Abstract
After a physical disturbance, such as a predatory stimulus, several marine organisms withdraw into a refuge to ensure survival. Consequently, they cannot engage in other essential activities. Re-emergence reflects a trade-off between avoidance of disturbance and lost feeding opportunities. The New Zealand sea anemone (Anthopleura aureoradiata) avoids predators by withdrawing its tentacles into its upper body. In a field experiment, responses of 480 A. aureoradiata to a physical disturbance were studied at three locations to assess the influence of the following factors on hiding time: physical contact with a neighbour; density of nearby conspecifics; total density per pool; and body size. Withdrawal times were significantly shorter for anemones in contact with a conspecific, occurring in high-density patches or of larger size. These patterns are consistent with rapid re-emergence being stimulated by competition for food from immediate neighbours and/or a lower risk of predation when in groups (i.e. a dilution effect), with larger size also potentially reducing vulnerability to predation.
Introduction
In response to a disturbance, such as wave action, physical contact with a conspecific or vegetation or a predatory stimulus, an animal may exhibit a morphological, ecological or behavioural reaction (Morse Citation1982; Brodie et al. Citation1991). For example, the anti-predator strategies of a motile species consist of avoidance, flight and camoflage (Broom & Ruxton Citation2005; Derby Citation2007). As sessile species cannot move, they often hide (Martin & Lopèz Citation1999). Hiding is one of the most common strategies in response to a physical disturbance, such as predation or wave action, and often involves retreating into a refuge (Dill & Fraser Citation1997). Typically, the refuge consists of a physical structure in the environment offering protection to the organism, but the withdrawal of vulnerable structures, such as the head or appendages, into the organism's main body also provides refuge against predation. Although use of a refuge guarantees safety, the prey knows little of the predator's activity while inside its refuge, yet it must decide when to re-emerge (Sih Citation1997; Hugie Citation2003). This decision is optimised by considering decreasing predation risk with time, but also by considering the cost of the loss of time that could have been spent maximising physiological functions through feeding (Martin & Lopèz Citation1999). Re-emerging too soon is risky for the prey (Guerra-Bobo & Brough Citation2010), yet remaining withdrawn for too long compromises future fitness, with less time available for mate finding, thus increasing the net costs of anti-predator behaviour (Martin & Lopèz Citation1999). Anti-predator hiding responses are therefore determined by the fine balance between these costs and benefits (Mauck & Harkless Citation2001). For filter-feeding animals avoiding potential predation by withdrawing, such as intertidal sea anemones or barnacles, this balance is particularly important as it significantly reduces feeding opportunities (Dill & Gillett Citation1991; Mauck & Harkless Citation2001). Few studies have examined how prey decide when to re-emerge and resume activity after an unsuccessful predator attack (Jennions et al. Citation2003).
The present study aims to determine whether there are relationships between habitat variables and withdrawal responses in the New Zealand sea anemone (Anthopleura aureoradiata). These intertidal cnidarians are often very abundant in rockpools; they can reach 20 mm in size (diameter of oral disk), though are generally smaller. Their main predators include starfish, crustaceans, gastropods, fish and polychaete worms (Ottaway Citation1977), and they ‘hide’ by retracting their tentacles when contacted by these predators or any other large physical object.
Higher anemone density increases competition, especially when there is physical contact between neighbours, as several individuals can potentially trap the same floating food particle. Therefore, higher density may favour early re-emergence to allow more time for feeding (Sebens Citation1981; Wood Citation1999). Furthermore, high density should decrease predation risk through ‘safety in numbers’ (i.e. via a dilution effect; Mauck & Harkless Citation2001). This has been observed in mud-crabs, fiddler crabs, cichlid fish and barnacles (Mauck & Harkless Citation2001; Guerra-Bobo & Brough Citation2010). Body size may also influence predation risk since larger individuals are more likely to be targeted as potential prey (Krause & Ruxton Citation2002; Jennions et al. Citation2003). Furthermore, larger individuals may be better competitors and can afford to be withdrawn for longer (Jennions et al. Citation2003). Consequently, smaller individuals with lower feeding ability may be forced to re-emerge sooner to compensate for the competitive disadvantage (Jennions et al. Citation2003). Alternatively, larger individuals may need to re-emerge sooner due to higher energy requirements, which necessitate longer feeding times (Sebens Citation1981). Finally, local factors can also influence anti-predator behavioural decisions, such as predation pressure or food abundance, and thus we investigated multiple study sites to determine whether observed patterns differ geographically, and to extend the generality of the conclusions. Although the influence of these or related factors on anti-predator tactics of barnacles and crabs have been investigated (Dill & Gillett Citation1991; Mauck & Harkless Citation2001; Jennions et al. Citation2003; Guerra-Bobo & Brough Citation2010), little work has been done to see if cnidarians, with their much simpler nervous system, make the same sophisticated adjustments to their hiding time.
In this study, we tested the hypotheses that: 1. withdrawal time is shorter in individuals that are in physical contact with neighbours and/or individuals living at high densities on either a local (neighbour) scale or at the scale of the total rock pool; and 2. that withdrawal time will increase with increasing sea anemone size. Together, tests of these hypotheses will determine whether anemones can adjust their hiding time to reflect the trade-off between safety and feeding.
Materials and methods
Data were collected at three different sites in between Picnic Point and Mahaka Point (Site 1;−46° 34.02′S, 169° 28.73′E, Site 2: 46° 34.06′S, 169° 28.73′E, Site 3; 46° 34.07′S, 169° 28.72′E) in the Catlins region, east coast of the South Island, New Zealand. The sites were all rocky shores located between 0 and 1 m above low water mark, with large platforms dotted with permanent shallow pools; they were visited at low tide over four autumn days in March 2011. At each site, data were collected in a systematic order to ensure that each rock pool was surveyed only once.
Each rock pool's surface area was estimated from measurements (to the nearest centimetre) of its dimensions. The total number of A. aureoradiata present in each pool was also recorded. In any given pool, a focal sea anemone was selected using a random number generator to reduce bias. Disk diameter of the fully open polyp was measured using calipers. After the behavioural test (see below), a 60 mm radius plastic hoop was placed around the selected anemone, using the focal individual as a centre point. Anemones that fell within the 60 mm radius were counted and used to calculate neighbour density. A 60 mm radius was chosen based on preliminary observations indicating that this corresponded to the typical area in which anemones clustered. Finally, we recorded whether or not the focal anemone was in physical contact with one or more neighbouring sea anemones.
The focal sea anemone was then disturbed in a standardised way. This was done by gently brushing all of the anemone's tentacles in a circular motion using a wooden prodder, acting as a physical disturbance to provoke a hiding response. Hiding duration was measured with a stopwatch and taken as the time between initial retreat, marked by the retraction of tentacles and slight invagination of the body, and re-emergence (i.e. when the oral disk became visible from directly above, due to re-extension of the tentacles).
This process was repeated on more than one sea anemone per pool for large pools (i.e. greater than 1 m2), but with a maximum of 12 anemones per pool. If the next focal sea anemone selected by the random number generator was in a clump that had already been disturbed, it was ignored and another one was selected. Only anemones that were initially open were used, allowing us to time their hiding response to a simulated predator threat starting from an undisturbed state. Consequently, individuals that were already withdrawn were excluded from the behavioural tests. This entire process was then repeated in 24–45 pools per site.
A t-test assuming unequal variance was performed to examine the difference in withdrawal time for anemones in physical contact with a neighbour and those not in contact. Ordinary least squares regressions and correlation coefficients were used to describe relationships between withdrawal time and density of neighbouring conspecifics (number of anemones within a 60 mm radius of the focal individual), and between withdrawal time and anemone body size (disk diameter). Finally, a one-way ANOVA was used to test for variation in withdrawal time with site. This analysis was performed using the statistics program R v.2.8.2.
Results
Withdrawal times were recorded for 480 A. aureoradiata within 109 pools. Anemone densities ranged from 1.39 to 11,071 anemones per m2. Time spent withdrawn as an anti-predator response ranged from 3.4 to 212.5 s, with a mean of 33.1 s.
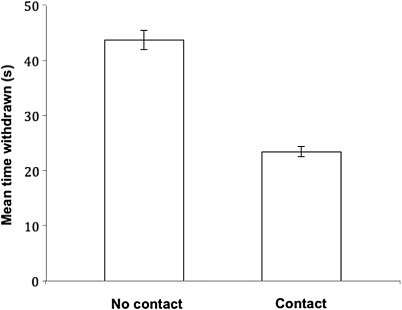
Overall, anemones in direct physical contact with at least one of their neighbours re-emerged faster after a physical disturbance than those without contact (t = 7.33, P < 0.001) (). The average withdrawal time for anemones not in contact with their neighbours was 43.7 s, nearly twice the mean time of 23.4 s for anemones in contact with neighbours.
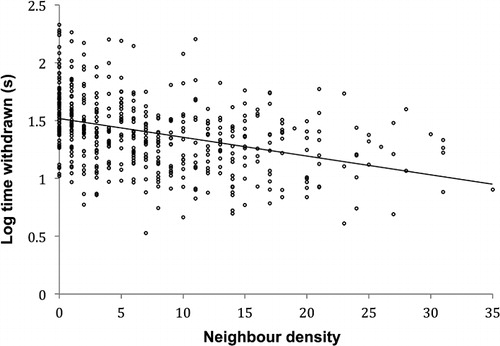
The number of individual anemones within a 60 mm radius of the focal anemone ranged from 0 to 41. There was a weak negative relationship between neighbour density and log withdrawal time (R2 = 0.15, F = 83.9, P < 0.001) ().
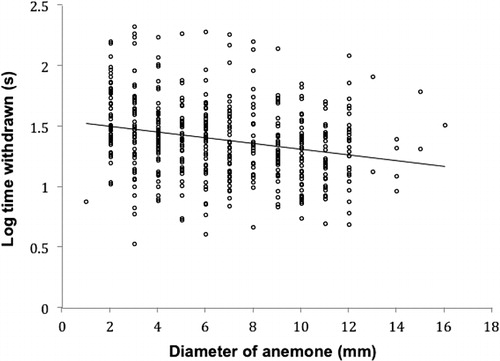
Anemone disk diameters ranged from 1 mm to 16 mm, and showed a very weak negative relationship with log withdrawal time (R2 = 0.06, F = 29.9, P < 0.001) ().
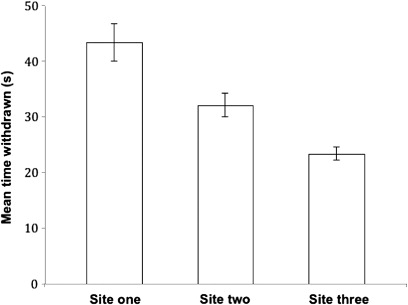
Finally, there were differences in withdrawal times between the three study sites (F = 479.6, P < 0.001). Wave exposure was not objectively quantified at each site; however, some differences were readily apparent at the time the study was conducted. Anemones from the site most exposed to waves (Site 3) re-opened faster than those from the other two sites (). In contrast, anemones from the least exposed site (Site 1) took almost twice as long to re-open following the stimulus.
Discussion
Feeding opportunities lost to competitors is one of the costs of prolonged withdrawal or self-protection (Mauck & Harkless Citation2001). In addition, neighbouring conspecifics can also diminish the risk of predation through a dilution effect (Mauck & Harkless Citation2001). Therefore, the immediate presence of conspecifics next to a focal A. aureoradiata should promote its rapid re-opening following a fright stimulus. Indeed, our results indicate that sea anemones in direct physical contact with at least one of their neighbours re-emerged almost twice as fast after a physical disturbance than those not in contact with their neighbours. Furthermore, anemones within denser patches of neighbours (within a 60 mm radius) re-emerged more rapidly after a physical disturbance than those within a less dense patch. This is consistent with our hypotheses that, for individuals either touching their neighbours or individuals with a high neighbour density, both increased competitive pressures and the dilution effect combine to favour shorter withdrawal times.
Contact with others or a high density of neighbours increases competition for suspended food particles (Sebens Citation1981; Wood Citation1999). Increased competition for resources decreases the net advantage of staying withdrawn, causing anemones to re-open faster and giving a shorter mean withdrawal time. Furthermore, an increase in neighbour density, especially for those with neighbour contact, should decrease predation risk resulting in shorter withdrawal times through safety in numbers (Ray & Stoner Citation1994; Hamilton Citation2004). This follows from the well-documented phenomenon whereby the risk of predation faced by any individual is diluted by membership in a large group, as the chance of being the selected victim is reduced, consequently lowering the benefit of protecting their tentacles for a long period (Ray & Stoner Citation1994; Hamilton Citation2004). In principle, this should apply to mobile predators that pick one prey item before moving on to other patches, such as the fish or larger crustaceans that prey on A. aureoradiata (Ottaway Citation1977), though it may not protect anemones against starfish, as starfish can still detect the anemone when withdrawn.
There was a significant negative relationship between hiding time and disk diameter, whereby larger sea anemones re-emerged quicker than smaller ones after a physical disturbance. This result contradicts our hypothesis (2) that larger individuals would have a longer hiding time as they are more likely to be targeted by predators and are better competitors than small ones, allowing them to withdraw for longer (Krause & Ruxton Citation2002; Jennions et al. Citation2003). As we lack data on the predators most active at our study sites, and their mode of feeding, it is possible that a larger size in itself might offer protection, and that smaller anemones are more susceptible to predation (Pineda & Escofet Citation1989). Larger anemones may be more difficult to dislodge and resist being eaten, decreasing their predation risk independently of their hiding behaviour (Berger & Shor Citation2009). Larger individuals have also been found to be better at regeneration (Karlson Citation1988), possibly making survival after an attack more likely. Furthermore, larger individuals may be more resilient to food deprivation (Krause et al. Citation1998). It has been found in some anemone species that larger individuals have higher energy requirements and thus need more feeding time (Sebens Citation1981), causing them to re-emerge more quickly. These factors can combine to decrease the predation risk and increase the benefit of re-emergence, resulting in shorter retreat time for larger anemones. Future studies could investigate whether increasing age itself results in shorter mean hiding times. Individuals that tend to re-emerge quicker may feed more; increasing their competitive advantage and achieving heightened survival, allowing them to reach a higher age and therefore size.
We only have approximate estimates of wave exposure at the different sites based on the conditions observed during the study. Sea anemones at the site most exposed to wave action (Site 3) appeared to re-open quicker than those from less exposed sites. Previous studies have shown that wave action can limit feeding time for intertidal organisms such as sea anemones (Sebens Citation2002). It is possible that anemones experiencing stronger wave action at Site 3 opened more quickly to compensate for this decrease in feeding efficiency. As all measurements were made at low tide, wave exposure at the time the measurements were made was the same across all sites. Therefore, if differences between sites were due to wave exposure then there must be habituation. Individuals more habituated to wave exposure may simply respond less to tactile stimulation. Hunger and food availability may also play a role in these withdrawal times (Dill & Fraser Citation1997; Martin Citation2001). As wave exposure was not objectively quantified it is unknown whether the site differences are related to wave action; however, it could be included in subsequent studies. Other physical factors, such as water temperature, tide level and salinity, may also affect the hiding behaviour of intertidal sea anemones, but the effect of the biological factors studied here are independent of these parameters.
Overall, neighbour contact and immediate neighbour density appeared to have the greatest effect on hiding time. This could be due to the dilution effect against predation and the intensity of competition peaking at high densities, especially when physical contact with conspecifics occurs. This may play a greater role in balancing the trade-off between safety and feeding than the anemone's body size. The dilution effect has been observed in other sessile organisms such as the northern rock barnacle, as well as mobile organisms such as cichlid fish, mud-crabs and fiddler crabs (Mauck & Harkless Citation2001; Guerra-Bobo & Brough Citation2010).
Acknowledgements
We thank Emily Frost, Tony Harland, Martin Krkosek, Alistair Senior and Jeff Vanderpham for their useful advice and, above all, Robert Poulin, without whom this work would not have been possible.
References
- Berger WH, Shor EN 2009. Ocean: reflections on a century of exploration. California, University of California Press. 536 p.
- Brodie ED, Formanowicz DR, Brodie ED 1991. Predator avoidance and antipredator mechanisms: distinct pathways to survival. Ethology, Ecology and Evolution 3: 73–77. 10.1080/08927014.1991.9525390
- Broom M, Ruxton GD 2005. You can run—or you can hide: optimal strategies for cryptic prey against pursuit predators. Behavioral Ecology 16: 534–540. 10.1093/beheco/ari024
- Derby CD 2007. Escape by inking and secreting: marine molluscs predators through a rich array of chemicals and mechanisms. Biological Bulletin 213: 274–289. 10.2307/25066645
- Dill LM, Fraser AHG 1997. The worm re-turns: hiding behavior of a tube-dwelling marine polychaete, Serpula vermicularis. Behavioral Ecology 8: 186–193. 10.1093/beheco/8.2.186
- Dill LM, Gillett JF 1991. The economic logic of barnacle Balanus glandula (Darwin) hiding behavior. Journal of Experimental Marine Biology and Ecology 153: 115–127. 10.1016/S0022-0981(05)80010-3
- Guerra-Bobo M, Brough TE 2010. Neighbour density, body size and anti-predator hiding time in the New Zealand mud-crab Austrohelice crassa. Journal of the Marine Biological Association of the United Kingdom 91: 691–694. 10.1017/S0025315410001049
- Hamilton IM 2004. Distance to neighbours influences the trade-off between hiding after disturbance and defending food patches in convict cichlids (Archocentrus nigrofasiatus). Behavioral Ecology 14: 807–817.
- Hugie DM 2003. The waiting game: a ‘battle of waits’ between predator and prey. Behavioral Ecology 14: 807–817. 10.1093/beheco/arg054
- Jennions MD, Blackwell PR, Murai M, Christy JH 2003. Hiding behaviour in fiddler crabs: how long should prey hide in response to a potential predator?Animal Behaviour 66: 251–257. 10.1006/anbe.2003.2190
- Karlson RH 1988. Size-dependent growth in two zoanthid species: a contrast in clonal strategies. Ecology 69: 1219–1232. 10.2307/1941277
- Krause J, Loader SP, McDermott J, Ruxton GD 1998. Refuge use by fish as a function of body length-related metabolic expenditure and predation risks. Proceedings of the Royal Society of Biological Sciences 265: 2373–2379. 10.1098/rspb.1998.0586
- Krause J, Ruxton GD 2002. Living in groups. New York, Oxford University Press. 210 p.
- Martin J 2001. When hiding from predators is costly: optimization of refuge use in lizards. Etología 9: 9–13.
- Martin J, Lopèz P 1999. When to come out from a refuge: risk-sensitive and state-dependent decisions in an alpine lizard. Behavioral Ecology 10: 487–492. 10.1093/beheco/10.5.487
- Mauck RA, Harkless KC 2001. The effect of group membership on hiding behaviour in the northern rock barnacle, Semibalanus balanoides. Animal Behaviour 62: 743–748. 10.1006/anbe.2001.1798
- Morse DH 1982. Behavioural mechanisms in ecology. Cambridge, MA, Harvard University Press. 392 p.
- Ottaway JR 1977. Predators of sea anemones. Tuatara 22: 213–220.
- Pineda J, Escofet A 1989. Selective effects of disturbance on populations of sea anemones from northern Baja California, Mexico. Marine Ecology Progress Series 55: 55–62. 10.3354/meps055055
- Ray M, Stoner AW 1994. Experimental analysis of growth and survivorship in a marine gastropod aggregation: balancing growth with safety in numbers. Marine Ecology Progress Series 105: 47–59. 10.3354/meps105047
- Sebens KP 1981. The allomentry of feeding, energetics, and body size in three anemone species. Biology Bulletin 161: 152–171. 10.2307/1541115
- Sebens KP 2002. Energetic constraints, size gradients, and size limits in benthic marine invertebrates. Integrative and Comparative Biology 42: 853–861. 10.1093/icb/42.4.853
- Sih A 1997. To hide or not to hide? Refuge use in a fluctuating environment. Trends in Ecology and Evolution 12: 375–376. 10.1016/S0169-5347(97)87376-4
- Wood R 1999. Reef evolution. New York, Oxford University Press. 432 p.