Abstract
Anomalies can arise when species-specific otolith equations are applied to congeneric species in analyses of seal prey remains. Opalfishes, Hemerocoetes, two spp., are the most numerous fish in the diet of New Zealand sea lions, Phocarctos hookeri, at their population base at the Auckland Islands. Opalfish identified from otoliths in sea lion prey remains accounted for one-third of prey items in this and two previous diet studies. This highlighted the need to estimate size of opalfish accurately. Equations to estimate fish size from otolith length differed between the two species because otoliths of H. morelandi were relatively smaller than otoliths of H. artus. However, otoliths of these species were indistinguishable and this precluded definitive estimates of opalfish length and mass from otoliths. In particular, misidentification of otoliths could create untenably large estimates for fish size. A combination of the equations for otolith size to fish size from the two species avoided this anomaly and superseded previously published equations.
Introduction
Opalfishes, Hemerocoetes, five spp. (Perciformes: Percophididae), are small (<20 cm) and slender bottom-dwelling marine fishes endemic to the continental shelf waters of the New Zealand region (Nelson Citation1979; Paulin et al. Citation2001). They frequent shell, pebble and sand substrates where they perch on their pelvic and anal fins (Francis Citation1996). Otoliths of opalfishes have been described and depicted by Schwarzhans (Citation1984). Opalfish, identified from their otoliths, were the smallest and most numerically abundant prey recorded from the two published studies of diet of the New Zealand sea lion (NZ sea lion), Phocarctos hookeri, at the Auckland Islands. They accounted for 37% of 3523 prey items recorded from 142 scats and 64 regurgitations (Childerhouse et al. Citation2001) and for 36% of 3627 prey items recorded from 121 stomach contents of animals incidentally killed in fisheries (Meynier et al. Citation2009). Video cameras attached to free-ranging NZ sea lions at the Auckland Islands showed that opalfish were taken as prey (Childerhouse et al. Citation2001), indicating primary occurrence in the diet rather than the secondary occurrence of small fish from the stomach contents of larger prey.
The NZ sea lion is the only pinniped species endemic to the New Zealand region and 86% of the entire population breeds at the sub-Antarctic Auckland Islands (Chilvers Citation2008; Robertson & Chilvers Citation2011). They forage across the continental shelf and shelf edge (e.g. Meynier et al. Citation2010; Chilvers et al. Citation2011; Leung et al. Citation2012). Incidental catches of NZ sea lions in trawl fisheries around the Auckland Islands are considered a major threat to the viability of the species (e.g. Chilvers Citation2008, Citation2012a,Citationb,Citationc; Robertson & Chilvers Citation2011; Breen et al. Citation2012). Their interactions with fisheries at the Auckland Islands may also extend to competition for resources, an aspect that can be assessed only with knowledge of the diet of the NZ sea lion (Robertson & Chilvers Citation2011; Riet-Sapriza et al. Citation2012).
Analysis of undigested prey remains in scats and regurgitations is the most widely used and least intrusive method for assessing the diets of seals (e.g. Jobling & Breiby Citation1986; Pierce & Boyle Citation1991). Biases resulting from these methods are well documented and largely a result of otoliths and other key diagnostic remains (e.g. bones) of depredated teleost fish being subject to digestive erosion whereas keratinous cephalopod beaks are not (e.g. Jobling & Breiby Citation1986; Pierce & Boyle Citation1991; Smale et al. Citation1995; Tollit et al. Citation1997, Citation2006). Despite these biases, which often confound analyses of seal diets from prey remains, they do not preclude spatial or temporal comparisons (Tollit et al. Citation2006).
In addition to estimates for frequency of occurrence and number of prey items, otoliths and other diagnostic bones of teleost fish can be used to estimate lengths and masses of fish consumed (e.g. Bowen Citation2000; Orr & Harvey Citation2001; Tollit et al. Citation2006). Two species of opalfishes frequent the continental shelf around the Auckland Islands: H. artus and H. morelandi (Nelson Citation1979). Meynier et al. (Citation2009) estimated the length and mass of opalfish represented by otoliths in the stomach contents of NZ sea lions from equations generated from a small sample of unidentified opalfish. In this study, we supersede these equations by generating species-specific power equations from relatively large numbers of H. artus and H. morelandi specimens collected around Auckland Islands.
Otolith shape is species specific, but the combination of intraspecific variation and shape similarities among some congeneric species may restrict identification to genus rather than species (e.g. Jobling & Breiby Citation1986; Smale et al. Citation1995; Caspar et al. Citation2006; Kemp et al. Citation2011). This becomes a problem when attempting to differentiate between otoliths of sympatric prey species. We investigated the impacts of misidentification of otoliths on length and mass estimates between the two species of opalfishes around the Auckland Islands. Although opalfishes are small, changes in equations to estimate fish size from otoliths could change estimates of their proportion by mass in the diet of NZ sea lions, which may have implications for the conservation management of sea lions.
Materials and methods
A reference collection of opalfish otoliths from H. artus and H. morelandi was collected by CL aboard fishing trawlers around the Auckland Islands during March and April in 1989 and 1995. Opalfish were measured and weighed fresh, with standard length (SL, cm) and fork length (FL, cm) to the nearest 0.1 cm and mass (FM, g) to the nearest 0.5 or 1 g. Otoliths were photographed through a Dino-Lite digital microscope. Paired left and right otoliths were measured separately to the nearest 0.01 mm with vernier callipers, with averages from pairs applied in equations. Otolith length (OL, mm) was measured parallel to the sulcus from anterior to posterior tips, and otolith height (OH, mm, following Smale et al. Citation1995) was measured from dorsal to ventral edges normal to OL. Dried pairs of otoliths were weighed together to the nearest 0.01 g with Mettler electronic scales and otolith mass (OM, mg) was taken as half the mass of the pair. Otolith robustness (OR, mg/mm) was calculated as mean OM (mg) divided by mean OL (mm) for each pair of otoliths, following Harvey (Citation1989) and Tollit et al. (Citation1997). Sample sizes for each measured parameter varied within each species because some fish were not weighed and one pair of H. morelandi otoliths were lost.
Power regressions of best fit were calculated using Microsoft Excel to deduce relationships among parameters for fish size and otolith size. The precision of constants in equations were presented to two significant figures. Tests for diferences between regression coefficients and between elevations followed Zar (Citation1999) and were applied to logarithmic transformations for interspecific comparisons of SL to FM and OL to SL. The linear regression through the origin and 95% prediction intervals (the likely spread of data) for the comparison of OL between paired left and right otoliths followed Zar (Citation1999).
To investigate the impact of different regressions on diet estimates, we used the measurements of otoliths from fresh scat samples collected from NZ sea lions through 2 weeks, 31 July–14 August 2010, on Enderby Island and on Auckland Island bordering Port Ross during a visit by the University of Otago research vessel Polaris II. Although scats are easily defined as the product of defecation, their quantification is vague and imprecise. Here a scat sample was designated to represent one or more fresh scats produced in close succession by one animal. This approach (rather than analysing each scat individually) avoided the possibility of remains from one prey item being represented in more than one scat. Samples were sieved (mesh size 0.7 mm) in water on site. Of the 113 scat samples collected, five (total five scats) lacked prey remains entirely and 108 (total 163 scats, mean 1.5 and range 1–5 scats per sample) contained identifiable prey remains. Opalfish were identified from their otoliths. Each opalfish otolith in each scat sample was assigned into one of five categories: left and right uneroded otoliths; left and right eroded otoliths; and otoliths too eroded to designate as left or right. After comparison with pristine otoliths from our reference collection, otoliths were designated uneroded if the measure for OL appeared unaffected by digestive erosion. The key feature for uneroded otoliths was a well-defined sulcus, following Tollit et al. (Citation1997). The total number of opalfish represented in each scat sample was estimated as the sum of paired otoliths, single left otoliths, single right otoliths, and half the total of otoliths that were too eroded to designate as left or right. The number of pairs of uneroded otoliths was estimated by pairing left and right otoliths with OL within the 95% prediction interval calculated from the reference collection. Eroded left and right otoliths were paired visually by size and state of erosion.
Meynier et al. (Citation2009) presented equations to estimate length and mass of opalfish represented by otoliths in the stomach contents of NZ sea lions incidentally killed in trawl fisheries around the Auckland Islands. Their data were derived from 10 frozen specimens of unidentified Hemerocoetes species (SL 14.3–19.2 cm) collected aboard a fishing trawler southeast of the Auckland Islands in April 2006. Fish length SL (mm) was estimated from OL (mm) from 20 otoliths with the linear regression SL=34.297+32.553OL and FM (g) was estimated from SL (mm) with the power regression FM=3×10−8SL3.9565 (Meynier et al. Citation2009). In the present study, the data used to derive these equations were analysed with SL presented in centimetres for comparison with analyses of H. artus and H. morelandi from our reference collection.
Results
Comparisons between the two species of opalfishes at Auckland Islands
In addition to large differences in the structure of the head described by Nelson (Citation1979), specimens of the two species of opalfishes from around the Auckland Islands were easily differentiated by a previously undescribed morphological feature, the colour of fins of fresh specimens: yellow for H. artus and red for H. morelandi. Maximum lengths from our fresh specimens, SL 20.8 cm from 47 H. artus and SL 14.5 cm from 36 H. morelandi, were similar to the respective 19.5 cm from 57 H. artus and 15.0 cm from 19 H. morelandi measured by Nelson (Citation1979). Analysis of records for the specimens from the Auckland Islands shelf documented by Nelson (Citation1979) highlighted a difference in depth distribution between the two species: depth range of 95–400 m (mean about 260 m from eight sites) for 18 H. artus and depth range of 20–180 m (mean about 85 m from 14 sites) for 53 H. morelandi.
The relationship between FM and SL from fresh specimens was similar for both species; H. artus FM=0.011SL2.78 (n=41, r 2=0.99, P<0.001, range SL 4.9–20.8 cm, range FM 1–50 g) and H. morelandi FM=0.021SL2.61 (n=30, r 2=0.96, P<0.001, range SL 6.5–14.5 cm, range FM 2.5–22 g) (). The regression coefficients of log transformations for these two equations were not significantly different (t=1.642, t 0.05(2),67=1.996, P>0.05) but the respective difference in elevations was highly significant (t=7.433, t 0.001(2),67=3.439, P<0.001). This indicated that the two species exhibited similar exponential rates of increase in FM with increase in fish length, but H. morelandi were heavier than H. artus for fish of the same length.
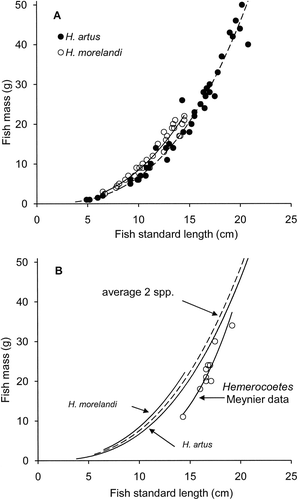
Application of midpoints between these two species-specific equations created a genus-specific estimate for opalfish mass at the Auckland Islands as FM=0.016SL2.68 () for application to SL estimates of ≤15.0 cm, the maximum recorded SL for H. morelandi. For SL entered as 15.0 cm, the genus-specific equation generated FM=20.7 g, 8% less than the 22.6 g generated by the equation for H. morelandi and 10% more than the 18.6 g generated by the equation for H. artus.
The equivalent equation applied for opalfish at the Auckland Islands by Meynier et al. (Citation2009) was FM=0.00031SL3.96 (n=10, r 2=0.90, P<0.001, range SL 14.3–19.2 cm, range FM 11–34 g), with all but the smallest fish longer than the longest recorded specimen of H. morelandi and therefore most probably H. artus (). However, the Meynier et al. (Citation2009) specimens tended to be lighter than H. artus specimens of the same length. When logarithmic transformations of equations for H. artus and the Meynier et al (Citation2009) were compared, both the regression coefficients (t=2.305, t 0.05(2),37=2.012, P>0.05) and the elevations (t=5.879, t 0.001(2),37=3.505, P<0.001) were both statistically different.
The relationship between FL and SL from fresh specimens was similar for both species; H. artus FL=1.12SL (n=47, r 2=1.00, P<0.001, range FL 4.2–22.6 cm) and H. morelandi FL=1.13SL (n=36, r 2=0.99, P<0.001, range FL 7.5–16.4 cm). Corresponding regression equations to estimate FM from FL were H. artus FM=0.008FL2.80 (n=41, r 2=0.99, P<0.001, range FL 5.6–22.6 cm) and H. morelandi FM=0.015FL2.61 (n=31, r 2=0.96, P<0.001, range FL 7.5–16.4 cm). Application of midpoints between these two species-specific equations created a genus-specific estimate for opalfish mass at the Auckland Islands as FM=0.011FL2.69 to be applied to fish shorter than 16.4 cm FL, the longest FL recorded for H. morelandi.
Comparisons of shape and dimensions of otoliths
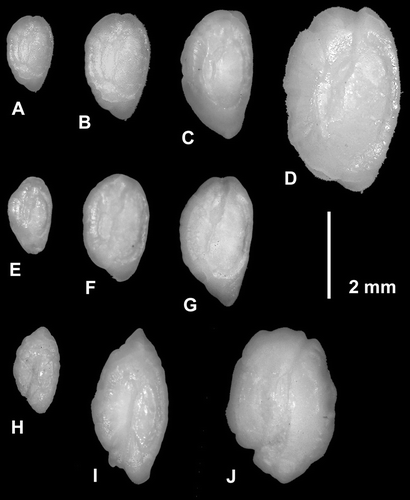
Otoliths of H. artus and H. morelandi lacked species-specific diagnostic differences in shape but differed in maximum length: OL 4.3 mm for H. artus and OL 3.0 mm for H. morelandi (). Consequently, opalfish otoliths with OL>3.0 mm could be designated H. artus but smaller otoliths could not be differentiated reliably between the two species. Otoliths of two other species on the Auckland Islands shelf had otolith shapes similar to opalfishes: deepwater triplefin (Matanui bathytaton) and giant stargazer (Kathetostoma giganteum). Both differed from opalfishes by sulcus shape and by the presence of a prominent anterior projection (rostrum) that was absent from opalfish otoliths ().
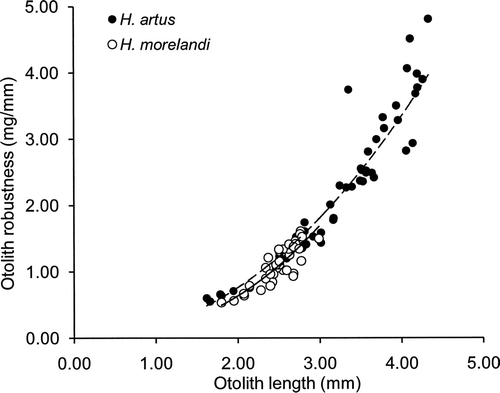
The two species of opalfishes could not be differentiated from relative dimensions of their otoliths. They had similar relationships between OH and OL, with H. artus OH=0.53OL1.11 (n=47, r 2=0.97, P<0.001, range OL 1.62–4.34 mm, range OH 0.96–2.84 mm) and H. morelandi OH=0.42OL1.33 (n=37, r 2=0.84, P<0.001, range OL 1.80–2.99 mm, range OH 0.96–1.74 mm) (); and similar relationships between OM and OL, H. artus OM=0.17OL3.13 (n=47, r 2=0.97, range OM 0.92–20.85 mg) and H. morelandi OM=0.12OL3.42 (n=37, r 2=0.87, range OM 0.97–4.48 mg) ().
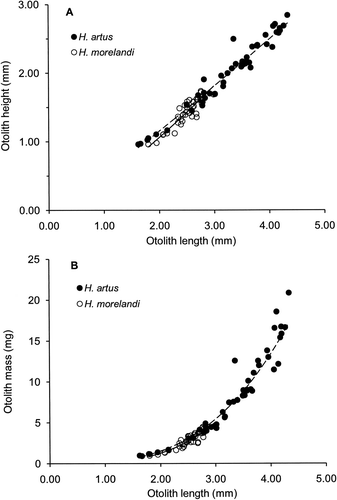
OR increased with OL for both species of opalfishes, with H. artus OR=0.17OL2.13 (n=47, r 2=0.95, P<0.001, range OR 0.55–4.81 mg/mm) and H. morelandi OR=0.12OL2.42 (n=37, r 2=0.78, P<0.001, range OR 0.54–1.61 mg/mm) (). The two species had similar values for OR through their range of overlap: 0.6–0.7 mg/mm for H. artus and 0.5–0.6 mg/mm for H. morelandi at OL 1.8 mm, increasing to 1.4–1.7 mg/mm for H. artus and 1.2–1.6 mg/mm for H. morelandi at OL 3.0 mm. OR for the largest H. artus otoliths increased to 4–5 mg/mm at OL 4.2–4.3 mm.
Estimates for fish length from otolith length
The relationship between fish SL and OL from fresh specimens was SL=2.13OL1.56 (n=47, r 2=0.94, P<0.001) for H. artus and SL=2.09OL1.84 (n=36, r 2=0.77, P<0.001) for H. morelandi (). The regression coefficients of logarithmic transformations for these two equations were not significantly different (t=1.596, t 0.05(2),79=1.990, P>0.05) but the respective difference in elevations was highly significant (t=8.940, t 0.001(2),79=3.416, P<0.001). This indicated that the two species exhibited similar exponential rates of increase in fish length with increase in OL but H. artus had longer otoliths than H. morelandi for fish of the same length.
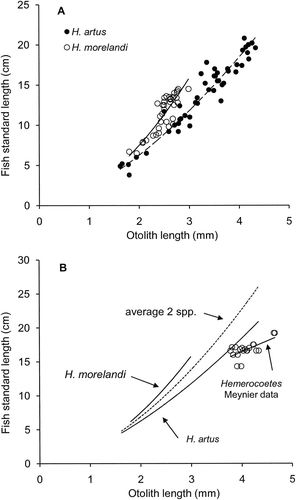
Midpoints between the two species-specific power regressions to calculate fish SL from OL created a genus-specific estimate for opalfish at the Auckland Islands as SL=2.09OL1.72 () for application to OL<3.00 mm, the maximum recorded OL for H. morelandi. For OL entered as 3.00 mm, the genus-specific equation generated SL=13.8 cm, 13% less than the 15.8 cm generated by the equation for H. morelandi and 17% more than the 11.8 cm generated by the equation for H. artus.
The equivalent equation applied to estimate fish SL from OL for opalfish at the Auckland Islands by Meynier et al. (Citation2009) was derived from 20 otoliths from 10 fish as a linear regression SL=3.43+3.26OL (n=20, r 2=0.46, P=0.001, range OL 3.81–4.66 mm, range SL 14.3–19.2 cm) with all otoliths longer than the maximum 2.99 mm recorded for H. morelandi () and so were probably from H. artus. In order to compare with equivalent data for H. artus, power regressions were applied to the average OL per pair of otoliths from Meynier et al. (Citation2009) and produced SL=5.22OL0.83 (n=10, r 2=0.44, P<0.05, range OL 3.85–4.65 mm, range SL 14.3–19.2 cm). When logarithmic transformations of equations for H. artus and Meynier et al (Citation2009) were compared, the regression coefficients of logarithmic transformations for these two equations were not significantly different (t=1.260, t 0.05(2),53=2.006, P>0.05) but the respective difference in elevations was statistically significant (t=3.368, t 0.01(2),53=2.670, P<0.01). This indicated that the opalfish analysed by Meynier et al. (Citation2009) and H. artus exhibited similar exponential rates of increase in fish length with increase in OL but fish lengths from the former were shorter than H. artus for otoliths of the same length.
Equations to estimate FL from OL were H. artus FL=2.41OL1.55 (n=47, r 2=0.94, P<0.001) and H. morelandi FL=2.36OL1.84 (n=37, r 2=0.79, P<0.001), where midpoints between these two equations produced an estimate of FL=2.36OL1.71 for application to OL≤2.99 mm, the maximum recorded OL for H. morelandi.
Pairing uneroded otoliths found in prey remains
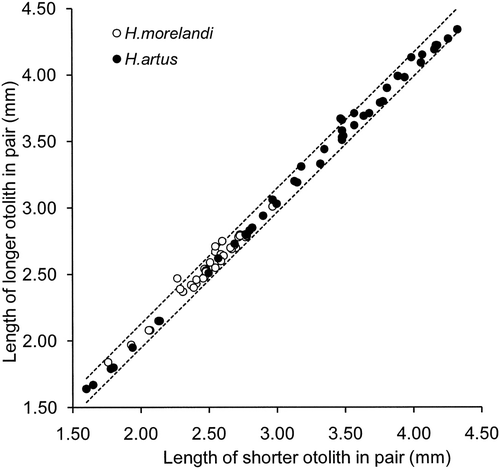
The relationship between lengths of the longer (y) and shorter (x) otoliths within a pair of otoliths for the combination of H. artus and H. morelandi in our reference collection indicated that they differed by an average 2% (: y=1.018x, n=81, r 2=1.00, P<0.001). The respective +95% prediction interval indicated that the likely range in difference in length for a pair of otoliths was 6% at 2.0 mm, 5% at 3.0 mm and 4% at 4.0 mm (). These values corresponded to a single measure of 0.2 mm applicable throughout the range in length of otoliths and so uneroded left and right opalfish otoliths found in prey remains were designated a pair if their lengths differed by <0.20 mm.
Analysis of uneroded opalfish otoliths from NZ sea lion scats
Opalfishes were the most numerically abundant teleost recorded in scats from NZ sea lions at the Auckland Islands, 31 July–14 August 2010. Their otoliths were found in 55 (51%) of 108 scat samples that contained prey remains and they accounted for 550 (34%) of 1631 prey items recorded. Uneroded otoliths accounted for 421 (77%) of these 550 opalfish, where 227 (54% of 421) were represented by pairs of otoliths, 83 (20%) by unpaired left otoliths and 111 (26%) by unpaired right otoliths. The mean length of these 421 measured pairs and single otoliths was 2.89 mm (SD 0.47, range 1.78–4.42 mm), with 132 (31% of 421) ≥3.00 mm that were considered to have originated from H. artus because they were too large to be H. morelandi (). The longest otolith, OL 4.42 mm, matched (2% longer) the maximum 4.34 mm recorded from our reference collection of H. artus otoliths.
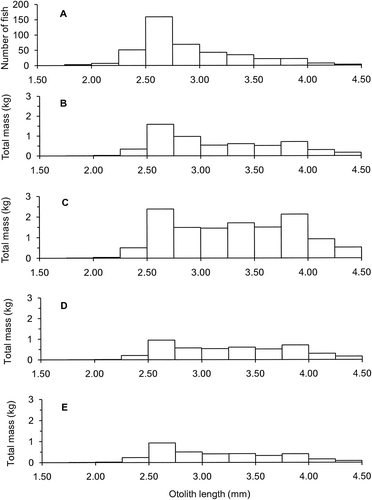
To illustrate the differences in quantifying fish length and FM from equations derived from different sources, four sets of regression equations were compared for the 421 opalfish represented by measured uneroded otoliths from the NZ sea lion prey remains in scats (, ). Values for mean mass and for total mass varied threefold among the four sources. The designated best estimates were calculated as the midpoints between equations for H. artus and H. morelandi for OL<3.00 mm and from equations for H. artus for larger otoliths (Figs. and ). For OL entered as 2.99 mm, the genus-specific equations generated FM=18.0 g, 35% less than the 27.7 g generated by the equation for H. morelandi and 73% more than the 10.4 g generated by the equation for H. artus. Application of equations from H. morelandi generated untenable results: the mean fish length SL 15.0 cm matched the maximum length recorded for this species, and the mean FM 30 g exceeded the maximum 22 g from specimens in our reference collection (). Mean fish lengths generated from equations for H. artus (SL 11.3 cm) and from equations in Meynier et al. (Citation2009) (SL 12.8 cm) were each within 10% of the best estimate (SL 12.2 cm). Mean FMs generated from H. artus (11.4 g) and from Meynier et al. (Citation2009) (8.0 g) were respectively 83% and 58% of the best estimate (13.7 g).
Table 1 Four sets of estimates for length and mass for the 421 opalfish represented by uneroded otoliths from New Zealand sea lion scats collected at the Auckland Islands, July–August 2010
Discussion
Otoliths of the two species of opalfishes that frequent the continental shelf around the Auckland Islands, H. artus and H. morelandi, could not be reliably differentiated visually or by relationships between linear measures and mass. Application of either set of species-specific equations to estimate fish size generated unrealistic results when applied to all opalfish otoliths found in NZ sea lion prey remains. In particular, application of equations for H. morelandi to the largest opalfish otoliths created fish 50% longer (32 cm cf. 21 cm) and 240% heavier (180 g cf. 50 g) than the largest opalfish in our reference collection. Otoliths from related species can by differentiated by chemical signatures or by growth analyses (Gauldie et al. Citation1991; Fablet et al. Citation2009; Kemp et al. Citation2011); however, these analyses are complex compared with identification by eye and therefore unlikely to be applied to otoliths extracted from prey remains of NZ sea lions.
OR (undigested OM divided by OL) exhibits an intraspecific increase with fish length (Harvey Citation1989; Tollit et al. Citation1997) and varied from 0.03 to 1.1 mg/mm among the 31 species investigated (Harvey Citation1989; Gales & Cheal Citation1992; Fea & Harcourt Citation1997; Tollit et al. Citation1997, Citation2007; Casper et al. Citation2006). The detection rates of otoliths recovered from scats of captive seals during feeding experiments typically increased with OR both among and within species, with an 80–90% recovery rate for the most robust otoliths (e.g. Tollit et al. Citation2007; Sweeney & Harvey Citation2011); although two studies found low recovery rates (<10%) regardless of robustness (Gales & Cheal Citation1992; Caspar et al. Citation2006). OR for opalfish increased with fish length and achieved higher values than those published for other species, with H. morelandi up to 1.4–1.7 mg/mm and H. artus up to 4–5 mg/mm. Feeding experiments have not been conducted with NZ sea lions and so the recovery rate of otoliths remains unknown. However, the recovery rate of opalfish otoliths was likely to be high because the majority had lengths that appeared unaffected by digestive erosion. These uneroded otoliths accounted for 77% of the 550 opalfish recorded from NZ sea lion scats at the Auckland Islands in 2010; a high proportion attributable to high OR.
Opalfish identified from otoliths accounted for 34% of prey items recorded from NZ sea lion scats at the Auckland Islands in 2010, similar to the 37% (Childerhouse et al. Citation2001) and 36% (Meynier et al. Citation2009) recorded previously. This numerical importance highlighted a necessity for accurate estimates of the size of opalfish represented in prey remains. In this study, two methods were developed to achieve this: first, all opalfish otoliths longer than 3.0 mm were assigned to H. artus because they were longer than the longest H. morelandi otoliths recorded from fresh specimens. Second, smaller otoliths could have been from either species and so genus-specific equations were created from the midpoints between species-specific equations. The application of genus-specific equations to the smaller otoliths generated large potential errors in estimates of FM when compared to the species-specific equations: up to 35% underestimate for H. morelandi and 73% overestimate for H. artus. Although they were the most numerous prey, the small size of opalfishes meant that the potentially large imprecision in estimates for mass fortuitously had little impact on the accuracy of estimates of the contribution of opalfishes towards NZ sea lion diet by mass. In the only assessments of diet by mass for NZ sea lions at the Auckland Islands, the contribution of opalfishes was only 2% from prey remains in stomach contents (Meynier et al. Citation2009) and <1% from quantitative fatty acid analysis (Meynier et al. Citation2010) from sea lions incidentally killed in trawl fisheries.
We recommend that the best estimate equations derived from a combination of the otolith size to fish size relationships from H. morelandi and H. artus supersede previous equations by Meynier et al. (Citation2009) to estimate length and mass of opalfish taken by sea lions at the Auckland Islands. The opalfish otoliths from Meynier et al. (Citation2009) were too large for H. morelandi but matched those in the reference collection from 47 fresh H. artus (SL 4.9–20.8 cm). However, fish lengths were shorter than for H. artus with the same OL, and FMs were lighter than for H. artus with the same fish length. These differences could be attributed to shrinkage and dehydration of frozen fish (‘freezer burn’) that can be avoided by freezing small fish in water rather than freezing them loose.
The inability to differentiate reliably between the two species of opalfishes by otolith shape precluded definitive estimates of fish length from otoliths. For example, Meynier et al. (Citation2009) concluded that juvenile NZ sea lions at the Auckland Islands ate smaller opalfish than did adults. However, fish lengths were deduced from OLs and so the difference may have reflected a difference in species composition rather than a difference in fish length. This example further emphasised anomalies that can arise when species-specific otolith equations are applied to congeneric species.
Acknowledgements
For the Auckland Islands trip in 2010, our logistics were funded by the Department of Marine Science, University of Otago and by Deepwater Group Ltd, with thanks to Richard Wells; with thanks to Polaris II crew (Bill Dickson, Phil Heseltine, Steve Little) for a safe trip; Will Rayment for organising the trip; Trudi Webster for assistance in scat collection; and the Department of Conservation, Invercargill (Pete McClelland, Doug Veint, Gilly Adam) for assistance with permitting and quarantine. We are grateful to Brett Gartrell, Massey University, for his helpful suggestions for improving the manuscript. CL thanks Ian Loveridge, Sanford Ltd, for permission to collect fish otoliths aboard trawlers, and the crews of the trawlers Dong Won 522 and Melilla for their hospitality.
References
- Bowen WD 2000. Reconstruction of pinniped diets: accounting for complete digestion of otoliths and cephalopod beaks. Canadian Journal of Fisheries and Aquatic Sciences 57: 898–905. 10.1139/f00-032
- Breen PA, Gilbert DJ, Starr PJ 2012. Comment on sea lion population viability analysis. Polar Biology 35: 1617–1618. 10.1007/s00300-012-1218-z
- Caspar RM, Gales NJ, Hindell MA, Robinson SM 2006. Diet estimation based on an integrated mixed prey feeding experiment using Arctocephalus seals. Journal of Experimental Marine Biology and Ecology 328: 228–239. 10.1016/j.jembe.2005.07.009
- Childerhouse S, Dix B, Gales N 2001. Diet of New Zealand sea lions (Phocarctos hookeri) at the Auckland Islands. Wildlife Research 28: 291–298. 10.1071/WR00063
- Chilvers BL 2008. New Zealand sea lion Phocarctos hookeri and squid trawl fisheries: bycatch problems and management options. Endangered Species Research 5: 193–204. 10.3354/esr00086
- Chilvers BL, Amey JM, Huckstadt LA, Costa DP 2011. Investigating foraging utilization distribution of female New Zealand sea lions, Auckland Islands. Polar Biology 34: 565–574. 10.1007/s00300-010-0915-8
- Chilvers BL 2012a. Population viability analysis of New Zealand sea lions, Auckland Islands, New Zealand's sub-Antarctics: assessing relative impacts and uncertainty. Polar Biology 35: 1607–1615. 10.1007/s00300-011-1143-6
- Chilvers BL 2012b. Using life-history traits of New Zealand sea lions, Auckland Islands to clarify potential causes of decline. Journal of Zoology (London) 287: 240–249. 10.1111/j.1469-7998.2012.00910.x
- Chilvers BL 2012c. Mortality rates clarified: reply to Breen et al. (2012). Polar Biology 35: 1619–1620. 10.1007/s00300-012-1219-y
- Fablet R, Chessel A, Carbini S, Benzinou A, de Pontual H 2009. Reconstructing individual shape histories of fish otoliths: A new image-based tool for otolith growth analysis and modeling. Fisheries Research 96: 148–159. 10.1016/j.fishres.2008.10.011
- Fea N, Harcourt R 1997. Assessing the use of faecal and regurgitate analysis as a means of determining the diet of New Zealand Fur Seals. In: Hindell MA, Kemper CM eds. Marine mammal research in the southern hemisphere volume 1: status, ecology and medicine. Chipping Norton, Surrey Beatty & Sons. Pp. 143–150.
- Francis M 1996. Coastal fishes of New Zealand. 2nd edition. Auckland, Reed Books.
- Gales NJ, Cheal AJ 1992. Estimating diet composition of the Australian sea-lion (Neophoca cinerea) from scat analysis: an unreliable technique. Wildlife Research 19: 447–456. 10.1071/WR9920447
- Gauldie RW, Coote G, Mulligan KP, West IF, Merrett NR 1991. Otoliths of deep water fishes: structure, chemistry and chemically-coded life histories. Comparative Biochemistry and Physiology 100A: 1–31. 10.1016/0300-9629(91)90179-G
- Harvey JT 1989. Assessment of errors associated with harbour seal (Phoca vitulina) faecal sampling. Journal of Zoology (London) 219: 101–111. 10.1111/j.1469-7998.1989.tb02569.x
- Jobling M, Breiby A 1986. The use and abuse of fish otoliths in studies of feeding habits of marine piscivores. Sarsia 71: 265–274.
- Kemp J, Swearer SE, Jenkins GP, Robertson S 2011. Otolith chemistry is more accurate than otolith shape in identifying cod species (genus Pseudophycis) in the diet of Australian fur seals (Arctocephalus pusillus doriferus). Canadian Journal of Fisheries and Aquatic Sciences 68: 1732–1743. 10.1139/f2011-088
- Leung ES, Chilvers BL, Nakagawa S, Moore AB, Robertson BC 2012. Sexual segregation in juvenile New Zealand sea lion foraging ranges: implications for intraspecific competition, population dynamics and conservation. PLoS ONE 7: 1–9.
- Meynier L, Mackenzie DDS, Duignan PJ, Chilvers BL, Morel PCH 2009. Variability in the diet of New Zealand sea lion (Phocarctos hookeri) at the Auckland Islands, New Zealand. Marine Mammal Science 25: 302–326. 10.1111/j.1748-7692.2008.00252.x
- Meynier L, Morel PCH, Chilvers BL, Mackenzie DDS, Duignan PJ 2010. Quantitative fatty acid signature analysis on New Zealand sea lions: model sensitivity and diet estimates. Journal of Mammalogy 91: 1484–1495. 10.1644/09-MAMM-A-299.1
- Nelson JS 1979. Revision of the fishes of the New Zealand genus Hemerocoetes (Perciformes: Percophididae), with descriptions of two new species. New Zealand Journal of Zoology 6: 587–599. 10.1080/03014223.1979.10428401
- Orr AJ, Harvey JT 2001. Quantifying errors associated with using fecal samples to determine the diet of the California sea lion (Zalophus californianus). Canadian Journal of Zoology 79: 1080–1087.
- Paulin C, Stewart A, Roberts C, McMillan P 2001. New Zealand fish: a complete guide. Wellington, Te Papa Press.
- Pierce GJ, Boyle PR 1991. A review of methods for the diet analysis in piscivorous marine mammals. Oceanography and Marine Biology 29: 409–486.
- Riet-Sapriza, FG, Duignan PJ, Chilvers BL, Wilkinson IS, Lopez-Villalobos N, Mackenzie DDS, MacGibbon A, Costa DP, Gales N 2012. Interannual and individual variation in milk composition of New Zealand sea lions (Phocarctos hookeri). Journal of Mammalogy 93: 1006–1016. 10.1644/11-MAMM-A-220.2
- Robertson B, Chilvers BL 2011. The population decline of the New Zealand sea lion Phocarctos hookeri: a review of possible causes. Mammal Review 41: 253–275. 10.1111/j.1365-2907.2011.00186.x
- Schwarzhans W 1984. Fish otoliths from the New Zealand Tertiary. New Zealand Geological Survey Report 113:1–269.
- Smale MJ, Watson G, Hecht T 1995. Otolith atlas of southern African marine fishes. Ichthyological Monographs of the J.L.B. Smith Institute of Ichthyology 1: 1–253.
- Sweeney JM, Harvey JT 2011. Diet estimation in California sea lions, Zalophus californianus. Marine Mammal Science 27: E279–E301. 10.1111/j.1748-7692.2010.00459.x
- Tollit DJ, Stewart MJ, Thompson PM, Pierce GJ, Santos MB, Hughes S 1997. Species and size differences in the digestion of otoliths and beaks: implications for estimates of pinniped diet composition. Canadian Journal of Fisheries and Aquatic Sciences 54: 105–119. 10.1139/f96-264
- Tollit DJ, Heaslip SG, Barrick RL, Trites AW 2007. Impact of diet-index selection and the digestion of prey hard remains on determining the diet of the Steller sea lion (Eumetopias jubatus). Canadian Journal of Zoology 85: 1–15. 10.1139/z06-174
- Tollit D, Heaslip S, Deagle B, Iverson S, Joy R, Rosen D, Trites A 2006. Estimating diet composition in sea lions: which technique to use? In: Trites AW, Atkinson SK, DeMaster DP, Fritz LW, Gelatt TS, Rea LD, Wynne KM eds. Sea lions of the world. Fairbanks, Alaska Sea Grant College Program, University of Alaska Fairbanks. Pp. 293–307.
- Zar JH 1999. Biostatistical analysis. 4th edition. Englewood Cliffs, NJ, Prentice Hall.