Abstract
This study examined whether element:Ca ratios within the otoliths of juvenile brown trout could provide accurate trace element signatures for specific natal tributaries, and attempted to match these to trace element natal signatures found within the otoliths of adult trout caught in the main stem rivers of the same catchment. The trace element signatures of juvenile trout otoliths were analysed from a sample of eight tributaries representing the main sub-catchments of the Motueka River catchment, New Zealand. Trace element signatures were determined using laser ablation inductively coupled plasma mass spectrometry, and differentiated using linear discriminant function analysis with an overall cross-validated classification success of 96.8%. Temporal stability in element:Ca ratios was investigated by repeat collections of juvenile fish over two years. Natal signatures from 11 of 23 adult trout sampled from the catchment main stems were matched to one of the eight tributary signatures showing recruitment sources to be spread relatively evenly throughout the catchment. This study demonstrates the potential of using otolith trace element analysis to determine the natal origins of freshwater fish within a catchment.
Introduction
Extensive ontogenetic migrations are a feature of the life history of many species of salmonid, including brown trout (Salmo trutta L.) (Klemetsen et al. Citation2003). In order to understand and effectively manage a wild (un-stocked) brown trout population within a river catchment, identification of the locations used for spawning and rearing of juveniles, and patterns of movement in juveniles and adults, is crucial. Brown trout life history can be described as having three distinct stages: spawning, juvenile rearing and maturity (Elliott Citation1994). Each stage has its own unique habitat requirements, often forcing the need for movement between areas that provide these requirements (Klemetsen et al. Citation2003). In a large catchment, smaller tributaries often provide the best conditions for spawning and rearing of juvenile brown trout, whilst the larger river channels provide the primary habitat for larger adult fish (Klemetsen et al. Citation2003). The extent to which different tributaries within a catchment contribute fish to the adult population of the main stem will vary depending on the conditions within a tributary and its context within the landscape (Kristensen et al. Citation2011).
Examining the distribution of trace elements within the otoliths of fish has emerged as a potentially powerful technique that can provide a natural tag, connecting fish to their environment (Brazner et al. Citation2004; Elsdon & Gillanders Citation2004). Otoliths are paired calcified structures used for the maintenance of equilibrium and hearing in all teleost fish (Payan et al. Citation2004). Otolith trace element analysis involves the examination of trace elements that are incorporated into discrete layers of calcium carbonate during the process of otolith biomineralisation, which occurs continuously as the fish ages (Campana Citation1999; Campana & Thorrold Citation2001). The ability to reconstruct the environmental histories of fish from their otolith chemistry relies on the concentration of elements in otoliths changing in relation to the environmental variables that the fish encounters, such as the ambient elemental concentrations in the water (Elsdon & Gillanders Citation2004). This enables an accurate assessment of fish movement through both time and space.
Otolith trace element analysis has been used to identify important recruitment areas for a number of marine species (Gillanders & Kingsford Citation2000, Citation2003). However, its application to the investigation of recruitment areas within freshwater provides a new challenge, relying on more subtle variation in the concentration of multiple trace elements (Wells et al. Citation2003), or between isotopes of trace elements (Barnett-Johnson et al. Citation2008) within the otoliths of freshwater fish. Among those elements examined, Sr:Ca and Ba:Ca ratios have been shown to be the most useful in examining patterns of movement in freshwater habitats (Wells et al. Citation2003).
The trace element composition of river water is primarily determined by its underlying bedrock geology (Kennedy et al. Citation2000; Wells et al. Citation2003; Friedrich & Halden Citation2008). Where significant geochemical variation exists within a catchment, it is likely that the otolith chemistry of fish from differing parts of the catchment will reflect local geochemistry; hence fish can be chemically linked to a specific area. By examining the chemical composition of otoliths taken from freshwater fish, it has been possible to connect these fish to particular freshwater sites with considerable accuracy (Thorrold et al. Citation1998; Kennedy et al. Citation1997, Citation2000, Citation2002; Wells et al. Citation2003; Brazner et al. Citation2004; Veinott & Porter Citation2005; Clarke et al. Citation2007). Because the first year of the brown trout life cycle is often spent in a natal stream (Klemetsen et al. Citation2003), it may be possible to use the otolith trace element signatures of juvenile trout collected from a range of tributaries to create a map chemically defining potential recruitment areas within a river catchment (Wells et al. Citation2003), assuming these signatures remain stable over time.
Recent advances in the spatial sampling abilities of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS), has made it possible to analyse discrete areas of otolith that correspond to various life history stages. For instance, the trace element signature of the juvenile region of an adult otolith, which corresponds to the period of otolith growth during residence in the natal or rearing habitat, can be identified and analysed separately from the rest of the otolith (Crook & Gillanders Citation2006; Humston et al. Citation2010). This approach has been used to identify the natal origins of adult fish populations in various fish species (e.g. Walther et al. Citation2008).
Hence, the objectives of this study were twofold: firstly, to assess whether the tributaries of a river catchment can be chemically defined by analysing the trace element content of the otoliths of juvenile trout found within them, and secondly, to attempt to chemically link adult trout caught in the larger main-stem river channels to their natal tributary. This was done by comparing the trace element signatures of the natal region of their otoliths to the trace element signatures obtained from the otoliths of juvenile trout sampled from tributaries.
Methods
Study site
The Motueka River catchment, located at the top of the South Island of New Zealand, drains an area of 2180 km2 (Basher Citation2003), terminating in Tasman Bay (). The underlying geology is complex, potentially resulting in a diverse range of water chemistries (Young et al. Citation2005). Beginning in the ultramafic rock of the Richmond Ranges, the Motueka River is joined from the east by a series of small and medium sized tributaries draining Moutere Gravels, and from the West by a series of generally much larger tributaries that drain both Moutere Gravels and mountainous terrain underlain by a complex assemblage of sedimentary and igneous rocks (Basher Citation2003) (). The adult brown trout populations of the Motueka River main stem and the Wangapeka River main stem, which is the largest tributary of the Motueka River (), are recognised as nationally outstanding and provide for the majority of recreational angling in the Nelson area (Nelson/Marlborough Fish and Game unpublished data). The population is reliant on natural recruitment with no stocking having occurred since the early 1900s, following the first introductions of brown trout to New Zealand. The population is not landlocked and has access to Tasman Bay although there is little evidence for diadromous migration beyond the marine influenced lower reaches of the river. The majority of the population is generally considered freshwater resident within the catchment (Nelson/Marlborough Fish and Game unpublished data). To what extent the trout populations of the Motueka River and the Wangapeka River are sustained by recruitment from their various tributaries is largely unknown.
Figure 1 Sampling locations within the Motueka River catchment. Abbreviated names represent tributaries from which juvenile brown trout were sampled; GR: Graham River, BA: Baton River, UW: Upper Wangapeka River, DA: Dart River, MO: Motupiko River, RA: Rainy River, BG: Blue Glen Stream and UM: Upper Motueka River. Numbers represent where each adult brown trout was caught.
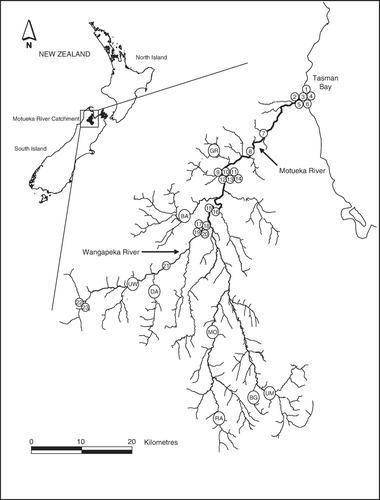
Table 1 Catchment area, flow, land cover types and rock types (Basher Citation2003) of each of the eight tributaries for which trace element signatures were generated.
Sample collection
Juvenile (young of year with a maximum length <100 mm) brown trout were collected from eight tributaries in February 2005 that were chosen to represent the major sub-catchments and known spawning areas present in the Motueka catchment (, ). To examine the recruitment of multiple cohorts of adult fish from these tributaries, the trace element signature of each tributary must be stable over time. To test this, four of the eight tributaries (Baton, Blue Glen, Dart and Rainy) were re-sampled in April 2005. All tributaries, apart from the Upper Motueka River, were re-sampled a third time in April 2006 (). During each sampling trip, five juvenile fish were caught using a pulsed DC Kainga EFM300 backpack electro shocker (NIWA Instruments, Christchurch, New Zealand). Once caught, fish were immediately euthanised in a lethal overdose of clove oil and later frozen before otolith removal.
Table 2 Sample dates and results of multivariate analysis of Sr:Ca, Rb:Ca, Ba:Ca and Mn:Ca ratios for each tributary over all sampling occasions.
Heads from 23 adult brown trout caught by anglers were collected throughout the 2007, 2008 and 2009 fishing seasons (fish length 41–61 cm; mean 53.9 cm). All adult trout were caught in either the Motueka River, downstream of its confluence with the Wangapeka River, or the Wangapeka River itself (). Once collected, fish heads were kept frozen until the otoliths were removed.
Otolith preparation
The saggital otoliths were extracted from both juvenile and adult trout. There is no evidence of any difference in the trace element concentrations between left and right otoliths (Campana et al. Citation1994), so one of each pair was prepared for analysis. Otoliths were cleaned ultrasonically of any biological tissue or contaminants for 5 min in MilliQ water (18 mΩ/cm) and left to dry under a laminar flow hood.
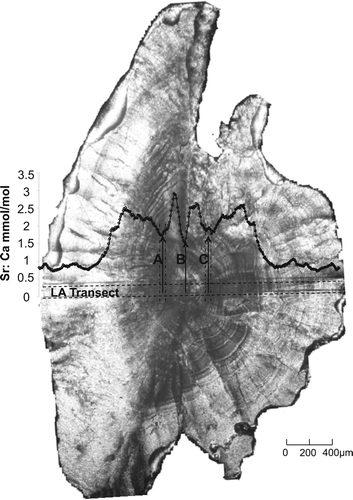
Juvenile otoliths were attached whole, distal surface (surface without the sulcal groove) up, to a glass slide prepared in advance with a thin coating of Crystal Bond™ thermal adhesive. Each adult otolith was secured sulcal surface up to the centre of a glass slide, embedded in a small drop of Crystal Bond™ thermal adhesive and ground using descending grades of wet/dry sandpaper lubricated with MilliQ water until the sulcal groove was removed. The otolith was then flipped distal surface up and again ground until its core was exposed, thus creating a thin section (). Once ground, the otolith sections were transferred onto an analysis slide. Prior to analysis, all otoliths were gently scrubbed under a constant flow of Milli-Q water to remove any further external contamination.
Figure 2 Photo showing sectioned adult otolith and ablated transect (LA Transect) highlighted by dashed horizontal lines. The element profile for Sr:Ca ratios is shown and represents Sr:Ca ratio levels as they relate to the ablated transect directly below it. Arrow marked ‘B’ indicates the primordium of the otolith, and the centre of the profile. Arrows marked ‘A’ and ‘C’ indicate the sections of transect and profile from which 50 µm of data was taken to generate the natal signature for the otolith (approximated by dashed vertical lines). Note that the natal signature also considers Ba:Ca, Rb:Ca and Mn:Ca ratios, but for clarity the element profiles for these elements are not shown.
Trace element analysis
Trace element levels within all otoliths were analysed by LA-ICP-MS using a custom built laser sampling system interfaced between an ArF (193 nm) Excimer laser (Lambda Physik LPX120i) and a Quadrupole ICPMS (Agilent 7500s) at the Research School of Earth Science, Australian National University. The system was calibrated to analyse the otoliths for magnesium (Mg), aluminium (Al), phosphorus (P), rubidium (Rb), manganese (Mn), barium (Ba) and strontium (Sr). A suite of elements were analysed to maximise the likelihood of achieving differentiation between tributaries, and elements were chosen based on those previously used to identify habitat specific element signatures in otoliths from freshwater fish (Wells et al. Citation2003; Brazner et al. Citation2004; Friedrich & Halden Citation2008).
For each juvenile otolith, the laser beam was focused at the centre of the distal surface directly above the primordium and a vertical transect was ablated through the entire width of the otolith (circular spot ablated at 20 Hz). For each adult otolith, a horizontal transect was ablated across the otolith section from the ventral to the dorsal edge and passing through the core (ablated at 20 Hz, at a speed of 1 mm/s) (). Adult otoliths required sectioning prior to analysis as a vertically drilled transect (as used on the juvenile otoliths to reduce sample preparation time) was not analytically feasible because of the error involved in deposition and element fractionation processes (Eggins et al. Citation1998).
In all cases, a carrier gas stream of He and Ar was used to transfer the ablated material from the sample chamber through a pulse-smoothing manifold into the ICP-MS where the specific quantities of each element were measured (see Eggins et al. Citation1998 for details of this system). Otoliths were analysed in batches of 10 with the international glass reference material NIST610 introduced at the start, end and between each batch to correct for drift in the instrument. Background samples, measured with the laser turned off, were analysed for 20 s prior to each standard and otolith transect. Data reduction followed established LA-ICP-MS protocols (after Longerich et al. 1996) using NIST610 for external calibration and 43Ca as the internal standard to correct for variation in mass ablation yield (Eggins et al. Citation2003). Mean background count rates measured with the laser off were subtracted from all measured element intensities (Eggins et al. Citation2003). All data was reduced in Excel spreadsheets, presented as element/Ca mmol/mol and then plotted against ablation distance (µm).
For each juvenile otolith, the first 5 µm of the vertically ablated transect was treated as a pre-ablation to remove any contamination. Element:Ca ratios were averaged from the subsequent 5 µm of the transect. This gave the mean element:Ca ratios from a small area close to the outside edge of each otolith representing otolith growth not long before fish collection.
For each adult otolith, the primordium of each otolith, as expressed in the horizontally ablated transect, was determined by assessing the symmetry in the element:Ca ratios over the whole transect and by the presence of a distinct spike in the Mn:Ca ratio at the core region (Brophy et al. Citation2004; Ruttenberg et al. Citation2005). Element:Ca ratios were averaged from two 50-µm sections of each transect (200 µm from the ventral and dorsal sides of the primordium) (), hereafter collectively referred to as the natal region, as each side of the core region should represent analogous otolith growth. These distances were chosen to give the mean element:Ca ratios from the two areas of the transect most likely to represent otolith material deposited soon after fry emergence into the natal tributary, and were determined by measuring the otolith radii of 10 recently emerged brown trout still showing evidence of a diminished yolk sac (mean fish length = 25.7 mm; mean otolith radius 177±25.4 µm).
Statistical analysis
Two-way analyses of variance (ANOVAs) were used to assess differences in the element:Ca ratios within the juvenile otoliths, among tributaries and sampling occasions. To characterise the element:Ca signatures of each tributary, element:Ca ratios that showed statistically significant differences among tributaries from the juvenile otoliths were entered into a linear discriminant function analysis (LDFA), grouped by the tributary from which they were sampled. To determine the accuracy of the classification generated by the LDFA, cross-validation of grouped cases was performed using a jack-knife procedure, which removes each sample from the data set and re-estimates the discriminant function based on the remaining samples. The resulting function is then used to classify the removed data point (Brazner et al. Citation2004). Any significant differences among group means were determined using Wilk's Lambda test of equality (Quinn & Keough Citation2002).
Average element:Ca ratios from the natal region of each adult otolith were added to the LDFA as ungrouped individuals. The analysis was used to classify these individuals into one of the eight tributary groups. To determine if an adult fish accurately matched a tributary group, the posterior probability for that individual (which suggests the most likely group membership) must have been greater than the mean of the posterior probabilities for the predicted group memberships of the juvenile fish minus two standard deviations. Also, the squared Mahalanobis distance from the group centroid for that individual (which gives a measure of how likely the predicted group membership is) must have been less than the mean of the squared Mahalanobis distances from the group centroids of the juvenile fish plus two standard deviations. To meet the assumptions of homogeneity of variance and normal distribution, all element:Ca ratios were Log10 transformed. All analyses were performed with the statistical package SPSS 13.0.
Results
One juvenile fish collected from the Dart River in April 2005 had a Sr:Ca ratio that was 3.02 standard deviations lower than the mean for that river. Comparison of this ratio with the Sr:Ca ratios from the Upper Wangapeka juveniles suggested that this fish was a recent immigrant into the Dart River from the Upper Wangapeka River, which joins the Dart River just 100 m downstream of the sampling site (). This fish was therefore considered an outlier and not included in the analyses.
Significant differences in element:Ca ratios among tributaries occurred for Sr:Ca (F=195.74; P<0.001), Mn:Ca (F=88.39; P<0.001), Rb:Ca (F=59.27; P<0.001) and Ba (F=44.83; P<0.001) (). Of these, Mn:Ca (F=14.57; P<0.001) and Ba:Ca (F=9.30; P<0.001) differed significantly among sampling occasions whereas Sr:Ca (F=1.56; P=0.216) and Rb:Ca (F=1.28; P=0.283) remained constant ().
Figure 3 Mean (±SE) element:Ca ratios from the otoliths of juvenile brown trout (n=5) from eight tributaries on each sampling occasion. Abbreviated names for the tributaries are described in .
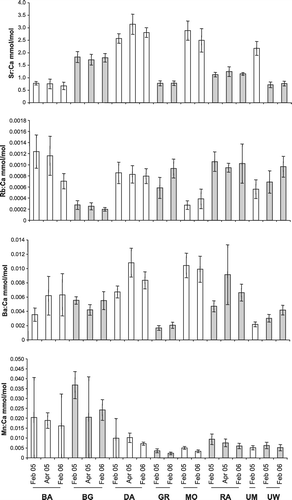
For each tributary (excluding the Graham, which was only sampled on one occasion), multivariate analysis of Sr:Ca, Mn:Ca, Rb:Ca and Ba:Ca ratios suggested some variation among sampling occasions especially for the Baton, Rainy and Upper Wangapeka tributaries ().
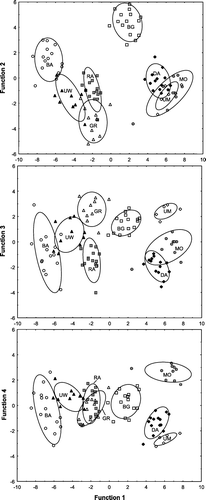
All element:Ca ratios from the juvenile otoliths that showed significant differences among tributaries were entered into a LDFA, where the Sr:Ca ratio was most statistically influential in the model (F=190.24), followed by Mn:Ca (F=67.46), Rb:Ca (F=46.91) and Ba:Ca (F=36.64). The first, second, third and fourth functions for this model accounted for 69.5%, 17.6%, 8.0% and 4.9% of the dispersion in the data set, respectively. The highest correlation between individual trace element:Ca ratios and canonical functions were, Sr:Ca and CV1 (0.784), Mn:Ca and CV2 (0.929), Ba:Ca and CV3 (0.849) and Rb:Ca and CV4 (0.653).
The LDFA resulted in the correct classification of 96.8% of cross-validated grouped cases. All individual fish sampled from the Blue Glen, Dart, Graham, Rainy and Upper Motueka tributaries classified to those tributaries with 100% accuracy. There was some misclassification within the Baton (93.3% accuracy), Motupiko (90% accuracy) and Upper Wangapeka groups (90% accuracy).
Plots of the discriminant functions showed that there was little overlap in group boundaries as defined by 95% confidence ellipses calculated around the group means (). Significant differences among group means for Sr:Ca (Wilk's λ = 0.06, F=190.24, P<0.001), Mn:Ca (Wilk's λ = 0.15, F=67.46, P<0.001), Rb:Ca (Wilk's λ = 0.21, F=46.91, P<0.001) and Ba:Ca (Wilk's λ = 0.25, F=36.64, P<0.001) confirmed strong differences among tributaries.
Figure 4 Trace element signatures obtained from the otoliths of juvenile brown trout collected from eight tributaries in the Motueka River Catchment. Groupings for each of the three plots are based on discriminant functions 2, 3 and 4 versus function 1; all obtained through linear discriminant function analysis of Sr:Ca, Rb:Ca, Ba:Ca and Mn:Ca ratios. Ellipses represent 95% confidence ellipses calculated around the group means. Abbreviated names for the tributaries are described in .
When entered into the LDFA, 11 of the 23 adult fish were able to be confidently assigned to one of the eight tributaries based on their posterior probability of most likely group membership (>0.786) and their squared Mahalanobis distance from that group centroid (<11.205) (). Of the 11 adult fish that matched a tributary signature, three fish matched the Dart signature, two fish matched the Baton signature, two fish matched the Rainy signature, two fish matched the Motupiko signature, one fish matched the Graham signature and one fish matched the Upper Wangapeka signature. No fish matched either the Upper Motueka or the Blue Glen signatures. Nine adult fish recruited from an upstream source and two from a downstream source. The greatest distance travelled by an individual fish from a tributary was estimated at 70 km and the smallest 5 km. The estimated mean distance travelled for all fish was 30 km.
Table 3 Capture location and predicted natal tributaries of the 23 adult brown trout sampled.
Discussion
The excellent cross-validated LDFA classification of juvenile trout to their tributaries suggests that the trace element signatures within their otoliths define this relationship remarkably well. Indeed, all but three of the eight tributaries sampled had a unique trace element signature so that 100% of the fish collected from that tributary grouped back to that tributary in the analysis. Where 100% classification could not be achieved, results were still significant enough to define these tributaries confidently to a reasonable level (90% and 93%). Consistent classification levels above 90% compare extremely well to similar studies using LDFA as a tool to classify otolith signatures within freshwater (Thorrold et al. Citation1998; Wells et al. Citation2003; Brazner et al. Citation2004; Veinott & Porter Citation2005; Clarke et al. Citation2007). The level of differentiation within the Motueka River catchment is among the most convincing published accounts of habitat trace element analysis, as demonstrated by the statistically significant differences in tributary groupings despite the relatively small number of juvenile trout sampled from each tributary. Furthermore, the data includes fish sampled on three occasions over 2 years from a relatively large number of tributaries. These results suggest that micro-sampling a section of otolith material recently produced (representative of 5 µm of otolith growth) will give an accurate assessment of the immediate tributary signature regardless of the exact mechanisms causing those signatures. Recent fish immigration from outside of the tributary of interest would result in a false habitat signature; however, this study suggests it may be possible to identify recent immigration as a distinct difference in the element:Ca data of an individual. A more troublesome scenario would result if that fish had moved among a number of tributaries before capture. This could result in a poorly defined habitat signature, as 5 µm of element:Ca data is likely to represent more than a week of otolith growth and potential movement. Mobile fish may also be identifiable by examining the element:Ca data, but more importantly, our results suggest that juvenile (less than 12 months of age) trout movement among tributaries at the river order sampled in this study is either uncommon or has minimal effect on tributary differentiation, as there were significant differences in tributary signatures. This approach also offers a relatively fast and cost effective method for the analysis of juvenile otoliths without the additional time involved in otolith sectioning.
Our results showed that by sampling a discrete area of adult brown trout otoliths representative of their natal habitat, and matching these signatures to tributary signatures, we can begin to examine catchment-wide recruitment patterns. Although this method has been suggested by a number of authors (Gillanders & Kingsford Citation2000, Citation2003; Veinott & Porter Citation2005), few have attempted it in practice. Notable exceptions include: Thorrold et al. (Citation2001) and Gillanders (Citation2002a) who examined adult connections to natal estuaries in marine species; Crook & Gillanders (Citation2006) who examined recruitment sources of freshwater carp within the Murray River catchment Australia; and Humston et al. (2010) who reported on tributary recruitment of small mouthed bass into a section of the James River, USA. However, to our knowledge this is the first time this method has been used to examine the recruitment patterns of multiple cohorts of adult fish throughout an entire catchment.
Of the 23 adult fish examined in this study, 11 could be confidently ascribed to one of the eight tributaries for which trace element signatures were generated. The recruitment patterns from these tributaries, although hindered by the relatively small sample size, does suggest that adult trout within the Motueka and Wangapeka River main stems recruit from up and downstream, and from localised and distant sources throughout the catchment. However, the utility of otolith trace element analysis as a tool for assessing trout recruitment requires an understanding of why 12 of the 23 adult trout sampled were unable to be matched to a natal source.
The natal regions of the adult trout otoliths were chosen to represent otolith material deposited during time spent in the natal tributary. Assuming that any maternal influences (Volk et al. Citation2000; Zimmerman & Reeves Citation2002) and core specific biomineralisation processes (Brophy et al. Citation2004; Ruttenberg et al. Citation2005) have been avoided (i.e. by beginning the assessment of the natal region 200 µm from the otolith primordium), error in the measured adult natal signature could result from fish movement within or among natal tributaries during the juvenile phase. This could potentially confuse the signature measured and hinder the ability of the LDFA to match adult fish to natal tributaries. Exactly how much time is represented by the natal region (i.e. a section of the otolith transect 200 µm from the otolith primordium, measuring 50 µm) needs to be accurately determined, and could be estimated by counting daily growth rings and referenced to hatch and emergence checks on thin highly polished otolith sections. Regardless, it is likely that the natal region corresponds to a period no longer than 6 months, immediately following emergence into the natal tributary. It is generally considered that the first year of the juvenile brown trout life cycle is often spent in the natal stream (Elliott Citation1994) as here the young fish are provided with a water velocity refuge, plentiful food and protection from predation by larger fish (Klemetsen et al. Citation2003). It is also possible that mortality amongst early migrants is likely to be high (Elliott Citation1994). Because of this, it is likely that the natal region of most adult trout, as it is calculated in this study, will accurately represent their natal stream.
Studies that have found significant inter-annual variation within the otoliths of fish from re-sampled locations (Gillanders Citation2002b; Schaffler & Winkelman Citation2008) often insist on the importance of tracing annual cohorts of adult fish to natal areas. In this study, we cannot be sure if our adult sample represents cohorts of the 2005 and 2006 spawning seasons. Our data suggest there is likely to be some variation in multi-element signatures within the tributaries of a catchment over time. Although Sr:Ca and Rb:Ca element ratios appear to be fairly stable, there was some variation in Mn:Ca and Ba:Ca element ratios among sampling occasions, which could affect the ability of the LDFA to predict the origin of adult fish. However, the temporal variation among element:Ca ratios and multi-element signatures was generally less than the variation among tributaries when significant differences in tributary chemistry were noted. Also, Sr:Ca element ratios were the most temporally stable of those element:Ca ratios measured and were also the most influential in the LDFA, reducing the effects on the analysis of the more temporally unstable element:Ca ratios. Furthermore, when data from all three sampling events was used to define the tributaries of the Motueka catchment, we still found a high differentiation among tributaries despite the inter- and intra-annual variation in some elements. This suggests that the effects of the trace element instability we saw over time probably had little influence on the development of tributary signatures and the ability of the LDFA to predict the origins of adult fish of multiple ages, sampled over multiple years. It also seems likely that the clear and consistent differences in catchment chemistry will either incorporate or override any effects on otolith chemistry caused by other influences that have been suggested to affect otolith trace element levels, such as temperature (Bath et al. Citation2000; Elsdon & Gillanders Citation2003, Citation2004) or diet (Sanchez-Jerez et al. Citation2002).
In this study, only eight tributaries of the Motueka River catchment were analysed for trace element signatures. Critiques of natal reconstructions using otoliths often emphasise the importance of sampling all possible areas of both recruitment and environmental trace element variability (Campana Citation2005; Elsdon et al. Citation2008). The logistics involved in thoroughly sampling a catchment the size of the Motueka make this goal difficult to achieve. When interpreting the LDFA assignment of adult fish to the eight tributaries, criteria based on the posterior probability of most likely group membership and the squared Mahalanobis distance from that group centroid were used in attempt to alleviate this problem. It was assumed theoretically possible for an adult fish to fall outside of a tributary grouping in discriminant space, having originated from outside of the eight tributaries sampled. More extensive sampling of juvenile trout throughout the catchment, thus creating habitat signatures for more tributaries (as well as for main stem locations), would almost certainly result in a greater proportion of adult fish being linked with particular spawning areas, as trout spawning and rearing in the Motueka River catchment occurs outside the eight tributaries sampled (Nelson/Marlborough Fish and Game unpubl. data). However, it is likely that the inclusion of further habitat signatures would result in a decrease in the overall classification success of the model (Barbee & Swearer Citation2007). Results from studies attempting to determine trace element variability over spatial scales are varied within the literature on otolith trace element analysis, and distance alone is often a poor determinant of the chemical variability of adjacent environments (Elsdon et al. Citation2008). Therefore, it would be hard to predict how much of a catchment needs to be sampled to achieve a reliable level of adult classification while maintaining tributary differentiation and this is likely to vary depending on the characteristics of a given catchment. The present study, using Blue Glen Stream as the smallest tributary sampled (catchment size 25 km2), suggests that tributaries contributing as little as 1% of a total catchment area can be differentiated. The diverse geology of the Motueka River catchment makes it an unusually good candidate for differentiating among tributaries when compared with other catchments. Nevertheless, this study also noted good separation between tributaries of homogenous geology; the Motupiko and Rainy tributaries, for example, are adjacent reaches of the Motupiko sub-catchment and are underlain by a Pliocene to early Pleistocene Moutere Gravel complex (Basher Citation2003). Both groups separated to 100% in the LDFA and the difference between these two tributaries was comparable to the greatest dissimilarity between any of the eight tributaries sampled. This confirms that significant differences in river element:Ca ratios can occur between river reaches separated by distances less than 10 km of river length (Humston et al. Citation2010) and highlights the apparent complexities involved in the development of trace element signatures. In some cases, it is possible that the influences of land use adjacent to a river system may be as important as geology in determining river chemistry.
When examining the origins of the 11 fish that could be matched to a natal tributary, our results suggest recruitment in the Motueka catchment can consist of long downstream migrations (i.e. Rainy River to the lower reaches of the Motueka River; adult trout number 2 travelled approximately 70 km), long upstream migrations (i.e. Baton River to South Branch of the Wangapeka River; adult trout number 22 travelled approximately 40 km) and relatively localised migrations (i.e. Dart River to the mid Wangapeka River; adult trout 21 travelled approximately 5 km), highlighting just how diverse the movements within a brown trout population can be. The average distance travelled by the adult trout in this study was approximately 30 km, roughly 30% of the total catchment length. However, the above distances do not consider all the possible movements made throughout the life of the fish examined, so for at least some individuals, their total lifetime movements may far exceed those distances stated above. The movements of adult trout captured in the Motupiko River (a sub-catchment of the Motueka River) have been examined by radio telemetry (Young et al. Citation2010). Movements over an 11-month period ranged from less than 100 m to greater than 40 km confirming that large-scale movements are a common strategy employed by some individuals in brown trout populations (Gowan et al. Citation1994). Furthermore, notwithstanding the small sample of adult trout, the results indicate that recruitment is evenly spread over several tributaries throughout the Motueka catchment, suggesting the population should be resilient to localised climatic and anthropomorphic disruptions.
In conclusion, our results suggest otolith trace element analysis offers a reliable and transferable tool for the chemical differentiation of natal tributaries within a river catchment. Moreover, following the comprehensive development of tributary signatures, useful information on the recruitment origins of the adult trout population can be predicted based on natal signatures found within their otoliths. Finally, understanding the patterns of recruitment within a river catchment can inform management decisions of the main stem fishery. Protection efforts can be prioritised for those tributaries that make a major contribution to main stem fish stocks. Also, this can enable an understanding of the effects of seasonal catchment events that have been suggested to influence long-term population dynamics, such as floods (Young et al. Citation2010). Given the plastic nature of the brown trout life history, catchment specific studies are likely to be necessary to understand fully how a population is maintained.
Acknowledgements
This project was funded by the University of Otago, the New Zealand Foundation for Research Science and Technology (Contract C09X0305), Fish & Game New Zealand Nelson/Marlborough Region and the generous donations of Brian Weatherhead. Mike Shelly, Charlotte Allen and Greg Yaxley are acknowledged for analytical support and Mike Palin for his helpful discussions on analytical logistics.
References
- Barbee , NC and Swearer , SE . 2007 . Characterising natal source population signatures in the diadromous fish Galaxias maculatus, using embryonic otolith chemistry . Marine Ecology Progress Series , 343 : 273 – 282 .
- Barnett-Johnson , R , Pearson , TE , Ramos , FC , Grimes , CB and McFarlane , RB . 2008 . Tracking natal origins of salmon using isotopes, otoliths, and landscape geology . Limnology and Oceanography , 53 : 1633 – 1642 .
- Basher LR 2003 The Motueka and Riwaka Catchments . Landcare Research Technical Report . Landcare Research , Lincoln, , New Zealand .
- Bath , GE , Thorrold , SR , Jones , CM , Campana , SE , McLaren , JW and Lam , JWH . 2000 . Strontium and barium uptake in aragonitic otoliths of marine fish . Geochimica et Cosmochimica Acta , 64 : 1705 – 1714 .
- Brazner , JC , Campana , SE and Tanner , DK . 2004 . Habitat fingerprints for Lake Superior coastal wetlands derived from elemental analysis of yellow perch otoliths . Transactions of the American Fisheries Society , 133 : 692 – 704 .
- Brophy , D , Jeffries , TE and Danilowicz , BS . 2004 . Elevated manganese concentrations at the cores of clupeid otoliths: possible environmental, physiological, or structural origins . Marine Biology , 144 : 779 – 789 .
- Campana , SE . 1999 . Chemistry and composition of fish otoliths: pathways, mechanisms and applications . Marine Ecology Progressive Series , 188 : 263 – 297 .
- Campana , SE . 2005 . Otolith science entering the 21st century . Marine and Freshwater Research , 56 : 485 – 489 .
- Campana , SE , Fowler , AJ and Jones , CM . 1994 . Otolith elemental fingerprinting for stock identification of Atlantic Cod (Gadus morhua) using laser ablation ICPMS . Canadian Journal of Fisheries and Aquatic Science , 51 : 1942 – 1950 .
- Campana , SE and Thorrold , SR . 2001 . Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations . Canadian Journal of Fisheries and Aquatic Sciences , 58 : 30 – 38 .
- Clarke , AD , Telmer , KH and Shrimpton , JM . 2007 . Habitat use and movement patterns for a fluvial species, the Arctic grayling, in a watershed impacted by a large reservoir: evidence from otolith microchemistry . Journal of Applied Ecology , 44 : 1156 – 1165 .
- Crook , DA and Gillanders , BM . 2006 . Use of otolith chemical signatures to estimate carp recruitment sources in the Mid-Murray River, Australia . River Research and Applications , 22 : 871 – 879 .
- Eggins , SM , Kinsley , LPJ and Shelly , JMG . 1998 . Deposition and element fractionation processes during atmospheric pressure laser sampling for analysis by ICP-MS . Applied Surface Science , 127–129 : 278 – 286 .
- Eggins , S , Grun , R , Pike , AWG , Shelly , M and Taylor , L . 2003 . 238U, 232Th profiling and U-series isotope analysis of fossil teeth by laser ablation-ICPMS . Quaternary Science Reviews , 22 : 1373 – 1382 .
- Elliott , JM . 1994 . Quantitative ecology and the brown trout , Oxford, , UK : Oxford University Press .
- Elsdon , TS and Gillanders , BM . 2003 . Relationship between water and otolith elemental concentrations in juvenile black bream Acanthopagrus butcheri . Marine Ecology Progressive Series , 260 : 263 – 272 .
- Elsdon , TS and Gillanders , BM . 2004 . Fish otolith chemistry influenced by exposure to multiple environmental variables . Journal of Experimental Marine Biology and Ecology , 313 : 269 – 284 .
- Elsdon , TS , Wells , BK , Campana , SE , Gillanders , BM , Jones , CM , Limburg , KE , Secor , DH , Thorrold , SR and Walther , BD . 2008 . Otolith chemistry to describe movements and life-history parameters of fishes: hypothesis, assumptions, limitations and inferences . Oceanography and Marine Biology: An Annual Review , 46 : 297 – 330 .
- Freiedrich , LA and Halden , NM . 2008 . Alkali element uptake in otoliths: A link between the environment and otolith microchemistry . Environmental Science and Technology , 42 : 3514 – 3518 .
- Gillanders , BM . 2002a . Connectivity between juvenile and adult fish populations: do adults remain near their recruitment estuaries? . Marine Ecology Progress Series , 240 : 215 – 223 .
- Gillanders , BM . 2002b . Temporal and spatial variability in elemental composition of otoliths: implications for determining stock identity and connectivity of populations . Canadian Journal of Fisheries and Aquatic Sciences , 59 : 669 – 679 .
- Gillanders , BM and Kingsford , MJ . 2000 . Elemental fingerprinting of otoliths of fish may distinguish estuarine ‘nursery’ habitats . Marine Ecology Progress Series , 201 : 273 – 286 .
- Gillanders , BM and Kingsford , MJ . 2003 . Spatial variation in elemental composition of otoliths of three species of fish (family Sparidae) . Estuarine Coastal and Shelf Science , 57 : 1049 – 1064 .
- Gowan , C , Young , MK , Fausch , KD and Riley , SC . 1994 . Restricted movement in resident stream salmonids: a paradigm lost? . Canadian Journal of Fisheries and Aquatic Sciences , 51 : 2626 – 2637 .
- Humston , R , Priest , BM and Hamilton , WC . 2010 . Dispersal between tributary and main-stem rivers by juvenile smallmouth bass using otolith microchemistry . Transactions of the American Fisheries Society , 139 : 171 – 184 .
- Kennedy , BP , Folt , CL , Blum , JD and Chamberlain , CP . 1997 . Natural isotope markers in salmon . Nature , 387 : 766 – 767 .
- Kennedy , BP , Blum , JD , Folt , CL and Nislow , KH . 2000 . Using natural strontium isotopic signatures as fish markers: methodology and application . Canadian Journal of Fisheries and Aquatic Sciences , 57 : 2280 – 2292 .
- Kennedy , BP , Klaue , A , Blum , JD , Folt , CL and Nislow , KH . 2002 . Reconstructing the lives of fish using Sr isotopes in otoliths . Canadian Journal of Fisheries and Aquatic Sciences , 59 : 925 – 929 .
- Klemetsen , A , Amundsen , P-A , Dempson , JB , Jonsson , B , Jonsson , N , O'Connell , MF and Mortensen , E . 2003 . Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus L.: a review of aspects of their life histories . Ecology of Freshwater Fish , 12 : 1 – 59 .
- Kristensen , EA , Closs , GP , Olley , R , Kim , J , Reid , M and Stirling , C . 2011 . Determining the spatial distribution of spawning by anadromous and resident brown trout Salmo trutta L using strontium content of eggs collected from redds , Ecology of Freshwater Fish in press .
- Longerich , HP , Kollmer , WE and Gunther , D . 1996 . Laser ablation inductively coupled mass-spectrometry transient signal data acquisition and analyte concentration calculation . Journal of Analytical and Atomic Spectrometry , 11 : 899 – 904 .
- Payan , P , De Pontual , H , Boeuf , G and Mayer-Gostan , N . 2004 . Endolymph chemistry and otolith growth in fish . Comptes Rendus Palevol , 3 : 535 – 547 .
- Quinn , GP and Keough , MJ . 2002 . Experimental design and data analysis for biologists , Cambridge : Cambridge University Press .
- Ruttenberg , BI , Hamilton , SL , Hickford , MJH , Paradis , GL , Sheehy , MS , Standish , JD , Ben-Tzvi , O and Warner , RR . 2005 . Elevated levels of trace elements in cores of otoliths and their potential for use as natural tags . Marine Ecology Progress Series , 297 : 273 – 281 .
- Sanchez-Jerez , P , Gillanders , BM and Kingsford , MJ . 2002 . Spatial variability of trace elements in fish otoliths: comparison with dietary items and habitat constitutes in seagrass meadows . Journal of Fish Biology , 61 : 801 – 821 .
- Schaffler , JJ and Winkelman , DL . 2008 . Temporal and spatial variability in otolith trace-element signatures of juvenile striped bass from spawning locations in Lake Texoma, Oklahoma-Texas . Transactions of the American Fisheries Society , 137 : 818 – 829 .
- Thorrold , SR , Jones , CM , Campana , SE , McLaren , JW and Lam , JWH . 1998 . Trace element signatures in otoliths record natal river of juvenile American shad (Alosa sapidissima) . Limnology and Oceanography , 43 : 1826 – 1835 .
- Thorrold , SR , Latkoczy , C , Swart , PK and Jones , CM . 2001 . Natal homing in a marine fish metapopulation . Science , 291 : 297 – 299 .
- Veinott , G and Porter , R . 2005 . Using otolith microchemistry to distinguish Atlantic salmon (Salmo salar) parr from different natal streams . Fisheries Research , 71 : 349 – 355 .
- Volk , EC , Blakley , A , Schroder , SL and Kuehner , SM . 2000 . Otolith chemistry reflects migratory characteristics of Pacific salmonids: Using otolith core chemistry to distinguish maternal associations with sea and freshwaters . Fisheries Research , 46 : 251 – 266 .
- Walther , BD , Thorrold , SR and Olney , JE . 2008 . Geochemical signatures in otoliths record natal origins of American shad . Transactions of the American Fisheries Society , 137 : 57 – 69 .
- Wells , BK , Rieman , BE , Clayton , JL , Horan , DL and Jones , CM . 2003 . Relationships between water, otolith, and scale chemistries of westslope cutthroat trout from the Coeur d'Alene River, Idaho: The potential application of hard part chemistry to describe movements in freshwater . Transactions of the American Fisheries Society , 132 : 409 – 424 .
- Young , RG , Quarterman , AJ , Eyles , RF , Smith , RA and Bowden , WB . 2005 . Water quality and thermal regime of the Motueka River: influences of land cover, geology and position in the catchment . New Zealand Journal of Marine and Freshwater Research , 39 : 803 – 825 .
- Young , RG , Wilkinson , J , Hay , J and Hayes , JW . 2010 . Movement and mortality of adult brown trout in the Motupiko River, New Zealand: effects of water temperature, flow and flooding . Transactions of the American Fisheries Society , 139 : 137 – 146 .
- Zimmerman , CE and Reeves , GH . 2002 . Identification of steelhead rainbow trout progeny in the Deschutes River, Oregon, revealed with otolith microchemistry . Transactions of the American Fisheries Society , 131 : 986 – 993 .