Abstract
Three species of Kareniaceae, Karenia brevis, Karenia brevisulcata and Karenia mikimotoi were assessed for toxicity to juvenile Chinook salmon (Oncorhynchus tshawytscha), snapper (Pagrus auratus) and six species of marine invertebrate larvae (GreenshellTM mussel, pacific oyster, paua [New Zealand abalone], sea slug, sea urchin and brine shrimp). Karenia brevis was ichthyotoxic to juvenile salmon with a LT50 of 0.75 h at 0.18 × 106 cells L–1 but juvenile snapper were less sensitive (LT50 of 4.5 h at 5.9 × 106 cells L–1). Karenia brevisulcata also showed differential toxicity to juvenile salmon and snapper with LT50 of 0.48 h at 11 × 106 cells L–1 and 7.5 h at 16 × 106 cells L–1 respectively. However, no acute symptoms were observed in the fish following 48 h exposure to K. mikimotoi at 3.4 × 106 cells L–1. Karenia brevis at the highest level tested (8.7 × 106 cells L–1) did not cause observable symptoms in any of the invertebrate larvae over 24 h. Karenia mikimotoi only affected paua larvae (LT50 10 h at 12 × 106 cells L–1). Karenia brevisulcata was highly toxic to mussel, sea urchin and paua larvae (LT50 1–6.1 h at 7.5–9.6 × 106 cells L–1), toxic to sea slug and oyster larvae (LT50 6 h at 80 × 106 cells L–1 and 9.2 h at 34 × 106 cells L–1 respectively) but did not affect brine shrimp. Analysis of test cultures by liquid chromatography-mass spectrometry confirmed that K. brevis produced brevetoxins (52 pg cell–1; mainly brevetoxin-2). Karenia brevisulcata produced brevisulcatic acids (BSXs) and the more toxic Karenia brevisulcata toxins (KBTs). Karenia mikimotoi did not produce brevetoxin, BSXs or KBTs. The present study confirms the broad threats that blooms of K. brevisulcata pose to marine ecosystems and aquaculture, and the hazards of K. brevis to finfish and K. mikimotoi to abalone respectively.
Introduction
Karenia species of unarmoured dinoflagellates often have been implicated in major fish-killing events world-wide, particularly in Florida and the Gulf of Mexico (Steidinger et al. Citation1998; Steidinger Citation2009). In New Zealand, major Karenia blooms have occurred on many occasions involving the toxic species: K. cf brevis, K. mikimotoi, K. selliformis. K. concordia and K. brevisulcata. In 1992–93, more than 180 cases of human illness that fitted the symptoms of neurotoxic shellfish poisoning (NSP) occurred after consumption of contaminated shellfish mainly collected from the coastline from Northland to the Bay of Plenty (Jasperse Citation1993; Chang et al. Citation1995; MacKenzie et al. Citation1995). High levels of the metabolites of brevetoxins (BTXs) were detected in shellfish samples collected at that time (Ishida et al. Citation1995; Murata et al. Citation1998; Morohashi et al. Citation1999). Karenia cf brevis (similar morphology to K. brevis and K. mikimotoi) was identified as the dominant species in the 1992–93 harmful algal bloom (HAB) (Chang Citation1995; MacKenzie et al. Citation1995; MacKenzie Citation2008). This species was considered the cause of the shellfish contamination and mass mortalities of marine fauna via production of ichthyotoxic brevetoxins but unfortunately the alga was not isolated at that time and brought into culture. However, a range of other Karenia species isolated from New Zealand waters have tested negative for brevetoxins by liquid chromatography-mass spectrometry (LC-MS)/MS (McNabb et al. Citation2006).
Karenia mikimotoi is one of the most common fish-killing toxic dinoflagellates in both the Atlantic and Pacific regions (Honjo Citation1995; Silke et al. Citation2005; Gentien et al. Citation2007; Zou et al. Citation2010). The cell concentration in a typical K. mikimotoi bloom can easily reach millions of cells per litre (1–20×106 cells L–1) (Silke et al. Citation2005; International Council for the Exploration of the Sea Citation2006; Davidson et al. Citation2009; Ulrich et al. Citation2010). This species was responsible for several severe HAB events in New Zealand waters in the past two decades and the development of some of these blooms have been characterised (Chang et al. Citation2003). Mass mortalities of fin-fish and eels were reported during a bloom dominated by K. mikimotoi (highest cell density > 2.1×106 cells L–1), which occurred at Te Puna Inlet, Northland, New Zealand in 2007 (Smith et al. Citation2007). No BTXs were detected in oysters collected from marine farms in the area using LC-MS/MS analysis for brevetoxin-2 (BTX-2) and brevetoxin-3 (BTX-3), and the fish kills were associated with mechanical gill damage or environmental stressors e.g. anoxia. Karenia mikimotoi produces several classes of compounds, including gymnocins, haemolytic glycolipids and polyunsaturated fatty acids, which have cytotoxic, haemolytic, and ichthyotoxic properties (Yasumoto et al. Citation1990; Satake et al. Citation2002; Marshall et al. Citation2005; Satake et al. Citation2005; Gentien et al. Citation2007). However, there is currently no comprehensive quantitative understanding of the roles of any of these compounds in marine mortalities caused by K. mikimotoi.
A severe HAB devastated almost all of the marine biota in Wellington Harbour, New Zealand, in 1998 and the dominant causative organism (maximum cell population 33.3×106 cells L–1) was isolated and described as K. brevisulcata (Chang 1999a,b; Chang et al. Citation2001). This species is similar to K. mikimotoi in morphology, but the cells are smaller (13–25 µm long) and the groove on the ventral surface of the epicone is shorter (etymology: Latin brevis=short, sulcatum=groove) (Chang Citation1999b). Over 500 cases of human respiratory illness were reported during the bloom from late January to early March, with symptoms including dry cough, severe sore throat, runny nose, skin and eye irritation, severe headaches and a facial sun-burn sensation (Chang Citation1999a). The respiratory distress resembled that caused by aerosolised BTX (Fleming et al. Citation2005) and was believed to be caused by direct exposure to sea-spray aerosols containing biotoxins or toxic algal cells (Chang et al. Citation2001; Cheng et al. Citation2005). The K. brevisulcata bloom was very damaging to a wide range of marine organisms including fish, invertebrates, macroalgae, and seaweeds (Chang Citation1999a). Ecosystem studies in Wellington Harbour confirmed the widespread and long-term damage caused to the benthos, particularly to marine invertebrate populations (Wear & Gardner Citation2001; Gardner & Wear Citation2006; Kröger et al. Citation2006).
The ongoing study of the toxins produced by K. brevisulcata (carried out in collaboration with the University of Tokyo, Japan) identified two suites of complex polyether compounds, including six to eight Karenia brevisulcata toxins (KBTs) with molecular weights of 1900–2200 Daltons and brevisulcatic acids (BSXs) with molecular weights 800–950 Daltons (Holland et al. 2012). The following toxins were isolated from mass cultures and could be detected by LC-MS (mass of molecular cation MH+) in solid phase extraction (SPE) extracts of cultures: KBT-F (2055) and KBT-G (2085); BSX-1 (917), BSX-2 (873), BSX-4 (899) and BSX-5 (855). Preliminary spectroscopic data show some structural similarities between KBTs and gymnocins and between BSXs and brevetoxin-A. BSX-1 (C49H72O16) and BSX-2 (C47H68O15) are lactone ring-opened derivatives of BSX-4 (C49H70O15) and BSX-5 (C47H66O14) respectively. These toxins were shown to be toxic in mouse and cell-based models with KBTs being significantly more active than BSXs. Lipophilic extracts of K. brevisulcata were active in the mouse neuroblastoma cytotoxicity assay (Truman et al. Citation2005; Truman Citation2007). However, the toxicities to marine organisms of K. brevisulcata and these compounds have not been established.
New Zealand coastal waters have been the home of several Karenia species that threaten our marine ecosystems and aquaculture (molluscan shellfish and cage fisheries). These toxic species appear similar under microscope, but they differ in the suites of toxins produced and their effects on marine species. In the present study, three toxic Karenia species were investigated for their toxic potencies against aquatic organisms including juveniles of two fish species and larvae of six invertebrate species. Cultures of the organisms were established for this purpose using both small-scale flasks and medium-scale carboys (Beuzenberg et al. 2011). The results from fish acute toxicology tests and invertebrate larvae survival bioassays are helpful in understanding the toxicity mechanisms of these related dinoflagellates. Algal toxins are often present in multiple forms in nature, which is important for understanding of their toxic potency and mechanism of action. For example, hydrolysis of BTXs can lead to ring-opened compounds that are hundreds-fold less cytotoxic than their parents (Roth et al. Citation2007). Bioassays combined with LC-MS determination of toxin production will contribute to evaluating the toxic potency and mode of action of biotoxins produced by K. brevisulcata in aquatic organisms. This research will provide aquaculture and fish farmers with some guidance should harmful Karenia species bloom in marine farming areas.
Methods
Microalgae cultures and growth conditions
The following algal species were used in the present study and are maintained in the Cawthron Institute Culture Collection of Micro-algae (CICCM): K. mikimotoi (CAWD 63; Hauraki Gulf, New Zealand NZ), K. brevisulcata (CAWD82; Wellington Harbour, NZ) and K. brevis (CAWD122; EPA-JR strain, Florida, USA). Karenia brevis was grown in modified L1 medium (Guillard & Hargraves Citation1993) at 25±1 °C. Karenia brevisulcata and K. mikimotoi were grown at 19±1 °C in selenium-enriched GP (Loeblich & Smith Citation1968; Beuzenberg et al. Citation2011) and GP50% medium respectively (Loeblich Citation1975). Mass cultures were grown in glass flasks (2 L) or plastic barrels (15 L) with 100 µmol m–2s–1 photon flux (14:10 h L:D) and sparged with filtered air (0.22 µm). All three species were grown for 18–22 days because previous studies showed maximum production of BSX toxins in K. breviculcata cultures occurred at c. 20 days before cell numbers collapsed. Respiratory masks were worn to prevent exposure to aerosolised cultures. The number of cells per litre of each mass culture (cell density) was calculated from micro-algal counts of 25 µL samples in Utermöhl chambers with 1 mL seawater/Lugols solution and using three random fields at 100× magnification (inverted microscopy, Olymplus TMT-2).
Fish acute toxicity tests
Juvenile Chinook salmon (Oncorhynchus tshawytscha; 50 g) were obtained from King Salmon Ltd., Marlborough Sounds and juvenile New Zealand snapper (Pagrus auratus; 60 g) from Plant and Food Ltd, Nelson. Both species were held in aquaria at the Cawthron Institute for 12 days to habituate them to laboratory conditions; feeding was ceased 1 day before exposure to Karenia cultures. Fish were transferred gently to cylindrical glass aquaria (four fish per aquarium per treatment) containing 10 L of seawater plus Karenia mass culture. The control treatment was seawater (salinity 32 ppt; ammonia stable at approximately 0.25 mg L–1). The aquaria were maintained in dim light at 18 °C. For the salmon experiment a single addition of culture was made to provide each of the cell densities (three for K. brevis and two for K. brevisulcata; ). For the snapper experiment, three stepwise additions of culture were made over several hours to provide sufficient cells to produce a response in this less sensitive species. Initial cell additions were to c. 10% of the final cell densities (). Stress responses in both species were observed and included colour change, dorsal fin position (relaxed or upright), balance, gasping, gill condition and panic. The health index was: healthy (6), slight loss of balance (5), agitation (4), gasping (3), leaping (2), extreme loss of balance (1), dead or moribund (0; fish floating, no response to touching). The experimental times were recorded for establishment of time-response curves. LT50 was defined as the time for 50% of the fish to become moribund as determined from semi-log plots of the data. When fish were showing acute stress, they were euthanised by transferring into a plastic bag and covering with crushed ice. Autopsies were immediately conducted. Animal Welfare Ethics approval was gained for all in vivo fish experiments from the Ministry of Agriculture and Forestry through the Nelson Marlborough Institute of Technology Bioethics Committee, under the New Zealand Animal Welfare Act (1993).
Table 1 Toxicity of Karenia species to juvenile Chinook salmon (Oncorhynchus tshawytscha) and juvenile New Zealand snapper (Pagrus auratus).
Invertebrate larvae bioassays
Brine shrimp (Artemia salina) cysts were obtained from a local pet shop and hatched at room temperature in filtered seawater; larvae were used at 24–48 h post-hatching. Larvae of Greenshell™ mussel (Perna canaliculus), Pacific oyster (Crassostrea gigas), sea urchin (Evechinus chloroticus) and paua (New Zealand abalone, Haliotis iris), were raised at the Glenhaven hatchery, Nelson, and used at 4–6 days. Sea slug larvae (Pleurobranchia maculata) were raised at the Cawthron Institute from adults collected from the Hauraki Gulf, New Zealand. Larvae (6–10 of each test species) were transferred with minimal carry-over of seawater, into wells of 24-well tissue culture plates (3.5 mL/well, Becten Dickinson, USA). Karenia cultures (18–22 days post-sub-culturing), sea water fractions from SPE extracts or individual BSX toxins in seawater were added at 1000 µL, 100 µL, 10 µL or 0 µL (control) per well following larval addition (, triplicate wells per treatment). Larvae were observed using an inverted microscope (Olymplus TMT-2) at six time intervals over 24 h and health scores were assigned: 0, death (larvae disintegrated, tissue appears amorphous and decayed); 1, moribund (cilia gone, larvae disintegrating, still some jerking movement); 2, severe stress (larvae located at bottom and retracted into cell but cilia movement still occurring inside shell); 3, stressed (larvae on bottom, cilia healthy but not moving); 4, static (larvae with cilia movement, but not swimming); 5, healthy (swimming larvae with healthy veligers). A mean health score at each exposure time was calculated for each well and then each treatment (triplicates; mean±SEM). The time-course of toxic effects was studied using plots of the mean health scores versus time. LT50 (median time for 50% of larvae being moribund) was calculated from quadratic curves fitted to the data points for individual larvae at specified cell concentrations. Estimates were also made of the LC50 (median cell concentration for 50% of larvae being moribund) for each species at specified observation times. For this purpose a mean health score of 2.5 was defined as 50% moribund.
Table 2 Toxic effects of fresh cultures (19–28 days) and solid phase extraction (SPE)1 extracts of Karenia species and of brevisulcatic acid (BSX)2 toxins, on invertebrate larvae3 (6 days old) over 1–24 h observation.
Chemical analyses
SPE extracts of cultures were tested for brevetoxins (BTX-1, BTX-2, BTX-3, BTX-B5 and their hydrolysed ring-opened derivatives) and brevisulcatic acids (BSX-1, BSX-2, BSX-4 and BSX-5) using a Waters Acquity Ultra-Performance Liquid Chromatography (UPLC) system coupled with a Waters-Micromass Quattro Premier XE mass spectrometer (MS) with electrospray ionisation (ESI). Separation was achieved using a Waters Acquity UPLC C8 BEH 1.7 µm 50×1.0 mm column plus 0.2 µm prefilter at 30 °C, eluted at 0.3 mL min–1 with linear gradient over 4 min from 90% A (5% acetonitrile + 10% buffer: 500 mM formic acid & 20 mM ammonia solution) plus 10% B (95% acetonitrile + 10% buffer) to 100% B. 100% B was held for 0.5 min, changing to 10% of B over 0.2 min, before returning to initial conditions. The ESI source was operated in positive-ion mode (ESI+) at 100 °C, capillary 4 kV, cone 70 V, nitrogen gas desolvation 800 L hr–1 (350 °C). Select ion recording (SIR) operating mode used channels for MH+ ions at 867.5, 885.5, 895.6, 897.6, 911.6, 913.6, 915.6 and 929.6 for BTXs and m/z 855.7, 873.7, 899.7 and 917.7 for BSXs. Concentrations were estimated from the area responses calibrated using BTX-2 for BSXs and BTX-1, BTX-2, BTX-3 and BTX-B5 for brevetoxins (standards supplied by CRMP, NRCC Halifax, NS). The LC-MS limits of detection (LODs) as determined from the signal-to-noise ratios (5:1) of toxin peaks were similar for BTXs and BSXs at c. 1 ng mL−1 solution for each toxin as injected into the LC-MS. These LODs correspond to 0. 075 µg L–1 algal culture.
Algal toxin extraction for LC-MS analysis and invertebrate bioassay
Mass culture samples and cell-free medium (control) were extracted using polymeric SPE cartridges (Strata-X 60 mg/3mL, Phenomonex, USA) pre-conditioned with methanol (3 mL) and Milli-Q purified water (3 mL). The SPE cartridges were loaded with culture or medium (50mL), washed with Milli-Q water (3 mL) and 20% methanol (3 mL) and eluted with methanol (3 mL). An aliquot of the methanol eluent (0.8 mL) was diluted to 1 mL with Milli-Q water for LC-MS determination of BTXs and BSX toxins. Aliquots of the methanol eluant (0.6 mL) were dried under a gentle flow of nitrogen (40 °C) and dissolved in sterile seawater (10 mL) to provide SPE extracts for the invertebrate larvae bioassays (equivalent concentration to raw cultures on a cell basis). Purified BSX-1, -2, -4 and -5 toxins from K. brevisulcata (Holland et al. Citation2012) were individually dissolved in sterile seawater to concentrations of 100 µg L–1.
Results
Algal cultures and toxin production
Karenia brevis
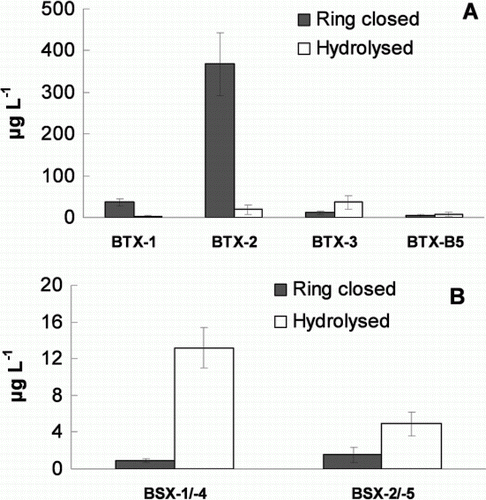
The mass culture used for fish and larvae assays was grown in a plastic carboy for 20 days and contained 8.7–9.3×106 cells L–1. The strain of K. brevis used in the present study was from Florida and produced four main BTXs which were each present in the SPE extracts as the parent toxin and the lactone ring-opened hydrolysis product (Abraham et al. Citation2006). The total concentration of these BTXs in the culture at 20 days was 490 µg L–1 (52 pg cell–1). BTX-2 was the predominant toxin (370 µg L–1). The ratios of parent to ring-opened BTX were 15 for BTX-1, 20 for BTX-2, 0.3 for BTX-3 and 0.6 for BTX-B5 (A).
Figure 1 Phycotoxins produced by Karenia brevis and Karenia brevisulcata—batch carboy cultures grown for 19–20 days and extracted by solid phase extraction for liquid chromatography-mass spectrometry determination; brevisulcatic acid (BSX) concentrations in brevetoxin-2 (BTX-2) response equivalents. A, Karenia brevis. B, Karenia brevisulcata. Columns and error bars depict the mean ± SEM (n = 3); note different scales on y-axes.
Karenia brevisulcata
An LC-MS method was validated to detect and quantify four BSXs in SPE extracts of cultures (Holland et al. Citation2012). The mass culture used for fish assays was grown in carboy for 19 days and contained 26×106 cells L–1 with a total concentration of BSXs of 12 µg L–1 (0.5 pg cell–1). The flask cultures of K. brevisulcata used for invertebrate larvae bioassays contained 84×106 cells L–1 with a total concentration of BSXs of 22 µg L–1 (0.26 pg cell–1). No BTXs were detected in SPE extracts of K. brevisulcata cultures. Preliminary spectroscopic and microchemical data show the BSXs have similarities to Type A BTXs in ring structure and include a carboxylic acid on the side chain (Holland et al. Citation2012). BSX-4 and BSX-5 are ring-closed lactone derivatives of BSX-1 and BSX-2 respectively and have appreciable activity as sodium channel agonists (Holland et al. Citation2012). The ratios of BSX-4 to BSX-1 and BSX-5 to BSX-2 were 0.05 and 0.1 respectively and thus the BSX toxins from K. brevisulcata were mainly present in the ring-opened, less active hydrolysed forms (B) in contrast to K. brevis where the ring-closed parent BTXs predominated. The KBTs are difficult to monitor by LC-MS owing to their complexity of structure, lack of standards and limitations in detection. However, preliminary data from large-scale isolations and LC-MS indicated that two of the key toxins, KBT-F and KBT-G, are present in K. brevisulcata cultures at similar concentrations to BSX-1 (Holland et al. 2012).
Karenia mikimotoi
The cultures used for fish and larvae assays were grown in carboys (5.4×106 cells L–1) and glass flasks (56–118×106 cells L–1) respectively for 28–30 days. No BTXs or BSXs were detected by LC-MS of SPE extracts. No other toxin determinations were attempted.
Ichthyotoxicity of Karenia microalgae
All fish survived after 48 h when sterile seawater (control) or K. mikimotoi (3.4×106 cells L–1) were added. Both K. brevisulcata and K. brevis were toxic to juvenile salmon and snapper. Panic, gasping and loss of balance were observed in all fish exposed to either K. brevis or K. brevisulcata with effects being apparent soon after exposure to cells. shows the time-response curves of juvenile salmon to two Karenia species. Exposure of salmon to K. brevis at 4.6×106 cells L–1 immediately led to a slight loss of balance. After 4 min, two fish completely lost balance and at 10 min three fish were euthanised. All fish had died or were euthanised by 18 min. Exposure of salmon to K. brevis at 0.9×106 cells L–1 led to stress symptoms at 2–18 min, including loss of balance, tilting, gasping, leaping and panicking. The four salmon were euthanised at 20, 25, 30 and 35 min, respectively. Exposure of salmon to K. brevisulcata at 11×106 cells L–1 led to loss of balance at 5 min in one fish, which died at 29 min. The other fish first started to gasp at 30 min and were euthanised at 40 min. At 1.3×106 cells L–1 salmon started gasping at 44 min and two fish lost balance. All fish had died or were euthanised by 112 min. Autopsy did not reveal any signs of damage or stress in the kidney, gill and liver of the experimental salmon. The LT50 for salmon in the presence of K. brevisulcata was 0.48 h with 11×106 cells L–1 and 0.93 h at 1.3×106 cells L–1 (). In comparison, K. brevis at 4.6, 0.9 and 0.18×106 cells L–1 had LT50 of 0.17, 0.42 and 0.75 h respectively ().
Figure 2 Responses of juvenile salmon (Oncorhynchus tshawytscha) to two Karenia species versus time: K. brevis 4.6 × 106 cells L–1 (▴) and K. brevis 0.9×106 cells L-1 (♦), K. brevisulcata 11×106 cells L-1 (□) and K. brevisulcata 1.3×106 cells L-1 (▪).
Notes: The points/bars depict the mean ± SEM (n = 4). Health index: 0, death; 1, extreme loss of balance; 2, leaping; 3, gasping; 4, agitation; 5, slight loss of balance; 6, healthy.
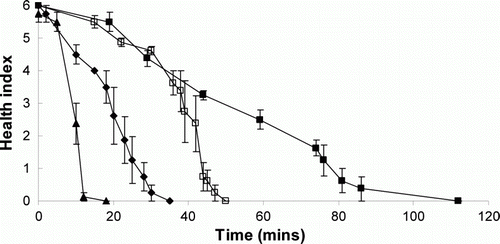
In snapper, the time course of toxic effects from K. brevis and K. brevisulcata were slower and only became evident at higher cell densities than for salmon. The LT50 for snapper were calculated as 4.5 h for K. brevis (6×106 cells L–1) and 7.5 h for K. brevisulcata (16×106 cells L–1; ). Autopsy of both fish species revealed that only the snapper exposed to K. brevisulcata exhibited any signs of damage, that being minor sloughing of the gill epithelial tissues.
Toxicity of Karenia to marine invertebrate larvae
Karenia brevis, K. brevisulcata and K. mikimotoi were tested for toxicity to the larvae of six invertebrate species (). No mortality or sub-lethal effects on brine shrimp were observed following exposure to any of the three Karenia species tested. Greenshell™ mussel, Pacific oyster, sea urchin, and sea slug were not significantly affected by K. brevis or K. mikimotoi at the highest cell densities of 8.7 and 56×106 cells L–1, respectively (). Paua larvae were not affected by K. brevis but were sensitive to K. mikimotoi (, A) with complete mortality after 24 h exposure to cell densities of 118×106 and 12×106 cells L–1.
Figure 3 Responses of marine invertebrate larvae (six days old) to Karenia cultures over 24 h. A, Response of paua (Haliotis iris) to K. mikimotoi: control (sterile seawater [×]); 12 × 106 cells L–1 (□) and 118 x 106 cells L–1 (♦). B, GreenshellTM mussel (Perna canaliculus). C, Pacific oyster (Crassostrea gigas). D, Sea slug (Pleurobranchia maculata). E, Sea urchin (Evechinus chloroticus). F, Paua. B–F, Responses of species to K. brevisulcata: control (sterile seawater [×]); 8.4 × 106 cells L-1 (□); 84 × 106 cells L-1 (♦).
Notes: Points and error bars depict the mean ± SEM (n = 3); health scores: 0, death; 1, moribund; 2, severe stress; 3, stress; 4, healthy but larvae static; 5, healthy and larvae swimming; median for 6–10 larvae in each of the triplicate wells.
![Figure 3 Responses of marine invertebrate larvae (six days old) to Karenia cultures over 24 h. A, Response of paua (Haliotis iris) to K. mikimotoi: control (sterile seawater [×]); 12 × 106 cells L–1 (□) and 118 x 106 cells L–1 (♦). B, GreenshellTM mussel (Perna canaliculus). C, Pacific oyster (Crassostrea gigas). D, Sea slug (Pleurobranchia maculata). E, Sea urchin (Evechinus chloroticus). F, Paua. B–F, Responses of species to K. brevisulcata: control (sterile seawater [×]); 8.4 × 106 cells L-1 (□); 84 × 106 cells L-1 (♦). Notes: Points and error bars depict the mean ± SEM (n = 3); health scores: 0, death; 1, moribund; 2, severe stress; 3, stress; 4, healthy but larvae static; 5, healthy and larvae swimming; median for 6–10 larvae in each of the triplicate wells.](/cms/asset/1b0f4b7a-ffa8-4b54-8a52-1f0a1d2c8fb9/tnzm_a_616210_o_f0003g.gif)
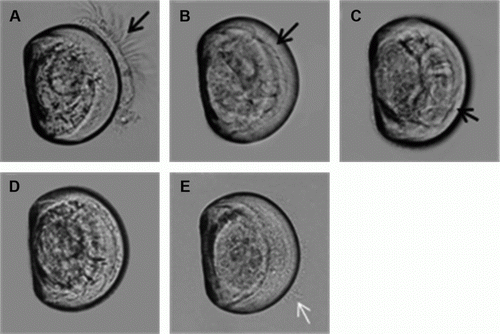
Karenia brevisulcata at a cell density of 84×106 cells L–1 severely affected Greenshell™ mussel, Pacific oyster, sea urchin, paua and sea slug larvae (, B–F) with sea urchin being the most sensitive and Pacific oyster the least sensitive. Karenia brevisulcata at 8.4×106 cells L–1 resulted in complete mortality of paua after 24 h (F), and almost complete mortality of sea urchin within 24 h (E) but there were no apparent effects on Pacific oyster (C) or sea slug (D). Greenshell™ mussel larvae did not appear to be affected by K. brevisulcata at this cell concentration after 0–1 h exposure to (health score 5, B; Score 4, A) but the larvae became stressed after 1–2 h (health score 3, B). At 2 h, most larvae retracted into their shell with cilia moving (health score 2, C), but some began disintegrating (health score 1, D). At 4 h, all larvae were moribund as identified by larval disintegration and damage to cilia (D). At 8 hr, larval death began to occur, and all larvae were dead by 24 h (health score 0, E). No symptoms were observed in any of the species after 24 h exposure to 0.84×106 cells L–1 ().
Figure 4 GreenshellTM mussel (Perna canaliculus) larvae (six days old) affected by Karenia brevisulcata during exposure over 24 h to 8.4 × 106 cells L–1. A, Healthy and static, score 4. B, Stressed, the velum started to disintegrate, and cilia withdrawn but healthy and moving, score 3. C, Severely stressed, larvae retracted into shell and cilia moving inside shell, score 2. D, Moribund, cilia gone, larvae disintegrating, still some jerking movement, score 1. E, Dead, disintegrated larvae appeared amorphous, decayed and jelly-like, and cells were being discharged from the dead animal, score 0.
Notes: Black arrow in A–C shows cilia and white arrow in E shows leaked tissue.
The analysis of the time-course data provided LT50 data (), which quantify the aforementioned observations at certain cell concentrations. In some cases the curve fitting was not possible owing to the health scores being mainly at the less useful extremes (0 or 5). In these cases estimates were made to provide approximate values (error±50%). It was not possible to derive accurate LC50 values by established data-fitting procedures because generally only three cell concentrations for each algal species were tested. However, the data were adequate to estimate approximate LC50 () for exposure times of 2 and 24 h. These toxicity measures confirmed that GreenshellTM mussel, sea urchin and paua larvae were severely affected by K. brevisulcata with 24 h LC50 of approximately 2×106 cells L–1. Pacific oyster and sea slug larvae were less affected with 24 h LC50 of approximately 10×106 cells L–1. Paua, but not other species, was similarly severely affected by K. mikimotoi (24 h LC50 of approximately 2×106 cells L–1).
Table 3 Toxicity of cultures of Karenia species to invertebrate larvae.
Toxicity of BSXs and SPE extracts of Karenia cultures against Greenshell™ mussel larvae
All larvae survived for 24 h with control extract, as did those challenged with SPE extracts of K. mikimotoi and K. brevis. The K. brevis extract (resuspended in seawater) contained high concentrations of BTXs (total 255 µg L–1 as determined by LC-MS) but no harmful effects were observed on the mussel larvae. Exposure of GreenshellTM mussel larvae to the SPE extract of a K. brevisulcata bulk culture (72×106 cells L–1 29 days old; total BSXs 31 µg L–1) gave a consistent increase in the severity of adverse symptoms with time (). The larvae exhibited stress after 2 h exposure to the extract. At 8 h, all larvae were moribund and at 24 h most were dead. The response curve was very similar to that for an older K. brevisulcata culture (56 days old; total BSXs 28 µg L–1), where motile, dividing cells had disappeared (). Toxicities of pure BSX toxins against GreenshellTM mussel larvae were also evaluated. No acute symptoms were observed following exposure to the four individual BSX toxins at concentrations in seawater of up to 100 µg L–1 ().
Figure 5 Responses of GreenshellTM mussel (Perna canaliculus) larvae to a 56-day K. brevisulcata mass culture without living cells □ and solid phase extraction extracts of 20–30-day Karenia mass cultures: K. mikimotoi (× [85 × 106 cells L–1]), K. brevis (□ [9 × 106 cells L–1]) and K. brevisulcata (♦ [72 × 106 cells L–1]).
Note: Points and error bars depict the mean ± SEM (n = 3).
![Figure 5 Responses of GreenshellTM mussel (Perna canaliculus) larvae to a 56-day K. brevisulcata mass culture without living cells □ and solid phase extraction extracts of 20–30-day Karenia mass cultures: K. mikimotoi (× [85 × 106 cells L–1]), K. brevis (□ [9 × 106 cells L–1]) and K. brevisulcata (♦ [72 × 106 cells L–1]). Note: Points and error bars depict the mean ± SEM (n = 3).](/cms/asset/5b3b2534-22ba-46a9-b38c-0d7ee142c560/tnzm_a_616210_o_f0005g.gif)
Discussion
HABs caused by dinoflagellates in the genus Karenia are increasing in frequency, intensity, duration and geographic distribution and are often associated with mass mortalities of marine vertebrates and invertebrates (Wear & Gardner Citation2001; Landsberg Citation2002; Magaña et al. Citation2003; Gentien et al. Citation2007). The toxic effects of Karenia vary depending on the species, strains, growth stage, test organisms and exposure conditions. Often it has been unclear which particular toxins have been present and what mode of action led to the observed effects in exposed organisms. In the present study, effects of three Karenia species on two commercial fish species and the larvae of six representative coastal invertebrate species underwent toxicity investigations. Karenia brevisulcata and K. mikimotoi have similarities to K. brevis in morphology (Haywood et al. Citation2004), but our LC-MS operated in SIR mode did not detect BTXs in cultures of the former two species, confirming previous data for New Zealand strains (McNabb et al. Citation2006). The LODs for BTXs were 0.075 ng mL–1 algal culture, similar to those reported for seawater (Backer et al. Citation2005).
Karenia mikimotoi caused no short-term effects on snapper at a concentration of 3.4×106 cells L–1, although this is at the higher end of concentrations reported for a fish-killing K. mikimotoi blooms in Ireland and New Zealand (0.5–4×106 cells L–1) (Silke et al. Citation2005; Smith et al. Citation2007). In a recent study of Kareniaceae ichthyotoxicity, live or lysed cells of K. mikimotoi (up to 10×106 cells L–1) were also found to be non-toxic to sheepshead minnow larvae (Cyprinodon variegatus) (Mooney et al. Citation2010). Karenia mikimotoi can produce several potentially toxic compounds, including gymnocins, haemolysins and reactive oxygen species (ROS) (Yasumoto et al. Citation1990; Satake et al. Citation2002; Haywood et al. Citation2004; Munday et al. Citation2004; Yamasaki et al. Citation2004; Satake et al. Citation2005). It is a cosmopolitan species and there may be a range of sub-species with varying toxicities and modes of action (Zou et al. Citation2010). The strain of K. mikimotoi used in the present study was isolated from the northern coast of New Zealand and is maintained in the CICCM. It is probably a gymnocin producer (A. Selwood, pers. comm.), but the levels, mode of action and uptake by marine vertebrates of gymnocins are still unclear. The cytotoxicity P388 cell bioassays showed that gymnocins were not highly cytotoxic (Satake et al. Citation2002; Satake et al. Citation2005). Yamasaki et al. (Citation2004) reported that K. mikimotoi isolated from Western Japan can produce ROS, similar to raphidophyceae flagellates involved in fish kills e.g. Chattonella marina and Heterosigma akashiwo (Yang et al. Citation1995; Ishimatsu et al. Citation1996; Oda et al. Citation1997). Zou et al. (Citation2010) indicated that ROS production depended on the K. mikimotoi strain and two other Japanese strains did not generate ROS. In the present study, we did not investigate ROS and haemolysins. Hallegraeff (Citation1995) classified K. mikimotoi as a red tide causative species that was non-toxic to humans, but harmful to fish and invertebrates through a gill-damage mechanism. However, we did not observe significant fish gill damage in snapper after 48 h exposure to K. mikimotoi. These results support the toxicity mechanism proposed in some previous studies: fish mortality in K. mikmotoi HABs is mainly because of environmental stressors resulting from high cell densities, such as depletion of dissolved oxygen (Landsberg Citation2002; Yamasaki et al. Citation2004; Silke et al. Citation2005), although roles for toxic agents have not been ruled out.
Karenia brevis and K. brevisulcata were both toxic to juvenile snapper and salmon. Karenia brevis is the representative red-tide causative dinoflagellate and has been responsible for many mass mortalities of fish over the decades (Magaña et al. Citation2003). HAB events involving this alga have been widespread and have affected hundreds of species of marine vertebrates and invertebrates (Landsberg Citation2002). Quick and Henderson (Citation1974) suggested exposure of K. brevis at 0.005–0.25×106 cells L–1 for days or weeks usually can trigger fish morbidities. In our study, salmon were killed in less than one hour after exposure to similar concentrations (0.18×106 cells L–1, ). The sensitivities of snapper and salmon to K. brevis were different. Exposure of juvenile fish to 4.6–5.9×106 cells L–1 resulted in LT50 of 4.5 h for snapper and 0.17 h for salmon. Salmon, therefore, seem to be more sensitive than snapper to K. brevis. The signs of intoxication observed in snapper and salmon, including panic and lose of balance, were similar to those reported previously for fish exposed to K. brevis (Baden Citation1989; Landsberg Citation2002). BTXs produced by K. brevis are potent ichthyotoxins (Baden Citation1989) and, as with mammals, the toxicity is strongly associated with binding to voltage-gated sodium channels leading to uncontrolled sodium flux and disruption of neuronal transmission. The toxic mechanism of K. brevis in fish is believed to relate to BTXs from cells or water being absorbed directly across the gill membranes of fish. Type A BTXs such as BTX-1 are more potent than Type B BTXs (BTX-2, BTX-3) (Baden Citation1989; Landsberg Citation2002; Abraham et al. Citation2006). Lethal concentrations for 50% of fish exposed for 24 h were 3–4 nM for BTX-1, 16–25 nM for BTX-2 and 10–37 nM for BTX-3 (Baden Citation1989). The concentration of BTXs in our fish bioassays with K. brevis ranged from 14–102 nM (0.1–2.0 nM BTX-1, 14–95 nM BTX-2, 0.1–4.0 nM BTX-3). The high concentrations of BTX-2 point to a major role for this toxin in our reported effects of K. brevis on fish and invertebrates.
The only reported K. brevisulcata bloom occurred in New Zealand during the austral summer of 1998 along the southeast coast of the North Island and in Wellington Harbour. Cell concentrations reached 33×106 cells L–1 and this red tide event severely impacted on the local marine ecosystem with mass mortalities of vertebrates and invertebrates (Chang Citation1999b; Wear & Gardner Citation2001; Gardner & Wear Citation2006; Kröger et al. Citation2006). In the in vivo bioassays with K. brevisulcata at c. 11–16×106 cells L–1 the LT50 was 7.5 and 0.5 h with juvenile snapper and salmon respectively. Both fish species showed similar signs of intoxication to those observed in the K. brevis bioassays. In the fish bioassays, only snapper exposed to K. brevisulcata for 7.5–10.5 h had minor disintegration of the gill filaments. The lack of observable gill damage in salmon may be because of the shorter experimental period (< 1 h). Fish gill damage by K. brevisulcata is not likely to be a major toxic mechanism leading to fish morbidity. While longer exposures of fish to lower cell concentrations would be of interest, bioethical considerations preclude such experiments.
Karenia brevisulcata produces two classes of novel complex polycyclic ether biotoxins: KBTs and BSX toxins (Holland et al. Citation2012). An LC-MS method was validated to detect and quantify four BSXs (B) in K. brevisulcata cultures which were dominated by the lactone ring-opened hydrolysis products BSX-1 and BSX-2 (B). In contrast, K. brevis cultures produced mainly the lactone ring-closed BTXs with only c. 10–20% of the ring-opened hydrolysis products (Abraham et al. Citation2006). The toxin productivity was also higher for K. brevis (52 pg cell–1) than K. brevisulcata (0.5 pg cell–1). It is probable that BSX-4 and BSX-5 are the parent toxins produced by K. brevisulcata and the hypothesis is that this species is more leaky or fragile than K. brevis, leading to higher toxin levels in the medium where hydrolysis of the lactone ring takes place (Holland et al. Citation2012). Ring-opened BTXs were much less potent agonists of sodium channels than their parent BTXs in receptor binding and cytotoxicity bioassays (Roth et al. Citation2007). Neuro-2A cytotoxicity assays reveal BSX-4 and BSX-5 have BTX-like sodium channel activity but at lower levels than BTX-2, and even more so for the predominant BSX-1 and BSX-2 (lactone ring-opened forms). The BSXs are also only weakly haemolytic (Holland et al. 2012). KBTs are 50–100-fold more potent in mouse bioassays (intraperitoneal administration) than BSXs and are also cytotoxic and haemolytic (Holland et al. 2012). These high molecular weight toxins are more difficult to monitor in cultures. However, some preliminary analytical data showed that concentrations of KBT-F and KBT-G in K. brevisulcata cultures were of similar order of magnitude to those of BSX-1 (Holland et al. 2012). The main contributors to toxicity in these bioassays and to fish kills in the Wellington Harbour bloom event may have been KBTs rather than BSXs.
Brine shrimp were unaffected by any of the three Karenia species tested. This confirms the data reported by Botes et al. (Citation2003) where A. salinas larvae were unaffected by exposure to a range of toxic dinoflagellates including K. brevis, K. mikimotoi and K. cristata (<10% mortality at 24 h for cell concentrations 1–10×106 cells L–1). This hardy organism, commonly used for ecotoxicology assessments, may not be sensitive to many algal toxins. However, seawater collected during the K. brevisulcata bloom event in 1998 did show quite strong effects on A. salinas (F.H. Chang, pers. comm.). It cannot be ruled out that the toxin profile has changed or the toxin quota has reduced in the strain of K. brevisulcata maintained by sub-culturing in the CICCM over the decade since it was collected.
No acute effects of K. brevis or K. mikimotoi were observed for the two bivalve mollusc species tested. However, Greenshell™ mussel larvae (five to six days old) gave a very consistent increase in the severity of symptoms over 24 h when exposed to K. brevisulcata with a well concentration of 8.4×106 cells L–1 (B). Pacific oyster larvae were less sensitive to K. brevisulcata, but still exhibited severe symptoms at high cell densities. The main reported effect of HABs on shellfish is reduced feeding, with mortality generally only occurring at very high cell densities where anoxia and other physical mechanisms may predominate. Shellfish appear well adapted to the common algal toxins and can accumulate them to levels that are harmful to predatory vertebrates, including humans. Therefore, the high toxicity of K. brevisulcata is unusual. The similar effects on mussel larvae of the lipohilic SPE extract of a younger mass culture (29 days old, equivalent well concentration 72×106 cells L–1) and an old K. brevisulacata mass culture (56 days old, living cell free) confirm that the toxic agents against invertebrates can be extracted from water and cells by absorption to polymeric material and that these toxins are still present in the medium after cells decay. BSXs were at similar concentrations in the older cell free culture and the SPE extract of a live culture (28 µg L–1 versus 31 µg L–1). However, no acute effects on Greenshell™ mussel larvae were observed following exposure to the four individual BSX toxins at a dose of 100 µg L–1. KBTs are also recovered from mass cultures by the SPE method (Holland et al. 2012). From their higher acute toxicity to mice and mammalian cells it is concluded that KBT toxins are likely to be the main toxic agents against invertebrates following exposure to K. brevisulcata. KBTs may also leak from into seawater medium from dead or damaged K. brevisulcata cells, similar to BSX toxins.
Paua larvae were also very sensitive to K. brevisulcata, supporting observations at the time of the 1998 bloom (Chang Citation1999a). Paua larvae were also the only invertebrate tested that was sensitive to K. mikimotoi (24 h LC50 of 2×106 cells L–1). Effects on paua were observed at a hatchery in Northland during a major bloom of K. mikimotoi in 2002 (F.H. Chang, pers. comm.). Botes et al. (2003) have reported bioassay data (24 h) for Haloitis midae larvae where K. mikimotoi gave a LC50 of 1.1×106 cells L–1 and K. brevis gave < 10% mortality at the highest concentration tested (< 10×106 cells L–1). These data for South African abalone larvae match our data for the New Zealand species. The South African study also included abalone spat, and this growth stage was very sensitive to all the Karenia species tested (Botes et al. 2003). There is increasing cultivation of abalone species world-wide and blooms of these Karenia species are, therefore, a threat to abalone aquaculture industries.
A K. breviusulcata bloom occurring near commercial aquaculture farms may directly cause unacceptable economic losses. For K. brevis, the early warning trigger for potential shellfish contamination and closure of growing areas for harvesting is when cell concentrations reach 5000 cells L–1 (Haywood et al. Citation2007). A trigger for K. brevisulcata is also required for bivalve mollusc farms and cage fisheries to provide early warning of potential damage from blooms of this unusual Karenia spp. Although accumulation of toxins in seafood from this species has not been established, the present data indicate that direct toxicity may be more of an issue, especially for juvenile salmon, shellfish and paua larvae. From the data gathered in the present study, a provisional trigger of 10,000 cells L–1 is recommended.
The present study confirms observations during bloom events that Karenia species are toxic to marine biota and that the toxicity is different between both the algal species and test organisms. Karenia brevisulcata has broad toxicity to fish and marine invertebrates and data is provided on the cell densities where harmful effects become pronounced. This organism is a very significant threat to coastal marine ecosystems and to aquaculture, with objective risks that are only lessened by the historical infrequency of blooms. The mechanisms of action for K. brevisulcata and its novel toxins against marine organisms will require further research.
Acknowledgements
Thanks to Kevin Heasman, Rod Asher, Tim Dodgshun and Aditya Kesarcodi-Watson for expert handling of fish; Ellie Watts, Bridget Alexander and Achim Janke for providing invertebrate larvae; and Veronica Beuzenberg and Krystyna Ponikla for assistance with algal cultures. Hoe Chang, NIWA, and Masayuki Satake, the University of Tokyo, provided expert advice and toxin standards. The study was supported by the NZ Foundation for Research Science & Technology, Contracts CAWX0703 and CAWX0804 (IIOF-Japan).
References
- Abraham , A , Plakas , SM , Wang , ZH , Jester , ELE , El Said , KR , Granade , HR , Henry , MS , Blum , PC , Pierce , RH and Dickey , RW . 2006 . Characterization of polar brevetoxin derivatives isolated from Karenia brevis cultures and natural blooms . Toxicon , 48 : 104 – 115 .
- Backer , LC , Kirkpatrick , B , Fleming , LE , Cheng , YS , Pierce , R , Bean , JA , Clark , R , Johnson , D , Wanner , A and Tamer , R . 2005 . Occupational exposure to aerosolized brevetoxins during Florida red tide events: effects on a healthy worker population . Environmental Health Perspectives , 113 : 644
- Baden , DG . 1989 . Brevetoxins: unique polyether dinoflagellate toxins . FASEB Journal , 3 : 1807 – 1817 .
- Beuzenberg V Mountfort D Holland PT Shi F MacKenzie AL 2011 . Optimization of growth and production of toxins by three dinoflagellates in photobioreactor cultures . Journal of Applied Phycology , published online , doi: 10.1007/s10811-011-9726-8.
- Botes , L , Smit , AJ and Cook , PA . 2003 . The potential threat of algal blooms to the abalone (Haliotis midae) mariculture industry situated around the South African coast . Harmful Algae , 2 : 247 – 259 .
- Chang FH 1995 . First records of Gymnodinium sp. nov. (cf. breve) (Dinophyceae) and other harmful phytoplankton species in the early 1993 blooms in New Zealand . Lassus P Erard E Gentien P Marcaillou C In: Harmful marine algal blooms . Paris , Lavoisier Science Publishers . Pp. 27 – 32 .
- Chang , FH . 1999a . A new species of Gymnodinium that caused the 1998 summer human respiratory syndrome and decimation of marine life in Wellington Harbour, New Zealand . Harmful Algae News , 19 : 3 – 4 .
- Chang , FH . 1999b . Gymnodinium brevisulcatum sp. nov. (Gymnodiniales, Dinophyceae), a new species isolation from the 1998 summer toxic bloom in Wellington Harbour, New Zealand . Phycologia , 38 : 377 – 384 .
- Chang , FH , Chiswell , SM and Uddstrom , MJ . 2001 . Occurrence and distribution of Karenia brevisulcata (Dinophyceae) during the 1998 summer toxic outbreaks on the central east coast of New Zealand . Phycologia , 40 : 215 – 222 .
- Chang , FH , Zeldis , J , Gall , M and Hall , J . 2003 . Seasonal and spatial variation of phytoplankton assemblages, biomass and cell size from spring to summer across the north-eastern New Zealand continental shelf . Journal of Plankton Research , 25 : 737 – 758 .
- Chang FH , MacKenzie AL , Till D , Hannah DJ , Rhodes L 1995 . The first toxic shellfish outbreaks and the associated phytoplankton blooms in early 1993 in New Zealand . Lassus P Erard E Gentien P Marcaillou C In: Harmful marine algal blooms . Paris , Lavoisier Science Publishers . Pp. 145 – 150 .
- Cheng , YS , Zhou , Y , Irvin , CM , Pierce , RH , Naar , J , Backer , LC , Fleming , LE , Kirkpatrick , B and Baden , DG . 2005 . Characterization of marine aerosol for assessment of human exposure to brevetoxins . Environmental Health Perspectives , 113 : 638 – 643 .
- Davidson , K , Miller , P , Wilding , TA , Shutler , J , Bresnan , E , Kennington , K and Swan , S . 2009 . A large and prolonged bloom of Karenia mikimotoi in Scottish waters in 2006 . Harmful Algae , 8 : 349 – 361 .
- Fleming , LE , Backer , LC and Baden , DG . 2005 . Overview of aerosolized Florida red tide toxins: exposures and effects . Environmental Health Perspectives , 113 : 618 – 620 .
- Gardner , JPA and Wear , RG . 2006 . Changes in subtidal macroinvertebrate community structure in Wellington Harbour (New Zealand) following a large-scale natural die-off . New Zealand Journal of Marine and Freshwater Research , 40 : 29 – 42 .
- Gentien , P , Lunven , M , Lazure , P , Youenou , A and Crassous , MP . 2007 . Motility and autotoxicity in Karenia mikimotoi (Dinophyceae) . Philosophical Transactions of the Royal Society B: Biological Sciences , 362 : 1937 – 1946 .
- Guillard , RRL and Hargraves , PE . 1993 . Stichochris immobilis is a diatom, not a chrysophyte . Phycologia , 32 : 234 – 236 .
- Hallegraeff GM 1995 . Harmful algal blooms: a global overview . Hallegraeff GM Adamson DM Cembella AD In: Manual on harmful marine microalgae . Paris , UNESCO . Pp. 1 – 22 .
- Haywood , AJ , Scholin , CA , Marin , R , Steidinger , KA , Heil , C and Ray , J . 2007 . Molecular detection of the brevetoxin-producing dinoflagellate Karenia brevis and closely related species using rRNA-targeted probes and a semiautomated sandwich hybridization assay . Journal of Phycology , 43 : 1271 – 1286 .
- Haywood , AJ , Steidinger , KA , Truby , EW , Bergquist , PR , Bergquist , PL , Adamson , J and MacKenzie , L . 2004 . Comparative morphology and molecular phylogenetic analysis of three new species of the genus Karenia (Dinophyceae) from New Zealand . Journal of Phycology , 40 : 165 – 179 .
- Holland PT Shi F Satake M Beuzenberg V McNabb P Munday R Truman P 2012 . Novel toxins produced by the dinoflagellate Karenia brevisulcata . Harmful Algae 13 : 47 – 57
- Honjo , T . 1995 . The biology and prediction of representative red tides associated with fish kills in Japan . Japanese Reviews of Fisheries Science , 2 : 65 – 104 .
- International Council for the Exploration of the Sea 2006 . Report of the ICES-IOC Working Group on Harmful Algal Bloom Dynamics (WGHABD) . ICES CM 2006/OCC:04. 47 p.
- Ishida , H , Nozawa , A , Totoribe , K , Muramatsu , N , Nukaya , H , Tsuji , K , Yamaguchi , K , Yasumoto , T , Kaspar , H and Berkett , N . 1995 . Brevetoxin B1, a new polyether marine toxin from the New Zealand shellfish, Austrovenus stutchburyi . Tetrahedron Letters , 36 : 725 – 728 .
- Ishimatsu , A , Oda , T , Yoshida , M and Ozaki , M . 1996 . Oxygen radicals are probably involved in the mortality of yellowtail by Chattonella marina . Fish Science , 62 : 836 – 837 .
- Jasperse JA 1993 . Marine toxins and New Zealand shellfish. Proceedings of a workshop on research issues, 10–11 June 1993 . The Royal Society of New Zealand Miscellaneous series 24 . Wellington , New Zealand , The Royal Society of New Zealand . 68 p.
- Kröger , K , Gardner , JPA , Rowden , AA and Wear , RG . 2006 . Long-term effects of a toxic algal bloom on subtidal soft-sediment macroinvertebrate communities in Wellington Harbour, New Zealand . Estuarine, Coastal and Shelf Science , 67 : 589 – 604 .
- Landsberg , JH . 2002 . The effects of harmful algal blooms on aquatic organisms . Reviews in Fisheries Science , 10 : 113 – 390 .
- Loeblich , AR . 1975 . A seawater medium for dinoflagellates and the nutrition of Cachonina Niei . Journal of Phycology , 11 : 80 – 86 .
- Loeblich , AR and Smith , VE . 1968 . Chloroplast pigments of the marine dinoflagellate Gyrodinium resplendens . Lipids , 3 : 5 – 13 .
- MacKenzie L 2008 . The ecobiology of the brevetoxin, ciguatoxin and cyclic imine producers . Botana LM In: Seafood and freshwater toxins: pharmacology, physiology and detection. 2nd edition . Boca Raton , FL , CRC Press . Pp. 433 – 467 .
- MacKenzie L , Rhodes LL , Till D , Chang FH , Kaspar H , Haywood A , Kapa J , Walker B 1995 . A Gymnodinium sp. bloom and the contamination of shellfish with lipid soluble toxins in New Zealand, Jan–April 1993 . Lassus P Erard E Gentien P Marcaillou C In: Harmful marine algal blooms . Paris , Lavoisier Science Publishers . Pp. 795 – 800 .
- Magaña , HA , Contreras , C and Villareal , TA . 2003 . A historical assessment of Karenia brevis in the western Gulf of Mexico . Harmful Algae , 2 : 163 – 171 .
- Marshall , JA , Ross , T , Pyecroft , S and Hallegraeff , G . 2005 . Superoxide production by marine microalgae: II. Towards understanding ecological consequences and possible functions . Marine Biology , 147 : 541 – 549 .
- McNabb , P , Rhodes , L , Adamson , J and Holland , P . 2006 . Brevetoxin—an elusive toxin in New Zealand waters . African Journal of Marine Science , 28 : 375 – 377 .
- Mooney BD , Hallegraeff GM , Place AR 2010 Ichthyotoxicity of four species of gymnodinioid dinoflagellates (Kareniaceae, Dinophyta) and purified karlotoxins to larval sheepshead minnow . Harmful Algae 9 : 557 – 562 .
- Morohashi , A , Satake , M , Naoki , H , Kaspar , HF , Oshima , Y and Yasumoto , T . 1999 . Brevetoxin B4 isolated from greenshell mussels Perna canaliculus, the major toxin involved in neurotoxic shellfish poisoning in New Zealand . Natural Toxins , 7 : 45 – 48 .
- Munday , R , Towers , NR , Mackenzie , L , Beuzenberg , V , Holland , PT and Miles , CO . 2004 . Acute toxicity of gymnodimine to mice . Toxicon , 44 : 173 – 178 .
- Murata , K , Satake , M , Naoki , H , Kaspar , HF and Yasumoto , T . 1998 . Isolation and structure of a new brevetoxin analog, brevetoxin B2, from Greenshell mussels from New Zealand . Tetrahedron , 54 : 735 – 742 .
- Oda , T , Nakamura , A , Shikayama , M , Kawano , I , Ishimatsu , A and Muramatsu , T . 1997 . Generation of reactive oxygen species by raphidophycean phytoplankton. Bioscience, Biotechnology . Biochemistry , 61 : 1658 – 1662 .
- Quick JA , Henderson GE 1974 . Effects of Gymnodinium breve red tide on fishes and birds: a preliminary report on behavior, anatomy, hematology and histopathology . Amborski RL Hood MA Miller RR In: Proceedings of the Gulf Coast Regional Symposium on Diseases of Aquatic Animals ,, Louisiana Sea Grant, Louisiana State University 16–17 August , . Pp. 85 – 113 .
- Roth , PB , Twiner , MJ , Wang , Z , Bottein Dechraoui , M-Y and Doucette , GJ . 2007 . Fate and distribution of brevetoxin (PbTx) following lysis of Karenia brevis by algicidal bacteria, including analysis of open A-ring derivatives . Toxicon , 50 : 1175 – 1191 .
- Satake , M , Shoji , M , Oshima , Y , Naoki , H , Fujita , T and Yasumoto , T . 2002 . Gymnocin-A, a cytotoxic polyether from the notorious red tide dinoflagellate, Gymnodinium mikimotoi . Tetrahedron Letters , 43 : 5829 – 5832 .
- Satake , M , Tanaka , Y , Ishikura , Y , Oshima , Y , Naoki , H and Yasumoto , T . 2005 . Gymnocin-B with the largest contiguous polyether rings from the red tide dinoflagellate, Karenia (formerly Gymnodinium) mikimotoi . Tetrahedron Letters , 46 : 3537 – 3540 .
- Silke J , O'Beirn F , Cronin M 2005. Karenia mikimotoi: an exceptional dinoflagellate bloom in western Irish waters, summer 2005 . Marine Environment and Health Series No. 21. 48 p .
- Smith KF , Rhodes LL , Marfell MJ , Zeewoldt CM , De Salas MF , Haywood AJ , Scholin CA 2007 . Massive Karenia mikimotoi bloom in Northland, New Zealand: use of traditional and molecular techniques for rapid identification of HAB species . Harmful Algae News : 1 – 3 .
- Steidinger , KA . 2009 . Historical perspective on Karenia brevis red tide research in the Gulf of Mexico . Harmful Algae , 8 : 549 – 561 .
- Steidinger KA , Vargo GA , Tester PA , Tomas CR 1998 . Bloom dynamics and physiology of Gymnodinium breve with emphasis on the Gulf of Mexico . In: Anderson DM Cembella AD Hallegraeff GM Physiological ecology of harmful algae blooms . >Berlin , Springer . Pp. 133 – 153 .
- Truman , P . 2007 . A cellular target for the lipophilic toxins from Karenia brevisulcata . Toxicon , 50 : 251 – 255 .
- Truman , P , Keyzers , RA , Northcote , PT , Ambrose , V , Redshaw , NA and Chang , HF . 2005 . Lipophilic toxicity from the marine dinoflagellate Karenia brevisulcata: use of the brevetoxin neuroblastoma assay to assess toxin presence and concentration . Toxicon , 46 : 441 – 445 .
- Ulrich , RM , Casper , ET , Campbell , L , Richardson , B , Heil , CA and Paul , JH . 2010 . Detection and quantification of Karenia mikimotoi using real-time nucleic acid sequence-based amplification with internal control RNA (IC-NASBA) . Harmful Algae , 9 : 116 – 122 .
- Wear , RG and Gardner , JPA . 2001 . Biological effects of the toxic algal bloom of February and March 1998 on the benthos of Wellington Harbour, New Zealand . Marine Ecology Progress Series , 218 : 63 – 76 .
- Yamasaki , Y , Kim , D-I , Matsuyama , Y , Oda , T and Honjo , T . 2004 . Production of superoxide anion and hydrogen peroxide by the red tide dinoflagellate Karenia mikimotoi . Journal of Bioscience and Bioengineering , 97 : 212 – 215 .
- Yang , CZ , Albright , LJ and Yousif , AN . 1995 . Oxygen-radical-mediated effects of the toxic phytoplankter Heterosigma carterae on juvenile rainbow trout Oncorhynchus mykiss . Disease of Aquatic Organisms , 23 : 101 – 108 .
- Yasumoto T Undersal B Aune T Hormazabal V Skulberg OM Oshima Y 1990 . Screening for hemolytic and ichthyotoxic components of Chrysochromulina polylepis and Gyodinium aurolum from Norwegian coastal waters . In : Granéli E Sundström B Edler L Anderson DM Toxic marine phytoplankton . New York , Elsevier . Pp. 436 – 440 .
- Zou , Y , Yamasaki , Y , Matsuyama , Y , Yamaguchi , K , Honjo , T and Oda , T . 2010 . Possible involvement of hemolytic activity in the contact-dependent lethal effects of the dinoflagellate Karenia mikimotoi on the rotifer Brachionus plicatilis . Harmful Algae , 9 : 367 – 373 .