ABSTRACT
A central question to any understanding of ecology is how animals use their habitat, and how habitat use is influenced by temporally changing features of the environment. Previous research on Hector's dolphins at Akaroa Harbour, New Zealand suggested that dolphins leave inshore, harbour environments during or after rough weather. To test this hypothesis, visual sightings (2000–2012) and acoustic detections (2007–2008) of Hector's dolphins in Akaroa Harbour were modelled to test for a relationship with swell height and swell direction. Sighting rates and acoustic detection rates in Akaroa Harbour were significantly lower on days after big swell events and in some linear models after swell events from the south. These results indicate that swell events influence Hector's dolphin movements in and around Akaroa Harbour. Possible reasons for this behaviour are diverse and need further investigation. However, this information can be used both to predict daily dolphin movement for conservation and research purposes, and to suggest how dolphins may react in future if extreme weather events are becoming frequent.
Introduction
Understanding habitat use, and teasing apart the physical and biological factors that influence animal distributions, are at the very core of ecology. Such studies are useful in predicting animal distributions in a changing environment (Matuszek & Beggs Citation1988; Marsden & Fielding Citation1999; Guisan & Thuiller Citation2005) and are, of course, important for area-based conservation initiatives (e.g. Bailey & Thompson Citation2009). At a small spatial scale, several studies of cetaceans have focused on associations with time of day or tide (Shane Citation1980; Würsig et al. Citation1991; Dawson et al. Citation2013) or phases of the moon (Benoit-Bird et al. Citation2009; Elwen et al. Citation2010). Other studies have investigated the effect of water temperature on dolphin's habitat use (Forney Citation2000; Neumann Citation2001). Fewer studies have examined responses to prey distribution or availability (e.g. Heithaus & Dill Citation2002; Torres et al. Citation2008), no doubt because quantifying prey distributions is very difficult. Surprisingly, very few have examined the effect of wave exposure (see Rayment et al. Citation2015, for an exception).
The habitat preferences of dolphins may be studied via visual surveys (e.g. Bailey & Thompson Citation2006; Izidoro & Le Pendu Citation2012) or passive acoustic monitoring (e.g. Akamatsu et al. Citation2010; Bailey et al. Citation2010). Both methods have their advantages and disadvantages. Data from visual surveys are likely to be biased by weather conditions and observer experience (Barlow Citation1988; Palka Citation1996; Hammond et al. Citation2002). However, detections can be made at relatively large distances and it is possible to quantify the number of dolphins in a group (Rayment et al. Citation2009a). In contrast, echolocation detectors typically have shorter detection ranges and are consequently prone to miss dolphins (Tougaard et al. Citation2006; Akamatsu et al. Citation2010; Elliott et al. Citation2012). Plus, echolocation detectors can usually only measure the presence or absence of dolphins. However, they can record around the clock and are less influenced by weather conditions (Dawson et al. Citation2013). Consequently, a combination of both methods provides an attractive option for investigating habitat utilisation by dolphins.
Hector's dolphin (Cephalorhynchus hectori) is endemic to the coastal waters of New Zealand (Baker Citation1987). It is the smallest of the delphinids, with a total length of 1.2–1.6 m (Slooten Citation1991). Population viability analyses indicate that Hector's dolphin abundance has been declining since 1970 (Slooten & Davies Citation2011). The major anthropogenic threats to the species are commercial and amateur gillnetting and commercial trawling (Dawson Citation1991; Dawson & Slooten Citation1993; Department of Conservation & Ministry of Fisheries Citation2007). The IUCN Red List of Threatened Species lists Hector's dolphin as endangered, with a decreasing population trend (Reeves et al. Citation2014).
High densities of Hector's dolphins are found in the coastal waters of Banks Peninsula, on the east coast of New Zealand's South Island (Dawson & Slooten Citation1993). Within Akaroa Harbour, dolphin distribution shows a strongly seasonal pattern, and can vary substantially from day to day (Dawson Citation1991; Rayment et al. Citation2010a; Dawson et al. Citation2013). For example, Dawson et al. (Citation2013) investigated the effects of time of day and tidal state on Hector's dolphin habitat use. For another, similarly coastal species, the Guiana dolphin (Sotalia guianensis), it has been suggested that use of near-shore habitats is negatively correlated with swell height, although sighting rates could be confounded by sighting conditions (Izidoro & Le Pendu Citation2012). The aim of this study is to investigate whether swell height and direction are important factors in explaining day-to-day variability in abundance of Hector's dolphins in Akaroa Harbour.
Materials and methods
Sighting data were collected on fixed standardised zigzag surveys of Akaroa Harbour described in Dawson (Citation1991), Rayment (Citation2008) and Rayment et al. (Citation2009a) (). The vessels used were either c. 6 m outboard powered open boats, surveying at 10–15 knots, or 9.5–15 m multihull yachts (under power) travelling at 7–10 knots. Survey tracks were recorded on a GPS-linked palmtop computer running custom written software. Each dolphin sighting was recorded on the computer, including information about group size, GPS position and time of encounter (Rayment et al. Citation2009a).
Figure 1. Example of a typical day of survey effort. Only dolphin sightings in the box inside Akaroa Harbour were used in this analysis.
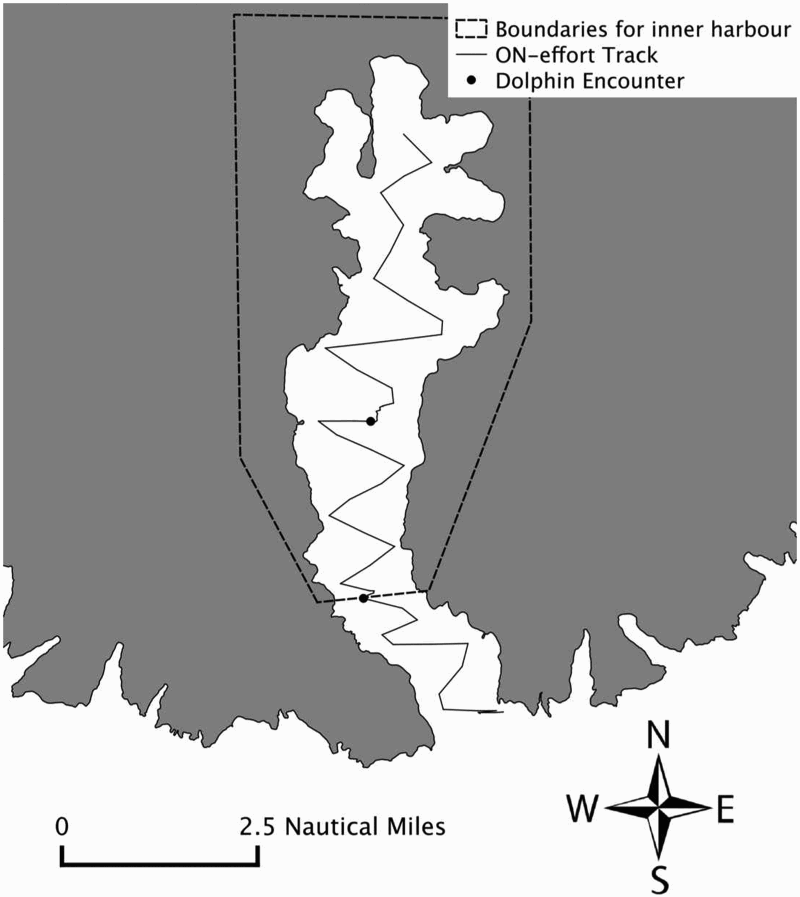
Because our question centred on whether dolphins leave Akaroa Harbour in certain swell conditions, we restricted our analysis to survey effort and sightings from well within the harbour (i.e. the inner and middle harbour; ). The analysis was further restricted to days with ≥5 nautical miles (n. miles) of survey effort within these boundaries. This restriction was necessary since there were a few surveys with an early abortion due to external reasons. Logically, these surveys mostly had no dolphin sightings, however there might have been some in the bay (false zeros).
Average sighting rates (number of dolphins sighted per n. mile of survey effort) within Akaroa Harbour were calculated for each day of boat-based observations from March 2000 to February 2012. Distance travelled was used as a measure of survey effort, rather than time spent surveying, because we typically slowed to conduct photo-ID when dolphins were encountered. Hence, a disproportionate amount of time was sometimes spent within a small area.
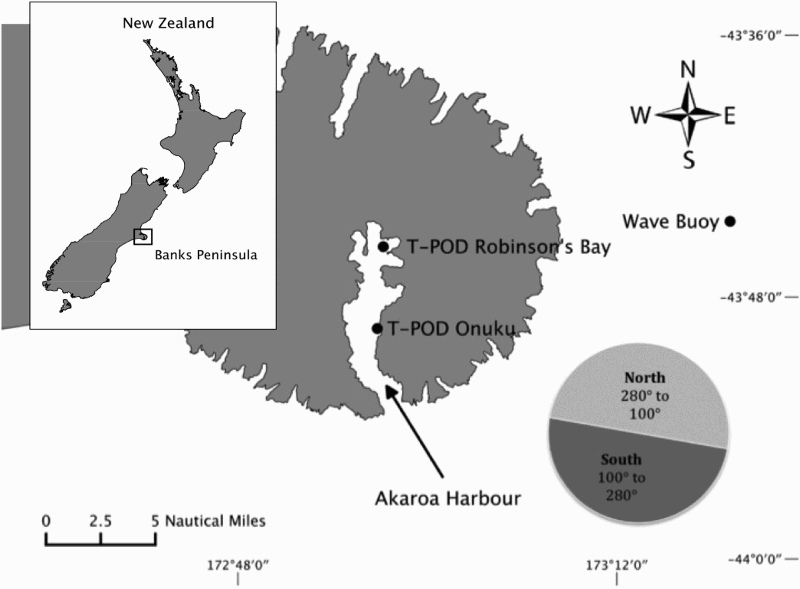
Passive acoustic monitoring data were gathered using T-PODs (Chelonia Ltd)—acoustic click detectors that have proved successful for monitoring the presence of Hector's dolphins (Rayment et al. Citation2010b) and showed a mean detection distance of 200 m (Rayment et al. Citation2009b). The T-POD data used here were from a study of seasonal movements within Akaroa Harbour in which three T-PODs were moored in the inner (Robinsons Bay), middle (Onuku) and outer harbour from 16 February 2007 until 18 February 2008 (Dawson et al. Citation2013) (). In this paper, we use the data from the two inner T-PODs (expressed as dolphin-positive minutes per day Rayment et al. Citation2010b) to augment the data from zigzag sighting surveys.
Figure 2. Map of Banks peninsula including Akaroa Harbour, the position of the wave buoy and the position of the T-PODs. Swell was separated by direction into swell that can run into Akaroa Harbour and swell that cannot. Therefore, swell from 280° to 100° was considered as swell from the north and swell from 100° to 280° as swell from the south.
The relationships between dolphin sighting rates or dolphin-positive minutes and environmental conditions were investigated using generalised least square (GLS) method models (e.g. Zuur et al. Citation2009). Nine models were built in total, three to explore the effect of swell height and direction on dolphin sighting rate, and six to examine the effect of swell height and direction on dolphin-positive minutes. Since the two T-PODs were moored in different locations in the harbour, we chose to model the dolphin-positive minutes separately. Furthermore, sighting rates and dolphin-positive minutes were modelled separately using swell information from three different days, as explained below. To account for the seasonal variation in dolphin abundance, month was implemented as an explanatory variable in the six dolphin-positive minutes models. This additional explanatory variable was not necessary for the sighting rate models. Due to a lack of winter sighting data, only summer data (October–March) were used for the sighting rate models.
Information about swell direction and swell height were obtained from a wave buoy (Environment Canterbury Citation2012) located about 9 n. miles east of Banks Peninsula (). Even though the swell was measured well outside Akaroa Harbour, it is obvious that the swell situation inside is related to the outside. However, swell can be a lot smaller inside especially on swells from the north. Unfortunately, the buoy did not register swell data on every day. Thus, not every day with dolphin data could be included in the analyses.
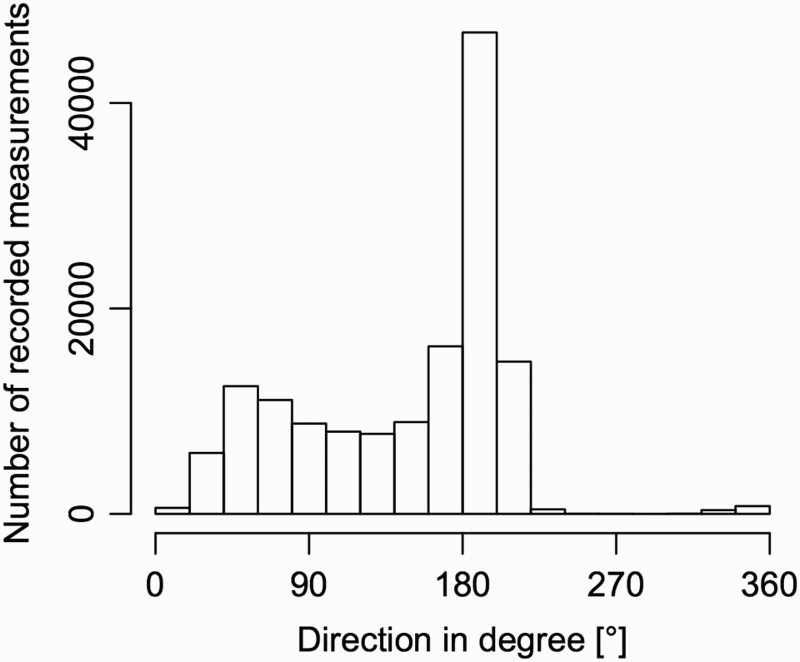
Swell data were aggregated per day (0000–2400 h). For swell height, the maximum wave height per day was used. For swell direction, each day was classified as having predominant swell from the north, south or with mixed swells. In order to distinguish swells that can run into the south-facing harbour from swells that cannot, swells from the directions between 280° to 100° were classified as from the north, whereas swells from 100° to 280° were classified as from the south (). Days with swell from both directions were classified as mixed swell periods. The pattern of swell directions around Banks Peninsula () shows a dominant peak between 140° and 220° and another smaller peak between 40° and 100°. This pattern shows that the dominant swell directions hitting the wave buoy are north-northwest and south-southwest.
Figure 3. Frequency of swell directions recorded by the wave buoy off Banks Peninsula from March 2000 to February 2012.
Swell height can affect the ability of researchers to spot dolphins and consequently directly affect sighting rate (Barlow Citation1988; Palka Citation1996; Hammond et al. Citation2002; Evans & Hammond Citation2004). Therefore, to minimise this observer effect we have only used sighting rate data when there was no swell ≥2 m recorded by the wave buoy during the boat surveys (between 0700 and 1600 h of the observation day). However, since T-PODs are not prone to observer effects in high swells, the dolphin-positive minutes were modelled versus swell conditions without any restrictions on swell height.
As mentioned earlier, we modelled both sighting rate and dolphin-positive minutes separately versus the swell conditions from three different days. Both the acoustic and visual data were modelled versus swell on the same day, 1 day prior and 2 days prior to the T-POD recording or boat-based observations. These different days were chosen to account for a possible temporal delay in the dolphins’ reaction to swell conditions.
For the sighting rate models, there were 259 days available with more than 5 n. miles of survey effort within Akaroa Harbour in the 12 year period between 1 March 2000 and 29 February 2012. Of these days, 213 days were during summer with swell information available. Of the 213 days, 119 had no swell ≥2 m recorded by the wave buoy during boat-based observation. At each of the two T-POD locations, there were data recorded from 353 days with T-POD and swell information.
Non-generalised linear regression models rely on normally distributed residuals (Zuur et al. Citation2009). However, both the sighting rate data and the T-POD data were rather negative binomially distributed. To satisfy this assumption of normality, the logarithm of sighting rate and the logarithm of dolphin-positive minutes were used as dependent variables. Furthermore, linear regression models rely on independent data points. However, in particular the T-POD data were temporally dependent. To account for temporal autocorrelation in the models an auto-regressive moving average (ARMA; p = 1, q = 1) error structure was implemented (Zuur et al. Citation2009). Another assumption of linear regression models is a homogeneous variance of the residuals throughout all values of the independent variables. Neither the sighting rate data nor the T-POD data had a homogeneous variance. However, this problem can be solved by allowing for different variances per variable value (Zuur et al. Citation2009). The variance in all models decreased with an increasing swell height and changed per month in the T-POD models. Therefore, an exponential variance structure based on swell height was implemented in all models and a fixed variance structure based on month was used in the T-POD models (Example R-Code S1). Statistical analyses were performed with the program R version 3.1.3 (R Core Team Citation2014). For data processing, analysis and graphics the packages plyr (Wickham Citation2011), reshape (Wickham Citation2007), car (Fox & Weisberg Citation2011), nlme (Pinheiro et al. Citation2014), ggpot (Wickham Citation2009), directlabels (Hocking Citation2013) and gridExtra (Auguie Citation2012) were used.
Results
Sighting rate
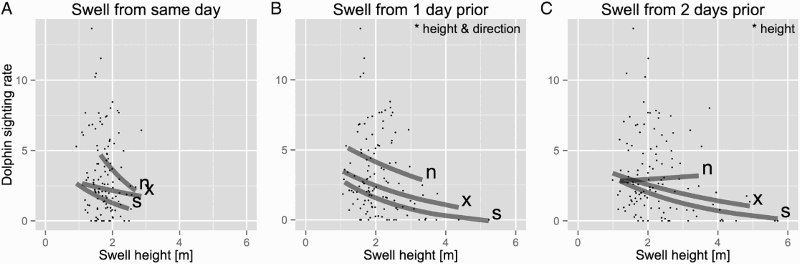
The linear models showed no significant effect of swell height on sighting rate on the same day as the boat-based observations (GLS, F1,115 = 1.85, P = 0.18). However, swell height 1 day prior (GLS, F1,115 = 10.00, P < 0.01) and 2 days prior (GLS, F1,115 = 10.19, P < 0.01) to the boat-based observations had a significant negative effect on the transformed sighting rate. The effect of swell direction on the transformed sighting rate was significant from 1 day prior to the boat-based observations (GLS, F2,115 = 6.20, P < 0.01). Sighting rates were lowest on days following southerly swells (). Swell direction from the same day (GLS, F2,115 = 2.74, P = 0.07) and 2 days prior (GLS, F2,115 = 1.77, P = 0.17) had no significant effect ().
Figure 4. Sighting rates of Hector's dolphins (Cephalorhynchus hectori) plotted against swell height and direction on: A, the same day; B, 1 day prior; and C, 2 days prior to the observation. Grey lines present the linear models based on different swell directions (n = north, x = mixed, s = south). Swell height of the same day as the boat observation had no significant effect on the transformed sighting rate. However, swell height 1 day prior and 2 days prior to the boat observation had a significant negative effect on the transformed sighting rate. The effect of swell direction on the transformed sighting rate was significant for swells from 1 day prior to the boat observation only. Swell direction of the same day and 2 days prior had no significant effect. Mixed swell directions are days with swell from both the north and the south.
T-POD data
The linear models for both Robinson's Bay and Onuku showed no significant effect of either swell height (Robinson's Bay: GLS, F1,338 = 1.32, P = 0.25; Onuku: GLS, F1,338 = 0.09, P = 0.77) or swell direction (Robinson's Bay: GLS, F2,338 = 2.17, P = 0.12; Onuku: GLS, F2,338 = 2.12, P = 0.12) from the same day as the transformed dolphin-positive minutes. However, swell height 1 day prior (Robinson's Bay: GLS, F1,338 = 9.97, P < 0.02; Onuku: GLS, F1,338 = 5.54, P = 0.02) and 2 days prior (Robinson's Bay: GLS, F1,338 = 16.05, P < 0.01; Onuku: GLS, F1,338 = 5.91, P = 0.02) to the recording day had a significant negative effect at both locations.
Swell direction had a significant effect from 1 day prior at Robinson's Bay only (GLS, F2,338 = 3.11, P < 0.05). Dolphin-positive minutes were fewest on days following southerly swells (). Swell direction was not significant in any of the other T-POD models (Robinson's Bay: 2 days prior: GLS, F2,338 = 0.06, P < 0.93; Onuku: 1 day prior: GLS, F2,338 = 1.08, P = 0.34; Onuku: 2 days prior: GLS, F2,338 = 0.46, P = 0.63; –).
Figure 5. Dolphin-positive minutes of Hector's dolphins (Cephalorhynchus hectori) recorded at Robinson's Bay plotted against swell height and swell direction of: A, the same day; B, 1 day prior; and C, 2 days prior to the T-POD recording. Grey lines present the linear models based on different swell directions (n = north, x = mixed, s = south). The linear models showed no significant effect of swell height from the same day on dolphin-positive minutes. Swell height 1 day prior and 2 days prior to the recording had a significant negative effect on the transformed dolphin-positive minutes. Only swell direction from 1 day prior to the T-POD recording had a significant effect on the positive minutes. Neither swell direction of the same day nor of 2 days prior to the recording showed a significant effect. Mixed swell directions are days with swell from both the north and the south.
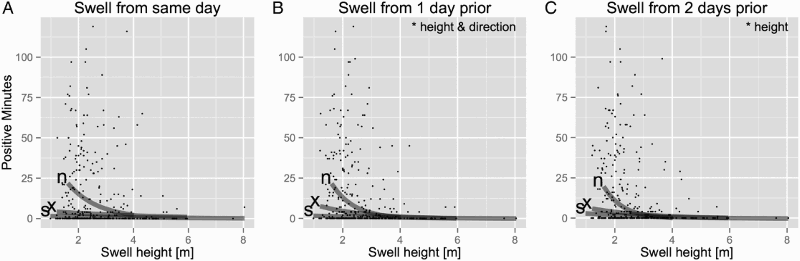
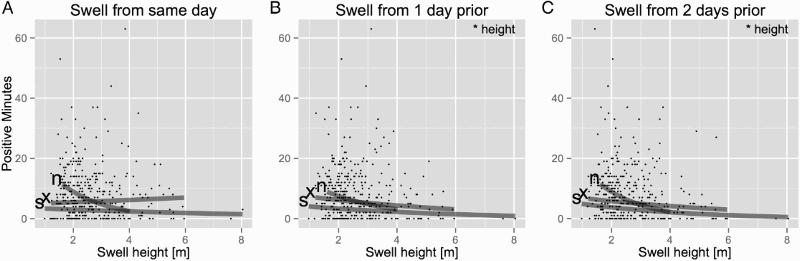
Figure 6. Dolphin-positive minutes of Hector's dolphins (Cephalorhynchus hectori) recorded at Onuku plotted against swell height and swell direction of: A, the same day; B, 1 day prior; and C, 2 days prior to the T-POD recording. Grey lines present the linear models based on different swell directions (n = north, x = mixed, s = south). The linear models showed no significant effect of swell height from the same day on dolphin-positive minutes. Swell height 1 day prior and 2 days prior to the recording had a significant negative effect on the transformed dolphin-positive minutes. Neither swell direction of the same day nor of 1 day prior nor of 2 days prior to the recording showed a significant effect at Onuku. Mixed swell directions are days with swell from both the north and the south.
Discussion and conclusions
The number of dolphin sightings inside Akaroa Harbour and the rate of acoustic detection at Robinson's Bay and Onuku were significantly affected by swell height on the two previous days. Fewer dolphins were sighted and acoustically detected after days of big swells. That the same pattern was evident in both the visual and acoustic data sets indicates that it is not an artefact of poor sighting conditions on days with significant swell.
The Guiana dolphin (S. guianensis) is found off South America and prefers a coastal habitat like the Hector's dolphin. Izidoro & Pendu (Citation2012) noticed in a visual quantitative analysis that this species was sighted significantly more often close to shore on days with small swells than on days with high swells. However, they could not prove that this swell effect on the sighting rate was not due to poor sighting conditions in higher waves (observer effect). However, by placing limits on environmental conditions and using acoustic detection data, this study has shown that the relationship between Hector's dolphin habitat use and swell is not due to compromised sighting ability.
These results differ from those observed in other studies (e.g. Sauter Citation1999; Karczmarski et al. Citation2000; Gannier & Petiau Citation2006) which found no effect of swell conditions on dolphin movements. Furthermore, studies such as Ersts & Rosenbaum (Citation2003), Elwen & Best (Citation2004) and Rayment et al. (Citation2015) showed contrary results. These studies suggest that some larger cetacean species actively choose more sheltered habitats at certain life stages, for example during calf rearing.
It is possible, even likely, that Hector's dolphins are not responding to swell directly, but are responding to a third factor (e.g. prey abundance or foraging success rate). Wave action is a major cause of increased turbidity (Uncles & Stephens Citation1997; Browne et al. Citation2013). Hector's dolphins feed on squid such as arrow squid (Nototodarus sp.) and on fish such as red cod (Pseudophycis bachus) and ahuru (Auchenoceros punctatus) (Miller et al. Citation2013). Large swell events could potentially increase turbidity more outside the sheltered harbour than inside, either attracting fish (Sazima Citation1986) or making it easier for dolphins to prey on species that have to rely on visual detection of predators and consequently have a decreased reaction distance in turbid water (Miner & Stein Citation1996). This idea is supported by Miller & Baltz (Citation2010) who found that bottlenose dolphins (Tursiops truncatus) showed increased foraging behaviour in turbid waters. The Heaviside's dolphin (Cephalorhynchus heavisidii) is closely related to the Hector's dolphin and shares its preference to coastal waters. Elwen et al. (Citation2010) observed higher densities of Heaviside's dolphins in areas exposed to swell compared with more protected areas. However, this result appears to be a general habitat preference rather than a small-scale movement depending on swell size.
Swell size from the same day as the visual and acoustic surveys had no significant effect in any model. One potential explanation is that there might be a lag between the onset of a swell event and its effect (e.g. increased turbidity). Another possible explanation would be that dolphins need some time to detect habitat changes plus some time to travel.
In every calculated model, the fitted sighting rate and the fitted acoustic detection rate were smallest on and after days with swell from the south and were particularly small at the inner-most location (Robinson's Bay). However, this effect was significant in two of nine models only. A possible effect of swell direction on Hector's dolphin movements could in part be due to the shape of Banks Peninsula. The southern side of the peninsula, which includes Akaroa Harbour, is likely to be sheltered from northerly swells. Therefore, any effect of swell height (on turbidity for example) will be more pronounced in southerly swell events.
The results of this paper raise the question why dolphin movement is triggered by the swell situation. Ample further research is necessary to understand the ecological connection between wave situation and dolphin movement. The information that dolphin movements are triggered by swell height and direction can be seen as pure ecological knowledge but could as well be used for scientific application and conservation. Being able to predict dolphin movement could make research on these animals more time and cost efficient. Gillnetting and commercial trawling is the major threat for the Hector's dolphins. Predictions on dolphin movements could decrease fisheries' bycatch by an opposed habitat use of fisherman and dolphins. If there are similar connections between swell situations and other marine mammals, it is likely that research and conservation of these species could also benefit from similar studies.
Even though visual observation and echolocation detectors rely on different methods to quantify dolphin habitat selection, both methods yielded comparable results in this study. This reinforces the conclusions of the study, and provides confidence that either measure provides an unbiased picture of habitat use by Hector's dolphins.
Supplementary data
R-Code S1. Model example (.R file).
R-Code S1. Model example (.R file).
Download R Objects File (411 B)Acknowledgements
Many thanks for the opportunity to work in the marine mammal research group of the University of Otago, including help from Anthony Davidson and Jennifer Turek. We thank Daryl Coup for the development of the Hector's dolphin database and Jörg Ganzhorn for his supervision from the University of Hamburg. We thank Environment Canterbury and Bruce Gabites for their support and for the wave buoy data.
Associate Editor: Dr Bruce Robertson.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Akamatsu T, Nakamura K, Kawabe R, Furukawa S, Murata H, Kawakubo A, Komaba M. 2010. Seasonal and diurnal presence of finless porpoises at a corridor to the ocean from their habitat. Mar Biol. 157:1879–1887. doi: 10.1007/s00227-010-1459-8
- Auguie B. 2012. gridExtra: functions in grid graphics. R package version 0.9.1.
- Bailey H, Clay G, Coates EA, Lusseau D, Senior B, Thompson PM. 2010. Using T-PODs to assess variations in the occurrence of coastal bottlenose dolphins and harbour porpoises. Aquat Conser Mar Freshw Ec. 20:150–158. doi: 10.1002/aqc.1060
- Bailey H, Thompson P. 2006. Quantitative analysis of bottlenose dolphin movement patterns and their relationship with foraging. J Anim Ecol. 75:456–465. doi: 10.1111/j.1365-2656.2006.01066.x
- Bailey H, Thompson PM. 2009. Using marine mammal habitat modelling to identify priority conservation zones within a marine protected area. Mar Ecol Prog Ser. 378:279–287. doi: 10.3354/meps07887
- Baker A. 1987. The status of hector's dolphin, Cephalorhynchus hectori (Van Beneden) in New Zealand waters. R Int Whal Comm. 28:331–334.
- Barlow J. 1988. Harbour porpoise, Phocoena phocoena, abundance estimation for California, Oregon and Washington. I. Ship Surveys. Fish Bull. 86:419–432.
- Benoit-Bird KJ, Dahood AD, Würsig B. 2009. Using active acoustics to compare lunar effects on predator-prey behavior in two marine mammal species. Mar Ecol Prog Ser. 395:119–135. doi: 10.3354/meps07793
- Browne NK, Smithers SG, Perry CT. 2013. Spatial and temporal variations in turbidity on two inshore turbid reefs on the great barrier reef, Australia. Coral Reefs. 32:195–210. doi: 10.1007/s00338-012-0965-1
- Dawson S, Fletcher D, Slooten E. 2013. Habitat use and conservation of an endangered dolphin. End Spe Res. 21:45–54. doi: 10.3354/esr00508
- Dawson SM. 1991. Incidental catch of Hector's dolphin in inshore gillnets. Mar Mammal Sci. 7:283–295. doi: 10.1111/j.1748-7692.1991.tb00103.x
- Dawson SM, Slooten E. 1993. Conservation of Hector's dolphins: the case and process which led to establishment of the banks peninsula marine mammal sanctuary. Aquat Conserv Mar Freshw Ec. 3:207–221. doi: 10.1002/aqc.3270030305
- Department of Conservation, Ministry of Fisheries. 2007. Hector's and Maui's dolphin threat management plan for public consultation. Wellington: DOC, MPI; [cited 2013 March 17]. Available from: www.fish.govt.nz/en-nz/Consultations/Archive/2008/Hectors+dolphins/Threat+Management+Plan.htm
- Elliott RG, Dawson SM, Rayment WJ. 2012. Optimizing T-pod settings and testing range of detection for bottlenose dolphins in doubtful sound, New Zealand. J Mar Biol Assoc UK. 92(Special Issue 08):1901–1907. doi: 10.1017/S002531541100035X
- Elwen SH, Best PB. 2004. Environmental factors influencing the distribution of southern right whales (Eubalaena australis) on the south coast of South Africa II: within bay distribution. Mar Mammal Sci. 20:583–601. doi: 10.1111/j.1748-7692.2004.tb01181.x
- Elwen SH, Thornton M, Reeb D, Best PB. 2010. Near-shore distribution of Heaviside's (Cephalorhynchus heavisidii) and dusky dolphins (Lagenorhynchus obscurus) at the southern limit of their range in South Africa. Afr Zool. 45:78–91. doi: 10.3377/004.045.0103
- Environment Canterbury. 2012. Wave buoy [Internet]. [cited 2012 June 15]. Available from: http://ecan.govt.nz/services/online-services/monitoring/coastal-monitoring/wave-buoy/Pages/Default.aspx
- Ersts PJ, Rosenbaum HC. 2003. Habitat preference reflects social organization of humpback whales (Megaptera novaeangliae) on a wintering ground. J Zool. 260:337–345. doi: 10.1017/S0952836903003807
- Evans PGH, Hammond PS. 2004. Monitoring cetaceans in European waters. Mammal Rev. 34:131–156. doi: 10.1046/j.0305-1838.2003.00027.x
- Forney KA. 2000. Environmental models of cetacean abundance: reducing uncertainty in population trends. Conserv Biol. 14:1271–1286. doi: 10.1046/j.1523-1739.2000.99412.x
- Fox J, Weisberg S. 2011. An {R} companion to applied regression. 2nd ed. Thousand Oaks, CA: Sage.
- Gannier A, Petiau E. 2006. Environmental variables affecting the residence of spinner dolphins (Stenella longirostris) in a bay of Tahiti (French Polynesia). Aquat Mammal. 32:202–211. doi: 10.1578/AM.32.2.2006.202
- Guisan A, Thuiller W. 2005. Predicting species distribution: offering more than simple habitat models. Ecol Lett. 8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x
- Hammond PS, Berggren P, Benke H, Borchers DL, Collet A, Heide-Jørgensen MP, Heimlich S, Hiby AR, Leopold MF, Øien N. 2002. Abundance of harbour porpoise and other cetaceans in the North Sea and adjacent waters. J Appl Ecol. 39:361–376. doi: 10.1046/j.1365-2664.2002.00713.x
- Heithaus MR, Dill LM. 2002. Food availability and tiger shark predation risk influence bottlenose dolphin habitat use. Ecology. 83:480–491. doi: 10.1890/0012-9658(2002)083[0480:FAATSP]2.0.CO;2
- Hocking TD. 2013. directlabels: Direct labels for multicolor plots in lattice or ggplot2. R package version 2013.6.15.
- Izidoro FB, Le Pendu Y. 2012. Estuarine dolphins (Sotalia guianensis) in Porto de Ilheus, Brazil: group characterization and response to ships. North-West J Zool. 8:232–240.
- Karczmarski L, Thornton M, Cockcroft VG. 2000. Daylight occurrence of humpback dolphins Sousa chinensis in Algoa Bay, South Africa. Afr J Ecol. 38:86–90. doi: 10.1046/j.1365-2028.2000.00215.x
- Marsden S, Fielding A. 1999. Habitat associations of parrots on the Wallacean islands of Buru, Seram and Sumba. J Biogeogra. 26:439–446. doi: 10.1046/j.1365-2699.1999.00308.x
- Matuszek J, Beggs G. 1988. Fish species richness in relation to lake area, pH, and other abiotic factors in Ontario lakes. Can J Fish Aquat Sci. 45:1931–1941. doi: 10.1139/f88-225
- Miller CE, Baltz DM. 2010. Environmental characterization of seasonal trends and foraging habitat of bottlenose dolphins (Tursiops truncatus) in northern Gulf of Mexico bays. Fish Bull. 108:79–86.
- Miller E, Lalas C, Dawson S, Ratz H, Slooten E. 2013. Hector's dolphin diet: the species, sizes and relative importance of prey eaten by Cephalorhynchus hectori, investigated using stomach content analysis. Mar Mammal Sci. 29:606–628.
- Miner JG, Stein RA. 1996. Detection of predators and habitat choice by small bluegills: effects of turbidity and alternative prey. T Am Fish Soc. 125:97–103. doi: 10.1577/1548-8659(1996)125<0097:DOPAHC>2.3.CO;2
- Neumann DR. 2001. Seasonal movements of short-beaked common dolphins (Delphinus delphis) in the north-western Bay of Plenty, New Zealand: influence of sea surface temperature and El Niño/La Niña. New Zeal J Mar Freshwat Res. 35:371–374. doi: 10.1080/00288330.2001.9517007
- Palka D. 1996. Effects of beafort sea state on the sightability of harbour porpoises in the gulf of Maine. Report Int Whal Comm. 46:575–582.
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. 2014. nlme: linear and nonlinear mixed effects models. R package version 3.1–117.
- R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available from: http://www.R-project.org
- Rayment W, Dawson S, Slooten E. 2010a. Seasonal changes in distribution of Hector's dolphin at Banks Peninsula, New Zealand: implications for protected area design. Aquat Conserv Mar Freshw Ec. 20:106–116.
- Rayment W, Dawson S, Slooten E. 2010b. Use of T-PODs for acoustic monitoring of Cephalorhynchus dolphins: a case study with Hector's dolphins in a marine protected area. End Spec Res. 10:333–339. doi: 10.3354/esr00189
- Rayment W, Dawson S, Slooten E, Bräger S, Fresne SD, Webster T. 2009a. Kernel density estimates of alongshore home range of Hector's dolphins at Banks Peninsula, New Zealand. Mar Mammal Sc. 25:537–556. doi: 10.1111/j.1748-7692.2008.00271.x
- Rayment W, Dawson S, Slooten L. 2009b. Trialling an automated passive acoustic detector (T-POD) with Hector's dolphins (Cephalorhynchus hectori). J Mar Biol Assoc UK. 89(Special Issue 05):1015–1022. doi: 10.1017/S0025315409003129
- Rayment W, Dawson S, Webster T. 2015. Breeding status affects fine-scale habitat selection of southern right whales on their wintering grounds. J Biogeogr. 42:463–474. doi: 10.1111/jbi.12443
- Rayment WJ. 2008. Distribution and ranging of Hector's dolphins: implications for protected area design [Unpublished PhD thesis]. Dunedin: University of Otago.
- Reeves RR, Dawson SM, Jefferson TA, Karczmarski L, Laidre K, O’Corry-Crowe G, Rojas-Bracho L, Secchi ER, Slooten E, Smith BD et al. 2013. Cephalorhynchus hectori [Internet]. The IUCN Red List of Threatened Species 2013; e.T4162A44199757. [cited 2014 May 21]. Available from: http://dx.doi.org/10.2305/IUCN.UK.2013-1.RLTS.T4162A44199757.en
- Sauter S. 1999. Spinner dolphins (Stenella longirostris) and environmental conditions. Hilo, HI: Department of Marine Science, University of Hawai'i at Hilo.
- Sazima I. 1986. Similarities in feeding behaviour between some marine and freshwater fishes in two tropical communities. J Fish Biol. 29:53–65. doi: 10.1111/j.1095-8649.1986.tb04926.x
- Shane S. 1980. Occurrence, movements, and distribution of bottlenose dolphin, Tursiops truncatus, in southern Texas. Fish Bull. 78:593–691.
- Slooten E. 1991. Age, growth, and reproduction in Hector's dolphins. Can J Zool. 69:1689–1700. doi: 10.1139/z91-234
- Slooten E, Davies N. 2011. Hector's dolphin risk assessments: old and new analyses show consistent results. J R Soc New Zeal. 42:49–60. doi: 10.1080/03036758.2011.606820
- Torres LG, Read AJ, Halpin P. 2008. Fine-scale habitat modeling of a top marine predator: do prey data improve predictive capacity. Ecol Appl. 18:1702–1717. doi: 10.1890/07-1455.1
- Tougaard J, Rosager Poulsen L, Amundin M, Larsen F, Rye J, Teilmann J. 2006. Detection function of T-PODs and estimation of porpoise densities. In: Leeney RH, Tregenza NJC, editors. Proceedings of the Workshop: static acoustic monitoring of cetaceans. Held at the 20th Annual Meeting of the European Cetacean Society, Gdynia, Poland, 2 April 2006. ECS Newsletter no. 46 (Special issue); p. 7–13.
- Uncles RJ, Stephens JA. 1997. Dynamic of turbidity in the tweed estuary. Estuar Coast Shelf Sci. 45:745–758. doi: 10.1006/ecss.1997.0232
- Wickham H. 2007. Reshaping data with the reshape package. J Stat Softw. 21(12):1–20. doi: 10.18637/jss.v021.i12
- Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York, NY: Springer.
- Wickham H. 2011. The split-apply-combine strategy for data analysis. J Stat Softw. 40:1–29.
- Würsig B, Cipriano F, Würsig M. 1991. Dolphin movement patterns: information from radio and theodolite tracking studies. In: Pryor K, Norris KS, editors. Dolphin societies: discoveries and puzzles. Berkeley, CA: University of California Press; p. 79–111.
- Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer-Verlag.
