Abstract
Samples of the kelp Durvillaea antarctica were placed in mesh bags on the upper shore of a southern New Zealand exposed sandy beach and monitored over 30 days to examine their decomposition and macrofaunal colonisation. Amphipoda, Staphylinidae, other Coleoptera and Diptera made up 99.9% of total macrofaunal abundance and biomass of the colonising fauna. Amphipoda and Staphylinidae abundance differed significantly between days of exposure. All main taxonomic groups, except other Coleoptera, differed in biomass between days of exposure and between seasons, with greater biomass in autumn than winter. Decomposition rate and moisture content of D. antarctica differed significantly between days of exposure and between seasons, whereas carbon content only differed significantly over time. Correlations between macrofaunal abundance and the decomposition rate, and total macrofaunal abundance and the C:N ratio, suggest that the macrofaunal community prefers older wrack, despite it being lower in nitrogen content then fresh kelp.
Introduction
Wave-exposed sandy beaches are dynamic ecosystems occurring from temperate to tropical latitudes (Rodil et al. Citation2007; Gonçalves et al. Citation2009). In temperate regions, food webs of exposed sandy beaches are supported by marine subsidies, such as stranded macrophytes (wrack), as little primary productivity occurs within the beach itself (Brown & McLachlan Citation1990; Colombini & Chelazzi Citation2003; Dugan et al. Citation2003; Lastra et al. Citation2008). Nutrients, such as carbon, nitrogen and phosphorus, are released to the surrounding sediments as macroalgae decomposes (Hanisak Citation1993). The changes in organic matter reflect the changes occurring to the carbon and nitrogen content during decomposition. For example, carbon content may decrease through respiration by microorganisms (Fourqurean & Schrlau Citation2003), while nitrogen compounds can be exported or imported, resulting in the C:N ratio in decaying seaweed to either decrease or increase over time (Rice & Tenore Citation1981; Pennings et al. Citation2000). The response to this nutrient enrichment of the sediment is species specific, with some taxa showing a positive response (Posey et al. Citation1999; Bolam et al. Citation2000; Rossi & Underwood Citation2002; Olabarria et al. Citation2010), whereas other species exhibit no significant change in abundance to such nutrient enrichment (Bolam et al. Citation2000; Rossi & Underwood Citation2002; Olabarria et al. Citation2010). Variations in the responses of taxa to the addition of wrack may be attributed to the different ways that wrack is utilised among taxa, i.e. as food and/or shelter, the sediment type and whether the wrack is on the sediment surface or buried (Rossi & Underwood Citation2002). For example, kelp that is buried will provide food for infauna (Rossi Citation2006), but will not be directly available to surface or near-surface grazers (Rossi Citation2007) such as talitrid amphipods (Inglis Citation1989). Wrack is the main food source for a range of invertebrates throughout their life cycle (Ince et al. Citation2007; Olabarria et al. Citation2009). For instance, on a south-western Australian sandy beach, Amphipoda, Diptera and Coleoptera fed on red and brown seaweeds, seagrasses and dune vegetation (Ince et al. Citation2007). Wrack also provides shelter against predators (Inglis Citation1989; Colombini & Chelazzi Citation2003) including birds, lizards, rodents and marine mammals (Polis et al. Citation1997; Hubbard & Dugan Citation2003). Processes are complex and variations in the responses of taxa to the addition of wrack may also be attributed to the amount and taxonomic composition of wrack, the origin of those species (native vs invasive), age of the wrack, buried wrack (anoxic conditions), and wrack defensive compounds (such as phenolic compounds).
The input of stranded macrophytes varies seasonally and between intertidal zones (Marsden Citation1991; Ochieng & Erftmeijer Citation1999; Dugan et al. Citation2003; Orr et al. Citation2005). Deposition rates vary due to the buoyancy characteristics of species that are stranded, the degree of exposure and the nearshore hydrodynamics (Orr et al. Citation2005). In New Zealand, Marsden (Citation1991) estimated an annual kelp input of <400 kg/m (average monthly input of 11.25 kg/5 m) (on Pegasus Bay, Canterbury), which is a low input compared with that found on South African beaches where the organic input has been found to exceed 2000 kg/m/year (Colombini & Chelazzi Citation2003).
Previous New Zealand research has focused mainly on the vertical distribution of species on the shore (MacIntyre Citation1963; Morton & Miller Citation1968; Marsden Citation1991; Stephenson Citation1998). There are two published studies carried out in Pegasus Bay (Canterbury, New Zealand), that focus on the spatial and temporal variation in macrofaunal communities that colonise and utilise stranded seaweed. Marsden (Citation1991) studied the composition and distribution of wrack up the shore, and the distribution of the talitrid sandhopper Bellorchestia quoyana as a response to substrate type (sand and macrophyte). A total of nine species of macrophyte were identified in the detritus on Pegasus Bay. Macrocystis pyrifera and D. antarctica were the two dominant wrack species, whereas the other seven macrophyte species constituted less than 16% of the total biomass. The amount of material stranded varied between seasons, and different species had variable distribution rates along the shore, with D. antarctica more abundant on the upper shore (Marsden Citation1991). Inglis (Citation1989) also focused on the upper shore and studied the colonisation and decomposition of stranded bladder kelp M. pyrifera on the upper shore over a period of 18 days.
Further research on the ecological role of stranded macrophytes on sandy shores is required, as there are implications for the associated fauna if stranded macrophytes are removed from beaches (Kirkman & Kendrick Citation1997). This study analysed the role of D. antarctica as a marine subsidy on exposed sandy shores over a 30-day period, using a field manipulation experimental design. The overall aims were (1) to quantify and analyse the colonisation of wrack by the macrofaunal community, and (2) to assess changes in the carbon and nitrogen content, and water content of the wrack (D. antarctica), during two sampling periods on the upper shore of an Otago exposed sandy beach. Durvillaea antarctica was chosen for this study due to its wide distribution and abundance (>80% of total macrophyte abundance and biomass) on the exposed shores of Otago (Hay Citation1994; Stevens et al. Citation2002; Garden & Smith Citation2011).
Methods
Study site
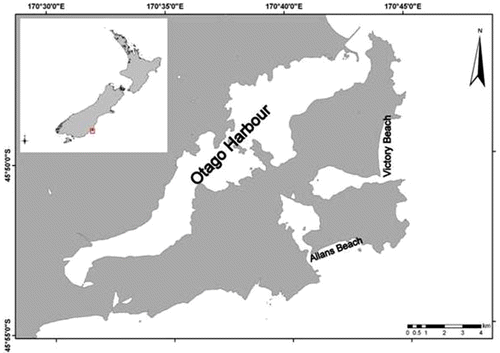
Victory Beach is a wave-exposed, east-facing sandy beach on the Otago Peninsula, on the east coast of the South Island, New Zealand (). The beach is 3.5 km long and backed by 2 km of sandflats and dunes (up to 10 m high) that run the length of the beach (Johnson Citation1992; Bishop & Hamel Citation1993; Wilson Citation2004).
Fieldwork
Attached, fresh D. antarctica was collected from a nearby rocky shore at Allans Beach, on the Otago Peninsula, one day prior to placing samples on Victory Beach (). Kelp was removed by prising the holdfast from the rock using a hammer and chisel. At the laboratory, the kelp was rinsed in seawater to remove any macrofauna before being blotted dry. Trellis mesh bags (37×38 cm) with 1×1-cm holes were filled with 1±0.1 kg wet weight D. antarctica fronds, and placed in a seawater holding tank until deployment in the field (within at least 12 h).
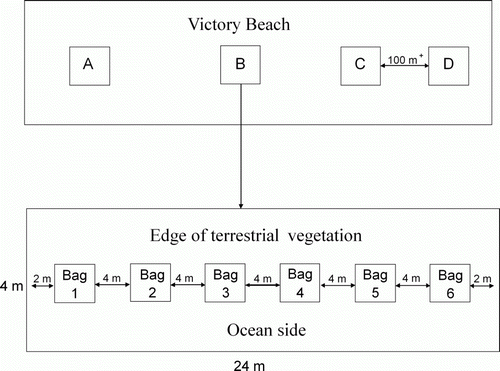
In late autumn (6 April to 5 May) and winter (21 June to 20 July) 2008, four sampling areas, each 24×4 m, were randomly selected across the entire length of Victory Beach but separated by a minimum of 100 m (). All areas were cleared of visible detritus prior to placing the mesh bags of D. antarctica. At each sampling area, six kelp samples were placed at 4-m intervals (to prevent interaction of macrofauna between kelp samples) along a 20-m transect that ran parallel to the upper shore (the edge of terrestrial vegetation). The mesh bags containing kelp were secured in place, using bamboo stakes and secured with cable ties. On day 1, 3, 6, 10, 20 and 30, kelp samples (n=4) were collected at random and were placed rapidly, along with any sand that was covering the kelp samples, into plastic bags and taken to the laboratory (see for sampling design). Some replicates were lost due to storms, resulting in a sample of three or less. The number of replicates per sampling event is indicated in the figure legends.
Figure 2 Study design showing the four sampling areas (A–D) along Victory Beach. Each area was separated by at least 100 m. Within each area, six mesh bags were placed at 4-m intervals along a 20-m transect. On sampling days 1, 3, 6, 10, 20 and 30, a bag was randomly selected from each sampling area and removed from the study site.
Laboratory analysis
Kelp samples were frozen at −20 °C for at least 48 h to euthanise associated macrofauna. Kelp samples were thawed for at least 2 h at 15 °C and sieved on a 1-mm mesh, along with any associated sand, to extract macrofauna. Macrofauna were preserved in 40% isopropanol (IPA), identified to the lowest taxonomic level possible and counted under a dissecting microscope. Macrofaunal samples were then rinsed in freshwater to remove excess IPA and oven dried at 60 °C for a minimum of 3 days, transferred to a desiccator for 1 h and weighed to the nearest 0.001 g to obtain dry weight.
Kelp samples were blotted dry, weighed (to the nearest 0.01 g), and placed in the drying oven at 60 °C for 5 days. The samples were then re-weighed to determine their moisture content. Decomposition rate was estimated by calculating the proportion of the initial weight remaining upon collection. To determine carbon and nitrogen content, dry kelp samples were ground to a fine powder. Subsamples of 2–3 mg of fine powder were analysed for percentage by dry weight of organic carbon and nitrogen content using a Carlo Erba Elemental Analyser EA 1108. The ratio of molar C:N for D. antarctica was then calculated.
Statistical analysis
Two-way ANOVAs (General Linear Model) were performed on the abundance and biomass of taxa to determine trends between sampling periods and days using SPSS 15 (IBM). The abundance and biomass of macrofaunal taxa were related to the percentage of original dry weight, percentage of moisture loss, the percentage of carbon and nitrogen, and the ratio of C:N of the wrack. Tukey's post hoc test was carried out to determine which days were significantly different in terms of the macrofaunal community, and Tamahane's post hoc test was used to determine which days were significantly different in terms of the percentage of kelp weight loss and moisture loss. To determine whether changes in the total macrofaunal abundance and biomass were influenced by changes in initial weight remaining, moisture content and the C:N ratio of D. antarctica, a multiple regression analysis (incorporating day as a factor for each analysis) was carried out using Minitab 14 (Minitab Inc.). Data were checked for homogeneity of variances using Levene's test, and log10(n+1) transformed when required. Species with an abundance of 10 or fewer individuals (over both sampling periods) were not included in the statistical analysis.
Results
Macrofaunal assemblages
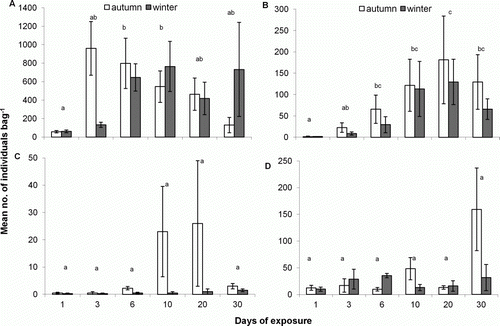
The macroinvertebrate community that colonised the transplanted samples of D. antarctica during autumn and winter comprised 20 taxa (identified to the lowest taxonomic level possible) (). The major taxa included Amphipoda, Staphylinidae, other Coleoptera and Diptera, which together constituted 99.9% of total macrofaunal abundance associated with the kelp samples (). Amphipoda were significantly more abundant than the three other taxa, whereas Staphylinidae and Diptera were both significantly more abundant than other Coleoptera (Tukey's test, P<0.01), but not significantly different in abundance to each other (Tukey's test, P>0.05).
Table 1 Total abundance (number and % of total community) and biomass (dry weight (g) and % of total community) of individual taxonomic groups associated with samples of Durvillaea antarctica on Victory Beach, Otago Peninsula
All four major taxa were present in the kelp after one day of exposure (). Subsequently, some taxa decreased in abundance, while others increased with days of exposure. For instance, amphipod abundance was lowest on day 1 compared with days 3 and 6 (Tukey's test, P<0.05) and then decreased after day 6 to day 30. In contrast, staphylinid abundance was highest on day 20 (Tukey's test, P<0.05). Diptera and other Coleoptera appeared more abundant after 10 days of sampling; however, there was no significant difference in abundance between days (ANOVA, P>0.05). There was no difference in abundance between autumn and winter for each of the four major taxa (two-way ANOVA, P>0.05) (). There was, however, a significant interaction effect between season and days for Amphipoda (F=2.66, P<0.05) and Diptera (F=2.60, P<0.05).
Figure 3 Mean number (±SE) of A, Amphipoda, B, Staphylinidae, C, other Coleoptera and D, Diptera (per kelp sample) over a period of 30 days during autumn (day 1–20, n=4; day 30, n=3) and winter (day 1–10, n=4; day 20, n=3; day 30, n=2) 2008, on Victory Beach. Significant differences between sampling days are denoted by dissimilar letters
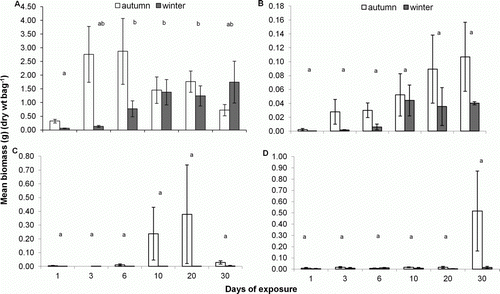
Similar trends were seen in the average biomass of the four major taxonomic groups, with the biomass of Staphylinidae, other Coleoptera and Diptera increasing with time of exposure, whereas amphipod biomass decreased over time in autumn (day 3, 2.76 g; day 30, 0.73 g) and increased over time in winter (day 1, 0.06 g; day 30, 1.75 g) (). The biomass of all four major taxa, except other Coleoptera, differed significantly between days of sampling and between autumn and winter, where biomass was on average greater in autumn than winter (two-way ANOVA, P<0.05) (). Amphipod biomass was lowest on day 1, which differed significantly from that of days 6, 10 and 20 (Tukey's test, P<0.05) (a). Staphylinid biomass increased after day 6, whereas dipteran biomass was constant throughout both sampling periods until day 30 (in autumn) when it reached its highest average biomass of 0.50 g (b and 4c). However, post hoc tests showed no significant differences between days for Staphylinidae and Diptera (Tamahane's test, P>0.05).
Figure 4 Mean biomass (g) (±SE) of A, Amphipoda, B, Staphylinidae, C, other Coleoptera and D, Diptera (per kelp sample) over a period of 30 days during autumn (day 1–20, n=4; day 30, n=3) and winter (day 1–10, n=4; day 20, n=3; day 30, n=2) 2008, on Victory Beach. Significant differences between sampling days are denoted by dissimilar letters
Kelp decomposition
Both the weight and moisture loss of D. antarctica differed significantly between autumn and winter and days of exposure (P<0.05). Decomposition was faster in autumn than winter, with the mean initial dry weight remaining ranging between 42% and 67% in autumn compared with 55–71% in winter (a). Similarly, the mean moisture content of kelp ranged between 57% and 66% in autumn and 71% and 81% in winter (b). The mean weight loss differed significantly between day 1 and day 3 of sampling in autumn (Tamahane's post hoc test, P=0.003). On day 3, the kelp samples had lost half of their initial weight (P<0.01) (a). The mean percentage of moisture content also decreased gradually, except on day 20, when the moisture content for both autumn (67%) and winter (81%) was higher than that for day 1 (66% in autumn and 74% in winter) (b). An ANOVA showed mean moisture content to differ significantly between days (F=4.82, P<0.01). Tamahane post hoc tests, however, did not detect differences (P>0.05).
Figure 5 Percentage (%) (±SE) of A, initial dry weight; B, initial moisture; and C, carbon content over a period of 30 days in autumn (day 1–20: n=4, day 30: n=3) and winter (day 1–10: n=4, day 20: n=3, day 30: n=2) on Victory Beach, on the Otago Peninsula.
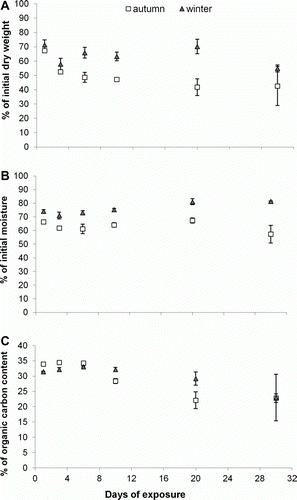
The mean percentage of organic carbon in D. antarctica samples ranged between 22.1% and 34.5% in autumn and 23.0% and 33.1% in winter. The mean percentage of nitrogen ranged between 0.78% and 1.14% in autumn and 0.84% and 1.26% in winter. There was no significant difference between seasons or days of exposure for mean percentage of nitrogen (P>0.05). Percentage of carbon did not vary between autumn and winter (F=0.78, P>0.05), but there was a significant decrease in the percentage of carbon with time of exposure (F=14.24, P<0.001) (c).
Relationships between macrofauna and kelp samples
Linear regression analyses showed that there was no significant relationship between moisture content and total macrofaunal abundance (R 2=0.00, P>0.05) or log biomass (R 2=0.045, P>0.05). Initial weight remaining, on the other hand, significantly influenced values of total macrofaunal log abundance (R 2=0.16, P=0.008) and macrofaunal log biomass (R 2=0.21, P<0.01) (). The following regression equations describe the log abundance for a given percentage of initial dry weight (see for the log biomass equation):
Figure 6 Relationship between the initial dry weight (%) of Durvillaea antarctica and the log macrofaunal biomass associated with samples on Victory Beach, during autumn and winter 2008.
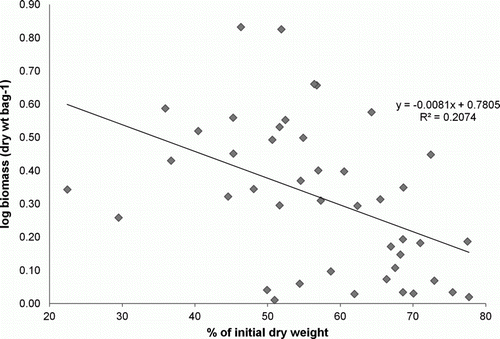
The regression equations indicate that log biomass and log macrofaunal abundance decreased with increasing initial weight of D. antarctica remaining. Both relationships are, however, weak as indicated by the low R 2 value of 16% for log abundance and 21% for log biomass (P<0.05) ().
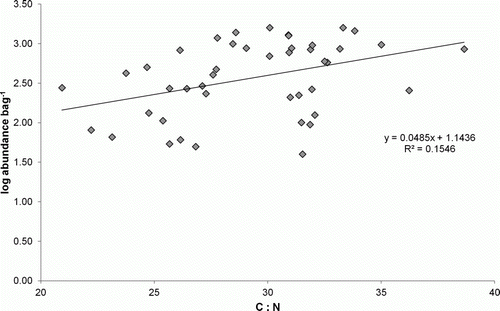
The C:N ratio of stranded D. antarctica did not differ significantly between seasons or days of exposure (ANOVA, P>0.05). Multiple regression showed, however, that total macrofaunal abundance was related to changes in C:N and the time the kelp had been stranded (R 2=0.44, P<0.05) but not by the time of year sampling took place (R 2=0.25, P>0.05). The total number of individuals associated with samples of stranded D. antarctica is given by the equation:
This relationship, however, is weak (R 2=0.44). The variable of interest for this study was the C:N of D. antarctica. depicts the linear relationship between C:N and the total macrofaunal abundance, where C:N increases with total macrofaunal abundance.
Figure 7 The relationship between the ratio of carbon to nitrogen and the log macrofaunal abundance associated with samples of Durvillaea antarctica on Victory Beach, over a 30-day period in autumn and winter 2008.
The total macrofauna log biomass associated with samples of D. antarctica was not influenced by changes in the C:N content of the kelp (R 2=0.37, P>0.05). It was, however, influenced by the time of year and the time during which the kelp had been stranded on the shore (R 2=0.37, P<0.05). The low R 2 value suggests that only 35% of the variance is explained by the regression equation (log biomass=0.026−0.170(season)+0.059(day)+0.004(C:N)).
Discussion
In temperate regions, food webs of exposed sandy shores are supported by marine subsidies (Brown & McLachlan Citation1990; Dugan et al. Citation2003; Colombini & Chelazzi Citation2003; Lastra et al. Citation2008). In the present study, stranded D. antarctica was shown to be ecologically important to marine and terrestrial macroinvertebrate species on the upper shore of an exposed sandy beach on the southeastern coast of New Zealand. Twenty taxa of invertebrates were identified on samples of D. antarctica. Four of these taxa dominated the macroinvertebrate community throughout a 30-day period in autumn and winter, namely Talitridae, Staphylinidae, other Coleoptera and Diptera (). Within a day of being stranded, the kelp was colonised by these four major taxa. The talitrid amphipod B. quoyana was the most common species associated with stranded D. antarctica. Overall, its percentage abundance was at least 5 times higher, and its percentage biomass was at least 12 times higher than the percentage abundance and biomass of Staphylinidae, other Coleoptera and Diptera. Bellorchestia quoyana accounted for 31–96% of the total macrofaunal abundance, and 53–98% of the total macrofaunal biomass during both sampling periods. Previous studies have also shown the macrofaunal community of stranded kelp to be dominated by amphipods. Talorchestia martensii accounted for 89% of total macrofauna community associated with wrack on Sar Uanle (Somalia) (Colombini et al. Citation2000). Similarly, Orchestia platensis accounted for 50% of the community abundance and 81% of community biomass across three New Hampshire habitats (Behbehani & Croker Citation1982), on a sandy beach in the Netherlands Talitrus saltator was the most abundant (22–24% of total community abundance) macrofaunal species associated with washed-up Zostera marina (Jedrzejczak Citation2002), and in Peru the macroinvertebrate biomass was dominated by Transorchestia chiliensis (average biomass of 48 mg per bag of Ulva) (Catenazzi & Donnelly Citation2007). Conversely, in another New Zealand study, Inglis (Citation1989) found Diptera to be the most abundant component (c. 48%) whereas talitrid (B. quoyana) abundance comprised 27% of the macrofaunal community associated with samples of M. pyrifera. The difference in findings may in part be due to the different collection methods used. Inglis (Citation1989) used insecticide, which ensured all wrack-associated fauna, including flying insects, did not escape when kelp samples were transferred into plastic bags for subsequent identification.
Amphipods are key colonisers and consumers of beach-cast algae (Griffiths & Stenton-Dozey Citation1981; Behbehani & Croker Citation1982; Marsden Citation1991; Colombini et al. Citation2000; Jedrzejczak Citation2002). In this study, amphipods were most abundant during the early stages of succession (days 3 and 6 of exposure), whereas staphylinid beetles (Blediotrogus guttiger and Macralymma punctiventre) occupied the wrack during later stages, peaking in abundance on day 20 of exposure. Inglis (Citation1989) obtained similar findings where B. quoyana reached its highest abundance within the first 3 days of sampling, while staphylinid beetles (Bledius spp.) were most abundant on day 18. Species composition has been found to change over time possibly due to the decay and desiccation of organic matter on sandy beaches (Catenazzi & Donnelly Citation2007; Ince et al. Citation2007). This may explain the early colonisation of D. antarctica by the talitrid amphipod, which requires a moist environment to thrive (Marsden Citation1991), whilst carnivorous coleopteran species are later colonisers as they prefer older, drier wrack (Griffiths & Stenton-Dozey Citation1981; Colombini et al. Citation2000) where there is an abundance of prey. Alternatively, the decrease in amphipod biomass and abundance over time may be a means of avoiding the influx of carnivorous staphylinid beetles, which prey on other invertebrates (Klimaszewski et al. Citation1996).
Abundance and biomass of other Coleoptera and Diptera tended to increase over time in the current study; however, this trend was not statistically significant (P>0.05). Other studies have demonstrated a significant change in the abundance and biomass of Coleoptera, dipteran adults and larvae on wrack over time (Inglis Citation1989; Chown Citation1996; Hodge & Arthur Citation1997; Catenazzi & Donnelly Citation2007; Garrido et al. Citation2008). However, these studies investigated only dipteran or coleopteran assemblages (Chown Citation1996; Hodge & Arthur Citation1997; Garrido et al. Citation2008), or the sampling method included the collection of meiofauna and associated larval stages (Inglis Citation1989; Catenazzi & Donnelly Citation2007) that were too small to be retained on 1-mm mesh sieves (Inglis Citation1989; Chown Citation1996). In this study, the biomass of Diptera differed significantly between sampling days, with highest biomass occurring during later stages. The change in dipteran biomass over time may be attributed to the hatching of newly laid eggs into pupae and then adults. For instance, in a study on dipteran assemblages on the north-east coast of England, adult abundance peaked at the beginning and end of the wrack cycle, with the adults found during later stages being the offspring of those that originally colonised the fresh wrack (Hodge & Arthur Citation1997).
Previous research on wrack-associated macrofauna has shown significant differences in abundance between summer and winter (Behbehani & Croker Citation1982; Persson Citation1999), where summer had the highest abundance of macrofauna. The availability of wrack on beaches varies seasonally (Marsden Citation1991) and, in turn, influences the abundance of individual macrofauna (Griffiths & Stenton-Dozey Citation1981; Behbehani & Croker Citation1982; Ince et al. Citation2007; Olabarria et al. Citation2007; Garrido et al. Citation2008). For instance, Behbehani & Croker (Citation1982) showed that the abundance of O. platensis decreased as the amount of available wrack decreased from summer to winter. The present study was carried out during autumn (April–May) and winter (June–July); however, the amount of wrack was kept consistent between the two sampling periods so any differences in biomass and/or abundance observed between seasons cannot be attributed to kelp availability. There was no seasonal difference in abundance of macroinvertebrates; however, there was a seasonal difference in biomass for all major taxa (Amphipoda, Staphylinidae, other Coleoptera and Diptera). All common taxa, except other Coleoptera, displayed higher average biomass in autumn, indicating species recruitment, a higher number of individuals and/or growth of individuals already present (Behbehani & Croker Citation1982; Persson Citation1999; Lastra et al. Citation2008). The latter is the most probable explanation, as there was no significant difference in abundance between seasons. Air temperature differed between sampling seasons with mean values of 12.4 °C and 8.3 °C for the autumn and winter sampling periods, respectively (data from nearby Portobello Marine Laboratory, 2008), and may be a contributing factor to seasonal differences in observed biomass. Previous research has shown higher growth rates for some macrofaunal species in warmer temperatures (Behbehani & Croker Citation1982; Persson Citation1999) due to increased consumption rates. The talitrid amphipod Talitrus saltator, for instance, consumed a greater amount of stranded Saccorhiza polyschides at 20 °C and 15 °C than at 10 °C (Lastra et al. Citation2008). Further studies into the particular effects of temperature and other environmental factors on the succession of macrofauna on wrack are, however, needed to examine such influences.
Stranded macrophytes act as a source of food (especially carbon, nitrogen and phosphorus) (Rice & Tenore Citation1981; Poore & Steinberg Citation1999; Rossi & Underwood Citation2002; Mateo Citation2010; Olabarria et al. Citation2010) for primary consumers on sandy shores (Chown Citation1996; Pennings et al. Citation2000; Hemmi & Jormalainen Citation2002; Lastra et al. Citation2008). The nutritional value of kelp can be assessed by the content of carbon, nitrogen and the C:N ratio. Durvillaea antarctica contained 22–35% carbon and 0.78–1.26% nitrogen (by dry weight). This agrees with previous research where the percentage of nitrogen ranged between 0.72% and 2.13% for nine species of stranded macrophytes in the northeast Pacific (Pennings et al. Citation2000), and the carbon and nitrogen content for eight species of brown algae, in Shark Bay (Australia), which ranged between 14% and 32% and 0.7% and 2.3%, respectively (Poore & Steinberg Citation1999).
In the current study, the C:N ratio ranged between 21 and 38 for both autumn and winter sampling, in agreement with previous research (Rice & Tenore Citation1981; Poore & Steinberg Citation1999; Pennings et al. Citation2000; McLeod unpublished data). For instance, the mean C:N ratio for fresh and aged D. antarctica was 48 and 47, respectively, and 21 and 28 for fresh and aged samples of Ecklonia during a sampling period of 16–22 days on Warrington Beach, southeastern New Zealand (McLeod unpublished data). Similar to this study, McLeod (unpublished data) found that the C:N ratio was not significantly different between days of exposure, which may be due to the short sampling period of 30 days or less. The C:N ratio was positively correlated with the total macrofaunal abundance (R 2=0.44, P<0.001) where macrofaunal abundance increased as the carbon content increased and the nitrogen content decreased. Further, the macrofaunal community associated with stranded D. antarctica increased over time, suggesting the macrofaunal community on Victory Beach prefer aged wrack over fresh kelp, despite it being lower in nitrogen content. This agrees with previous research on the amphipods Peramphithoe parmerong (Poore & Steinberg Citation1999), Melagorchestia californiana and Traskorchestia traskiana, and the isopod Ligia pallasii (Pennings et al. Citation2000), which did not show a preference for a nitrogen-rich food. These consumers select food based on the content of defensive compounds, where decreased concentrations make the wrack more palatable (Poore & Steinberg Citation1999; Pennings et al. Citation2000). It is recommended that future research investigate the content of defensive compounds in fresh and aged kelp samples to determine whether it influences macrofaunal assemblages associated with wrack on New Zealand sandy shores.
Conclusion
A field manipulation study that experimentally added D. antarctica samples to the upper shore of an exposed sandy beach on the southeast coast of New Zealand showed that the presence of wrack enhanced the abundance of some species (the amphipod B. quoyana and Staphylinidae) but not others (Diptera and other Coleoptera), suggesting some taxonomic groups use wrack opportunistically for food and/or shelter. Correlations between macrofaunal abundance and the decomposition rate, and total macrofaunal abundance and the C:N ratio, suggest that the macrofaunal community prefers older wrack despite it being lower in nitrogen content then fresh kelp.
Acknowledgements
We thank fellow Marine Science students and Jean-Philippe Dufour for help with field sampling, Anthony Harris of the Otago Museum for help with species identification, the Campbell Macroanalytical Laboratory, University of Otago, for carbon and nitrogen analysis, and the University of Otago for a Postgraduate Publishing Bursary.
References
- Behbehani , MI and Croker , RA . 1982 . Ecology of beach wrack in Northern New England with special reference to Orchestia platensis . Estuarine Coastal and Shelf Science , 15 : 611 – 620 .
- Bishop , G and Hamel , A . 1993 . From sea to silver peaks , Dunedin : McIndoe Publishing .
- Bolam , SG , Fernandes , TF , Read , P and Raffaelli , D . 2000 . Effects of macroalgal mats on intertidal sandflats: an experimental study . Journal of Experimental Marine Biology and Ecology , 249 : 123 – 137 .
- Brown , AC and McLachlan , A . 1990 . Ecology of sandy shores , 2nd edition , Amsterdam : Elsevier .
- Catenazzi , A and Donnelly , MA . 2007 . Role of supratidal invertebrates in the decomposition of beach-cast green algae Ulva sp . Marine Ecology Progress Series , 349 : 33 – 42 .
- Chown , SL . 1996 . Kelp degradation by Paractora trichosterna (Thomson) (Diptera: Helcomyzidae) at sub-Antarctic South Georgia . Polar Biology , 16 : 171 – 178 .
- Colombini , I , Aloia , A , Fallaci , M , Pezzoli , G and Chelazzi , L . 2000 . Temporal and spatial use of stranded wrack by the macrofauna of a tropical sandy beach . Marine Biology , 136 : 531 – 541 .
- Colombini , I and Chelazzi , L . 2003 . Influence of marine allochthonous input on sandy beach communities . Oceanography and Marine Biology: an Annual Review , 41 : 115 – 159 .
- Dugan , JE , Hubbard , DM , McCrary , MD and Pierson , MO . 2003 . The response of macrofauna communities and shorebirds to macrophyte wrack and subsidies on exposed sandy beaches of southern California . Estuarine Coastal and Shelf Science , 58 : 25 – 40 .
- Fourqurean , JW and Schrlau , JE . 2003 . Changes in nutrient content and stable isotope reatios of C and N during decomposition of seagrasses and mangrove leaves along a nutrient availability gradient in Florida Bay, USA . Chemistry and Ecology , 19 : 373 – 390 .
- Garden , CJ and Smith , AB . 2011 . The role of kelp in sediment transport: Observations from southeastern New Zealand . Marine Geology , 281 : 35 – 42 .
- Garrido , J , Olabarria , C and Lastra , M . 2008 . Colonization of wrack by beetles (Insecta: Coleoptera) on a sandy beach of the Atlantic Coast . Vie et Milieu-Life and Environment , 58 : 223 – 232 .
- Gonçalves , SC , Anastácio , PM , Pardal , MA , Gardoso , PG , Ferreira , SM and Marques , JC . 2009 . Sandy beach macrofaunal communities on the western coast of Portugal – Is there a steady structure under similar exposed conditions? . Estuarine, Coastal and Shelf Science , 81 : 555 – 568 .
- Griffiths , CL and Stenton-Dozey , J . 1981 . The fauna and rate of degradation of stranded kelp . Estuarine Coastal and Shelf Science , 12 : 645 – 653 .
- Hanisak , MD . 1993 . Nitrogen release from decomposing seaweeds: species and temperature effects . Journal of Applied Phycology , 5 : 175 – 181 .
- Hay CH 1994 . Durvillaea (Bory) . In : Biology of economic algae . The Hague : SPB Academic Publishing . 353 – 384 .
- Hemmi , A and Jormalainen , V . 2002 . Nutrient enhancement increases performance of a marine herbivore via quality of its food alga . Ecology , 83 : 1052 – 1064 .
- Hodge , S and Arthur , W . 1997 . Asymmetric interactions between species of seaweed fly . Journal of Animal Ecology , 66 : 743 – 754 .
- Hubbard , DM and Dugan , JE . 2003 . Shorebird use of an exposed sandy beach in southern California . Estuarine Coastal and Shelf Science , 58 : 41 – 54 .
- Ince , R , Hyndes , GA , Lavery , PS and Vanderklift , MA . 2007 . Marine macrophytes directly enhance abundances of sandy beach fauna through provision of food and habitat . Estuarine Coastal and Shelf Science , 74 : 77 – 86 .
- Inglis , G . 1989 . The colonization and degradation of stranded Macrosystis pyrifera (L.) C. Ag. by the macrofauna of a New Zealand sandy beach . Journal of Experimental Marine Biology and Ecology , 125 : 203 – 217 .
- Jedrzejczak , MF . 2002 . Stranded Zostera marina L. vs wrack fauna community interactions on a Baltic sandy beach (Hel, Poland): a short-term pilot study. Part II. Driftline effects of succession changes and colonisation of beach fauna . Oceanologia , 44 : 367 – 387 .
- Johnson PN 1992 . The sand dune and beach vegetation inventory of New Zealand. II, South Island and Stewart Island . Scientific Report No. 16 . Christchurch, DSIR Land Resources .
- Klimaszewski , J , Newton , A F JR and Thayer , MK . 1996 . A review of the New Zealand rove beetles (Coleoptera: Staphylinidae) . New Zealand Journal of Zoology , 23 : 143 – 160 .
- Kirkman , H and Kendrick , G.A. 1997 . Ecological significance and commercial harvesting of drifting and beach-cast macro-algae and seagrasses in Australia: a review . Journal of Applied Phycology , 9 : 311 – 326 .
- Lastra , M , Page , HM , Dugan , JE , Hubbard , DM and Rodil , IF . 2008 . Processing of allochthonous macrophyte subsidies by sandy beach consumers: estimates of feeding rates and impacts on food resources . Marine Biology , 154 : 163 – 174 .
- MacIntyre , RJ . 1963 . The supra-littoral fringe of New Zealand sand beaches . Transactions of the Royal Society of New Zealand , 1 : 89 – 103 .
- Marsden , ID . 1991 . Kelp–sandhopper interactions on a sand beach in New Zealand. I. Drift composition and distribution . Journal or Experimental Marine Biology and Ecology , 152 : 61 – 74 .
- Mateo , M . 2010 . Beach-cast Cymodocea nodosa along the shore of a semienclosed bay: sampling and elements to assess its ecological implications . Journal of Coastal Research , 26 : 283 – 291 .
- Morton J Miller M 1968 . The New Zealand Seashore . Auckland, Collins .
- Ochieng , CA and Erftemeijer , PLA . 1999 . Accumulation of seagrass beach cast along the Kenyan coast: a quantitative assessment . Aquatic Botany , 65 : 221 – 238 .
- Olabarria , C , Lastra , M and Garrido , J . 2007 . Succession of macrofauna on macroalgal wrack of an exposed sandy beach: effects of patch size and site . Marine Environmental Research , 63 : 19 – 40 .
- Olabarria , C , Incera , M , Garrido , J , Rodil , IF and Rossi , F . 2009 . Intraspecific diet shift in Talitrus saltator inhabiting exposed sandy beaches . Estuarine, Coastal and Shelf Science , 84 : 282 – 288 .
- Olabarria , C , Incera , M , Garrido , J and Rossi , F . 2010 . The effect of wrack composition and diversity on macrofaunal assemblages in intertidal marine sediment . Journal of Experimental Marine Biology and Ecology , 396 : 18 – 26 .
- Orr , M , Zimmer , M , Jelinski , ED and Mews , M . 2005 . Wrack deposition on different beach types: spatial and temporal variation in the pattern of subsidy . Ecology , 86 : 1496 – 1507 .
- Pennings , SC , Carefoot , TH , Zimmer , M , Danko , JP and Ziegler , A . 2000 . Feeding preferences of supralittoral isopods and amphipods . Canadian Journal of Zoology , 78 : 1918 – 1929 .
- Persson , L-E . 1999 . Growth and reproduction in two brackish water populations of Orchestia gammarellus (Amphipoda: Talitridae) in the Baltic Sea . Journal of Crustacean Biology , 19 : 53 – 59 .
- Polis , GA , Anderson , WB and Holt , RD . 1997 . Toward an intergration of landscape and food web ecology: the dynamics of spatially subsidized food webs . Annual Review of Ecology and Systematics , 28 : 289 – 316 .
- Poore , AGB and Steinberg , PD . 1999 . Preference–performance relationships and effects of host plant choice in an herbivorous marine amphipod . Ecological Monographs , 69 : 443 – 464 .
- Posey , MH , Alphin , TD , Cahoon , L , Lindquist , D and Becker , ME . 1999 . Interactive effects of nutrient additions and predation on infaunal communities . Estuaries , 22 : 785 – 792 .
- Rice , DL and Tenore , KR . 1981 . Dynamics of carbon and nitrogen during the decomposition of detritus derived from estuarine macrophytes . Estuarine, Coastal and Shelf Science , 13 : 681 – 690 .
- Rodil , IF , Lastra , M and López , J . 2007 . Macroinfauna community structure and biochemical composition of sedimentary organic matter along a gradient of wave exposure in sandy beaches (NW Spain) . Hydrobiologia , 579 : 301 – 316 .
- Rossi , F and Underwood , AJ . 2002 . Small-scale disturbance and increased nutrient as influences on intertidal macrobenthic assemblages: experimental burial of wrack in different intertidal environments . Marine Ecology Progress Series , 241 : 29 – 39 .
- Rossi , F . 2006 . Small-scale burial of macroalgal detritus in marine sediments: effects of Ulva spp. on the spatial distribution of macrofauna assemblages . Journal of Experimental Marine Ecology and Biology , 332 : 84 – 95 .
- Rossi , F . 2007 . Recycle of buried macroalgal detritus in sediments: use of dual-labelling experiments in the field . Marine Biology , 150 : 1073 – 1081 .
- Stephenson G 1998 . Macroinfauna of Wanui Beach, Gisborne-impact of beach protection structures . Conservation Advisory Science Notes No. 212 . Wellington : Department of Conservation .
- Stevens , CL , Hurd , CL and Smith , MJ . 2002 . Field measurement of the dynamics of the bull kelp Durvillaea antarctica (Chamisso) Heriot . Journal of Experimental Marine Biology and Ecology , 269 : 147 – 171 .
- Wilson , B . 2004 . Day walks of Dunedin & coastal Otago , Auckland : Reed Publishing .