Abstract
Banded kōkopu body patterns are visible in 6-month-old individuals, are unique and remain unchanged over an extended time period. This allows for reliable individual identification and was confirmed by known age captive individuals and from individuals re-trapped in the wild. Body pattern types were specific to particular habitats, with lighter more spotted individuals found in forested habitats compared with darker more striped individuals found in backwater habitats. Breeding condition, which was determined by substantial weight loss through the expression of genital products, was confirmed by fin colour change, visibility of gonads through the body wall, the presence of a pectoral patch and post spawning change of body shape.
Introduction
The banded kōkopu (Galaxias fasciatus Gray, 1842) is an endemic New Zealand species considered to be affected by habitat loss/alteration, barriers to upstream migration and invasive fish (McDowall Citation1990; Townsend & Crowl Citation1991; Crowl et al. Citation1992). Detailed knowledge of the biology of the species is lacking; spawning biology is poorly understood and based on a few observations from a variety of geographical localities (Mitchell & Penlington Citation1982; Mitchell Citation1991; Charteris et al. Citation2003). Although the recent ranking of this species is Not Threatened (Allibone et al. Citation2010), few data are available on the ecological health of banded kōkopu populations over an extended period and none from an urban stream.
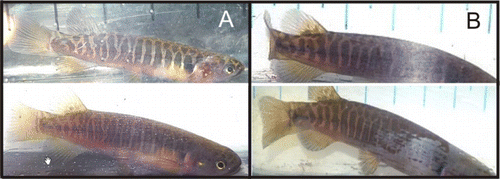
The general body colour and pattern of banded kōkopu has been described by McDowall (Citation1970). The body is a dark purplish grey colour anteriorly, banded mostly laterally with a series of narrow pale vertical bands. Lateroventrally the trunk colouration alters quite abruptly to a dull pale brown; the extent of this colouration is variable between individuals. There is a dark blue/black blotch above and behind the pectoral fin base, which has varying prominence between individuals. The fins are uniform pale orange/yellow colour and there is no obvious other contrasting colour. The origin of the anal fin is in line with that of the dorsal fin; this is one way of telling this species apart from a young giant kōkopu (Galaxias argenteus Gmelin; McDowall Citation1970).
Individual recognition is an important tool for monitoring the health of populations. The technique of implanting identification tags into a fish's body has some limitations (Charteris et al. 2003; West et al. Citation2005). It is invasive and potentially influences survival, particularly of small, young individuals (West et al. Citation2005), which are likely to show the most growth/change over an extended period. Non-intrusive waterproof markings can be used (RM Allibone, pers. comm. 2012), but these wear off with time and rely on regular recapture of marked individuals before this happens.
Photographs of patterns unique to individuals have been used to accurately and reliably identify individuals of a variety of taxa (Emery & Wydaski Citation1987; Würsig & Jefferson Citation1990 and references therein). With improved digital technology, these images can be obtained quickly, with no processing, are easily stored, adjusted and retrieved on digital media. Photographs also allow a retrospective examination of individuals as new information becomes available during a long-term study; something that one-off tagging of fish, with no other visible record, does not. No previous studies of banded kōkopu have investigated whether photographs can be used to identify individuals using their unique banding patterns.
Auckland is the largest city in New Zealand and the study stream is in a heavily urbanised area. With continued growth and expansion of cities and industry, an understanding of the biology of native fish in urban catchments is vital to ensuring the survival of future populations in these areas. This paper presents a technique used to identify individual fish from an urban population of banded kōkopu in the unnamed stream system that runs through Le Roy's Bush on Auckland's North Shore from August 2009 to June 2012.
Methods
Study area
The study was carried out in the unnamed stream that flows from Le Roy's Bush (36°48′56.81″S, 174°44′21.87″E) into the sea (hereafter referred to as Le Roy's Stream), which is about 1.2 km long (). There is a large waterfall (about 15 m high) about a third of the way from the source along the main channel where the stream runs through native bush.
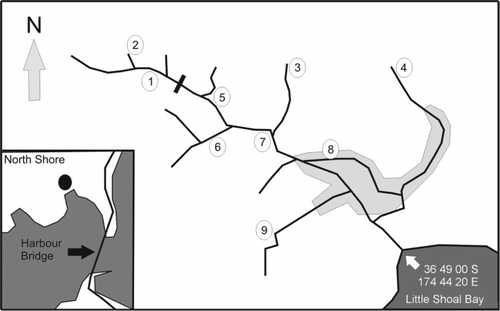
This section of the stream is characterised by many pools of different sizes and depths, a reasonable slope and flow, no macrophytes, little sediment (except during floods), small rapids and waterfalls. It has extensive overhanging vegetation and flood debris trapped at various points along its length and is a forested stream sensu lato Baker and Smith (Citation2007) (sites1, 2, 3, 4; ); a section of riffle habitat sensu lato Baker and Smith (Citation2007) (sites 5, 6; ) occurs below the main waterfall. Further downstream is a slow flowing section with a negligible slope and is a backwater sensu lato Baker and Smith (Citation2007) (sites 7, 8, 9; ) with a large sediment load, a deep muddy bottom and shade, restricted to areas where suitable trees exist. Closer to the sea there is no shade, depth is very shallow during low water flows and the stream system is characterised by a raupo Typha angustifolia swamp and high densities of the invasive mosquitofish (Gambusia affinis Baird and Girard, 1853).
Sampling protocol
The stream was sampled weekly from August 2009 to June 2012 using between six and nine bottom set collapsible minnow traps (with a 100 mm diameter entrance and enclosed in fine cloth gauze, baited with Sanitarium MarmiteTM).
A clear polycarbonate box (1.8 L click-clack boxTM) with a close fitting waterproof lid, filled with water from the pool where the trap was set, was used to examine the captured fish. The top and bottom was painted black and a graduated scale, marked in 1 cm lengths was drawn on one side, which was painted white. The box, lid and water were weighed on a battery-powered portable digital kitchen scale, accurate to 0.1 g, prior to the fish being added. The scale was then tared, the fish quickly removed from the trap, placed in the click-clack box and put back on the scale to determine its weight, to the nearest gram. Care was taken to ensure that no water was displaced from the click-clack box when the fish was inserted, as this would have affected the resulting weight.
The weight was recorded and both sides of the fish were photographed using a digital Nikon TM SLR camera fitted with a 18–55 mm lens and polarising filter. For larger fish, this was sometimes not possible as they were too big to turn around in the box, so every effort was made to ensure that a good quality photograph of one side was obtained. The fish was then released back to its capture location. The process for each individual took less than 5 min, during which time it spent less than 10 s out of water.
The photographs were sharpened using Picture Project TM software. Analyses of the photographs allowed the total length of the fish to be determined, to the nearest cm along the scale, and the pattern of spots and stripes along the sides of the fish to be recorded. The locality, date, catchment and weight information for each fish was also recorded, cross-referenced with the photograph and stored for future reference.
Every fish had a unique pattern of stripes and/or spots which were used to identify individuals that were re-trapped. Matching the stripe/spot pattern with the photographic record was relatively easy after capture and the technique allowed for a quick process time with minimal stress and intrusion for the individuals concerned. If there was some doubt about the identity of individual fish, they were classified as new individuals on the database.
Eight juvenile banded kōkopu were removed from the stream in October 2009 and grown out in an aquarium to determine the reliability of this identification technique. These fish were photographed: initially; when their body patterns started to appear; once the patterns were discernable; and then annually so that any changes to their body patterns could be documented. These individuals provided a reliable reference for detecting changes in body condition, development of gonads, subtle changes in the fin colour and the development of breeding condition. Changes in the captive individuals suggested that similar changes might be underway for the field population, and the database of photographs of the fish in the stream was then searched to confirm this.
Breeding
Dead fish retrieved from the stream between March and May (n=6) were checked against the photographic database and dissected to determine their reproductive status and sex. Localities known to contain large (>140 mm total length, TL) fish were regularly sampled in December, February to July 2010; April to July 2011 and April to May 2012. Particular attention was paid to the ventral surface of the photographed fish to determine the presence/absence of eggs or developing testes. Individuals were not squeezed to express reproductive products at any time. Photographs taken of these fish were compared over time to generate visual information that would indicate breeding condition and if and when they had spawned. Fish that had indications of vent abrasion/damage (Hopkins Citation1979) and/or showed an indented ‘step’ between the paired pelvic and anal fins when examined in the field were assumed to have spawned. This was confirmed, where possible, by a comparison of weight of the same individual between capture events; large weight loss generated by a loss of genital products (Hopkins Citation1979) suggested that these individuals had spawned.
Results
Body pattern of captive fish
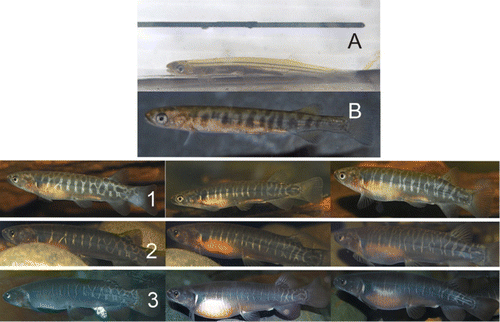
The body patterns of the aquarium fish were just discernible at the time of capture and they were almost completely transparent. The patterns started to become obvious after a month in captivity (about 4 months old, assuming they were born in July) and after 3 months in captivity (about 6 months old) they were sufficiently distinct to enable individuals to be identified with confidence (). During this time they grew substantially and once the body pattern had developed it remained unchanged, irrespective of body size, and was a reliable indicator of individual identity 3 years later (). Some bands became narrower and indistinct (), and some disappeared altogether, but the area between the dorsal and caudal fin retained the pattern consistently and could be used as a reliable method for the identification of individuals over a long time period (). Photographs taken of wild-caught individuals confirmed the stability of the body pattern over time (). Some large individuals caught in the stream showed extensive body patterning () and no fading between capture events.
Body patterns of stream fish
Banded kōkopu showed a wide variety of patterns and background colours; those adults living in the backwater habitats had a darker dorsal colouration when compared with those living in the forested habitats (). There was a trend for individuals from the same habitats to have similar body stripe patterns. Fish living in forested habitats had more spots in their body patterns (81%, n=106, χ2=41.09, d.f.=1, P<0.0001) than individuals from the backwater habitat (19%, n=47,χ2=18.75, d.f.=1, P<0.0001; ). This indicates that the body patterns on the fish in the different habitats were not distributed uniformly. It was possible to predict, with some accuracy, the habitat from which an unknown individual fish came by looking at its body pattern. Dark individuals dorsally with no spots in their body patterns came from the backwater, whereas lighter fish dorsally with spots in their body pattern came from forested habitats. This was an unexpected result.

Age and breeding condition
Individuals showing colour in the form of spots/smudges on the dorsal fin were a minimum of 6 months old. After this age, all individuals had spots/colour present at the base of the dorsal fin ().
Baseline post breeding condition was determined in August 2009 when the study began and individuals largely conformed to the description in McDowall (Citation1970). Of importance is the dark blue/black blotch above and behind the pectoral fin base. Anterior to this patch is a pale contrasting mark (, D). The fins are uniform pale orange/yellow colour and there are no obvious other contrasting colours on the fish at all.
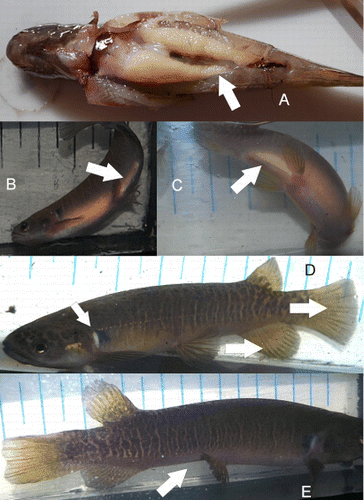
In February, the initiation of breeding condition in individuals became visible and this was accompanied by a steady increase in body weight (K Hustler unpublished data). The development of a contrasting pale area between pelvic and anal fin corresponded with the development of gonads and this was confirmed by the dissection of dead individuals retrieved from the stream (A). At the same time, there was a change in the colour of the posterior edges of the pelvic, anal and caudal fins, which showed a distinct darker edge (D). The extent of this varied between individuals but was easily visible in all breeding fish.
In April and May, all of the above characters were very well developed. In some individuals the pectoral patch was very prominent (B, 5C), and individuals had a ‘fat’ feel when briefly handled between trap and click-clack box. Distension of the vent, disappearance/reduction of the pale patch between pelvic and anal fins, reduction of contrast in fin colour, and a ‘hollow’ look, confirmed that spawning had occurred. The loss of weight of breeding individuals during this time coincided with these changes. The ‘hollow’ look identified in individuals in the stream as having spawned (E) was confirmed after the captive females took on this appearance after shedding large numbers of eggs in mid September.
By June, the vent of many individuals was distended and there was a significant weight loss, presumably attributable to loss of genital products at this time (Hopkins Citation1979; K Hustler unpublished data). There were still traces of enlarged gonads visible through the body wall of some individuals. From July onwards there was a reduction of gonads visible through the body wall and a return to a uniform fin colour and a less ‘fat feel’ of the fish.
Time of breeding
Individual fish which were re-trapped and which had lost 20% or more of their previously recorded body weight were assumed to have spawned in the vicinity. The loss of weight attributable to the loss of genital products (Hopkins Citation1979) began at the end of April and extended through May and into June (K Hustler unpublished data).
After spawning the abdomen appeared to be hollow and not in line with the ventral surface of the fish (E); this was a visual indication of spawning. This was not confirmed until the captive fish shed their eggs in September 2011, but on retrospective examination, was visible in the photographs taken of adult fish in the stream in May. In 2010, spawning took place between 7 and 29 May. Fish photographed on 7 May 2010 showed large numbers of eggs visible through the body wall. All large fish photographed on 29 May 2010 showed the hollow in their abdomen and a few showed vent abrasion. In 2011, spawning occurred a bit later in the year, with some fish showing the hollow in the last week in May, while all fish had the hollow in July. There was no sampling in June, due to the heavy rains that had occurred, and this is probably when most of the spawning took place. Spawning in stream 3 () probably occurred on 9 May 2012 when a large rainstorm resulted in higher than usual water levels. An adult female caught after this showed a 32% decrease in body weight compared to her weight at the end of April.
Discussion
Individual recognition
This is the first demonstration that body patterns can be used to identify individual banded kōkopu. Individual recognition through body markings is advantageous over conventional implanted identification tags as it is non-intrusive, is not dependent on fish size and is likely to be permanent for the life of the fish. The number of individuals captured multiple times over the course of this study indicates that the sampling protocol had no detrimental effect on fish survival.
The body patterns of banded kōkopu are individually unique. The development of body patterns within an estimated 6 months of hatching is slightly less than that reported by Mitchell (Citation1991) who observed pattern development of captive reared banded kōkopu at 9 months of age. There are minor changes to the pattern with time (lines change in diameter, spots change in shape), but the overall pattern remains consistent and allows individuals to be easily identified over long time periods. Small individuals retained their body pattern in spite of growing significantly between capture events. For individuals (>15 cm TL) that had not grown between capture events, the body pattern remained unchanged for 500 days and more. Body patterns have been used to confirm individual adult giant kōkopu initially tagged for identification (David & Stoffels Citation2003; Hansen & Closs Citation2005, Citation2009). The change in body pattern from juvenile to adult in giant kōkopu is so significant that without an alternative permanent mark younger individuals would not be recognisable over a long-term study (K Hustler, pers. obs. 2010).
The use of the click-clack box allowed a comprehensive examination of the fish to be undertaken, without removing it from the water for an extended period or administering anaesthetic. The measurements are not 100% accurate due to issues with parallax and the position of the fish in the container, but they are accurate enough to determine change in size over a long time period. It was possible to examine all the photographs retrospectively, and particularly in the light of new information as a result of changes observed in the captive individuals. This was particularly enlightening with the development of gonads, the two tone colour of the fins, the pectoral patch, the post spawning change in the shape of the abdomen and the development of markings on the dorsal fins in individuals more than a year old. The photographic database was invaluable in determining growth data, particularly from individuals that were small when initially captured.
Breeding
The change in fin colour and presence of the pectoral patch as an indication of breeding condition have not apparently been published before. Visibility of enlarged gonads through the body wall, and the change in abdomen shape, have been observed by many researchers working on galaxiids as an indication of breeding (RM Allibone, pers. comm. 2012). All of these morphological changes may have been exaggerated by the use of a polarising filter and flash photography but, with practice, they can all be seen in the field, in the click-clack box, without photographs. Darkening of the fins is present in shortjaw kōkopu (http://www.youtube.com/watch?v=01PWIfqtJGU), so might be more widespread in galaxiids than previously reported, and is a useful and easily seen indicator of banded kōkopu individuals in breeding condition.
The size of breeding fish recorded by Mitchell and Penlington (Citation1982) was between 80 (male) and 110 mm TL (female). Hopkins (Citation1979) reports breeding at 4 years old but does not elaborate on body size. The smallest fish found to be in breeding condition in Le Roy's Stream were between 95 (male) and 110 mm TL (female), conforming to the data in Mitchell and Penlington (Citation1982). Gonads were visible through the body wall in individuals ranging from 100 mm to 220 mm TL in Le Roy's Stream between April and June, showing that the breeding population is made up of individuals of a variety of sizes and all probably 2 or more years old.
Effect of habitat
Individuals from the backwater habitats were generally darker above and had stripe patterns that were consistently different from those individuals in the forested habitats. The reasons for this are unknown, but are perhaps a reflection of different selection pressures operating in each habitat. The factors governing pattern development in banded kōkopu are unknown, and the possibility exists that the impact of local environmental conditions play a part in the type of pattern developed by an individual fish. If so, this would need to occur soon after the fish has entered a particular habitat, as by 6 months old the patterns are already established. When the captive fish were removed from a forested stream they were golden coloured juveniles with no markings; but they developed markings typical of forest individuals reflecting the pattern type typical of this habitat.
Mitchell (Citation1991) recorded spawning of banded kōkopu in tidal habitats and this would be a possibility for the backwater population in Le Roy's Stream. The waterfalls on the forested part of the stream preclude the regular movement of adult fish to the tidal habitats for breeding, and they are sedentary and breed in the pools above the waterfalls (K Hustler unpublished data). The significantly different number of fish with/without spots in the forested/backwater habitats suggests that different selection pressures are operating which is reflected by the body patterns of the individuals that survive to adulthood in each habitat. The other possibility is that the dispersal of juvenile fish in this stream is not random. This is an interesting avenue for further investigation and has implications for the conservation of this species.
Acknowledgements
Megan Beard (WAICARE) and Monique Zwann (ENVIROSCHOOLS) of the then North Shore City Council helped with permissions and permits. Keith Salmon sourced funding from the ARC Coastal Enhancement Fund grant to the Le Roy's Bush and Little Shoal Bay Management Committee. Peter Crossley assisted with discussion, fieldwork and materials. Craig Hustler, Abby Hustler, Andrew Bailey, Michael Duxfield, Scott Neville, Paloma Serville, Hussain Sabori, Emma Henare, John Blomfield and Jordan Grey assisted with fish capture. Georgina Hewlett and Ian McLaren provided logistical support. Daniel Basset and Richard Allibone critiqued an earlier draft of the manuscript. Two anonymous reviewers made helpful suggestions, which improved the manuscript.
References
- Allibone RM, David BO, Hitchmough R, Jellyman DJ, Ling N, Ravenscroft P, et al. 2010. Conservation status of New Zealand freshwater fish, 2009. New Zealand Journal of Marine and Freshwater Research 44: 271–287. 10.1080/00288330.2010.514346
- Baker CF, Smith JP 2007. Habitat use by banded kokopu (Galaxias fasciatus) and giant kokopu (G. argenteus) co-occurring in streams draining the Hakarimata Range, New Zealand. New Zealand Journal of Marine and Freshwater Research 41: 25–33. 10.1080/00288330709509893
- Charteris SC, Allibone RM, Death RG 2003. Spawning site selection, egg development, and larval drift of Galaxias postvectis and G. fasciatus in a New Zealand stream. New Zealand Journal of Marine and Freshwater Research 37: 493–505. 10.1080/00288330.2003.9517184
- Crowl TA, Townsend CR, McIntosh AR 1992. The impact of introduced brown and rainbow trout on native fish: the case of Australasia. Reviews in Fish Biology and Fisheries 2: 217–241. 10.1007/BF00045038
- David BO, Stoffels RJ 2003. Spatial organization and behavioural interaction of giant kokopu (Galaxias argenteus) in two stream pools differing in fish density. New Zealand Journal of Marine and Freshwater Research 37: 315–322. 10.1080/00288330.2003.9517169
- Emery L, Wydoski R 1987. Marking and tagging of aquatic animals: an indexed bibliography. Washington, DC, US Fish & Wildlife Service. 57 p.
- Hansen EA, Closs GP 2005. Diel activity and home range size in relation to food supply in a drift-feeding stream fish. Behavioral Ecology 16: 640–648. 10.1093/beheco/ari036
- Hansen EA, Closs GP 2009. Long-term growth and movement in relation to food supply and social status in a stream fish. Behavioral Ecology 20: 616–623. 10.1093/beheco/arp039
- Hopkins CL 1979. Reproduction in Galaxias fasciatus Gray (Salmoniformes: Galaxiidae). New Zealand Journal of Marine and Freshwater Research 13: 225–230. 10.1080/00288330.1979.9515797
- McDowall RM 1970. The galaxiid fishes of New Zealand. Bulletin of the Museum of Comparative Zoology, Harvard University 139: 341–432.
- McDowall RM 1990. New Zealand freshwater fishes: a guide and natural history. Auckland, Heinemann Reed. 553 p.
- Mitchell CP 1991. Deposition of Galaxias fasciatus eggs with Galaxias maculatus eggs at a tidal site. New Zealand Journal of Marine and Freshwater Research 25: 201–205. 10.1080/00288330.1991.9516471
- Mitchell CP, Penlington BP 1982. Spawning of Galaxias fasciatus Gray (Salmoniformes: Galaxiidae). New Zealand Journal of Marine and Freshwater Research 16: 131–133.
- Townsend CR, Crowl TA 1991. Fragmented population structure in a native New Zealand fish:an effect of introduced brown trout? Oikos 61: 347–354. 10.2307/3545242
- West D, Jowett IG, Richardson J 2005. Growth, diet, movement, and abundance of adult banded kokopu (Galaxias fasciatus) in five Coromandel, New Zealand streams. New Zealand Journal of Marine and Freshwater Research 39: 915–929. 10.1080/00288330.2005.9517362
- Würsig B, Jefferson TA 1990. Methods of photo-identification of small cetaceans. In: Hammond PS, Mizroch SA, Donovan GP eds. Individual recognition of cetaceans: use of photo-identification and other techniques to estimate population parameters. Report of the International Whaling Commission Special Issue 12. Pp. 43–52.