Abstract
The diatom genus Pseudo-nitzschia blooms throughout New Zealand's coastal waters. More than a decade of monitoring data were analysed in this study and seasonality was a significant factor in the appearance of Pseudo-nitzschia blooms. Austral spring and summer were the main bloom periods, although there were regional differences. Between January 2000 and August 2011, 8.4% of 29,000 seawater samples analysed for Pseudo-nitzschia exceeded the voluntary trigger level for biotoxin testing (10×104 cells l−1), and these were analysed by fluorescent in situ hybridisation (FISH) assays. Pseudo-nitzschia fraudulenta and P. pseudodelicatissima (low domoic acid [DA] producers) were the dominant bloom formers throughout New Zealand (each contributing 25%) followed by the highly toxic P. australis (10%). Shellfish flesh testing for DA was triggered on 8477 occasions between 2000 and 2011, but no samples exceeded the regulatory limit (20 mg/kg); the maximum concentration was 13 mg/kg (Marlborough Sounds, mid-winter 2010).
Introduction
Shellfish were first monitored in New Zealand for the biotoxin domoic acid (DA) in 1993, following a major neurotoxic shellfish poisoning event (Jasperse Citation1993). DA itself was not implicated in the event, which was caused by a massive Karenia bloom, but as DA was a regulated toxin internationally it was included in the New Zealand biotoxin monitoring programme established as a result of that event. The commercial harvest (which included exported product) came under both New Zealand's Shellfish Quality Assurance programme and the first New Zealand Shellfish Standard (Busby Citation1992). A phytoplankton monitoring programme was also established in 1993 and it included voluntary trigger levels for the genus Pseudo-nitzschia (H. Peragallo), i.e. cell concentrations at which shellfish harvesting would voluntarily cease until results of analyses for biotoxins were available. The voluntary trigger levels were determined collaboratively by industry representatives, regulators and researchers, and were based on the known DA concentrations (pg/cell) produced by the various Pseudo-nitzschia species ().
Table 1 Domoic acid (DA) production determined for Pseudo-nitzschia isolates from sites throughout New Zealand (analysed by LC-MS or HPLC-UV).
The non-commercial (or recreational) shellfish harvest was also monitored for biotoxins from 1993 and a review was commissioned in 1996 by the Ministry of Health (Wilson & Sim Citation1996). Amnesic shellfish poisoning (ASP) was assessed as being ‘theoretically possible’ in New Zealand, but of low risk. Scallops were noted as being prone to DA contamination as Pseudo-nitzschia cells may sink and enter the benthic scallop beds (Rhodes et al. Citation1998a). For example, the most toxic phase for P. australis is the stationary phase and empty frustules of this species were detected (standard error mean, SEM) in digestive glands of Northland scallops between January 1993 and July 1996, when high DA concentrations were recorded (Rhodes Citation1994; Rhodes et al. Citation1998a). To reduce costs, management of non-commercial areas became focused on areas with a known biotoxin issue (Rhodes et al. Citation2001a).
In 1997, in commercially regulated areas with no history of biotoxin events, shellfish testing was reduced to fortnightly or monthly, but with mandatory weekly phytoplankton sampling (P. Busby, Ministry of Primary Industries [MPI] Food Safety, pers. comm. 2012). If Pseudo-nitzschia trigger levels were exceeded a shellfish sample was analysed for DA within 24–48 h. Between 1993 and 1999 the percentage of whole shellfish samples that contained DA exceeding the regulatory levels was only 0.2% of 19,000 samples analysed, and biotoxin testing was triggered by 2.4% of the>9000 seawater samples analysed for Pseudo-nitzschia (Hay et al. Citation2000). Specifications for the regulated control of bivalve molluscan shellfish were published in 2006 (NZFSA Citation2006), and this has helped ensure continued access of product to world markets.
The turnaround time for biotoxin analyses between 1993 and 2001 was considerably longer than for the phytoplankton analyses, results of which were reported within 24 h of sample receipt. To increase the value of the phytoplankton data, fluorescent in situ hybridisation (FISH) assays, to determine the species present, were carried out on request if Pseudo-nitzschia levels increased above 5×104 cells l−1. The FISH assays were accredited in 1996 (NZS/ISI/IEC 17025) and accepted as a risk assessment tool (although not a regulatory tool) in the New Zealand Marine Biotoxin Management Plan. Analysis of 200 FISH assays, carried out for both research and commercial testing between 1997 and 1999, determined that P. pungens Grunow ex Cleve and P. fraudulenta (Cleve) Hasle constituted 70% of the total Pseudo-nitzschia detected during that time. Interestingly, 16% of assays carried out specifically for commercial sites were dominated by the highly toxic P. australis Frenguelli and 40% by non-toxic species with the balance comprised of low-toxin producers dominated by P. fraudulenta (Rhodes et al. Citation2001b).
Other species of Pseudo-nitzschia recorded between 1993 and 1999 in New Zealand waters included P. multiseries Hasle (Hasle), P. turgidula (Hustedt) Hasle, P. pseudodelicatissima (Hasle) Hasle emend. Lundholm, Moestrup et Hasle, P. delicatissima (Cleve) Heiden and P. americana (Hasle) Fryxell in Hasle. Six strains of P. americana were identified (Lundholm et al. Citation2002) and tested for DA by HPLC-UV (Rhodes et al. 1996, 1998a, b) and immunoassay (Garthwaite et al. Citation1998a, Citationb) and all were below the detection limit of the analytical methods. Similarly, all strains of P. cf. heimii Manguin and P. multistriata (Takano) Takano tested at that time were negative for DA ().
By 2000, FISH assays were being used regularly in the non-commercial monitoring programme to determine whether shellfish samples should be taken for toxin analysis. On the basis of the data generated from the previous decade of research, trigger levels for Pseudo-nitzschia were relaxed in 2006 to 10×104 cells l−1 for P. australis, P. pungens and P. multiseries, and to 50×104 cells l−1 for all other Pseudo-nitzschia species (NZFSA Citation2006). One problem for the phytoplankton monitoring risk assessments has been the variable FISH response to Pseudo-nitzschia cells that fall within the ‘delicatissima complex’. The complex now includes P. delicatissima, P. pseudodelicatissima, P. multistriata, P. calliantha Lundholm, Moestrup et Hasle, P. caciantha Lundholm, Moestrup et Hasle and P. cuspidata (Hasle) Hasle emend. Lundholm, Moestrup et Hasle (Lundholm et al. Citation2003). In this study, species in the ‘delicatissima complex’ detected in New Zealand coastal waters have been identified using transmission electron microscopy (TEM) and/or DNA sequencing. Once cultures were established, their toxin production was ascertained.
Despite the assessed low risk of ASP occurring in New Zealand, DA has been detected in shellfish on occasion. The aims of this review were therefore to elicit quantitative data regarding the occurrence of Pseudo-nitzschia species and DA in New Zealand, and to ascertain whether any seasonal, geographic or environmental trends could be determined for Pseudo-nitzschia blooms.
Methods
Sampling, isolation and culture
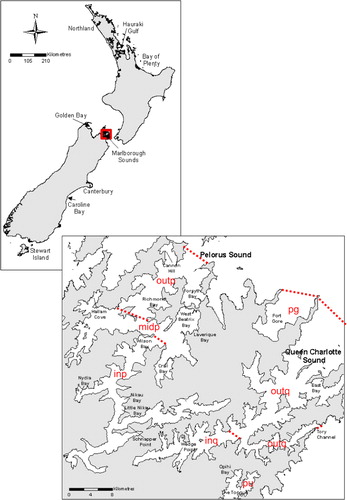
Samples were collected with hose or bottle samplers for routine phytoplankton monitoring programmes by approved samplers (MPI certificated) from c. 70 sites around New Zealand's coastline (). Pseudo-nitzschia cells were isolated from untreated sea water (modified micro-pipettes, f/2 medium; Guillard Citation1975) and enumerated from Lugol's iodine-treated seawater samples. Analyses were carried out by Cawthron Institute's Micro-algae Laboratory using Utermöhl chambers (Utermöhl Citation1958) and inverted light microscopes (Olympus, IMT2). Risk assessments (based on total Pseudo-nitzschia species cell concentrations and maximum potential DA concentrations per cell) were provided to regulators and marine farm managers within 24 h of sample receipt.
Clonal cultures were incubated at 100 µmol m−2 s−1 (PAR, 14:10 h light:dark), 19 °C and maintained in the Cawthron Institute Culture Collection of Micro-algae. Clonal isolates were stressed by removal of silicate from medium for production of DA (3 d growth in f/2 minus Na2SiO3.5H2O, 30 ml in 50 ml plastic pottles; Biolab, New Zealand), then a further 9–14 d growth (40% inoculum; 100 ml f/2 –Si in 200 ml plastic pottles). A cell count was obtained and cultures frozen until analysis for DA.
Identification
Fluorescent in situ hybridisation assays targeting species-specific large-subunit ribosomal RNA (LSU rRNA) were used to identify cultured Pseudo-nitzschia isolates as previously described (Scholin et al. Citation2003; Miller & Scholin Citation1996). The method was previously validated for New Zealand coastal waters by SEM of isolates following FISH assays (Holland Citation2001).
Pseudo-nitzschia cells were prepared for TEM by settling cultured cells in 50 ml KIMAX tubes, removing culture medium and adding hydrochloric acid (10 ml, 1 h), washing (distilled water) and adding sulphuric acid (2 ml) and potassium permanganate solution (10 ml, 16 h) then adding oxalic solution until the colour was dispelled. The cells were settled and washed several times per day for 3 d (Hendey Citation1974) and were then loaded onto grids for species confirmation (400 mesh copper grids pre-coated with parlodion). A Philips CM12 transmission microscope was run at 80 kv (University of Auckland, New Zealand) and images captured on a Gatan model 792 BioScan camera.
DNA extraction, polymerase chain reaction (PCR) amplification, and sequencing
Cultures of Pseudo-nitzschia spp. were grown to mid-exponential phase and pelleted by gentle centrifugation (10 ml, 542×g, 15 min, 20 °C) for DNA sequencing. Genomic DNA was extracted from the resultant pellet using DNeasy Plant Mini Kits (Qiagen, Hilden, Germany), following the manufacturer's instructions. The D1–D3 fragment of the large subunit ribosomal DNA (LSU rDNA) was amplified using the primers D1R-F (Scholin et al. Citation1994) and D3B-R (Nunn et al. Citation1996). PCR products were purified with AxyPrepTM PCR clean-up kit (Axygen Biosciences, CA, USA) and sequenced (both directions), using the PCR primers (Waikato University DNA Sequencing Facility, Hamilton, New Zealand). Sequence chromatograms were examined visually and base-calling errors corrected manually using BioEdit Sequence Alignment Editor (Hall Citation1999). Sequences were compared to those in GenBank using BLAST online software (http://blast.ncbi.nlm.nih.gov/Blast.cgi/).
Chemistry
DA analyses were carried out using LC-MS as part of a multi-residue method for shellfish toxins (Biotoxin Lab, Cawthron Institute) as described previously (Holland et al. Citation2003, Citation2005). Level of detection: 0.05 mg/kg.
Data sources and analysis
Quantifications of: 1) Pseudo-nitzschia spp.; 2) Pseudo-nitzschia spp. using FISH assays; and 3) DA concentrations were obtained by analysing data from samples collected between 2000 and 2011. Additional environmental data for selected sites were obtained from the Marlborough Shellfish Quality Programme's routine monitoring dataset, which included weekly depth averaged temperature and salinity for the period 2003–10. To identify seasonal patterns, samples were classified into spring (September–November), summer (December–February), autumn (March–May) and winter (June–August). Average phytoplankton abundances were calculated and mapped if samples shared the same recorded location. The proportion of samples exceeding the voluntary trigger level (10×104 cells l−1) was estimated and mapped by site and season. DA concentrations and the proportion of samples exceeding the regulatory level (20 mg/kg) were also mapped.
Species composition from data generated by FISH assays was provided as the percentage of the total Pseudo-nitzschia cell count. To reveal spatial and seasonal variation, the mean contribution of each species was calculated by site and season. A generalised linear model (GLM; Rhodes & Jiang Citation2011; Rhodes et al. Citation2012) was used to explore potential relationships of environmental variables with the concentration of Pseudo-nitzschia spp. in Forsyth Bay, Marlborough Sounds.Footnote1
Two GLMs were constructed following the steps outlined in Zuur et al. (Citation2010) to explore relationships between Pseudo-nitzschia cell concentration in seawater, DA concentration in shellfish, and environmental variables. Firstly, a logistic GLM was constructed to assess if independent variables (sample date, location [strata], latitude, longitude, temperature and equivalent freshwater depth [efd]) could be used to predict the exceedance of the voluntary trigger level for Pseudo-nitzschia cell concentrations. Efd was used in the analysis because the depth-averaged salinity was not comparable between times (the depth varied between samples) and is calculated as:
The second model, a Gaussian GLM, investigated possible correlation between Pseudo-nitzschia cell concentration and environmental variables on DA concentrations. The DA dataset was matched with the Pseudo-nitzschia dataset and then with the environmental dataset. A total of 482 samples from nine sites (2003 to 2011) were available for the analysis.
A forward selection procedure was used to construct both GLM models with the lowest Akaike Information Criterion (AIC; Akaike Citation1974) used for the model selection criteria. The performance of the Gaussian GLM was determined by analysis of the variance explained by the model using the coefficient of determination (R2). The performance of the logistic model was determined through assessment of the area under a ROC curve (AUROC) and was defined using the traditional academic point system, where AUROC score ranges are: 0.90–1.00=excellent; 0.80–0.90=good; 0.70–0.80=fair; 0.60–0.70=poor; 0.50–0.60=fail (Swets Citation1988).
Results and discussion
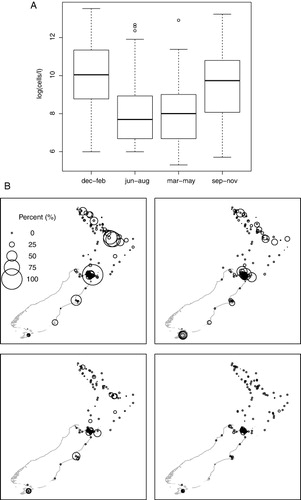
Pseudo-nitzschia was first recorded in New Zealand over 50 years ago (Crosby & Ferguson Wood Citation1959). Between 1994 and 1999, 66% of the regular weekly phytoplankton monitoring samples contained Pseudo-nitzschia cells, but only 2% of samples containing Pseudo-nitzschia resulted in closures to harvesting shellfish (Hay et al. Citation2000). Between January 2000 and September 2011, blooms occurred at sites throughout New Zealand, although significant spatial variability was evident. Greatest cell concentrations were recorded in the Bay of Plenty and at some sites in the Marlborough Sounds (Figure 2b; Rhodes & Jiang Citation2011). Blooms commonly occurred from spring to the end of summer, although in the Bay of Plenty there was a decreasing trend from spring to the end of autumn. Further south, at Taylors Mistake (Canterbury) and Stewart Island, peak concentrations were recorded in May (late autumn). High concentrations of Pseudo-nitzschia were recorded in the Marlborough Sounds in winter (Figure 2a, b) and, overall, season correlated with increased Pseudo-nitzschia blooms (Rhodes & Jiang Citation2011; Rhodes et al. Citation2012).
Triggers of bloom development
From 2000 to 2011, 8.2% of a total of 29,251 samples analysed for Pseudo-nitzschia exceeded the voluntary trigger level (10×104 cells l−1) with the most frequently sampled sites situated in the Marlborough Sounds. The variables of season, temperature and salinity were plotted against Pseudo-nitzschia concentrations, and season had a significant influence (Figure 2a; Rhodes & Jiang Citation2011). It is probable that this was due to changing day length and/or light intensity, although spring turnover may have had an influence in some areas. Neither temperature nor salinity had a significant effect.
Results of data subjected to GLM analyses
The GLM model included the four predictor variables of strata, season, temperature and latitude, which explained 6.5% of the total variation in the data. Variation of Pseudo-nitzschia cell concentration exceedances of the voluntary threshold was more related to the strata and season than to temperature or efd. The performance of the model was confirmed to be poor as the AUROC was calculated as 0.66.
The DA Gaussian GLM showed location and season had no significant effect on DA concentration. Pseudo-nitzschia cell concentrations had a significant effect (as expected) on DA concentrations; temperature and latitude also displayed some significant effects. However, while the performance of this Gaussian GLM model was better than the logistic model (R2=13.64), it was still judged to be poor.
Pseudo-nitzschia species detected in New Zealand coastal waters
The globally occurring genus was reviewed recently (Lelong et al. Citation2012; Trainer et al. Citation2012) and 12 of the 37 currently described species of Pseudo-nitzschia have been identified in New Zealand waters. Contributions of individual species to total abundance varied by site and by season (; Rhodes & Jiang Citation2011). In the Marlborough Sounds, P. fraudulenta showed a higher contribution in autumn, whereas P. australis had the highest overall contribution in the eastern Bay of Plenty in spring; however, the dominant species altered year by year.
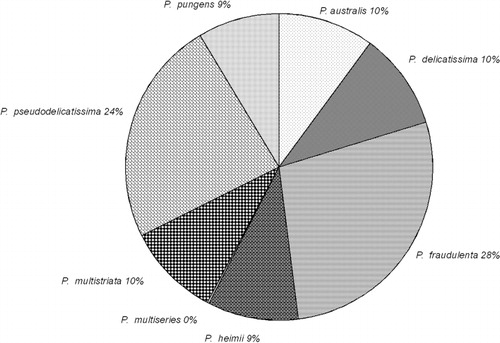
Data derived from the analysis of 1827 FISH assays were available from January 2005 to August 2011. P. americana was not included in the analyses as all isolates tested to date have proved non-toxic, although it has been detected at many sites around New Zealand. Eight species were identified from the combined data with P. fraudulenta (28%) and P. pseudodelicatissima (24%) being the most abundant (). Both are considered low risk species, with low concentrations of DA production per cell (c. 0.12 pg cell−1) being determined for New Zealand isolates (; Rhodes et al. Citation1996; Rhodes Citation1998). P. delicatissima (low risk) and the non-toxic P. cf. heimii each contributed 10%, as did the highly toxic P. australis (≤ 35 pg DA cell−1). P. pungens and P. multiseries, which comprised the balance, have non-toxic as well as toxic strains, but risk assessments were based on their known DA production potential (). Globally, 14 Pseudo-nitzschia species have been shown to produce measurable DA (Lelong et al. Citation2012; Trainer et al. Citation2012); in New Zealand, eight species are known to produce DA.
Analyses of the phytoplankton data for all commercial sites from January 2000 to August 2011 showed that, in the North Island's Bay of Plenty, toxic P. multistriata blooms occurred in August–September. In the South Island, non-toxic blooms of P. pungens were common throughout Pelorus Sound and Port Underwood (Marlborough Sounds) from May to September, with P. cf. heimii and P. pseudodelicatissima also being present in Pelorus Sound. In Queen Charlotte Sound, P. fraudulenta bloomed from late autumn through to spring. Further south, in Akaroa (Canterbury), mixed blooms of the non-toxic P. cf. heimii and the toxic P. australis were detected during May. At Big Glory Bay, Stewart Island, P. americana bloomed in May, followed in August–September by mixed blooms of P. australis, P. pseudodelicatissima and P. fraudulenta.
In a discrete study carried out between September and December 2011, Pseudo-nitzschia cells were isolated from untreated routine commercial monitoring samples where concentrations exceeded the voluntary trigger level. Isolates were identified by FISH assays, followed by DNA sequence analyses and/or TEM, and toxin production (pg/cell) was determined (LC-MS). P. pseudodelicatissima and P. cf. heimii each comprised 25% of the occurrences, with P. australis, P. fraudulenta and P. multistriata each contributing 13% to the total. P. pungens and P. delicatissima each contributed 5%; the species P. multiseries was not detected. P. australis had quantifiable concentrations of DA ranging from 0.5 to 2.4 pg cell−1, but only trace levels were detected in isolates of P. fraudulenta, P. multistriata and P. pseudodelicatissima. P. delicatissima, P. pungens and P. cf. heimii were non-toxic. This four-month dataset mirrored the results of the analysis of the long-term dataset. Pseudo-nitzschia species were also present on many occasions below the trigger levels, but the data confirmed the low risk of DA causing human illness in New Zealand. The sites at which the voluntary trigger levels were exceeded were: Northland and inner Marlborough Sounds (austral spring), and Hauraki Gulf, outer Marlborough Sounds and Big Glory Bay (austral summer).
Pseudo-nitzschia multistriata strains analysed in 2000 (Hay et al. 2000) were non-toxic (Rhodes et al. Citation2000), but further strains isolated from Collingwood, Golden Bay, and from Wilson's Bay (Marlborough Sounds), have since been shown to produce maximum concentrations of DA of 1.5 pg cell−1 (L. Rhodes, unpubl. data; ). No annual trend was detected for the toxic P. australis, although there was a peak in cell numbers during 2008 at Port Fitzroy, Hauraki Gulf. Overall, there was no evidence of an increase in Pseudo-nitzschia blooms during the decade of data analysed.
There are many recently described Pseudo-nitzschia species which fall within the ‘delicatissima complex’ and some for which the FISH probes used in this study were not specific. For example, P. pseudodelicatissima is now considered to be a group of five species (P. cuspidata, P. calliantha, P. calliantha2, P. caciantha and P. pseudodelicatissima) and P. delicatissima a group of three species (P. delicatissima, P. delicatissima2 and P. dolorosa; Amato et al. Citation2007; Lelong et al. Citation2012). Isolates which had a positive response to the P. pseudodelicatissima and P. delicatissima probes were therefore further examined by TEM and/or DNA sequencing.
The species in the ‘delicatissima complex’ so far isolated from New Zealand coastal waters and identified by TEM are P. delicatissima, P. pseudodelicatissima, P. cuspidata (Hobart-5 type), P. calliantha and P. caciantha. Partial LSU sequences were also amplified from cultures of unidentified Pseudo-nitzschia spp. and were used as query sequences in BLAST searches of the GenBank non-redundant database. Strain CAWB114 (GenBank accession number JQ776550) had the highest homology with P. calliantha (GenBank accession numbers EF642973 and EF566011: query coverage=100%, E-value=0.0, percent identity=97%) and TEM investigation and comparison with the literature (Lundholm et al. Citation2003) confirmed the identification. For strain CAWB111 (GenBank accession number JQ776549) the highest homology was with P. multistriata (GenBank accession number AF417654: query coverage=99%, E-value=0.0, percent identity=99%). P. multistriata is often included in the ‘delicatissima’ complex, but it actually cross-reacts with the P. australis specific probe in the FISH assay. However, due to its characteristic sigmoid shape and the fact that it also produces DA this has not been a problem for DA risk assessments.
For strain CAWB106 (GenBank accession number JQ776548) the highest homology was with both P. cf. subpacifica (GenBank accession numbers AF417642–AF417644: query coverage=99%, E-value=0.0, percent identity=99%) and P. cf. heimii (GenBank accession number AF440777: query coverage=98%, E-value=0.0, percent identity=99%). Pseudo-nitzschia cf. heimii was originally identified as P. heimii by FISH assay, but a re-evaluation of SEMs and TEMs obtained from isolates collected in 1997 and 1998 suggests that the correct morphological identification is P. subpacifica (Hasle) Hasle (). The isolates did have similarities with P. heimii (length and width of frustules and the central interspace), but the number of striae and fibulae in 10 µm most closely fitted the description of P. subpacifica (). All isolates had eight poroids per µm and were non-toxic, which also closely matched the description of P. cf. subpacifica (from Portugal, Spain and Hong Kong) as discussed by Lundholm et al. (Citation2002). As the records are historical and cultures are no longer maintained in the CICCM no sequence data is available. The isolates were tested by HPLC-UV while extant and were non-toxic. A more recent isolate from Akaroa, Canterbury (May 2011), also tested positive with the FISH probe for P. heimii, but when the LSU rRNA region of the DNA was sequenced and the data analysed it was found to be 99% similar to both P. heimii and P. subpacifica. Morphologically this isolate was almost identical to the four isolates from 1997–98 already discussed. It is clear that the published descriptions of P. heimii and P. subpacifica will need revisiting in the future. For continuity in the data for the analyses in this study, the term P. cf. heimii is used. A definitive analysis of these species referred to in the historical data is no longer possible.
Table 2 Comparison of morphometric data obtained for four New Zealand isolates of Pseudo-nitzschia cf. heimii (as determined by fluorescent in situ hybridisation assay; species specific probe heD2-2) with published descriptions* of species in the ‘Nitzschia seriata’ complex (Hasle Citation1965, Citation1975; Hasle & Syvertsen Citation1996).
Domoic acid in New Zealand, 2000–11
A total of 8477 samples were available for DA analysis from 1 January 2000 to 17 August 2011 and the measured DA concentrations ranged from 1 to 13 mg/kg. The highest DA levels were from samples collected at three sites in the Marlborough Sounds region (Cannon Hill, Richmond Bay and Brightlands Bay) in July 2010; all had a DA level of 13 mg/kg. From the full datasets, 1898 samples were identified where there were records for both Pseudo-nitzschia and DA concentrations. Pseudo-nitzschia concentrations ranged from 2.0×102 to<1.7×106 cells l−1, while the maximum DA concentration recorded was 13 mg/kg. The two variables were significantly correlated (as expected) with a Pearson correlation of 0.27 (t=12, d.f.=1896, P<0.001). No shellfish flesh samples exceeded the DA regulatory limit (20 mg/kg) at any time from January 2000 to August 2011, and no illness related to DA has ever been reported in New Zealand or been related to exported product.
The analysis of the 1993–99 data for DA indicated that of c. 19,000 samples tested only 0.2% exceeded the regulatory level (Hay et al. Citation2000). Currently, the regulatory level for DA and epiDA combined continues to be 20 mg/kg in shellfish, a level established by international regulatory authorities (Codex Citation2006).
The analytical methods have a much faster turnaround time since the replacement of HPLC-UV based detection methods with LC-MS methods for DA and epiDA (validated and approved in 2001; Holland et al. Citation2003). With the introduction of LCMS analyses risk assessments were available to shellfish harvest managers and regulators before the phytoplankton data. The shellfish industry pays for the regulatory biotoxin monitoring in New Zealand and, in the interests of cost savings, there are now few requests for FISH assays. However, the assays are regularly used for the non-commercial programme and the reduction in sampling and testing of shellfish and greater reliance on the micro-algae data has substantially reduced public health monitoring costs.
Conclusion
In summary, Pseudo-nitzschia blooms are a common feature of New Zealand's coastal waters, although the species present are predominantly non-toxic and pose a low risk of human illness. The most common toxic Pseudo-nitzschia species in New Zealand (and the most toxic isolate analysed so far) is P. australis, which can be readily detected by FISH assays. The fast turnaround of biotoxin test results has ensured that there have been no DA-related illnesses associated with New Zealand shellfish products. Currently, under the Animal Products Notice, samples must be submitted to analytical laboratories within 24 h of sampling and be tested and confirmed within 24 h of receipt at that laboratory. In fact, no illnesses have been reported globally since the first cases of ASP in Canada in 1987 due to effective monitoring programmes, although wildlife continues to be impacted. The cumulative effects of DA exposure in sea mammals have been evidenced (Goldstein et al. Citation2008) and potential chronic exposure effects in humans requires further investigation (Lefebvre & Robertson Citation2010).
In New Zealand, Pseudo-nitzschia may bloom at any time of the year, with an increase in overall likelihood during the austral spring and summer, although there are minor regional differences in the timing of blooms. When environmental temperature and salinity data were analysed for the Marlborough Sounds region, there was no strong evidence to suggest that these factors influenced the occurrence of Pseudo-nitzschia blooms or DA production. There were regional differences in the exceedances of the Pseudo-nitzschia trigger levels, which were highest in the Bay of Plenty sites. P. fraudulenta followed by P. pseudodelicatissima, both low risk species, were the most common bloom formers, with the highly toxic P. australis contributing 10% of the total abundance. Despite the occurrence of high Pseudo-nitzschia spp. counts in some regions, no shellfish exceeded the DA regulatory limit and no ASP illnesses have been reported.
Acknowledgements
Cawthron Institute Biotoxin and Micro-algae Lab clients, including MAF Food Safety and Ministry of Health, kindly allowed the use of their results. Shellfish industry clients included: Bluff Farmed Oysters, Cloudy Bay Clams, Coromandel Marine Farmers Assoc., Challenger Scallop Enhancement Co. Ltd., Houhora Farms Shellfish Farms Assoc., Marlborough Shellfish Quality Programme, Nelson Bay Clam Co., Nelson Ranger Fishing Co., NIWA (Christchurch), New Zealand Geoduck Ltd., New Zealand Mussel Industry Council, Port Fitzroy Marine Farms, Southern Seas Marine Farm, Southern Clams Ltd., Talley's Group Ltd., Tasman Bay Mussels Ltd., Whangaroa Delivery Centre. Thanks to Adrian Turner, University of Auckland, for the TEMs to support species identifications. Thanks also to Krystyna Ponikla for curation of isolates in the Cawthron Institute Culture Collection of Micro-algae, Stef Naldi, Catherine Moisan and Paul Barter for accessing historical databases, and Joy Chou for FISH assays. NIWA (Christchurch) are also thanked for their provision of Marlborough Sounds’ environmental data used in the study. The research for this review was supported by the Ministry of Science and Innovation (New Zealand) funded Seafood Safety Programme (contract no. CAWXO703).
Notes
1 Additional data associated with the modelling section of this article can be accessed from the Cawthron Institute library as Cawthron Reports, 2035a (2011) and b (2012).
References
- Akaike H 1974. A new look at the statistical model identification. IEEE Transactions on Automatic Control19: 716–723. 10.1109/TAC.1974.1100705
- Amato A, Kooistra WHCF, Ghiron JHL, Mann DG, Proschold T, Montressor M 2007. Reproductive isolation among sympatric cryptic species in marine diatoms. Protist158: 193–207. 10.1016/j.protis.2006.10.001
- Busby P 1992. New Zealand industry agreed implementation standard 005: shellfish quality assurance circular. Wellington, New Zealand, Fishing Industry Inspection and Certification Council (FIICC).
- Chang FH 1995. The first records of Gymnodinium sp. nov. (cf breve) (Dinophyceae) and other harmful phytoplankton species in the early 1993 blooms in New Zealand. In: Lassus P, Arzul G, Erard-Le Denne E, Gentien P, Marcaillou-le Baut C eds. Harmful marine algal blooms. Paris, Lavoisier, Intercept Ltd. Pp. 27–32.
- Codex 2006. Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme. Codex Committee on Fish and Fishery Products, 28th session, Beijing, China, 18–22September 2006. ftp:ftp.fao.org/Codex/ccffp28/fp2806ae.pdf
- Crosby LH, Ferguson Wood EJ 1959. Studies on Australian and New Zealand diatoms. 11. Normally epontic and benthic genera. Transactions of the Royal Society of New Zealand86: 1–58.
- Garthwaite I, Ross KM, Miles CO, Hansen R, Foster D, Towers NR 1998a. An immunoassay for determination of domoic acid in water samples and shellfish. In: Reguera B, Blanco J, Fernandez ML, Wyatt T eds. Harmful algae. Spain, Xunta de Galicia, and Paris, Intergovernmental Oceanographic Commission of UNESCO. Pp. 559–562.
- Garthwaite I, Ross KM, Miles CO, Hansen R, Foster D, Wilkins AL, Towers NR 1998b. Polyclonal antibodies to domoic acid, and their use in immunoassays for domoic acid in seawater and shellfish. Natural Toxins6: 93–104. 10.1002/(SICI)1522-7189(199805/08)6:3/4<93::AID-NT15>3.0.CO;2-9
- Goldstein T, Mazet JA, Zabka TS, Langlois G, Colegrove KM, Silver M, Bargu S, van Dolah F, Leighfield T, Conrad PA, Barakos J, Williams DC, Dennison S, Haulena M, Gulland FM 2008. Novel symptomology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. Proceedings of the Royal Society Biological Sciences Series B275: 267–276. 10.1098/rspb.2007.1221
- Guillard RRL 1975. Culture of phytoplankton for feeding marine invertebrates. In: Smith WH, Chanley MH eds. Culture of marine invertebrate animals. New York, Plenum Press. Pp. 29–60.
- Hall TA 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series41: 95–98.
- Hasle GR 1965. Nitzschia and Fragilariopsis species studied in the light and electron microscopes. II. The group Pseudo-nitzschia. Skrifter utgitt av Det Norske Videnskaps-Academi i Oslo. 1. Matematisk-Naturvidenskapelig Klasse. Ny Serie18: 1–45.
- Hasle GR 1975. Some living marine species of the diatom family Rhizosoleniaceae. Beiheft zur Nova Hedwigia53: 99–140.
- Hasle GR, Syvertsen EE 1996. Marine diatoms. In: Tomas CR ed. Identifying marine phytoplankton. San Diego, USA, Academic Press. Pp. 5–385.
- Hay BE, Grant CM, McCoubrey D-J 2000. A review of the marine biotoxin monitoring programme for non-commercially harvested shellfish. Part 1: Technical Report. Prepared for the New Zealand Ministry of Health by AquaBio Consultants Ltd. Auckland, New Zealand, New Zealand Ministry of Health. 47p
- Hendey NI 1974. The permanganate method for cleaning freshly gathered diatoms. Microscopy32: 423–426.
- Holland P 2001. Validation of whole-cell RNA gene probes for identification of Pseudo-nitzschia species. Cawthron Report for the New Zealand Ministry of Agriculture and Forestry. Cawthron report No. 659. 4 p.
- Holland PT, McNabb P, Rhodes LL, Selwood AI, Neil T 2003. Amnesic shellfish poisoning toxins in New Zealand shellfish—detection of a novel domoic acid isomer using a newly validated LC-MS/MS method. In: Villalba A, Reguera B, Romalde JL, Beiras R. eds. Molluscan shellfish safety. Consellería de Pesca e Asuntos Marítimos da Xunta de Galicia, and Paris, Intergovernmental Oceanographic Commission of UNESCO. Pp. 29–42.
- Holland PT, Selwood AI, Mountfort DO, Wilkins AL, McNabb P, Rhodes LL, Doucette GJ, Mikulski CM, King KL 2005. Isodomoic acid C, an unusual amnesic shellfish poisoning toxin from Pseudo-nitzschia australis. Chemical Research in Toxicology18: 814–816. 10.1021/tx0496845
- Hollander M, Wolfe DA 1973. Non-parametric statistical methods. London, John Wiley & Sons. 503 p.
- Jasperse JA 1993. Marine toxins and New Zealand shellfish. Proceedings of a workshop on research issues, 10–11 June 1993. The Royal Society of New Zealand, Misc. Series. 24 p.
- Lefebvre KA, Robertson A 2010. Domoic acid and human exposure risks: a review. Toxicon56: 218–230. 10.1016/j.toxicon.2009.05.034
- Lelong A, Hégaret H, Soudant P, Bates SS 2012. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: revisiting previous paradigms. Phycologia51: 168–216. 10.2216/11-37.1
- Lundholm N, Daugbjerg N, Moestrup O 2002. Phylogeny of the Bacillariaceae with emphasis on the genus Pseudo-nitzschia (Bacillariophyceae) based on partial LSU rDNA. European Journal of Phycology37: 115–134. 10.1017/S096702620100347X
- Lundholm N, Moestrup Ø, Hasle GR, Hoef-Emden K 2003. A study of the Pseudo-nitzschia pseudodelicatissima/cuspidata complex (Bacillariophyceae): what is P. pseudodelicatissima? Journal of Phycology39: 797–813. 10.1046/j.1529-8817.2003.02031.x
- Miller PE, Scholin CA 1996. Identification of cultured Pseudo-nitzschia (Bacillariophyceae) using species-specific LSU rRNA-targeted fluorescent probes. Journal of Phycology32: 646–655. 10.1111/j.0022-3646.1996.00646.x
- Nunn GB, Theisen BF, Christensen B, Arctander P 1996. Simplicity-correlated size growth of the nuclear 28S ribosomal RNA D3 expansion segment in the crustacean order Isopoda. Journal of Molecular Evolution42: 211–223. 10.1007/BF02198847
- NZFSA (New Zealand Food Safety Authority) 2006. Animal Products (Regulated Control Scheme-Bivalve Molluscan Shellfish) Regulations 2006 and the Animal Products (Specifications for Bivalve Molluscan Shellfish) Notice 2006. Wellington, NZFSA, Ministry of Agriculture and Forestry. 77 p.
- Rhodes LL 1994. Prymnesiophytes of New Zealand's coastal waters: taxonomy, physiology and ecology. Unpublished PhD thesis, Massey University, New Zealand.
- Rhodes LL 1998. Identification of potentially toxic Pseudo-nitzschia (Bacillariophyceae) in New Zealand coastal waters, using lectins. New Zealand Journal of Marine and Freshwater Research32: 536–544. 10.1080/00288330.1998.9516842
- Rhodes LL, White D, Syhre M, Atkinson M 1996. Pseudo-nitzschia species isolated from New Zealand coastal waters: domoic acid production in vitro and links with shellfish toxicity. In: Yasumoto T, Oshima Y, Fukuyo Y eds. Harmful and toxic algal blooms. Paris, Intergovernmental Oceanographic Commission of UNESCO. Pp 155–158.
- Rhodes LL, Scholin C, Garthwaite I 1998a. Pseudo-nitzschia in New Zealand and the role of DNA probes and immunoassays in refining marine biotoxin monitoring programmes. Natural Toxins6: 105–111. 10.1002/(SICI)1522-7189(199805/08)6:3/4<105::AID-NT13>3.0.CO;2-9
- Rhodes LL, Scholin C, Garthwaite I, Haywood A, Thomas A 1998b. Domoic acid producing Pseudo-nitzschia species educed by whole cell DNA probe-based and immunochemical assays. In: Reguera B, Blanco J, Fernandez ML, Wyatt T eds. Harmful algae. Xunta de Galicia, and Paris, Intergovernmental Oceanographic Commission of UNESCO. Pp. 274–277.
- Rhodes L, Adamson J, Scholin C 2000. Pseudo-nitzschia multistriata (Bacillariophyceae) in New Zealand. New Zealand Journal of Marine and Freshwater Research34: 463–467. 10.1080/00288330.2000.9516948
- Rhodes LL, Mackenzie AL, Kaspar HF, Todd KE 2001a. Harmful algae and mariculture in New Zealand. ICES Journal of Marine Science58: 398–403. 10.1006/jmsc.2000.1023
- Rhodes L, Scholin C, Tyrrell J, Adamson J, Todd K 2001b. The integration of DNA probes into New Zealand's routine phytoplankton monitoring programmes. In: Hallegraeff GM, Blackburn SI, Bolch CJ, Lewis RJ eds. Harmful algal blooms. Paris, Intergovernmental Oceanographic Commission of UNESCO. Pp. 429–432.
- Rhodes LL, Jiang W 2011. Review of Pseudo-nitzschia and domoic acid in New Zealand, 2000–2011. Report funded by New Zealand Ministry for Science and Innovation. Cawthron Report No. 2035a. 28 p.
- Rhodes LL, Jiang W, Knight B, Adamson J, Smith K, Langi V, Edgar M, Munday R 2012. The genus Pseudo-nitzschia (Bacillariophyceae) in New Zealand: a review of the last decade's research achievements and monitoring data. Report funded by New Zealand Ministry for Science and Innovation. Cawthron Report No. 2035b. 22 p.
- Scholin CA, Herzog M, Sogin ML, Anderson DM 1994. Identification of group and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. Journal of Phycology30: 999–1011. 10.1111/j.0022-3646.1994.00999.x
- Scholin C, Vreiling E, Peperzak L, Rhodes L, Rublee P 2003. Detection of HAB species using lectin, antibody and DNA probes. In: Hallegraeff GM, Anderson DM, Cembella AD eds. Manual on harmful marine micro-algae. Paris, UNESCO Publishing. Pp. 131–164.
- Swets JA 1988. Measuring the accuracy of diagnostic systems. Science240(4857): 1285.10.1126/science.3287615
- Trainer VL, Bates SS, Lundholm N, Thessen AE, Adams NG, Cochlan WP, Trick CG 2012. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae14: 271–300.
- Utermöhl H 1958. Zur vervollkommung der quantitativen phytoplankton methodik. (Towards a perfection of quantitative phytoplankton methodology.) Mitteilungen der Internationale Vereinigung für Theoretische und Angewandte Limnologie9: 1–38.
- Wilson N, Sim J 1996. Review of the New Zealand marine biotoxin monitoring programme data. Report for Public Health Group, New Zealand Ministry of Health. 50 p.
- Zuur AF, Ieno EN, Elphick CS 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution1: 3–14.