ABSTRACT
The grounding of the MV Rena in 2011 necessitated urgent entry of salvage vessels into New Zealand. Two, a cargo barge and tug, had been idle in Australia before mobilisation and had well-developed biofouling assemblages that included non-indigenous species new to New Zealand. Surveillance for the species was initiated in Tauranga harbour, Astrolabe Reef and Mōtītī Island, where the vessels had operated. Response personnel were trained to recognise and report the organisms and active surveys were implemented, including reinspection of the vessels after treatment of the biofouling, dive and shoreline searches, and trapping. Although none of the risk species was detected, the biosecurity risk from the vessels changed during the response, from initially acting as a vector for species new to New Zealand to later transporting non-indigenous species from coastal ports to the arena of operations. The study highlights a need for better integration of biosecurity risk management into forward planning for maritime incidents.
Introduction
The grounding or sinking of a vessel presents a range of threats to marine environments. These can include the direct mechanical impacts of the grounding and associated debris on marine habitats and organisms (Marshall & Edgar Citation2003; Work et al. Citation2008; Lirman et al. Citation2010); liberation of oil (Edgar & Barrett Citation2000; Peterson et al. Citation2003; Yamamoto et al. Citation2003) and other contaminants from the ship's cargo (Brondi et al. Citation1981; Dollar & Grigg Citation1981; Hawkins et al. Citation1991) or its antifouling coatings (Negri et al. Citation2002; Smith et al. Citation2003); and the effects of clean-up efforts themselves on ecological assemblages (Pezeshki et al. Citation2000).
An often overlooked risk is the inadvertent release of stowaway pest organisms. Although the phenomenon of ‘rat spill’—the introduction of rodents to isolated islands from shipwrecks—is relatively well known (Ebbert et al. Citation2007), response and recovery plans for serious maritime accidents rarely consider the potential for the introduction of other types of risk organism such as non-indigenous weeds, insects or marine organisms, despite their association with shipping (Carlton & Geller Citation1993; Stanaway et al. Citation2001; Mack Citation2003; Work et al. Citation2005).
Non-indigenous marine organisms are spread by shipping, within ballast water and as biofouling attached to submerged surfaces of vessels (Ruiz et al. Citation1997). Foundering of a vessel can increase the risk that these organisms will establish local populations. Organisms attached to or within the stricken vessel may be released or shed offspring into the surrounding environment (Wotton et al. Citation2004; Wanless et al. Citation2010). Other vessels brought in to assist with the response can, themselves, transport risk organisms to the site from overseas or elsewhere within the region. Over time, the surfaces of wrecks can also be important beach-heads for the proliferation of non-indigenous species and their spread to surrounding natural habitats (Clapin & Evans Citation1995; LaValle et al. Citation1999; Sheehy & Vik Citation2010; Sampaio et al. Citation2012).
The grounding of the 38,788 GT container vessel, MV Rena, on Astrolabe Reef (Otaiti), New Zealand on 5 October 2011 triggered a whole-of-government response to manage the environmental effects of the incident (Schiel et al. Citation2016). Vessels mobilised to the site during the course of the response and salvage included offshore supply ships and tugs, a bunkering tanker, a naval fuel tanker, shear leg and cargo barges, oil recovery vessels, mussel barges, naval patrol vessels and a heavy crane ship (Murdoch Citation2013). Some entered New Zealand waters under urgency to assist. During this phase, the Ministry for Primary Industries (MPI) provided support to assess biosecurity risks from the vessels arriving from overseas (McDonald et al. Citation2012). Two in particular were identified as potentially high risk: a cargo barge and its support tug, which were brought in to remove containers from the stricken Rena. Slow-speed service vessels, such as barges and tugs, can accumulate heavy biofouling because they spend long periods at anchor or laid-up. Their slow steaming speed also means that many organisms can survive the relatively low sheer stress experienced during voyages (Lewis et al. Citation2006; Coutts et al. Citation2010; Hopkins & Forrest Citation2010).
The barge and tug were mobilised from Port Curtis, Australia (23°49′48″S, 151°15′10″E) on 15 October 2011. Prior to that, they had been idle and heavily ballasted in Port Curtis for up to 2 years (McDonald et al. Citation2012). Based on the information provided to MPI on the antifouling coatings and dry docking history, MPI advised that the vessels be inspected for macrofouling upon arrival to New Zealand. Here, we describe the inspection and attempted treatment of these vessels to mitigate biosecurity risks and surveys implemented in environments surrounding the incident site to determine if non-indigenous species associated with them established local populations.
Materials and methods
Inspection and treatment of the barge and tug
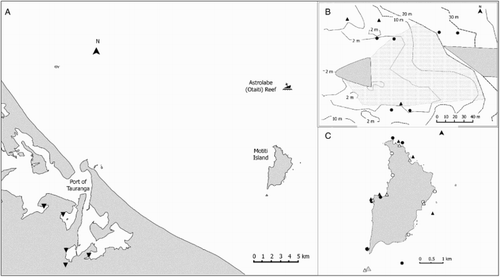
The tug (length 29 m, beam 9 m, GRT 254 mt) and cargo barge (length 85.3 m, beam 24.4 m, DWT 6000 mt, GRT 2772 mt) arrived in the Port of Tauranga on 27 October 2011 and remained there until 16 November 2011, when containers began to be removed from the Rena. During salvage operations, the vessels moved between the Port of Tauranga, Astrolabe Reef and Mōtītī Island (), where they occasionally anchored during unfavourable weather or down-time.
Figure 1. A, Area of operations of the barge and tug in Tauranga harbour, Mōtītī Island and Astrolabe Reef denotes the locations of pitfall trapping; B, Location of dive searches at Astrolabe Reef in May (▴) and October (●) 2013. The shaded area depicts the bow and stern sections of the Rena. The debris zone is shown as stipple; C, Locations of dive (●) and shoreline searches at Mōtītī in May (▴) and October (○) 2013.
Inspections of the vessels’ hulls were undertaken in the Port of Tauranga shortly after their arrival (28 October 2011), with the primary focus being an inspection of the barge. Divers surveyed four longitudinal transects along the port side of the barge's hull, from stern to bow. Each transect was c. 4 m wide and spaced c. 2 m apart. Safety concerns prevented divers inspecting the starboard side of the barge while it was secured to the wharf. Video was taken along two transects and representative samples were collected of biofouling encountered. No samples were obtained from the mid-point transect as water currents were too strong to retain the specimens safely. A full bow-to-stern transect (3 m width) was also surveyed above the waterline, in a visible zone of biofouling that had been submerged when the barge was ballasted (the ‘boot top’; ). Samples were also collected from heavily fouled ‘niche’ areas that included the footholds located towards the stern. An opportunistic visual inspection along the waterline was also made of the tug and any visible biofouling was collected. Fourteen bulk samples were removed from the barge and a single specimen of red alga was collected from the tug. Each sample was preserved and identified by taxonomic experts.
Figure 2. Zone of barnacle fouling where the barge was previously ballasted, taken from stern to bow. Footholds are visible on the side of the barge. This zone was sampled during the horizontal waterline transect.

The red alga was provisionally identified as a non-indigenous species from the genus Grateloupia that had not previously been reported from New Zealand. This genus contains a number of invasive species (D'Archino Citation2011). MPI subsequently commissioned treatment of the tug using low-pressure hot water and manual removal of the alga by divers (Stratford Citation2011). To minimise the impact of the treatment on salvage operations, the treatment occurred on 12–13 December 2011, when the tug was not required for operations. No treatment was undertaken of the barge.
The tug was reinspected on 24 August 2012 to evaluate the effectiveness of the heat treatment on the biofouling on the hull. Five transects were surveyed along the length of the hull. Transects were located at the waterline on both sides of the vessel, and at intervals of 1 m below the starboard waterline (along the vertical hull), 2 m below the waterline (along the hull and bilge keel) and 3 m below waterline (along the keel). In addition, the propeller, rudder and sea chest gratings were each inspected. A separate visual inspection was also made of the portside waterline of the barge. Strong currents prevented divers from undertaking a full inspection. Six samples were removed from the tug by hand and a single sample was collected from the barge.
Surveillance for marine pests
Because non-indigenous species were found on the tug and barge, it was decided to implement additional monitoring. This included enhanced passive surveillance, extending existing surveillance, trapping within Tauranga harbour and dive surveys at the locations where these vessels were active. This included within Tauranga harbour, at Astrolabe Reef and Mōtītī Island, where the barge was often moored during salvage operations. Details of each are described below.
Enhancing passive surveillance
A workshop on 20 September 2012 provided training in identification of high-risk marine pests to contractors and volunteers involved in the salvage and environmental recovery. Preserved specimens, colour images and identification guides were used to demonstrate key diagnostic features of species that are the target of the National Marine High Risk Site Surveillance (NMHRSS, see below) and five risk species identified from inspections of the barge and tug. These were the mangrove crabs, Metopograpsus latifrons and M. frontalis, the bivalves, Patro australis and Neotrapezium sublaevigatum, and the red alga Grateloupia sp. filicina-type. Identification guides developed for these species were disseminated to all participants. The workshop also outlined procedures for preserving and reporting suspect specimens encountered during the clean-up and recovery.
Extending the NMHRSS
The NMHRSS is undertaken every 6 months in Tauranga harbour. Its primary objective is to detect incursions to New Zealand of five non-indigenous organisms listed on the New Zealand Unwanted Organisms Register: the Northern Pacific seastar, Asterias amurensis; European shore crab, Carcinus maenas; the marine aquarium weed, Caulerpa taxifolia; Chinese mitten crab, Eriocheir sinensis; and Asian clam, Potamocorbula amurensis. It is also tasked with detecting incursions by other non-indigenous organisms not known to be present in New Zealand and detecting range extensions by species that have already established populations in parts of New Zealand (Morrisey et al. Citation2013). The surveys use a risk-based sampling design, with sample effort allocated from a survey grid relative to the distribution of suitable habitat and likely locations of first arrival for the five primary target species (Inglis et al. Citation2006; Morrisey et al. Citation2013). Sampling includes a combination of baited crab traps (n = 240 sets per survey), unbaited crab shelters (n = 24 sets), benthic sled tows (n = 100), visual searches by divers (n = 30 sites) and shoreline searches (n = 25 sites).
NMHRSS surveys of Tauranga harbour in September 2012 and January 2013 were also tasked with detecting the five risk species recorded from inspections of the barge and tug (described above). An extra 15 sites were also surveyed by divers within the Port of Tauranga and Mount Maunganui Mātaitai (a reserve surrounding Mount Maunganui that recognises traditional Māori fishing grounds that are important for customary food gathering) and 20–31 additional shoreline sites were searched on each survey. The shoreline searches were of intertidal habitats close to the port that were suitable for the five risk species found on the vessels; namely, mangroves, intertidal oyster reefs, shell debris and artificial structures (wharf piles, pontoons and breakwalls).
Trapping for M. frontalis and M. latifrons
Grapsid mangrove crabs can be difficult to sample because they are very mobile and may be cryptic or in burrows when inactive. Pitfall traps are the most effective way to estimate their abundance and diversity (Salgado-Kent & McGuinness Citation2006). We established two trapping grids, each containing five pitfall traps, at four mangrove sites in Tauranga harbour: Matua, Otumoetai, Fraser Cove and Welcome Bay (a). The sites were chosen for their proximity to areas where the barge and tug had operated in the Port of Tauranga. The traps were constructed of plastic plant pots (c. 15 cm diam. × 17 cm deep) with a 1 mm mesh attached to the bottom to allow drainage. Funnels were fitted to the tops to prevent escape. Each trap was buried so that the rim was flush with the surface of the mud. Traps were deployed after spring tides when the crabs are most active (Frusher et al. Citation1994). They were cleared after 24 h and 48 h. Captured crabs were removed, identified in situ (where possible) and any specimens resembling M. frontalis or M. latifrons were photographed and retained for expert identification.
Two trapping surveys were implemented: between 31 October and 2 November 2012 and between 11 and 13 March 2013. On each occasion, timed visual searches (10 min) were also undertaken within the mangrove fringe for any tree-climbing crabs that resembled M. frontalis and M. latifrons.
Surveys of Astrolabe Reef and Mōtītī Island
Surveys of Mōtītī Island were targeted at locations and habitats where the barge and tug had operated or moored. They included artificial structures (pilings, jetties and a fishing boat wreck) and natural reef habitats. Sites for survey were chosen after consultations with Te Ahikaaroa o Mōtītī who provided advice on the anchoring locations and areas of concern for kaimoana. A hui at the island's marae before implementation of the surveys outlined the purpose of the surveys and provided training in identification of the species. Members of the Mōtītī Island community accompanied the field team during the surveys to assist with access to sites and the shoreline searches.
Searches of the shoreline were made at six intertidal locations between 11 April and 10 May 2013 and again on 3 October 2013 (c). Two of the intertidal sites were searched by divers in April 2013 because of unfavourable tides. Dive searches were also undertaken at six subtidal locations during each survey period.
Dive searches were made at three locations near the wreck site on Astrolabe Reef in May 2013 and in October 2013. The sites were chosen in consultation with the salvors to ensure they were close to the wreck site, but would not jeopardise the divers or interfere with ongoing salvage operations. The locations varied slightly between the two surveys because of restrictions around the operating salvage vessel. In May, all sites were located on rocky reef at the edge of the debris field. Each site was searched for 20 min by a pair of divers (b). Transects (20 m) were laid along the contours of the reef at depths of 10–15 m and at 25 m. A 10 min search was also completed in macroalgal habitats near the reef top (c. 5 m depth). In October, the sites were located at 5 m, 15 m depth and in the debris field at 20 m depth. Two 30 m transects were surveyed in the debris field and scoured reef top. Wreck debris (aluminium ingots, scrap metal, wire, steel) was also inspected for the target species and recent settlement of biofouling.
Results
Inspection and treatment of the barge and tug
During the initial survey in October 2011, biofouling covered c. 90% of the ‘boot top' on the portside of the barge. The fouling consisted mostly of dead barnacles, mussels (Modiolus sp. and Mytilus sp.) and oysters (principally Crassostrea gigas). However, organisms located closer to the unballasted waterline and/or in the recessed footholds were still alive at the time of the survey. This area also contained encrusting bryozoans, hydroids and Anomiidae oysters. Biofouling on the submerged hull of the barge was patchy and covered <5% of the available surface. It consisted mostly of small patches of hydroids and acorn barnacles. Fourteen taxa were identified from the samples (). These included 11 non-indigenous taxa, only one of which (C. gigas) was known to be established in New Zealand waters. Six of the taxa had not previously been recorded in New Zealand ().
Table 1. Species recorded during inspections of the barge and tug in October 2011 and August 2012. Species that are first records for New Zealand are highlighted in bold.
Biofouling along the waterline of the tug consisted predominantly of filamentous green algae and small patches of a non-indigenous species of Grateloupia that had not previously been recorded from New Zealand and which was subsequently identified as G. subpectinata (Nelson et al. Citation2013; ).
The barge remained heavily fouled when it was reinspected in August 2012. Biofouling along the waterline comprised mostly algae, barnacles, encrusting bryozoans and bivalves (). None of the non-indigenous species recorded in the initial survey was recorded. Instead, the assemblage was dominated by native New Zealand species (seven taxa) and two non-indigenous species—the kelp Undaria pinnatifida and bryozoan Tricellaria catalinensis—that are established in New Zealand ports and harbours ().
Reinspection of the tug 8 months after the hot-water treatment revealed patches of filamentous green algae, dead/decomposing hydroids and goose barnacles at the waterline (). Grateloupia subpectinata was still present in patches along both sides of the vessel, as were large U. pinnatifida sporophytes. The hull was also fouled with hydroids, oysters and mussels, particularly along the dry dock support strips on the keel (). The assemblage was dominated by native taxa (13 species) and six non-indigenous species that are present in New Zealand ports (). The bilge keel on the starboard side was heavily fouled with decomposing hydroids and algae, living barnacles and hundreds of the native crab, Guinusia chabrus (). The sea chest was also heavily fouled with hydroids and mussels. Following the detection of G. subpectinata on the tug during the August 2012 inspection, MPI undertook a second response assessment and appropriate action was subsequently taken (in this case in-water cleaning using a contained system whereby all biofouling material removed was captured).
Additional surveillance in Tauranga
The two NMHRSS surveys of Tauranga harbour were completed in September 2012 and January 2013. None of the NMHRSS primary target species nor any of the five risk species from the vessels was detected. Undaria pinnatifida was recorded at two dive sites at the western end of the Port of Tauranga in September 2012 and at three dive sites and two shore search locations in this area in January 2013.
Pitfall trapping for mangrove crabs
The pitfall traps were effective at capturing mangrove crabs, with an average of between 16–80 crabs caught per trap in each survey. In total, more crabs (n = 2404 individuals) were caught during the sampling in March 2013 than in November 2012 (n = 1812; ANOVA: F1,4 = 12.3, P = 0.003). This was due mostly to greater abundance of the native stalk-eyed mud crab, Hemiplax hirtipes, in March (ANOVA: F1,4 = 39.8, P = 0.0001). All of the crabs caught were native species, with the most abundant being the burrowing mud-crab, Austrohelice crassa (67% of individuals caught) and H. hirtipes (29%). No Metopograpsus frontalis or M. latifrons were observed in the traps or during timed searches.
Targeted dives and shoreline surveys
None of the NMHRSS target species or the five risk species from the barge or tug was found in dive surveys and shoreline searches of Mōtītī Island and Astrolable Reef. Six suspect samples of red alga collected during the surveys of Mōtītī were subsequently identified as native species: Pterocladia lucida, Melanthalia abscissa, Gigartina clavifera, Plocamium costatum and Delisea elegans. Two samples of red alga collected from Astrolabe Reef in May 2013 and a single red alga collected in October 2013 were identified as Plocamium costatum and Pterocladia lucida, respectively.
Discussion and conclusions
Numerous recent studies have highlighted the potential for invasive species to be introduced into sensitive marine environments by maritime accidents. For example, Wanless et al. (Citation2010) described the stranding of a heavily fouled decommissioned submersible petroleum platform on the remote island of Tristan da Cunha. At least 62 taxa recorded from the platform were not indigenous to the archipelago. In New Zealand, the invasive alga U. pinnatifida was successfully removed from a fishing trawler that sank at the Chatham Islands (Wotton et al. Citation2004). More recently, debris from a vessel wrecked in the Pacific that drifted close to the northern coastline of New Zealand in 2007 contained up to 76 taxa, including 28 non-indigenous species (Williams et al. Citation2008). Comparable reports of the transport of non-indigenous species by vessel debris have also occurred on the west coast of North America following the 2011 Japanese tsunami (Calder et al. Citation2014). In the Galapagos Islands, Marshall & Edgar (Citation2003) observed dense blooms of filamentous algae and the fouling hydroids Ectopleura media and Pennaria disticha surrounding the grounded coastal tanker, Jessica. Although it was initially feared that E. media had been introduced by the accident, it was subsequently shown to be indigenous to the Galapagos. Its rapid proliferation on the wreckage and surrounding bare substratum was, however, characteristic of opportunistic biofouling organisms.
Our study shows that the biosecurity risks associated with maritime accidents are not restricted to the prone vessel. Salvage and support vessels brought into the arena of operations may, themselves, bring unwanted species. The barge and tug surveyed on arrival in New Zealand contained a range of species that were not present in New Zealand waters. This included the mangrove crabs Metopograpsus latifrons and M. frontalis and the red alga Grateloupia subpectinata.
Although the mangrove crabs have a largely tropical, Indo-Pacific distribution, it is possible they may be able to survive in northern New Zealand. Neither species has a track record of invasion and, to our knowledge, this is the first time they have been recorded outside their natural geographic range. Their potential impacts on New Zealand native species and environments are unclear. Both are opportunistic omnivores although Poon et al. (Citation2010) suggested that M. frontalis may be an important predator within mangrove ecosystems of Hong Kong. Unlike native New Zealand mangrove crabs, both species are tree climbers. Metopograpsus latifrons is a specialised climber that often climbs into the mangrove canopy during high tide (Fratini et al. Citation2005), whereas M. frontalis is more likely to occur among the roots and lower trunk.
Grateloupia subpectinata is probably a native of the northwest Pacific (Japan, Korea and China) and appears to have been introduced to Britain, France and Australia (Nelson et al. Citation2013). The variable morphology of this genus, particularly the finely branched forms, makes identification difficult and as a consequence, the biology and ecology of individual species is not well resolved. Grateloupia species have formed nuisance blooms in the past. On exposed coasts in Japan, G. subpectinata tends to grow in tide pools in the mid to lower intertidal, whereas in calm coastal environments it is restricted to the lower intertidal (Faye et al. Citation2004).
Because of the size of the salvage barge, there were few options available to remove the biofouling from it once it had entered the country. An attempt to remove biofouling from the tug using low-pressure hot water did not eliminate G. subpectinata. Repeat surveys of the vessels also showed how the nature of the biosecurity risk to environments in the area of salvage operations changed over the course of the response as the composition of the biofouling assemblage on them changed. When the vessels were inspected after almost a year of operations, biofouling assemblages on their hulls were dominated by native taxa and by non-indigenous species (U. pinnatifida, Grateloupia turuturu and Tricellaria catalinensis) that were already present in some New Zealand ports, but which had not yet invaded offshore islands and reefs surrounding the wreck site. Establishment of U. pinnatifida on natural reefs is often mediated by disturbances that remove the existing canopy of native algae or grazers (Valentine & Johnson Citation2003). Scouring of the reef top at Astrolabe Reef by the bow of the Rena could provide an opportunity for these species to gain a foothold. Given the longevity of the gametophyte stage of U. pinnatifida (Schaffelke et al. Citation2005), continued monitoring of the wreck site is recommended to guard against this eventuality.
Active surveillance in Tauranga harbour, Mōtītī Island and Astrolabe Reef over 12 months did not detect any of the species of concern. However, given the size of the arena of operations for the vessels, the level of surveillance effort that could be achieved in that time was modest and we would encourage ongoing vigilance. While implementing the surveys, we also sought to increase the knowledge and capability of others involved in the Rena response to enhance passive surveillance for the potential pests. However, by itself, surveillance cannot prevent unwanted species from becoming established and efforts to remove them once they have arrived are technically difficult and costly. Provisions need to be made to include the assessment of biosecurity risk within the emergency response planning for similar transportation accidents so that risks can be identified early and mitigation strategies put in place. These should include provisions for vessels operating in the response zone to have effective biofouling and ballast water management plans (IMO Citation2009) to ensure that the environmental consequences of the accident are not compounded by the introduction of harmful species from other coastal or international locations. In the time since these inspections were undertaken, MPI has released a Craft Risk Management Standard (CRMS) for vessel biofouling, which sets the permissible level of hull fouling and acceptable measures for meeting the requirements for vessels entering New Zealand waters. This standard will become compulsory in 2018.
Acknowledgements
Preparation of the manuscript was supported by the National Institute of Water and Atmospheric Research under Coasts and Oceans Programme 6: 2014/15 SCI. We would like to thank the following people for their assistance in implementing the surveys: Caine Taiapa and students from Te Māuri Moana Partnership, Rangi Butler (Te Ahikaaroa o Mōtiti), Bradley Tong, Ian Clark and Ajay Prasad (Resolve Salvage and Fire Ltd), Richard Calvert and Peter Stratford (AsureQuality), Dane Buckthought, Rod Budd, Caroline Chin, Scott Edhouse, Weno Iti, Crispin Middleton, Don Morrisey, Darren Parsons, Apanui Skipper and Caroline Williams (NIWA).
Guest Editor: Dr Philip Ross.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Brondi M, Dall'aglio M, Ghiara E, Mignuzzi C, Tiravanti G. 1981. Environmental studies on lead alkyl release in sea water by the Cavtat wreck. Sci Total Environ. 19:21–31. doi: 10.1016/0048-9697(81)90115-7
- Calder DR, Choong HH, Carlton JT, Chapman JW, Miller JA, Geller J. 2014. Hydroids (Cnidaria: Hydrozoa) from Japanese tsunami marine debris washing ashore in the northwestern United States. Aquat Invasions. 9:425–440. doi: 10.3391/ai.2014.9.4.02
- Carlton JT, Geller JB. 1993. Ecological roulette: the global transport of nonindigenous marine organisms. Science. 261:78–82. doi: 10.1126/science.261.5117.78
- Clapin G, Evans DR. 1995. The status of the introduced marine fanworm Sabella spallanzanii in Western Australia: a preliminary investigation. Technical Report No. 2. Hobart, TAS: CSIRO Centre for Research on Introduced Marine Pests (CRIMP).
- Coutts ADM, Piola RF, Hewitt CL, Connell SD, Gardner JP. 2010. Effect of vessel voyage speed on survival of biofouling organisms: implications for translocation of non-indigenous marine species. Biofouling. 26:1–13. doi: 10.1080/08927010903174599
- D'Archino R. 2011. A non-indigenous species of Grateloupia sp. with affinities to G. filicina complex (Halymeniales, Halymeniaceae). Marine Exotic Species Note. 62:1–4.
- Dollar SJ, Grigg RW. 1981. Impact of a kaolin clay spill on a coral reef in Hawaii. Mar Biol. 65:269–276. doi: 10.1007/BF00397121
- Ebbert SM, Sowls AL, Byrd GV. 2007. Alaska's rat spill response program. In: Witmer GM, Pitt WC, Fagerstone KA, editors. Managing vertebrate invasive species: proceedings of an international symposium. Fort Collins, CO: USDA/APHIS/WS, National Wildlife Research Center; p. 332–337.
- Edgar GJ, Barrett NS. 2000. Impact of the Iron Baron oil spill on subtidal reef assemblages in Tasmania. Mar Pollut Bull. 40:36–49. doi: 10.1016/S0025-326X(99)00101-0
- Faye EJ, Wang HW, Kawaguchi S, Shimada S, Masuda M. 2004. Reinstatement of Grateloupia subpectinata (Rhodophyta, Halymeniaceae) based on morphology and rbcL sequences. Phycol Res. 52:59–67. doi: 10.1111/j.1440-1835.2004.tb00316.x
- Fratini S, Vannini M, Cannicci S, Schubart CD. 2005. Tree-climbing mangrove crabs: a case of convergent evolution. Evol Ecol Res. 7:219–233.
- Frusher S, Giddins R, Smith T. 1994. Distribution and abundance of grapsid crabs (Grapsidae) in a mangrove estuary: effects of sediment characteristics, salinity tolerances, and osmoregulatory ability. Estuar Coast. 17:647–654. doi: 10.2307/1352412
- Hawkins JP, Roberts CM, Adamson T. 1991. Effects of a phosphate ship grounding on a Red Sea coral reef. Mar Pollut Bull. 22:538–542. doi: 10.1016/0025-326X(91)90892-V
- Hopkins GA, Forrest BM. 2010. A preliminary assessment of biofouling and non-indigenous marine species associated with commercial slow-moving vessels arriving in New Zealand. Biofouling. 26:613–621. doi: 10.1080/08927014.2010.502963
- IMO. 2009. Development of international measures for minimizing the transfer of invasve aquatic specis through bio-fouling of ships. Research review—bio-fouling as a mechanism for invasive aquatic species transfer. London: Sub-committee on Bulk Liquids and Gases, BLG 13/INF.3, International Maritime Organization.
- Inglis GJ, Hurren H, Gust N, Oldman J, Fitridge I, Floerl O, Hayden B. 2006. Surveillance design for early detection of unwanted exotic marine organisms in New Zealand. Biosecurity New Zealand Technical Paper No. 2005-17. Wellington: Ministry of Agriculture and Forestry.
- LaValle PD, Brooks A, Lakhan VC. 1999. Zebra mussel wastes and concentrations of heavy metals on shipwrecks in western Lake Erie. J Great Lakes Res. 25:330–338. doi: 10.1016/S0380-1330(99)70741-0
- Lewis P, Bergstrom D, Whinam J. 2006. Barging in: a temperate marine community travels to the subantarctic. Biol Invasions. 8:787–795. doi: 10.1007/s10530-005-3837-6
- Lirman D, Gracias N, Gintert B, Gleason ACR, Deangelo G, Dick M, Martinez E, Reid RP. 2010. Damage and recovery assessment of vessel grounding injuries on coral reef habitats by use of georeferenced landscape video mosaics. Limnol Oceanogr: Methods. 8:88–97. doi: 10.4319/lom.2010.8.0088
- Mack RN. 2003. Global plant dispersal, naturalization, and invasion: pathways, modes, and circumstances. In: Ruiz GM, Carlton JT, editors. Invasive species: vector and management strategies. Washington, DC: Island Press; p. 3–30.
- Marshall PA, Edgar GJ. 2003. The effect of the Jessica grounding on subtidal invertebrate and plant communities at the Galápagos wreck site. Mar Pollut Bull. 47:284–295. doi: 10.1016/S0025-326X(03)00157-7
- McDonald S, Kluza D, Wilkens S. 2012. Identifying post-border risks: surveillance of barge finds unwanted marine hitch-hikers. Surveillance. 39(2): 32–34.
- Morrisey D, Seaward K, Inglis G. 2013. Marine high-risk site surveillance: annual report for all ports and marinas 2012/2013 (Project 12099). Technical Paper 2013/55. Wellington: Ministry for Primary Industries.
- Murdoch S. 2013. Independent review of Maritime New Zealand's response to the MV Rena incident on 5 October 2011. Wellington: Maritime New Zealand.
- Negri AP, Smith LD, Webster NS, Heyward AJ. 2002. Understanding ship-grounding impacts on a coral reef: potential effects of anti-foulant paint contamination on coral recruitment. Mar Pollut Bull. 44:111–117. doi: 10.1016/S0025-326X(01)00128-X
- Nelson WA, Kim Su Y, D'Archino R, Boo Sung M. 2013. The first record of Grateloupia subpectinata from the New Zealand region and comparison with G. prolifera, a species endemic to the Chatham Islands. Bot Mar. 56:507–513. doi: 10.1515/bot-2013-0059
- Peterson CH, Rice SD, Short JW, Esler D, Bodkin JL, Ballachey BE, Irons DB. 2003. Long-term ecosystem response to the Exxon Valdez oil spill. Science. 302:2082–2086. doi: 10.1126/science.1084282
- Pezeshki SR, Hester MW, Lin Q, Nyman JA. 2000. The effects of oil spill and clean-up on dominant US Gulf coast marsh macrophytes: a review. Environ Pollut. 108:129–139. doi: 10.1016/S0269-7491(99)00244-4
- Poon DYN, Chan BKK, Williams GA. 2010. Spatial and temporal variation in diets of the crabs Metopograpsus frontalis (Grapsidae) and Perisesarma bidens (Sesarmidae): implications for mangrove food webs. Hydrobiologia. 638:29–40. doi: 10.1007/s10750-009-0005-5
- Ruiz GM, Carlton JT, Grosholz ED, Hines AH. 1997. Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent and consequences. Amer Zoo. 37:621–632. doi: 10.1093/icb/37.6.621
- Salgado-Kent C, McGuinness KA. 2006. A comparison of methods for estimating relative abundance of grapsid crabs. Wet Ecol Manag. 14:1–9. doi: 10.1007/s11273-004-5075-6
- Sampaio CL, Miranda RJ, Maia-Nogueira R. 2012. New occurrences of the nonindigenous orange cup corals. Check List. 8:528–530.
- Schaffelke B, Campbell ML, Hewitt CL. 2005. Reproductive phenology of the introduced kelp Undaria pinnatifida (Phaeophyceae, Laminariales) in Tasmania, Australia. Phycologia. 44:84–94. doi: 10.2216/0031-8884(2005)44[84:RPOTIK]2.0.CO;2
- Schiel DR, Ross PM, Battershill CN. 2016. Environmental effects of the MV Rena shipwreck: cross-disciplinary investigations of oil and debris impacts on a coastal ecosystem. New Zeal J Mar Freshwat Res. 50:1–9.
- Sheehy DJ, Vik SF. 2010. The role of constructed reefs in non-indigenous species introductions and range expansions. Ecol Eng. 36:1–11. doi: 10.1016/j.ecoleng.2009.09.012
- Smith LD, Negri AP, Philipp E, Webster NS, Heyward AJ. 2003. The effects of antifoulant-paint-contaminated sediments on coral recruits and branchlets. Mar Biol. 143:651–657. doi: 10.1007/s00227-003-1107-7
- Stanaway M, Zalucki M, Gillespie P, Rodriguez C. 2001. Pest risk assessment of insects in sea cargo containers. Aust J Entomol. 40:180–192. doi: 10.1046/j.1440-6055.2001.00215.x
- Stratford P. 2011. Biosecurity Operations Report: treatment of fouling on the tug boat, PB Katea. Ministry of Agriculture and Forestry, Investigation Number 2750. Report prepared for MAF. Tauranga, Bay of Plenty: AsureQuality Ltd.
- Valentine JP, Johnson CR. 2003. Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. J Exp Mar Biol Ecol. 295:63–90. doi: 10.1016/S0022-0981(03)00272-7
- Wanless R, Scott S, Sauer WH, Andrew TG, Glass JP, Godfrey B, Griffiths C, Yeld E. 2010. Semi-submersible rigs: a vector transporting entire marine communities around the world. Biol Invasions. 12:2573–2583. doi: 10.1007/s10530-009-9666-2
- Williams R, Gould B, Christian S. 2008. Shipwrecks–an international biosecurity risk? Surveillance. 35:4–6.
- Work TM, Aeby GS, Maragos JE. 2008. Phase shift from a coral to a corallimorph-dominated reef associated with a shipwreck on Palmyra Atoll. PLoS ONE. 3(8):1–5. doi:10.1371/journal.pone.0002989.
- Work TT, McCollough DG, Cavey JF, Komsa R. 2005. Arrival rate of nonindigenous insect species into the United States through foreign trade. Biol Invasions. 7:323–332. doi: 10.1007/s10530-004-1663-x
- Wotton D, O'Brien C, Stuart M, Fergus D. 2004. Eradication success down under: heat treatment of a sunken trawler to kill the invasive seaweed Undaria pinnatifida. Mar Pollut Bull. 49:844–849. doi: 10.1016/j.marpolbul.2004.05.001
- Yamamoto T, Nakaoka M, Komatsu T, Kawai H, Ohwada K, Marine Life Research Group of Takeno. 2003. Impacts by heavy-oil spill from the Russian tanker Nakhodka on intertidal ecosystems: recovery of animal community. Mar Pollut Bull. 47:91–98. doi: 10.1016/S0025-326X(03)00051-1
