Abstract
In this study, the distribution and occurrence of backswimmers in 21 fishless ponds was analysed in the Nahuel Huapi National Park (Patagonia, Argentina). We performed laboratory experiments to study the impact of different developmental stages of the endemic backswimmer Notonecta vereertbruggheni on typical co-occurring prey. We recorded three species of backswimmers N. vereertbruggheni, Notonecta virescens and Notonecta fazi. A seasonal study in Fantasma pond showed that adults of N. vereertbruggheni colonise and reproduce in the pond in spring and summer, co-occurring with other macroinvertebrates and endemic tadpoles. Predation experiments explored the impact of N. vereertbruggheni on two large co-occurring prey, the calanoid copepod Parabroteas sarsi and the tadpoles of Pleurodema thaul. The backswimmer consumed more copepods than tadpoles when prey was offered separately. Selectivity experiments demonstrated that the copepods were preferred over the tadpoles. Backswimmers may impact the typical assemblages of organisms found in fishless ponds of Patagonia during spring and summer.
Introduction
Predation and competition are major interactions affecting the dynamics and structure of prey communities (Murdoch et al. Citation1984; Sih et al. Citation1985; Schneider & Frost Citation1996; Wellborn et al. Citation1996; Hero et al. Citation1998). In temporary fishless ponds, aquatic insects, spiders and salamanders are usually the top predators (Heyer et al. Citation1975; Wellborn et al. Citation1996; Wilbur Citation1987, Citation1997; Jara Citation2008a,Citationb). In fact, in some pond communities, predation by several macroinvertebrates like notonectids (backswimmers), belostomatids and dragonflies may control the size structure and species composition (Williams Citation1987; Blaustein et al. Citation1995; Hössler et al. Citation1995; Herwig & Schindler Citation1996; Spencer et al. Citation1999; Cobbaert et al. Citation2010; Hampton et al. Citation2000). Backswimmers in particular are well known for their voracious predatory habits and their ability to exploit many prey types such as rotifers, crustaceans, mosquito larvae, tadpoles and small fish (Cronin & Travis Citation1986; Streams Citation1987a; Hampton et al. Citation2000; Mazzucconi Citation2008; Gilbert & Diéguez Citation2010). Furthermore, they often attack other predators like damselflies and dragonflies that eventually exceed many times their own size. Additionally, the predation pressure exerted by notonectids can induce numerical as well as developmental, morphological and behavioural shifts in their prey (Black Citation1993; Repka et al. Citation1994; Jara & Perotti Citation2010). In particular, Notonecta species are typically ambush predators that remain still, perched on aquatic vegetation, and seize moving prey or organisms stranded in the water surface (Gittelman Citation1974; Streams Citation1987a). Prey detection in backswimmers is both visual and mechanical (Schwind Citation1980; Peckarsky Citation1982; Streams Citation1982; Savage Citation1989). Visual detection is relevant particularly for catching small prey and thus, prey size may determine day–night differences in their foraging efficiency (Diéguez & Gilbert Citation2003). Backswimmers catch the prey using their anterior and middle legs, pierce it with the rostrum, inject with digestive enzymes and suck the liquefied contents.
In Northwestern Patagonia, the dynamics and impact of predatory invertebrates on aquatic communities have been poorly studied. In small lakes and temporary fishless ponds, ciliates, turbellarians, large copepods and water mites are the primary invertebrates preying on zooplanktonic prey, including rotifers, cladocerans and copepods (Vega Citation1995; Diéguez & Balseiro Citation1998; Trochine et al. Citation2006, Citation2008). These predators co-occur seasonally with several other carnivorous insect species such as notonectids, belostomatids, diving beetles and odonate larvae, which are known to consume tadpoles (Jara & Perotti Citation2009, Citation2010) as well as zooplanktonic prey (Gilbert & Diéguez Citation2010).
Notonectids are widespread in Patagonia and most of the species present are endemic to this region (Bachmann Citation1962, Citation1963; Mazzuconi 2008; Melo Citation2009). In the Nahuel Huapi National Park (NHNP), four species of the genus Notonecta have been recorded: N. peruviana Hungerford, N. vereertbruggheni Hungerford, N. virescens Blanchard and N. fazi Hungerford (Mazzucconi Citation2008). In this park adult notonectids have been found in temporary ponds and pools during spring and summer (Jara Citation2010). The endemic backswimmer N. vereertbruggheni is known to prey on tadpoles of the anurans Pleurodema thaul and Pleurodema bufoninum (Jara & Perotti Citation2010) while the younger nymphs consume zooplankton (Gilbert & Diéguez Citation2010). Although Notonecta has the potential to exploit a wide range of prey type and size, up to the moment there is no evidence about its impact on the structure of Patagonian pond communities.
The objectives of this study were to analyse the occurrence of backswimmers in shallow ponds located inside NHNP and their potential impact on co-occurring prey, such as crustaceans and tadpoles. For this purpose we surveyed 21 ponds located at different altitude on a longitudinal transect of 40 km, comprising high altitude water bodies in the west and piedmont pools in the east. In each location we recorded notonectid species and potential prey such as macroinvertebrates and tadpoles. Furthermore, we analysed the life cycle of the endemic backswimmer N. vereertbruggheni in Fantasma pond, a fishless temporary piedmont system. Finally, we studied the feeding and selectivity of N. vereertbruggheni on two prey species, the copepod Parabroteas sarsi and tadpoles of P. thaul, which co-occur in Fantasma Pond during spring and summer. These experiments were performed under light and dark conditions in order to determine whether prey type along with light availability could influence the foraging efficiency of backswimmers.
Materials and methods
Field sampling
This research was conducted in 21 ponds located in Nahuel Huapi National Park (NHNP), Northwestern Patagonia, Argentina (). The study area is located within a sharp (40 km) east–west precipitation gradient (800–1500 mm), which largely determines the water regime and pond duration. The ponds studied are located in different landscape units within the NHNP, including steppe and transitional zones, Andean–Patagonian forest and Sub-Antarctic forest (). These shallow ponds are fishless and include temporary, semi-temporary and permanent water bodies. Ponds located at piedmont (<906 m a.s.l) are located mostly in urban and suburban areas, while high altitude ponds (>1400 m a.s.l) are located in remote zones of the park ().
Figure 1 Map of the study area (Nahuel Huapi National Park, Patagonia, Argentina). Numbers refer to pond location: 1, Llao-Llao; 2, Fantasma; 3, Virgen de las Nieves; 4, Pinar de Festa; 5, Teleférico; 6, Mallín Ñireco; Ñireco 1 and 2; 7, Los Patos and Verde; 8, Refugio de Jesús; 9, Bernal 1 to 6; 10, Ñirihuau 1 to 4.
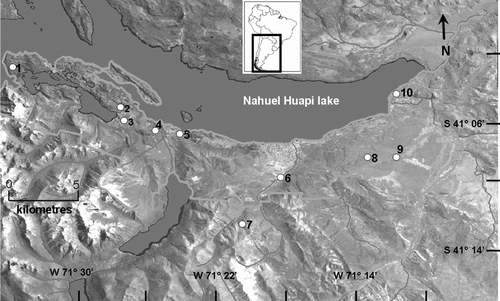
Table 1 Environmental features of 21 shallow fishless ponds inside Nahuel Huapi National Park (NHNP, Patagonia, Argentina).
All the ponds were visited monthly from September to December 2008. At each sampling occasion, depth, temperature, pH, conductivity and dissolved oxygen were measured using multi-parameter probes (HI 9828). The presence of aquatic vegetation was recorded at each pond. Two types of samples were taken in order to: (1) characterise the biotic assemblages of the ponds, and (2) collect backswimmers, copepods and tadpoles for their use in feeding experiments. Macroinvertebrate and amphibian species were collected in the littoral and in the open water areas of the ponds by sweeping 10 longitudinal sections of 2 m with a dip-net (36×25 cm; 300 µm mesh). Zooplankton samples were collected using a Schindler–Patalas trap (12 l) in the open water, while littoral samples were taken using a 5-l van Dorn bottle, and filtered through 45-µm mesh net. Tadpole samples were preserved using formaldehyde 10% while zooplankton was preserved with Lugol's solution.
Macroinvertebrates including notonectids were identified to the lowest taxonomic level after Bachmann (Citation1963, Citation1998) and Mazzuconi (2008). Backswimmer instars were identified following Angrisano (Citation1982) and quantified separately. The amphibian species were identified following Cei (Citation1980) and their larval stages after Gosner (Citation1960). The different stages were pooled in the categories ‘vulnerable‘ (stages 23 to 27) and ‘invulnerable‘ (stages older than 28) following Jara & Perotti (Citation2010).
In particular, the assemblage composed by N. vereertbruggheni, the tadpoles of P. thaul and large calanoid copepods observed in Fantasma Pond (García Citation2010; Jara Citation2010) was considered amenable for the study of the life cycle of this backswimmer and its predatory impact on tadpoles and co-occurring crustaceans. For this purpose, two stations were set up in Fantasma pond, one in the littoral and one in the open-water, which were visited and sampled monthly from September to December 2008. Macroinvertebrates and tadpoles were collected using a dip-net as described before, poured into a shallow plastic container and counted in situ. Also, a separate sample was collected and preserved in order to confirm species identification, to estimate the abundance and to obtain the total body length (TL).
Laboratory experiments
Predation experiments were conducted in the laboratory and involved the incubation of different stages of the backswimmer N. vereertbruggheni, the calanoid copepod P. sarsi and the tadpoles of P. thaul obtained from Fantasma pond. These species were selected for the feeding experiments because they are the larger organisms co-occurring with notonectids during spring and summer in Fantasma pond.
Feeding trials were set up to analyse the consumption of the copepod P. sarsi and tadpoles of P. thaul by different stages of Notonecta, separately. Another set of experiments tested for the selectivity of different stages of Notonecta on mixed copepod and tadpole prey. Dark and light treatments were applied to feeding and selectivity trials in order to evaluate the effect of light availability on prey consumption and to infer potential light-dependent periodicity in the use of different prey type.
The feeding experiments were performed using four different stages of Notonecta as predators; instar II (mean TL±1SEM = 4.9±0.23 mm), III (6.7±0.14 mm), V (8.4±0.6 mm) and adults (11.5±0.7 mm), incubated separately with P. sarsi adults (4.87±0.08 mm) and with newly hatched tadpoles of P. thaul stage 25 (11±0.1 mm). Each replicate contained six tadpoles or 10 copepods and one backswimmer. These prey numbers were equivalent in terms of biomass based on prey wet weights.
Selectivity experiments were set up to analyse the preference of different instars (II, III, V and adult) of N. vereertbruggheni on a copepod–tadpole assemblage, under light and dark. Each replicate contained 10 copepods, six tadpoles and one backswimmer.
The following general conditions were applied to all the experimental assays. Backswimmers, copepods and tadpoles were collected from Fantasma pond on November 2008, using a dip net and were taken to the laboratory within 1 h of collection. The prey and predators were incubated at 18±1 °C for 24 h before conducting the experiments. Parabroteas sarsi was maintained in 5-l buckets filled with pond water with natural co-occurring prey (rotifers, copepods and cladocerans). Tadpoles were incubated in 5-l flat plastic containers filled with pond water and fed with a suspension of the algae Chlamydomonas reinhardtii and Scenedesmus sp. from laboratory cultures. Backswimmers were incubated in 5-l flat plastic containers filled with filtered (50-µm mesh) pond water deprived of food to ensure 24 h of starvation before setting up the experiments. For each prey, a total of 50 replicates were set up in 1000-ml plastic beakers filled with 700 ml of natural pond water. Prey were placed in each of the 50 containers; one backswimmer was added to 10 of the replicates while 10 containers without the predator served to control for natural mortality of the prey. The experiment was run separately with instars II, III, V and adults. Light and dark treatments were run simultaneously in two separate compartments inside an environmental chamber; in one compartment light was supplied by two fluorescent lamps (Philips daylight, TLT 40W/54RS) while the other was kept dark with a cover of aluminium foil. A cylindrical wooden rod was put in each replicate in order to serve as a perch for the predator. After 2 h, the number of prey remaining alive was recorded in each replicate. This exposure period was established following the results of preliminary trials and corresponded to the time at which 50% of the prey was consumed.
Analytical procedures
The correspondence of environmental variables and notonectid species occurrence in 21 ponds inside the NHNP was analysed by means of Canonical Correspondence Analysis (CCA). Calculations were performed by the program CANOCO (ter Braak Citation1988). The data set analysed was based on qualitative and quantitative samples. Forward selection was used for adding environmental variables to the model. The significance of the ordination axes was assessed by Monte Carlo permutations.
The abundance of N. vereertbruggheni in the littoral and open water of Fantasma pond was compared using the non-parametric Mann–Whitney test. The ingestion rate (IR) of Notonecta on copepods and tadpoles when prey was exposed separately, was calculated as the number of prey consumed (initial prey minus final prey) divided by the exposure time and predator abundance, and was expressed as prey/predator/h (Hampton Citation2004). Two-way analysis of variance (ANOVA) was applied to study the effects of the instar (II, III, V and adult) and light availability (light and dark) on the IR of Notonecta on copepods and tadpoles (Zar Citation1999).
The preference index α was calculated for each instar of Notonecta from the results of the selectivity experiments after Manly (Citation1974) and Chesson (Citation1978) as follows:
where N i and N j are the initial and final number of copepods in the experiment; R i and R j are the initial and final number of tadpoles in the experiment; K is the number of prey species. αi ranges between 0 and 1 and thus α = 0.5 indicates neutral selectivity, α values >0.5 indicate positive selection while values <0.5 denote negative selection. This index is appropriate for experiments in which prey is not replaced after consumption. Three-way ANOVA was applied to evaluate the effect of light availability (light and dark), prey type and predator instar on prey selectivity (α).
All the statistical analyses were conducted using the software SPSS (9.0). When significant effects of main factors were found, post hoc multiple comparisons were performed applying the Holm–Sidăk test (HS; Zar Citation1999).
Results
Distribution of notonectid species in ponds of NHNP
Eleven of the 21 ponds surveyed inside the NHNP had aquatic vegetation (Table 1). The aquatic vegetation was found to be variable and included submerged and floating hydrophytes and rushes in the littoral zone. In shallower ponds, different species of rushes were found covering almost all the surface. In general, the ponds had conductivity values below 130 µS/cm coinciding with pH deviating slightly from neutrality. However, higher values of conductivity up to ~700 µS/cm were recorded in two ponds along with more alkaline conditions (Table 1). Temperature varied widely during spring and summer, and also on a daily basis, with low temperatures in the morning (as low as 4 °C) and high values in the afternoon (up to 30 °C). Concomitantly, dissolved oxygen (O2) varied broadly; however, the values were never below 5 mg/l, reflecting the action of the strong winds characteristic of the area.
The survey of ponds revealed the occurrence of several species of predatory insects. The most common were waterbugs, beetle larvae, dragonflies and damselflies, as have been previously recorded also by Jara & Perotti (Citation2010). Notonectids in particular, were found inhabiting 12 of the 21 ponds studied. Three species of backswimmers of the genus Notonecta were recorded, N. vereertbruggheni and N. fazi (adults up to 14 mm) and N. virescens (adults up to 9 mm). Notonecta vereertbruggheni was the most widespread species in the study area occurring in ~50% of the surveyed ponds, while N. virescens and N. fazi occurred in less than 20% of the ponds ().
The CCA performed using the environmental variables pond area, depth, altitude, pH, conductivity and vegetation revealed that altitude by itself determined the occurrence of notonectid species. However, depth, vegetation and conductivity contributed as well to the first axis accounting overall for 97% of the total variance (). A Monte Carlo unrestricted permutation test on the first eigenvalue indicated that the altitude was significantly correlated with the first axis (; ).
Figure 2 Plot of Axis 1 and 2 of the Canonical Correspondence Analysis applied to study the effects of environmental variables on the distribution of notonectid species in 21 ponds inside the Nahuel Huapi National Park (Patagonia, Argentina). Environmental variables are indicated by arrows. Numbers and black triangles refer to ponds: 1, Llao-Llao; 2, Fantasma; 3, Virgen de las Nieves; 4, Pinar de Festa; 5, Teleférico; 6, Mallín Ñireco; Ñireco 1 and 2; 7, Los Patos and Verde; 8, Refugio de Jesús; 9, Bernal 1 to 6; 10, Ñirihuau 1 to 4. Grey triangles indicate notonectid species.
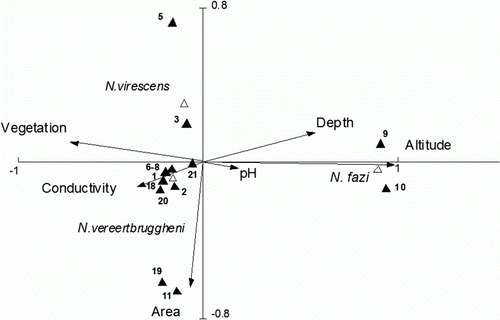
Table 2 Results of the Canonical Correspondence Analysis (CCA) performed to study the distribution of notonectids in ponds belonging to Nahuel Huapi National Park.
Overall, N. fazi was the only backswimmer species present in the high altitude ponds Los Patos and Verde, which are characterised by comparatively greater depths and low conductivities. Notonecta vereertbruggheni was present in 10 piedmont ponds, co-occurring with N. virescens in three of them. Other predatory insects occurred along with backswimmers. The most common were waterbugs (Belostoma bifoveolatum), beetle larvae (Rhantus antarcticus, Tropisternus and Lancetes sp.) and odonate larvae (Rhionaeschna variegata, Erythrodiplax connata) and Cyanallagma interruptum).
Other components of pond communities were amphibians, with a total record of six species. Pleurodema thaul was found in eight ponds located in areas of mixed Nothofagus forests and in the transition to the steppe. Hylorina sylvatica and Batrachyla taeniata were present exclusively in the westernmost pond Llao-Llao, while Atelognathus nitoi was restricted to the high altitude pond Verde and was observed occasionally in Los Patos pond. Pleurodema bufoninum and Rhinella spinulosa occurred in steppe ponds and transitional areas (). Crustaceans, particularly calanoid copepods, cladocerans and amphipods were found in most ponds. The zooplankton community of the ponds was dominated by calanoid copepods of the genus Boeckella: B. gracilis, B. gracilipes, B. brevicaudata, B. antiqua and the large predator P. sarsi. The cyclopoid Acanthocyclops robustus was also present in some ponds along with cladocerans such as Daphnia spp., Ceriodaphnia dubia and Simocephalus serrulatus.
Life cycle of Notonecta vereertbruggheni in Fantasma pond
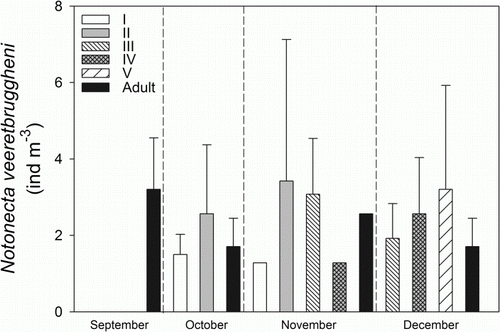
During 2008, the adults of N. vereertbruggheni were captured with the dip net from September to the end of December in Fantasma pond. In September, the adults of Notonecta attained a total density of 3 ind/m3 (). Instars I and II were observed in October along with adults at a density of 4.5 ind/m3. In November, the greatest total density of 9 ind/m3 was recorded, with the overlap of instars I, II, III, IV and the adults. In December the instars III, IV, V and adults were present in the pond at a total density of 7.5 ind/m3 (). The distribution of Notonecta in the pond assessed during daytime in November, showed a higher abundance of backswimmers in the littoral (15.4±3.3 ind/m3) compared with the open water (2.6±1.4 ind/m3) (Mann–Whitney, U=93.5, P=0.001). Furthermore, the density of nymphs was higher in the littoral (12.8±6 ind/m3) than in the open water (2.6±4.5 ind/m3). In contrast, the adults occurred exclusively inside the vegetation belt in the littoral zone of the pond, where they attained a lower density than the nymphs (2.6±4 ind/m3).
Figure 3 Seasonal abundance of Notonecta vereertbruggheni in Fantasma pond during 2008. The bars indicate density (mean±1SEM; ind/m3) of N. vereertbruggheni. Maximum body length of the different instars of Notonecta: I = 2 mm, II = 3.9 mm, III = 5.4 mm, IV = 8.9 mm, V = 9.8 mm, adult = 14 mm.
In Fantasma pond, N. vereertbruggheni co-occurred with the predatory beetle larvae of Lancetes sp. and Rhantus antarcticus, the flatworm Mesostoma ehrenbergii, and a crustacean assemblage dominated by calanoid and cyclopoid copepods. The most common crustaceans in the pond during spring were the calanoids P. sarsi and Boeckella gracilis, the cyclopoid Acanthocyclops robustus, the cladoceran Simocephalus serrulatus and the amphipod Hyalella curvispina.
Pleurodema thaul was the only amphibian present in the pond (). The tadpole stages 24 to 28 (body lengths ranging from 7 to 20 mm) of this frog have been reported as vulnerable to predation by Notonecta and other aquatic insects, while older developmental stages are invulnerable to insect predators (Jara & Perotti Citation2010). The vulnerable stages 24 to 28 had higher abundances during October and occurred in patches in the littoral zone attaining densities up to 30 ind/m3. During November and December, most of the tadpoles were older than stage 28, and therefore not vulnerable to predation.
Laboratory experiments
The survivorship of tadpoles and copepods in the feeding experiments was 100% in the controls indicating that natural mortality was negligible. Feeding experiments revealed that older nymphs of Notonecta had higher IRs than younger ones, independent of the prey type (a, b).
Figure 4 Ingestion rate (IR) (mean±1SEM; prey/pred./h) of different developmental stages of Notonecta vereertbruggheni on: a, the calanoid copepod Parabroteas sarsi and b, newly hatched tadpoles of Pleurodema thaul. IRs were calculated from laboratory feeding experiments in light and dark treatments.
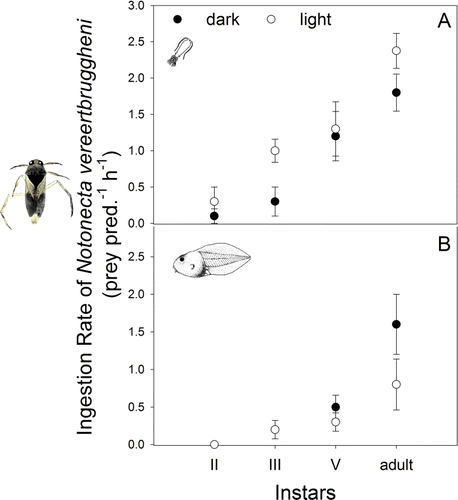
The IR of Notonecta on the copepod P. sarsi increased with the backwimmer instar (F 3, 38=20.6, P<0.001) and was higher in the light compared with the dark treatment (F 1, 38 = 4.97, P<0.03; a).
The consumption rate of the adults of Notonecta on tadpole prey was significantly higher than those shown by its nymphs (F 3, 39=12.8, P<0.001). Instar II did not feed on this prey regardless of the light treatment, while instars III and V had lower consumption rates than the adults (P<0.001). Interestingly, tadpoles were consumed by the adult backswimmers with higher rates in the dark than in light treatment (P<0.01) (b).
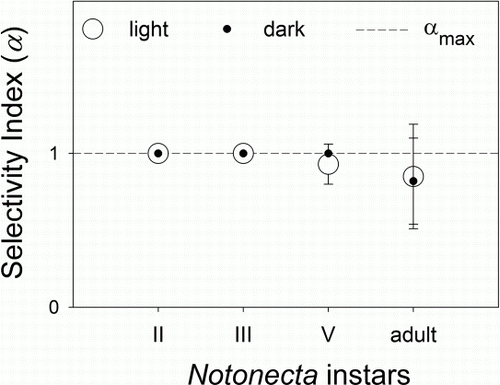
In the selectivity experiment, the different instars of backswimmer selected almost exclusively the copepod, neglecting the tadpoles (F 1, 75=508.05, P<0.001). Instars II and III exclusively selected the copepod (α = 1), regardless of the light availability (P=0.22). Instar V and the adults also preferred copepods, although they were also able to kill tadpoles, but at a lower rate and regardless of the light treatment (P=1) ().
Figure 5 Selectivity index (α) of Notonecta vereertbruggheni on Parabroteas sarsi calculated from mixed prey experiments exposing P. sarsi and newly hatched tadpoles of Pleurodema thaul, in light and dark treatments (mean±1SEM). The dashed line indicates maximum α (α=1). Values close to 1 indicate total preference for P. sarsi.
Discussion
Three species of backswimmers of the genus Notonecta, N. vereertbruggheni, N. fazi and N. virescens, occurred during spring and summer in 12 out of the 21 ponds surveyed inside the NHNP (Patagonia). Notonecta fazi was found exclusively in the two high altitude ponds surveyed, Verde and Los Patos, characterised by comparatively lower conductivity and greater depth. The most widespread species, N. vereertbruggheni, occurred in nine piedmont ponds studied, co-occurring in three shallow and vegetated piedmont ponds with N. virescens (). Several authors have found a strong association of Notonecta with aquatic vegetation (Streams & Newfield 1972 ; Hampton Citation2004). Aquatic plants play an important role in the reproduction of backswimmers since they have been observed to use macrophyte stems to insert or glue their eggs (Rice Citation1954).
The co-occurrence of Notonecta virescens and N. vereertbruggheni could be explained by the habitat complexity provided by aquatic vegetation in piedmont ponds inside the NHNP. Vegetation increases habitat complexity thereby providing refuge from predators and promoting co-occurrence. In particular the co-occurrence of notonectids has been reported to increase competition for shared prey and intraguild predation (Streams Citation1992a, Citationb; Svensson et al. Citation2000; Hampton Citation2004). The different body sizes of N. vereertbruggheni (up to 14 mm) and N. virescens (up to 9 mm), may also facilitate their co-occurrence since it may determine differential prey use, diminishing diet overlap, as has been pointed out by several authors (Streams Citation1974, Citation1992a; Svensson et al. Citation2000).
The study of the life cycle of N. vereertbruggheni in Fantasma pond revealed that migrant adult notonectids colonised the pond in spring and reproduced during October. The assemblage of developmental stages of N. vereertbruggheni reached its maximum complexity from November throughout December, when all nymphs were found co-occurring with the adults. The adults of N. vereertbruggheni were found exclusively in the littoral zone of the pond during daytime, perching on the vegetation, while the nymphs occurred also in the open water. This spatial segregation seems to be a common behavioural pattern in several notonectid species to avoid overlap (Fox Citation1975a, Citationb; Murdoch & Sih Citation1978; Streams & Shubeck Citation1982; Streams Citation1987b; Gilbert et al. Citation1999; Hampton Citation2004). In Fantasma pond, the higher abundance of notonectids in the littoral may be due to the presence of aquatic vegetation, which provides them with refuge against other insect predators. Although, the availability of their prey was higher in the open water, P. sarsi and newly hatched tadpoles were more abundant inside the vegetation. Thus, backswimmers appear to respond to factors other than simple prey availability when selecting optimal habitat inside the pond as has been suggested in previous studies (Hampton Citation2004). Habitat heterogeneity and the particular habitat use by different interacting stages or species may determine different levels of competition, intraguild predation and cannibalism in backswimmers (Bennett & Streams Citation1986; Sih Citation1987; Hampton Citation2004).
In Fantasma pond, N. vereertbruggheni co-occur with several groups of organisms including rotifers (Gilbert & Diéguez Citation2010), crustaceans (García Citation2010), insects and tadpoles (Jara & Perotti Citation2010; ), which may be considered potential prey given the fact that notonectids can access a wide variety of organisms (Cooper et al. Citation1985; Blaustein Citation1998). Several studies have pointed out that backswimmers actively select larger crustacean prey such as copepods and cladocerans (Cooper Citation1983; Cooper et al. Citation1985; Streams Citation1994; Gilbert et al. Citation1999). By exerting size selective predation, backswimmers are important organisers of community structure, strongly reducing larger pelagic or neustonic species, and indirectly favouring smaller and less competitive species (Scott & Murdoch Citation1983; Blaustein Citation1998; Hampton et al. Citation2000).
In Fantasma pond, the large calanoid copepod P. sarsi (up to 5 mm) and the newly hatched tadpoles of P. thaul (7–20 mm) are the largest prey occurring with several stages of N. vereertbruggheni during spring. The feeding experiments performed in the laboratory showed that different stages of N. vereertbruggheni were able to feed on the copepod while only stages III, V and adults fed on tadpoles. The backswimmer removed up to 3 copepods/pred./h and up to 1.5 tadpoles/pred./h (a, b). Light availability did not affect the IR of Notonecta on tadpoles; however, when the prey was Parabroteas the IR was comparatively lower in the dark. This suggests that visual detection is involved in foraging on copepods while mechanical cues may be enough for successful detection of tadpole prey. In fact, the instar V and the adults of N. vereertbruggheni preyed on tadpoles at a higher rate in the dark treatment (a, b).
Furthermore, Notonecta showed marked preference towards the copepod (0.8 < α<1) when presented with copepod and tadpoles together in selectivity experiments. Instar II and III exclusively selected the copepod (), while the remaining developmental stages of Notonecta also preyed on tadpoles, although with a lower selectivity coefficient. Preference for copepods was not affected by light availability and thus, it can be inferred that they may be preferentially consumed regardless the light availability during day and night (). The feeding efficiency of notonectids on small crustacean prey has been observed previously to depend on light availability (Streams Citation1982; Diéguez & Gilbert Citation2003).
Differences observed in the IR and selectivity may be due to prey palatability, size, conspicuousness and behaviour. Both prey used seem to be palatable as they were not rejected after capture. Tadpoles used in the experiments were up to 11 mm of total body size, more than two-fold larger than the copepods. Tadpole size may have constrained notonectid feeding, as apparently occurred in the case of the instar II. Also, the tadpoles remained still in the bottom of the experimental vessels when the predator was present, thus reducing their conspicuity. This behaviour has been observed to protect this prey from different predators such as waterbugs, dragonfly naiads and beetle larvae (Jara & Perotti Citation2010).
The higher consumption rate and selectivity of Notonecta on Parabroteas may reflect the fact that its size is readily accessible for all the instars, even though larger instars of the backswimmer fed more efficiently on the copepods, perhaps suggesting a more favourable predator–prey size relationship. Parabroteas is bright red coloured and about 5 mm of body length, and therefore can be considered a conspicuous prey, both for visual or mechanically oriented predators. This copepod can elicit jumps of several times its body length when encountered and can be considered highly evasive. However, this swimming behaviour is likely to enhance conspicuousness for Notonecta as reflected by the results of our experiments in the dark.
As far as we know, P. sarsi and the flatworm Mesostoma ehrenbergii are the only predators that have been considered to impact on other invertebrates in Fantasma pond (Vega Citation1998; Trochine et al. Citation2008). However, our results show that all nymphs of N. vereertbruggheni prey up on Parabroteas and thus, when notonectids are present in the pond (September–December) they may add complexity to the trophic interactions. Recently, Gilbert & Dieguez (Citation2010) suggested that predation pressure by Notonecta and Parabroteas may drive the population cycle of the rotifer Brachionus calyciflorus, which may also apply to other co-occurring prey, such as cladocerans, and copepods.
Overall, our results indicate that backswimmers are common in shallow piedmont and high altitude ponds inside the NHNP (Patagonia, Argentina). In particular, N. vereertbruggheni is the most common species. Predation by notonectids in fishless Patagonian ponds could influence the size structure and species abundance of pond communities during spring and summer when they are present at higher abundances.
Acknowledgements
This research was performed under the institutional animal care guidelines established by the Bureau for National Parks of Argentina (APN). This institution also granted the permissions to collect samples and living animals in ponds within the Nahuel-Huapi National Park (APN Nos. 498–730). Subsecretaria de Medio Ambiente of San Carlos de Bariloche authorised the samplings in Fantasma pond. We are grateful to S Mazzucconi and J Muzzon who kindly identified the backswimmers and macroinvertebrate species. C Queimaliños advised us with the Canonical Correspondence Analysis. This work was supported by UNComa B166, FONCyT PICT 01205, PICT 0381 and CONICET fellowship to FG Jara.
References
- Angrisano , EB . 1982 . Biología de algunas Notonectidae argentinas (Insecta, Heteroptera) . Physis , 40 : 121 – 132 .
- Bachmann , AO . 1962 . Apuntes para una hidrobiología Argentina. V. Los hemípteros acuáticos de los parques nacionales Lanín, Nahuel Huapi y Los Alerces y zonas vecinas (Insecta-Hemiptera) . Physis , 23 : 103 – 107 .
- Bachmann , AO . 1963 . Apuntes para una hidrobiología Argentina. VI. Los Hemiptera Cryptocerata de la Patagonia extracordillerana . Physis , 24 : 35 – 37 .
- Bachmann AO 1998 . Heteroptera acuáticos . In: Morrone JJ and Coscarón S (dir.) Biodiversidad de artrópodos argentinos. Una perspectiva biotaxonómica . La Plata , , Argentina , Ediciones Sur . Pp. 163 – 180 .
- Bennet , DV and Streams , FA . 1986 . Effects of vegetation on Notonecta (Hemiptera) distribution with and without fish . Oikos , 46 : 62 – 69 . doi: 10.2307/3565381
- Black , AR . 1993 . Predator-induced phenotypic plasticity in Daphnia pulex: life-history and morphological responses to Notonecta and Chaoborus . Limnology and Oceanography , 38 : 986 – 996 . doi: 10.4319/lo.1993.38.5.0986
- Blaustein , L . 1998 . Influence of the predatory backswimmer, Notonecta maculata, on invertebrate community structure . Ecological Entomology , 23 : 246 – 252 . doi: 10.1046/j.1365-2311.1998.00138.x
- Blaustein , L , Kotler , BK and Ward , D . 1995 . Direct and indirect effects of a predatory backswimmer (Notonecta maculata) on community structure of desert temporary pools . Ecological Entomology , 20 : 311 – 318 . doi: 10.1111/j.1365-2311.1995.tb00462.x
- Cei , JM . 1980 . Amphibians of Argentina. Monitore Zoologico Italiano . Italian Journal of Zoology , 2 : 1 – 609 .
- Chesson , J . 1978 . Measuring preference in selective predation . Ecology , 59 : 211 – 215 . doi: 10.2307/1936364
- Cobbaert , D , Bayley , SE and Greter , JL . 2010 . Effects of a top invertebrate predator (Dytiscus alaskanus; Coleoptera: Dytiscidae) on fishless pond ecosystems . Hydrobiologia , 644 : 103 – 114 . doi: 10.1007/s10750-010-0100-7
- Cooper , SD . 1983 . Selective predation on cladocerans by common pond insects . Canadian Journal of Zoology , 61 : 879 – 886 . doi: 10.1139/z83-115
- Cooper , SD , Smith , DW and Bence , JR . 1985 . Prey selection by freshwater predators with different foraging strategies . Canadian Journal of Fisheries and Aquatic Science , 42 : 1720 – 1732 . doi: 10.1139/f85-216
- Cronin , JT and Travis , J . 1986 . Size-limited predation on larval Rana areolata (Anura: Ranidae) by two species of backswimmer (Insecta: Hemiptera: Notonectidae) . Herpetologica , 42 : 171 – 174 .
- Diéguez , MC and Balseiro , EG . 1998 . Colony size in Conochilus hippocrepis: defensive adaptation to predator stage sizes . Hydrobiologia , 388 : 421 – 425 .
- Diéguez , MC and Gilbert , JJ . 2003 . Predation by Buenoa macrotibialis (Insecta, Hemiptera) on zooplankton: effect of light on selection and consumption of prey . Journal of Plankton Research , 25 : 759 – 769 . doi: 10.1093/plankt/25.7.759
- Fox , LR . 1975a . Some demographic consequences of food shortage for the predator Notonecta hoffmanni . Ecology , 56 : 868 – 880 . doi: 10.2307/1936297
- Fox , LR . 1975b . Factors Influencing cannibalism, a mechanism of population limitation in the predator Notonecta hoffmanni . Ecology , 56 : 933 – 941 . doi: 10.2307/1936303
- García RD 2010 . Ciclo de vida del copépodo depredador Parabroteas sarsi Calanoida , , Centropagidae : Impacto del canibalismo en la población de la Laguna Fantasma .
- Gilbert , JJ and Diéguez , MC . 2010 . Low crowding threshold for induction of sexual reproduction and diapause in a Patagonian rotifer . Freshwater Biology , 55 : 1705 – 1718 . doi: 10.1111/j.1365-2427.2010.02455.x
- Gilbert , JJ , Burns , CW and Gilbert , CC . 1999 . Summer distribution patterns of the backswimmer, Anisops wakefieldi (Hemiptera: Notonectidae), in a New Zealand Pond . New Zealand Journal of Marine and Freshwater Research , 33 : 661 – 672 . doi: 10.1080/00288330.1999.9516909
- Gittelman , SH . 1974 . Locomotion and predatory strategy in backswimmers . American Midland Naturalist , 92 : 496 – 500 . doi: 10.2307/2424316
- Gosner , KL . 1960 . A simplified table for staging anuran embryos on larvae with notes on identification . Herpetologica , 16 : 183 – 190 .
- Hampton , SE . 2004 . Habitat overlap of enemies: temporal patterns and the role of spatial complexity . Oecologia , 138 : 475 – 484 . doi: 10.1007/s00442-003-1446-6
- Hampton , SE , Gilbert , JJ and Burns , CW . 2000 . Direct and indirect effects of juvenile Buenoa macrotibialis (Hemiptera: Notonectidae) on the zooplankton of a shallow pond . Limnology and Oceanography , 45 : 1006 – 1012 . doi: 10.4319/lo.2000.45.4.1006
- Hero , JM , Gascon , C and Magnusson , WE . 1998 . Direct and indirect effects of predation on tadpole community structure in the Amazon rainforest . Austral Journal of Ecology , 23 : 474 – 482 . doi: 10.1111/j.1442-9993.1998.tb00755.x
- Herwig , BR and Schindler , DE . 1996 . Effects of aquatic insect predators on zooplankton in fishless ponds . Hydrobiologia , 324 : 141 – 147 . doi: 10.1007/BF00018175
- Heyer , WR , McDiarmid , RW and Weigmann , DL . 1975 . Tadpoles, predation and pond habitats in the tropics . Biotropica , 7 : 100 – 111 . doi: 10.2307/2989753
- Hössler , J , Maier , G and Tessenov , U . 1995 . Some notes on the ecology of a german Branchipus schaefferi population (Crustacea: Anostraca) . Hydrobiologia , 298 : 105 – 112 . doi: 10.1007/BF00033805
- Jara , FG . 2008a . Differential vulnerability of Physalaemus pustulosus tadpole size classes to predation by the water spider Thaumasia sp. (Physauridae) . Amphibia-Reptilia , 29 : 432 – 437 . doi: 10.1163/156853808785111977
- Jara , FG . 2008b . Tadpole–odonate larvae interactions: influence of body size and diel rhythm . Aquatic Ecology , 42 : 503 – 509 . doi: 10.1007/s10452-007-9110-6
- Jara FG 2010 . Plasticidad fenotípica en anuros Patagónicos de los géneros Pleurodema y Rhinella: respuestas al hidroperíodo y a los depredadores . Doctoral thesys. San Carlos de Bariloche. Universidad Nacional del Comahue, Centro Regional Universitario Bariloche .
- Jara , FG and Perotti , MG . 2009 . La rana de cuatro ojos en la laguna Fantasma de Bariloche . Desde la Patagonia Difundiendo Saberes , 6 : 10 – 15 .
- Jara , FG and Perotti , MG . 2010 . Risk of predation and behavioral response in three anuran species: influence of tadpole size and predator type . Hydrobiologia , 644 : 313 – 324 . doi: 10.1007/s10750-010-0196-9
- Manly , BFJ . 1974 . A model for certain types of selection experiments . Biometrics , 30 : 281 – 294 . doi: 10.2307/2529649
- Mazzucconi , SA . 2008 . Notonectidae . Biodiversidad de Artrópodos Argentinos , 2 : 209 – 221 .
- Melo , MC . 2009 . Biodiversity of aquatic and semiaquatic Heteroptera (Hemiptera) from Argentinean Patagonia . Revista de la Sociedad Entomológica Argentina , 68 : 177 – 185 .
- Murdoch , WW and Sih , A . 1978 . Age-dependent interference in a predatory insect . Journal of Animal Ecology , 47 : 581 – 592 . doi: 10.2307/3802
- Murdoch , WW , Scott , MA and Ebsworth , P . 1984 . Effects of the general predator, Notonecta (Hemiptera) upon a freshwater community . Journal of Animal Ecology , 53 : 791 – 808 . doi: 10.2307/4660
- Peckarsky , BL . 1982 . Aquatic insect predator–prey relations . BioScience , 32 : 261 – 266 . doi: 10.2307/1308532
- Repka , S , Ketola , M and Walls , M . 1994 . Specificity of predator-induced neck spine and alteration in life history traits in Daphnia pulex . Hydrobiologia , 294 : 129 – 140 . doi: 10.1007/BF00016853
- Rice , LA . 1954 . Observations on the biology of ten notonectid species found in the Douglas Lake, Michigan Region . American Midland Naturalist , 51 : 105 – 132 . doi: 10.2307/2422216
- Savage , AA . 1989 . Adults of the British aquatic Hemiptera Heteroptera. A key with ecological notes . Freshwater Biological Association, Scientific Publication , 50 : 1 – 173 .
- Schneider , DW and Frost , TM . 1996 . Habitat duration and community structure in temporary ponds . Journal of the North American Benthological Society , 15 : 64 – 86 . doi: 10.2307/1467433
- Schwind , R . 1980 . Geometrical optics of the Notonecta eye: adaptations to optical environment and way of life cycle . Journal of Comparative Physiology , 140 : 59 – 68 . doi: 10.1007/BF00613748
- Scott , MA and Murdoch , WW . 1983 . Selective predation by the backswimmer Notonecta . Limnology and Oceanography , 28 : 352 – 366 . doi: 10.4319/lo.1983.28.2.0352
- Sih , A . 1987 . Prey refuges and predator–prey stability . Theoretical Population Biology , 31 : 1 – 12 . doi: 10.1016/0040-5809(87)90019-0
- Sih , A , Crowley , P , McPeek , M , Petranka , J and Strohmeier , K . 1985 . Predation, competition and prey communities: a review of field experiments . Annual Review of Ecology and Systematics , 16 : 269 – 311 . doi: 10.1146/annurev.es.16.110185.001413
- Spencer , M , Blaustein , L , Schwartz , SS and Cohen , JE . 1999 . Species richness and the proportion of predatory animal species in temporary freshwater pools: relationships with habitat size and permanence . Ecological letters , 2 : 157 – 166 . doi: 10.1046/j.1461-0248.1999.00062.x
- Streams FA 1974 . Size and competition in Connecticut Notonecta . In: Beard RL , 25th Anniversary Memiors of the Connecticut Entomological Society . New Haven , CT , Connecticut Entomological Society . Pp. 215 – 225 .
- Streams , FA . 1982 . Spatial structure and intraspecific interactions in Notonecta populations . Environmental Entomology , 11 : 652 – 659 .
- Streams , FA . 1987a . Foraging behavior in a notonectid assemblage . American Midland Naturalist , 117 : 353 – 361 . doi: 10.2307/2425977
- Streams , FA . 1987b . Within-habitat spatial separation of two Notonecta species: interactive vs. noninteractive resource partitioning . Ecology , 68 : 935 – 945 . doi: 10.2307/1938365
- Streams , FA . 1992a . Intrageneric predation by Notonecta (Hemiptera: Notonectidae) in the laboratory and in nature . Annals of the Entomological Society of America , 85 : 265 – 273 .
- Streams , FA . 1992b . Age-dependent foraging depths of two species of Notonecta (Heteroptera: Notonectidae) breeding together in a small pond . Aquatic Insects , 14 : 183 – 191 . doi: 10.1080/01650429209361481
- Streams , FA . 1994 . Effect of prey size on attack components of the functional response by Notonecta undulata . Oecologia , 98 : 57 – 63 . doi: 10.1007/BF00326090
- Streams , FA and Newfield , S . 1972 . Spatial and temporal overlap among breeding populations of New England Notonecta. University of Connecticut Occasional Papers . Biological Science Series , 2 : 139 – 157 .
- Streams , FA and Shubeck , TP . 1982 . Spatial structure and intraspecific interactions in Notonecta populations . Environmental Entomology , 11 : 652 – 659 .
- Svensson , B , Tallmark , B and Petersson , E . 2000 . Habitat heterogeneity, coexistence and habitat utilization in five backswimmer species (Notonecta spp.; Hemiptera, Notonectidae) . Aquatic Insects , 22 : 81 – 98 . doi: 10.1076/0165-0424(200004)22:2;1-P;FT081
- ter Braak CJF 1988 . CANOCO a FORTRAN program for canonical community ordination by partial detrended canonical correspondence analysis, principal components analysis and redundancy analysis (version 2.1) . Report LWA-88-02 . Wageningen , , The Netherlands , Agricultural Mathematics Group .
- Trochine , C , Modenutti , B and Balseiro , E . 2006 . Influence of spatial heterogeneity on predation by the flatworm Mesostoma ehrenbergii (Focke) on calanoid and cyclopoid copepods . Journal of Plankton Research , 28 : 267 – 274 . doi: 10.1093/plankt/fbi071
- Trochine , C , Balseiro , E and Modenutti , B . 2008 . Zooplankton of fishless ponds of Northern Patagonia: insights into predation effects of Mesostoma ehrenbergii . International Review of Hydrobiology , 93 : 312 – 327 . doi: 10.1002/iroh.200711011
- Vega , MPA . 1995 . Morphology and defensive structures in the predator–prey interaction: an experimental study of Parabroteas sarsi (Copepoda, Calanoida) with different cladoceran prey . Hydrobiologia , 299 : 139 – 145 . doi: 10.1007/BF00017565
- Vega , MPA . 1998 . Impact of Parabroteas sarsi (Copepoda: Calanoida) predation on planktonic cladocerans in a pond of the Southern Andes . Journal of Freshwater Ecology , 13 : 383 – 389 . doi: 10.1080/02705060.1998.9663635
- Wellborn , GA , Skelly , DK and Werner , EE . 1996 . Mechanisms creating community structure across a freshwater habitat gradient . Annual Review of Ecology and Systematics , 27 : 337 – 363 . doi: 10.1146/annurev.ecolsys.27.1.337
- Wilbur , HM . 1987 . Regulation of structure in complex systems: experimental temporary pond communities . Ecology , 68 : 1437 – 1452 . doi: 10.2307/1939227
- Wilbur , HM . 1997 . Experimental ecology of food webs: complex systems in temporary ponds . Ecology , 78 : 2279 – 2302 . doi: 10.1890/0012-9658(1997)078[2279:EEOFWC]2.0.CO;2
- Williams DD 1987 . The ecology of temporary waters . Timber Press , Portland, Oregon .
- Zar JH 1999 . Biostatistical analysis , 4th edition . Upper Saddle River , NJ , Prentice Hall .