1998; Parsons et al. Citation2003; Gerloff et al. Citation2008). In small cetaceans, the collection of faeces is directly related to environmental conditions and is not suitable in poor visibility waters or where strong currents are present. Both skin swabbing and collection of faeces also tend to provide poor quality or low quantity DNA samples, which can affect the reliability of some genetic analyses (e.g. microsatellites). Furthermore, blubber for toxicology, pregnancy or stable isotopes analyses cannot be obtained using these methods (Parsons et al. Citation2003).
A biopsy system consists of a small dart projected from an airgun, crossbow or veterinary capture rifles (Lambertsen Citation1987; Kasamatsu et al. Citation1991; Palsbøll et al. Citation1991; Weinrich et al. Citation1991; Barrett-Lennard et al. Citation1996; Larsen Citation1998). These systems are considered ‘invasive’ because they penetrate the epidermis, leaving behind a small wound. With one notable exception (Bearzi Citation2000), however, remote biopsy sampling can be characterised as ‘minimally intrusive’ in terms of the reported low level of short-term reactions and long-term responses.
Observations of reactions by large whales have shown only short-term, low-level of behavioural disturbance and no evidence of long-term impact to habitat use or reproduction (Weinrich et al. Citation1991; Clapham & Mattila Citation1993; Brown et al. Citation1994; Gauthier & Sears Citation1999; Hooker et al. Citation2001; Best et al. Citation2005; Cantor et al. Citation2010). Observations of reactions by small cetaceans (e.g. delphinids) are similar, reporting short-term and minor behavioural reactions (Aguilar & Nadal Citation1984; Barrett-Lennard et al. Citation1996; Weller et al. Citation1997; Krützen et al. Citation2002; Parsons et al. Citation2003; Gorgone et al. Citation2008; Jefferson & Hung Citation2008; Kiszka et al. Citation2010). Biopsy sampling wounds usually heal quickly with no reported physiological complications (Weller et al. Citation1997; Krützen et al. Citation2002; Parsons et al. Citation2003).
The one reported exception to the safe collection of biopsy samples was the death of a common dolphin (Delphinus delphis, Bearzi Citation2000), sampled with a biopsy dart designed for the larger killer whale (Orcinus orca, Barrett-Lennard et al. Citation1996). The death appeared to have been caused by the dart penetrating beyond the stop, causing vertebral trauma, or as a result of stress related to the efforts to assist the dolphin (Bearzi Citation2000).
A significant improvement in biopsy sampling of small cetaceans is the system of Krützen et al. (Citation2002). This system uses a modified veterinary gun to propel the dart. The advantage of using a rifle is that it is more accurate than a crossbow, which is vital when sampling small, fast-moving dolphins that are frequently encountered in tight groups. Furthermore, the system allows modification of the velocity (i.e. air pressure) at which the dart leaves the barrel depending on the distance to the animal and its size (Krützen et al. Citation2002). The main improvements in the safety of the dart used by Krützen et al. (Citation2002) are a wider-barrel body made of lighter polycarbonate to spread the impact over a wider area and therefore, reduce the risk of injury by penetration (Krützen et al. Citation2002).
Here, we report short-term reactions to biopsy sampling from bottlenose dolphins in two populations of New Zealand: the Bay of Islands and Doubtful Sound. A project investigating the genetic structure of the New Zealand populations (Tezanos-Pinto et al. Citation2009) was initiated in 2003 using the biopsy sampling system designed by Krützen et al. (Citation2002). At the time, there was public concern regarding biopsy sampling the Doubtful Sound subpopulation because of the perception that dolphins in this region were ‘unusually sensitive to invasive techniques’ (Currey et al. Citation2009). This perception resulted in part, from a report that Doubtful Sound dolphins displayed strenuous reactions after a suction cup tag attempt, at both the individual and group level (Schneider et al. Citation1998). Given the environmental and ecological differences between the Bay of Islands and Doubtful Sound, we investigated the possibility that dolphins in these subpopulations may react differently to the biopsy sampling technique. In the Bay of Islands, we also investigated long-term responses to biopsy sampling through the analysis of resighting rates and capture probabilities using a long-term photo-identification database.
Materials and methods
Study area
Biopsy sampling surveys were conducted in the Bay of Islands (35°12′S, 174°10′E) from 3 June 2003 to 1 May 2006 using primarily a 4.7-m inflatable boat powered by a 50-hp outboard engine. Biopsy sampling surveys were conducted in Doubtful Sound/Patea (45°15′S, 166°51′E) from 23 October to 5 November 2004 using a rigid Stabi-craft boat equipped with a 90-hp outboard engine.
Biopsy sampling was also conducted in the Hauraki Gulf (Auckland) and Marlborough Sounds (Tezanos-Pinto et al. Citation2009), but in these regions, data were collected by several researchers. In order to avoid potential inter-observer biases, we have included only data collected by the two authors.
Biopsy sample collection
Skin samples were collected by the authors using a remote biopsy sampling system (PAXARMS, Timaru, New Zealand; Krützen et al. Citation2002). A biopsy attempt was defined as a dart fired from the biopsy gun aimed at a dolphin, regardless of contact or collection of a sample. A targeted dolphin was defined as the individual at whom the dart was aimed to. Biopsy sampling targeted only adult dolphins and took place in reasonable weather conditions (<Beaufort 4). An ‘adult’ was defined as a fully grown dolphin measuring 3–3.5 m in length (Constantine Citation2002). An attempt was made to photo-identify the targeted dolphin during biopsy sampling, although this was only partially successful. Additionally, a mounted video camera attached to the biopsy gun was used to record sampling attempts and to assist with individual identification and behavioural reactions. Biopsy samples were collected in the absence of permitted tour-boats and suspended for 30–45 min if dolphins showed any avoidance to the research boat.
A protocol designed specifically for individual identification consisted of a photographer standing immediately adjacent to the biopsy sampler, photographing the individual that was being biopsy sampled. Photo-identification of non-targeted dolphins was not conducted during biopsy sampling. Data collected after a sampling attempt included: date, time, hit or failed attempt, air pressure used, photograph frame number, biopsy dart number, group size and composition, GPS position, place and side of the body hit by the dart, distance to the dolphin, individual and behavioural reactions (events and states; see below) before and after the sampling attempt and the duration of the response. If the identity of the targeted dolphin was known, this was also recorded.
Behavioural reactions to biopsy sampling
Individual and group reactions to biopsy sampling were ranked from 0 for no reaction to 5 for a sustained group reaction, as modified from Krützen et al. (Citation2002) and Schneider et al. (Citation1998, ). Particular attention was given to potential for group reactions given the previous observations of strenuous reaction to suction cap tags placed on bottlenose dolphins in Doubtful Sound (Schneider et al. Citation1998).
Table 1 Hierarchical classification of individual and group behavioural reactions of bottlenose dolphins to biopsy sampling (adapted from Schneider et al. 1998; Krützen et al. 2002)
Behavioural data were only collected by the two authors to avoid potential inter-observer biases. Behavioural data collected included both events and states before and after biopsy sampling attempts from both the targeted individual and the group. Group reactions included the immediate response of the dolphins next to the targeted individual (within less than two body lengths of the targeted individual). Behavioural events for individuals and groups included: no reaction, startle, flinch, dive, tail slap or flick, speed burst, high-speed surfacing, leaps, multiple leaps and moved away from the boat. These were recorded for 5 min post-biopsy.
The behavioural state of the group was also observed before and after a sampling attempt and defined as: socialising, foraging, resting, slow travelling, travelling, fast travelling and milling (Constantine et al. Citation2004). Observations of behavioural states were collected over periods ranging from 10 min to 5 h post-biopsy (mean = 67 min, mode = 21 min).
Evaluation of long-term responses in the Bay of Islands
The potential for long-term responses in the Bay of Islands was evaluated by comparing the resighting rates of dolphins before and after biopsy sampling using a simple ratio based on total sighting events. A second approach conducted consisted in estimating the capture probabilities of biopsy sampled versus non-biopsy sampled dolphins in MARK (White & Burnham Citation1999). Dolphins were individually identified by photographs (Tezanos-Pinto Citation2009) and microsatellite genotyping (Tezanos-Pinto, unpubl. data).
For the analyses of resighting rates, we considered the sighting records of individually identified dolphins in the Bay of Islands (Tezanos-Pinto Citation2009) before and after biopsy sampling (i.e. non-standardised sighting data). A Mann–Whitney U-test was implemented to detect potential differences in the sighting data before and after biopsy sampling. A ‘sighting’ refers to an individual identification photograph obtained during an encounter with a uniquely identified dolphin (ID) and the associated data collected during such an encounter. To standardise the sighting rates, we considered the date of the sampling and the total opportunities for sightings before and after this date. This is because a dolphin sampled late in the study would have fewer opportunities to be resighted after the sampling. A sighting ratio was calculated for each dolphin by summing the number of sightings of the individual before being biopsy sampled, and dividing by the total number of sightings collected of all dolphins up to the sampling event (Sb). Similarly, the sighting ratio for each dolphin after biopsy sampling was calculated by summing the number of sightings of the individual after sampling and dividing by the total number of sighting events collected of all dolphins after the sampling date up to the last survey (Sa). When multiple samples were collected from the same dolphin, the date of the first sampling was used to estimate the sighting ratio. A sign test was implemented to detect potential differences in the sighting ratio of dolphins before and after being biopsy sampled.
Analyses conducted in MARK included annual data collected during 2003–05, a period of consistent research effort (Tezanos-Pinto Citation2009). To evaluate if biopsied dolphins presented differences in capture probabilities compared with non-biopsy sampled dolphins, we allowed for the effects of a ‘biopsy group’ (biopsied dolphin or non-biopsy sampled dolphin). Given the variable residency patterns among bottlenose dolphins observed in the Bay of Islands (Constantine Citation2002; Tezanos-Pinto Citation2009), a ‘residency group’ option was built considering the residency pattern of each dolphin as previously defined by Tezanos Pinto (2009). The residency pattern of each dolphin was assigned considering one sighting per lunar month; according to this, occasional visitors were sighted 2–8 lunar months and frequent users sighted ≥9 lunar months (Tezanos-Pinto Citation2009). Transient animals (sighted only 1 lunar month during the study period) were excluded from this analysis. The selection procedure consisted in choosing a model with the lowest corrected Akaike Information Criterion (AICc) score (Hurvich & Tsai Citation1989), which is a combination of the number of parameters and the maximum likelihood estimate of the model (Cooch & White Citation2005). The significance of difference between two nested models was tested with chi-squared test on MARK (i.e. likelihood-ratio-test; Cooch & White Citation2005). A significant difference between models means that: (1) there is a significant increase in deviance with the reduction in the number of parameters, such that the reduced model fits significantly less well and (2) the parameter(s) involved contribute significantly to variation in the data (Cooch & White Citation2005).
Results
A total of 65 surveys were conducted in the Bay of Islands during which 215 biopsy sampling attempts (i.e. in which a dart was fired at a dolphin) occurred. This provided data on 187 individual and 145 group behavioural reactions to biopsy sampling. Reactions not recorded occurred at the beginning of the study when the sampling protocol was being developed. Dolphins were biopsied at estimated distances of 1–15 m (n=138, mean = 7.10 m, SD = 3.81). Overall, 73% attempts (n=156) resulted in contact with the dolphin; of which 144 (92%) provided enough DNA for genetic analyses of sex and mtDNA control region. Of the hits that did not provide a tissue sample, three cases were caused by the dart breaking upon impact and sinking before being retrieved, two cases the biopsy tip was empty when the dart was retrieved and seven cases were caused by the dart sticking and being subsequently lost. When darts were stuck, biopsy sampling was interrupted and the dolphin's behaviour was observed until the dart detached. Of the darts that stuck, six cases resulted in detachment within 5 min of being hit, whereas in one case the dart remained stuck for 1 h 40 min (). In all cases, the darts did not penetrate beyond the stop (i.e. beyond the 9-mm length of the tip) but, rather, were attached superficially to the skin by the tip of the dart.
Table 2 Description of individual (IR) and group behavioural reactions (GR) of bottlenose dolphins in the Bay of Islands when a dart remained stuck; including the time elapsed until the dart detached and the time the dolphin was observed post-biopsy
A total of nine surveys were conducted in Doubtful Sound in which there were 39 biopsy attempts, providing information on 38 individual and 39 group behavioural reactions. One individual reaction was recorded as a tail flick; however, heavy rain at the time of sampling prevented a detailed observation (e.g. if the dolphin also dove, startled, etc. …) and therefore, this reaction was excluded from the analyses. Dolphins were sampled at estimated distances of 2–20 m (n=32, mean = 10.1 m, SD = 6.97). Overall, 46% of attempts resulted in contact with the dolphins (n=18); of which 17 samples (94%) provided adequate tissue for genetic analyses of sex and mtDNA control region.
Individual behavioural reactions
Of the 225 observed individual reactions in the total dataset, 99% resulted in mild reactions with only two attempts (1%) representing moderate reactions (level 3) characterised by a single vertical jump. All individual reactions were momentary, lasting <1 min. The majority of individual reactions observed in both the Bay of Islands and Doubtful Sound were level 1 (72% and 68% respectively; ). There were no individual reactions of level 4 or 5 (strenuous reactions). Given the small sample size of some reactions (e.g. level 3) individual reactions were grouped as ‘no reaction’ (level 0 and 1) and ‘mild–moderate’ (level 2 and 3). There were no significant differences when comparing individual reactions between populations (Fisher exact test, P=0.42). We examined potential changes in behaviour from biopsies that hit (n=174) with those that missed (n=51) for both populations combined. We found no significant difference in reactions (Fisher exact test, P=0.28; ), suggesting that dolphins responded the same way to a hit as a miss.
Table 3 Level of behavioural responses (LR) of bottlenose dolphins (T. truncatus) in the Bay of Islands (BOI) and Doubtful Sound (DS) to remote biopsy sampling; including individual, group and responses to hit and misses
Group behavioural reactions
A total of 184 group behavioural reactions to biopsy sampling were recorded; most observations were collected in the Bay of Islands (n=145) and the rest in Doubtful Sound (n=39). Of these 184 observations, 24% were level 0; 72% were level 1 and 4% were level 2. There were no group reactions of level 3, 4 or 5. All group reactions were instant, lasting <1 min. Most of the group reactions observed in both the Bay of Islands and Doubtful Sound were level 1 (70% and 74% respectively, ). Because of small sample size, group reactions were grouped as ‘no reaction’ (level 0) and ‘mild reactions’ (level 1 and 2). There were no significant differences in group reactions between populations (Fisher exact test, P=0.99). Overall, we found no significant difference in reactions (Fisher exact test, P=0.99), suggesting that dolphins responded the same way to a hit as a miss.
Behavioural states of groups
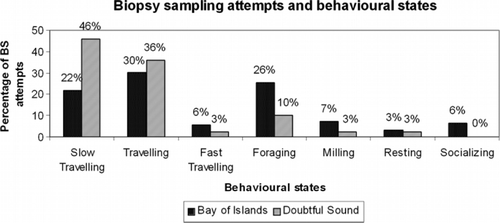
Following sampling, we observed no changes of behavioural states in Doubtful Sound and only in four occasions (3%) we observed changes in behavioural states in the Bay of Islands. Behavioural states that changed included transitions from milling to foraging (n=1), from resting to slow travelling (n=1), from foraging to travelling (n=1) and from slow travelling to travelling (n=1). However, given the low number of transitions, it is possible that these may be natural occurring events rather than a consequence of sampling. Overall, in both locations most biopsy sampling attempts were conducted during travelling (slow, travelling or fast travelling) followed by foraging and milling ().
Evaluation of long-term individual responses in the Bay of Islands
In the total of 144 biopsy samples collected from 3 June 2003 to 1 May 2006, 54 (including multiple sampling) were collected from 40 individual dolphins identified from photo-identification records and/or microsatellite genotyping (). Of these 40 individuals, 29 were sampled once, eight sampled twice and three sampled three times. To look for long-term responses (e.g. long-term avoidance), we compared the total number of photo-identification sightings of these individuals before and after the biopsy events and the standardised ratios of these sightings, adjusted by the date of sampling and total photo-identification effort (see Methods). The frequency of sightings events for these individual dolphins varied from 0 to 31 before sampling and 0 to 42 after sampling. Using the non-standardised sighting data, there was no significant difference in the resighting rates of dolphins before and after sampling: 23 dolphins had more sightings before sampling, 15 had more sightings after sampling and two had a tie (Mann–Whitney U-test; adjusted H=1.952, df = 1, P=0.162). Using the standardised sighting data, the direction of the trend was reversed (i.e. higher sighting ratios after sampling) but the difference was still not significant: 15 dolphins had lower sighting ratios before sampling, 20 had higher sighting ratios after sampling and five were tied (sign test, P=0.130; ).
Table 4 Summary of sighting events for 40 individually identified dolphins that were biopsy sampled (BS) in the Bay of Islands from 2002–06
We also addressed the question of long-term responses by estimating recapture probabilities using the program MARK. To help reduce the underlying heterogeneity of residency patterns in the Bay of Islands (Constantine Citation2002; Tezanos-Pinto Citation2009), we used annual resighting records stratified into ‘frequent users’ and ‘occasional users’. Further stratification included a ‘biopsy effect’ (whether dolphins were or not biopsy sampled). The best model selected in MARK indicated that the effect of ‘residency’ (i.e. if a dolphin was a frequent user or occasional visitor) explained most of the variation in capture probabilities rather than the effect of being (or not) biopsy sampled (Model 1 compared with model 3, . Likelihood-ratio-test, chi-square = 11.03, df = 2, P<0.004). The capture probability of frequent users biopsy sampled (Cap. P=0.999, SE=0.006, n=24) was not significantly different than those frequent users not biopsy sampled (Cap. P=0.997, SE = 0.014, n=17; two-tailed P=0.885); similarly the capture probability of occasional visitors biopsy sampled (Cap. P=0.841, SE=0.008, n=15) was not significantly different from those occasional visitors not-biopsy sampled (Cap. P=0.818, SE=0.097, n=72; two-tailed P=0.914).
Table 5 Cormack Jolly Seber open population models conducted in MARK to estimate capture probabilities of bottlenose dolphins sighted annually in the Bay of Islands from 2003–05
Wound healing in the Bay of Islands
In the Bay of Islands, it was possible to monitor the healing of biopsy wounds for 10 individuals photographed subsequently from a minimum of 3 days to a maximum of 7 months after sampling. Fresh wounds presented a dark spot of relatively small size that turned white after 4–5 days post-biopsy (). We observed no bleeding of a biopsy wound at the time of sampling or during healing. Healing seemed to occur without complication and no apparent signs of infection or swelling were observed. In Doubtful Sound, surveys occurred during a 2-week period only and therefore monitoring of wound healing was not possible.
Discussion
Individual and group behavioural reactions
Our results support previous research indicating little apparent behavioural reactions to biopsy sampling in bottlenose dolphins at either the individual or group level (, Weller et al. Citation1997; Krützen et al. Citation2002; Parsons et al. Citation2003; Gorgone et al. Citation2008). Most individual reactions observed here were mild as evidenced by startle (speed burst or dive), tail flick or slap. Startle reactions to biopsy sampling have commonly been reported in other species (e.g. Whitehead et al. Citation1990) and seem to be a part of the behavioural repertoire of bottlenose dolphins as a response to external stimuli. Startle reactions are also observed during vessel approaches, changes in vessel speed, presence of predators and interactions with other dolphins (G. Tezanos-Pinto pers. obs.; Weller et al. Citation1997). Close boat approaches elicit behavioural responses in dolphins (e.g. Constantine et al. Citation2004; Bejder Citation2005; Lusseau Citation2006a); therefore, it is possible that some of the observations reported here are a response to the boat approach, the sampling procedure or both.
Table 6 Comparison of individual reactions of bottlenose dolphins (Tursiops sp.) to biopsy sampling including results from this study
Although we observed no category 4 reaction during our standard survey, the opportunistic collection of samples during a survey in the Hauraki Gulf (Auckland) conducted on the 5 March 2004 resulted in a biopsy sampled bottlenose dolphin displaying a series of vertical jumps (i.e. leaps, N. Wiseman, K. Stockin, pers. comm.) when a dart stuck (reaction level 4). This individual was part of a group of 40–50 (five of which were previously biopsied and displayed only mild reactions). The dart did not detached from the dolphin but remained attached superficially to the skin (i.e. the dart did not penetrate beyond the 9-mm length of the tip). This dolphin was subsequently resighted in the area during 2005 and 2006, based on photo-identification (Tezanos-Pinto unpubl. data). It seems likely the strenuous reaction observed may have been caused by the animal trying to dislodge the dart from its body. However, we did not observe such strong reaction when darts remained stuck on bottlenose dolphins in the Bay of Islands. Although leaps are part of the normal behavioural repertoire of bottlenose dolphins, particularly during social interactions and foraging (Acevedo-Gutierrez Citation1999; Lusseau Citation2006b), there may be individual specific differences as a reaction to a dart that does not dislodge. We would speculate that animals habituated to an open-water (i.e. oceanic) environment may attempt to dislodge the dart from their body, as they do with remoras (Remora sp.) or cookie-cutter sharks (Isistius sp.); whereas dolphins habituated to a coastal environment have no such experience and therefore do not attempt to dislodge a stuck dart. Potential causes for sticking of darts identified included insufficient recoil resulting from our efforts to minimise the force of the dart, as we adjust the pressure gauge for different distances, or blunt dart tips. These can be minimised increasing the force (i.e. air pressure), using a stronger charge and sharpening the dart tips before each survey.
As reported in other studies, group reactions observed were mild in all cases in both populations. A typical situation observed included several dolphins simultaneously surfacing near the research boat, when the targeted animal was struck by the dart it reacted by accelerating with a speed burst and the dolphin closest to it reacted with a startle. This pattern of group reaction has also been observed in Galveston, South Carolina, Georgia (USA) and Shark Bay (Weller et al. Citation1997; Krützen et al. Citation2002; Gorgone et al. Citation2008).
Evaluation of long-term responses in the Bay of Islands
Analyses of individual capture probabilities between biopsied and non-biopsy sampled dolphins in the Bay of Islands showed no differences between groups, indicating that long-term residency patterns were not affected by biopsy sampling. Similarly, analyses of resighting rates of bottlenose dolphins indicated no changes after dolphins were sampled. Dolphins continued using the area after being biopsy sampled, approaching the research boat and allowing individual photo-identification. Eleven dolphins that were sampled twice and three that were sampled three times (confirmed by photo-identification and microsatellite markers; Tezanos-Pinto unpubl. data) were subsequently resighted and easily approached during research surveys. Similarly, in Shark Bay, Krützen et al. (Citation2002) reported that biopsy sampled dolphins did not appear to alter their long-term behaviour after being biopsy sampled and were still easily approachable later during systematic surveys.
Behavioural reactions to biopsy sampling in Doubtful Sound
There were differences in the hit success rate between the Bay of Islands and Doubtful Sound (73% vs 46%, respectively). It appears that environmental factors mostly explained location differences in success rate since both authors (CSB and GTP) collected samples in both subpopulations. Water visibility in Doubtful Sound is very poor (Gibbs Citation2001) and therefore, predicting the surfacing patterns of dolphins is difficult in comparison with the Bay of Islands, resulting in reduced time for aiming. Additionally, some surveys in Doubtful Sound were conducted under rainy conditions, which may have resulted in reduced efficiency.
The observed mild reactions of the Doubtful Sound dolphins to biopsy sampling were similar to those observed in the Bay of Islands and other bottlenose dolphin populations (; Krützen et al. Citation2002; Parsons et al. Citation2003; Gorgone et al. Citation2008). This contrasts with the reported strenuous group reactions to previous suction cup tagging (Schneider et al. Citation1998). In this regard, concerns raised by some about the ‘unusual sensitivity of the Doubtful Sound population to invasive techniques’ (Currey et al. Citation2009) were not supported. It seems more likely that the strong reactions observed during the suction cup tagging could be explained by important differences between the two techniques. We speculate that the suction tag is likely to be perceived by the dolphin as a foreign object attached to the body, whereas biopsy sampling results in only momentary contact as it rebounds off the skin. As such, coastal dolphins do not react as if they are attempting to remove and/or dislodge a foreign object.
Conclusion
Our results from bottlenose dolphins in New Zealand support previous observations that the small, lightweight dart developed by Krützen et al. (Citation2002) is both safe and effective. We observed only relatively low levels of short-term reactions in both populations. Furthermore, we detected no evidence of differences in residency patterns or resighting rates before and after sampling of known individuals in the Bay of Islands, indicating that long-term aversive responses are unlikely.
Acknowledgements
Funding for this project was provided by the Northland Marine Mammal Trust; Department of Conservation (Northland); J. Watson Conservation Trust from the Royal Forest and Bird Society; Postgraduate Tuition Fee Bursary (University of Auckland), the Whale and Dolphin Adoption Project and PBRF Funding from the School of Biological Sciences, University of Auckland to GTP; Marsden Fund of the Royal Society of New Zealand to CSB. Biopsy samples were collected under permit to CSB from the New Zealand Department of Conservation and animal ethics protocols AEC/02/2002/R9 and AEC/02/2005/R334 from the University of Auckland.
Logistic support was provided by the Department of Conservation (Northland and Te Anau), especially T. Beauchamp (DoC Northland), R. Kemper, B. Masser (DoC Te Anau). We thank the following for assistance in fieldwork: P. Gay, P. Norris, C. Paterson, N. Baker, R. Gosh and B. Woodward (Doubtful Sound); S. Wells, D. Pouwels, F. Mourão, J. Brueggeman, K. McLeod, E. Newcombe, A. Fleming, C. Clark, D. Heimeier, J. Jackson, M. Oremus, S. Caballero, M. Richtlen and E. Carroll (Bay of Islands). Thanks to R. Constantine for providing access to the Bay of Islands catalogue, the stakeholders of the Hauraki Gulf catalogue for providing access to the catalogue and S. Lavery for providing support and working facilities at his laboratory. Thanks to N. Wiseman and K. Stockin for providing information regarding biopsy sampling in the Hauraki Gulf. Thanks to the Department of Conservation (Te Anau, Southland) for assistance and for providing an independent observer of the sampling procedure in Doubtful Sound. K. Stockin, M. Oremus, D. Heimeier, C. Olavarría and R. Albertson reviewed an early version of the manuscript. S. Childerhouse and an anonymous reviewer provided valuable comments that improved the quality of this work.
References
- Acevedo-Gutierrez , A . 1999 . Aerial behaviour is not a social facilitator in bottlenose dolphins hunting in small groups . Journal of Mammalogy , 80 : 768 – 776 .
- Aguilar , A and Nadal , J . 1984 . Obtención de biopsias hipodérmicas de cetáceos en libertad . Investigación Pesquera , 48 : 23 – 29 .
- Amos B , Hoelzel AR 1990 . DNA fingerprinting cetacean biopsy samples for individual identification . In : Hammond PS , Mizroch SA , Donovan GP . Individual recognition of cetaceans: use of photo-identification and other techniques to estimate population parameters. Cambridge, International Whaling Commission, Special Issue 12.
- Baker , CS , Palumbi , SR , Lambertsen , RH , Weinrich , MT , Calambokidis , J and O'Brien , SJ . 1990 . Influence of seasonal migration on geographic distribution of mitochondrial DNA haplotypes in humpback whales . Nature , 344 : 238 – 240 .
- Barrett-Lennard , LG , Smith , TG and Ellis , GM . 1996 . A cetacean biopsy system using lightweight pneumatic darts, and its effect on the behavior of killer whales . Marine Mammal Science , 12 : 14 – 27 .
- Bearzi , G . 2000 . First report of a common dolphin (Delphinus delphis) death following penetration of a biopsy dart . Journal of Cetacean Research and Management , 2 : 217 – 221 .
- Bejder L 2005 . Linking short and long-term effects of nature-based tourism on cetaceans . Unpublished PhD thesis, Dalhousie University, Canada.
- Best , P , Reeb , D , Rew , MB , Palsbøll , PJ , Schaeff , C and Brandão , A . 2005 . Biopsying southern right whales: their reactions and effects on reproduction . Journal of Wildlife Management , 69 : 1171 – 1180 .
- Bilgmann , K , Griffiths , O , Allen , J and Möller , L . 2008 . A biopsy pole system from bow-riding dolphins: sampling success, behavioural responses and test for sampling bias . Marine Mammal Science , 23 : 218 – 225 .
- Brown , MR , Corkeron , P , Hale , PT , Schultz , KW and Bryden , MM . 1994 . Behavioral responses of east Australian humpback whales Megaptera novaeangliae to biopsy sampling . Marine Mammal Science , 10 : 391 – 400 .
- Caballero , S , Jackson , J , Mignucci-Giannoni , AA , Barrios-Garrido , H , Beltrán-Pedreros , S , Montiel-Villalobos , MG , Robertson , KM and Baker , CS . 2008 . Molecular systematics of South American dolphins Sotalia: sister taxa determination and phylogenetic relationships, with insights into a multi-locus phylogeny of the Delphinidae . Molecular Phylogenetics and Evolution , 46 : 252 – 268 .
- Caballero , S , Trujillo , F , Vianna , J , Barrios-Garrido , H , Montiel , M , Beltrán-Pedreros , S , Marmontel , M , Santos , M , Rossi-Santos , M Santos , F . 2007 . Taxonomic status of the genus Sotalia: species level ranking for ‘Tucuxi’ (Sotalia fluviatilis) and ‘Costero’ (Sotalia guianensis) dolphins . Marine Mammal Science , 23 : 358 – 386 .
- Cantor M , Cachuba T, Fernandes L, Engel M 2010 . Behavioural reactions of wintering humpback whales (Megaptera novaeangliae) to biopsy sampling in the western South Atlantic . Journal of the Marine Biological Association of the United Kingdom . doi: 10.1017/S0025315409991561 .
- Clapham , PJ and Mattila , DK . 1993 . Reactions of humpback whales to skin biopsy sampling on a West Indies breeding ground . Marine Mammal Science , 9 : 382 – 391 .
- Constantine R 2002 . The behavioural ecology of the bottlenose dolphins (Tursiops truncatus) of Northeastern New Zealand: a population exposed to tourism . Unpublished PhD thesis, The University of Auckland, Auckland, New Zealand.
- Constantine , R , Brunton , D and Dennis , T . 2004 . Dolphin-watching tour boats change bottlenose dolphin (Tursiops truncatus) behaviour . Biological Conservation , 117 : 299 – 307 .
- Cooch E White GC 2005 Program MARK. ‘A gentle introduction’ Colorado State University , Fort Collins , CO .
- Currey , R , Dawson , SM , Slooten , E , Schneider , K , Lusseau , D , Boisseau , O , Haase , P and Williams , JA . 2009 . Survival rates of declining bottlenose dolphins in Doubtful Sound, New Zealand: an information theoretic approach to assessing the role of human impacts . Aquatic Conservation: Marine and Freshwater Ecosystems , 19 : 658 – 670 .
- de Stephanis , R , García-Tíscar , S , Verborgh , P , Esteban-Pavo , R , Pérez , S , Minvielle-Sebastia , L and Guinet , C . 2008 . Diet of the social groups of long-finned pilot whales (Globicephala melas) in the Strait of Gibraltar . Marine Biology , 154 : 603 – 612 .
- Duffield DA , Wells RS 1991 . The combined application of chromosome, protein and molecular data for the investigation of social unit structure and dynamics in: Tursiops truncatus . In : Hoelzel AR . Genetic Ecology of Whales and Dolphins, Report of the International Whaling Commission 13 . Pp. 155 – 169 .
- Fossi , MC , Marsili , L , Casini , S , Bearzi , G , Politi , E , Zaradelli , M and Panigada , S . 2000 . Skin biopsy of Mediterranean cetaceans for the investigation of interspecies susceptibility to xenobiotic contaminants . Marine Environmental Research , 50 : 517 – 521 .
- Frère , C , Krzyszczyk , E , Patterson , EM , Hunter , S , Ginsburg , A and Mann , J . 2010 . Thar she blows! A novel method for DNA collection from cetacean blow . PLoS Biology , 5 : e12299
- Gauthier , J and Sears , R . 1999 . Behavioral response of four species of balaenopterid whales to biopsy sampling . Marine Mammal Science , 15 : 85 – 101 .
- Gerloff , U , Schlötterer , C , Rassamann , K , Rambold , I , Hohmann , G , Fruth , B and Tautz , D . 2008 . Amplification of hypervariable simple sequence repeats (microsatellites) from excremental DNA of wild living bonobos (Pan paniscus) . Molecular Ecology , 4 : 515 – 518 .
- Gibbs , MK . 2001 . Aspects of the structure and variability of the low-salinity-layer in Doubtful Sound, a New Zealand fiord . New Zealand Journal of Marine and Freshwater Research , 35 : 59 – 72 .
- Gorgone , AM , Haase , P , Griffith , ES and Hohn , A . 2008 . Modelling response of target and nontarget dolphins to biopsy darting . Journal of Wildlife Management , 72 : 926 – 932 .
- Harlin , AD , Würsig , B , Baker , CS and Markowitz , T . 1999 . Skin swabbing for genetic analysis: application to dusky dolphins (Lagenorhynchus obscurus) . Marine Mammal Science , 15 : 409 – 425 .
- Herman , DP , Ylitalo , GM , Robbins , J , Straley , JM , Gabriele , CM , Clapham , PJ , Boyer , RH , Tilbury , KL , Pearce , RW and Krahn , MM . 2009 . Age determination of humpback whales Megaptera novaeangliae through blubber fatty acid compositions of biopsy samples . Marine Ecology Progress Series , 392 : 277 – 293 .
- Hooker , SK , Baird , RW , Al-Omari , S , Gowans , S and Whitehead , H . 2001 . Behavioral reactions of northern bottlenose whales (Hyperoodon ampullatus) to biopsy darting and tag attachment . Fishery Bulletin , 99 : 303 – 308 .
- Hurvich , M and Tsai , C . 1989 . Regression and time series model selection in small samples . Biometrika , 76 : 297 – 307 .
- Jefferson , TA and Hung , SK . 2008 . Effects of biopsy sampling on Indo-Pacific humpback dolphins (Sousa chinensis) in a polluted coastal environment . Aquatic Mammals , 34 : 310 – 316 .
- Kasamatsu , F , Iwata , S and Nishiwaki , S . 1991 . Development of biopsy skin sampling system for fast swimming whales in pelagic waters . Report of the International Whaling Commission , 41 : 555 – 557 .
- Kiszka , JJ , Simon-Bouhet , B , Charlier , F , Pusineri , C and Ridoux , V . 2010 . Individual and group behavioural reactions of small delphinids to remote biopsy sampling . Animal Welfare , 19 : 411 – 417 .
- Krützen , M , Barre , LM , Möller , LM , Heithaus , MR , Simms , C and Sherwin , WB . 2002 . A biopsy system for small cetaceans: darting success and wound healing in Tursiops spp . Marine Mammal Science , 18 : 863 – 878 .
- Krützen , M , Mann , J , Heithaus , MR , Connor , R , Bejder , L and Sherwin , W . 2005 . Cultural transmission of tool use in bottlenose dolphins . Proceedings of the National Academy of Sciences , 102 : 8939 – 8943 .
- Lambertsen , RH . 1987 . A biopsy system for large whales and its use for cytogenetics . Journal of Mammalogy , 68 : 443 – 445 .
- Larsen F 1998 . Development of a biopsy system primarily for use on large cetaceans . Report SH/50/O15 presented at the International Whaling Commission, Oman, 16–20 May
- Lusseau , D . 2006a . The short-term behavioural reactions of bottlenose dolphins to interactions with boats in Doubtful Sound, New Zealand . Marine Mammal Science , 22 : 804 – 818 .
- Lusseau , D . 2006b . Why do dolphins jump? Interpreting the behavioural repertoire of bottlenose dolphins (Tursiops sp.) in Doubtful Sound, New Zealand . Behavioural Processes , 73 : 257 – 265 .
- Oremus , M , Poole , MM , Steel , D and Baker , CS . 2007 . Isolation and interchange among insular spinner dolphin communities in the South Pacific revealed by individual identification and genetic diversity . Marine Ecology Progress Series , 336 : 275 – 289 .
- Palsbøll PJ , Larsen F, Hansen ES 1991 . Sampling of skin biopsies from free-ranging large cetaceans in West Greenland: development of new biopsy tips and bolt designs . In : Hoelzel AR . Genetic ecology of whales and dolphins. Cambridge, International Whaling Commission. Special Issue 13 . Pp. 71 – 79 .
- Parsons , KM , Durban , JW and Claridge , DE . 2003 . Comparing two alternative methods for sampling small cetaceans for molecular analysis . Marine Mammal Science , 19 : 224 – 231 .
- Parsons , KM , Dallas , JF , Claridge , DE , Durban , JW , Balcomb , KC , Thompson , PM and Noble , LR . 1999 . Amplifying dolphin mitochondrial DNA from faecal plumes . Molecular Ecology , 8 : 1766 – 1768 .
- Rosenbaum , HC , Weinrich , MT , Stoleson , SA , Gibbs , JP , Baker , CS and Desalle , R . 2002 . The effect of differential reproductive success on population genetic structure: correlations of life history with matrilines in humpback whales of the Gulf of Maine . Journal of Heredity , 93 : 389 – 399 .
- Schneider , K , Baird , RW , Dawson , SM , Visser , I and Childerhouse , S . 1998 . Reactions of bottlenose dolphins to tagging attempts using a remote deployed suction-cup tag . Marine Mammal Science , 14 : 316 – 324 .
- Taberlet , P and Waits , LP . 1998 . Non-invasive genetic sampling . Trends in Ecology and Evolution , 13 : 26 – 27 .
- Tezanos-Pinto G Population structure, abundance and reproductive parameters of bottlenose dolphins (Tursiops truncatus) in the Bay of Islands (Northland, New Zealand) Unpublished PhD thesis, The University of Auckland, Auckland, New Zealand .
- Tezanos-Pinto G Baker CS Russell K Martien KK Baird RW Stone G Mignucci-Giannoni A Caballero S Endo T others A worldwide perspective on the population structure and genetic diversity of bottlenose dolphins (Tursiops truncatus) in New Zealand Journal of Heredity 2009 100 11 24
- Valenzuela , L , Sironi , M , Rowntree , V and Seger , J . 2009 . Isotopic and genetic evidence for culturally inherited site fidelity to feeding grounds in southern right whales (Eubalaena australis) . Molecular Ecology , 18 : 782 – 791 .
- Weinrich , MT RH Lambertsen Baker , CS MR Schilling Belt , C 1991 . Behavioural responses of humpback whales (Megaptera novaeangliae) in southern gulf of Maine to biopsy sampling , in AR Hoelzel , GP Donovan Genetic ecology of whales and dolphins . Cambridge : The International Whaling Commission , Pp. 91 – 97 .
- Weller , DW , Cockcroft , VG , Würsig , B , Lynn , SK and Fertl , D . 1997 . Behavioral responses of bottlenose dolphins to remote biopsy sampling and observations of surgical biopsy wound healing . Aquatic Mammals , 23 : 49 – 58 .
- White , GC and Burnham , KP . 1999 . Program MARK: survival estimation from populations of marked animals . Bird Study , 46 : 120 – 139 .
- Whitehead , H , Gordon , J , Mathews , EA and Richard , KR . 1990 . Obtaining skin samples from living sperm whales . Marine Mammal Science , 6 : 316 – 326 .