ABSTRACT
A contemporary approach to the assessment of metal pollutants in aquatic environments has been to measure contaminant concentrations in biological indicator species (mussels, oysters). However, for environments in which such indicator species do not occur naturally and cannot be deployed, alternative approaches for monitoring trace-metal pollution are required. Three years after the 2011 grounding of the MV Rena, diffusive gradients in thin films (DGT) samplers were deployed at Otaiti (the offshore reef where the Rena grounded) to assess levels of waterborne trace-metal contamination. Although the probability of detecting waterborne contaminants within a dynamic open ocean reef environment would seem low, due to mixing and dilution, our analyses confirmed the presence of copper (≤0.43 vs ≤0.27 nmol kg−1), iron (≤6.3 vs ≤4.2 nmol kg−1), aluminium (≤8.9 vs ≤1.3 nmol kg−1), zinc (≤2.8 vs ≤2.0 nmol kg−1) and manganese (≤0.11 vs ≤0.09 nmol kg−1) at elevated concentrations in the Rena debris field relative to reference sites. The results demonstrate the utility of DGTs as a tool for measuring waterborne contaminants and suggest further research is required to characterise the contaminant plume and determine what effect it might have on the recovery of Otaiti's impacted biological communities.
Introduction
Oceanic environments worldwide have been impacted by the introduction of a range of anthropogenically generated organic and inorganic contaminants (Han et al. Citation2002; Islam & Tanaka Citation2004). Of the different types of contaminants entering marine ecosystems, metals are of particular concern because of their potential to affect marine organisms even at low concentrations (Duruibe et al. Citation2007; Boyd Citation2010; He et al. Citation2013). Metals exist naturally within the ocean, both in the water column and in sediments (Bruland et al. Citation2013; Dimitrakakis et al. Citation2014), hence any assessment of anthropogenically mediated impacts of metals needs to be set in the context of natural background levels. Although many metals, for example copper (Cu), nickel (Ni) and iron (Fe), are used by marine biota in trace quantities for metabolic processes, in excess, they can cause a range of lethal and sublethal effects (Rainbow Citation2002). Increasingly, there is concern about the consequences of anthropogenic metal contamination and a desire to better understand the pathways by which metals can be transported into marine ecosystems (Han et al. Citation2002; McIlgorm et al. Citation2011; He et al. Citation2013).
Shipwrecks are one potential mechanism by which comparatively large quantities of anthropogenic metals can enter coastal ecosystems (Dimitrakakis et al. Citation2014; Ross et al. Citation2016). Ship groundings often result in severe but localised ecological damage caused by the physical disturbance of a ship-grounding event and subsequent movement of wreckage (Jones Citation2007). More widespread and long-lasting effects may result from the discharge of oils, antifouling biocides and other contaminants derived from the ship's structure (Jones Citation2007; Dimitrakakis et al. Citation2014). For cargo ships, the range of potential contaminants is greater as these vessels may transport commercial and industrial chemicals and materials, raw minerals, oils, paints, manufactured goods, agricultural and horticultural produce and personal belongings. Metals are present in almost every component of a ship's structure (Dimitrakakis et al. Citation2014) and while it is likely that shipwreck-derived metals will influence the physiochemical and biological status of the water column adjacent to a wreck site, few studies have examined this possibility (Prego & Cobelo-Garcia Citation2004; Dimitrakakis et al. Citation2014). The benthic boundary layer, the layer of water immediately above the seafloor, is arguably one of the most critical microhabitats in any reef system. It is through this medium that the settling larvae of reef-associated organisms must transition to recruit to the reef ecosystem (Whitman & Reidenbach Citation2012). Furthermore, it is also within this zone that most food is consumed by sedentary reef species (Vedel Citation1998). After a shipwreck, research is generally focused on the fate and effects of discharged hydrocarbons. Consequently, little is known of the fate or effects of non-fuel contaminants, particularly metals derived from vessels or their cargo (Prego & Cobelo-Garcia Citation2004; Jones Citation2007; Dimitrakakis et al. Citation2014). The lack of information about how these contaminants may influence ecological processes near the seafloor is of some concern.
The traditional approach to measuring metals in aquatic environments is the collection of water or sediment samples for chemical analysis to provide a point-in-time assessment of contaminants at a sampling location (Brown et al. Citation1974). A more contemporary approach has been to measure bioavailable contaminant concentrations in organisms that are considered ‘sentinel species’ or ‘biological indicators’ (Goldberg et al. Citation1978; Melwani et al. Citation2014). Suspension feeding bivalves are widely used as a key monitoring species (Webb & Keough Citation2002; Schintu et al. Citation2008; Sondergaard et al. Citation2014). However, for environments in which bioindicator taxa do not naturally occur and cannot easily be deployed, possibly due to depth, exposure or logistical considerations, alternative approaches to the monitoring of trace-metal pollution are required. One such alternative is the use of passive sampling devices, for example, diffusive gradients in thin films (DGT) (Davison & Zhang Citation1995; Webb & Keough Citation2002; Schintu et al. Citation2008; Sondergaard et al. Citation2014; de Souza et al. Citation2014).
DGT samplers are simple, inexpensive (£12.50 per unit) and readily available devices for in situ passive water sampling. DGT samplers designed for metal analysis consist of a 25 mm diameter plastic disc containing a layer of chelating resin beneath a layer of polyacrylamide hydrogel which is exposed to the environment through a membrane filter and a 20 mm diameter window. DGT samplers work by permitting metal ions to diffuse freely through the hydrogel layer to be captured in the chelating resin (Davison & Zhang Citation1995). Mass transport of metals into the DGT is controlled by diffusion across the well-defined hydrogel layer, allowing quantification of contaminant concentrations and speciation based on Fick's law (Davison & Zhang Citation1995). A time-integrated metal concentration from deployment can then be analysed from a pre-concentrate of dissolved trace metals (Schintu et al. Citation2008; Sherwood et al. Citation2009; Sondergaard et al. Citation2014). These properties are particularly useful when monitoring pollutants in environments where water chemistry is temporally variable (Turner et al. Citation2014). On account of these properties, DGTs have been used on a number of occasions to quantify the labile, dissolved fraction of trace metals in freshwater and marine environments (Zhang & Davison Citation1995; Webb & Keough Citation2002; Buzier et al. Citation2006; Schintu et al. Citation2008; Sherwood et al. Citation2009; Hartland et al. Citation2011; Sondergaard et al. Citation2014; de Souza et al. Citation2014; Turner et al. Citation2014).
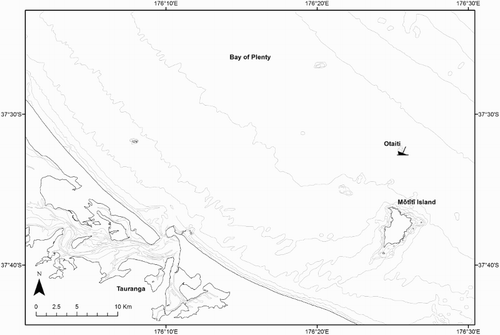
The grounding of the MV Rena on Otaiti (Astrolabe Reef, Bay of Plenty, New Zealand; Schiel et al. Citation2016; ) on 5 October 2011 provided an opportunity to assess levels of waterborne trace-metal contamination after a real-world environmental disaster and to test the utility of DGTs for measuring trace-metal contamination in high-energy offshore environments. Although DGTs are commonly used in marine settings (Webb & Keough Citation2002; Schintu et al. Citation2008; Sherwood et al. Citation2009; Sondergaard et al. Citation2014), DGT deployments either on shipwrecks or in high-energy open ocean environments have not previously been documented. At least 200 tonnes of heavy fuel oil (HFO380) were discharged from the Rena shipwreck into the Bay of Plenty (Transport Accident Investigation Commission Citation2014). Hundreds of shipping containers carried by the Rena were also lost overboard and in some instances broke open, spilling their contents into the waters around the grounding site (Beca Citation2014; Maritime New Zealand Citation2014; Ross et al. Citation2016; Schiel et al. Citation2016). The Rena subsequently broke into two pieces resulting in the loss of further oil, cargo and ship debris.
Figure 1. Map of the Bay of Plenty showing the location of the MV Rena on Otaiti (Astrolabe Reef), Mōtītī Island and Tauranga.
Almost 3 years after the grounding and eventual sinking of the Rena, a halo of ship and container debris spanning c. 10,000 m2 remains on Otaiti surrounding the wreckage. The debris includes metals from the ship's structure as well as scrap metal, aluminium ingots and c. 21,000 kg of granulated copper (Beca Citation2014). Ross et al. (Citation2016) recorded greater concentrations of metals including cadmium (Cd), chromium (Cr), Cu, Ni, tin (Sn) and zinc (Zn) in sediments from Otaiti compared with reference sites. Although the bioavailability of these contaminants is unknown, detection of Cu, Sn, organotins and Zn at elevated concentrations in some biota at Otaiti suggests a degree of bioavailability and transmission through the food chain (Ross et al. Citation2016). Similarly, the effects of Rena debris on the chemistry of the overlying water column are also unknown. Addressing this uncertainty is of some importance as the presence of Rena-derived bioavailable trace metals in the water column would imply an additional pathway by which marine life might be impacted by the Rena grounding, with possible implications for the recovery of Otaiti's biological communities.
Here we present a study in which DGT passive samplers, as well as measurements of total metals in solution, are used to assess trace-metal concentrations in the water column at Otaiti. DGTs were deployed at Otaiti and nearby Mōtītī Island for a period of 7 days to assess the contribution of the Rena shipwreck and debris to near seafloor water chemistry. Although the replication of sampling achieved in the study falls short of what might be desired under ideal circumstances, the context within which this research was conducted is that of a real-world shipwreck and salvage operation. Access to Otaiti was limited by the weather, politics, the logistical considerations of working 25 km offshore and the continually evolving schedule of the ship's salvors. Deployments could only occur in areas where salvage activities were not being conducted and within a weather window for which swells, with the potential to damage or displace DGT units, were not expected. As such, this piece of work should be treated as a precursor to future assessments of water chemistry at Otaiti and an exploration of the potential for DGTs to be used to measure trace-metal contaminants under similar circumstances. To date, this study has been the only attempt to quantify the effects of the Rena shipwreck on Otaiti's water quality.
Methodology
Study site and field procedures
Sampling was carried out in three areas: the Rena debris field; northwestern Otaiti (away from the debris field; ); and Mōtītī Island (). Although not the ideal location for comparison, northwest Mōtītī Island was chosen as a control site due to logistical constraints (boat access and suitable weather windows) which precluded timely access to other, possibly more ideal, offshore reef systems. Mōtītī Island is similar to Otaiti in its ecology and the oceanographic conditions it experiences (Heath Citation1982). Although Mōtītī was impacted by oil and some container debris immediately after the grounding, it was deemed a viable location for comparison as almost 3 years had passed since the oil spill and because the focus of this study was on metals rather than hydrocarbon pollutants.
Figure 2. Outer reef (OR1–3) and debris field (DF1–3) sampling sites (grey ovals) on Otaiti (Astrolabe Reef) where DGTs were deployed and water samples collected. The approximate position of the bow and stern sections of the Rena are indicated in grey. A dashed line indicates the area designated in June 2013 as the main Rena debris field.
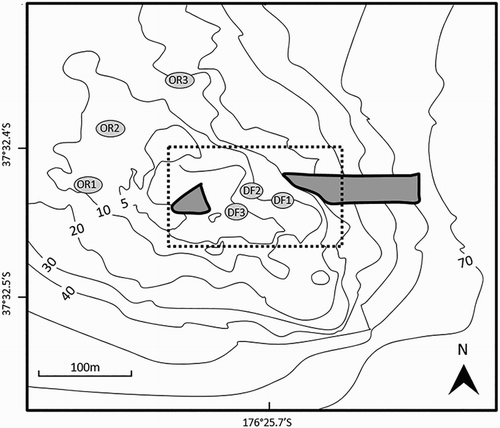
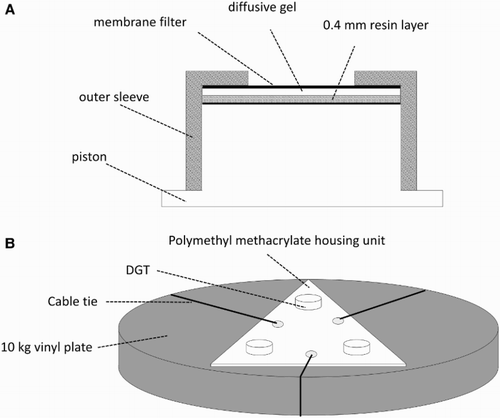
Within each of the three areas examined, three sampling sites were selected for DGT deployment. Sites were selected to avoid DGT deployment in areas of scheduled salvage activity. Standard open pore DGT units for the measurement of cations in solution were used in this research (Chelex-100 resin; 0.4 mm thick hydrogel; 0.45 µm cellulose nitrate membrane filter; DGT Research Ltd) as described by Zhang & Davison (Citation1995). DGT samplers were installed in triplicate into triangular polymethyl methacrylate housing units which were cable tied to 10 kg vinyl plates (). DGT housing units were deployed by divers to depths of between 11.5 m and 16.5 m at three sites within the debris field (DF1, DF2 and DF3; ), three sites on the northwestern slope of Otaiti (hereafter referred to as the outer reef; OR1, OR2 and OR3; ) and to the northwestern side of Mōtītī Island ().
Figure 3. A, cross section of a standard open pore DGT sampler (Chelex-100 resin; 0.4 mm thick; 0.45 µm cellulose nitrate membrane filter); B, triangular polymethyl methacrylate DGT housing unit cable tied to a 10 kg vinyl plate.
DGT samplers were deployed from 29 June to 6 July 2014. After the 7-day deployment period, DGT units were recovered, removed from their housings, rinsed with deionised water, placed in clean polyethylene bags and stored in a cooler bin on salt ice. Once on land, DGT units were refrigerated at 4 °C prior to laboratory processing which occurred within 48 h.
Spot sampling of total and dissolved metals was conducted on 29 June at the time of DGT deployment. Water samples were collected at each site from 25 cm above the seafloor by divers using two 1.2 L Sistema containers. Containers were cleaned with sample water before collection and then rinsed with deionised water between collections. These water samples were taken to quantify the total concentration of dissolved metals in the water column at each site. The water samples provide a means of validating the DGT results by providing an upper-limit on the total metal contamination of the water column at this site. This method differs from the DGT technique that measures only the labile metal species present in the water (Zhang & Davison Citation1995). This difference is important because the concentrations measured by DGT correspond more closely to the concentration of bioavailable metals, since a large proportion of the total metals in solution will be chemically inert due to the formation of organic complexes and adsorption on to suspended particles.
For each site, 10 mL of water from one of the 1.2 L Sistema containers was passed through a 0.45 µm filter into a sterile 15 mL falcon tube and another 400 mL of water was transferred into a 500 mL Nalgene bottle. In the second water sample container, a YSI Model 85 handheld multimeter was used to measure temperature, salinity, dissolved oxygen and conductivity, and pH was measured using a ‘DoubleTestr pH10’. These physiochemical properties were also measured, using the same methodology, at the time of DGT retrieval. The above procedures were performed on board the dive support vessel immediately after collection. Disposable latex gloves were worn throughout and all equipment was rinsed with seawater thrice and then deionised water before and after each sample was taken. As with the DGT units, water samples were stored in a cooler bin on salt ice then refrigerated at c. 4 °C before laboratory analysis.
Sample preparation
Water samples
Filtered water samples were acidified with ultrapure HNO3 to a final concentration of 2% before analysis by RJ Hill Laboratories on a Perkin Elmer quadrupole ICP-MS in dynamic reaction cell mode. Concentrations of total aluminium (Al), Cd, Cr, cobalt (Co), Cu, Fe, lead (Pb), manganese (Mn), Ni and Zn were reported with detection limits in the range of 1.87 (Cd) to 500 nmol kg−1 (Al; ). Although analysis by a more sensitive method, for example a pre-concentration of trace metals prior to ICP-MS, would have resulted in reduced detection limits, pre-concentration methods were not accessible at the time this research was conducted.
Table 1. Total solution concentrations (nmol kg−1) of trace metals for sites within the debris field, outer reef and Mōtītī Island sampling areas.
DGT samplers
DGT caps were removed and the DGT resin layer extracted and placed in a 15 mL falcon tube using plastic tweezers (pre-cleaned in dilute nitric acid). One millilitre of 1 M ppt grade HNO3 was added to the falcon tube to elute metals from the binding gel. Falcon tubes were left capped for 48 h at 4 °C. A further 4 mL of deionised water was then added to dilute the sample in preparation for ICP-MS analysis at the University of Waikato (Zhang & Davison Citation1995).
Calculations
The concentrations of DGT-labile metals present in the water column over the deployment period were calculated using the following equation (Zhang & Davison Citation1995):
where M is the mass of metal absorbed on the resin (found by equating the analysis of an acid extract, and assuming the gel volume is 0.16 mL with an elution factor of 0.8 for metals); Δg is the thickness of the diffusive gel (0.4 mm) and the filter membrane (0.13 mm); D is the diffusion coefficient of metal ions at a given water temperature (in this case calculated as the mean of deployment and retrieval temperature measurements); t is the deployment duration (in seconds) and A is the exposure area of the diffusive layer (3.14 cm2). Ideally, water temperatures would have been logged continuously and D calculated from the mean temperature as measured over the entire deployment period. However, the approach taken here, of averaging deployment and retrieval temperatures, is consistent with methods used for deployments of DGTs elsewhere (Webb & Keough Citation2002; Schintu et al. Citation2008; de Souza et al. Citation2014). The diffusion coefficient was based on freshwater so it would be expected that values of D in seawater would be lower due to the formation of a range of inorganic complexes with slightly greater molar mass than the equivalent aquo complexes (Zhang & Davison Citation1995). Thus, the values of DGT metal concentrations (CDGT) reported here are conservative. It should also be noted that since DGTs measure the concentration of metal ions able to diffuse through the membrane and hydrogel, the true total concentration of metals in solution is predicted to be greater than the concentration measured via DGT. Indeed, strong scavenging of many metals by surface adsorption to particles and complexation by organic ligands is likely. This combination of factors suggests that the DGT method will underestimate the total concentration of many metals and metalloids in the water column (Davison & Zhang Citation2011; Knutsson et al. Citation2014).
Statistical methods
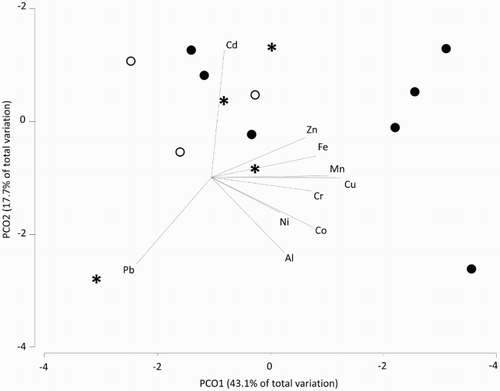
A permutational multivariate analysis of variance (PERMANOVA) using square-root transformation and Euclidean similarity resemblance matrices was performed in PRIMER 7 to test for differences in DGT metal concentrations among sampling areas (debris field vs outer reef vs Mōtītī Island). A principal component analysis (PCO), also performed in Primer 7, was then used to visualise differences in DGT metal concentrations across sampling areas. The PCO technique for environmental data is correlation based, comparing the Euclidean distance between data points with the corresponding dissimilarity in variable structure (Anderson et al. Citation2008). PCO was performed to explore the similarity of sites based on chemical properties and to determine which metals drove the greatest variance in the data set as a whole ().
Figure 4. Two-dimensional PCO ordination of DGT data from sampling sites in the Otaiti debris field (●), Otaiti outer reef (○) and Mōtītī Island (*). Principal component axis 1 (PCO1) accounts for 43.1% and axis 2 (PCO2) 17.7% of total variance in the data.
From the PCO ordination it was clear that the metal profiles of DGTs deployed at the easternmost debris field sites, DF1 and DF2, differed from those at all other sites across Otaiti. Consequently, a second PERMANOVA was performed with a revised grouping of sites to compare DGT metal concentrations between these two sites (DF1 and DF2), other sites across Otaiti (DF3, OR2 and OR3) and Mōtītī Island. Spatial variation in the univariate data was examined again using PERMANOVA to identify specific metals responsible for differences among these groups.
The comparability of data generated through spot water samples vs DGT samplers was investigated through regressions of total trace metals against CDGT values. This analysis was performed in Statistica version 12 and could only be conducted for analytes recorded above ICP-MS detection limits in two or more water samples.
Results
Water samples—total dissolved metals
Water physiochemical variables at the start and end of the deployment period ranged from 7.8–8.2 for pH; 15.1–16.4 °C for temperature; 31–32.4 ppt for salinity; 5.5–9.3 mg/L dissolved oxygen; and 38.5–47.6 mS for conductivity. Total concentrations of dissolved metals in water samples were almost entirely below the detection limits for direct ICP-MS analysis (). Only Fe, Al, Mn and Cu were recorded above detection limits. Fe was recorded in all debris field water samples between 208 and 1074 nmol kg−1, in two outer reef samples (OR1 and OR2) at 136 and 91 nmol kg−1 and in one Mōtītī Island sample at 109 nmol kg−1 (). Mn (25 nmol kg−1) and Cu (20 nmol kg−1) were only recorded at DF1. Al was only recorded at DF1 (667 nmol kg−1) and DF2 (519 nmol kg−1).
CDGT—bioavailable metal flux
At the end of the deployment period there were only light signs of biofouling of the DGT diffusion membranes. Unfortunately, of the 27 DGT samplers deployed 13 were damaged, possibly by the turbulent hydrodynamic conditions experienced by DGTs on Otaiti or through grazing by reef biota. These damaged DGT units were missing their membrane, hydrogel and resin layers and therefore could not be analysed. This left 14 DGTs providing usable data at sites DF1 (n = 3), DF2 (1), DF3 (3), OR2 (2), OR3 (1), MI1 (1) and MI3 (3). Unfortunately the loss of this many samplers does reduce our ability to differentiate between spatial patterns of water chemistry vs manufacturing differences in the DGTs as sources of variability in the data set (Kreuzeder et al. Citation2015). However, the information generated by the remaining samplers does provide an indication of the time-integrated flux of metals from contaminated sediments and debris to the water column.
All metal analytes were present in all analysed DGTs above the detection limits for ICP-MS analysis. Analyte concentrations varied both within and among sites and areas ( and ). For the analytes Fe, Al, Mn, Cu, Zn and Cr, the mean CDGT was greater in the debris field compared with the outer reef or Mōtītī Island (; ). In contrast, Pb and Co concentrations were greatest at Mōtītī Island (). Despite the apparent CDGT differences between sampling areas, PERMANOVA of the multivariate data set indicated that differences in the CDGT profile of the samplers between areas (debris field vs outer reef vs Mōtītī) were marginally non-significant (P = 0.077).
Figure 5. DGT-available metal concentrations (CDGT) ± SE (nmol kg−1) for the three sampling areas ( and ); debris field (DF), outer reef (OR) and Mōtītī Island (MI).
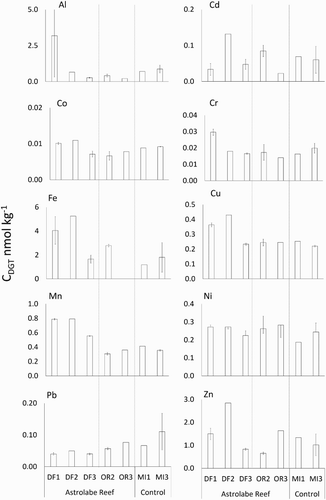
Table 2. Pearson correlation vectors, mean ± SE and maximum trace-metal concentrations (nmol kg−1) for DGTs deployed at debris field, outer reef and Mōtītī Island sampling sites.
The two-dimensional PCO ordination accounted for 60.8% of the total variance in the data (): 43.1% of the variation was explained by the first principal component axis (PCO1) and 17.7% by the second axis (PCO2). Cu was the analyte most correlated with PCO1 (r = 0.93) followed by Mn (r = 0.82), Fe (r = 0.74), Co (r = 0.71), Cr (r = 0.71), Zn (r = 0.65), Pb (r = −0.53), Al (r = 0.51) and Ni (r = 0.48; ). Cd (r = 0.89), Pb (r = −0.61) and Al (r = −0.54) were the only analytes strongly correlated with PCO2. In the PCO ordination, the majority of samples from all locations (debris field, outer reef and Mōtītī Island sites) cluster together on the upper left corner of the ordination. The four debris field samples from sites DF1 and DF2 are clearly separated from this main cluster and are positioned towards the right side of the ordination due to increased concentrations of PCO1 correlated metals (Zn, Fe, Mn, Cu, Cr, Ni, Co and Al). Once our a priori arrangement of sites within sampling areas was revised, based on the PCO analysis ([DF1 and DF2] vs [DF3, OR2 and OR3] vs Mōtītī), PERMANOVA of the multivariate data set showed significant differences between these new groupings (P = 0.002) on account of the elevated metal concentrations recorded in DGTs deployed at sites DF1 and DF2. Cu (up to 0.43 nmol kg−1; P = 0.004) and Mn (up to 0.80 nmol kg−1; P = 0.002) concentrations were significantly elevated in debris field DGTs, whereas Zn concentrations at these sites were on average greater but were marginally non-significant (up to 2.8 nmol kg−1; P = 0.072; ). Al was also recorded at high concentrations in debris field DGTs (up to 8.9 nmol kg−1) although overall, the difference between areas was not significant ().
Determining the comparability of trace-metal data derived from water sampling and DGT methods was hindered by the fact that total concentrations of dissolved metals were almost entirely below detection limits for most water samples (Table S1). The analysis could be conducted for only two analytes. Fe was recorded at six of nine sites, and Al was recorded at sites DF1 and DF3 (). There was no correlation between total metal and CDGT concentrations for either metal (Fe: r2 = 0.21, P > 0.05, n = 12; Al: r2 = 0.18, P > 0.05, n = 4).
Discussion
DGT samplers were deployed at Otaiti to assess the level of waterborne trace-metal contamination in a high-energy offshore environment 3 years after a real-world environmental disaster. The probability of detecting waterborne contaminants within a dynamic open ocean reef environment would seem low, as metals released into the water column from ship debris would be rapidly mixed and diluted. However, the results presented here indicate the presence of significantly elevated concentrations of waterborne Cu and Mn, and higher concentrations of Al, Fe and Zn at sites within the Rena debris field. The results also demonstrate the utility of DGTs for providing a time-integrated measure of waterborne trace-metal contaminants. By comparison, Fe was the only trace metal consistently measured in spot water samples while Al, Mn and Cu were only measurable in 11%–22% of water samples ().
The lack of correlation between water sample and DGT measured metal concentrations is perhaps not surprising. Water samples represent a single point in time. These samples were collected under conditions where swell and current were effectively non-existent. The DGT data were gathered over a 7-day period under a variety of sea states. It is possible that some contaminants are only mobilised from the sediment to the water column under certain hydrodynamic conditions.
The chemical profile of DGTs deployed at two of the three debris field sites (DF1 and DF2) differed significantly from DGTs deployed at all other sites in this study. DF1 and DF2 also yielded the highest recorded concentrations for all elements measured except for Pb and Ni which were present at greater concentrations at Mōtītī Island. These results are consistent with our knowledge of sediment contamination at Otaiti. Sites DF1 and DF2 were located in a valley running east to west along the northeastern slope of Otaiti ( and S1). This is the valley in which the Rena became lodged on 5 October 2011, the area that received the greatest quantity of ship and cargo debris once the Rena’s hull split in two (PM Ross, pers. obs.) and the area in which greatest metal contamination of sediments has been recorded (Figure S1). DF1 and DF2 are also in close proximity (c. 60 m) to the position at which a significant quantity (up to 21,000 kg) of granulated copper (from the ship's cargo) was deposited on the northeastern slope of Otaiti (Figure S1; Ross et al. Citation2016), explaining the greater copper concentrations recorded in these DGTs. The third debris field site, DF3, for which DGT metal concentrations were significantly lower, is located to the southwest of DF1 and DF2 and is elevated above much of the ship debris in an area where sediment contaminant levels are lower (Figure S1). DF3 is also further removed, by an additional 20–30 m, from the main copper deposit (Ross et al. Citation2016). The concentrations of metals recorded in sediments in the vicinity of DF3 are less than those recorded at DF1 and DF2 and this is reflected in the DGT data. In contrast to DF1 and DF2, concentrations of metals recorded in DGTs at Otaiti outer reef sampling sites were comparable to those recorded at Mōtītī Island, our reference site. Together, these results indicate: that concentrations of DGT-labile metals in the water column vary over relatively small spatial scales (<10s of metres); that proximity to specific components of the debris field, including contaminated sediments, may influence adjacent water chemistry; and that the most severe effects of Rena-derived contaminants on water chemistry will probably be restricted to the immediate vicinity of the debris field. For future studies, with greater spatial sampling resolution and lower rates of DGT attrition, it should be possible to compare sediment and water trace-metal concentrations, gain a better understanding of the spatial scales at which water quality varies and describe a zone of influence for specific contaminants or components of the Rena debris field.
Rena-derived contaminants including Cu, Sn, Zn, Cr, Ni, polycyclic aromatic hydrocarbons and organotins are present at elevated concentrations in debris field sediments (Ross et al. Citation2016). The detection of Cu at elevated concentrations in whelks (Dicathais orbita and Haustrum haustorium) and the presence of Sn and antifouling-derived organotins at low levels in some urchins, rock lobster, gastropods and fishes indicates a degree of bioavailability (Ross et al. Citation2016). However, it is uncertain whether these organisms have been exposed to water soluble contaminants, have consumed contaminants through their diet or have come into direct contact with contaminated substrates. The fact that only Cu and Mn were recorded in DGTs at significantly elevated concentrations is of some interest given the elevated concentrations of other metals in Otaiti sediment (Ross et al. Citation2016). It is not clear whether our failure to detect a significant effect of these contaminants on water chemistry is a consequence of differences in their solubility (or DGT availability), the duration of our DGT deployment or the high rate of DGT failure experienced and consequent loss of statistical power.
The failure of almost half the DGTs deployed is of some concern, particularly as the cause of these failures is uncertain. The waters of Otaiti are dynamic, with currents up to 0.66 m s−1 and significant wave heights of up to 8 m (Robertson Citation2014). High turbulence or currents appear to be the most plausible explanation for the failures experienced here, although some sort of interference by reef biota cannot be ruled out. For the most part, published DGT deployments have been in less exposed locales (Webb & Keough Citation2002; Buzier et al. Citation2006; Schintu et al. Citation2008; Sherwood et al. Citation2009; Sondergaard et al. Citation2014) and to the best of our knowledge there is no information available to indicate DGT tolerances to turbulence or wave action. For future studies it would be wise to utilise a DGT deployment system that provides the units with a greater degree of protection. At the same time, it would be prudent to deploy additional DGTs to ensure a suitable sample size can be obtained, in the event of some DGT attrition, to permit a robust statistical analysis. Where repeated deployments are made in a location or environment, it is likely that researchers will quickly determine rates of DGT attrition and plan accordingly.
The metal concentrations recorded using DGTs for Otaiti and Mōtītī Island are consistent with values previously reported for New Zealand oceanic waters (Dickson & Hunter Citation1981) and are therefore not likely to be high enough to cause significant adverse ecological effects. The comparison with Dickson & Hunter (Citation1981) provides a degree of confidence in our results. However, it is important to note that Dickson & Hunter (Citation1981) measured total concentrations in solution rather than the smaller DGT-labile fraction measured here; and that both the number and spatial distribution of DGTs deployed for this study were insufficient to fully determine the extent and magnitude of the effect of Rena-derived contaminants on Otaiti water chemistry. Where DGTs have previously been used to assess coastal waters influenced by industry (smelting, mining and oil refineries), Cu concentrations in the order of 1.62 nmol kg−1 (de Souza et al. Citation2014) and 1.28 nmol kg−1 (Schintu et al. Citation2008) have been reported. Cu concentrations recorded at Otaiti (up to 0.43 nmol kg−1), although elevated above our reference sites (0.23 ± 0.01 nmol kg−1 ± s.e.), fall in the lower spectrum of values reported for these contaminated locations. While metals such as Cu can adversely affect marine organisms when present above ambient concentrations, different marine taxa will accumulate contaminants at different rates, with different physiological effects and therefore biological significance (Rainbow Citation2002).
As a consequence of the Rena grounding, biological communities across much of the Rena debris field have been severely disturbed, or removed in their entirety. Our results suggest that these ecological changes are more likely to be due to physical disturbances or the chemical contamination occurring in the aftermath of the grounding, rather than being a consequence of the waterborne chemical environment measured here, almost 3 years after the grounding. The recovery of impacted biological communities will to a large extent be dependent on recruitment from outside the disturbed areas. Understandably, there are concerns, among stakeholders and the public, that chemical contamination could impede biological recovery as contaminants such as Cu and Zn can decrease fertilisation success and inhibit recruitment and survival of invertebrate larvae (Labare et al. Citation1997; Johnston & Keough Citation2002; Hill et al. Citation2013). However, based on the trace-metal concentrations recorded here, recruitment inhibition is unlikely. Further sampling and assessment of biological thresholds to toxicity will be required to adequately test this hypothesis.
Clearly, the Rena grounding has significantly impacted the water chemistry of Otaiti, with small but statistically significant contamination evident almost 3 years after the grounding. Determining whether these changes are also ecologically significant will require further observation and experimentation. Additional research is required to properly characterise the nature of waterborne contaminants and their distribution across Otaiti. This could be achieved through additional DGT deployments and an experimental design that incorporates greater spatio-temporal resolution and a vertical sampling component. Ecotoxicity bio-assays, utilising relevant native species, will increase our understanding of how the propagules and later life stages of key habitat-forming species respond to Rena contaminants (or combinations of contaminants), and aid in assessing and predicting the possible ecological consequences of waterborne Rena-derived contaminants.
Supplementary data
Table S1. A comparison of detection limits for discrete water samples and DGT samplers.
Figure S1. Map of Astrolabe Reef showing the position of on-reef sediment sampling stations. A, The concentrations of copper recorded in sediments relative to the Australian and New Zealand Environment and Conservation Council (ANZECC) Interim Sediment Quality Guideline (ISQG) threshold concentrations. For copper, the ISQG-low concentration is 65 mg kg−1 and ISQG-high is 270 mg kg−1. B, the concentrations of zinc recorded in sediments relative to ANZECC ISQG threshold concentrations. For zinc, the ISQG-low concentration is 200 mg kg−1 and ISQG-high is 410 mg kg−1.
Figure S1. Map of Astrolabe Reef showing the position of on-reef sediment sampling stations.
Download JPEG Image (392.5 KB)Table S1. A comparison of detection limits for discrete water samples and DGT samplers.
Download MS Word (12.8 KB)Acknowledgements
We would like to thank two anonymous reviewers for their comments and suggestions which greatly improved this manuscript, University of Waikato technicians David Culliford and Rex Fairweather, and the Rena owners and insurers, Beca, Pacific Divers and TMC for assisting with and facilitating fieldwork. Thanks to the University of Waikato Chemistry Laboratory and RJ Hill Laboratories for sample analysis.
Guest Editor: Professor David Schiel.
Disclosure statement
Phil Ross is a Research Fellow at the University of Waikato. During the time that this research was conducted, Ross was contracted both by the Bay of Plenty Regional Council and by the owner (Daina Shipping Co.) and insurer (P & I Services) of the MV Rena to conduct environmental monitoring and report on monitoring results. Ross was called as an expert witness for the owner and insurer of the MV Rena at the Resource Consent hearing in November 2015.
Additional information
Funding
References
- Anderson MJ, Gorley RN, Clarke KR. 2008. PERMANOVA+ for primer: guide for software and statistical methods. Plymouth, UK: PRIMER-E.
- Beca. 2014. The Rena project [Internet]. [cited 2014 Jun]. Available from: http://www.renaproject.co.nz/
- Boyd RS. 2010. Heavy metal pollutants and chemical ecology: exploring new frontiers. J Chem Ecol. 36:46–58. doi: 10.1007/s10886-009-9730-5
- Brown CW, Lynch PF, Ahmadjian M. 1974. Monitoring Narragansett Bay oil spills by infrared spectroscopy. Env Sci Tec. 8:669–670. doi: 10.1021/es60092a009
- Bruland KW, Middag R, Lohan MC. 2013. Controls of trace metals in seawater. In: Turekian KK, Holland HD, editors. Treatise on geochemistry. Volume 1–16. 2nd ed. Oxford: Elsevier; p. 19–51.
- Buzier R, Tusseau-Vuillemin MH, Mouchel JM. 2006. Evaluation of DGT as a metal speciation tool in wastewater. Sci Total Environ. 358:277–285. doi: 10.1016/j.scitotenv.2005.09.051
- Davison W, Zhang H. 1995. In-situ speciation measurements of trace components in natural-waters using thin-film gels. Nature. 367:546–548. doi: 10.1038/367546a0
- Davison W, Zhang H. 2011. Progress in understanding the use of diffusive gradients in thin films (DGT)—back to basics. Environ Chem. 9:1–13. doi: 10.1071/EN11084
- Dickson RJ, Hunter KA. 1981. Copper and nickel in surface waters of Otago Harbour. New Zeal J Mar Freshwat Res. 15:475–480. doi: 10.1080/00288330.1981.9515939
- Dimitrakakis E, Hahladakis J, Gidarakos E. 2014. The “Sea Diamond” shipwreck: environmental impact assessment in the water column and sediments of the wreck area. Int J Environ Sci Te. 11:1421–1432. doi: 10.1007/s13762-013-0331-z
- Duruibe JO, Ogwuegbu MOC, Egwurugwu JN. 2007. Heavy metal pollution and human biotoxic effects. Int J Phys Sci. 2:112–118.
- Goldberg ED, Bowen VT, Farrington JW, Harvey G, Martin JH, Parker PL, Risebrough RW, Robertson W, Schneider E, Gamble E. 1978. The Mussel Watch. Environ Conserv. 5:101–125. doi: 10.1017/S0376892900005555
- Han FXX, Banin A, Su Y, Monts DL, Plodinec MJ, Kingery WL, Triplett GE. 2002. Industrial age anthropogenic inputs of heavy metals into the pedosphere. Naturwissenschaften. 89:497–504. doi: 10.1007/s00114-002-0373-4
- Hartland A, Fairchild IJ, Lead JR, Zhang H, Baalousha M. 2011. Size, speciation and lability of NOM-metal complexes in hyperalkaline cave dripwater. Geochimica Et Cosmochimica Acta. 75:7533–7551. doi: 10.1016/j.gca.2011.09.030
- He B, Yun ZJ, Shi JB, Jiang GB. 2013. Research progress of heavy metal pollution in China: sources, analytical methods, status, and toxicity. Chin Sci Bull. 58:134–140. doi: 10.1007/s11434-012-5541-0
- Heath RA. 1982. A review of the physical oceanography of the seas around New Zealand. New Zeal J Mar Freshwat Res. 19:79–124. doi: 10.1080/00288330.1985.9516077
- Hill NA, Simpson SL, Johnstone EL. 2013. Beyond the bed: effects of metal contamination on recruitment to bedded sediments and overlying substrata. Environ Pollut. 173:182–191. doi: 10.1016/j.envpol.2012.09.029
- Islam MS, Tanaka M. 2004. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar Pollut Bull. 48:624–649. doi: 10.1016/j.marpolbul.2003.12.004
- Johnston EL, Keough MJ. 2002. Direct and indirect effects of pollution events on marine hard substrate assemblages. Ecol Appl. 12:1212–1228.
- Jones RJ. 2007. Chemical contamination of a coral reef by the grounding of a cruise ship in Bermuda. Mar Pollut Bull. 54:905–911. doi: 10.1016/j.marpolbul.2007.02.018
- Knutsson J, Rauch S, Morrison GM. 2014. Estimation of measurement uncertainties for the DGT passive sampler used for determination of copper in water. Int J Anal Chem. 2014:1–7. doi: 10.1155/2014/389125
- Kreuzeder A, Santner J, Zhang H, Prohaska T, Wenzel WW. 2015. Uncertainty evaluation of the diffusive gradients in thin films technique. Env Sci Tec. 49:1594–1602. doi: 10.1021/es504533e
- Labare ML, Coon SL, Matthias C, Weiner RM. 1997. Magnification of tributyl tin toxicity to oyster larvae by bioconcentration in biofilms of Shewanella colwelliana. Appl Environ Microb. 63:4107–4110.
- Maritime New Zealand. 2014. Response to the Rena grounding [Internet]. [cited 2014 Jul]. Available from: http://www.maritimenz.govt.nz/Environmental/Responding-to-spills-and-pollution/Past-spill-responses/Rena-response.asp
- McIlgorm A, Campbell HF, Rule MJ. 2011. The economic cost and control of marine debris damage in the Asia-Pacific region. Ocean Coastal Manage. 54:643–651. doi: 10.1016/j.ocecoaman.2011.05.007
- Melwani AR, Gregorio D, Jin Y, Stephenson M, Ichikawa G, Siegel E, Crane D, Lauenstein G, Davis JA. 2014. Mussel Watch update: long-term trends in selected contaminants from coastal California, 1977–2010. Mar Pollut Bull. 81:291–302. doi: 10.1016/j.marpolbul.2013.04.025
- Prego R, Cobelo-Garcia A. 2004. Cadmium, copper and lead contamination of the seawater column on the Prestige shipwreck (NE Atlantic Ocean). Anal Chim Acta. 524:23–26. doi: 10.1016/j.aca.2004.03.032
- Rainbow PS. 2002. Trace metal concentrations in aquatic invertebrates: why and so what? Environ Pollut. 120:497–507. doi: 10.1016/S0269-7491(02)00238-5
- Robertson W. 2014. Natural character assessment: proposal to leave the remains of the MV Rena on the Astrolabe Reef. Tauranga, New Zealand: Beca.
- Ross PM, Battershill CN, Loomb C. 2016. The wreck of the MV Rena: spatio-temporal analysis of ship-derived contaminants in the sediments and fauna of Astrolabe Reef. New Zeal J Mar Freshwat Res. 50:87–114.
- Schiel DR, Ross PM, Battershill CN. 2016. Environmental effects of the MV Rena shipwreck: cross-disciplinary investigations of oil and debris impacts on a coastal ecosystem. New Zeal J Mar Freshwat Res. 50:1–9.
- Schintu M, Durante L, Maccioni A, Meloni P, Degetto S, Contu A. 2008. Measurement of environmental trace-metal levels in Mediterranean coastal areas with transplanted mussels and DGT techniques. Mar Pollut Bull. 57(6–12):832–837. doi: 10.1016/j.marpolbul.2008.02.038
- Sherwood JE, Barnett D, Barnett NW, Dover K, Howitt J, Ii H, Kew P, Mondon J. 2009. Deployment of DGT units in marine waters to assess the environmental risk from a deep sea tailings outfall. Anal Chim Acta. 652:215–223. doi: 10.1016/j.aca.2009.06.009
- Sondergaard J, Bach L, Gustavson K. 2014. Measuring bioavailable metals using diffusive gradients in thin films (DGT) and transplanted seaweed (Fucus vesiculosus), blue mussels (Mytilus edulis) and sea snails (Littorina saxatilis) suspended from monitoring buoys near a former lead-zinc mine in West Greenland. Mar Pollut Bull. 78:102–109. doi: 10.1016/j.marpolbul.2013.10.054
- de Souza JM, Menegario AA, de Araujo MAG, Francioni E. 2014. Measurements of labile Cd, Cu, Ni, Pb, and Zn levels at a northeastern Brazilian coastal area under the influence of oil production with diffusive gradients in thin films technique (DGT). Sci Total Environ. 500–501:325–331. doi: 10.1016/j.scitotenv.2014.08.117
- Transport Accident Investigation Commission (TAIC). 2014. Container ship MV Rena grounding on Astrolabe Reef, 5 October 2011. Marine Inquiry Final Report 11–204. Wellington: TAIC; [cited 2016 Mar 30]. Available from: http://www.taic.org.nz/ReportsandSafetyRecs/MarineReports/tabid/87/ctl/Detail/mid/484/InvNumber/2011-204/language/en-US/Default.aspx?SkinSrc=%5BG%5Dskins%2FtaicMarine%2Fskin_marine
- Turner GSC, Mills GA, Bowes MJ, Burnett JL, Amos S, Fones GR. 2014. Evaluation of DGT as a long-term water quality monitoring tool in natural waters; uranium as a case study. Env Sci Process Impact. 16:393–403. doi: 10.1039/c3em00574g
- Vedel A. 1998. Phytoplankton depletion in the benthic boundary layer caused by suspension-feeding Nereis diversicolor (Polychaeta): grazing impact and effect of temperature. Mar Ecol-Prog Ser. 163:125–132. doi: 10.3354/meps163125
- Webb JA, Keough MJ. 2002. Measurement of environmental trace-metal levels with transplanted mussels and diffusive gradients in thin films (DGT): a comparison of techniques. Mar Pollut Bull. 44:222–229. doi: 10.1016/S0025-326X(01)00244-2
- Whitman ER, Reidenbach MA. 2012. Benthic flow environments affect recruitment of Crassostrea virginica larvae to an intertidal oyster reef. Mar Ecol Prog Ser. 463:177–191. doi: 10.3354/meps09882
- Zhang H, Davison W. 1995. Performance-characteristics of diffusion gradients in thin-films for the in-situ measurement of trace-metals in aqueous-solution. Anal Chem. 67:3391–3400. doi: 10.1021/ac00115a005
