ABSTRACT
Mysids form a large biomass and mediate the benthic–pelagic coupling of numerous estuaries in southern New Zealand. An intra-annual (2011–2012) field survey in the breeding seasons (i.e. austral spring followed by summer) of mysids Tenagomysis chiltoni and Tenagomysis novaezealandiae, examining the body and brood sizes, was conducted. Samples were collected from six open and eight intermittently open/closed estuaries along the Otago coastline. Brood size of gravid females of T. chiltoni was not significantly related to their body size. Brood size of gravid females of T. novaezealandiae showed a significantly positive relationship with body size in spring but not in summer. It appears that brood size of gravid mysids do not always necessarily depend on body size, but may be influenced by breeding season (e.g. spring) and estuarine typology (e.g. intermittently open/closed).
Introduction
In cold temperate regions key features of ectothermic life histories, such as maximum body size at maturity, fecundity (e.g. brood size), and production of cohorts per year, are often adjusted to suit variable seasonal temperatures (Stearn Citation1992). Animals may delay or accelerate maturity, with implications for adult body size and hence fecundity, to maximise use of favourable seasonal (e.g. warmer and productive climate) conditions (Sibly & Atkinson Citation1994). A larger maternal body enables a female to carry a large number of broods but spawning frequency declines (Shine Citation1988), whereas some females reach maturity earlier at a smaller body size, enabling them to increase the number of cohorts per year, but producing smaller broods as a consequence (Stearn Citation1992; Atkinson Citation1995). These fecundity strategies have been studied across a wide range of estuarine crustacea (crabs, copepods), but there have been no detailed studies for mysids (Mauchline Citation1980; Hines Citation1982; Fukui & Wada Citation1986; Jeong et al. Citation2007; Ramarn et al. Citation2012).
Small estuaries in southern New Zealand receive limited fresh water inputs, and their mouths can remain closed for periods ranging from days to years (Lill Citation2010). During the closed state, nutrients running off from urban and agricultural activities accumulate in the lower reaches, which at times turn these small estuaries into eutrophic environments (Lill Citation2010). During spring and summer water temperatures rise, hence the productivity of these small estuaries increases (Lill Citation2010). Such warm and productive estuarine environments accelerate growth rates of resident crustacea, influencing timing of maturity and maternal body size at maturity (Hines Citation1982; Fukui & Wada Citation1986; Väinölä & Vainio Citation1998; Jeong et al. Citation2007; Paul & Closs Citation2014). For numerous temperate mysids of the Northern Hemisphere, the carrying capacity of broods generally increases with the increase in maternal body size (Mauchline Citation1988; Mees et al. Citation1994; Takahashi & Kawaguchi Citation2004). An attempt is made here to assess if these patterns apply to the mysids of Tenagomysis spp.
In southern New Zealand, the estuarine mysids Tenagomysis chiltoni and T. novaezealandiae are euryhaline and tolerate wide abiotic fluctuations (Paul et al. Citation2013). Adults of T. chiltoni inhabit hypo-saline upper reaches of open estuaries and tidal lakes (Jones et al. Citation1989), although high mortality of their juveniles may occur in winter (Paul et al. Citation2013). Tenagomysis novaezealandiae dominates near the mouth of numerous small intermittently open/closed eutrophic estuaries of Otago, with occasional presence in upper reaches (Lill Citation2010; Lill et al. Citation2010). Gravid T. chiltoni females attain a significantly larger body size at maturity compared to gravid T. novaezealandiae (Jones et al. Citation1989; Lill et al. Citation2010). Tenagomysis chiltoni females mostly breed in spring (occasional small summer cohorts are evident in small eutrophic estuaries) after a period of prolonged winter growth to maturity (i.e. 6 to 8 months), but gravid females of T. novaezealandiae breed from late spring till mid autumn, and reach maturity relatively earlier than T. chiltoni females (i.e. within 3 to 4 months; Lill et al. Citation2010). Considering such contrasts in survival, growth, timing of maturity and breeding frequency, we examined the brood and body sizes of gravid females of these species to better understand their reproductive strategies, and relationships to their respective habitat conditions. For that, we conducted a field survey focused on the body–brood size relationships of gravid females of T. chiltoni and T. novaezealandiae across six open and eight small intermittently open/closed estuaries in the Otago region of southern New Zealand. We predicted brood size of these species would increase with maternal body size. We expected that mysids from intermittently open/closed estuaries would be generally larger and more fecund due to the relatively higher productivity of these systems compared to open estuaries (Lill Citation2010).
Methods
Collection of gravid mysids
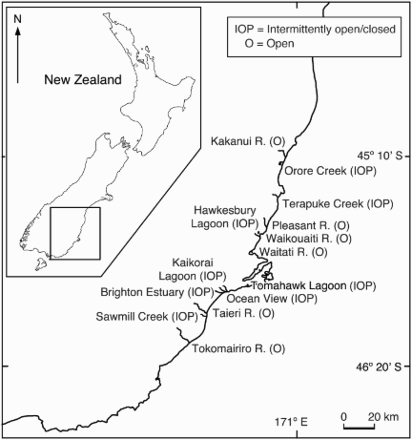
Gravid females of T. chiltoni and T. novaezealandiae were collected from six large open and eight small intermittently open/closed estuaries along the Otago coast of southern New Zealand (; ) in 2011–2012 during the breeding seasons (i.e. the austral spring and summer). Gravid mysids were collected from upstream and downstream estuarine reaches, and an effort was made to catch at least 10 gravid females (total catch from up and downstream) of T. chiltoni and T. novaezealandiae from every sampled estuary (). Due to the within-estuary distribution pattern of T. chiltoni and T. novaezealandiae (Jones et al. Citation1989; Lill et al. Citation2010), gravid T. chiltoni were largely sampled from upstream whereas T. novaezealandiae were mostly from downstream (). In each sampling season estuaries were sampled at dark during low tide. Each sampling site was waded for 5 min with a 250 µm plankton net to collect mysids from the top of the benthic layer of the respective sampling sites. Gravid T. chiltoni were difficult to catch irrespective of season and type of estuaries, which at times led to unequal sample sizes (). After the sampling, individuals were fixed immediately in formalin (10%), transported to the laboratory in 1 L sampling bottles, and stored at −20 C for later processing. Identification (species level) and sexual stage (i.e. gravid carrying brood pouch) determination were done by following Lill et al. (Citation2010).
Table 1. Sampling locations and sample sizes of Tenagomysis chiltoni and Tenagomysis novaezealandiae gravid females caught during the field survey in 2011/12 in southern New Zealand.
Estimate of fecundity
Population fecundity estimates (i.e. ‘brood size’) were based on a randomly selected subsample of gravid females collected from each sampling site within and across estuaries. Individuals were first sorted into species and sex and stage of life history (i.e. gravid females; Lill et al. Citation2010), brood pouches dissected, and all eggs, larvae and eyed larvae inside the brood pouch were counted. All eggs, larvae and eyed larvae were then summed to estimate the ‘brood’ of each individual mysid. Standard length (SL = distance between midpoint of eye to end of 6th abdominal somite) of these individuals was recorded (± 0.01 mm) under a microscope fitted with a graticule.
Dataset arrangement
We ran separate analyses for T. chiltoni and T. novaezealandiae since we had no hypothesis which looked at inter-specific comparisons between these two species. Firstly, the relationship between the maternal body size (mm) and brood size (total number of eggs and different stages of larvae) of gravid T. chiltoni was analysed (only for spring cohorts inhabiting open estuaries). A similar approach was followed for individuals of T. novaezealandiae, but in this case the dataset was divided into samples collected from (i) ‘Open’ and (ii) ‘Intermittently open/closed’ estuaries. Then the respective datasets were subdivided for ‘Season’ (i.e. austral spring and summer).
Due to the low sample sizes the following samples were excluded from data analysis. For example, in spring, the eight gravid T. chiltoni individuals caught from the Hawkesbury Lagoon were the only specimens of this species collected from the intermittently open/closed estuaries sampled, and in summer, only four gravid T. chiltoni were caught from all the estuaries sampled (two from Waitati River, and one each from Kakanui and Waikouaiti River) (). All other individuals collected (see ) during the survey were included in the analysis.
Data analysis
During data analysis, brood size of gravid females of T. chiltoni and T. novaezealandiae were regressed against their body size (mm). The response variable (i.e. ‘brood size’) exhibited an over-dispersed Poisson distribution. Further random effects (i.e. Random variable = 1 | ‘sampling site’ in this case) were required on the intercepts of the regressions for the hierarchical nature of the sampling design. For this purpose, the ‘glmmadmb’ function of glmmADMB package of CRAN R.3.2.2 for Windows was used with an ‘nbionm1’ family, which approximates a quasi-Poisson distribution (R Development Core Team Citation2015). Mann-Whitney U-tests (body size data were not Gaussian (Shapiro-Wilk Test: P < 0.001)) were conducted to compare body sizes of mysids between austral spring and summer (only for T. novaezealandiae). Mann-Whitney U tests (independent two groups) were conducted to evaluate if the body sizes of gravid females of T. novaezealandiae from open estuaries differed from those that were caught from intermittently open/closed estuaries. All the confidence intervals were set at 95%, Z (a measure of standard deviation), U (Mann-Whitney) and P values were reported, and analyses performed using R.3.2.2 for Windows (R Development Core Team Citation2015).
Results
Body–brood sizes of Tenagomysis chiltoni
Gravid females caught from open estuaries in spring ranged from 8.7 mm to 15.3 mm in length, with a median of 13.3 mm. Their brood size had a range of 5 to 25, with a median of 16. However, brood size was not significantly related to their body size (Z = 0.22; P = 0.8) (; ).
Figure 2. Brood and body size relationships of gravid females of Tenagomysis chiltoni sampled during austral spring (2011) from the open estuaries in southern New Zealand.
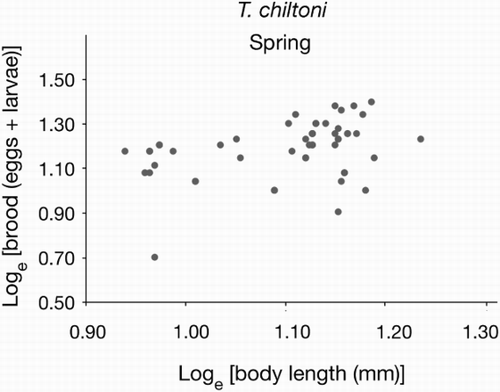
Table 2. Regressions of brood size vs. body size (mm) of gravid females of Tenagomysis chiltoni and Tenagomysis novaezealandiae caught during 2011/12 field survey in the estuaries of southern New Zealand.
Body–brood sizes of Tenagomysis novaezealandiae
Spring
Brood size had a range of 4 to 30, and a median of 10.5 for gravid T. novaezealandiae collected from open estuaries (A). Their body size range was 6.5 mm to 10.2 mm, with a median of 8 mm in length (B). Gravid females caught from intermittently open/closed estuaries had a brood size range of 3 to 39 with a median of 13 (C), and body size range was 6 mm to 12 mm in intermittently open/closed estuaries, with a median of 7.25 (D). There was no significant difference (U = 1155; P = 0.06) in body size of the gravid females caught from two different estuary types (B,D). However, brood size of gravid females caught from open estuaries was significantly smaller (U = 2301.5; P = 0.0007) than those caught from intermittently open/closed estuaries (A,C). Brood and body size relationships were significantly positive (Z = 3.05; P = 0.002) for individuals collected from open estuaries (A; ) as well as for those sampled from intermittently open/closed estuaries (Z = 8.28; P < 0.001) (A; ).
Figure 3. Brood and body sizes of gravid females of Tenagomysis novaezealandiae. A, Median and quartile ranges of brood size in open estuaries; B, median and quartile ranges of body size in open estuaries; C, median and quartile ranges of brood size in intermittently open/closed estuaries; D, median and quartile ranges of body size in intermittently open/closed estuaries. Samples were collected from upstream and downstream of six open and eight intermittently open/closed estuaries. Number of gravid females collected from each sampling site is c. 10.
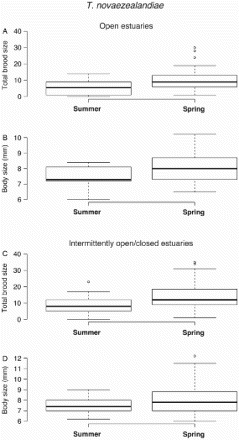
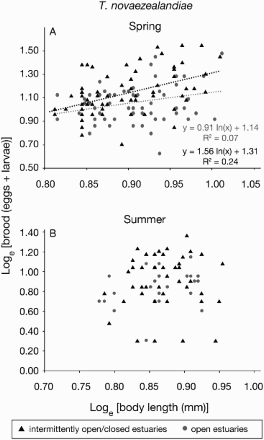
Figure 4. Brood and body size relationships of gravid females of Tenagomysis novaezealandiae. A, Sampled during austral spring (2011); B, sampled during austral summer (2012). Samples were collected from upstream and downstream of six open and eight intermittently open/closed estuaries. Number of gravid females collected from each sampling site is c. 10. Regression lines were not added where relationships were not statistically significant (α = 0.05).
Summer
Gravid T. novaezealandiae females caught from open estuaries had a brood size range of 2 to 14, and a median of 7.5 (A), whereas their body size was between 6 mm to 8.4 mm, with a median of 7.3 mm (B). In intermittently open/closed estuaries the range of brood size was 2 to 23, and a median of 9.5 (C), which is significantly higher (U = 1386.5, P = 0.043) than open estuaries (A,C). Body size was 6.1 mm to 9 mm, and median of 7.35 mm, in intermittently open/closed estuaries (D), which was not significantly different (U = 338, P = 0.95) from those gravid individuals sampled from open estuaries (B,D). Brood and body size relationships were not significant for samples that were collected from open estuaries (Z = 1.78; P = 0.07) as well as intermittently open/closed estuaries (Z = −0.19; P = 0.85) (B; ).
Discussion
Brood sizes (i.e. total number of eggs and larvae) of gravid females of T. chiltoni did not significantly increase with the increase of their body sizes. Brood sizes of gravid females of T. novaezealandiae collected from large open and small intermittently open/closed estuaries were significantly related to their body sizes in spring, but not in summer. Spring cohorts of T. novaezealandiae gravid females had a larger body size than their summer cohorts, like T. tasmania of southern Australia (Fenton Citation1992). Body sizes of gravid T. novaezealandiae collected from open estuaries were not significantly different than those collected from small intermittently open/closed estuaries. In spring, gravid females of T. novaezealandiae collected from small intermittently open/closed estuaries carried significantly larger broods than those individuals of large open estuaries, but this was not observed in summer.
In temperate estuaries, mysids, copepods, amphipods and crabs of overwintering spring generations exhibit larger maternal bodies and produce larger broods than their summer generations (Fenton Citation1992; Mees et al. Citation1994; Jeong et al. Citation2007; Viegas et al. Citation2012). This is possibly because, during spring, enhanced primary production enables females to reach maturity at their maximum body size; consequently they can carry larger broods in their pouch (Hines Citation1982; Toda et al. Citation1982; Fenton Citation1992; Väinölä & Vainio Citation1998; Fockedey et al. Citation2005, Citation2006; Jeong et al. Citation2007). Results showed spring cohorts of gravid T. novaezealandiae adhere to such patterns (i.e. body–brood size relationships are often linked in a positively significant manner), which is applicable for other temperate mysids like Neomysis spp. of the Northern Hemisphere (Fockedey et al. Citation2005, Citation2006). In contrast, relationships between body and brood size of gravid T. chiltoni and summer cohorts of gravid T. novaezealandiae were not significant. This is uncommon for temperate mysid species, but not unprecedented (Mauchline Citation1980, Citation1988); for example, the brood size of the tropical mysid Acanthomysis thailandica does not depend on body size (Ramarn et al. Citation2012). The size range of T. novaezealandiae gravid females collected during summer was narrow and less than 10 mm. According to Mauchline (Citation1980, Citation1988), if the body size of a mysid species is smaller than 10 mm then the relationship between brood and body size may not be significant. Another possibility is that abortion of eggs and/or larvae during sampling or preservation (although this has never been investigated for mysids in detail) led to the underestimation of brood sizes of T. chiltoni in spring and T. novaezealandiae in summer.
Temperate mysids and similar estuarine crustaceans are known to adjust their body and brood sizes depending on the temperature and productivity that are related to specific seasons and habitats (Hines Citation1982; Väinölä & Vainio Citation1998; Lill et al. Citation2010). For example, high mortality of juvenile mysids, crabs and copepods in winter often forces them to adopt contrasting fecundity strategies for optimising their fecundity outputs (Hines Citation1982; Toda et al. Citation1982; Atkinson Citation1995; Fockedey et al. Citation2005, Citation2006). Tenagomysis novaezealandiae were smaller and carried smaller broods in summer compared to spring, a trend which has also been observed among Neomysis spp. and in Mysis spp. which inhabit estuaries at higher latitudes of the Northern Hemisphere (Mees et al. Citation1994; Väinölä & Vainio Citation1998; Fockedey et al. Citation2005, Citation2006). It is possible that the larger body size of overwintering cohorts of mysids may alleviate some of the energetic costs of osmoregulation by altering the volume to surface ratio (Paul et al. Citation2013, Citation2014, Citation2015). We speculate that such energy saving strategies may facilitate the direction of extra resources into larger brood production. For example, in small intermittently open/closed estuaries, T. novaezealandiae gravid females carried larger broods in spring than in summer. During spring, the primary production of small intermittently open/closed estuaries of temperate South Africa and New Zealand remains high, which can boost egg production of mysids and similar crustaceans (Froneman Citation2001, Citation2004; Lill Citation2010; Lill et al. Citation2010). Only four gravid females of T. chiltoni were sampled during the austral summer of 2012. Consistent with our study, Lill et al. (Citation2010) found a small second cohort of T. chiltoni in the intermittently open/closed Kaikorai Lagoon. However, Kaikorai Lagoon is a highly productive habitat, particularly when closed for long periods, as it receives considerable nutrient-rich urban runoff (Lill Citation2010). This may indicate that, if the estuaries are sufficiently eutrophic and warm, the fecundity of mysids may be enhanced either through larger broods or by production of multiple cohorts within a short period of time. However, the actual mechanism behind such a fecundity boost is poorly studied for mysids. We suggest that the seasonal variation in productivity of specific types of estuaries could be responsible. Thus, we predict that in lower latitudes temperate mysids such as T. chiltoni may produce multiple cohorts every year. Such a prediction could be readily tested by comparison of populations across a broader range of estuaries and latitudinal range.
Acknowledgements
We thank Mrs Kajal Paul, Mr Antoine Serriere, Ms Charlyne Ribeyrolles, Ms Alicia Catlin, for assistance in the field, and Ken Miller for graphical work. Thanks to the Department of Zoology of University of Otago, Nelson Mandela Metropolitan University, and DST-NRF Shallow Water Research Chair of South Africa for institutional support.
Associate Editor: Dr Richard Taylor.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Atkinson D. 1995. Effects of temperature on the size of aquatic ectotherms: exceptions to the general rule. J Therm Biol. 20:61–74. doi: 10.1016/0306-4565(94)00028-H
- Fenton GE. 1992. Population dynamics of Tenagomysis tasmaniae Fenton, Anisomysis mixta australis (Zimmer) and Paramesopodopsis rufa Fenton from south-eastern Tasmania (Crustacea: Mysidacea). Hydrobiologia. 246:173–193. doi: 10.1007/BF00005696
- Fockedey N, Ghekiere A, Bruwiere S, Janssen CR, Vincx M. 2006. Effect of salinity and temperature on the intra-marsupial development of the brackish water mysid Neomysis integer (Crustacea: Mysidacea). Marine Biol. 148:1339–1356. doi: 10.1007/s00227-005-0160-9
- Fockedey N, Mees J, Vangheluwe M, Verslycke T, Janssen CR, Vincx M. 2005. Temperature and salinity effects on post-marsupial growth of Neomysis integer (Crustacea: Mysidacea). J Exp Marine Biol Ecol. 326:27–47. doi: 10.1016/j.jembe.2005.05.005
- Froneman PW. 2001. Seasonal changes in zooplankton biomass and grazing in a temperate estuary, South Africa. Estuar Coast Shelf S. 52:543–553. doi: 10.1006/ecss.2001.0776
- Froneman PW. 2004. Zooplankton community structure and biomass in a southern African temporarily open/closed estuary. Estuar Coast Shelf S. 60:125–132. doi: 10.1016/j.ecss.2003.12.002
- Fukui Y, Wada K. 1986. Distribution and reproduction of four intertidal crabs in Tonda River Estuary, Japan. Mar Ecol-Prog Ser. 30:229–241. doi: 10.3354/meps030229
- Hines AH. 1982. Allometric constraints and variables of reproductive effort in brachyuran crabs. Marine Biol. 69:309–320. doi: 10.1007/BF00397496
- Jeong SJ, Yu OH, Suh HL. 2007. Life history and reproduction of Jassas latteryi (Amphipoda, Ischyroceridae) on a sea grass bed (Zostera marina L.) in Southern Korea. J Crustacean Biol. 27:65–70. doi: 10.1651/S-2739.1
- Jones MB, Greenwood JG, Greenwood J. 1989. Distribution, body size, and brood characteristics of four species of mysids (Crustacea: Peracarida) in the Avon-Heathcote Estuary, New Zealand. New Zeal J Mar Fresh. 23:195–199. doi: 10.1080/00288330.1989.9516356
- Lill AWT. 2010. The ecology of intermittently closed estuaries of Otago, South Island, New Zealand [PhD. Thesis]. Dunedin: University of Otago.
- Lill AWT, Lal A, Closs GP. 2010. Life history and reproduction of two abundant mysids (Mysidacea: Mysidae) in an intermittently open New Zealand estuary. Mar Freshwater Res. 61:633–641. doi: 10.1071/MF09085
- Mauchline J. 1980. The biology of mysids. Adv Marine Biol. 8:3–369.
- Mauchline J. 1988. Egg and brood sizes of oceanic pelagic crustaceans. Mar Ecol-Prog Ser. 43:251–258. doi: 10.3354/meps043251
- Mees J, Abdulkerim Z, Hamerlynck O. 1994. Life-history, growth and production of Neomysis integer in the Westerchelde estuary (SW Netherlands). Mar Ecol-Prog Ser. 109:43–57. doi: 10.3354/meps109043
- Paul S, Closs GP. 2014. Effects of salinity and food quality on the growth of sub-adult mysids of Tenagomysis spp.: a laboratory study. Aquat Ecol. 48:229–235. doi: 10.1007/s10452-014-9478-z
- Paul S, Krkosek M, Probert PK, Closs GP. 2013. Osmoregulation and survival of two mysids of Tenagomysis from southern estuaries of New Zealand. Mar Freshwater Res. 64:340–347. doi: 10.1071/MF12316
- Paul S, Probert PK, Closs GP. 2015. Oxygen consumption of adult females of Tenagomysis spp. (Crustacea: Mysida) in relation to body size and salinity. New Zeal J Mar Fresh. 49:390–397. doi: 10.1080/00288330.2015.1027712
- R Development Core Team [Internet]. 2015. R: a language and environment for statistical computing; [cited 2016 Jan 20]. Available from: www.r-project.org
- Ramarn T, Chong VC, Hanamura Y. 2012. Population structure and reproduction of the mysid shrimp Acanthomysis thailandica (Crustacea: Mysidae) in a tropical mangrove estuary, Malaysia. Zool Stud. 51:768–782.
- Shine R. 1988. The evolution of large body size in females—a critique of Darwin’s fecundity advantage model. Am Nat. 131:124–131. doi: 10.1086/284778
- Sibly RM, Atkinson D. 1994. How rearing temperature affects optimal adult size in ectotherms. Funct Ecol. 8:486–493. doi: 10.2307/2390073
- Stearn SC. 1992. The evolution of life histories. Oxford: Oxford University Press; p. 1–262.
- Takahashi K, Kawaguchi K. 2004. Reproductive biology of the intertidal and infralittoral mysids Archaeomysis kokuboi and A. japonica on a sandy beach in NE Japan. Mar Ecol-Prog Ser. 283:219–231. doi: 10.3354/meps283219
- Toda H, Takahashi M, Ichimura S. 1982. Abundance and life-history of Neomysis intermedia Czerniawsky in Lake Kasumiguara. Hydrobiologia. 93:31–39. doi: 10.1007/BF00008096
- Väinölä R, Vainio JK. 1998. Distributions, life cycles and hybridization of two Mysis relicta group species (Crustacea, Mysida) in the northern Baltic Sea and Lake Båven. Hydrobiologia. 368:137–148. doi: 10.1023/A:1003237829779
- Viegas ISC, Marques F, Bessa A, Primo L, Martinho F, Azeiteiro UM, Pardal MA. 2012. Life history strategy of a southern European population of brown shrimp (Crangon crangon L.): evidence for latitudinal changes in growth phenology and population dynamics. Marine Biol. 159:33–43. doi: 10.1007/s00227-011-1787-3