Abstract
Calanoid copepods of the genus Boeckella and Parabroteas (P. sarsi) are dominant in zooplankton communities of Patagonian lakes and ponds. This study addresses the occurrence of sexual size dimorphism in calanoid copepods of the genus Boeckella and in the monospecific genus Parabroteas occurring in 12 lakes and ponds of Patagonia (Argentina). The morphometric analysis performed showed that in all the species studied the female is larger than the male, although the difference in size among sexes was found to be variable between species. Interestingly, all species showed significant intraspecific stability in their sexual size dimorphism despite potential differences in their environments regarding predation pressure, environmental stability (permanent or temporary waters) and altitude distribution of populations. Finally, we discuss the potential implications of sexual dimorphism in terms of resource use and vulnerability to predation among other environmental forces which may play a role in promoting larger female to male size.
Introduction
Some studies propose that sexual dimorphism evolves to reduce intraspecific competition for food (intersexual niche divergence or ecological sexual dimorphism) and that it is not associated directly with selection on reproductive traits (Ralls Citation1976; Slatkin Citation1984; Shine Citation1989; Andersson Citation1994). In several species, the selective processes producing sexual differences result in dimorphism of total body length (sexual size dimorphism [SSD]). SSD is a widespread phenomenon among different groups of animals (Fairbairn Citation1997; Blanckenhorn Citation2005). In particular, female-biased SSD (i.e. females larger than males) predominates among invertebrates (Teder & Tammaru Citation2005; Esperk et al. Citation2007). Planktonic copepods are not exceptional in this regard, with females usually being larger and their SSDs being related to certain environmental variables, such as salinity, temperature and ultraviolet radiation, among other factors (Bayly Citation1978; Gilbert & Williamson Citation1983; Maly Citation1983; Grad & Maly Citation1988; Maly & Maly Citation1999). The higher relative size of female copepods has been hypothesised to be due to the greater investment in reproduction and offspring in the female, particularly in the species that carry the eggs during their development (Gilbert & Williamson Citation1983). Large females are more fecund (Cole Citation1966; Corkett & McLaren Citation1969; Hopkins Citation1977; Maly Citation1983), but they may reach maturity slower than small females or males (Gilbert & Williamson Citation1983; Nishikawa & Maly Citation1996). Some females store most of the energy for reproduction during their development, so the adult final size represents the maximum potential resource base that can be put towards reproduction (Gilbert & Williamson Citation1983).
The male is the active partner and its primary role is geared towards the location and encounter of the female (Ohtsuka & Huys Citation2001). Thus the larger females may exhibit a greater mating success compared to smaller ones (Maly Citation1978). However, the fact that larger males also tend to exhibit greater mating success could minimise any positive selection of sexual dimorphism in copepods (Gilbert & Williamson Citation1983). Some authors found that low food availability can produce a reduction in the male length, which may enhance food availability for females (Maly Citation1978; Gilbert & Williamson Citation1983). According to Bayly (Citation1978), the strongest dimorphism is found in species that occur exclusively in temporary waters where interspecific competition is likely to be low. In such conditions, a pronounced sexual dimorphism would enhance resource use by allowing access to an exceptionally wide range of particle sizes.
In inland waters from southern South America, the zooplankton is characterised by the frequent occurrence and/or dominance of copepods of the family Centropagidae (Soto & Zúñiga Citation1991; Modenutti et al. Citation1998; De los Ríos & Contreras Citation2005; Reissig et al. Citation2006; Adamowicz et al. Citation2010). This condition differs from that of lakes and ponds in the northern hemisphere, in which cladocerans and cyclopoid copepods codominate the zooplankton assemblages (Williamson Citation1984; Wong & Sprules Citation1985; Maier Citation1990). The genus Boeckella comprises 40 species distributed worldwide, of which 17 have been recorded in Argentina (Bayly Citation1992a; Menu-Marque & Zuñiga Citation1994; Adamowicz et al. Citation2007). Parabroteas sarsi is the largest centropagid copepod known and is found co-occurring with Boeckella in southern South America.
The Andean Patagonian region contains thousands of temporary and permanent ponds and lakes (Iriondo Citation1989). The zooplankton assemblages occurring in such ecosystems are dominated by endemic centropagids of the genus Boeckella (Modenutti et al. Citation1998; Menu-Marque et al. Citation2000; Marinone et al. Citation2006). They inhabit a remarkable diversity of habitats that range from small temporary pools to large and deep lakes coexisting with several invertebrate species as well as with endemic and introduced fishes.
In this study we address the occurrence of sexual dimorphism in nine centropagid species and describe the pattern of this trait. We discuss the potential relevance of sexual dimorphism in Boeckella spp. and Parabroteas sarsi as an adaptation to endure fluctuating environmental conditions, including resource limitation and predation, as well as other factors.
Methods
Study sites and sampling
The study was carried out in 12 Patagonian lakes and ponds, located between 38°58′–48°38′S and 70°22′–71°17′W (), sampled during different seasons from 2005 to 2010. Most of these lakes and ponds are shallow, with maximum depths less than 12 m (). Lake Rivadavia is a deep lake with a maximum depth of 147 m. Five of the studied ponds are temporary and fishless, whereas the other seven lakes and ponds are permanent (; ). Lakes Rivadavia and Morenito, and Ocho Pond have fish, while the other set of lakes are fishless.
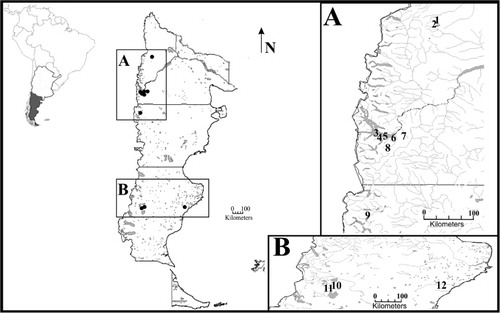
Table 1 Characteristics of sampled lakes and ponds and distribution of Boeckella species.
Conductance was measured with multiparameter probes. Zooplankton samples were collected with a 25 cm diameter plankton net of 55 µm mesh by horizontal tows at a central area of the ponds. The tows were pooled into one sample. In lakes Rivadavia and Morenito, zooplankton was collected with a 12 L Schindler-Patalas trap at different depths. The water from each depth was filtered through a 55 µm mesh net and pooled. All samples were preserved in 10% formalin. Copepods were separated from the samples under the microscope (Olympus SZ61 and Olympus SZ30). Adult copepods were identified to species level, following Bayly (Citation1992b), and separated by sex. Two morphological parameters were measured on each individual from about 50–70 copepods per sex: the prosomal length (PL, from the anterior margin up to the end of the prosome) and the total length (TL, from the anterior margin of the prosome to the insertion of the caudal setae). In the case of the species from Lake Rivadavia, only TL was measured. In some cases, where abundance was low, the measurements were performed on at least 30 individuals per sex.
Data analysis
Mean lengths and standard deviations of morphometric parameters recorded in each sex were obtained for the different species studied. Pearson correlation was applied to assess the relationship between PL and TL of each population. Comparisons of means among sexes within each population and among populations were performed using the non-parametric Mann-Whitney test. The non-parametric Kruskal-Wallis test was applied to compare the somatic measurements of males and females when more than two populations of the same species were available (P. sarsi).
The SSD was calculated using the mean of lengths, as follow:
Where ♀PL=mean female prosomal length; ♂PL=mean male prosomal length; ♀TL=mean female total length; and ♂TL=mean male total length. In the case of copepods from Lake Rivadavia, the SSD was estimated with the total length (♀TL/♂TL). Linear regression was applied to assess the SSD in centropagids using log-transformed data.
Results
The morphometric analysis performed in this study indicated that, except in B. antiqua and males of P. sarsi from Fantasma and Jesús ponds (Mann-Whitney test, P > 0.05; Kruskal-Wallis test, P > 0.05, respectively), the size of males and females of the remaining species showed inter-population variation (Mann-Whitney test, P <0.001 in all cases). Parabroteas sarsi, the largest species, showed significant variation among populations, with the sizes of the copepods from Lake Rivadavia being significantly smaller than those from Fantasma and Jesús ponds (). Boeckella brevicaudata was the largest Boeckella studied, while B. michalsenii was the smallest species (). All the Patagonian species of Centropagidae analysed showed a female-biased dimorphism, with the females always being larger than the males (, ). SSD varied strongly between species. The greatest relative size difference between females and males was found in B. gracilis and the smallest was recorded in B. gracilipes (SSD = 1.4 and c. 1, respectively; ; ). Larger species such as P. sarsi and B. brevicaudata, were found to be slightly dimorphic (SSD c. 1.06). The SSDs were relatively constant within species, despite there being intraspecific size differences among individuals from different environments (; ). TL had a high positive correlation with PL (Pearson correlation, R = 0.995, P<0.001, n=2251) so the use of either measurement in Boeckella could be a helpful tool to describe their SSD.
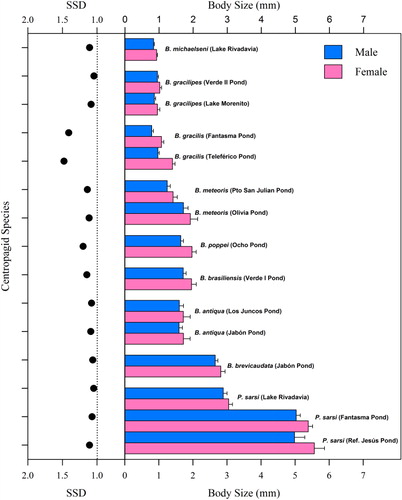
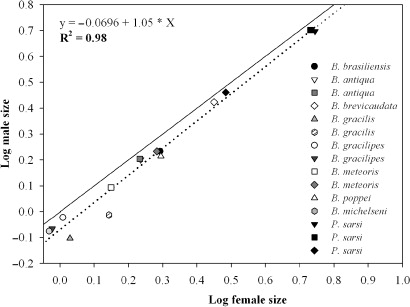
Table 2 Sexual size dimorphism and relationships between body lengths of centropagid species.
Discussion
In general, the size of males and females was found to be highly variable within species. In the case of P. sarsi, greater size difference was recorded among copepods from different lakes. Intraspecific plasticity in body size may be advantageous in the co-occurrence of centropagid species because it may prevent food niche overlap, thereby reducing competition for resources. However, other interactions, such as predation, may promote size divergence among separate populations. In the case of P. sarsi, the size difference found among the populations studied could be attributed to fish predation pressure. In Lake Rivadavia the predation pressure exerted by a fish assemblage composed of native and introduced species may explain the smaller relative size of P. sarsi as compared to the conspecifics present in the fishless system, Fanstasma Pond (Reissig et al. Citation2004). In other species, size differences could be attributed to differences in environmental conditions. For example, the body size of copepods has been shown to depend on temperature (Escribano & McLaren Citation1992; Van der Have & de Jong Citation1996; Atkinson & Sibly Citation1997; Hansen et al. Citation2010) and food conditions (Gilbert & Williamson Citation1983).
Overall, the observed intraspecific size fluctuations recorded in P. sarsi, B. antiqua, B. gracilis and B. meteoris suggest that absolute body size, by itself, is of limited taxonomic value in centropagid copepods ().
The size differences between females and males were significant in all the species studied, with females being larger than the males. This constant pattern may indicate an adaptive advantage, perhaps reflecting that the production of offspring in the females demands a comparatively greater energy investment which is afforded by comparatively greater resource storage (Gilbert & Williamson Citation1983). In particular, B. gracilipes was found to be the least dimorphic species (SSD = 1.044), together with B. michaelseni, the smallest species in this study. Interestingly, the two larger centropagid species analysed, P. sarsi and B. brevicaudata, also had low SSDs. In contrast, the greatest dimorphism was recorded in B. gracilis (1.477), which typically inhabits highly temporally dynamic shallow lakes in Patagonia (Trochine et al. Citation2008; Adamowicz et al. Citation2010; De los Rios-Escalante et al. Citation2010). The remarkable dimorphism in B. gracilis could be related to reproduction. This species has been found to have long copulation periods, which may last for several days (Trochine et al. Citation2005; R.D. Garcia, pers. obs. 2010). In this case, it may be hypothesised that extended copulation periods could represent an energetic cost for the females, which could be attenuated by a size reduction in the male. Besides, Grad and Maly (Citation1988) suggested that a pronounced SSD, such as observed in Diaptomus, may involve a cost because the small male would require more time to place the spermatophores properly in the female. If one sex carries the other one during courtship or mating, or if mating pairs remain attached for prolonged periods, the mating success of males may depend significantly on their relative size to their mates (Adams & Greenwood Citation1987; Fairbairn Citation1990, Citation1997; Lovich & Gibbons Citation1992).
This study shows that the SSDs of the centropagid species studied are variable, even though the most frequent values of SSD fall between 1.0 and 1.2. Bayly (Citation1978) suggested that environmental seasonality could be a factor influencing SSD, showing that the SSDs were higher in temporary ponds than in permanent environments for several Boeckella species from New Zealand and Australia. This hypothesis seems not to apply in the case of the Boeckella studied here because most of the species can be found either in permanent or temporary environments (Reissig et al. Citation2006; this study). Perhaps the most difficult problem for testing the hypothesis outlined by Bayly (Citation1978) is to determine which species are found exclusively in temporary or permanent waters, given the huge number of temporary and permanent environments in Patagonia and the tendency of Boeckella to occur in all of them. For example, we know through personal observations that B. brevicaudata and B. antiqua inhabit only temporary environments during winter, while the largest freshwater copepod, P. sarsi can be found in temporary ponds as well as in deep lakes (Vega Citation1999; Reissig et al. 2004, 2006; De los Ríos & Rivera Citation2008).
Interestingly, the SSDs were relatively constant within species of centropagids () despite size differences between populations, such as observed in populations from shallow fishless ponds or lakes with complex fish assemblages. The intraspecific stability in SSD observed could indicate genetic rather than ecological control of SSDs, as was suggested previously by Bayly (Citation1978). Further, Jersabek et al. (Citation2007) postulated that populations of Arctodiaptomus alpinus from fishless alpine lakes exhibit higher SSD (c. 1.4) than those of lowland species (c. 1.2). Among centropagid species B. gracilipes inhabits a wide environmental gradient from deep piedmont lakes to high altitude ponds (Menu-Marque et al. Citation2000). Remarkably B. gracilipes was the least dimorphic species recorded in this study (SSD = 1.044), contrasting with the findings of Jersabek et al. (Citation2007) in diaptomid copepods. Centropagids seem to bear low to null intraspecific differences in SSD among populations in contrasting environments. In this way, the SSD may qualify as a more suitable morphometric variable for taxonomic purposes than body size, because of its intraspecific stability.
In crustaceans, body size is a major factor determining the potential egg mass (Jensen Citation1958; McLaren Citation1965; Dagg Citation1976). Particularly in copepods, larger females tend to have larger clutches (Smyly Citation1968; Maly Citation1973) which may represent an advantageous reproductive trait. Nevertheless, environmental variables, such as temperature and food availability, also play an important role in determining egg production potential (Dagg Citation1978; Jamieson & Burns Citation1988). Furthermore, predation pressure may influence the body size of co-existing potential prey, perhaps favouring larger females that can produce and carry more eggs, but not so large so as to increase their vulnerability to predation (Hall et al. Citation1976; DeFrenza et al. Citation1986). In contrast, predation may be influenced by the degree of dimorphism, as it may be adaptive for both males and females to achieve a size that minimises the loss by predation. Also, predation may threaten reproductive success through the differential removal of one sex, as may be the case when the males become small enough to fall within the feeding range of a planktonic predator (Dodson Citation1974) or when the females become large enough to be eaten by a nektonic predator (insect or fish; Bayly Citation1978). Only a few studies have analysed the susceptibilities of male and female copepods to predators but, ultimately, the outcome of sex-dependent predation appears to be related to the assemblage of predators occurring in nature (Maly Citation1970; Gerritsen Citation1978; Cooper & Goldman Citation1980; Trochine et al. Citation2005).
In southern Argentina, the zooplankton assemblages are characterised by the dominance of endemic calanoid copepods of different species, which have a central ecological role in pelagic food webs of large and deep lakes as well as in shallow ponds (Menu-Marque et al. Citation2000; Hansson & Tranvik Citation2003; Reissig et al. Citation2004, Citation2006; Trochine et al. Citation2008; Lancelotti et al. Citation2009). Most boeckellids have a tendency to exploit larger food items towards adulthood. Small species of Boeckella, such as B. gracilipes, B. gracilis and B. michaelseni, feed on phytoplankton and small ciliates while larger species, such as B. brevicaudata and B. antiqua, appear to be able also to access larger food items. In contrast, P. sarsi shifts its diet, being herbivorous in naupliar stages, omnivorous in early copepodid stages and carnivorous from copepodite IV to adulthood. Food niche differences have been reported between species of Boeckella and among developmental stages of P. sarsi; however, as far as we know, there is no evidence of food niche divergence among sexes based on their size difference. Due to the ecological relevance of centropagid copepods in lake foodwebs of Antarctic, Patagonian and Andean lakes, it could be hypothesised that SSD could increase complexity in pelagic food webs if size differences between sexes are sufficiently large to segregate food niches according to sex. Furthermore, there is a lack of information regarding SSDs in these copepods in relation to predation pressure by invertebrates or fish, such that females and males of similar size would have similar vulnerability to predation while females larger than males would imply differences in vulnerability depending on the predator type and selectivity. In this context, our results show a first record of the presence of dimorphic pairs within centropagid species, which may increase the complexity of interactions within their habitats. Further investigations are needed to determine the ecological implications of the observed patterns of dimorphism in Patagonian systems.
Acknowledgements
This research was supported by Universidad Nacional del Comahue (B-001). The authors would like to thank C. Pozzi, P. Naim and J. Lancelotti for collecting samples from ponds in Santa Cruz Province (Argentina). Thanks also to M.C. Diéguez for helpful comments on the manuscript. R.D. García and P.E. García are CONICET Fellows, and Mariana Reissig is a CONICET Researcher.
References
- Adamowicz SJ, Menu-Marque SA, Halse SA, Topan JC, Zemlak TS, Hebert DN, Witt JDS 2010. The evolutionary diversification of the Centropagidae (Crustacea, Calanoida): a history of habitat shifts. Molecular Phylogenetics and Evolution 55: 418–430.
- Adamowicz SJ, Menu-Marque SA, Hebert P, Purvis A 2007. Molecular systematics and patterns of morphological evolution in the Centropagidae (Copepoda: Calanoida) of Argentina. Biological Journal of the Linnean Society 90: 279–292.
- Adams J, Greenwood PJ 1987. Loading constraints, sexual selection and assortative mating in peracarid Crustacea. Journal of Zoology 211: 35–46.
- Andersson M 1994. Sexual selection. Princeton, NJ, Princeton University Press. 599 p.
- Atkinson D, Sibly RM 1997. Why are organisms usually bigger in colder environments? Making sense of a life-history puzzle. Trends in Ecology and Evolution 12: 235–239.
- Bayly IAE 1978. Variation in sexual dimorphism in nonmarine Calanoid copepods and its ecological significance. Limnology and Oceanography 23: 1224–l228.
- Bayly IAE 1992a. Fusion of the genera Boeckella and Pseudoboeckella (Copepoda) and revision of their species from South America and sub-Antartic islands. Revista Chilena de Historia Natural 65: 17–63.
- Bayly IAE 1992b. The non-marine Centropagidae (Copepoda: Calanoida) of the world. Guides to the identification of the microinvertebrates of the continental waters of the world. The Netherlands, SPB Academic Publishing BV.
- Blanckenhorn WU 2005. Behavioral causes and consequences of sexual size dimorphism. Ethology 111: 977–1016.
- Cole GA 1966. Contrasts among calanoid copepods from permanent and temporary ponds in Arizona. The American Midland Naturalist Journal 76: 351–368.
- Cooper SD, Goldman CR 1980. Opossum shrimp (Mysis relicta) predation on zooplankton. Canadian Journal of Fisheries and Aquatic Sciences 37: 909–919.
- Corkett CJ, McLaren IA 1969. Egg production and oil storage by the copepod Pseudocalanus in the laboratory. Journal of Experimental Marine Biology and Ecology 3: 90–105.
- Dagg MJ 1976. Complete carbon and nitrogen budgets for the carnivorous amphipod Calliophts laeviuscuhts (Kroyer). Internationale Revue gesamten Hydrobiologie 61: 297–357.
- Dagg MJ 1978. Estimated, in situ, rates of egg production for the copepod Centropages typicus (Kroyer) in the New York bight. Journal of Experimental Marine Biology and Ecology 34: 183–196.
- De los Ríos P, Contreras P 2005. Salinity level and occurrence of centropagid copepods (Crustacea, Copepoda, Calanoida) in shallow lakes in Andes mountains and Patagonian plains, Chile. Polish Journal of Ecology 53: 445–450.
- De los Ríos P, Rivera R 2008. On the geographic distribution of Parabroteas sarsi (Mrázek, 1901) (Copepoda, Calanoida). Anales Instituto Patagonia (Chile) 36: 75–78.
- De los Ríos-Escalante P, Carreño E, Hauenstein E, Vega M 2010. An update of the distribution of Boeckella gracilis (Daday, 1902) (Crustacea, Copepoda) in the Araucania region (38°S), Chile, and a null model for understanding its species associations in its habitat. Latin American Journal of Aquatic Research 38: 507–513.
- DeFrenza J, Kirner RJ, Maly EJ, Van Leeuwen HC 1986. The relationships of sex size ratio and-season to mating intensity in some calanoid copepods. Limnology and Oceanography 31: 491–496.
- Dodson SI 1974. Zooplankton competition and predation: an experimental test of the size efficiency hypothesis. Ecology 55: 605–613.
- Escribano R, McLaren IA 1992. Influence of food and temperature on lengths and weights of two marine copepods. Journal of Experimental Marine Biology and Ecology 159: 77–88.
- Esperk T, Tammaru T, Nylin S, Teder T 2007. Achieving high sexual size dimorphism in insects: females add instars. Ecological Entomology 32: 243–256.
- Fairbairn DJ 1990. Factors influencing sexual size dimorphism in temperate water striders. American Naturalist 136: 61–86.
- Fairbairn DJ 1997. Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Annual Review of Ecology and Systematics 28: 659–687.
- Gerritsen J 1978. Intra-specific swimming patterns and predation of planktonic copepods. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 20: 2531–2536.
- Gilbert J, Williamson C 1983. Sexual dimorphism in zooplankton (Copepoda, Cladocera and Rotifera). Annual Review of Ecology and Systematics 14: 1–33.
- Grad G, Maly EJ 1988. Sex size ratios and their influence on mating success in a calanoid copepod. Limnology and Oceanography 33: 1629–1634.
- Hall DJ, Threlkeld ST, Burns CW, Crowley PH 1976. The size efficiency hypothesis and the size structure of zooplankton communities. Annual Review of Ecology and Systematics 7: 177–208.
- Hansen BW, Drillet G, Kozmér A, Madsen KV, Pedersen ME, Sorensen TF 2010. Temperature effects on copepods egg hatching: does acclimatization matter?Journal of Plankton Research 32: 305–315.
- Hansson L, Tranvik L 2003. Food webs in sub-Antarctic lakes: a stable isotope approach. Polar Biology 26: 783–788.
- Hopkins CCE 1977. The relationship between maternal body size and clutch size, development time, and egg mortality in Euchaeta norvegica (Copepoda: Calanoida) from Loch Etive, Scotland. Journal of the Marine Biological Association of the UK 57: 723–733.
- Iriondo M 1989. Quaternary lakes of Argentina. Palaeogeography, Palaeoclimatology and Palaeoecology 70: 81–88.
- Jamieson C, Burns CW 1988. The effects of temperature and food on copepodite development, growth and reproduction in three species of Boeckella (Copepoda; Calanoida). Hydrobiologia 164: 235–257.
- Jensen JP 1958. The relation between body size and number of eggs in marine Malacostrakes. Meddelelser fra Danmarks Fiskeri-Og Havundersogelser. NY Series 19: 25.
- Jersabek C, Luger M, Schabetsberger R, Grill S, Strickler JR 2007. Hang on or run? Copepod mating versus predation risk in contrasting environments. Oecologia 153: 761–773.
- Lancelotti JL, Pozzi LM, Yorio, PM, Diéguez MC, Pascual MA 2009. Fishless shallow lakes of Southern Patagonia as habitat for waterbirds at the onset of trout aquaculture. Aquatic Conservation: Marine and Freshwater Ecosystem 19: 497–505.
- Lovich JE, Gibbons JW 1992. A review of techniques for quantifying sexual size dimorphism. Growth Development & Aging 56: 269–281.
- Maier G 1990. Coexistence of the predatory cyclopoids Acanthocyclops robustus (Sars) and Mesocyclops leuckarti (Claus) in small eutrophic lake. Hydrobiologia 198: 185–203.
- Maly EJ 1970. The influence of predation on the adult sex ratios of two copepod species. Limnology and Oceanography 15: 566–573.
- Maly EJ 1973. Density, size, and clutch of two high altitude diaptomid copepods. Limnology and Oceanography 18: 840–848.
- Maly EJ 1978. Some factors influencing size of Diaptomus shoshone. Limnology and Oceanography 23: 835–837.
- Maly EJ 1983. Some further observations on diaptomid body size and clutch size relationships. Limnology and Oceanography 28: 148–152.
- Maly EJ, Maly MP 1999. Body size and sexual size dimorphism in calanoid copepods. Hydrobiologia 391: 173–179.
- Marinone MC, Menu-Marque S, Añón Suárez D, Dieguez MC, Perez P, De Los Ríos P, Soto D, Zagarese HE 2006. UV radiation as a potential driving force for zooplankton community structure in Patagonian lakes. Photochemistry and Photobiology 82: 962–971.
- McLaren IA 1965. Some relationships between temperature and egg size, body size, development rate and fecundity of the copepod Pseudocalanus. Limnology & Oceanography 10: 528–538.
- Menu-Marque SA, Morrone J, Locascio De Mitrovich C 2000. Distributional patterns of the South American species of Boeckella (Copepoda, Centropagidae): a track analysis. Journal of Crustacean Biology 20: 262–272.
- Menu-Marque SA, Zuñiga L 1994. Boeckella diamantina n. sp. (Calanoida, Centropagidae), from a high Andean lake in Mendoza, Argentina. Hydrobiologia 292/293: 81–87.
- Modenutti BE, Balseiro EG, Queimaliños CP, Añon Suárez D, Diéguez MC, Albariño RJ 1998. Structure and dynamics of food webs in Andean lakes. Lakes & Reservoirs: Research and Management 3: 179–186.
- Nishikawa TS, Maly EJ 1996. Factors influencing the degree of sexual size dimorphism within and among calanoid copepod species. Oecologia 107: 490–497.
- Ohtsuka S, Huys R 2001. Sexual dimorphism in calanoid copepods: morphology and function. Hydrobiologia 453/454: 441–466.
- Ralls K 1976. Mammals in which females are larger than males. Quarterly Review of Biology 51: 245–76.
- Reissig M, Modenutti BE, Balseiro EG, Queimaliños CP 2004. The role of the predaceous copepod Parabroteas sarsi in the pelagic food web of a large deep Andean lake. Hydrobiologia 524: 67–77.
- Reissig M, Trochine C, Queimaliños CP, Balseiro EG, Modenutti BE 2006. Impact of fish introduction on planktonic food webs in lakes of the Patagonian Plateau. Biological Conservation 132: 437–447.
- Shine R 1989. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Quarterly Review of Biology 64: 419–461.
- Slatkin M 1984. Ecological causes of sexual dimorphism. Evolution 38: 622–630.
- Smyly WJP 1968. Number of eggs and body size in Diaptomus grucilis Sars in the English Lake District. Oikos 19: 323–338.
- Soto D, Zúñiga L 1991. Zooplankton assemblages of Chilean temperate lakes: a comparison with North American counterparts. Revista Chilena de Historia Natural 64: 569–581.
- Teder T, Tammaru T 2005. Sexual size dimorphism within species increases with body size in insects. Oikos 108: 321–334.
- Trochine C, Balseiro EG, Modenutti BE 2008. Zooplankton of fishless ponds of Northern Patagonia: insights into predation effects of Mesostoma ehrenbergii. International Review of Hydrobiology 93: 312–327.
- Trochine C, Modenutti BE, Balseiro EG 2005. When prey mating increases predation risk: the relationship between the flatworm Mesostoma ehrenbergii and the copepod Boeckella gracilis. Archiv für Hydrobiologie 163: 555–569.
- Van der Have TM, de Jong G 1996. Adult size in ectotherms: temperature effects on growth and differentiation. Journal of Theoretical Biology 183: 329–340.
- Vega M 1999. Life-stage differences in the diet of Parabroteas sarsi (Daday) (Copepoda, Calanoida): a field study. Limnologica 29: 186–190.
- Williamson CE 1984. Laboratory and field experiments on the feeding ecology of the freshwater cyclopoid copepod, Mesocyclops edax. Freshwater Biology 14: 575–585.
- Wong CK, Sprules WG 1985. Size-selective feeding by the predatory copepod Epischura lacustris. Canadian Journal of Aquatic Sciences 42: 189–193.