Abstract
Two-year-old New Zealand geoducks (Panopea zelandica) were conditioned within combinations of three water temperatures (7–8, 11–12 and 16–17 °C) and three feeding rations (10,000, 50,000 and 100,000 cells mL−1 of Chaetoceros mulleri and Isochrysis galbana) for 73 days. Similar percent matured and dry condition index values were observed among temperatures. However, significantly higher dry gonadosomatic indices (GSIdw) were recorded at 8 and 12 °C. Although no difference was detected in percentage of spawned individuals and connective tissue occupation indices, a higher percent matured were recorded when fed 10,000 and 50,000 cells mL−1. A reference group conditioned in pond water became matured 2 months later than the other nine experimental groups, but GSIdw were similar. To maximise reproductive output, we suggest that 2-year-old P. zelandica may be conditioned in pond water for a month and then in 8 or 12 °C seawater with 50,000 cells mL−1.
Introduction
The New Zealand geoduck clam (Panopea zelandica) has been identified as a new potential species to add to the country's aquaculture exports, which are expected to reach NZ$1 billion by 2025 (Carter Citation2012). While this species has an excellent potential to achieve a high commercial value, there are many obstacles that need to be overcome before reliable production and markets can be established. Unlike other bivalve species, such as Pacific oysters (Crassostrea gigas) and Greenshell™ mussels (Perna canaliculus), P. zelandica spat cannot be obtained commercially from the wild. Thus, a reliable geoduck hatchery production must be established to supply on-growing farmers. In addition, hatchery production depends on a consistent source of broodstock, which is problematic for geoduck since this species has a long maturation period with one spawning peak per year (Gribben et al. Citation2004). An added problem is that P. zelandica females may produce few eggs (about 2 million eggs per female; Le Viet Dung, pers. obs.), as is the case with P. generosa females, which produce about 2–10 million eggs (Beattie Citation1994). With these bottlenecks to overcome, it is clear that early research efforts to commercialise this species need to be focused on broodstock conditioning and larval production. Specifically, temperature and feeding ration are well-known to affect broodstock conditioning of shellfish.
It is well-known that temperature has an important influence on the reproductive development of bivalves (Bayne Citation1976; Utting & Millican Citation1997; Helm et al. Citation2004). For geoducks, reproductive cycles have been shown to have a strong association with season for the Pacific species P. generosa (Andersen Citation1971; Goodwin Citation1976; Sloan & Robinson Citation1984; Campbell & Ming Citation2003), the Southern species P. abbreviata (van der Molen et al. Citation2007) and the Cortes species P. globosa (Calderón-Aguilera et al. Citation2010a). This seasonal reproductive behaviour suggests a strong influence of water temperature, although the particular patterns are species-specific. For example, the gametogenic development of P. generosa starts in late autumn and winter when water temperatures are low (8 °C), and spawning occurs in spring/summer when water temperatures rise to 11–12 °C (Sloan & Robinson Citation1984). However, gametogenesis of P. globosa begins in mid-autumn, when the water temperature is warmer (> 26 °C), and gametes are released during cooler periods (16 °C) (Calderón-Aguilera et al. Citation2010a). Conversely, ripe P. abrreviata females are found throughout the year, and higher proportions of spawning individuals are encountered during the colder months (van der Molen et al. Citation2007). Recently, Marshall et al. (Citation2012) found that P. generosa held at 7 and 11 °C displayed a more advanced state of reproductive development than individuals maintained at 15 and 19 °C. For P. zelandica, water temperature has been suggested to modulate the time to maturity as well as spawning (Gribben et al. Citation2004). In the only study to investigate wild P. zelandica, Gribben et al. (Citation2004) suggested that gamete production was 3 months longer at cooler sites (Shelly Bay; 11–15 °C) compared with warmer sites (Kennedy Bay; 15–19 °C) in northern New Zealand (Gribben et al. Citation2004). These studies suggest that temperature may have an important effect on reproductive conditioning of P. zelandica for aquaculture purposes. However, specific studies to identify the effect of temperature on maturity and spawning of P. zelandica have been absent until now. Gribben & Hay (Citation2003) attempted to spawn wild broodstock from Shelly Bay and Kennedy Bay in the laboratory, but those attempts failed. Therefore, establishing the appropriate temperature for gonadal growth and development in P. zelandica was considered to be the first critical step to establishing successful reproductive conditioning protocols for this species.
In addition to temperature, food quality and quantity have been shown to be deterministic factors in bivalve conditioning, since gonad development and maturation depend on sufficient and essential nutrient requirements (Utting & Spencer Citation1991). Conditioning experiments with P. generosa have shown that rations of 4.0 × 109 and 5.6 × 109 cells−1geoduck−1day−1 of a mixture of Chaetoceros mulleri and Isochrysis galbana (T-strain) achieved the highest survival and maturation rates for this species (Marshall Citation2012). In addition, Marshall (Citation2012) tested the efficiency of three different microalgal species (Isochrysis sp., C. muelleri and Dunaliella tertiolecta), and one mixed diet (Isochrysis sp. plus C. mulleri), on reproductive development of P. generosa, but was unable to detect any differences based on the quality of these feeds. Furthermore, García-Esquivel et al. (Citation2013) found that P. globosa could be matured successfully with Isochrysis sp. clone T-ISO as the sole diet at a ration of 16.4 × 109 cells−1geoduck−1day−1. However, optimal broodstock feeding requirements are strongly species-specific, and this information is lacking for P. zelandica. For practical purposes, broodstock conditioning of bivalve species has tended to use microalgal diets that are both readily available and have shown some success for other species. One such diet is a combination of Chaetoceros sp. (rich in DHA) and Isochrysis sp. (rich in EPA), which has been used successfully with bivalves such as the Pacific geoduck P. generosa (Marshall Citation2012) and the Pacific oyster C. gigas (Helm et al. Citation2004). However, appropriate rations may vary greatly from species to species, since utilisation of glycogen reserves for lipid synthesis during vitellogenesis also differ from species to species (Gabbott Citation1983).
External factors, mainly temperature and feeding ration, have been demonstrated to play an important role in the scope for growth of marine bivalves (Bayne & Newell Citation1983; MacDonald & Thompson Citation1986; Bayne & Hawkins Citation1990; Thompson et al. Citation1996). Ultimately, identifying the correct ration in relation to a specific temperature (i.e. scope for growth) is critical. However, no study combining the effects of temperature and food ration on reproduction has been carried out for geoducks. Thus, the aim of this study is to investigate the effects of water temperature and food ration on the conditioning of P. zelandica broodstock for aquaculture purposes.
Methods and materials
Source of broodstock
Two-year-old geoducks, which were grown from gametes at the Cawthron Institute, Nelson, New Zealand, were utilised as broodstock.The animals used were produced from the same set of parents, and were reared under the same culturing conditions. This common history provided reduced variability in reproductive condition due to season, location, age and genetic make-up.
The geoducks were dug up from the nursery tank (0.8 m height × 1 m diameter with a 0.5m sand layer), and immediately cleaned of biofoulings (mostly ascidians). One hundred individuals of similar shell lengths (the anterior-posterior axis of the right valve) and wet weights were selected. Mean (± SD) shell lengths were 57.8 ± 5.5 mm and mean (± SD) wet weights were 44.8 ± 9.4 g. These were relatively small individuals since it was expected that this information could be used to speed up the breeding process in the future.
Production of microlgae
Monocultures of two microalgal strains that are cultivated at the Cawthron Institute, Nelson, were selected for this experiment: Isochrysis galbana (T-ISO); and Chaetoceros mulleri. The microalgae were cultivated according to standard hatchery practices in 40 L plastic bags (Kaspar et al. Citation2014). The cultures were axenic and continuous in Conway medium. The culture conditions included: temperature of 20–23 °C; continuous light at 750–850 lx from cold white fluorescent tubes; salinity of 35 ppt pasteurised water at 95 °C; and aeration of 2.4 L min−1 with CO2 addition at 0.2% v:v. For C. mulleri, silicates (30 mL of a 30 g L−1 stock solution) were added directly to the bags every 2 days.
Conditioning experiment
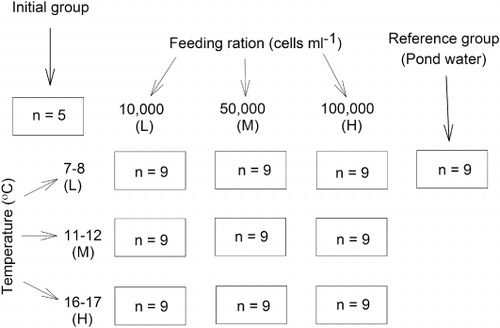
Geoducks were step-wise acclimatised at 8, 12 and 16 °C for 3 weeks. During the acclimatisation phase the geoducks were continuously fed at a ration of 35,000–40,000 cells mL−1 (C. mulleri:I. galbana = 1:1 by cell count). The broodstock conditioning experiment involved a completely randomised factorial design, with three water temperatures (7–8, 11–12 and 16–17 °C) and three feeding rations (10,000, 50,000 and 100,000 cells mL−1 of a 1:1 mixture of C. mulleri and I. galbana) (). These temperature levels were selected based on the temperature range for wild populations, and feeding rations were selected according to previous clearance rate experiments for 2-year-old P. zelandica (Le Viet Dung, unpubl. data). Salinity was 35 ppt throughout the experiment. Each of the nine treatments included three replicate tanks, each with three geoducks inside. A separate reference group of nine geoducks were exposed to pond water to generally compare the experimental animals to those grown in more ‘natural’ conditions. However, the reference group was not included in the statistical analyses. The condition period lasted for 73 days. The experimental set up included a flow-through system in which water and microalgae flowed at a rate of 160 mL min−1 from header tanks to the experimental tanks (10 L PVC tanks). Each of the 27 tanks contained three geoducks, placed vertically inside individual 400 mL plastic bottles containing 300 g of sand from the nursery tank (grain size 1–2.4 mm in diameter). These sand bottles mimicked the natural substrate conditions, and allowed for minimal handling disturbance to the animals.
Sampling
Five geoducks were sacrificed at day 0 for histological gonad analysis, and wet weight and dry weight measurements. At days 36 and 73, another three randomly selected geoducks per treatment, respectively, were sacrificed for histological and morphometric analyses. The remaining geoducks were induced to spawn by thermal shock on day 73 following procedures in Helm et al. (Citation2004). The percent spawning individuals was calculated for each treatment.
Histological analysis
A section gonad tissue was cut from the right side of the posterior end of the visceral mass of each geoduck (Marshall et al. Citation2012). The histological samples were prepared following Howard & Smith (Citation1983). Briefly, the gonads were fixed in a formalin solution for 48 h and then transferred to 70% ethanol for storage. The samples were cut into four to five subsections, dehydrated using a series of ascending ethanol solutions and eventually embedded in paraffin wax. Embedded gonad samples were sectioned to a 5 µm thickness, stained with hematoxylin-eosin, and mounted on slides for examination under a microscope (Olympus BX41).
Sex was determined by the presence of oocytes or spermatocytes. The gonadal stages were classified as early active, late active, ripe, partially spent or spent/resorbed according to the most dominant stage of at least 10 randomly chosen follicles in each slide (Gribben et al. Citation2004). The stages were then grouped into two categories: mature (late active, ripe, partially spent) or immature (spent/resorbed, early active) (Marshall et al. Citation2012).
Connective tissue occupation index
Connective tissue occupation index (COI) is defined as the ratio of connective tissue surface area divided by the total gonadal surface area (including connective tissue, follicles and vacuoles, but excluding digestive gland). This index was used as an indicator of gonad degeneration, and allowed for comparisons among treatments regardless of sex (Marshall et al. Citation2012). The region of connective tissue was determined colorimetrically using image processing software, ImageJ (http://rsb.info.nih.gov/ij/index.html). Images were filtered by convolve and Gaussian blur. Subsequently their thresholds were adjusted in 8-bit greyscale (0 = black, 255 = white). Within that colour scale, connective tissue and the follicle were distinguished. The COI was the average of the area fraction of 40 fields (each field 48,070 µm2) from each gonad histological sample. This method was adapted from Marshall et al. (Citation2012).
Condition index
Condition index (CI), which indicates the meat condition, was calculated as:
Gonadosomatic index
The gonadosomatic index (GSI) which correlated with reproductive cycles of wild P. generosa (Sloan & Robinson Citation1984) and P. zelandica (Gribben et al. Citation2004), was calculated as:
Statistical analyses
Due to the low number of animals available for this experiment, the number of observation units per replicate was low. Thus, non-parametric two-way ANOVA tests based on permutations (unrestricted with 999 random permutations and a seed integer of 5) were used to test the interaction effect and main effects of temperature and feeding ration on CI, GSI, COI, maturation and spawn for each sampling event. If the interaction effect or the main effects were significant, a pair-wise comparison among treatments was analysed with a t-test. The CI and GSI data did not need transformation to satisfy statistical requirements, but the COI data were arcsine transformed. The maturation and spawning data were converted to presence/absence. Non-parametric two-way ANOVAs were conducted on Bray-Curtis distances calculated from raw and transformed data using the FORTRAN program PERMANOVA (Anderson Citation2001). The permutation tests were valid regardless of the number of variables or the nature of their individual distributions or correlations (Anderson Citation2001).
Results
Results from the conditioning experiment indicate that there was no significant difference among temperature or feeding ration treatments for geoduck wet (CIww) and dry (CIdw) weights after the first 36 days of the experiment (; ). However, after 73 days, there was a general trend of increasing geoduck CIww and CIdw with increasing feeding ration for the medium (11–12 °C) and high (16–17 °C) temperature treatments. A different pattern was observed at low temperatures (7–8 °C), where a medium feeding ration (50,000 cells mL−1) resulted in the highest geoduck CIww and CIdw values of 416.4 ± 28.6% and 109.1 ± 8.9%, respectively. These values were similar to those of geoducks conditioned in pond water (reference group), which were 391.7 ± 2.5% and 113.2 ± 12.1% for CIww and CIdw, respectively. These values were not as high (although not significantly different) as those of individuals sampled at the start of the experiment (day 0), which were 461.0 ± 54.7% and 122.4 ± 10.6%, respectively for CIww and CIdw. Two-way ANOVAs on Bray-Curtis distances analyses resulted in significant temperature and food ration treatments and interaction for CIww and a significant food ration treatment and interaction for CIdw ().These results reflect the different trends of feeding ration effects among temperatures.
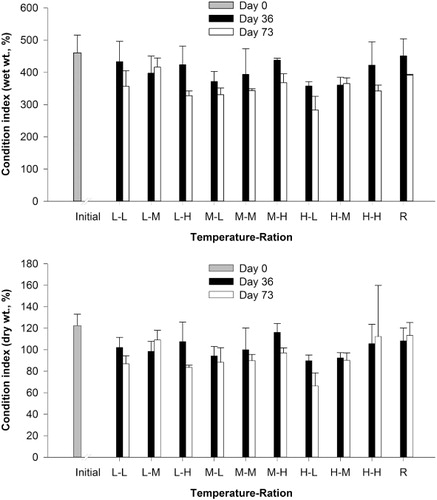
Table 1 Nonparametric two-way ANOVA on Bray-Curtis distances for reproductive indices of 2-year-old P. zelandica after 36 and 73 days of conditioning in three water temperatures (L = low, 7–8 °C; M = medium, 11–12 °C; H = high, 16–17 °C), three feeding rations (10,000, 50,000 and 100,000 cells mL−1). Bold P values indicate significant tests.
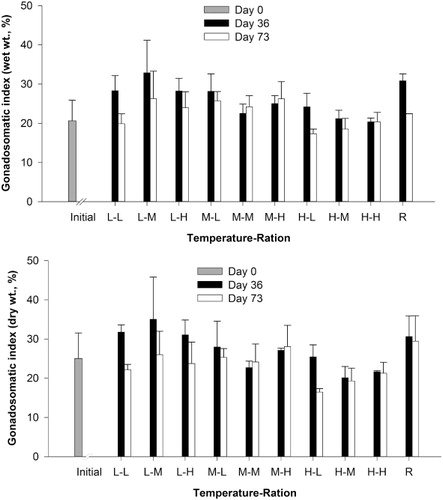
Geoduck gonadosomatic analyses resulted in decreasing GSIww and GSIdw values with increasing temperature after the first 36 days (; ). The highest values of 29.8 ± 5.4% and 32.6 ± 6.0% for GSIww and GSIdw, respectively, were observed in geoducks conditioned at low temperatures. These values were similar to those of geoducks in the reference group, which were 30.9 ± 1.8% and 30.6 ± 5.2% for GSIww and GSIdw, respectively. However, after 73 days, the highest GSIww and GSIdw values were observed in geoduck conditions at medium temperatures (25.4 ± 3.0% and 25.8 ± 4.1%, respectively), while those of geoducks in the reference were 22.4 ± 0.1% and 29.4 ± 6.5% for GSIww and GSIdw, respectively. Conversely, GSIww and GSIdw values for geoducks sampled on day 0 were 20.7 ± 5.2% and 25.0 ± 6.5%, respectively. Results from two-way ANOVAs on Bray-Curtis distances analyses indicate significant differences among temperatures and non-significant differences among food rations and interactions for both GSIww and GSIdw after 36 and 73 days ().
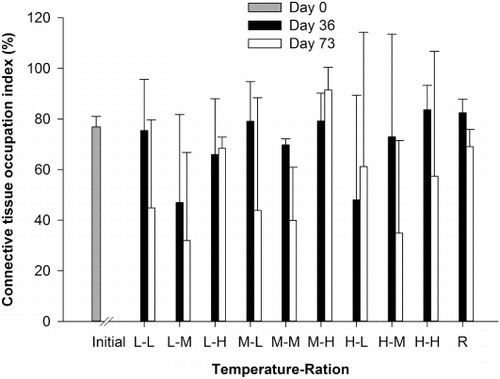
The COI did not differ significantly among temperature or feeding ration treatments after 36 days or 73 days (; ). On day 36, the high temperature and high feeding ration treatment had the highest COI values (83.7 ± 9.6%), while the lowest COI values were observed in the low temperature and medium feeding ration group (47.0 ± 34.8%). After 73 days, the low temperature and medium feeding ration group still had the lowest COI values (32.0 ± 34.9%), and the highest values were recorded in the medium temperature and high feeding ration (91.5 ± 8.9%). The COI of the reference group on day 73 and initial group were 82.4 ± 5.4% and 76.8 ± 4.2%, respectively ().
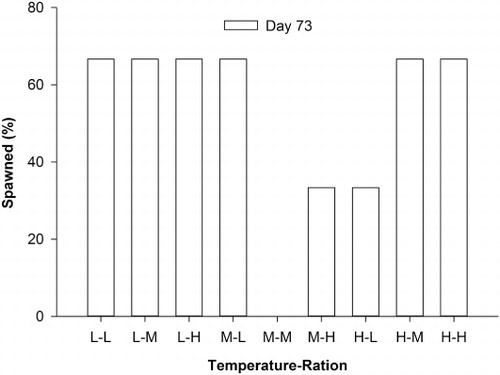
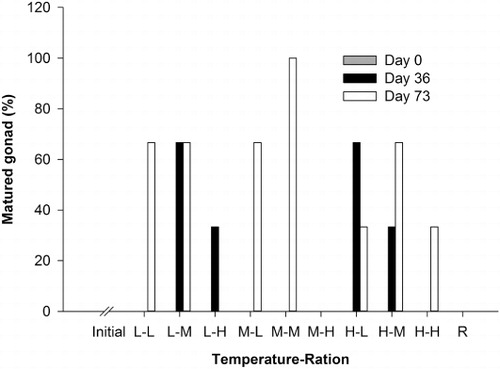
The percentage of mature gonads was not significantly different among temperature and feeding ration treatments after 36 days (; ), but the feeding ration alone was significant for the 73 day dataset (). Geoducks fed medium feeding rations had a significantly higher percentage (77.8%) of mature gonads than those fed low and high feeding rations after the 73 day experiment (). The reference group had the lowest percentages (0%) of mature gonads compared to all feeding ration groups. The percentages of geoducks which spawned after the 73 day conditioning experiment were not significantly different among temperature and feeding ration treatments (; ).
Discussion
To determine which temperature and feeding ration were more appropriate for geoduck broodstock conditioning, we based our examination of the dataset on two priorities. The first priority was evidence of maturity and spawning. The second priority was the reproductive development indicators (GSIs and CIs). The GSI has been correlated with reproductive state in wild populations of P. generosa (Sloan & Robinson Citation1984) and P. zelandica (Gribben et al. Citation2004). Condition indices are indicators of stress or sexual activity, and low values of these indices indicate that a major biological effort has been expended, either as maintenance energy under poor environmental conditions or disease, or in the production or release of gametes (Lucas & Beninger Citation1985). Although wet and dry GSIs and CIs were measured, we based our interpretations on GSIdw and CIdw, since the use of the dry indices in bivalves eliminates the bias due to water content fluctuations of whole tissue samples (Lucas & Beninger Citation1985; Gribben et al. Citation2004; Marshall et al. Citation2012).
Results from our study indicate that, although high temperatures were acceptable for conditioning geoducks, low and medium temperatures were more appropriate to condition 2-year-old P. zelandica broodstock. Based on the percentage of mature gonads, spawned geoducks and COI values, there was no difference in maturity among all three temperature levels. However, results from GSIdw analyses indicate that geoducks had a higher gonad material ratio when exposed to medium and low temperatures. GSIdw values on days 36 and 73 at medium (25% and 25%, respectively) and low (32% and 23%, respectively) temperatures were higher than GSIdwvalues at high (22% and 19%, respectively) temperatures. In addition, CIdw values were not different among the three temperatures and the dry weight gonad:shell weight ratio of the high temperature treatment was significantly higher than that of the low and medium temperature treatments (data not shown). These results confirmed that the differences in GSIdw values were due to the development of reproductive products.
There is limited information on the effect of temperature on gonad development of P. zelandica. However, previous studies on the reproductive cycle of wild geoducks support our result that geoducks may become mature when exposed to either high or low temperatures (Gribben et al. Citation2004; van der Molen et al. Citation2007). Gametogenic development of P. zelandica from Kennedy Bay started at low temperatures (11 °C), while that of P. zelandica from Shelly Bay started at high temperatures (18 °C) (Gribben et al. Citation2004). Also, P. abbreviata were found ripe for most of the year when exposed to a temperature range of 8 to 20 °C (van der Molen et al. Citation2007). The lack of significant influence of a particular temperature window on the percentage of mature gonads in our study also has been observed in other bivalve species. Toba & Miyama (Citation1995) concluded that temperature between 10 and 27 °C was not a limiting factor in the reproductive development of Ruditapes philippinarum. Also, if the amount of food ingested was similar, then temperature differences of 4 °C did not influence the rate of gonadal development of R. philippinarum (Delgado & Camacho Citation2007). In addition, Navarro et al. (Citation2000) rejected the effect of temperature within the range studied (16–20 °C) on the gonadal development of Argopecten purpuratus. Moreover, an increase in temperature within 2–15 °C did not speed up gametogenesis in the mussel Mytilus edulis, except at temperatures close to their lower LT50 (Gosling Citation2003). In fact, our temperature range was similar to that of Marshall et al. (Citation2012), who found no difference in the percentage of mature gonads of P. generosa at a range between 7 and 15 °C, but found a difference when the exposure was 19 °C.
For many marine species, food rather than temperature is the major factor determining the gametogenesis length (Gosling Citation2003). Our study showed that medium feeding rations were the most appropriate for conditioning 2-year-old P. zelandica, while the low rations were also acceptable. According to the percentage of mature gonads, medium and low feeding rations (55.6% and 77.8%) were superior to high rations (11.1%). However, based on the percentage of spawned geoducks, COI and GSIdw values, there was no difference among the three feeding rations. Furthermore, CIdw values indicate that geoducks exposed to medium feeding rations had more meat ratio (96.4%) than those exposed to low feeding rations (80.5%).
Previous studies of conditioning other geoduck species support our results that feeding ration influences gametogensis development. However, results from feeding ration values among studies are widely different and difficult to compare. To make a feasible comparison among studies, we standardised the feeding ration units in Marshall (Citation2012) and our studies to percentage dry weight of algae/dry weight of tissue. Marshall (Citation2012) used a range of rations: 0.8 × 109; 4.0 × 109; 5.6 × 109; 7.2 × 109; and 10 ×1 09 cells geoduck−1day−1, which were equivalent to 0.02%, 0.1%, 0.14%, 0.18% and 0.25% dry tissue weight. Our feeding rations (10,000, 50,000 and 100,000 cells mL−1) were equivalent to 0.58 × 109, 2.88 × 109 and 5.76 × 109 cells geoduck−1 day−1, respectively. These values were equivalent to 0.33%, 1.65% and 3.3% dry tissue weight, respectively. Marshall (Citation2012) found that a 0.25% ration caused P. generosa mortality, while the high and low rations (0.18% and 0.02%) resulted in less gonadal material than those in the medium rations (0.14% and 0.1%). In addition, P. globosa could become mature when exposed to a 0.31% dry tissue weight feeding ration without compromising survival (García-Esquivel et al. Citation2013). On the other hand, our study showed that the most appropriate feeding ration was 1.6%, and a feeding ration of 0.33% was acceptable. We also observed the least number of mature P. zelandica at a feeding ration of 3.3%. Another potential difference for the result differences among studies could be the size of the animals studied. Our specimens were on average 44 g of live wet weight, while Marshall (Citation2012) and García-Esquivel et al. (Citation2013) used 1200–1600 g live wet weight animals. Smaller animals have proportionally higher metabolic demands than larger animals (Hamburger et al. Citation1983; Brougrier et al. Citation1995) and therefore require proportionally more food. The rapid growth of wild geoducks during the first few years (Goodwin & Pease Citation1989; Gribben & Creese Citation2005; Calderón-Aguilera et al. Citation2010b; Pérez-Valencia & Aragón-Noriega Citation2013) supports this interpretation since adult geoducks require energy for only maintenance and reproductive growth, while juvenile geoducks still utilised energy for maintenance, reproductive growth and additional somatic growth.
In comparison with other bivalve species (i.e. oysters, clams, scallops) reported by Helm et al. (Citation2004), the appropriate feeding ration for geoduck conditioning in our study and previous studies was lower (0.1%–1.6%) than theirs (2%–6%). There may be two reasons for successful maturation at low feeding rations: 1. lower metabolic demands of larger animals (mentioned above); and 2. the fact that geoducks belong to a bivalve group which relies on endogenous reserves for gamete development (Bayne Citation1976; Goodwin Citation1976). In other words, most bivalves may store reproductive energy reserves in the mantle, digestive gland (Dridi et al. Citation2007) and adductor muscles (Devauchelle & Mingant Citation1991; Ngo et al. Citation2006), while geoducks also store energy in their massive muscular siphon. Evidence for the use of the siphon as a reserve for gonad development also was observed in P. generosa, which despite being starved for 3 weeks, had thick gonad layers in the visceral mass (Marshall Citation2012). Similarly, our observations with 3-year-old P. zelandica indicate that they became ripe after being starved for 1 month at 19 °C and re-fed at 16 °C in the following month. Some males even spawned after the first starvation month.
It also is noteworthy that the pond water group in our study had among the highest values in both CIdw and GSIdw. However, they did not mature until December, 2 months after the end of this experiment. This indicates that the pond water provided enough nutrients for geoducks to accumulate energy for gonad development, but there was a missing ‘trigger’ to start gametogenesis earlier.
Based on the results of our study, we suggest that P. zelandica may be conditioned successfully with a two-phase strategy. When geoducks have yet to start gametogenesis in winter (June–August), they may be provided a medium feeding ration equivalent to 50,000 cells mL−1 from pond water in a flow-through system. The objective of this approach is to boost the levels of food reserves that will later be mobilised to gamete development. Following 4 to 6 weeks of a medium ration with a cold pond water temperature regime, geoducks may be transferred into hatchery conditions where temperature is gradually increased or decreased (1 to 2 °C per day) to either 8 or 12 °C and a mixture of I. galbana and C. mulleri (1:1) is provided at ration 50,000 cells mL−1. The objective of this conditioning approach is to trigger the gametogenesis process.
In conclusion, the temperature range provided to 2-year-old geoducks did not significantly influence the gametogenic development of P. zelandica. Nonetheless, 8 and 12 °C were preferable to maximise the development of gonad material. In contrast, feeding rations significantly influenced gonad maturation, with more matured geoducks at low and medium ration. However, a feeding ration of 50,000 cells mL−1 was more favourable due to the higher meat ratio. Despite having a maintained high CI, the maturation of the reference group occurred later than the experimental groups, which provides encouraging information for geoduck conditioning under laboratory conditions. Overall, this study contributes to the knowledge of conditioning juvenile geoducks as a broodstock source. The implications would be that a shorter period may be needed to spawn juveniles rather than adults produced along a selective breeding programme. Finally, future investigations may focus on testing the effects of extreme temperatures and a wider range of feeding rations on the gametogenic process of P. zelandica. After a series of conditioning trials, we also recognised the limitations of having both the scarcity of broodstock for this species and the intrinsically high variability of gonad development among individuals. Thus, we have set out to develop a non-invasive method using magnetic resonance imaging (MRI) techniques to determine the sex and gonad stage of geoducks so that we can perform measurements on the same stage of individuals over time.
Acknowledgements
We are thankful for the technical assistance of Ellie Watts and Steve Webb at the Cawthron Institute. This paper was greatly improved through discussions with the Aquaculture Biotechnology Group (AUT University) and the Geoduck Group (Cawthron Institute). This project was funded by the Cultured Shellfish Programme (MBIE contracts CAWS0802, CAW1315). Logistical support was provided by the School of Applied Sciences, Auckland University of Technology. This project is part of a PhD thesis, which was supported by a New Zealand Aid scholarship awarded to Dung Viet Le. We appreciate the comments and suggestions of four anonymous reviewers, which improved the quality of the manuscript.
References
- Andersen AM 1971. Spawning, growth and spatial distribution of the geoduck clam, Panopea generosa Gould, in Hood Canal, Washington. Unpublished PhD thesis. Washington, University of Washington. 147 p.
- Anderson MJ 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46.
- Bayne BL 1976. Marine mussels: their ecology and physiology. Cambridge, Cambridge University Press. 523 p.
- Bayne BL, Newell C 1983. Physiological energetics of marine molluscs. In: Saleuddin ASM, Wilbur KM eds. The Mollusca. Vol. 4, Physiology Part 1. New York, NY, Academic Press. Pp. 407–515.
- Bayne BL, Hawkins AJS 1990. Filter feeding in bivalve molluscs: controls on energy balance. In: Mellinger J, Truchot JP, Lahlou B eds. Comparative physiology, animal nutrition and transport processes. I. nutrition in wild and domestic animals. Basel, Karger. Pp. 70–83.
- Beattie JH 1994. Serial spawning of the geoduck clam (Panopea abrupta). Journal of Shellfish Research 14: 227.
- Brougrier S, Geairon P, Deslous-Paoli JM, Bacher C, Jonquières G 1995. Allometric relationships and effects of temperature on clearance and oxygen consumption rates of Crassostrea gigas (Thunberg). Aquaculture 134: 143–154. 10.1016/0044-8486(95)00036-2
- Bureau D, Station P 2003. Age, size structure and growth parameters of geoducks (Panopea abrupta, Conrad 1849) from seven locations in British Columbia sampled in 2001 and 2002. Canadian Technical Report of Fisheries and Aquatic Sciences. 29 p.
- Calderón-Aguilera LE, Aragón-Noriega EA, Reyes-Bonilla H, Paniagua-Chavez CG, Romo-Curiel AE, Moreno-Rivera VM 2010a. Reproduction of the Cortes geoduck Panopea globosa (Bivalvia: Hiatellidae) and its relationship with temperature and ocean productivity. Journal of Shellfish Research 29: 135–141. 10.2983/035.029.0107
- Calderón-Aguilera LE, Aragón-Noriega EA, Hand CM, Moreno-Rivera VM 2010b. Morphometric relationships, age, growth and mortality of the geoduck clam Panopea generosa, along the Pacific coast of Baja California, Mexico. Journal of Shellfish Research 29: 319–326. 10.2983/035.029.0206
- Campbell A, Ming MD 2003. Maturity and growth of the Pacific geoduck clam, Panopea abrupta, in southern British Columbia, Canada. Journal of Shellfish Research 22: 85–90.
- Carter D 2012. The Government's aquaculture strategy and five-year action plan to support aquaculture. Wellington, Ministry for Primary Industries.
- Delgado M, Pérez Camacho A 2007. Influence of temperature on gonadal development of Ruditapes philippinarum (Adams and Reeve, 1850) with special reference to ingested food and energy balance. Aquaculture 264: 398–407. 10.1016/j.aquaculture.2006.11.009
- Devauchelle N, Mingant C 1991. Review of the reproductive physiology of the scallop, Pecten maximus, applicable to intensive aquaculture. Aquatic Living Resources 4: 41–51. 10.1051/alr:1991004
- Dridi S, Romdhane MS, Elcafsi M 2007. Seasonal variation in weight and biochemical composition of the Pacific oyster, Crassostrea gigas in relation to the gametogenic cycle and environmental conditions of the Bizert lagoon, Tunisia. Aquaculture 263: 238–248. 10.1016/j.aquaculture.2006.10.028
- Gabbott PA 1983. Developmental and seasonal metabolic activities in marine molluscs. In: Hochachka PW ed. The mollusca, environmental biochemistry and physiology vol 2. New York, Academic Press. Pp. 165–217.
- García-Esquivel Z, Valenzuela-Espinoza E, Buitimea MI, Searcy-Bernal R, Anguiano-Beltrán C, Ley-Lou F 2013. Effect of lipid emulsion and kelp meal supplementation on the maturation and productive performance of the geoduck clam, Panopea globosa. Aquaculture 396–399: 25–31. 10.1016/j.aquaculture.2013.02.012
- Goodwin C, Pease B 1989. Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (Pacific Northwest) – Pacific geoduck clam. Biological Report. 14 p.
- Goodwin L 1976. Observations on spawning and growth of subtidalgeoducks (Panopea generosa, Gould). Proceedings of the National Shellfisheries Association 65: 49–58.
- Gosling E 2003. Bivalve mollusc: biology, ecology, and culture. Oxford, Fishing New Books. 455 p.
- Gribben PE, Creese RG 2005. Age, growth, and mortality of the New Zealand geoduck clam, Panopea zelandica (Bivalvia: Hiatellidae) in two North Island populations. Bulletin of Marine Science 77: 119–135.
- Gribben PE, Hay BE 2003. Larval development of the New Zealand geoduck Panopea zelandica (Bivalvia: Hiatellidae). New Zealand Journal of Marine and Freshwater Research 37: 231–239. 10.1080/00288330.2003.9517161
- Gribben PE, Helson J, Jeffs AG 2004. Reproductive cycle of the New Zealand geoduck, Panopea zelandica, in two north island populations. The Veliger 47: 53–65.
- Hamburger K, Møhlenberg F, Randløv A, Riisgård HU 1983. Size, oxygen consumption and growth in the mussel Mytilus edulis. Marine Biology 75: 303–306. 10.1007/BF00406016
- Helm MM, Bourne N, Lovatelli A 2004. Hatchery culture of bivalves: a practical manual. Rome, FAO Fisheries Technical Paper. 177 p.
- Howard DW, Smith CS 1983. Histological techniques for marine bivalve mollusks. NOAA Technical Memorandum NMFS-F/NEC-25. Woods Hole, MA, National Oceanic and Atmospheric Administration.
- Kaspar HF, Keys EF, King N, Smith KF, Kesarcodi-Watson A, Miller MR 2014. Continuous production of Chaetoceros calcitrans in a system suitable for commercial hatcheries. Aquaculture 420–421: 1–9. 10.1016/j.aquaculture.2013.10.021
- Lucas A, Beninger PG 1985. The use of physiological condition indices in marine bivalve aquaculture. Aquaculture 44: 187–200. 10.1007/BF00428653
- MacDonald BA, Thompson RJ 1986. Influence of temperature and food availability on the ecological energetics of the giant scallop Placopecten magellanicus. III. Physiological ecology, the gametogenic cycle and scope for growth. Marine Biology 93: 37–48. 10.1007/BF00428653
- Marshall R 2012. Broodstock conditioning and larval rearing of the geoduck clam (Panopea generosa Gould, 1850). Unpublished PhD thesis. Vancouver, University of British Columbia. 227 p.
- Marshall R, McKinley RS, Pearce CM 2012. Effect of temperature on gonad development of the Pacific geoduck clam (Panopea generosa Gould, 1850). Aquaculture 338–341: 264–273. 10.1016/j.aquaculture.2012.01.004
- Navarro J, Leiva G, Martinez G, Aguilera C 2000. Interactive effects of diet and temperature on the scope for growth of the scallop Argopecten purpuratus during reproductive conditioning. Journal of Experimental Marine Biology and Ecology 247: 67–83. 10.1016/S0022-0981(00)00140-4
- Ngo TTT, Kang S, Kang D, Sorgeloos P, Choi K 2006. Effect of culture depth on the proximate composition and reproduction of the Pacific oyster, Crassostrea gigas from Gosung Bay, Korea. Aquaculture 253: 712–720. 10.1016/j.aquaculture.2005.09.009
- Pérez-Valencia SA, Aragón-Noriega EA 2013. Age and growth of the Cortes geoduck Panopea globosa (Dall, 1898) in the Upper Gulf of California. Indian Journal of Geo-Marine Science 42: 201–205.
- Sloan NA, Robinson SMC 1984. Age and gonad development in the geoduck clam Panopea abrupta (Conrad) from southern British Columbia, Canada. Journal of Shellfish Research 4: 131–137.
- Thompson R, Newell R, Kennedy V, Mann R 1996. Reproductive processes and early development. In: Kennedy V, Newell R, Eble A eds. The eastern oyster Crassostrea virginica. Maryland, Maryland Sea Grant Books. Pp. 355–370.
- Toba M, Miyama Y 1995. Influence of temperature on sexual maturation in Manila clam Ruditapes philippinarum. Aquaculture Science 43: 305–314.
- Utting SD, Spencer BE 1991. The hatchery culture of bivalve mollusc larvae and juveniles. Laboratory Leaflet no. 68. Lowestoft, MAFF Directorate of Fisheries. 31 p.
- Utting SD, Millican PF 1997. Techniques for the hatchery conditioning of bivalve broodstocks and the subsequent effect on egg quality and larval viability. Aquaculture 155: 45–54. 10.1016/S0044-8486(97)00108-7
- van der Molen S, Kroeck M, Ciocco N 2007. Reproductive cycle of the southern geoduck clam, Panopea abbreviata (Bivalvia: Hiatellidae), in north Patagonia, Argentina. Invertebrate Reproduction & Development 50: 75–84. 10.1080/07924259.2007.9652230
- Walne PR 1976. Experiments on the culture in the sea of the butterfish Venerupis decussata L. Aquaculture 8: 271–381. 10.1016/0044-8486(76)90119-8