Abstract
We review the contributions from research conducted through the Leigh Marine Laboratory (LML) since its establishment in 1962, to the understanding of the biology and ecology of the rock lobster, Jasus edwardsii. The number of publications (103 since 1978), their breadth of subject matter, and their frequency of citation indicate the influence of this research effort. The development of an underwater population sampling tool at the LML has been key to the research on lobster distribution, abundance, size structure and sex ratios. These, in turn, allowed significant insights into the impact of fishing on lobster populations, population recovery in marine reserves, the mating system of lobsters, and the cascading effects of lobster removal on reef communities. Other key research has focused on the planktonic larval and post-larval stages. It is likely that J. edwardsii has received a greater level of ecological research effort through the LML than any other species. The location of the LML on a stretch of rocky coastline with year-round easy access to the field, and the availability of a rebuilding population of J. edwardsii in an adjacent marine reserve, allowed coupled laboratory and field research to rapidly develop without the confounding effects of fishing. The LML is well placed to address outstanding questions about lobster biology and ecology, and to further contribute to the conservation and management of this important resource.
Introduction
Three species of spiny lobsters, commonly known as rock lobsters or crayfish, occur in the waters around New Zealand. The red rock lobster (Jasus edwardsii) occurs around the entire coastline of mainland New Zealand and its offshore islands from the Three Kings Islands in the north, to the Chatham Islands in the east and the northern tip of the Auckland Islands in the south, as well as around the coasts of southern Australia (Booth Citation2011). The other two species have much more restricted distributions. The packhorse rock lobster (Sagmariasus verreauxi) is largely restricted to the region from East Cape to the northernmost parts of North Island, and eastern temperate Australia. The deep-water lobster (Projasus parkeri) is restricted to the slopes of seamounts between 200 and 600 m depth (Booth Citation2011).
By far the most important lobster species in New Zealand waters is J. edwardsii. It is the most abundant large benthic predator on coastal reefs and plays an important role in rocky reef ecosystem functioning (Pinkerton et al. Citation2008; CitationBeaumont et al. in press, Eddy et al. unpublished data). In both New Zealand and temperate Australia, this species supports valuable coastal fisheries (Booth Citation2006). In the early 1960s, when the Leigh Marine Laboratory (LML) was first established, the understanding of the ecology of J. edwardsii was poor. This was despite the species supporting commercial fisheries for over 100 years and being fished by Māori for centuries prior. CitationMacDiarmidet al. (in press) have recently compiled a history of evidence for the state and exploitation of marine fish and invertebrate resources, including lobsters, in the Hauraki Gulf from 1769 onwards. They found that the earliest accounts by Europeans indicate the ease at which Māori fished for rock lobsters. Elsdon Best (Citation1929, p. 52) in his report Fishing methods and devices of the Maori noted that:
Crayfish are numerous on many parts of the rocky coast-line, and so furnished quite an important food-supply to the natives. They were taken largely by means of a lobster-pot, termed a taruke, and also often by hand.
Best (Citation1929, p. 52) recounts Captain Cook's description from 1769:
These [crayfish] we also brought everywhere to the northward in great quantities of the natives, who catch them by diving near the shore and finding out where they lie with their feet.
Almost 100 years later, at Great Mercury Island, Cameron Buchanan, aged 14, observed Māori catching crayfish ‘of which there are quite a lot in the seaweed that fringes the beaches and reefs around the Island’ in an identical manner:
The Maori felt for the crayfish with their feet, then reached down and caught them by their feelers and threw them onto the beach. In about 20 minutes, they caught about 12–15 crayfish (Anon. Citation1977, p. 8–9).
Similarly, Roddy Matheson, the farm owner from whom the University of Auckland purchased the land on which the LML sits, recounted to Bill Ballantine, the laboratory's first director, how in the 1920s–1940s he had regularly caught lobsters by spearing them with a pitchfork from the rocks on the shore below the farmhouse, and sent sugar sacks of them by steamer for sale in Auckland (Bill Ballantine, pers. comm. 7 December 2012). Easy access to rock lobsters in shallow water continued in parts of the Hauraki Gulf into the 1950s. CitationMaxwell and MacDiarmid (in press) report an oral interview from a man who, as a child, lived on the southeast coast of Great Barrier Island:
Crayfish used to be around the rocks there. Dad caught them with a net and a pole and he would just put the net down and wait 10 minutes and lift it up and he'd have half a dozen.
Another man recalled holidaying as a child on Arid Island off the east coast of Great Barrier Island in the mid-1950s (Maxwell & MacDiarmid in press):
When I was going to boarding school I used to go to Arid Island in the holidays and the people there used to feed their dogs on crayfish because it was the only reliable source of food they could get readily each day. They'd just walk down the beach lift the pot up and take a cray out for the dogs.
About all that was understood of the ecology of rock lobsters at this time was based on the seasonal waxing and waning of lobster populations near shore where they could be easily observed, their entry into baited traps, and occasional large catches by bottom trawlers on open ground well away from reefs. It was known that lobsters became soft-shelled and moulted at particular times of the year, and that females brood an external clutch of eggs from autumn to spring (MacDiarmid et al. in press). Anderton (Citation1906, p. 483) summarised the known information about rock lobsters as follows:
It is generally supposed to be of sedentary habits, and to live for a great part of its life within a very restricted area. Large hauls of them are occasionally taken in the trawl some distance from shore, and on a sandy bottom. The fishermen say they are then on the move, and it would appear that at certain periods a great migratory movement takes place from one part of the coast to another.
In 1938 the Sea Fisheries Investigation Committee summarised the known effects on fisheries as follows (MacDiarmid et al. in press):
There are two seasons for the taking of crayfish from these waters, one from July to September with a short break in October, and another from November till after Christmas. The crayfish-men follow the crayfish out when they leave the inshore rocks and migrate to the sand and kelp bottom.
A lack of fundamental knowledge about the biology and ecology of lobsters was acknowledged by the Sea Fisheries Investigation Committee which recommended, ‘a study be made of the habits, size, sex groups, and migrations of the crayfish, the results being the basis for future legislation’ (MacDiarmid et al. in press). After a 10-year delay caused by World War II, these studies got underway in the late 1940s when Cedric Alec Bradstock completed a two-year study of J. edwardsii on Wellington's south coast (Bradstock Citation1948), then lapsed until the 1960s when Bob Street began his investigations into the biology and ecology of lobsters around southern New Zealand (Street Citation1969), and accelerated in the 1970s with the appointment of additional scientists by the then Ministry of Agriculture and Fisheries (Booth Citation2011). At this stage, active underwater ecological research on a wide variety of reef flora and fauna was well underway through the LML (see other papers in this special issue), stimulated by the establishment in 1975 of the adjacent Cape Rodney to Okakari Point (CROP) Marine Reserve, New Zealand's first no-take marine reserve. It was inevitable that at some stage attention would turn to rock lobsters.
Here, we review the contributions from research conducted through the LML to the understanding of the biology and ecology of J. edwardsii, the most important lobster species in New Zealand waters, numerically, ecologically and commercially. We have taken a broad and inclusive approach to the research we consider to fall within the ambit of this review. We have included any ecological research on lobsters undertaken using LML facilities, supervised by LML staff, or authored by LML staff or graduates during their career (after leaving the University of Auckland) if it stemmed directly from research initiated at LML. We included information from journal publications, books, book chapters, published and unpublished reports, and student theses.
Lobster ecology publications since 1962
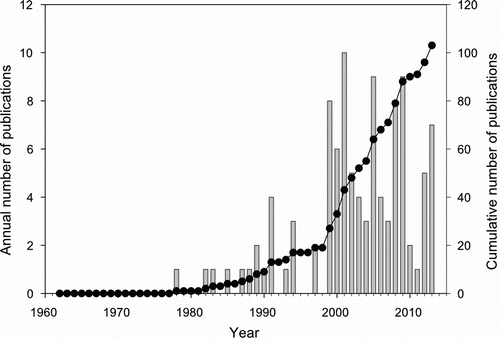
We found 103 publications in seven broad categories on the ecology of rock lobsters stemming from research conducted through the LML (). The distribution of publications was uneven across these areas of inquiry, with the highest number relating to trophic interactions and cascades. The highest number of citations for any publication was 268 for the publication by Babcock et al. (Citation1999) on changes in community structure in temperate marine reserves.
Table 1 Number of publications, highest cited paper and number of citations for the highest cited paper by rock lobster research area for publications undertaken through the LML 1962–2012.
For the first 16 years after the establishment of the LML, rock lobsters were not studied. Not until 1978 when Tony Ayling published his report detailing the results of the initial survey of the CROP Marine Reserve did research on lobster ecology get underway (). Thereafter, research rapidly increased, with seven publications in the 1980s, 19 in the 1990s, 61 in the 2000s, and 15 so far this decade.
Distribution and abundance
Work through the LML was the first to describe densities of J. edwardsii and document spatial and temporal variation in its distribution over a range of scales. Lobster abundance and distribution had previously been inferred from captures in baited pots or traps, but was potentially biased because of variation in trap entry by lobsters due to an individual's moult status, reproductive state, gender or body size (Ziegler et al. Citation2002a, Citationb, 2004; Ihde et al. Citation2006), competition (Frusher & Hoenig Citation2001) or density-dependent increases in the effects of these factors (Ziegler et al. Citation2003). Another problem is that the smallest scale of distribution possible to describe using baited traps is the distance over which a trap attracts lobsters during the period it is set, possibly 100 m or more (Jernakoff & Phillips Citation1988; Aedo & Arancibia Citation2003). To reduce this bias Ayling (Citation1978) pioneered the use in New Zealand of the visual underwater strip transect, whereby scuba divers count and estimate the size of lobsters in a defined area of seafloor. MacDiarmid (Citation1987, Citation1991) refined this technique by using different transect sizes and by collecting additional information about lobster sex, moult and reproductive status, den cohabitation and behaviour. This enabled description of seasonal changes in lobster depth distribution in relation to moulting and reproductive schedules (MacDiarmid Citation1991), gender, size and seasonal patterns of cohabitation (Gabites Citation1990; MacDiarmid Citation1994; Kelly et al. Citation1999), population size (e.g. MacDiarmid Citation1987), and comparison of population density and size distribution among protected and fished localities throughout New Zealand (Cole et al. Citation1991; MacDiarmid & Breen Citation1992; Kelly et al. Citation2000; Shears et al. Citation2006; Pande et al. Citation2008; Freeman et al. Citation2012a).
This research has shown that in areas of low fishing intensity, the density of lobsters and egg production per unit area can reach high levels. For example, in the Long Island–Kokomohua Marine Reserve in the Marlborough Sounds, rock lobsters currently occur at mean densities of 1200 ± 200 (SEM) per ha of reef (Freeman et al. Citation2012a). In contrast, in fished areas adjacent to marine reserves densities are typically much lower at between 20 and 200 ± 40 (SEM) lobsters per ha (Freeman et al. Citation2012a). In an underwater transect survey of kelp habitats in the Hauraki Gulf, CitationBeaumont et al. (in press) found lobsters in all four reserves surveyed, but at only one of the six fished localities assessed. Similarly, Kelly et al. (Citation2000) described much higher abundance and biomass of legal-sized lobsters and egg production in northern marine reserves than in adjacent fished areas. Furthermore, Freeman et al.(Citation2012a) found that the abundance of sublegal juveniles also increased within New Zealand marine reserves, indicating enhanced settlement, post-settlement survival or migration of juvenile lobsters into reserves.
Within coastal ecosystems, red rock lobsters are not evenly distributed but are more abundant in complex habitats that provide daytime shelter and a variety of microhabitats for nocturnal foraging (MacDiarmid Citation1987). Within habitats, rock lobsters are patchily distributed with patch location dependent in part on the uneven distribution of suitable daytime dens, and den composition dependent on lobster size, sex, and moult and reproductive state (MacDiarmid Citation1987, Citation1991, Citation1994). Field surveys show that red rock lobsters are solitary as newly settled juveniles and become social and aggregate as they grow larger (MacDiarmid Citation1994; Butler et al. Citation1999). Butler et al. (Citation1999) demonstrated that there is a size-specific increase in the response of larger juveniles to the chemical cues of larger lobsters which facilitates these life-stage changes in aggregation. Field experiments confirmed that aggregation is selectively advantageous by increasing the survival of larger but not smaller juveniles (Butler et al. Citation1999). In adult red rock lobsters the tendency to cohabit with other lobsters varies between males and females and the time of year. Mature males tend to aggregate with other males during their moulting season in spring, and den separately from other males during the mating season when males compete for access to females. In contrast, mature females are most highly aggregated over summer and slightly less so over the egg-bearing stage in winter (MacDiarmid Citation1994). During the peak period of mating activity (May–June), the majority of post-moult, un-mated females cohabit with a large mature male, resulting in as many as 16–20 females cohabiting with a single large male (MacDiarmid Citation1994).
Reproduction
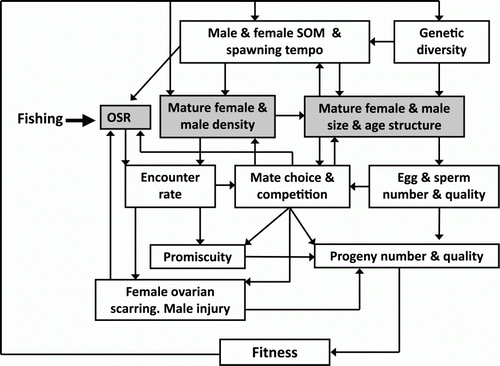
Research carried out through the LML has been important in elucidating many aspects of the mating system of J. edwardsii and spiny lobsters generally (). This has enabled very detailed insights into how exploitation may affect egg and sperm production, and thus the production of fertilised eggs and larvae (Kittaka & MacDiarmid Citation1994; MacDiarmid & Kittaka Citation2000; MacDiarmid & Sainte-Marie Citation2006).
Figure 2 Diagram of the effects of exploitation on the mating system of Jasus edwardsii. Fishing directly modifies the density, size and age structure, and the operational sex ratio (OSR) of the population of mature individuals (shaded boxes). These modifications alter the number and possibly the quality of the eggs and sperm on offer by individual lobsters, the encounter rate of potential mates, and the opportunity for mate choice, mate competition, and promiscuity that all play a part in affecting the number and quality of progeny produced by an individual within a mating season. Lack of choice or too much competition may drive some individuals to migrate from the area, affecting lobster population density, size and age structure, and OSR. During competition for mates some males may be injured and some females may never encounter a preferred male during her 10-day mating window, thus withdrawing from mating and leading to ovarian scarring as unused ripe eggs are absorbed (MacDiarmid et al. Citation1999). Both factors affect OSR. The number and quality of progeny produced by successful individuals may lead to population compensatory responses such as changes in size at onset of maturity (SOM) and reproductive tempo. If these changes are heritable and widespread the population genetic structure may change over time. Demonstrated effects are denoted by solid lines while hypothetical effects are denoted by dashed lines. Source: adapted from MacDiarmid & Sainte-Marie Citation2006.
One common approach to managing crustacean fisheries, where characteristically few data are available on the age-structure or stock-recruit relationships of populations, has been to focus on eggs-per-recruit (Caddy Citation2004). This approach typically concentrates on the abundance, size-structure and size-specific fecundity of females to ensure sufficient breeding females remain in the population to sustain recruitment at average historical levels (Caddy Citation2004). This focus ignores the abundance of males and assumes implicitly that sperm production never limits female breeding success (MacDiarmid & Sainte-Marie Citation2006).
The first suggestion that male reproductive output should also be considered in any lobster species stemmed from research carried out in the CROP Marine Reserve. MacDiarmid (Citation1989b) hypothesised that vas deferens wet weight was highly variable amongst same sized males due to the males being sampled at different intervals after a mating event, and that finite sperm production limited the number of eggs a male could fertilise over the short term. MacDiarmid and Butler (Citation1999) confirmed experimentally that in two genera of spiny lobsters (Jasus and Panulirus) size-dependent male sperm supply limits the size of clutches brooded by females. Further laboratory experiments indicated that post-moult un-mated females are choosy, selecting the largest male to mate with (MacDiarmid et al. Citation2000; MacDiarmid & Sainte-Marie Citation2006), and that females compete for access to the preferred male (MacDiarmid et al. unpublished data). Raethke et al. (Citation2004) identified the role of olfactory, visual and tactile cues used by both males and females during courtship and mate choice. MacDiarmid and Stewart (Citation2005a) observed that male but not female mate choice in J. edwardsii is influenced by the number of potential mates in a den.
This focus on population sperm supply led to the first attempts to determine the size at onset of maturity of males in J. edwardsii using internal (MacDiarmid Citation1989b) and external (MacDiarmid & Sainte-Marie Citation2006) criteria. Whether fertilisation in J. edwardsii occurred internally, as in clawed lobsters, or externally, like other spiny lobsters, was also investigated and confirmed that in this species the male extrudes a short lived spermatophore that the female immediately uses to fertilise her eggs externally (MacDiarmid Citation1988).
Sperm limitation in the red rock lobster in part stems from the limited regeneration of sperm supplies during the 6-week mating season (Mauger Citation2001). Accordingly, males ration their sperm supplies by depositing a spermatophore to match the size of each female they mate (MacDiarmid & Butler Citation1999; Mauger Citation2001). This rationing has its limits. Laboratory experiments, where males were provided with new mates until their sperm supply was exhausted, indicated that large 3 kg males could mate and successfully fertilise the eggs from a combined female biomass of 17 kg in a single 6-week mating season, while small, just mature males could mate with a combined female biomass of just 1 kg (MacDiarmid & Stewart Citation2005b).
Larval release, diet, behaviour, settlement
Research conducted through the LML has also contributed to a deeper understanding of the long planktonic larval phase of J. edwardsii and settlement of the post-larval puerulus back on coastal reefs 1 to 2 years later. This phase starts with egg hatching. Over the course of the extended egg brooding period (MacDiarmid Citation1989a) females accumulate on the deep seaward edge of coastal reefs and may venture across the sandflats to form offshore aggregations (MacDiarmid Citation1991; Kelly et al. Citation1999). Between late September and late October females hatch their larvae at dawn each day for 3–5 days (MacDiarmid Citation1985). The initial naupliosoma larvae swim vigorously towards the surface and within 30 min of hatching moult into stage 1 phyllosoma larvae which remain positively phototactic and form surface swarms. Foster (unpublished data) indicated the variable fate of the surface swarms of phyllosoma larvae hatched on successive days; on one day the swarms were swept inshore and were preyed upon by planktivorous reef fish, while on the other the newly hatched larvae were swept offshore (Buckeridge & Newman Citation1994). MacDiarmid (Citation1985) noted that the typically low catches of stage 1 phyllosoma found in previous studies may be due to the non-coincidence of sampling and patches of larvae, caused in part by the simultaneous hatching of larvae from aggregated females at dawn.
The phyllosoma larvae progress through at least 11 distinct stages during their 1–2 year planktonic phase well offshore (Phillips & McWilliam Citation1986; Booth & Phillips Citation1994). Little is known about the natural prey of J. edwardsii phyllosoma, but observational, morphological and physiological studies carried out through the LML indicate that they are opportunistic feeders, capable of grasping prey items using their pereiopods (Phleger et al. Citation2001; Cox & Bruce Citation2002; Cox & Johnston Citation2003; Jeffs et al. Citation2004; Jeffs Citation2007). Early larval stages have mouthparts suited to soft or fleshy prey items, and their pereiopods contain hydrodynamic and chemosensory setae which are used in tactile and chemically mediated prey capture. Gut morphology and enzyme activities suggest that phyllosoma are capable of digesting a wide range of prey types. A review of phyllosoma diets strongly suggested that gelatinous zooplankton and some small crustaceans are their main prey, and that there are pronounced changes in diet between mid and late stages (Jeffs Citation2007). Current research at the LML using polymerase chain reaction (PCR) enrichment techniques may identify prey species (O'Rorke et al. Citation2012). Late-stage phyllosomes accumulate large amounts of lipid, which appears important for fuelling the non-feeding puerulus stage that vigorously swims inshore to settle on coastal reefs 1–2 years after hatching (Jeffs & Holland Citation2000; Jeffs et al. Citation2001a, Citationb; Wells et al. Citation2001; Jeffs Citation2007). A model of the energetics of swimming in the puerulus stage of J. edwardsii (Wilkin & Jeffs 2011) suggested that the biochemical energy stores previously measured in pueruli (Jeffs et al. Citation1999) are marginal, with a significant proportion of pueruli having insufficient energy stores to complete the migration across the shelf. This suggests recruitment to benthic phases could be affected if currents cause the available energy of pueruli to be expended before they can reach the coast.
Possible mechanisms used by J. edwardsii post-larvae to find the coast include orientation towards nearshore sources of underwater sound, water chemistry, magnetic fields, celestial cues, hydrodynamic cues or electrosense (Jeffs et al. Citation2005). Of these, underwater sound appears to be the most likely orientation cue at distances more than a few kilometres offshore (Montgomery et al. Citation2006). Monitoring of the settlement of J. edwardsii pueruli in the CROP Marine Reserve with the use of artificial crevice collectors by MacDiarmid (Citation1987) confirmed the generally very low levels of recruitment of juveniles to reefs in northeastern New Zealand (Booth & McKenzie Citation2009).
Moulting and growth
Prior to the 1960s, much of what was known about moulting and growth in J. edwardsii from New Zealand was inferred from overseas studies of related species. However, over the following 20 years, a concerted effort was made to inform the management of fisheries for this species by describing (among other attributes) the growth rates and moult cycle of lobsters in central and southern New Zealand. Studies by Street (Citation1969), Saila et al. (Citation1979), McKoy and Esterman (Citation1981), Annala and Bycroft (Citation1985, Citation1988) and McKoy (Citation1985) provided the first detailed descriptions of growth rates and moulting seasonality. These studies described significant variability in growth within and among populations, a result of variability in moult increment and frequency.
Researchers from the LML have made significant contributions to what is known of the life history of J. edwardsii, building on the foundation established by these earlier studies. The proximity of the LML to an unfished wild population of spiny lobsters in the CROP Marine Reserve, where there was easy access to high densities and a wide size range of lobsters, enhanced the ability to study such life history aspects. MacDiarmid (Citation1987, Citation1989a) used diver sampling and laboratory observations to describe moulting behaviour and periodicity in J. edwardsii, noting the opportunity at Leigh for both in situ and in vitro resolution of these ecological questions. MacDiarmid's study confirmed latitudinal variability in the timing of moulting, egg-laying and incubation period. MacDiarmid (Citation1989a) also found that moulting occurred predominantly at night in shallow water and that the timing of moulting and egg-laying was related to lobster size. Surveys of lobster depth distribution, size and sex structure provided further information on the effects of seasonal moulting on lobster distribution and abundance (MacDiarmid Citation1991).
Tank-based studies of juvenile J. edwardsii at the LML have provided valuable information on the growth rates of captive individuals, with implications for the aquaculture of this species. Water temperature and diet appear to be key factors affecting juvenile growth (Berry Citation1997; Hooker et al. Citation1997; Radford et al. Citation2007). Oliver and MacDiarmid (Citation2001) confirmed that the blood refractive index (BRI) reliably estimated the nutritional condition of juvenile lobsters but that moult phase also needed to be taken into consideration.
The first New Zealand study to compare the growth rates of lobsters from fished and unfished populations (Freeman Citation2008; Freeman et al. Citation2012b) was undertaken on the east coast of the North Island. This study used growth data from tagged lobsters and suggested that growth rates were density-dependent and affected by fishing, possibly associated with repeated handling of sublegal-sized individuals.
Movement
In the early 1980s, knowledge about the movements of J. edwardsii was based mainly on observations and tagging carried out around the South Island of New Zealand (see McKoy Citation1983) and studies on other spiny lobster species such as Panulirus argus (e.g. Herrnkind Citation1969; Herrnkind & McLean Citation1971). Information suggested that J. edwardsii was a highly mobile species with small males and immature females routinely undertaking large scale, counter-current migrations (Street Citation1969). Little was known about finer scale (<5 km) foraging and seasonal movement patterns, the underlying reasons for J. edwardsii movements, or movements in northern New Zealand. Research carried out through the LML since the 1980s using passive and acoustic tagging, daily and monthly depth-stratified population surveys, and logging of den occupancy has provided a detailed account of those topics.
Tagging and population surveys carried out in the CROP Marine Reserve over a three-year period between 1982 and 1985 demonstrated that J. edwardsii undertake predictable seasonal movements between depth strata, with adult males and females moving up and down the reef at different times of the year (MacDiarmid Citation1991). MacDiarmid (Citation1991) concluded that movement patterns were related to the annual timing of moulting, reproductive and feeding activities.
On finer time and spatial scales, tagged lobsters were found to remain within a relatively small area, with their movements being strongly influenced by local topography (MacDiarmid et al. Citation1991). Detailed observations over 24-hour periods indicated that lobsters did not move up and over ridges or large boulders during nocturnal foraging. The greatest overnight distance moved by any of 254 tagged lobsters observed during the study was 99 m, with a median total distance of 41 m and median foraging range of 24 m. Forty per cent of lobsters returned to the same shelter on consecutive days, and those that did not return remained within a median distance of 16.8 m from the original shelter. Much of the variation in daily activity of female J. edwardsii occurred during the female moulting and mating season. Just prior to moulting, most females were active and exhibited low den fidelity, possibly due to the females actively selecting shelters suitable for moulting or mating, or the males that guarded them (MacDiarmid et al. Citation1991). Male activity also changed during the mating season, when mature males became aggressive towards one another and competed for shelters. The largest male lobsters guarded shelters containing the most females, while smaller males sought to increase their reproductive output by moving among shelters, day and night, in search of unguarded receptive females.
Passive tagging confirmed that J. edwardsii in the CROP Marine Reserve displayed a high degree of site fidelity (Kelly & MacDiarmid Citation2003). Twenty-one per cent of tagged lobsters (n=323) re-sighted between 1983 and 1985 maintained an association with a 15 ha reef. A high degree of site fidelity was also displayed by 32 mature J. edwardsii tracked with acoustic tags for up to 1 year within the CROP Marine Reserve and Tāwharanui Marine Park (Kelly Citation1999a). Twenty-one per cent never left their 100 m diameter home site, and 56% of those that did move away eventually returned. Periods away from the primary home site ranged from 1 to 103 days, with most lobsters spending < 40 days away in a continuous period.
Despite a high level of site fidelity, passive and active tagging have shown that J. edwardsii undertake seasonal movements to offshore sandflats beyond the CROP and Tāwharanui Marine Reserve boundaries (Kelly et al. Citation1999; Kelly & MacDiarmid Citation2003). A commercial catch survey carried out between 1995 and 1997 showed that these movements maintained catch rates around the CROP Marine Reserve at levels similar to nearby coastal areas. However, catch characteristics around the reserve were more variable due to the movement of lobsters to and from offshore areas where traps were set (Kelly et al. Citation2002).
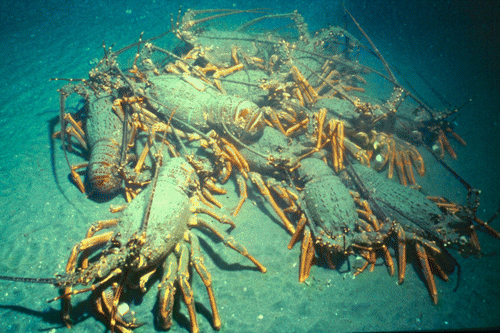
Approximately 17% of 212 re-sighted lobsters tagged in and around the CROP Marine Reserve from 1994 to 1996 had moved across the seaward boundary of the reserve (Kelly & MacDiarmid Citation2003). Further acoustic tagging showed that lobsters moving onto the sandflats remained there for days to weeks, forming diurnal defensive aggregations of up to 200 lobsters (). Lobsters clustered into tight aggregations with those on the perimeter facing outward. When threatened, masses of antennae were used to fend off potential threats. Rather than fleeing, individuals would commonly respond aggressively and often move forward to confront the threat (Kelly et al. Citation1999). The proportion of males and females in offshore aggregations displayed strong seasonality, which was related to cycles in reproduction (mating and egg brooding), moulting and food consumption (Kelly et al. Citation1999). Male behaviour underwent a marked change at the end of the mating season, with large male lobsters switching from aggressively defending inshore dens and potential mates from other males, to forming male groups in offshore areas (Kelly et al. Citation1999). The proportion of males in offshore aggregations, and male food consumption rates, both peaked in winter immediately after the mating season, and again later in the year after males had moulted. In contrast, peak food consumption rates by females occurred soon after moulting and egg extrusion, but there was a lag in the proportion of females recorded in offshore aggregations until the end of the 3–4 month egg-brooding period.
Figure 3 Aggregation of male Jasus edwardsii on sandflats approximately 1 km offshore of coastal reefs in the CROP Marine Reserve. An acoustic tagged male (middle left) enabled the aggregation to be located.
Movements of J. edwardsii have also been investigated in and around the Te Tapuwae o Rongokako Marine Reserve, on the east coast of New Zealand's North Island, where 921 of 5225 tagged lobsters were recaptured in baited traps (Freeman et al. Citation2009). The median distances moved by male and female lobsters in this area were comparable with those from the CROP Marine Reserve, but varied slightly among size classes. The greatest median distance moved was 202 m by males over 70 mm tail width. The greatest median distance for females was 45 m by individuals between 60 and 69.9 mm tail width. Maximum distances ranged up to 4.5 km in smaller male size classes. Clear seasonality was not apparent on lobster movements, in particular for females, which was possibly due to an artefact of the sampling method and, in particular, seasonal variation in catchability. Around 98% of recaptures came from the same reef where tagging occurred, suggesting infrequent movement among reefs. A negative trend was apparent between proportion of reef within the protected area and the average density and size of lobsters on them. This was consistent with lobsters moving out of the reserve and being lost to fishing on the reefs that straddled the reserve boundary.
Trophic interactions and cascades
Prey
Adult J. edwardsii are a large and conspicuous component of the natural reef ecosystem in northeastern New Zealand. Their potential influence on other components of the reef community has been a major theme for research on trophic interactions and community dynamics (Babcock et al. Citation1999; Shears & Babcock Citation2002, Citation2003; Salomon et al. Citation2008; Shears et al. Citation2008; Babcock et al. Citation2010). Experiments, surveys and field observations demonstrated that the grazing urchin Evechinus chloroticus (and a variety of gastropods) were consumed by J. edwardsii, with the maximum prey size increasing with lobster size (Andrew & MacDiarmid Citation1991). Lobsters >130 mm carapace length (CL) were capable of consuming urchins of all sizes, while smaller lobsters took progressively smaller urchins. Gut sampling indicates that urchins are not a preferred prey item of J. edwardsii and that urchins comprise only a small proportion of their diet (Kelly et al. unpublished data). Kelly et al. (unpublished data) have undertaken a comprehensive study of J. edwardsii diet, comparing samples obtained from the northern boundary of the CROP Marine Reserve to those from Wellington's southern coast. They found that J. edwardsii consume a broad range of prey, including molluscs, crustaceans, annelid worms, macroalgae, echinoderms, sponges, bryozoans, fish, foraminifera and brachiopods. The highest frequencies of occurrence in lobster guts were for three mytilid bivalves, which were present in nearly half of the lobster guts containing food and comprised 40% of gut content the volume. The trochid gastropods, Cantharidus purpureus and Coelotrochus viridis, also appear to be important prey, found in > 30% of the guts containing food. Crustacean remains were found in 66% of the guts containing food. A variety of decapods, ostracods, amphipods and barnacles were distinguishable, with decapods comprising >50% of crustacean material. The frequency of occurrence of E. chloroticus in the guts of lobsters was fairly consistent among locations and times, found in 10% ± 2 (SEM) of lobsters with food in their guts and in 9% of all lobsters sampled.
Dense populations of lobsters may also exhibit quite high levels of cannibalism—presumably larger individuals eating smaller ones, or intermoult animals preying on just moulted individuals (Freeman Citation2008). Using stable isotope signatures, it was estimated on average 39% ± 2.5 (SEM) of the diet of lobsters within the Te Tapuwae o Rongokako Marine Reserve came from other lobsters. In lower density populations of the surrounding areas only 16.4% ± 0.9 (SEM) of lobster diet was met through cannibalism. Likewise, Kelly et al. (unpublished data) found that lobster remains frequently occurred in gut samples of lobsters. Video observations revealed that cannibalism accounted for 16% of observed predation events (Oliver et al. Citation2005).
As in many other lobster species, macroalgae comprise a significant dietary component for J. edwardsii, with fleshy green, brown and red algae occurring in 26% of the 255 stomachs examined by Kelly et al. (unpublished data) that contained food and 40%–60% of lobster stomachs sampled by Freeman (Citation2008). Kelly et al. (unpublished data) also recorded some coralline algae in the diet of lobsters sampled in northeastern New Zealand, while Freeman (Citation2008) found that this group occurred in 90% of the lobsters sampled. Moreover, Freeman (Citation2008) also found that coralline turf was less abundant where lobsters were abundant and inferred that their grazing has a significant influence on turf populations. Consumption of macroalgae does not appear to have been due to a limited supply of animal prey, as has been suggested for other lobster species (Edgar Citation1990), indicating that J. edwardsii may obtain specific nutritional benefits from its consumption (Freeman Citation2008).
It has been difficult to determine the significance of fish in the guts of lobsters as fish carcasses are often used as bait in traps, which lobsters can readily enter, feed within and later escape. However, data obtained from inside the CROP Marine Reserve, where baited traps are absent, indicates that fish do constitute a natural, although relatively unimportant (<2% occurrence and stomach volume), component of lobster diet (Kelly et al. unpublished data). In contrast, Freeman (Citation2008) found no fish in the diet of lobsters sampled from within the Te Tapuwae o Rongokako Marine Reserve. In nearby fished areas, however, she found fish bait species comprised about 45% of the diet of lobsters.
Kelly et al. (Citation1999) found that feeding rates by adult J. edwardsii fluctuates seasonally in relation to moulting and reproductive cycles. Males ceased feeding before and during the mating season in April–June, and during their moulting season in October. Female moulting and mating cycles are synchronised, so they only stop feeding once per year between April and June. In the intervening periods males and females move offshore and forage for bivalves, gastropods and crustaceans on the sandflats several kilometres seaward of the deep reef edge (Kelly & MacDiarmid Citation2003; Langlois et al. Citation2005a; Langlois et al. Citation2006a, Citationb). Here they form large diurnal aggregations but forage nocturnally (Kelly et al. Citation1999). Some females move onto the sandflats during spring to hatch their larvae, forming similar diurnal aggregations and may forage during this time. The mean size of lobsters in offshore aggregations is greater than the mean size of the reef population (Kelly et al. Citation1999), but some smaller adults forage nocturnally on sandflats adjacent to the reef, returning to shelter on the reef by day. This can depress the abundance and size structure of near reef bivalve populations (Langlois et al. 2005a, Citation2006a, Citationb).
Predators
Relatively little is known about predation on lobsters, with most information coming from incidental observations and tethering experiments on juvenile lobsters. Despite its limitations, tethering juvenile lobsters in the field has provided useful information on predation. Using this approach, 10 species of fish and an octopus have been identified as juvenile predators, including the diurnal active species such as the banded wrasse (Notolabrus fucicola), spotty (Notolabrus celidotus), blue cod (Parapercis colias), snapper (Pagrus auratus) and leatherjacket (Meuschenia scaber), and nocturnal species such as bastard cod (Pseudophycis barbata), rock cod (Lotella rhacinus), hiwihiwi (Chironemus marmoratus), red-banded perch (Hypoplectrodes huntii), long-tailed stingray (Dasyatis thetidis) and octopus (Pinnoctopus cordiformis) (Kington Citation1999; Bassett et al. Citation2008). Small lobsters < 25mm CL were found to be most vulnerable to predators, with individuals > 30 mm less susceptible (Kington Citation1999). Oliver et al. (Citation2005), using tethering devices that recorded the time of predation and video surveillance, found a peak in predation within 2 hours after release of juvenile lobsters but that a secondary peak at dawn coincided with the emergence of diurnal predators. Predation of juvenile J. edwardsii may also be modulated by aggregation. Butler et al. (Citation1999) found that aggregation did not increase the survival of small newly settled lobsters, but larger juveniles survived better in groups. Two studies suggest that when fish predators are present, juvenile lobsters reduce activity and spend less time foraging, thus increasing survival but potentially at the cost of decreased growth (Oliver et al. Citation2006; Mislan & Babcock Citation2008).
Records of adult lobsters in the guts of fish are rare, occurring in bronze whaler sharks (Carcharhinus brachyurus), rig (Mustelus lenticulatus), hāpuku (Polyprion oxygeneios), snapper and blue cod (Ayling & Cox Citation1982; Francis Citation2001; M. Francis, NIWA, pers. com. 10 March 2007). Red rock lobsters were found in the guts of only two out of 450 banded wrasse caught at Kaikoura (Denny & Schiel Citation2001), and were not found in the guts of any of the 50 species of rocky reef fishes sampled around Goat Island, which included 23 snapper (Russell Citation1983).
Octopus (Pinnoctopus cordiformis) is widely acknowledged as an important predator of trapped adult spiny lobsters. In the Leigh area, between August 1995 and September 1996, 1.6% (n=101) of 6277 lobsters caught in commercial traps were eaten by octopus (Kelly unpublished data). Octopus has also been observed preying on adult lobsters in the wild (Kelly et al. Citation1999; Kelly unpublished data).
Ecosystem modelling
Rock lobsters were important components of four ecosystem models recently constructed of reef systems around New Zealand; one qualitative model (CitationBeaumont et al. in press), and three mass balance models (Pinkerton et al. Citation2008; CitationPinkerton in press; CitationPinkerton et al. in press; Eddy et al. unpublished data).
CitationBeaumont et al. (in press) used qualitative modelling to explore relationships among ecosystem components in the CROP Marine Reserve. The model results agreed with observations in a number of northern New Zealand marine reserves where an increase in the abundance and size of lobsters and piscine predators of macroinvertebrates coincided with a gradual decrease in urchin density and an increase in algal cover (Babcock et al. Citation1999; Shears & Babcock Citation2002, Citation2003; Salomon et al. Citation2008; Babcock et al. Citation2010). However, the low degree of model predictability indicates that other, perhaps infrequent, events, such as intermittent strong settlement by one or several components or toxic algal events, may direct the immediate future of the local ecosystem (e.g. Shears et al. Citation2008). The effects of a simultaneous change in the abundance of three predatory groups on other ecosystem components, either due to fishing or protection in marine reserves, indicated that the effects were due mainly to lobsters and piscivores; the response to a change in abundance of snapper was neutral (CitationBeaumont et al. in press).
A mass balance trophic model of the Te Tapuwae o Rongokako Marine Reserve suggested that lobsters were responsible for substantial predation on the grazing, predatory, algal associated and infaunal invertebrate groups Pinkerton et al. (Citation2008). Eddy et al. (unpublished data) used a mass balance ecosystem-based modelling approach to analyse the trophic relationships of reef and adjacent soft sediment habitat species on Wellington's south coast at the time of establishment of the Taputeranga Marine Reserve. They then compared this model to reconstructed historical ecosystem states and found that over the last 60 years the commercial fishery has reduced lobster biomass by about 75%, with significant impacts on the organisation and function of the entire nearshore ecosystem. Similarly, CitationPinkerton (in press) constructed mass balance trophic models of the Hauraki Gulf shelf ecosystem (including coastal reefs) for five time periods over the last 1000 years. The models suggested that lobsters were the third most ecologically important benthic invertebrate group in the Hauraki Gulf before human arrival but, with the decrease in lobster biomass since 1950, have declined to the least important.
All four models indicate the key role played by rock lobsters in unfished coastal reef ecosystems where they are the dominant benthic predator. Three of the models (CitationBeaumont et al. in press; Eddy et al. unpublished data; CitationPinkerton in press) indicate that, at present levels of fishing, this role has been greatly diminished. In these areas of low biomass, lobsters could be considered to be ecologically extinct.
Fisheries and conservation
The location of the LML adjacent to New Zealand's first no-take marine reserve has provided the opportunity to study the response of spiny lobsters to protection and fishing. Lobster populations within the CROP Marine Reserve were surveyed at the time of the reserve's establishment (Ayling Citation1978), with further surveys undertaken periodically since (Cole et al. Citation1991; MacDiarmid & Breen Citation1992; Kelly Citation2000a; Kelly et al. Citation2000; Haggitt & Mead Citation2009a; Freeman et al. Citation2012a). Dedicated PhD programmes related to lobsters and marine reserves (MacDiarmid Citation1987; Kelly Citation1999a) provided some of the first data on the response of previously-harvested species to protection in marine reserves, highlighting the effects of exploitation on the lobster abundance, population size structure, and sex ratios—key attributes of the lobster mating system and trophic dynamics.
LML researchers have also been an integral part of marine reserve monitoring in New Zealand, particularly in northeastern New Zealand, but also more generally. Kelly et al. (Citation2000) presented the first New Zealand study of the response of lobsters to protection in multiple marine reserves. Further studies by LML researchers have demonstrated not only the magnitude and timing of recovery in a range of marine reserves (Kelly Citation1999b, Citation2000b; Haggitt & Mead Citation2009a, Citationb; Pande et al. Citation2008; Diaz Guisado et al. Citation2012; Davidson et al. Citation2002), but have also provided insight into the mechanisms driving the spatial and temporal variability in the response of lobsters to protection (Freeman et al. Citation2012a). The transect methodology first introduced by Ayling (Citation1978) and later modified (e.g. MacDiarmid Citation1991, Kelly et al. Citation2000) for northeastern New Zealand is now used across New Zealand's marine reserve network (Freeman et al. Citation2012a).
The connectivity between fished and unfished populations, studied primarily through tagging studies and surveys of spatial patterns in abundance, has provided insight into the potential impacts of marine reserves on surrounding fisheries. Kelly et al. (Citation2002) estimated the dollar value of lobster migration from the CROP Marine Reserve by comparing catch per unit effort from baited traps set near the marine reserve to catches further from the reserve. Freeman et al. (Citation2009) used the movement patterns of tagged lobsters to demonstrate that the distribution of rocky reef drove the cross-marine reserve boundary movement of lobsters and influenced the abundance of lobsters on reefs within the reserve. Kelly and MacDiarmid's (2003) tagging study showed that lobsters in CROP Marine Reserve were vulnerable to fishing effort in adjacent areas through their seasonal inshore–offshore migrations, which resulted in lobsters crossing the offshore boundary of the reserve. This prompted the setting of offshore boundaries further from shore in more recently established New Zealand marine reserves.
Comparisons of lobster population abundance and size structure in no-take versus partially protected areas indicated the impact of recreational fishing (Shears et al. Citation2006).
LML researchers have utilised marine reserves as a tool for assessing the effects of fishing on lobsters. Studies have addressed issues such as the ecological role of lobsters (Langlois et al. Citation2005a, Citation2005b; Langlois Citation2005), predation (Mislan & Babcock Citation2008), growth (Freeman et al. Citation2012b) and disease (Freeman & MacDiarmid Citation2009). Many of the studies reviewed in earlier sections, are also relevant to fisheries management and have been used in stock assessments and assessments of fishery impacts (e.g. Breen Citation2005). Studies undertaken by LML researchers have also provided valuable insight into the aquaculture and restocking potential of J. edwardsii through a focus on the feeding behaviour and dietary requirements of lobsters in captivity (Sheppard Citation2001; Sheppard et al. Citation2002; Cox Citation2004; Devey Citation2004; Fordyce Citation2004; Simon & James Citation2007; Simon & Jeffs Citation2008; Simon Citation2009a, Citationb, Citationc, Citationd, Citatione; Jeffs Citation2010).
Future research needs
This review of research conducted through the LML suggests directions for further research to address outstanding questions about the biology and ecology of J. edwardsii. Only a handful of research undertaken through the LML has focused on lobster moulting and growth, in part because growth is difficult to measure in wild lobsters. As lobsters periodically shed their exoskeleton they do not lay down growth rings in the hard parts of their body. Thus, studies of growth are usually dependent on the capture–recapture of tagged individuals, a time consuming and expensive process. New insights into density-dependent effects on growth potential may be possible by focusing instead on instantaneous measures of nutritional state, such as the blood refractive index (Oliver & MacDiarmid Citation2001), or determining how fast a population of juveniles cycles through a complete moult cycle by monitoring the proportion of individuals in post-moult, inter-moult or pre-moult stages via pleopod moult staging (e.g. Oliver Citation2000).
Lobster movement patterns demand more attention from both an ecological and fisheries perspective. The interaction of lobsters with coastal ecosystems is mediated through the movement of lobsters across habitats in search of preferred prey species, but we still have outstanding questions about the mechanism lobsters use for spatial orientation in complex habitat. The commercial fishery uses baited traps to attract lobsters during nocturnal foraging, but the distance over which lobsters are attracted is unknown, as is the pattern of movement by lobsters towards the trap and interactions with other lobsters. As lobster stock status is monitored on a catch per pot lift basis (Ministry of Fisheries Science Group Citation2011), answers to these questions are important to fishery management. Egg-brooding females, undersized or soft-shell lobsters are legally required to be returned to the water immediately after a trap is hauled, but there is a poor understanding of how the movement patterns of these lobsters are affected by being trapped and handled. Can these lobsters navigate back to their original home range? Do they remain trap shy for a period? Is foraging and growth compromised? Given the high levels of repeated handling of lobsters by the commercial fishery (Ministry of Fisheries Science Group Citation2011), these questions have important economic implications.
Rock lobsters, unlike fish, have lengthy well-defined periods of the year when they are not actively foraging. Consequently, lobster density cannot simply be equated to predation pressure. To better understand the trophic role of lobsters more information is required on lobster diet, especially for smaller size classes, the importance of bait in traps to lobsters nutrition, levels of cannibalism, use of intertidal and offshore resources, and flexibility in feeding schedules.
The recovery of lobster populations in New Zealand marine reserves, though initially unexpected because it was thought that rates of emigration would be too high from the relatively small protected areas (Ballantine Citation1989, Langlois & Ballantine Citation2005), is now recognised as a consequence of protection of formerly exploited populations (Langlois & Ballantine Citation2005; Babcock et al. Citation2010). Yet there may be some indirect effects on lobsters occurring. Further investigation is required to ascertain any link between adult lobster densities and patterns of puerulus settlement, recruitment and juvenile mortality rates in and around marine reserves.
The management of fishing in New Zealand is becoming more ecosystem focused (Ministry of Fisheries Science Group Citation2011). An important emerging question is: to what extent can lobster biomass be removed from coastal ecosystems before their important ecological role is significantly diminished? To address this question a better understanding of the relationship between lobster biomass and reef community structure is required.
Conclusions
It is clear that research carried out through the LML has made a substantial contribution to understanding the biology and ecology of J. edwardsii, and rock lobsters in general. The number of publications, their breadth of subject matter and their frequency of citation indicate the influence of this research effort. It is likely that J. edwardsii has received a greater level of ecological research effort through the LML than any other species. Why is this so? We suggest that the establishment of the LML in 1962, near the start of underwater observation and experimentation using scuba, its location on a stretch of rocky coastline with year-round easy access to the field, and the availability of a rebuilding population of J. edwardsii in the adjacent CROP Marine Reserve, allowed coupled laboratory and field research to rapidly develop from 1978 without the confounding effects of fishing. As outlined above, many important questions about rock lobster biology and ecology remain. The natural advantages enjoyed by the LML, coupled with its strong history of lobster research, means it is well placed to address these questions and contribute to the conservation and management of this important resource.
Acknowledgements
We thank Kendall Clements and John Montgomery for the invitation to submit this review. We thank two anonymous referees for making substantial improvements to the paper.
References
- Aedo G , Arancibia H 2003 . Estimating attraction areas and effective fishing areas for Chilean lemon crab (Cancer porteri) using traps . Fisheries Research 60 : 267 – 272 . doi: 10.1016/S0165-7836(02)00177-7
- Anderton T 1906 . Observations on New Zealand fishes made at the Portobello Marine Fish-hatchery . Transactions and Proceedings of the New Zealand Institute 39 : 477 – 496 .
- Andrew NL , MacDiarmid AB 1991 . Interrelations between sea urchins and spiny lobsters in northeastern New Zealand . Marine Ecology Progress Series 70 : 211 – 222 . doi: 10.3354/meps070211
- Annala JH , Bycroft BL 1985 . Growth rate of juvenile rock lobsters (Jasus edwardsii) at Stewart Island, New Zealand . New Zealand Journal of Marine and Freshwater Research 19 : 445 – 455 . doi: 10.1080/00288330.1985.9516109
- Annala JH , Bycroft BL 1988 . Growth of rock lobsters (Jasus edwardsii) in Fiordland, New Zealand . New Zealand Journal of Marine and Freshwater Research 22 : 29 – 41 . doi: 10.1080/00288330.1988.9516275
- Anon . 1977 . Ahuahu (Great Mercury Island): Memoirs of Cameron Buchanan 1859–1873 , Mercury Bay Historical Society , Whitianga .
- Ayling AM 1978 . Okakari Point to Cape Rodney Marine Reserve: a biological survey . University of Auckland Leigh Marine Laboratory .
- Ayling T , Cox GJ 1982 . Collins guide to the sea fishes of New Zealand . Collins Publishers , Auckland . 343
- Babcock RC , Kelly S , Shears NT , Walker JW , Willis TJ 1999 . Changes in community structure in temperate marine reserves . Marine Ecology Progress Series 189 : 125 – 134 . doi: 10.3354/meps189125
- Babcock RC , Shears NT , Alcala AC , Barrett NS , Edgar GJ , Lafferty KD , et al. 2010 . Decadal trends in marine reserves reveal differential rates of change in direct and indirect effects . Proceedings of the National Academy of Sciences 107 : 18256 – 18261 . doi: 10.1073/pnas.0908012107
- Ballantine WJ 1989 . Marine reserves: lessons from New Zealand . Progress in Underwater Science 13 : 1 – 14 .
- Bassett DK , Jeffs AG , Montgomery JC 2008 . Identification of the predators of early juvenile spiny lobsters using a novel photographic tethering device . Marine and Freshwater Research 59 : 1079 – 1083 . doi: 10.1071/MF08036
- Beaumont J , MacDiarmid A , Morrison M , McKenzie A , Abraham E , Taylor R , et al. in press . Rocky reef ecosystems—how do they function? Integrating the roles of primary and secondary production, biodiversity, and connectivity across coastal habitats . Final research report for project ZBD2005–09 , Ministry for Primary Industries , Wellington , , New Zealand .
- Berry AR 1997 . Growth aspects of cultured New Zealand rock lobster Jasus edwardsii (Decapoda: Palinuridae) (Hutton) . Unpublished MMarSc thesis . Auckland, New Zealand, University of Auckland . 173
- Best E 1929 . Fishing methods and devices of the Maori . Dominion Museum Bulletin 12 , Wellington .
- Booth JD 2006 . Jasus species . In : Phillips B . Lobsters: biology, management, aquaculture and fisheries . Blackwell , Oxford , , UK . 340 – 358 .
- Booth JD 2011 . Spiny lobsters through the eyes of the giant packhorse Wellington , New Zealand , Victoria University Press . 256
- Booth JD , McKenzie A 2009 . Strong relationships between levels of puerulus settlement and recruited stock abundance in the red rock lobster (Jasus edwardsii) in New Zealand . Fisheries Research 95 : 161 – 168 . doi: 10.1016/j.fishres.2008.08.009
- Booth JD , Phillips BF 1994 . Early life history of spiny lobster . Crustaceana 66 : 271 – 294 . doi: 10.1163/156854094X00035
- Bradstock CA 1948 . A study of the marine spiny crayfish, Jasus lalandii (Milne-Edwards) . Unpublished MSc thesis . Wellington , New Zealand , Victoria University of Wellington . 79
- Breen PA 2005 . Managing the effects of fishing on the environment: what does it mean for the rock lobster (Jasus edwardsii) fishery? New Zealand Fisheries Assessment Report 2005/53 , Ministry of Fisheries , Wellington , , New Zealand .
- Buckeridge JS , Newman WA 1994 . Brian Arthur Foster 10 July 1942–26 June 1992 . Crustaceana 66 : 247 – 252 . doi: 10.1163/156854094X00738
- Butler MJ , MacDiarmid AB , Booth JD 1999 . The cause and consequence of ontogenetic changes in social aggregation in New Zealand spiny lobsters . Marine Ecology Progress Series 188 : 179 – 191 . doi: 10.3354/meps188179
- Caddy JF 2004 . Current usage of fisheries indicators and reference points, and their potential applications to management of fisheries for marine invertebrates . Canadian Journal of Fisheries and Aquatic Sciences 61 : 1307 – 1324 . doi: 10.1139/f04-132
- Cole RG , Ayling TM , Creese RG 1991 . Effects of marine reserve protection at Goat Island, northern New Zealand . New Zealand Journal of Marine and Freshwater Research 24 : 197 – 210 . doi: 10.1080/00288330.1990.9516415
- Cox SL 2004 . Feeding biology and artificial diet development for the culture of Jasus edwardsii and Jasus verreauxi spiny lobster Phyllosomas . Unpublished PhD thesis . Auckland New Zealand University of Auckland . 231
- Cox SL , Bruce MP 2002 . Feeding behaviour and associated sensory mechanisms of stage I-III phyllosoma of Jasus edwardsii and Jasus verreauxi . Journal of the Marine Biological Association of the United Kingdom 82 : 1 – 5 . doi: 10.1017/S002531540200512X
- Cox SL , Johnston DJ 2003 . Feeding biology of spiny lobster larvae and implications for culture . Reviews in Fisheries Science 11 : 89 – 106 . doi: 10.1080/713610924
- Davidson RJ , Villouta E , Cole RG , Barrier RGF 2002 . Effects of marine reserve protection on spiny lobster (Jasus edwardsii) abundance and size at Tonga Island Marine Reserve, New Zealand . Aquatic Conservation: Marine and Freshwater Ecosystems 12 : 213 – 227 . doi: 10.1002/aqc.505
- Denny CM , Schiel DR 2001 . Feeding ecology of the banded wrasse Notolabrus fucicola (Labridae) in southern New Zealand: prey items, seasonal differences and ontogenetic variation . New Zealand Journal of Marine and Freshwater Research 35 : 925 – 933 . doi: 10.1080/00288330.2001.9517054
- Devey MA 2004 . Dietary lipid and fatty acid requirements of the juvenile red rock lobster Jasus edwardsii: a formulation of artificial diets for aquaculture . Unpublished MSc thesis . Auckland New Zealand University of Auckland .
- Diaz Guisado D , Cole RG , Davidson RJ , Freeman DJ , Kelly S , MacDiarmid A , et al. 2012 . Comparison of methodologies to quantify the effects of age and area of marine reserves on the density and size of targeted species . Aquatic Biology 14 : 185 – 200 . doi: 10.3354/ab00391
- Edgar GJ 1990 . Predator-prey interactions in seagrass beds. I The influence of macro-faunal abundance and size structure on the diet and growth of the western rock lobster Panulirus cygnus George . Journal of Experimental Marine Biology and Ecology 139 : 1 – 22 . doi: 10.1016/0022-0981(90)90034-A
- Fordyce MS 2004 . Potential protein sources for use in an artificial diet for juvenile southern spiny lobsters, Jasus edwardsii . Unpublished MSc thesis. Auckland , University of Auckland . 110
- Francis M 2001 . Coastal fishes of New Zealand, an identification guide . Auckland , , New Zealand , Reed Books . 103
- Freeman DJ 2008 . The ecology of spiny lobsters (Jasus edwardsii) on fished and unfished reefs . Unpublished PhD thesis . Auckland, New Zealand, University of Auckland . 306
- Freeman DJ , Breen PA , MacDiarmid AB 2012b . Use of a marine reserve to determine the direct and indirect effects of fishing on growth in a New Zealand fishery for the spiny lobster Jasus edwardsii . Canadian Journal of Fisheries and Aquatic Sciences 69 : 894 .
- Freeman DJ , MacDiarmid AB 2009 . Healthier lobsters in a marine reserve: effects of fishing on disease incidence in the spiny lobster, Jasus edwardsii . Marine and Freshwater Research 60 : 140 – 145 . doi: 10.1071/MF08091
- Freeman DJ , MacDiarmid AB , Taylor RB 2009 . Habitat patches that cross marine reserve boundaries: consequences for the lobster Jasus edwardsii . Marine Ecology Progress Series 388 : 159 – 167 . doi: 10.3354/meps08122
- Freeman DJ , MacDiarmid AB , Taylor RB , Davidson RJ , Grace RV , Haggitt TR , et al. 2012a . Trajectories of spiny lobster Jasus edwardsii recovery in New Zealand marine reserves: is settlement a driver? Environmental Conservation 39 : 295 – 304 . doi: 10.1017/S037689291200015X
- Frusher SD , Hoenig JM 2001 . Impact of lobster size on selectivity of traps for southern rock lobster (Jasus edwardsii) . Canadian Journal of Fisheries and Aquatic Science 58 : 2482 – 2489 . doi: 10.1139/f01-181
- Gabites BP 1990 . Shelter related distribution of the lobster Jasus edwardsii . Unpublished MSc thesis , University of Auckland , Auckland , , New Zealand . 110
- Haggitt TR , Mead S 2009a . Cape Rodney to Okakari Point Marine Reserve and Tawharanui Marine Park monitoring programme: May 2009 survey . Auckland , , New Zealand , Coastal and Aquatic Systems Ltd .
- Haggitt TR , Mead S 2009b . Te Whanganui a Hei Marine Reserve benthic and lobster monitoring programme: May–June 2009 survey . Auckland , , New Zealand , Coastal and Aquatic Systems Ltd .
- Herrnkind W 1969 . Queuing behaviour of spiny lobsters . Science 164 : 1425 – 1427 . doi: 10.1126/science.164.3886.1425
- Herrnkind WF , McLean R 1971 . Field studies of homing, mass emigration and orientation in the spiny lobster, Panulirus argus . Annals of the New York Academy of Sciences 188 : 359 – 377 . doi: 10.1111/j.1749-6632.1971.tb13109.x
- Hooker SH , Jeffs AG , Creese RG , Sivaguru K 1997 . Growth of captive Jasus edwardsii (Hutton) (Crustacea: Palinuridae) in north-eastern New Zealand . Marine and Freshwater Research 48 : 903 – 909 . doi: 10.1071/MF97156
- Ihde TF , Frusher SD , Hoenig JM 2006 . Do large rock lobsters inhibit smaller ones from entering traps? A field experiment . Marine and Freshwater Research 57 : 665 – 674 . doi: 10.1071/MF05238
- Jeffs A 2007 . Revealing the natural diet of the phyllosoma larvae of spiny lobster . Bulletin of Fisheries Research Agency (Japan) 20 : 9 – 13 .
- Jeffs A 2010 . Status and challenges for advancing lobster aquaculture . Journal of the Marine Biological Association of India 52 : 320 – 326 .
- Jeffs AG , Chiswell SM , Booth JD 2001a . Distribution and condition of pueruli of the spiny lobster Jasus edwardsii offshore from north-east New Zealand . Marine and Freshwater Research 52 : 1211 – 1216 . doi: 10.1071/MF01182
- Jeffs AG , Holland RC 2000 . Swimming behaviour of the puerulus of the spiny lobster, Jasus edwardsii (Hutton, 1875) (Decapoda, Palinuridae) . Crustaceana 73 : 847 – 856 . doi: 10.1163/156854000504859
- Jeffs AG , Montgomery JC , Tindle CT 2005 . How do spiny lobster post-larvae find the coast? New Zealand Journal of Marine and Freshwater Research 39 : 605 – 617 . doi: 10.1080/00288330.2005.9517339
- Jeffs AG , Nichols PD , Bruce MP 2001b . Lipid reserves used by the pueruli of the spiny lobster Jasus edwardsii in crossing the continental shelf of New Zealand . Comparative Biochemistry and Physiology A Comparative Physiology 129 : 305 – 311 . doi: 10.1016/S1095-6433(00)00348-2
- Jeffs AG , Nichols PD , Mooney BD , Phillips KL , Phleger CF 2004 . Identifying potential prey of the pelagic larvae of the spiny lobster Jasus edwardsii using signature lipids . Comparative Biochemistry and Physiology B Comparative Biochemistry 137 : 487 – 507 . doi: 10.1016/j.cbpc.2004.02.003
- Jeffs AG , Willmott ME , Wells RMG 1999 . The use of energy stores in the puerulus of the spiny lobster, Jasus edwardsii across the continental shelf of New Zealand . Comparative Biochemistry and Physiology A Comparative Physiology 123A : 351 – 357 .
- Jernakoff P , Phillips BF 1988 . Effect of a baited trap on the foraging movements of juvenile western rock lobsters Panulirus cygnus George . Australian Journal of Marine and Freshwater Research 39 : 185 – 192 . doi: 10.1071/MF9880185
- Kelly S 1999a . Marine reserves and the spiny lobster, Jasus edwardsii . Unpublished PhD thesis . Auckland New Zealand University of Auckland . 185
- Kelly S 1999b . 1999 lobster survey of Te Awaatu (The Gut) Marine Reserve , Doubtful Sound. Unpublished report for the Department of Conservation. Wellington, New Zealand, Department of Conservation. 18 p.
- Kelly S 2000a . Cape Rodney to Okakari Point Marine Reserve lobster monitoring programme: May 2000 survey . Auckland , , New Zealand , Coastal and Aquatic Systems Ltd . 25
- Kelly S 2000b . 2000 Lobster survey of the Cathedral Cove (Te Whanganui a Hei) Marine Reserve . Auckland , , New Zealand : Coastal and Aquatic Systems Ltd . 21
- Kelly S 2001 . Temporal variation in the movement of the spiny lobster Jasus edwardsii . Marine and Freshwater Research 52 : 323 – 331 . doi: 10.1071/MF00028
- Kelly S , MacDiarmid AB 2003 . Movement patterns of mature spiny lobsters, Jasus edwardsii, from a marine reserve . New Zealand Journal of Marine and Freshwater Research 37 : 149 – 158 . doi: 10.1080/00288330.2003.9517153
- Kelly S , MacDiarmid AB , Babcock RC 1999 . Characteristics of spiny lobster, Jasus edwardsii, aggregations in exposed reef and sandy areas . Marine and Freshwater Research 50 : 409 – 416 . doi: 10.1071/MF98126
- Kelly S , Scott D , MacDiarmid AB 2002 . The value of a spillover fishery for spiny lobsters around a marine reserve in northern New Zealand . Coastal Management 30 : 153 – 166 . doi: 10.1080/089207502753504689
- Kelly S , Scott D , MacDiarmid AB , Babcock RC 2000 . Spiny lobster, Jasus edwardsii, recovery in New Zealand marine reserves . Biological Conservation 92 : 359 – 369 . doi: 10.1016/S0006-3207(99)00109-3
- Kington SW 1999 . Factors influencing the on-growing and restocking of Jasus edwardsii . Unpublished MSc thesis. Auckland, New Zealand, University of Auckland . 78
- Kittaka J , MacDiarmid AB 1994 . Breeding . In : Phillips BF , Cobb JS , Kittaka J . Spiny lobster management: current situation and perspectives . Oxford , , UK , Blackwell . 384 – 401 .
- Langlois TJ 2005 . Influence of reef-associated predators on adjacent soft-sediment communities . Unpublished PhD thesis. Auckland, New Zealand, University of Auckland . 161
- Langlois TJ , Anderson MJ , Babcock RC 2005a . Reef-associated predators influence adjacent soft-sediment communities . Ecology 86 : 1508 – 1519 . doi: 10.1890/04-0234
- Langlois TJ , Anderson MJ , Babcock RC 2006a . Inconsistent effects of reefs on different size classes of macrofauna in adjacent sand habitats . Journal of Experimental Marine Biology and Ecology 334 : 269 – 282 . doi: 10.1016/j.jembe.2006.02.001
- Langlois TJ , Anderson MJ , Babcock RC , Kato S 2005b . Marine reserves demonstrate trophic interactions across habitats . Oecologia 147 : 134 – 140 . doi: 10.1007/s00442-005-0148-7
- Langlois TJ , Anderson MJ , Brock M , Murman G 2006b . Importance of rock lobster size-structure for trophic interactions: choice of soft-sediment bivalve prey . Marine Biology 149 : 447 – 454 . doi: 10.1007/s00227-005-0238-4
- Langlois TJ , Ballantine WJ 2005 . Marine ecological research in New Zealand: developing predictive models through the study of no-take marine reserves . Conservation Biology 19 : 1763 – 1770 . doi: 10.1111/j.1523-1739.2005.00278.x
- MacDiarmid AB 1985 . Sunrise release of larvae from the palinurid rock lobster Jasus edwardsii . Marine Ecology Progress Series 21 : 313 – 315 . doi: 10.3354/meps021313
- MacDiarmid AB 1987 . The ecology of Jasus edwardsii (Hutton) (Crustacea: Palinuridae) . Unpublished PhD thesis. Auckland, New Zealand, University of Auckland . 169
- MacDiarmid AB 1988 . Experimental confirmation of external fertilisation in the southern temperate rock lobster Jasus edwardsii (Hutton) (Decapoda: Palinuridae) . Journal of Experimental Marine Biology and Ecology 120 : 277 – 285 . doi: 10.1016/0022-0981(88)90007-X
- MacDiarmid AB 1989a . Moulting and reproduction of the spiny lobster Jasus edwardsii (Decapods: Palinuridae) in northern New Zealand . Marine Biology 103 : 303 – 310 . doi: 10.1007/BF00397263
- MacDiarmid AB 1989b . Size at onset of maturity and size-dependent reproductive output of female and male spiny lobsters Jasus edwardsii (Hutton) (Decapoda, Palinuridae) in northern New Zealand . Journal of Experimental Marine Biology and Ecology 127 : 229 – 243 . doi: 10.1016/0022-0981(89)90076-2
- MacDiarmid AB 1991 . Seasonal changes in depth distribution, sex ratio and size frequency of spiny lobster Jasus edwardsii on a coastal reef in northern New Zealand . Marine Ecology Progress Series 70 : 129 – 141 . doi: 10.3354/meps070129
- MacDiarmid AB 1994 . Cohabitation in the spiny lobster Jasus edwardsii (Hutton, 1875) . Crustaceana 66 : 341 – 355 . doi: 10.1163/156854094X00071
- MacDiarmid AB , Breen PA 1992 . Spiny lobster population change in a marine reserve . In : Battershill CN , Schiel DR , Jones GP , Creese RG , MacDiarmid AB . Second International Temperate Reef Symposium . Auckland , , New Zealand . 47 – 56 .
- MacDiarmid AB , Butler MJ 1999 . Sperm economy and limitation in spiny lobsters . Behavioural Ecology and Sociobiology 46 : 14 – 24 . doi: 10.1007/s002650050587
- MacDiarmid A , Cleaver P , Stirling B in press . Historical evidence for the state and exploitation of marine fish and invertebrate resources in the Hauraki Gulf and along the Otago-Catlins shelf 1769–1950 . Ministry for Primary Industries . 615
- MacDiarmid AB , Hickey B , Maller RA 1991 . Daily movement patterns of the spiny lobster Jasus edwardsii (Hutton) on a shallow reef in northern New Zealand . Journal of Experimental Marine Biology and Ecology 147 : 185 – 205 . doi: 10.1016/0022-0981(91)90182-V
- MacDiarmid AB , Kittaka J 2000 . Breeding . In : Phillips BF , Kittaka J . Spiny lobsters: fisheries and culture . Blackwell , Oxford , , UK . 485 – 507 .
- MacDiarmid AB , Sainte-Marie B 2006 . Reproduction . In : Phillips B . Lobsters: biology, management, aquaculture and fisheries . Oxford , , UK , Blackwell . 45 – 77 .
- MacDiarmid A , Stewart R 2005a . Male mating capacity in the New Zealand red rock lobster . The Lobster Newsletter 18 : 7 .
- MacDiarmid A , Stewart R 2005b . Male but not female choice is influenced by numbers of potential mates in a den . The Lobster Newsletter 18 : 8 – 9 .
- MacDiarmid AB , Stewart R , Oliver M 2000 . Mate choice in rock lobsters . Seafood New Zealand August : 38 – 39 .
- MacDiarmid AB , Stewart R , Oliver M , Mauger J 1999 . Female rock lobsters are vulnerable to mate availability . Seafood New Zealand July : 45 – 47 .
- Mauger JW 2001 . Sperm depletion and regeneration in the spiny lobster Jasus edwardsii . Unpublished MSc thesis. Auckland, New Zealand, University of Auckland . 100
- Maxwell K , MacDiarmid AB in press . Oral histories of marine fish and shellfish state and use in the Hauraki Gulf and along the Otago-Catlins coast 1940–2008 . Wellington , , New Zealand , Ministry for Primary Industries .
- McKoy JL 1983 . Movements of rock lobsters, Jasus edwardsii (Decapoda: Palinuridae), tagged near Stewart Island, New Zealand . New Zealand Journal of Marine and Freshwater Research 17 : 357 – 366 . doi: 10.1080/00288330.1983.9516011
- McKoy JL 1985 . Growth of tagged rock lobsters (Jasus edwardsii) near Stewart Island, New Zealand . New Zealand Journal of Marine and Freshwater Research 19 : 457 . doi: 10.1080/00288330.1985.9516110
- McKoy JL , Esterman DB 1981 . Growth of rock lobsters (Jasus edwardsii) in the Gisborne region, New Zealand . New Zealand Journal of Marine and Freshwater Research 15 : 121 – 136 . doi: 10.1080/00288330.1981.9515905
- Ministry of Fisheries Science Group 2011 . Report from the mid-year fisheries assessment plenary, November 2011: stock assessments and yield estimates . Unpublished report held in NIWA Greta Point library , Wellington , , New Zealand . 355
- Mislan KAS , Babcock RC 2008 . Survival and behaviour of juvenile red rock lobster, Jasus edwardsii, on rocky reefs with varying predation pressure and habitat complexity . Marine and Freshwater Research 59 : 246 – 253 . doi: 10.1071/MF07116
- Montgomery JC , Jeffs AG , Simpson S , Meekan M , Tindle C 2006 . Sound as an orientation cue for the pelagic larvae of reef fish and decapod crustaceans . Advances in Marine Biology 51 : 143 – 196 .
- Oliver MD 2000 . Predicting moult frequency, moult increment, and nutritional condition in spiny rock lobsters, Jasus edwardsii (Decapoda: Palinuridae) (Hutton) . Unpublished MSc thesis. Christchurch, New Zealand, University of Canterbury . 76
- Oliver MD , MacDiarmid AB 2001 . Blood refractive index and ratio of weight to carapace length as indices of nutritional condition in juvenile rock lobsters (Jasus edwardsii) . Marine and Freshwater Research 52 : 1395 – 1400 . doi: 10.1071/MF01030
- Oliver MD , MacDiarmid AB , Stewart R , Gardner C 2006 . Spiny lobster population enhancement: moderation of emergence behaviour of juvenile Jasus edwardsii reared in captivity . New Zealand Journal of Marine and Freshwater Research 40 : 605 – 613 . doi: 10.1080/00288330.2006.9517449
- Oliver MD , Stewart R , Mills D , MacDiarmid AB , Gardner C 2005 . Stock enhancement of rock lobsters (Jasus edwardsii): timing of predation on naïve juvenile lobsters immediately after release . New Zealand Journal of Marine and Freshwater Research 39 : 391 – 397 . doi: 10.1080/00288330.2005.9517320
- O'Rorke R , Lavery S , Jeffs AG 2012 . PCR enrichment techniques to identify the diet of predators . Molecular Ecology Resources 12 : 5 – 17 . doi: 10.1111/j.1755-0998.2011.03091.x
- Pande A , MacDiarmid AB , Smith PJ , Davidson RJ , Cole RG , Freeman DJ , et al. 2008 . Marine reserves increase the abundance and size of blue cod and rock lobster . Marine Ecology Progress Series 366 : 147 – 158 . doi: 10.3354/meps07494
- Phillips BF , McWilliam PS 1986 . The pelagic phase of spiny lobster development . Canadian Journal of Fisheries and Aquatic Sciences 43 : 2153 – 2163 . doi: 10.1139/f86-264
- Phleger CF , Nelson MM , Mooney BD , Nichols PD , Ritar AJ , Smith GG , et al. 2001 . Lipids and nutrition of the southern rock lobster, Jasus edwardsii, from hatch to puerulus . Marine and Freshwater Research 52 : 1475 – 1486 . doi: 10.1071/MF01071
- Pinkerton MH in press . Trophic modelling of a New Zealand marine shelf ecosystem since AD 1000 . New Zealand Aquatic Environment and Biodiversity Report .
- Pinkerton MH , Lundquist CJ , Duffy CAJ , Freeman DJ 2008 . Trophic modelling of a New Zealand rocky reef ecosystem using simultaneous adjustment of diet, biomass and energetic parameters . Journal of Experimental Marine Biology and Ecology 367 : 61 – 266 . doi: 10.1016/j.jembe.2008.09.022
- Pinkerton MH , Lundquist CJ , Jones E , MacDiarmid AB in press . Benthic invertebrates: trophic modelling of Hauraki Gulf . Appendix 4 in Pinkerton MH, Trophic modelling of a New Zealand marine shelf ecosystem since AD 1000. New Zealand Aquatic Environment and Biodiversity Report .
- Radford CA , Marsden ID , Davison W , Jeffs AG 2007 . Effects of dietary carbohydrate on growth of juvenile New Zealand rock lobsters, Jasus edwardsii . Aquaculture 273 : 151 . doi: 10.1016/j.aquaculture.2007.09.021
- Raethke N , MacDiarmid AB , Montgomery JC 2004 . The role of olfaction during mating in the southern temperate spiny lobster Jasus edwardsii . Hormones and Behavior 46 : 311 – 318 . doi: 10.1016/j.yhbeh.2004.04.005
- Russell BC 1983 . The food and feeding habits of rocky reef fish of northeastern New Zealand . New Zealand Journal of Marine and Freshwater Research 17 : 121 – 145 . doi: 10.1080/00288330.1983.9515991
- Saila SB , Annala JH , McKoy JL , Booth JD 1979 . Application of yield models to the New Zealand rock lobster fishery . New Zealand Journal of Marine and Freshwater Research 13 : 1 – 11 . doi: 10.1080/00288330.1979.9515775
- Salomon AK , Shears NT , Langlois TJ , Babcock RC 2008 . Cascading effects of fishing can alter carbon flow through a temperate coastal ecosystem . Ecological Applications 18 : 1874 – 1887 . doi: 10.1890/07-1777.1
- Shears NT , Babcock RC 2002 . Marine reserves demonstrate top-down control of community structure on temperate reefs . Oecologia 132 : 131 – 142 . doi: 10.1007/s00442-002-0920-x
- Shears NT , Babcock RC 2003 . Continuing trophic cascade effects after 25 years of no-take marine reserve protection . Marine Ecology Progress Series 246 : 1 – 16 . doi: 10.3354/meps246001
- Shears NT , Babcock RC , Salomon AK 2008 . Context-dependent effects of fishing: variation in trophic cascades across environmental gradients . Ecological Applications 18 : 1860 – 1873 . doi: 10.1890/07-1776.1
- Shears NT , Grace RV , Usmar NR , Kerr V , Babcock RC 2006 . Long-term trends in lobster populations in a partially protected vs. no-take marine park . Biological Conservation 132 : 222 – 231 . doi: 10.1016/j.biocon.2006.04.001
- Sheppard JK 2001 . Development of an artificial diet for New Zealand spiny lobster Jasus edwardsiiaquaculture . Unpublished MSc thesis. Auckland, New Zealand, University of Auckland . 134
- Sheppard JK , Bruce MP , Jeffs AG 2002 . Optimal feed pellet size for culturing juvenile spiny lobster Jasus edwardsii (Hutton, 1875) in New Zealand . Aquaculture Research 33 : 913 – 916 . doi: 10.1046/j.1365-2109.2002.00730.x
- Simon CJ 2009a . Advancing the nutrition of juvenile spiny lobster, Jasus edwardsii, in aquaculture . Unpublished PhD thesis. Auckland, New Zealand, University of Auckland . 188
- Simon CJ 2009b . Digestive enzyme response to natural and formulated diets in cultured juvenile spiny lobster, Jasus edwardsii . Aquaculture 294 : 271 – 281 . doi: 10.1016/j.aquaculture.2009.06.023
- Simon CJ 2009c . The effect of carbohydrate source, inclusion level of gelatinised starch, feed binder and fishmeal particle size on the apparent digestibility of formulated diets for spiny lobster juveniles, Jasus edwardsii . Aquaculture 296 : 329 – 336 . doi: 10.1016/j.aquaculture.2009.08.032
- Simon CJ 2009d . Feed intake and its relation to foregut capacity in juvenile spiny lobster, Jasus edwardsii . New Zealand Journal of Marine and Freshwater Research 43 : 195 – 203 . doi: 10.1080/00288330909509993
- Simon CJ 2009e . Identification of digestible carbohydrate sources for inclusion in formulated diets for juvenile spiny lobsters, Jasus edwardsii . Aquaculture 290 : 275 – 282 . doi: 10.1016/j.aquaculture.2009.02.026
- Simon CJ , James PJ 2007 . The effect of different holding systems and diets on the performance of spiny lobster juveniles, Jasus edwardsii (Hutton, 1875) . Aquaculture 266 : 166 – 178 . doi: 10.1016/j.aquaculture.2007.02.050
- Simon CJ , Jeffs A 2008 . Feeding and gut evacuation of cultured juvenile spiny lobsters, Jasus edwardsii . Aquaculture 280 : 211 – 219 . doi: 10.1016/j.aquaculture.2008.05.019
- Street RJ 1969 . The New Zealand crayfish Jasus edwardsii (Hutton) . Fisheries Technical Report 30: 1–24 . Wellington , New Zealand Marine Department .
- Wells RMG , Lu J , Hickey AJR , Jeffs AG 2001 . Ontogenetic changes in enzyme activities associated with energy production in the spiny lobster, Jasus edwardsii . Comparative Biochemistry and Physiology B Comparative Biochemistry 130 : 339 – 347 . doi: 10.1016/S1096-4959(01)00439-0
- Wilkins JL , Jeffs AG 2011 . Energetics of swimming to shore in the puerulus stage of a spiny lobster: can a postlarval lobster afford the cost of crossing the continental shelf? Limnology and Oceanography: Fluids and Environments 1 : 163 – 175 . doi: 10.1215/21573698-1504363
- Ziegler PE , Frusher SD , Johnson CR 2003 . Space-time variation in catchability of southern rock lobster Jasus edwardsii in Tasmania explained by environmental, physiological and density-dependent processes . Fisheries Research 61 : 107 – 123 . doi: 10.1016/S0165-7836(02)00240-0
- Ziegler PE , Frusher SD , Johnson CR , Gardner C 2002a . Catchability of the southern rock lobster Jasus edwardsii. I Effects of sex, season and catch history . Marine and Freshwater Research 53 : 1143 – 1148 .
- Ziegler PE , Haddon M , Frusher SD , Johnson CR 2004 . Modelling seasonal catchability of the southern rock lobster Jasus edwardsii by water temperature, moulting and mating . Marine Biology 145 : 179 – 190 .
- Ziegler PE , Johnson CR , Frusher SD , Gardner C 2002b . Catchability of the southern rock lobster Jasus edwardsii. II Effects of size . Marine and Freshwater Research 53 : 1149 – 1159 .