Abstract
Hydrographic and water quality surveys of the Motueka River and its river plume were conducted during a moderate flood event (peak flow of 420 m3/s) to assess the source and fate of faecal contaminants transported into Tasman Bay. Escherichia coli (E. coli) and enterococci concentrations in the river were up to 10000 and 7300 Most Probable Number (MPN)/100 ml during peak flow, respectively. A coastal survey revealed a shallow low-salinity plume that extended at least 6 km into Tasman Bay and the region's largest aquaculture management areas (AMAs). Mussels within the influence of the river plume, including those collected within an AMA, had E. coli and enterococci concentrations as high as 1300 and 2200 MPN/100 g tissue, respectively. Application of microbial source tracking markers using end-point PCR assays identified the presence of faecal contamination from ruminant animals (cows, sheep) in water and mussel samples. The detection of ruminant faecal contamination within shellfish located 6 km offshore highlights the close connection between land use and the quality of New Zealand's highly valued coastal resources.
Introduction
Rivers are conduits for the delivery of land-derived pollution and associated contaminants into the marine environment. New Zealand's highly valued shellfish resources [e.g. recreational and customary harvest areas for cockles (Austrovenus stutchburyi) and pipis (Paphies australis) and commercial aquaculture areas for Greenshell™ mussels (Perna canaliculus) and Pacific oysters (Crassostrea gigas)] are often situated in close proximity to coastal rivers. Consequently the majority of these areas are frequently (and in some cases permanently) contaminated by land-derived sources of faecal contaminants, which leads to loss of revenue related to harvest closures, export bans and product recalls (Industry representatives, pers. comm.). Faecal contamination of aquatic systems is, in many cases, positively correlated with antecedent rainfall (e.g. Ahn et al. Citation2005; Krometis et al. Citation2007; Brodie et al. Citation2010; McKergow & Davies-Colley Citation2010), indicating that contaminants can be washed from terrestrial environments or possibly released from sewerage overflows and transported by receiving waters. The sustainability of New Zealand's shellfish resources will likely be impacted by the nature and intensity of surrounding land use and the physical behaviour of coastal river plumes that transport contaminants into the marine environment (Warrick et al. Citation2007; Reifel et al. Citation2009).
The current understanding of faecal contamination in aquatic environments in New Zealand is largely based on freshwater systems. Internationally there has been an increasing number of studies on the persistence and fate of faecal microorganisms in estuaries (e.g. Geary & Davies Citation2003; Fries et al. Citation2008); however, in situ information on the transport and fate of faecal contaminants in the marine environment remains limited (Ahn et al. Citation2005; Bonkosky et al. Citation2009; Svejkovsky et al. Citation2010). Human faecal contamination is directly relevant to health risk (NRC Citation2004) and carries highly host-specific human pathogens such as enteric viruses. Other sources contributing to high bacterial concentrations that impact coastal resources in New Zealand likely include untreated faecal material directly deposited on the land from c. 50 million ruminant animals (Livestock statistics; NZ Ministry for Agriculture and forestry), and these sources can also carry pathogens (e.g. Campylobacter, Cryptosporidium; Schueler & Holland Citation2000). Nonetheless, there remains a paucity of information on the extent to which different faecal contaminant sources or land uses contribute to contamination problems. Questions also remain about the spatial scale of effects of land-derived sources of faecal contamination on the marine environment, where significant dilution occurs.
As a research contribution to the Motueka River Integrated Catchment Management Programme and using a ‘ridgetops to sea’ approach, we aimed to describe the extent to which a moderate flood event (peak flow of 420 m3/s) leads to faecal contamination in the Motueka River and the resulting river plume, which is known to influence a large portion of Tasman Bay. Tasman Bay is a shallow and highly productive water body; aquaculture for Greenshell™ mussels is currently being developed in three aquaculture management areas (AMAs) that collectively represent an area of 15 km2. Following heavy rainfall, the river produces extensive low-salinity plumes that can extend more than 18 km offshore and encompass the area of the AMAs (Tuckey et al. Citation2006). During the flood event, we conducted a hydrographic survey of the Motueka River and the river plume in order to investigate the source and fate of faecal contaminants in Tasman Bay. Water and mussel samples collected between the river mouth and the AMAs were analysed by conventional, culture-dependent methods for enumerating faecal indicator bacteria (FIB). Concentrations of FIB such as Escherichia coli can indicate levels of faecal contamination but not the source of contamination. In order to investigate faecal contamination derived from ruminant animals and humans, we applied a suite of molecular markers to identify the presence of host-specific faecal microorganisms in water and shellfish samples.
Methods
Site description
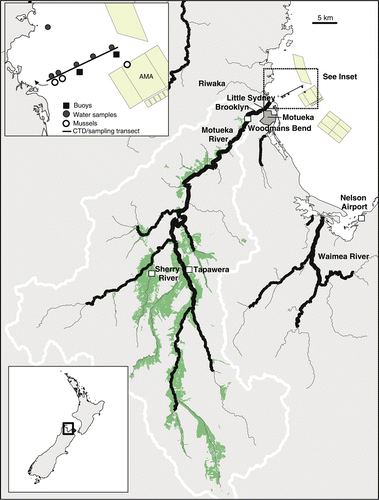
The Motueka River flows in a northerly direction for 110 km and drains a catchment area of 2180 km2 (including the 105-km2 Riwaka catchment) into Tasman Bay (). Mean annual rainfall for the catchment as a whole is 1600 mm and can vary considerably across the catchment as a function of topography (Basher Citation2003; McKergow & Davies-Colley Citation2010). The catchment is largely rural, with a mixture of native forest (35%), planted pine forest (25%) and pastoral land (19%) representing the majority of the landscape. Urbanised areas are limited to the township of Motueka (population of about 7000) and represent only 0.14% of the catchment area (Basher Citation2003). Historically, there was a small estuary within the delta region of the river; however, as a result of development, the river channel has been narrowed and shifted within the lower reaches, causing the river to flow more directly into Tasman Bay with little estuarine influence (Basher Citation2003). Smaller rivers that flow into estuaries prior to entering the bay (Riwaka, Waimea, Maitai and Wakapuaka Rivers) also contribute to freshwater inflows to Tasman Bay (Gillespie et al. Citation2011).
Figure 1 Map of study area showing the Motueka River Catchment, river sampling locations, weather stations at Tapawera and the Nelson Airport and locations of boat surveys and monitoring buoys. Land used for pastoral farming is shaded in green. The aquaculture management areas (AMAs) are shown as is the area of the catchment classified as high producing grassland for pastoral farming (shaded area; data from Land Cover Database, NZ Ministry for the Environment). The small line with arrow in the inset shows the path of GPS drifters.
Field sampling
Meteorological parameters
Rain and wind data, collected 1 week prior to, during and 1 week following a rain event that occurred between 26 and 30 April 2009, was obtained through the NIWA CliFlo website (cliflo.niwa.co.nz). Rainfall data was taken from the Tapawera weather station and wind data from the Nelson Airport located c. 30 km east of the Motueka River mouth (). Although these variables show a high degree of spatial variability, these locations were chosen to provide a general picture of rainfall in ‘mid catchment’ and winds measured near Tasman Bay and close to sea level, which are most relevant to processes occurring in the Bay.
Motueka River sampling
River flow data was obtained from a network of flow gauges managed by Tasman District Council. River water sampling using auto-samplers (ISCO 3700) was conducted between 28 and 30 April 2009 at two locations in the Motueka catchment: (1) the Sherry River, a tributary river located in the upper Motueka catchment and surrounded by pastoral grazing land; and (2) Woodmans Bend, located in the lower main stem of the Motueka River (). Auto-samplers were triggered based on river flows (triggered at 10 and 40 m3/s at the Sherry and Woodman's Bend sites, respectively). River flows were estimated using measures of the water level and a state-discharge rating in order to capture the rise and fall of the main hydrograph. Water samples (1-l) were collected in sterile polycarbonate bottles at 1-h intervals. In order to minimise costs, a total of 10 and 15 samples that best represented the hydrograph were retained for analysis of faecal bacteria from the Sherry and Woodmans Bend sites, respectively. Turbidity was measured at both sites every 15 min by an optical backscatter sensor (Greenspan TS100 at Woodmans Bend and Greenspan TS1200 at the Sherry; 0–2000 NTU for both).
In addition to auto-sampling, triplicate 1-l water samples were manually collected just following the peak of the flood in Brooklyn Stream, which flows through a low-density housing area and joins the Motueka River downstream of Woodmans Bend, and within Little Sydney Stream, which is a small stream that flows parallel to the Motueka River and through a small residential area (). Water samples were collected in these locations in order to assess faecal contamination entering the river that would not have been captured at the Woodmans Bend auto-sampling station. All water samples were kept cold on ice and were processed within 24 h of collection for FIB and microbial source tracking (MST) molecular markers.
Tasman Bay sampling
Temporal and spatial sampling of the water column in Tasman Bay was conducted through the use of moored sensor arrays and a boat survey, respectively. Two buoy-mounted sensor arrays were moored between the river mouth and the region of the AMAs. One mooring was situated a distance of 6 km from the river mouth and serves as a state-of-environment (SOE) site for the Tasman District Council. Two weeks prior to the storm event, a second replicate sensor array was placed 3 km from the river mouth equidistant between the river mouth and the SOE buoy (). Both moorings were outfitted with an acoustic current meter, conductivity–temperature–depth (CTD) sensors and a turbidity sensor (Falmouth Scientific Instruments) that were placed at a depth of 3 m below mean sea level.
Surface and sub-surface solar radiation measurements were recorded with Odyssey light sensors (Dataflow Systems Pty Ltd., Christchurch, NZ) deployed 2 weeks prior to the event. One light sensor was deployed above the water's surface on the SOE buoy, and one was placed on each of the two buoys at 3 m depth (attached to the external frame of the CTD). Each recorder was programmed to collect integrated light data over 10-min scan periods. Recorders were calibrated prior to deployment using a Licor light sensor and data were converted to units of µmol quanta/m2/s1. No visible fouling of the light sensors was observed when they were retrieved the week following the flood event.
A synoptic boat survey was conducted on 30 April 2010 between 08:00 and 14:00; 2 days following the peak of the flood in the river. The lag in sampling allowed for both adequate development of the river plume into the bay and suitable sea conditions to conduct field sampling. The boat survey was conducted along a 6-km transect located between the river mouth and approximately 0.5 km beyond the SOE buoy location (). Salinity, temperature, turbidity and irradiance was measured along the transect using a CTD (Seabird SBE-19) that was placed within a remotely operated glider housing. The CTD was towed at a speed of 3 knots while being continuously raised and lowered within the top 12 m of the water column. For visualisation of the data, a two-dimensional plane of salinity, temperature, turbidity and irradiation along the main transect was created through linear interpolation of the data. In order to assess surface water current direction during the survey, two sets of three drifters were released approximately 1.2 km from the river mouth. Each drifter recorded position every 15 s using Garmin GPS units placed within a small surface float that was attached to a drogue (water sail) submerged to 1 m depth.
Water samples for analysis of FIB and MST markers were collected at five locations along the main transect, the drop-off and pick-up locations of the GPS drifters, and a location approximately 4.5 km from the river mouth but closer to the shoreline, approximately 1 km off Kaiteriteri Beach (). At each location, 1-l polycarbonate containers (Nalgene) were filled at 1 m depth using an extension pole with grip. Three replicate samples were collected at each location and were stored in a chilly bin and processed immediately upon return to the laboratory (within 6 h of collection).
In order to track contamination in shellfish, we deployed six moored cages (plastic mesh size 20 mm) of mussels located between 1.5 and 6 km distance from the river mouth along the transect. The mussels within the cages were a mixture of Greenshell™ (P. canaliculus) and blue mussels (Mytilis edulis) collected from surface floats within the AMA on 6 April 2009 (c. 3 weeks prior to the flood event surveyed). Greenshell™ mussels were low in abundance along the surface floats and including blue mussels ensured there was enough tissue for analysis of FIB and molecular markers. We lost four of the mussel moorings during the storm event and were only able to retrieve the cages located at 1.5 and 2 km distance. In order to assess the extent of contamination in mussels at the distance of the AMAs, we collected M. edulis and Greenshell™ mussels that colonised a surface float within the central AMA at approximately 6 km distance from the river mouth (). Approximately 24 mussels (20–60 mm in length) were collected from each of the baskets and the surface float the day of the boat survey, and 24 more were collected a week later to assess the extent to which the mussels remained contaminated following the event. Roughly equivalent numbers of each mussel species were included in each composite sample for analysis of FIB concentrations in order to minimise any potential variation related to differing clearance rates. A composite of approximately 12 mussels (wet weight equivalent of about 100 g tissue) was used for FIB analysis. MST analyses were conducted on samples of both Greenshell™ mussels and M. edulis from the mooring at 1.5 km distance, and for Greenshell™ mussels and M. edulis at the mooring at 2 km distance and the AMA, respectively.
Sample analyses
Faecal indicator bacteria
Individual water samples were assayed for E. coli and enterococci using Colilert®-18 and Enterolert™, respectively (IDEXX Laboratories, Westbrook, ME, USA). Mussel samples (1-, 0.1- and 0.01-g portions of homogenised flesh and liquid) were analysed using a five-tube fermentation technique, where the E. coli numbers were estimated using sample treatment, growth media and procedures as described in NZFSA Method for Enumeration of Escherichia coli in bivalve molluscan shellfish (NZFSA Citation2008) and enterococci densities were identified in mussels as specified in AgResearch MIRINZ Meat Industry Microbiological Methods (AgResearch MIRINZ Citation2005). Samples in which bacterial levels were beneath the detection limit were treated as half the detection limit (e.g. Most Probable Number (MPN) <10=5 MPN) to calculate the arithmetic means.
Microbial source tracking markers
Water and mussel samples were analysed in singlet for the presence/absence of MST markers. The microbial community was isolated from water samples using a membrane filtration technique. The pH of the water sample was adjusted to 3.5 prior to filtration to facilitate binding of human polyomaviruses to the filter (McQuaig et al. Citation2006). A 200-ml sample of water from Tasman Bay samples and 100–200-ml sub-sample of water from river water samples was filtered through a 0.45-µm pore size membrane filter (mixed cellulose ester, 47 mm diameter) (Toyo Roshi Kaisha, Ltd, Tokyo, Japan). After filtration, the filter was folded using sterile forceps, and immediately placed into PowerBead tubes (Mo Bio Laboratories, Inc., Carlsbad, CA, USA) for DNA extraction.
Mussel samples were washed and scrubbed under running water before being shucked with a sterile knife. Three to five grams of digestive gland tissue were recovered from each sample, homogenised using a sterile scalpel and placed in sterile 50-ml Falcon tubes. An equal volume (volume per weight) of phosphate-buffered saline solution (PBS; Sigma-Aldrich Inc., St. Louis, MO, USA) was added and the mixture was vortexed at maximum speed for 3 min. The homogenate was left to settle for 10–15 min after which 250 µl of liquid supernatant was removed to PowerBead tubes (Mo Bio Laboratories) for DNA extraction.
DNA was extracted from all water and mussel samples using PowerSoil™ DNA extraction Kit (Mo Bio Laboratories) according to the modified manufacturer's protocol, where sample homogenisation was performed on Mini-Beadbeater-8 (Biospec, Bartlesville, OK, USA) for 2 min. Extracted DNA was tested for the presence of general, human- and ruminant-associated Bacteroidales [GBac (Bac32), HBac (HF183) and RBac (CF128); Bernhard & Field Citation2000a, Citationb]. Two additional human markers included the human Methanobrevibacter smithii nifH gene [HMbS (nifH); Ufnar et al. Citation2006] and human polyomaviruses (HPyV), JCV and BKV (McQuaig et al. Citation2006).
The PCR primers and protocols as described in the original publications (listed above) were used, except for Gbac, RBac, HBac and HMbS markers, which were analysed using protocols detailed in McQuaig et al. (Citation2009) and Leskinen et al. (Citation2010). The detection protocol for HPyV marker was modified from McQuaig et al. (Citation2006); 45 amplification cycles were used and primers were as specified in McQuaig et al. (Citation2009). No additional nested amplification step was performed. Platinum® Blue PCR Supermix (Invitrogen, Carlsbad, CA, USA) was used to amplify the HPyV marker, while GoTaq® Green Master Mix (Promega, Madison, WI, USA) was used for amplification of all other markers. Divalent cation concentrations were not modified (default Mg2+ concentration was 1.5 mM). Additional PCR additives were not used. After 45 cycles, PCR products were sized on 2% agarose gel stained in ethidium bromide solution (0.5 µg/ml) and visualised under UV excitation. Positive, negative and extraction controls were included with all sample runs. The extraction control consisted of an aliquot of DNA-free water that was subjected to all procedures experienced by the samples, including membrane filtration if applicable. All samples with a PCR-negative result were subsequently seeded with positive control material to identify any samples in which inhibition of the PCR occurred. The detection limit for water samples was approximately 15 gene copies per 100 ml. Approximately 330–530 gene copies per digestive gland were identified as a theoretical lower detection limit for the MST marker assays, assuming the ability to detect a single gene copy in the PCR reaction and 100% DNA recovery during each extraction step. As no sample expressed any significant level of inhibition, further DNA dilutions and PCR tests were not conducted.
Suspended solids
To compliment turbidity data, water samples (1-l), collected near the river mouth at the location where GPS drifters were released and retrieved, were analysed for suspended solids (both inorganic and organic). Well-mixed samples were filtered through a weighed, glass-fibre filter. The filter was dried at 105°C and re-weighed to determine concentration of total suspended solids. The filter was then ignited at 550°C and re-weighed to determine the volatile (organic) component (APHA Citation2005 method 2540 D and E).
Results
River flows and water quality
Total rainfall at Tapawera during the event surveyed was 55 mm; daily rainfall was 30.2, 11.7 and 0.8 mm on 28, 29 and 30 April 2009, respectively. Rainfall resulted in a rapid increase in river flow that was first observed in the Sherry River located in the upper catchment, and subsequently downstream in the lower Motueka River. There were two peaks in river flow, with a maximum flow of 25 and 420 m3/s in the Sherry and Motueka Rivers, respectively (). These river flows are indicative of a moderate flood event; an annual flood event in the Motueka River is 993 m3/s (based on long-term gauging at Woodstock located approximately 20 km upstream from Woodmans Bend).
Figure 2 Rainfall and river water conditions over the rain event surveyed. A, Daily rainfall accumulation at Tapawera. B, River flows, turbidity and faecal indicator bacteria in the Sherry River during the event. C, River flows, turbidity and faecal indicator bacteria in the Motueka River at Woodmans Bend.
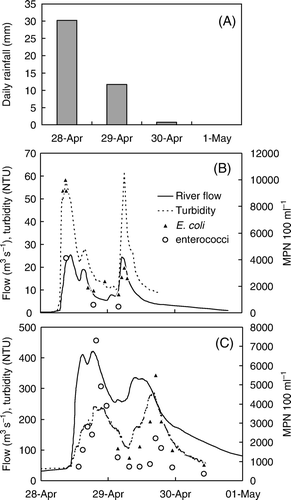
The increase in river flow closely coincided with increases in both turbidity and concentrations of FIB (). Turbidity increased to a maximum of 62 NTU in the Sherry River, and to a maximum of 414 NTU downstream at the Woodmans Bend sampling site. Concentrations of E. coli in the Sherry River were as high as 10,000 MPN/100 ml during peak flow, and down to 1400 MPN/100 ml during periods of lower flows. Enterococci concentrations from the Sherry River ranged between 455 and 4100 MPN/100 ml.
The first seven samples collected from Woodmans Bend during the initial peak in river flow were removed from the data set because of probable analytical error in FIB analysis. Peak concentrations of E. coli at Woodmans bend were 100 to 1000 times higher than those observed during base flows (McKergow & Davies-Colley Citation2010). Concentrations of E. coli peaked at 5500 MPN/100 ml during the second rise in river flow (). Enterococci concentrations at Woodmans Bend reached a maximum concentration of 7300 and 2200 MPN/100 ml during the first and second rise in river flow, respectively.
Concentrations of E. coli and enterococci in the Brooklyn and Little Sydney Streams were lower than those observed in the Sherry and the main river and ranged between 119 and 146 MPN/100 ml and between 306 and 573 MPN/100 ml, respectively. As was the case for other river samples, enterococci concentrations were lower than those for E. coli and ranged between 85 and 98 MPN/100 ml in the Brooklyn Stream and between 52 and 108 MPN/100 ml in the Little Sydney Stream. Turbidity in the Brooklyn and Little Sydney streams was comparatively lower than measured in the main river and averaged 4.1 and 11.3 NTU, respectively. As was the case for the Sherry and Woodmans Bend sites, high turbidity corresponded with high FIB concentrations in both the Little Sydney Stream and Brooklyn Stream.
Response in Tasman Bay to weather and river discharge
Turbidity measured at the buoys situated 3 and 6 km from the river mouth coincided with an increase in wind speeds (). Wind was directed from the northeast during the event and peaked at 16 m/s (). Tidal amplitude during and shortly following the event ranged between 2.6 to 3.2 m and noise in the pressure sensor during the event was consistent with increased waves observed in Tasman Bay during the period of high wind. Turbidity decreased with distance from the river mouth and the period of elevated turbidity preceded the advance of freshwater discharge into the bay.
Figure 3 Weather conditions, river flows and water quality conditions in Tasman bay between 22 April and 7 May 2009. A, Rainfall measured at Tapawera and wind speed measured at the Nelson Airport. B, River flow and turbidity measured at Woodmans Bend. C, Turbidity (NTU) measured at c. 3 m depth at the two moorings located at 3 and 6 km distance from the river mouth and the tide signal as measured by a pressure-depth sensor mounted on the buoy at 3 km. D, Salinity and water temperature measured at the two buoys. E, Incoming and sub-surface (3 m) irradiance measured at the buoy located 6 km from the river mouth.
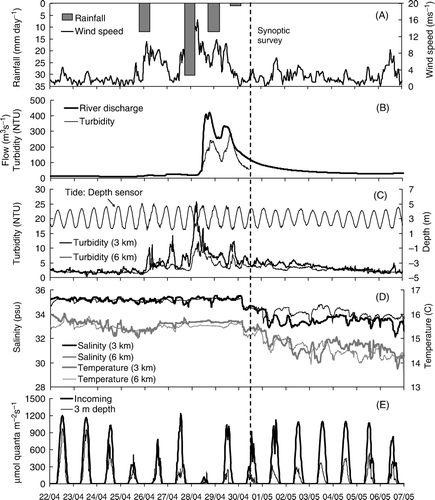
Time series of salinity and temperature measured at the two buoys revealed the persistence of the river plume the week following the flood event. Salinity at 3 m depth was reduced to 32.3 and 33.5 PSU at 3 and 6 km distance from the river mouth, respectively. Water temperature corresponded with salinity, and was reduced from a high of about 15.8°C prior to the event, to a low of 13.8° and 14.0°C at a distance of 3 and 6 km, respectively. Both salinity and temperature remained relatively constant at the two sites during the weather event when winds were high, indicating vertical mixing of the water column likely associated with increased wave action ().
The amount of light attenuation at 3 m depth was considerably greater following the flood event than prior to the event (bottom panel in ). Up to at least 5 days after the boat survey, the amount of light reaching the sensor on a comparatively sunny day was approximately half that reaching the same sensor prior to the development of the river plume.
Although turbidity at 3 m depth declined shortly following the rainfall and high winds (), a persistent layer of turbid water revealed a conspicuous river plume in Tasman Bay during the synoptic survey conducted 48 h following the commencement of river flooding (). This suspended sediment layer was oriented near the surface in the upper few meters of the water column and according to the in situ data had only a marginal effect on the gradually decreasing turbidity levels at 3 m depth ().
Figure 4 Photograph collected during the synoptic survey on 30 April 2009, 2 days after the main rain event, showing the river plume (left) as a layer of suspended sediment at the water surface.

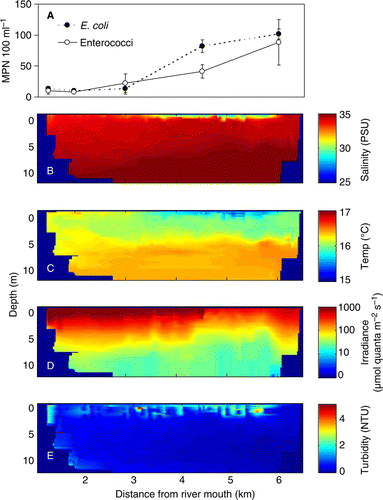
The synoptic CTD survey conducted 2 days following the peak rainfall revealed a buoyant plume extending offshore to a distance of approximately 6 km and within the regions of the AMAs (). Salinity near the surface ranged between 28 and 32 PSU at a distance of 3 to 6 km offshore, respectively. The region of low salinity corresponded with reduced water temperatures, increased turbidity and reduced photosynthetically active radiation (). Turbidity near the surface during the survey, although elevated, was lower than the peak in turbidity measured during the period of high rainfall and wind (). At the time of the survey, the salinity measured at the buoys by sensors at 3 m depth had only just begun to detect water with a reduced salinity of about 34.5 PSU ().
Figure 5 Results from the synoptic boat survey conducted on 30 April 2009 between 08:00 and 14:00. A, Faecal indicator bacteria concentrations in surface water (1 m depth) as a function of distance from the river mouth. B–E, vertical (0 to 12 m depth) cross sections of interpolated salinity, temperature, light and turbidity between 1 km from the river mouth (left in each panel) and just past the buoy situated 6 km from the river mouth.
The path of GPS drifters released near the river mouth revealed the influence of the tide on the movement of surface waters near the river mouth. The drifters moved approximately 1.2 km toward the river mouth during an incoming tide and had begun to head alongshore upon retrieval. Despite the small-scale flow pattern in surface waters observed with the drifters, the plume was spatially extensive. The observation of surface sediments associated with low salinity water () revealed the presence of the plume throughout much of the central and eastern AMA regions and extending along the Western Tasman Bay shore into Abel Tasman National Park.
Tasman Bay water and shellfish quality
Patterns of FIB in surface water samples coincided with the region most influenced by the river plume (). Concentrations of E. coli and enterococci in Tasman Bay water samples ranged between <10 and 110 MPN/100 ml and between <10 and 146 MPN/100 ml, respectively. Highest concentrations were observed at the sampling location furthest from the river mouth and within the region of the AMAs. A single sample collected closer to the western shore along Kaiteriteri (approximately 4.5 km from the river mouth) had concentrations of E. coli (109 MPN/100 ml) and enterococci (52 MPN/100 ml) that were similar to those measured between 4 and 6 km distance along the main transect. Surface salinity at this location was 31 PSU.
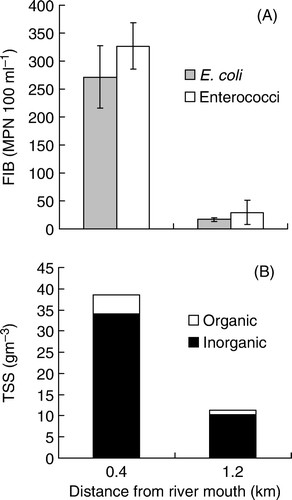
FIB concentrations in water samples collected at the location where GPS drifters were released (approximately 1.2 km from the river mouth) ranged between 10 and 20 MPN/100 ml and 5 and 73 MPN/100 ml for E. coli and enterococci, respectively. Samples collected within 0.4 km of the river mouth ranged between 161 and 327 MPN/100 ml for E. coliand between 282 and 410 MPN/100 ml for enterococci (). As was the case for river water samples, higher concentrations of FIB coincided with higher levels of suspended solids ( bottom panel).
Figure 6 A, Mean (±1SE) faecal indicator bacteria (FIB) concentrations, and B, total suspended solids for surface water samples (n=3) collected at the point where drifters were released (1.2 km from the river mouth) and where they were retrieved (0.4 km from the river mouth).
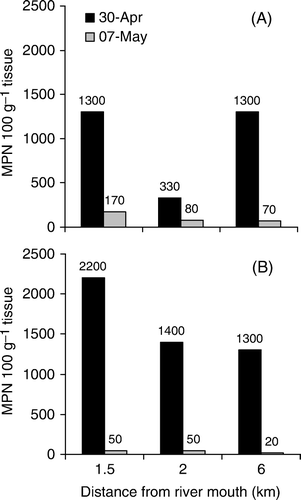
Mussels showed little FIB contamination prior to the flood event (E. coli <20 MPN/100 g tissue; composite of 12 mussels). On 30 April 2009 and following exposure to the river plume, concentrations of E. coli and enterococci in mussels varied between 330 and 1300 MPN/100 g tissue and between 1300 and 2200 MPN/100 g tissue, respectively (). On 7 May 2009 (1 week following the event), the FIB concentrations in mussels were much lower, ranging between 70 and 170 MPN/100 g tissue and 20 and 50 MPN/100 g for E. coli and enterococci, respectively. With the exception of E. coli on 30 April, concentrations of FIB declined with distance from the river mouth on both sampling occasions.
Microbial source tracking
Results from MST analyses of river water samples confirmed the presence of faecal contamination. A strong signal of the general Bacteroidales (GBac) marker, a non-specific marker of faecal contamination, was found in all river water samples tested for MST markers (). The ruminant Bacteroidales (RBac) marker was found in all river samples, with the exception of two of the samples from Little Sydney stream, which is located in the lower catchment in a low-density residential area and largely removed from pastoral farming. No human markers were detected in the river water samples.
Table 1 The number of samples that tested positive for the presence of general and ruminant Bacteroidales markers and the three human markers over the number of samples analysed in water and mussel samples.
The detection of MST markers in Tasman Bay water samples was primarily limited to samples collected nearest the river mouth at the location where the GPS drifters were retrieved and FIB concentrations were greatest (). The general Bacteroidales (GBac) marker was found in all three replicate water samples, and the ruminant Bacteroidales (RBac) marker was found in two of the three samples at this location. The GBac marker was also found in one of the two water samples analysed from those collected 6 km off shore.
All mussel samples collected during the boat survey tested positive for the presence of both the GBac and RBac markers (), indicating that faecal contaminants from ruminant animals were transported at least 6 km from the river mouth. Mussel samples collected 7 days later on 7 May from 1.5 and 2 km distance from the river mouth tested positive for the GBac marker and negative for the RBac marker (Data not shown). No human markers were detected in any of the Tasman Bay water or mussel samples.
Discussion
A ‘ridge tops to sea approach’ enabled demonstration of the close connection between land use in a coastal catchment and downstream impacts of land use activities on shellfish resources within developing AMAs. Studies on the source and fate of faecal contaminants generally focus on smaller spatial scales and few have covered the full catchment footprint of land-derived faecal contamination on downstream marine systems. Our results corroborate studies that have illustrated the role of river plumes in sediment and contaminant transport (Forrest et al. 2007; Korajkic et al. Citation2009; Reifel et al. Citation2009; Brodie et al. Citation2010) and provide further support that rivers and the plumes that form during flood events are effective conduits for land-derived contaminants into coastal waters.
Our findings support those of McKergow & Davies-Colley (Citation2010), which demonstrated that the bulk of faecal loading in the Motueka River, and therefore into Tasman Bay, occurs during storm events. The event surveyed occurred following a prolonged dry period, with the only significant rainfall and river flows above 40 m3/s occurring nearly 2 months prior on 1 March 2009 (234 m3/s). During dry conditions when river flows and concentrations of faecal bacteria are low, it is unlikely that shellfish in the AMAs are at risk of contamination from land-based contamination sources. This is largely because the AMAs lie within a well flushed embayment (Tuckey et al. Citation2006) and are located at least 6 km away from river inputs. In addition, dry periods would be accompanied by high levels of solar radiation, which is the primary factor regulating mortality of faecal microbes (Gameson & Gould Citation1975; Wilkinson et al. Citation2011). Nonetheless, the fact that faecal contaminants from the land can be tracked into mussels within the region's largest AMA during moderate flooding highlights a need to understand the mechanisms and environmental conditions that lead to a greater likelihood of faecal contamination and subsequently higher risk of pathogens within shellfish. Further surveys combining analyses of FIB and MST markers will also assist in determining whether successive floods result in a decline in faecal contamination in the AMAs because of flushing of the landscape and river stores of bacteria (Muirhead et al. Citation2004; McKergow & Davies-Colley Citation2010; Svejkovsky et al. Citation2010; Wilkinson et al. Citation2011).
Detectable levels of faecal bacteria in surface waters alongside the presence of a molecular marker specific to ruminant gut bacteria in mussels offshore demonstrates that the issue of faecal contamination of our freshwater waterways extends well beyond the land–sea interface. Data collected from moored instrumentation also indicate that the biophysical effects of the river plume, including reductions in surface salinity, water temperature and subsurface irradiation, persisted for at least a week following the flood event. The presence of the general Bacteroidales marker (an obligate anaerobe associated with the faeces of many animals) in mussel samples up to a week following river flooding provides evidence of residual bacteria at low concentrations being discharged by the river and concentrated by the mussels.
The observed correspondence between levels of faecal bacteria, turbidity and suspended solids suggests potential interactive effects of sediment and faecal contaminant loading into Tasman Bay. As identified by Noble et al. (Citation2004), a number of factors other than salinity (such as water temperature, solar radiation, source of contamination) likely have a major influence on mortality rates of faecal bacteria. Reductions in solar radiation and water temperature were found to be positively correlated to decreased inactivation rates of faecal bacteria (Noble et al. Citation2004). Consequently, the observed shading provided by the fine sediment plume at the surface would reduce mortality associated with exposure to solar radiation, thereby prolonging survival and enhancing the distance free-living or particle-bound bacteria can be transported.
A large portion of faecal bacteria associated with runoff and carried into marine waters is bound to particles (Characklis et al. Citation2005; Fries et al. Citation2006; Krometis et al. Citation2007). The suspended solids contained inorganic sediments as well as organic material, which would provide habitat for microbes (Lyons et al. Citation2007, Citation2010) and also enhance their settlement and subsequent consumption by mussels deeper in the water column or shellfish along the bottom (De Luca-Abbott et al. Citation2000). Mussels at 1.5 and 2 km distance were positioned at a depth of 3 m below the surface; hence they were beneath the main influence of the low-salinity plume when sampled. This provides preliminary evidence that mussels were contaminated through consumption of bacteria attached to settling particles. The effect of fine sediments on sub-surface irradiance in surface waters and the settling of contaminated particles identify an important link between sedimentation, faecal microbes and shellfish contamination (Korajkic et al. Citation2009). Mussels farmed in the AMAs are typically suspended at depths between 5 and 20 m in order to take advantage of mid-water chlorophyll maxima that frequently occur in Tasman Bay (MacKenzie & Adamson Citation2004). Although suspended deeper than the main influence of buoyant river plumes, further research will assist in determining whether contamination of subsurface shellfish cultures is enhanced by the consumption of settling particles and associated microbes.
Current water quality and shellfish sanitation monitoring is based on FIB. Indicator bacteria such as E. coli and enterococci are associated with all warm-blooded animals and can persist in the environment. Consequently, E. coli concentrations are poorly correlated to pathogen risk and provide no information on the source of contamination (Stoeckel & Harwood Citation2007; Hartz et al. Citation2008; Ishii and Sadowsky Citation2008; Yamahara et al. Citation2009). The use of molecular markers allowed identification of pastoral grazing animals as a source of faecal contamination in shellfish within the area influenced by the river plume. It is likely that other types of wildlife and domestic animals also contributed to faecal contamination; however, the very high concentrations of enterococci and E. coli coupled with the strong signal of the ruminant Bacteroidales (RBac) marker in both Sherry and Motueka River samples, and current land use patterns indicate that pastoral farming (dairy, beef and sheep) is likely the main source of contamination. The Sherry River catchment accounts for only 3.8% of the total Motueka River catchment area, and (after accounting for die-off during transport) contributes an estimated 8% of the total bacteria loads measured downstream at the Woodmans Bend sampling location (Wilkinson et al. Citation2011). Pastoral farming is also carried out in lower reaches of the catchment and it is likely that these areas contribute a larger portion of the total faecal contamination transported into Tasman Bay. Sampling within the catchment at a higher spatial resolution during flood events in conjunction with MST techniques will assist in identifying areas of the catchment that contribute the greatest to contamination in the AMAs.
Although the RBac marker has a high level of specificity (c. 85%) for ruminant animals in New Zealand (Kirs et al. Citation2011), the marker has some cross-reactivity with other animals such as pigs, kangaroos, wallabies and possums (Gourmelon et al. Citation2007; Kirs et al. Citation2011). Furthermore, one of 11 human faecal samples tested positive for this marker in a recent study conducted in New Zealand (Kirs et al. Citation2011). In most cases, the cross-reactivity detected for material extracted from raw faecal samples is limited, with only a few individuals being positive and the marker concentration being low and likely diluted below detection limits once released. Furthermore, animals such as kangaroos and wallabies as well as pig farms are not present in the Motueka catchment. Although possums roam freely throughout the study area, high water quality and low E. coli concentrations in upper reaches of the Motueka Catchment in areas of native bush and planted forest (Young et al. Citation2005) suggest that the contribution of these sources are likely minimal compared with ruminant animals grazing in grasslands adjacent to the river where water quality is degraded.
To confirm the presence of ruminant contamination further, we subsequently screened the extracted DNA from the same mussel samples by real-time PCR for a different ruminant marker (described in Shanks et al. 2008) that is highly specific (validation against 115 faecal samples collected from different regions of North and South Island revealed 95% specificity and 93% sensitivity and have indicated high ruminant marker concentrations in the raw ruminant faecal material; Kirs, unpublished data). This marker, which targets a different region of 16s RNA gene of Bacteroidales, was detected in all mussel samples tested, providing further confirmation of ruminant animals as a source of contamination.
Wilkinson et al. (Citation2011) predicted that a large portion of faecal contaminants can be transported a distance of 50 km downstream in the Motueka River. The detection of FIB of ruminant origin in the mussels offshore indicates that at least a portion of the faecal contamination is derived from the middle to upper reaches of the catchment and therefore originates at least 50 km upstream. Markers indicating a human contamination source were not detected, which indicates either that human-derived faecal contaminants are not present in the system, or that they are at very low, undetectable concentrations. Disease-causing organisms such as Campylobacter and Cryptosporidium can originate from ruminants (Wilson et al. Citation2008); however, further research is required to understand fully the links between these sources of contamination and human health risk. The results highlight that contamination events in the AMAs that lead to breaches of shellfish sanitation standards are not associated with significant human inputs, but rather pastoral farming in the catchment.
Confirmation that activities on land can directly influence the quality of aquaculture resources highlights a need to improve land use management practices. For example, fencing of pastoral areas and planting of vegetated riparian buffer strips should be advocated throughout coastal catchments to limit microbial contamination risk to shellfish resources. Simultaneous, rapid growth of pastoral farming and the aquaculture industry in New Zealand will be difficult to achieve when we consider that aquaculture, which is critically dependent on clean growing waters, is downstream of the adverse effects of pastoral farming. These implications highlight the importance of considering land–sea connections and river plume processes in ecosystem-based planning and management of aquaculture resources.
Implications for shellfish harvest
Harvest closures in Tasman Bay are currently based on the understanding of relationships between river flows and subsequent faecal bacteria loading and the possible likelihood of contamination offshore. Closures in the AMA off Motueka are managed around average river flows over a 24-h period, with <150 m3/s leading to no closure, and flows greater than 150, 200, 310 and 730 m3/s leading to closures of 1, 2, 4 and 7 days, respectively (J. Wilson, pers. comm.). The event sampled in this study would have resulted in a 4-day harvest closure; however, based on our data (), this duration may not have been sufficient for levels in mussels to drop below 230 MPN/100 g tissue, which is the current sanitation threshold for harvesting shellfish.
Figure 7 A, E. coli and B, enterococci concentrations in mussels (composite of Perna canaliculus and Mytilis edulis) shortly following the flood event (30 April 2009) and 1 week later (7 May 2009). Mussels were collected from cages suspended at c. 3 m depth at 1.5 and 2 km distance from the river mouth, and from surface buoys at 6 km distance in one of the aquaculture management areas (AMAs).
For the 12 months between the event surveyed and 30 April 2010, there were a total of five events above 310 m3/s, three events between 200 and 310 m3/s, and three events between 150 and 200 m3/s based on a rolling 24-h average. These floods would have lead to a total closure duration of 29 days, which represents 8% of the total time over the year. Although a relatively small percentage of the total time, the timing of closures could have a large impact on the farms if it occurred when mussels are reaching peak condition. The ability to time harvesting with peak mussel condition is critical to maximising product value. If the farm is closed for a period of time that includes a spawning event the mussels could rapidly lose condition before harvesting is allowed.
While perhaps considered a conservative approach, closures based on river flow discharge are not ideal since there are likely a number of factors that influence the extent of contamination risk for the AMAs in Tasman Bay. Accurate prediction of faecal contaminant loading for the Motueka River is possible (McKergow & Davies-Colley Citation2010; Wilkinson et al. Citation2011); however, forecasting the concentration and fate of faecal contaminants in coastal waters is more difficult. Upon exiting the confines of the river, the discharged freshwater forms an expansive buoyant plume that mixes with underlying seawater and is susceptible to movement associated with currents, which will be largely influenced by wind and tides (Warrick et al. Citation2007; Svejkovsky et al. Citation2010). The recent deployment of moored sensor arrays for the purpose of real-time water quality monitoring within the vicinity of the AMAs will assist in further understanding river plume behaviour and managing harvest closures.
Acknowledgements
This research was carried out under contract CO9X0014 to the New Zealand Foundation for Research Science and Technology. Technical support by Eric Goodwin and Aaron Quarterman, Cawthron, was greatly appreciated. Cawthron's Internal Investment Fund contributed to the development and validation of MST markers used in the study and supported collaboration with Professor Valerie J. Harwood.
References
- Ahn , JH , Grant , SB , Surbeck , CQ , Digiacomo , PM , Nezlin , NP and Jiang , S . 2005 . Coastal water quality impact of stormwater runoff from an urban watershed in southern California . Environmental Science and Technology , 39 : 5940 – 5953 .
- AgResearch MIRINZ 2005 . Indicator organisms in natural waters and effluents . Section 12.2 Enterococci in meat industry microbiological methods. 4th edition. Hamilton.
- APHA 2005 . Standard Methods for the Examination of Water and Wastewater, A joint publication of the American Public Health Association (APHA), the American Water Works Association (AWWA), and the Water Environment Federation .
- Basher L 2003 . The Motueka and Riwaka catchments . Lincoln, Canterbury, Land Care Research .
- Bernhard , AE and Field , KG . 2000a . A PCR assay to discriminate human and ruminant feces on the basis of host differences in Bacteroides-Prevotella genes encoding 16S rRNA . Applied and Environmental Microbiology , 66 : 4571 – 4574 .
- Bernhard , AE and Field , KG . 2000b . Identification of nonpoint sources of fecal pollution in coastal waters by using host-specific 16S ribosomal DNA genetic markers from fecal anaerobes . Applied and Environmental Microbiology , 66 : 1587 – 1594 .
- Bonkosky , M , Hernandez-Delgado , EA , Sandoz , B , Robledo , IE , Norat-Ramirez , J and Mattei , H . 2009 . Detection of spatial fluctuations of non-point source fecal pollution in coral reef surrounding waters in southwestern Puerto Rico using PCR-based assays . Marine Pollution Bulletin , 58 : 45 – 54 .
- Brodie , J , Schroeder , T , Rohde , K , Faithful , J , Masters , B , Dekker , A , Brando , V and Maughan , M . 2010 . Dispersal of suspended sediments and nutrients in the Great Barrier Reef lagoon during river-discharge events: conclusions from satellite remote sensing and concurrent flood-plume sampling . Marine and Freshwater Research , 61 : 651 – 664 .
- Characklis , GW , Dilts , MJ , Simmons , OD , Likirdopulos , CA , Krometis , LAH and Sobsey , MD . 2005 . Microbial partitioning to settleable particles in stormwater . Water Research , 39 : 1773 – 1782 .
- De Luca-Abbott , S , Lewis , GD and Creese , RG . 2000 . Temporal and spatial distribution of enterococcus in sediment, shellfish tissue, and water in a New Zealand Harbour . Journal of Shellfish Research , 19 : 423 – 429 .
- Forrest , BM , Gillespie , PA , Cornelisen , CD and Rogers , KM . 2007 . Multiple indicators reveal river plume influence on sediments and benthos in a New Zealand coastal embayment . New Zealand Journal of Marine and Freshwater Research , 41 : 13 – 24 .
- Fries , JS , Characklis , GW and Noble , RT . 2006 . Attachment of fecal indicator bacteria to particles in the Neuse River Estuary, NC . Journal of Environmental Engineering – ASCE , 132 : 1338 – 1345 .
- Fries , JS , Characklis , GW and Noble , RT . 2008 . Sediment-water exchange of Vibrio sp. and fecal indicator bacteria: implications for persistence and transport in the Neuse River Estuary, North Carolina, USA . Water Research , 42 : 941 – 50 .
- Gameson A , Gould D 1975 . Effects of solar radiation on the mortality of some terrestrial bacteria in sea water . In : Gameson A . Discharge of sewage from sea outfalls . Pergamon Press , Oxford Pp. 209 – 219 .
- Geary , PM and Davies , CM . 2003 . Bacterial source tracking and shellfish contamination in a coastal catchment . Water Science and Technology , 47 : 95 – 100 .
- Gillespie P , Forrest R , Knight B , Cornelisen C , Young R 2011 . Variation in nutrient loading from the Motueka River into Tasman Bay, New Zealand, 2005–2009: implications for shellfish growth . New Zealand Journal of Marine and Freshwater Research 45 497 – 512 .
- Gourmelon , M , Caprais , MP , Segura , R , Le Mennec , C , Lozach , S , Piriou , JY and Rince , A . 2007 . Evaluation of two library-independent microbial source tracking methods to identify sources of fecal contamination in French estuaries . Applied Environmental Microbiology , 73 : 4857 – 4866 .
- Hartz , A , Cuvelier , M , Nowosielski , K , Bonilla , TD , Green , M , Esiobu , N , McCorquodale , DS and Rogerson , A . 2008 . Survival potential of Escherichia coli and Enterococci in subtropical beach sand: implications for water quality managers . Journal of Environmental Quality , 37 : 898 – 905 .
- Ishii , S and Sadowsky , MJ . 2008 . Escherichia coli in the environment: Implications for water quality and human health . Microbes and Environments , 23 : 101 – 108 .
- Kirs , M , Harwood , JH , Fidler , AE , Gillespie , PA , Fyfe , WA , Blackwood , AD and Cornelisen , CD . 2011 . Source tracking faecal contamination in an urbanised and a rural waterway in the Nelson–Tasman region, New Zealand . New Zealand Journal of Marine and Freshwater Research. , 45 : 43 – 58 .
- Korajkic , A , Badgley , BD , Brownell , MJ and Harwood , VJ . 2009 . Application of microbial source tracking methods in a Gulf of Mexico field setting . Journal of Applied Microbiology , 107 : 1518 – 1527 .
- Krometis , LAH , Characklis , GW , Simmons , OD , Dilts , MJ , Likirdopulos , CA and Sobsey , MD . 2007 . Intra-storm variability in microbial partitioning and microbial loading rates . Water Research , 41 : 506 – 516 .
- Leskinen , SD , Brownell , M , Lim , DV and Harwood , VJ . 2010 . Hollow-fiber ultrafiltration and PCR detection of human-associated genetic markers from various types of surface water in Florida . Applied Environmental Microbiology , 76 : 4116 – 4117 .
- Lyons , M , Lau , Y , Carden , W , Roberts , S , Smolowitz , R , Vallino , J and Allam , B . 2007 . Characteristics of marine aggregates in shallow-water ecosystems: Implications for disease ecology . Ecohealth , 4 : 406 – 420 .
- Lyons , MM , Ward , JE , Gaff , H , Hicks , RE , Drake , JM and Dobbs , FC . 2010 . Theory of island biogeography on a microscopic scale: organic aggregates as islands for aquatic pathogens . Aquatic Microbial Ecology , 60 : 1 – 13 .
- MacKenzie , L and Adamson , J . 2004 . Water column stratification and the spatial and temporal distribution of phytoplankton biomass in Tasman Bay, New Zealand: implications for aquaculture . New Zealand Journal of Marine and Freshwater Research , 38 : 705 – 728 .
- McKergow , LA and Davies-Colley , RJ . 2010 . Stormflow dynamics and loads of Escherichia coli in a large mixed land use catchment . Hydrological Processes , 24 : 276 – 289 .
- McQuaig , SM , Scott , TM , Harwood , VJ , Farrah , SR and Lukasik , JO . 2006 . Detection of human-derived fecal pollution in environmental waters by use of a PCR-based human polyomavirus assay . Applied and Environmental Microbiology , 72 : 7567 – 7574 .
- McQuaig , SM , Scott , TM , Lukasik , J , Paul , JH and Harwood , VJ . 2009 . Quantification of human polyomaviruses JCV and BKV by Taqman® quantitative PCR and comparison to other water quality indicators in water and fecal samples . Applied and Environmental Microbiology , 75 : 3379 – 3388 .
- Muirhead , RW , Davies-Colley , RJ , Donnison , AM and Nagels , JW . 2004 . Faecal bacteria yields in artificial flood events: quantifying in-stream stores . Water Research , 38 : 1215 – 1224 .
- National Research Council (NRC) 2004 . Indicators for Waterborne Pathogens . Washington, DC , National Academies Press .
- Noble , RT , Lee , IM and Schiff , KC . 2004 . Inactivation of indicator micro-organisms from various sources of faecal contamination in seawater and freshwater . Journal of Applied Microbiology , 96 : 464 – 472 .
- NZFSA 2008 . Enumeration of Escherichia coli in bivalve molluscan shellfish . NSFSA Method. Version 8, 061108. New Zealand Food and Safety Authority .
- Reifel , KM , Johnson , SC , DiGiacomo , PM , Mengel , MJ , Nezlin , NP , Warrick , JA and Jones , BH . 2009 . Impacts of stormwater runoff in the Southern California Bight: relationships among plume constituents . Continental Shelf Research , 29 : 1821 – 1835 .
- Schueler T , Holland H 2000 . Microbes and urban watersheds: concentrations, sources and pathways . In : The practice of watershed protection: techniques for protecting our nation's streams, lakes, rivers, and estuaries . Centre for Watershed Protection . Pp. 554 – 563 .
- Shanks , OC , Atikovic , E , Blackwood , AD , Lu , J , Noble , RT , Domingo , JS , Seifring , S , Sivaganesan , M and Haugland , RA . 2008 . Quantitative PCR for detection and enumeration of genetic markers of bovine fecal pollution . Applied Environmental Microbiology , 74 : 745 – 752 .
- Stoeckel , DM and Harwood , VJ . 2007 . Performance, design, and analysis in microbial source tracking studies . Applied and Environmental Microbiology. , 73 : 2405 – 2415 .
- Svejkovsky , J , Nezlin , NP , Mustain , NM and Kum , JB . 2010 . Tracking stormwater discharge plumes and water quality of the Tijuana River with multispectral aerial imagery . Estuarine Coastal and Shelf Science , 87 : 387 – 398 .
- Tuckey , BJ , Gibbs , MT , Knight , BR and Gillespie , PA . 2006 . Tidal circulation in Tasman and Golden Bays: implications for river plume behaviour . New Zealand Journal of Marine and Freshwater Research , 40 : 305 – 324 .
- Ufnar , JA , Wang , SY , Christiansen , JM , Yampara-Iquise , H , Carson , CA and Ellender , RD . 2006 . Detection of the nifH gene of Methanobrevibacter smithii: a potential tool to identify sewage pollution in recreational waters . Journal of Applied Microbiology , 101 : 44 – 52 .
- Warrick , JA , DiGiacomo , PM , Welsberg , SB , Nezlin , NP , Mengel , M , Jones , BH , Ohlmann , JC , Washburn , L , Terrill , EJ and Farnsworth , KL . 2007 . River plume patterns and dynamics within the Southern California Bight . Continental Shelf Research , 27 : 2427 – 2448 .
- Wilkinson RJ , McKergow LA , Davies-Colley RJ , Ballantine DJ , Young RG 2011 . Modelling E. coli from livestock in the Motueka and Sherry Rivers in the South Island, New Zealand . New Zealand Journal of Marine and Freshwater Research 45 369 – 393 .
- Wilson , DJ , Gabriel , E , Leatherbarrow , AJ , Cheesbrough , J , Gee , S , Bolton , E , Fox , A , Fearnhead , P , Hart , CA and Diggle , PJ . 2008 . Tracing the source of campylobacteriosis . PLoS Genetics , 4 : e 1000203
- Yamahara , KM , Walters , SP and Boehm , AB . 2009 . Growth of enterococci in unaltered, unseeded beach sands subjected to tidal wetting . Applied Environmental Microbiology , 75 : 1517 – 1524 .
- Young , RG , Quarterman , AJ , Eyles , RF , Smith , RA and Bowden , WB . 2005 . Water quality and thermal regime of the Motueka River: influences of land cover, geology and position in the catchment . New Zealand Journal of Marine and Freshwater Research , 39 : 803 – 825 .