Abstract
Brown trout (Salmo trutta) are known to have effects on multiple trophic levels in New Zealand streams, but their impacts on lower trophic levels are less well understood within lentic systems. We examined the effects of brown trout removal using rotenone on zooplankton and phytoplankton community composition in the Upper Karori Reservoir, New Zealand. Significant shifts were observed in zooplankton and phytoplankton composition following removal of brown trout from the reservoir. Shifts in zooplankton community composition did not occur immediately following trout removal (February), but instead followed the likely timing of galaxiid spawning (July). The removal of brown trout likely resulted in reduced predation pressure on galaxiids. A major change occurred in the zooplankton community with the dominance shifting from larger crustaceans to smaller rotifers, indicating an increased predation pressure from the larval native galaxiid. A delayed response in zooplankton community composition change indicates rotenone was not the direct cause of this. A major shift in phytoplankton community composition occurred immediately following trout removal. This was not consistent with the trophic cascade hypothesis of reduced grazing pressure from larger zooplankton due to increased galaxiid predation as a result of brown trout removal.
Introduction
Trophic cascades occur when a species has effects at more than one trophic level, and are commonly observed following addition or removal of a predator from an ecosystem. Such effects can be observed at population, community or ecosystem levels (e.g. Pace et al. Citation1999; Simon & Townsend Citation2003), and are widespread across a variety of ecosystem types (Pace et al. Citation1999; Shurin et al. Citation2002). Trophic cascades are often observed in lakes, where they are commonly documented as biomass changes between trophic levels. In such cascades, planktivorous fish or mysids feed on and reduce the abundances of large zooplankton species (Ellis et al. Citation2011). Large herbivorous zooplankton species, such as Daphnia and calanoid copepods, have a greater impact on phytoplankton abundance than small zooplankton species, as they are more efficient feeders and consume a broader range of sizes and morphologies of algae. Removal of large zooplankton species can lead to an increase in algal biomass (e.g. Burns Citation1968; Hall et al. Citation1976; Bergquist et al. Citation1985; Mourelatos & Lacroix Citation1990). The observation of ‘three level’ food chains is relatively common, whereby following fish removal, zooplankton importance is increased, and phytoplankton biomass decreases. In some instances, effects can be observed on four trophic levels; for example, the addition of piscivorous fish reduces planktivorous fish abundance, allowing large-bodied zooplankton (i.e. crustaceans) to dominate, which in turn lowers phytoplankton biomass (Tronstad et al. Citation2010). Less commonly appreciated are the cascading effects on community composition (Cochran-Stafira & von Ende Citation1998). However, additions or increases in planktivorous fish can shift dominance from larger towards smaller zooplankton individuals (e.g. from Daphnia to small cladocerans, such as Bosmina, or rotifer species; Ellis et al. Citation2011). Rotifers have been observed to be outcompeted or killed by larger crustaceans in both experimental and field-based studies, and reductions in larger species by predators can enable smaller zooplankton to dominate (e.g. Gilbert & Stemberger Citation1985; Vanni Citation1986; MacIsaac & Gilbert Citation1991).
Trout have effects on multiple trophic levels, and these have been particularly well studied in stream ecosystems (Townsend Citation2003). Brown trout (Salmo trutta) have established non-indigenous populations in a number of Southern Hemisphere locations, including New Zealand, Australia, Southern Africa and the Falkland Islands (McDowall Citation2003). In New Zealand, brown trout were introduced to the South Island in 1867, and are now the most widely distributed non-indigenous fish species in the country (McDowall Citation1990). In New Zealand, differences in invertebrate community composition and depressions in invertebrate abundance have been recorded among streams with and without brown trout, and trout presence is associated with increased periphyton biomass (Flecker & Townsend Citation1994; McDowall Citation2003; Townsend Citation2003). Brown trout have had negative effects on native non-migratory galaxiids that spend their entire life in freshwater, both through predation and competition (McIntosh et al. Citation2010). In streams, non-migratory galaxiids are now typically restricted to trout-free reaches above waterfalls and other barriers which prevent trout migration (Townsend & Crowl Citation1991; Eby et al. Citation2006; McDowall Citation2006; Jellyman & McIntosh Citation2008). A comparison of streams with native fish and trout showed that algal assemblages in streams containing trout were dominated by erect taxa that are more susceptible to grazing, presumably due to a reduction in grazing pressure by native fish species (Biggs et al. Citation2000).
While brown trout effects are well appreciated in New Zealand streams, they are not as well understood within lakes. In the central North Island, rainbow trout (Oncorhynchus mykiss) predation has resulted in large declines in lake stocks of kōaro (Galaxias brevipinnis). Consequently, the more resilient smelt (Retropinna retropinna) have been stocked as trout forage (Rowe et al. Citation2003). Townsend (Citation2003) observed that a small brown trout in a laboratory aquarium consumed 135 Galaxias fry in a day, indicating a strong potential predation pressure. In South America, larvae and juveniles of inanga (Galaxias maculatus) prey on crustacean zooplankton (Modenutti et al. Citation1993); as such, effects on the community composition of lower trophic levels are expected to arise following the addition or removal of brown trout in lakes. Here, we examine the effects of trout removal on the community composition of zooplankton and phytoplankton in Upper Karori Reservoir, New Zealand, following rotenone treatment.
Methods
Upper Karori Reservoir
The Upper Karori Reservoir (41°17′56″ S 174°44′39″ E) is situated within the Kaiwharawhara catchment, Wellington, New Zealand. The catchment contains two reservoirs. The Lower Reservoir was formed when a large earth dam was built in 1874, and in 1908 a large concrete dam was built, forming the Upper Karori Reservoir. The maximum depth of the reservoir was approximately 20 m and it had an impounding capacity of 284 million L. The Upper Karori Reservoir was partially emptied in 1992 following an engineer's report that found it did not have ‘sufficient factors of safety’ to cover the risk of an earthquake on the Wellington fault line, which runs through both dams (Astwood & Fell Citation2012). The current maximum depth of the reservoir is 9 m (de Winton Citation2013). Populations of brown trout and banded kōkopu (Galaxias fasciatus) have been land-locked within the catchment since the development of these reservoirs.
Rotenone in the form of cube-root-slurry was added to the Upper Karori Reservoir and its catchment streams on 22 and 23 February 2011. One hundred and fifty-nine banded kōkopu were caught and held in upstream refuges during rotenone treatment, and re-released into the stream once rotenone concentrations decreased below levels deemed to be toxic to this species. During rotenone treatment, 480 (range 46–273 mm) brown trout were removed from the catchment streams and 54 were removed from the reservoir (range 165–460 mm; Pham et al. Citation2013). One year following the eradication of brown trout from the catchment, many juvenile banded kōkopu were observed entering the streams flowing into the reservoir (Pham et al. Citation2013). As banded kōkopu spawn in late autumn and early winter (Mitchell & Penlington Citation1982), spawning would have occurred after rotenone dosing, allowing juveniles to rear in the reservoir and return to the tributaries (Pham et al. Citation2013).
Sampling, laboratory methods and data analysis
Zooplankton were collected from 22 November 2010 to 23 April 2012, initially at two-weekly intervals (until May 2011), and approximately monthly for the final year. Eight samples were collected prior to rotenone addition, and 16 post-application. For zooplankton, 4 L samples were collected at 0 m and 5 m depths using a Van Dorn sampler. The samples were filtered (40 µm mesh) and the filtrate stored in Falcon tubes (50 mL) containing 70% ethanol. For analyses, depth samples were combined to give a single sample per day. Samples were counted in aliquots under a dissecting microscope at c. 30× magnification on a gridded Perspex tray until at least 300 individuals were encountered, or the whole sample was completed. Individuals were identified to the lowest level practical (usually species). Phytoplankton samples (c. 500 mL) were collected at two depths (0 m and 5 m) from 9 November 2010 to 23 April 2012. Nine samples were collected prior to trout eradication, and 16 after. Subsamples (100 mL) for algal enumeration were preserved immediately with 1% Lugol's iodine. Phytoplankton identification and enumeration were carried out using an inverted Olympus microscope (CKX41, Olympus, Wellington, New Zealand) and Utermöhl settling chambers (Utermöhl Citation1958).
Non-metric multi-dimensional scaling (nMDS) and analysis of similarities (ANOSIM, Primer v6.1.13; Primer-E Ltd 2009), based on Bray-Curtis similarities, were used to examine changes over time in zooplankton and phytoplankton community composition in the Upper Karori Reservoir. Data were log(x + 1) transformed to down-weight the influence of dominant species. To reduce the influence of rare species potentially sampled by chance, only zooplankton species found in three or more samples were included. ANOSIM (using 999 permutations) was applied to the similarity matrix to test for statistically significant changes in zooplankton and phytoplankton community composition between samples collected before and after rotenone treatment, and before and after the expected recruitment of larval banded kōkopu into the Upper Karori Reservoir. In the absence of data on the appearance of larvae, we estimated larvae to enter the reservoir in early July. This is based on a timing of early June for banded kōkopu spawning, as observed in tributaries of the Waihi Reservoir, and a further 30 days before eggs from the Waihi tributary hatched (Mitchell & Penlington Citation1982). As the dominance of large and small zooplankton may change due to the influence of rotenone and/or predation, Fisher's exact tests were used to determine if the likelihood of dominance of rotifers and crustaceans changed before and after trout removal, and before and after the timing of probable galaxiid spawning (QuickCalcs, Graphpad Software).
Results
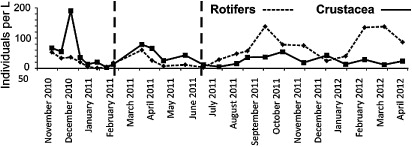
Thirty-one zooplankton taxa were identified during the study (). Crustaceans typically numerically dominated the community at the beginning of the study, being the most abundant taxon in 10 of the first 12 samples (up to the start of July), including six of eight prior to rotenone addition (). Post July 2011, rotifers dominated 10 of 12 samples. A Fisher's exact test indicated that crustaceans were no more likely to dominate community composition prior to trout removal than afterwards (two-tailed P = 0.193). However, using a timing of July for recruitment of larvae into the reservoir, Fisher's exact test indicated that crustaceans were more likely to dominate the community prior to banded kōkopu recruitment, and rotifers after (two-tailed P = 0.003). The nMDS ordination for zooplankton community composition illustrated a significant difference in zooplankton community compositon before and after rotenone treatment (, upper plot) as indicated by ANOSIM results (Global R = 0.212, P = 0.021). A significant difference in zooplankton community composition before and after the probable galaxiid spawning date (Global R = 0.209, P = 0.008) was also evident in the nMDS ordination (, lower plot).
Table 1 Zooplankton taxa observed in the Upper Karori Reservoir (22 November 2010 to 23 April 2012).
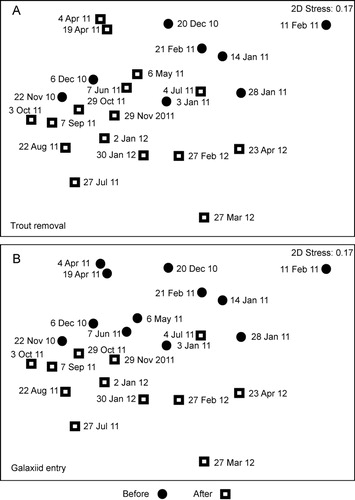
Twenty-three phytoplankton taxa were identified during the study (). The nMDS ordination for phytoplankton illustrated a significant difference in community composition before and after trout removal (, upper plot) as indicated by ANOSIM results (Global R = 0.624, P = 0.001). The nMDS showed a less clear pattern before and after the probable galaxiid spawning date (, lower plot), although ANOSIM results indicated phytoplankton community composition significantly differed before and after July (Global R = 0.179, P = 0.010).
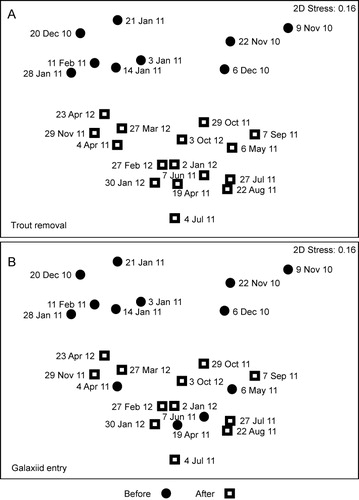
Table 2 Phytoplankton taxa observed in the Upper Karori Reservoir (9 November 2010 to 23 April 2012).
Discussion
Significant shifts were observed in zooplankton and phytoplankton community composition following the removal of brown trout from the Upper Karori Reservoir in February 2011. For zooplankton, the response did not occur immediately. Instead, a shift from crustacean to rotifer dominance occurred several months following trout removal, after July 2011, coinciding with the expected arrival of banded kōkopu larvae into the reservoir. Although we do not have direct measures of the abundances of banded kōkopu, Pham et al. (Citation2013) noted a large increase in recruitment of juveniles to the catchment streams from the Upper Karori Reservoir 1 year following the rotenone application. In that study, banded kōkopu in the streams were dominated by adults prior to trout removal, with only one juvenile collected among 81 individuals. One year post-trout eradication, around 75% of the > 200 banded kōkopu caught were juveniles, indicating a very large increase in recruitment from the reservoir. Limited data exists on the diet of larval and juvenile banded kōkopu. However, Ots & Eldon (Citation1975) found banded kōkopu larvae to average 9.1 mm when newly hatched on 26 June, and by 5 July they were able to feed on Artemia nauplii. Rowe & Dean (Citation1998) found juvenile banded kōkopu (close to 40 mm) readily feed on Daphnia in experimental tanks. Of related species, a large proportion of the diet of juvenile kōaro is planktonic prey, particularly Daphnia (Rowe et al. Citation2002). Similarly, in a South American lake, feeding of inanga on zooplankton indicated that prey preferentially select copepod nauplii as larvae, and cladocerans once above 15 mm. Although rotifers were observed as an additional food item, inanga did not actively select them as prey (Modenutti et al. Citation1993). In the Upper Karori Reservoir, smaller rotifer species generally increased in abundance throughout the study. Feeding on crustaceans likely explains some of the early suppression of crustaceans approximately 1 month following the expected arrival of larvae, and the proceeding relatively rapid change from crustacean to rotifer dominance. Larger zooplankton, such as copepods and cladocerans, are more efficient feeders than smaller species such as rotifers (Hall et al. Citation1976; Mourelatos & Lacroix Citation1990). Both experimental (e.g. Gilbert Citation1988; MacIsaac & Gilbert Citation1991; Nandini et al. Citation2002) and field (Vanni Citation1986; Balvert et al. Citation2009) studies have demonstrated that the presence of cladocerans, in particular, can have profound negative effects on the occurrence of smaller species such as rotifers. For example, following the invasion of Daphnia galeata into Lake Puketirini, New Zealand, many rotifer species were extirpated (Balvert et al. Citation2009). Our results are similar to the observations of Tronstad et al. (Citation2010), who noted that cladocerans increased in importance in Yellowstone Lake, California, following introduction of lake trout (Salvelinus namaycush), which preyed on the zooplanktivorous Yellowstone cutthroat trout (Oncorhynchus clarkia bouvieri). However, our results differ from studies where trout are added to fishless alpine lakes, where trout, by consuming large zooplankton species, enable smaller zooplankton to increase in abundance (e.g. Eby et al. Citation2006). In our study, it is likely that galaxiids have a greater net effect on larger zooplankton than trout, such that trout preying on galaxiids reduce the overall predation pressure on large zooplankton. The shift observed in zooplankton composition immediately following trout removal was more subtle than might be expected if trout had a major direct effect on zooplankton species.
A shift in species composition was observed for phytoplankton in Upper Karori Reservoir immediately following trout removal. Such changes are commonly the result of a direct change in grazing pressure due to changes in zooplankton composition (Ellis et al. Citation2011). For example, Ellis et al. (Citation2011) found changes in zooplankton community composition, and concomitant changes in phytoplankton composition, following introduction of opossum shrimp (Mysis diluviana) to a lake in Montana, USA. They observed declines in large zooplankton species (e.g. cladocerans and adult copepods) and increases in smaller zooplankton (e.g. rotifer species), and increases or decreases in the biomass and/or densities of eight phytoplankton families. However, the marked shift in phytoplankton composition in our study was rapid following trout removal by rotenone treatment, but occurred prior to the more delayed shifts in the zooplankton community. Overall, our results were more congruent with phytoplankton composition shifts being determined by trout removal. A possible explanation is that trout removal altered the nutrient dynamics in the reservoir, affecting phytoplankton composition. Large fish excrete nitrogen and phosphorus at greater rates than small fish (Schindler & Eby Citation1997). Due to smaller fish having larger population sizes, it is difficult to predict the net effect of a shift from trout to galaxiids. However, once trout were removed from the reservoir, a delay existed prior to galaxiid spawning; this suggests that fish would have been absent in the reservoir, or existed in very low numbers, and therefore a probable overall reduction in nutrient recycling and availability would have occurred. Several authors have demonstrated that phytoplankton taxa respond to nutrients recycled by fish, in experiments in the absence of the fish themselves, which may explain some of the apparent top-down effects commonly attributed to them (e.g. Vanni & Layne Citation1997; Vanni & Findlay Citation1990; Attayde & Hansson Citation2001). Further, phytoplankton compositional changes in response to fish-mediated nutrient releases have been observed to be stronger than phytoplankton exposed to contrasting zooplankton assemblages (i.e. through grazing effects of small versus large zooplankton; Vanni & Layne Citation1997). High nutrient release rates have been observed for inanga, due to excretion and egestion under experimental and field conditions (Reissig et al. Citation2003). Recovery of galaxiid populations in the lake may thus increase nutrient recycling in the reservoir, returning phytoplankton composition towards pre-trout removal through time. The change in phytoplankton community due to changes in nutrient dynamics remains a hypothesis here, however, as we do not have data on nutrient species concentrations and dynamics in the reservoir during our study period. In addition, although the nMDS ordination shows clear differences in phytoplankton community composition before and after rotenone addition, we cannot rule out that the changes in algal assemblages, which can be inherently variable, were not due to an unrelated, stochastic event.
An alternative explanation for the observed patterns is that the rotenone applied to the reservoir had direct toxic effects on the zooplankton and phytoplankton communities. Several studies have shown that rotenone has immediate lethal effects on crustacean zooplankton, including extirpation of species that took months or years to recover (e.g. Kiser et al. Citation1963; Beal & Anderson Citation1993). Such direct effects are unlikely to have caused the patterns observed in the current study, as the effects on zooplankton communities were not immediately apparent. Anderson (Citation1970) found that long-term variations in composition and abundance of zooplankton following rotenone application are likely due to changes in competition and predation pressures, rather than to direct effects of rotenone itself. Our results, with a delayed zooplankton response, suggest this to be the case. Although changes in phytoplankton composition occurred immediately following rotenone treatment, phytoplankton are not known to be affected by rotenone directly (e.g. Brown & Ball Citation1943).
Removal of brown trout from New Zealand lakes may have a number of implications for lake management. Removal of trout is likely to ultimately reduce grazing pressure by zooplankton on phytoplankton. Although our results focused on compositional changes, a decrease in abundances of crustacean zooplankton and their associated grazing pressure, may ultimately enhance algal biomass, leading to possible blooms. Larger zooplankton, such as Ceriodaphnia and Boeckella, are known to be more efficient feeders on algae than small species such as rotifers (e.g. Hall et al. Citation1976), which dominated post-trout removal. For example, Balvert et al. (Citation2009) showed that, following the establishment of Daphnia galeata in a New Zealand lake, Secchi transparency was greatly increased; the opposite may occur if population sizes of large zooplankton are reduced. Furthermore, in waterways such as the Upper Karori Reservoir, large non-indigenous zooplankton are more likely to establish than in natural lakes (Banks & Duggan Citation2009; Parkes & Duggan Citation2012). Boeckella minuta, an Australian calanoid copepod with non-indigenous populations in a number of constructed waters in New Zealand, was found to be reduced in abundance following trout removal in Upper Karori Reservoir; this suggests that healthy populations of native fish species, in the absence of trout, might reduce the probabilities of initial establishment of non-native zooplankton species into reservoirs (i.e. provide ‘biotic resistance’; Taylor & Duggan Citation2012). In addition, by reducing the abundances of the non-indigenous zooplankton species at invaded sites, the opportunity for, and propagule supply of, individuals dispersing from invaded reservoirs will be lowered, reducing the probabilities of their establishment in other water bodies.
Acknowledgements
ICD and SAW thank the New Zealand Ministry of Business, Innovation and Employment (UOWX0505; Lake Biodiversity Restoration) for funding. The rotenone operation was led by Zealandia and the Department of Conservation (DOC) with DOC providing resourcing and numerous staff, David Moss being the foremost. We thank John Wood for zooplankton and phytoplankton sample collection and Zealandia staff for assistance throughout this project. We thank the two anonymous referees who made comments that improved our manuscript.
Associate Editor: Dr Joanne Clapcott.
References
- Anderson RS 1970. Effects of rotenone on zooplankton communities and a study of their recovery patterns in two mountain lakes in Alberta. Journal of the Fisheries Research Board of Canada 27: 1335–1356.
- Astwood K, Fell G 2012. Karori water supply dams and reservoirs. IPENZ Engineering Heritage Register Report. Wellington, IPENZ. 28 p.
- Attayde JL, Hansson L-A 2001. The relative importance of fish predation and excretion effects on planktonic communities. Limnology and Oceanography 46: 1001–1012.
- Balvert SF, Duggan IC, Hogg ID 2009. Zooplankton seasonal dynamics in a recently filled mine pit lake: the effect of non-indigenous Daphnia establishment. Aquatic Ecology 43: 403–413.
- Banks CM, Duggan IC 2009. Lake construction has facilitated calanoid copepod invasions in New Zealand. Diversity and Distributions 15: 80–87.
- Beal DL, Anderson RV 1993. Response of zooplankton to rotenone in a small pond. Bulletin of Environmental Contamination and Toxicology 51: 551–556.
- Bergquist AM, Carpenter SR, Latino JC 1985. Shifts in phytoplankton size structure and community composition during grazing by contrasting zooplankton assemblages. Limnology and Oceanography 30: 1037–1045.
- Biggs BJF, Francoeur SN, Huryn AD, Young R, Arbuckle CJ, Townsend CR 2000. Trophic cascades in streams: effects of nutrient enrichment on autotrophic and consumer benthic communities under two different fish predation regimes. Canadian Journal of Fisheries and Aquatic Sciences 57: 1380–1394.
- Brown CJD, Ball RC 1943. An experiment in the use of derris root (rote-none) on the fish and fish-food organisms of third sister lake. Transactions of the American Fisheries Society 72: 267–284.
- Burns CW 1968. The relationship between body size of filter-feeding Cladocera and the maximum size particle ingested. Limnology and Oceanography 13: 675–678.
- Cochran-Stafira DL, von Ende CN 1998. Integrating bacteria into food webs: studies with Sarracenia purpurea inquilines. Ecology 79: 880–898.
- Eby LA, Roach WJ, Crowder LB, Stanford JA 2006. Effects of stocking-up freshwater food webs. Trends in Ecology and Evolution 21: 576–584.
- Ellis BK, Stanford JA, Goodman D, Stafford CP, Gustafson DL, Beauchamp DA et al. 2011. Long-term effects of a trophic cascade in a large lake ecosystem. Proceedings of the National Academy of Sciences 108: 1070–1075.
- Flecker AS, Townsend CR 1994. Community-wide consequences of trout introduction in New Zealand streams. Ecological Applications 4: 798–807.
- Gilbert JJ 1988. Suppression of rotifer populations by Daphnia: a review of the evidence, the mechanisms and the effects on zooplankton community structure. Limnology and Oceanography 6: 1286–1303.
- Gilbert JJ, Stemberger RS 1985. Control of Keratella populations by interference competition from Daphnia. Limnology and Oceanography 30: 180–188.
- Hall DJ, Threlkeld ST, Burns CW, Crowley PH 1976. The size-efficiency hypothesis and the size structure of zooplankton communities. Annual Review of Ecology, Evolution and Systematics 7: 177–203.
- Jellyman PG, McIntosh AR 2008. The influence of habitat availability and adult density on non-diadromous galaxiid fry settlement in New Zealand. Journal of Fish Biology 72: 143–156.
- Kiser RW, Donaldson JR, Olson PR 1963. The effect of rotenone on zooplankton populations in freshwater lakes. Transactions of the American Fisheries Society 92: 17–24.
- MacIsaac HJ, Gilbert JJ 1991. Discrimination between exploitative and interference competition between Cladocera and Keratella cochlearis. Ecology 72: 924–937.
- McDowall RM 1990. When galaxiid and salmonid fishes meet—a family reunion in New Zealand. Journal of Fish Biology 37: 35–43.
- McDowall RM 2003. Impacts of introduced salmonids on native galaxiids in New Zealand upland streams: a new look at an old problem. Transactions of the American Fisheries Society 132: 229–238.
- McDowall RM 2006. Crying wolf, crying foul, or crying shame: alien salmonids and a biodiversity crisis in the southern cool-temperate galaxioid fishes? Reviews in Fish Biology and Fisheries 16: 233–422.
- McIntosh AR, McHugh PA, Dunn NR, Goodman JM, Howard SW, Jellyman PG et al. 2010. The impact of trout on galaxiid fishes in New Zealand. New Zealand Journal of Ecology 34: 195–206.
- Mitchell CP, Penlington BP 1982. Spawning of Galaxias fasciatus Gray (Salmoniformes: Galaxiidae). New Zealand Journal of Marine and Freshwater Research 16: 131–133.
- Modenutti BE, Balseiro EG, Cervellini PM 1993. Effect of the selective feeding of Galaxias maculatus (Salmoniformes, Galaxiidae) on zooplankton of a South Andes lake. Aquatic Sciences 55: 65–75.
- Mourelatos S, Lacroix G 1990. In situ filtering rates of Cladocera: effect of body length, temperature and food concentration. Limnology and Oceanography 35: 1101–1111.
- Nandini S, Sarma SSS, Hurtado-Bocanegra MD 2002. Effect of four species of Cladocerans (Crustacea) on the population growth of Brachionus patulus (Rotifera). Acta Hydrochimica et Hydrobiologica 30: 101–107.
- Ots J-P, Eldon GA 1975. Downstream movement of fry of Galaxias fasciatus Gray. New Zealand Journal of Marine and Freshwater Research 9: 97–99.
- Pace ML, Cole JJ, Carpenter SR, Kitchell JF 1999. Trophic cascades revealed in diverse ecosystems. Trends in Ecology and Evolution 14: 483–488.
- Parkes SM, Duggan IC 2012. Are zooplankton invasions in constructed waters facilitated by simple communities? Diversity & Distributions 18: 1199–1210.
- Pham L, West D, Closs GP 2013. Reintroduction of a native galaxiid (Galaxias fasciatus) following piscicide treatment in two streams: response and recovery of the fish population. Ecology of Freshwater Fish 22: 361–373.
- Reissig M, Queimaliños CP, Balseiro EG 2003. Effects of Galaxias maculatus on nutrient dynamics and phytoplakton biomass in a North Patagonian oligotrophic lake. Environmental Biology of Fishes 68: 15–24.
- Rowe DK, Dean TL 1998. Effects of turbidity on the feeding ability of the juvenile migrant stage of six New Zealand freshwater fish species. New Zealand Journal of Marine and Freshwater Research 32: 21–29.
- Rowe D, Graynoth E, James G, Taylor M, Hawke L 2003. Influence of turbidity and fluctuating water levels on the abundance and depth distribution of small, benthic fish in New Zealand alpine lakes. Ecology Freshwater Fish 12: 216–227.
- Rowe DK, Konui G, Christie KD 2002. Population structure, distribution, reproduction, diet, and relative abundance of koaro (Galaxias brevipinnis) in a New Zealand lake. Journal of the Royal Society of New Zealand 32: 275–291.
- Schindler DE, Eby LA 1997. Stoichiometry of fishes and their prey: implications for nutrient recycling. Ecology 78: 1816–1831.
- Shurin JB, Borer ET, Seabloom EW, Anderso K, Blanchette CA, Broitman B et al. 2002. A cross-ecosystem comparison of the strength of trophic cascades. Ecology Letters 5: 785–791.
- Simon KS, Townsend CR 2003. Impacts of freshwater invaders at different levels of ecological organisation, with emphasis on salmonids and ecosystem consequences. Freshwater Biology 48: 982–994.
- Taylor CM, Duggan IC 2012. Can biotic resistance be utilized to reduce establishment rates of non-indigenous species in constructed waters? Biological Invasions 14: 307–322.
- Townsend CR 2003. Individual, population, community, and ecosystem consequences of a fish invader in New Zealand streams. Conservation Biology 17: 38–47.
- Townsend CR, Crowl TA 1991. Fragmented population structure in a native New Zealand fish: an effect of introduced brown trout? Oikos 67: 347–354.
- Tronstad LM, Hall RO Jr, Koel TM 2010. Introduced lake trout produced a four-level trophic cascade in Yellowstone Lake. Transactions of the American Fisheries Society 139: 1536–1550.
- Utermöhl H 1958. Zur vervollkommnung der quantitativen phytoplankton-methodik. Mitteilungen der Internationale Vereinigung für Theoretische und Angewandte Limnologie 9: 1–38.
- Vanni MJ 1986. Competition in zooplankton communities: suppression of small species by Daphnia pulex. Limnology & Oceanography 31: 1039–1056.
- Vanni MJ, Findlay DL 1990. Trophic cascades and phytoplankton community structure. Ecology 71: 921–937.
- Vanni MJ, Layne CD 1997. Nutrient recycling and herbivory as mechanisms in the ‘top-down’ effect of fish on algae in lakes. Ecology 78: 21–40.
- de Winton M 2013. LakeSPI survey of the Upper Karori Reservoir. NIWA Client Report HAM2013-041. Hamilton, NIWA.
