Abstract
The large dog cockle, Tucetona laticostata (Quoy and Gaimard, 1835), is widely but sporadically distributed throughout coastal waters of Hauraki Gulf. One location at which this species proves common is around Otata Island, one of a series of islands in the ‘Noises’ complex in Hauraki Gulf, where it resides partially buried in gravel and rhodoliths in shallow water, at 5–15 m depth. The shells of T. laticostata collect in large post-mortem deposits in an area ramping from the sea bed off southwestern Otata Island. Seasonal variation in and benthic macroinvertebrate composition of taxon assemblages within and outside T. laticostata/rhodolith mounds and gravel are described. Both benthic invertebrate mean taxon richness and abundance within T. laticostata/rhodolith habitat are higher than in gravel; spatial and temporal variation in these communities is reported. Anthropogenic threats to structurally complex T. laticostata shell and rhodolith-based biogenic substrata are discussed.
Introduction
The marine environment around Auckland, throughout Hauraki Gulf has been modified by development, discharge, spoil and munitions disposal, channel deepening and dredging, pollution, fisheries, recreational activities, and waves of incursion of marine invasive species (Thrush et al. Citation1995, Citation1998; Hayward et al. Citation1997). However, without baseline data, neither the individual nor cumulative effects of these disturbances on marine biodiversity can be quantified. Subtidal biodiversity throughout this region has been reported on few occasions, and at few locations, with notable exceptions being Rangitoto Channel (Powell Citation1937; Dodd Citation2009), eastern Waiheke Island (Wong Citation2009; Wong & O'Shea Citation2010), and at select sites throughout parts of the greater Hauraki Gulf (McKnight Citation1969; Hayward Citation1982; Hayward et al. Citation1986a, Citationb; Gowing et al. Citation1997; Morrison et al. Citation2003; Dewas Citation2008).
Macroinvertebrate density and species richness are considered to positively correlate with structure (e.g. Crowder & Cooper Citation1982; Diehl Citation1992; Bostrom & Bonsdorff Citation1997; Warwick et al. Citation1997; Grabowski & Powers Citation2004), although in New Zealand this relationship has not been well studied. Of all coastal, subtidal substrata in Hauraki Gulf, shell gravels have probably received the least ecological attention, with few accounts of Recent flora and fauna associated with them available (e.g. Hayward Citation1982; Hayward et al. Citation1986a, Citationb), or limited to generalised accounts of species assemblages referred to as associations or formations, such as those of Powell (Citation1937). The influence of shell and structure on sea-bed biodiversity has otherwise received limited domestic attention, except for studies on the horse mussel Atrina zelandica (Gray, 1835) and its influence on aspects of benthic biodiversity (Cummings et al. Citation1998; Norkko et al. Citation2001; Grange et al. Citation2003; Hewitt et al. Citation2005).
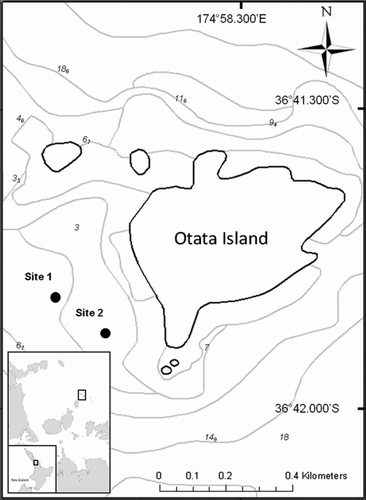
Around Otata Island, one of a group of islands and rocks comprising the Noises complex within Hauraki Gulf (), the sea bed between depths of about 5 and 15 m comprises gravel, interspersed with mounds of shells of the large, robust-valved dog cockle, Tucetona laticostata (Quoy and Gaimard, 1835), and free-living coralline algae, rhodoliths; these mounds represent islands of hard substrata in otherwise less topographically complex benthic habitat (Kidwell Citation1986), and are colonised by diverse epibiont communities and myriad encrusting and errant invertebrates. The structure afforded by the adjacent gravel appears less complex, with fewer epibionts, encrusting taxa, articulated or otherwise intact T. laticostata valves, and rhodoliths apparent (). This location therefore presented an opportunity to compare the composition of species assemblages in two adjacent, visually contrasting, largely biogenic habitat types.
Figure 2 Sea bed off southwestern Otata Island, 7 m, with contrasting T. laticostata/rhodolith and gravel habitats.

The contribution of shell of T. laticostata to the sea bed, corresponding change in sea-bed structure and thus the potential influence of T. laticostata on benthic invertebrate species diversity has not been studied, although it has been inferred for invertebrate communities off Spirits Bay, northernmost New Zealand (Cryer et al. Citation2000). We have been unable to locate any local study that has demonstrated the importance of rhodoliths to sea-bed biodiversity, although many references to these coralline algae elevating species diversity can be found in international literature (e.g. Keegan Citation1974; De Grave Citation1999; De Grave & Whitaker Citation1999; Stellar et al. Citation2003).
The primitive, palaeotaxodont hinge and robust shell of T. laticostata renders it resistant to damage, even in the high-energy environments within which both this species lives and its shells accumulate. Although T. laticostata may live for approximately 100 years (sensu Ramsay et al. Citation2000), their valves can persist for thousands of years in the sea bed. The disarticulated valves of T. laticostata collected from the Wanganui shelf have been carbon-dated at 9170±210 years BP (Gillespie et al. Citation1998), and those from surface, relictual deposits off the Bay of Plenty have been dated at 35,800 ± 2250 years BP (Beu Citation2004). It is quite possible that the shells of dead T. laticostata persist on the sea-bed surface in an articulated state for decades, if not hundreds of years once encrusting communities have established upon and within the valves, although no supporting radiocarbon data are available.
Herein novel data are presented that detail differences in benthic invertebrate community structure inside and outside of mounds of T. laticostata shell and secondarily rhodoliths, at two depths off Otata Island. The implications of these findings to conservation of coastal biodiversity are discussed. We consider these findings significant and worthy of reporting here, despite recognised limitations in our site, depth and sample replication, given the dearth of information on the patterns in distribution, diversity and abundance of coastal invertebrates in New Zealand.
Methods
Two sites were monitored within the recognised bathymetric distribution of T. laticostata off southwestern Otata Island: site 1, 7 m, 36°41.300′S, 174°58.227′E; and site 2, 5 m, 36°41.878′S, 174°58.301′E (). From January to September 2007, 83 SCUBA-collected core samples were collected from T. laticostata shell/rhodolith habitat and adjacent gravel. The corer, of 12.5 cm diameter, was pushed into the sediment to a depth of 10 cm. Cores were bagged underwater, then aboard the vessel fixed in 5% formalin–seawater solution immediately after collection. Following fixation, all samples were sieved over 2- and 0.5-mm Endecott sieves; the coarser (2-mm) fraction was sorted by eye, and the finer fraction (0.5-mm) beneath a binocular dissecting microscope.
Many taxa could not be identified to species. In such instances, taxa were identified to the lowest common denominator, or enumerated unknown, with the exception of all Amphipoda, Asellota, Cumacea, Oligochaeta, Nematoda, Nemertea, Platyhelminthes and most Porifera—the latter treated as single taxonomic units. A voucher set of all identified taxa has been accessioned into the biological collections of Auckland University of Technology; all bulk invertebrate samples have been similarly archived. Full biological data are available online (www.Monalisa.ac.nz).
To characterise the sediments within each of these two visually contrasting habitat types, an additional 12 cores were collected, six from each habitat, three from each site. Sediment grain-size analysis was conducted as described in Higgins (Citation1988). Each sample was placed on a stack of 10 sieves of different mesh size (). Environmental difference using sediment grain-size was measured by the Mantel test using cosine similarity as described in Paradis (Citation2011).
Table 1 Average dry weights and standard error of material retained in each sieve (x≥40 mm; 40 mm < x≥33.5 mm; 33.5 mm < x≥20 mm; 20 mm < x≥10 mm; 10 mm < x≥6 mm; 6 mm < x≥3 mm; 3 mm < x≥2.12 mm; 2.12 mm < x≥1.50 mm; 1.50 mm < x≥0.63 mm; 0.63 mm < x≥0.11 mm; x<0.11 mm) for each sample group.
To determine whether assemblages of species occurring within and between habitats at 5 and 7 m depth, and over the duration of sampling, were significantly different, three null hypotheses were generated: (Ho) that no difference exists in the benthic-invertebrate community structure of gravel and T. laticostata/rhodolith habitat at 5 and 7 m; (Ho) that no seasonal difference exists in the benthic-invertebrate community structures found within gravel and T. laticostata/rhodolith habitat at 5 and 7 m; and (Ho) that no difference exists in the benthic-invertebrate community structures found within gravel and T. laticostata/rhodolith habitat at 5 and 7 m. To do so, similarity matrices comparing 41 and 42 samples collected from both 5 and 7 m, respectively, were built.
Most statistical analysis was carried out using PRIMER (version 6) as described in Clarke & Warwick (Citation1994). Indices of total individuals (N ), total ‘taxa’ (S ), Margalef's index of ‘taxon’ richness (d ) were computed using the DIVERSE dialog in PRIMER. For comparing assemblage structure between sites, depths and seasons, taxon abundances were square-root-transformed to down-weight the effect of numerically dominant taxa, and similarity matrices were calculated with the Bray–Curtis similarity index. Two-way crossed analysis of similarity (ANOSIM; Clarke & Green Citation1988) and multiple pairwise comparisons were used to test for differences in taxon assemblages between the two habitats. ANOSIM uses a multivariate test that calculates similarities between groups; the R value ranges between −1 and +1, with the closer this value is to 0 indicating the higher the similarity between and within groups. SIMPER was used to examine individual taxon contribution (up to 90%) to average similarity within each identified taxon assemblage (Clarke & Warwick Citation1994) after a square-root transformation to downweight the contribution of numerically dominant taxa.
Results
Grain-size distributions (phi scale, ϕ) of sediments are provided in . Differences were apparent between sediments from T. laticostata/rhodolith-characterised substrata and those of gravels (), using all data from each of the 10 mesh sizes, and in median quartile and skewness between those two habitats (). There were significant differences between the two habitats as the Mantel test resulted in a P-value strictly under 0.05.
Table 2 Characteristic granulometric indices for each sediment type: median, lower and upper quartiles, inclusive sorting coefficient and graphic skewness values.
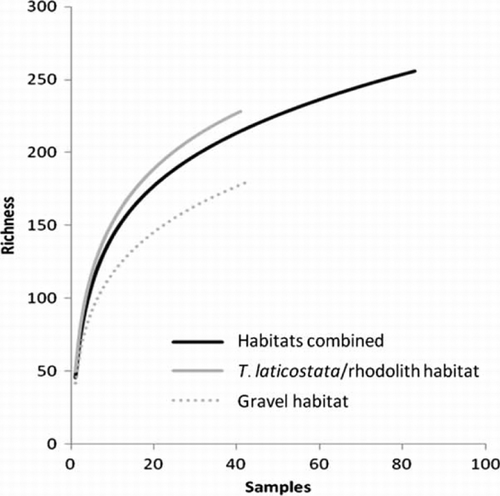
Significant differences in taxon assemblages within T.laticostata/rhodolith and gravel habitat at both 5 m and 7 m depths were apparent, but the taxon assemblages found within each of these substrata did not differ significantly between the two depths (); accordingly biological data for gravels at 5 and 7 m, and for T. laticostata/rhodolith samples at 5 and 7 m, were combined for survey seasons to determine whether significant temporal differences existed in their respective assemblage structures. Neither taxon accumulation curve prepared for T. laticostata/rhodolith nor gravel habitat reached an asymptote (), although the combined accumulation curve for these substrata most closely did, given nearly 50% of taxa were common to the two habitat types (). Mean numbers of taxa and individuals almost always were higher within T. laticostata/rhodolith than gravel habitat ().
Figure 4 Means of total taxa (S ), total individuals (N ), and Margalef's index of taxon richness (d ), by site, habitat and season, with standard error bars.
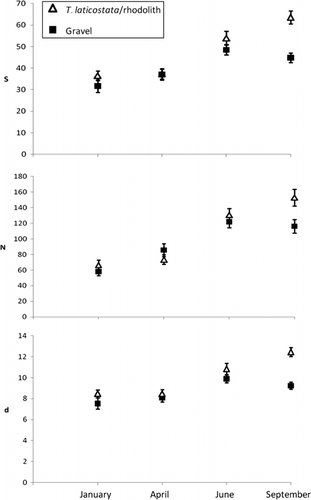
Table 3 Analysis of similarity (ANOSIM) R-values for each hypothesis tested.
Table 4 SIMPER results (cut-off for low contributions: 50.00%) for habitat types combined, square-root transformed data average dissimilarity: 58.74.
Discussion
Ecology of the survey environment: southwestern Otata Island
The sediment grain-size characteristics of the two habitat types, coarse and very coarse sands/gravels, the latter characterised by T. laticostata shell and rhodolith surface deposits, differ significantly in their overall sediment grain-size composition. These two habitat types are instantly, visually discernable underwater (). As a consequence, we suggest the presence or absence of the complex structure offered by T. laticostata shells and associated rhodoliths plays a major role in shaping taxon assemblage, but unfortunately we cannot differentiate the effects of T. laticostata shells from that of, if any, associated rhodoliths.
At the level of species, or lowest practicable but regrettably not-necessarily common taxonomic unit, using transformed data, the taxonomic composition and relative abundance of taxa differed considerably between the two habitat types. Of interest is the low density of live T. laticostata in samples; this bivalve contributes little to overall similarity between samples in the habitat type characterised by extensive deposits of its shells, although in terms of biomass it is likely to be the most important taxon in the region.
Although those taxa that characterise the two habitats, T. laticostata/rhodolith and gravel, have been shown to differ in statistical terms, the reality is that the same taxa occur in both habitats, albeit at different absolute and relative abundances (). More widely distributed gravels may function as a reservoir of species, the density of which can increase in local patches of greater structure, such as afforded by T. laticostata and rhodolith habitat. With approximately 40% of taxa presently being known only from one or other substratum type, many taxa known from single or very few cores only, and with taxon accumulation curves reaching no asymptote for either substratum type, the biological significance of the statistical significance in assemblage structure within these two habitats could be minor.
Implications of this research
Biogenic reefs, solid structures formed by accumulation of organisms rising from the sea bed, create habitat distinct from that of the surrounding sea bed (Kidwell Citation1986; Holt et al. Citation1998). In this study, the valves of T. laticostata and associated rhodoliths create habitat that appears to define the taxa associated with them, although our data, as presented here, cannot differentiate any effects of rhodoliths on sea-bed taxon richness and abundance from those of T. laticostata shell. Currently the Department of Conservation, Ministry of Fisheries Marine Protected Area (MPA) draft classification (May 2007) recognises only bryozoan and rhodolith beds, sponge gardens, tube-worm mounds and cold-water corals as biogenic substrata in New Zealand waters (CitationDOC/MFish 2007). We would advocate for inclusion of T. laticostata shell mounds in this biogenic reef classification system, as these mounds are larger than those of known rhodolith beds throughout Hauraki Gulf.
If the patterns we report for T. laticostata in Hauraki Gulf occur at greater scales throughout New Zealand, then fisheries for this species, and shell fragmentation or removal as a consequence of dredging and anchorage, are likely to significantly, negatively impact associated benthic assemblages of species. Remarkably, McKnight (Citation1968) reported that bivalve shell deposits, including valves of T. laticostata, were unfavourable for the development of a rich infauna, although he did recognise that such shell deposits support large epifaunal communities. It is a shame that the assemblages of species that occur in these coastal gravels have been subject to such limited taxonomic research; this certainly limits our ability to determine how unique their taxonomic composition is relative to other better-studied sediment types, such as muds.
Acknowledgements
We would like to thank Drs Kay Vopel, Robin Hankin and Barbara Breen, and Ms Emma Beatson (all AUT) for their help with statistics, GIS and field work; Nik Hannam and the Dewas family for additional field assistance that enabled this study to proceed; and Bruce Marshall, Museum of New Zealand Te Papa Tongarewa, for providing information on the Recent distribution of T. laticostata throughout Hauraki Gulf; we would also like to acknowledge Drs Lindsey White (AUT), Martin Cryer (NZ Ministry of Fisheries) and one anonymous referee for valuable advice on an earlier draft of this manuscript. Funding and facilities of the Earth & Oceanic Sciences Research Institute, Auckland University of Technology, enabled this research to proceed.
References
- Beu , AG . 2004 . Marine Mollusca of oxygen isotope stages of the last 2 million years in New Zealand. Part 1: Revised generic positions and recognition of warm-water and cool-water migrants . Journal of the Royal Society of New Zealand , 34 : 111 – 265 .
- Bostrom , C and Bonsdorff , E . 1997 . Community structure and spatial variation of benthic invertebrates associated with Zostera marina (L.) beds in the northern Baltic Sea . Journal of Sea Research , 37 : 153 – 166 .
- Clarke , KR and Green , RH . 1988 . Statistical design and analysis for a ‘biological effects’ study. Biological effects of pollutants . Results of a practical workshop , 46 : 213 – 226 .
- Clarke KR , Warwick RM 1994 . Change in marine communities: an approach to statistical analysis and interpretation . Plymouth, Plymouth Marine Laboratory, Natural Environment Research Counci
- Crowder , LB and Cooper , WE . 1982 . Habitat structural complexity and the interaction between bluegills and their prey . Ecology , 63 : 1802 – 1813 .
- Cryer , M , O'Shea , S , Gordon , D , Kelly , M , Drury , J , Morrison , M , Hill , A , Saunders , H , Shankar , U , Wilkinson , M and Foster , G . 2000 . Distribution and structure of benthic invertebrate communities between North Cape and Cape Reinga . Final research report for Ministry of Fisheries research project ENV9805 Objectives , 1–4 : 1 – 154 .
- Cummings , VJ , Thrush , SF , Hewitt , JE and Turner , SJ . 1998 . The influence of the pinnid bivalve Atrina zelandica [sic] (Gray) on benthic macroinvertebrate communities in soft-sediment habitats . Journal of Experimental Marine Biology and Ecology , 228 : 227 – 240 .
- De Grave , S . 1999 . The influence of sedimentary heterogeneity on within maerl bed differences in infaunal crustacean community. Estuarine . Coastal and Shelf Science , 49 : 153 – 163 .
- De Grave , S and Whitaker , A . 1999 . Benthic community re-adjustment following dredging of a muddy-maerl matrix . Marine Pollution Bulletin , 38 : 102 – 108 .
- Dewas SEA 2008 . Benthic-invertebrate diversity of Tucetona laticostata (Mollusca: Bivalvia) biogenic substrata in Hauraki Gulf . Unpublished MAppSc thesis, Auckland University of Technology.
- DOC/MFish 2008 . Department of Conservation and Ministry of Fisheries . Marine protected areas classification, protection standard and implementation guidelines.
- Dodd S 2009 . The role of non-indigenous benthic macrofauna in the diet of snapper (Pagrus auratus) . Unpublished MAppSc thesis, Auckland University of Technology.
- Diehl , S . 1992 . Fish predation and benthic community structure: the role of omnivory and habitat complexity . Ecology , 73 : 1646 – 1661 .
- Gillespie , JL , Nelson , CS and Nodder , SD . 1998 . Post-glacial sea-level control and sequence stratigraphy of carbonate-terrigenous sediments, Wanganui shelf, New Zealand . Sedimentary Geology , 122 : 245 – 266 .
- Gowing L , Priestley S , Kennedy P 1997 . Monitoring the Hauraki Gulf DISPOSAL Site using REMOTS® and other established sampling techniques . Combined Australasian Coastal Engineering and Ports Conference, Christchurch
- Grabowski , JH and Powers , SP . 2004 . Habitat complexity mitigates trophic transfer on oyster reefs . Marine Ecology Progress Series , 277 : 291 – 295 .
- Grange , KR , Tovey , A and Hill , AF . 2003 . The spatial extent and nature of the bryozoan communities at Separation Point, Tasman Bay . New Zealand Ministry of Fisheries, Marine Biodiversity Biosecurity Report , 4 : 7 – 9 .
- Hayward , BW . 1982 . Foraminifera and Ostracoda in nearshore sediments, Little Barrier Island, northern New Zealand . Tane , 28 : 53 – 62 .
- Hayward , BW , Grace , RV and Francis , MP . 1986a . Sediments and benthos of northeastern Great Barrier Island, New Zealand . Journal of the Royal Society of New Zealand , 16 : 347 – 355 .
- Hayward , BW , Grace , RV and McCallum , J . 1986b . Soft bottom macrobenthos and sediments off the Broken Islands, northern New Zealand . Tane , 31 : 85 – 103 .
- Hayward , BW , Stephenson , AB , Morley , M , Riley , JL and Grenfell , HG . 1997 . Faunal changes in Waitemata Harbour sediments, 1930s–1990s . Journal of the Royal Society of New Zealand , 27 : 1 – 20 .
- Hewitt , JE , Thrush , SF , Halliday , J and Duffy , C . 2005 . The importance of small-scale habitat structure for maintaining beta diversity . Ecology , 86 : 1619 – 1626 .
- Higgins , RP and Thiel , H . 1988 . Introduction to the study of meiofauna , Washington , DC : Smithsonian Institution Press .
- Holt TJ Rees EI Hawkins SJ Seed R Biogenic Reefs An overview of dynamic and sensitivity characteristics for conservation management of marine SACs. Dunstaffnage, Scottish Association for Marine Science (UK Marine SACs Project). IX 1998
- Keegan , BF . 1974 . The macrofauna of maerl substrates on the west coast of Ireland . Cahiers de Biologie Marine , 15 : 513 – 530 .
- Kidwell , SM . 1986 . Taphonomic feedback in Miocene assemblages: testing the role of dead hardparts in benthic communities . Palaios , 1 : 239 – 255 .
- McKnight , DG . 1968 . Recent and relict molluscan faunas from off Cape Farewell . New Zealand Journal of Marine and Freshwater Research , 2 : 708 – 711 .
- McKnight , DG . 1969 . Infaunal benthic communities of the New Zealand continental shelf . New Zealand Journal of Marine and Freshwater Research , 3 : 409 – 444 .
- Morrison M Drury J Shankar U Middleton C Smith M A broad scale, soft sediment habitat assessment of the Hauraki Gulf. Unpublished report, National Institute of Water & Atmospheric Research Ltd (NIWA) prepared for the Department of Conservation. NIWA Client Report AKL2003-64 2003
- Norkko , A , Hewitt , JE , Thrush , SF and Funnell , GA . 2001 . Benthic-pelagic coupling and suspension feeding bivalves: linking site-specific sediment flux and biodeposition to benthic community structure . Limnology and Oceanography , 46 : 2067 – 2072 .
- Paradis E Bolker B Claude J Cuong HS Desper R Durand B Dutheil J Heibl OGC Lawson D Lefort V Legendre P Lemon J Noel Y Nylander J Opgen-Rhein R Schliep K Strimmer K de Vienne D Analysis of phylogenetics and evolution: package ‘ape’ Version 2.7 (http://www.r-project.org/) 2011 107 – 108
- Powell , AWB . 1937 . Animal communities of the sea-bottom in Auckland and Manukau Harbours . Transactions and Proceedings of the Royal Society of New Zealand , 66 : 354 – 401 .
- Powell AWB New Zealand Mollusca: marine, land, and freshwater shells 1979
- Ramsay , K , Kaiser , MJ , Richardson , CA , Veale , LO and Brand , AR . 2000 . Can shell scars on dog cockles (Glycymeris glycymeris L.) be used as an indicator of fishing disturbance? . Journal of Sea Research , 43 : 167 – 176 .
- Stellar , DL , Riosmena-Rodríguez , RR , Foster , MS and Roberts , CA . 2003 . Rhodolith bed diversity in the Gulf of California: the importance of rhodolith structure and consequences of disturbance . Aquatic Conservation: Marine and Freshwater Ecosystems , 13 : S5 – S20 .
- Thrush , SF , Hewitt , JE , Cummings , VJ and Dayton , PK . 1995 . The impact of habitat disturbance by scallop dredging on marine benthic communities: what can be predicted from the results of experiments? . Marine Ecology Progress Series , 129 : 141 – 150 .
- Thrush , SF , Hewitt , JE , Cummings , VJ , Dayton , PK , Cryer , M , Turner , SJ , Funnell , GA , Budd , RG , Milburn , CJ and Wilkinson , MR . 1998 . Disturbance of the marine benthic habitat by commercial fishing: impacts at the scale of the fishery . Ecological Applications , 8 : 866 – 879 .
- Warwick , RM , McEvoy , AJ and Thrush , SF . 1997 . The influence of Atrina zelandica (Gray) on meiobenthic nematode diversity and community structure . Journal of Experimental Marine Biology and Ecology , 214 : 231 – 247 .
- Wong KLC The effects of green shelled mussel mariculture on benthic communities in Hauraki Gulf Unpublished MAppSc thesis, Auckland University of Technology 2009
- Wong , KLC and O'Shea , S . 2010 . Sediment macrobenthos off eastern Waiheke Island, Hauraki Gulf, New Zealand . New Zealand Journal of Marine and Freshwater Research , 44 : 149 – 165 .