ABSTRACT
Rivers receiving acid mine drainage (AMD) are frequently depauperate in fish and impacts may extend long distances downstream. AMD inputs may form chemical barriers for migratory species and isolate fish in unimpacted headwaters. We investigated the response of a diadromous fish, kōaro (Galaxias brevipinnis), to remediation of an AMD tributary in a 5th order river in New Zealand. A 2005 survey indicated limited recruitment of kōaro in the river likely due to the chemical barrier of AMD. By 2010, water treatment in the contaminated tributary had raised pH from a median value of 4.3 to 6 and reduced metals in the lower river, notably aluminium from a median of 2.48 to 0.41 mg/L. In 2012, kōaro density had increased by an order of magnitude relative to 2005. Furthermore, a greater proportion of juvenile fish were present. These results indicate that large-scale remediation of discharges can reverse the impacts of AMD on fish migration.
Introduction
Globally, acid mine drainage (AMD) has extirpated fish from many waterways and degraded populations in others (Stoertz et al. Citation2002; Harding Citation2005; Cravotta et al. Citation2010; Henry et al. Citation2012). AMD can impact fish through acute and chronic toxicity, smothering by sediment and indirect effects of degraded habitat and food resources (Greig et al. Citation2010). Furthermore, fish populations in unimpacted headwaters, upstream of mining impacts, can become isolated (Stoertz et al. Citation2002; Cravotta et al. Citation2010). This may particularly affect species that migrate to sea to complete their life cycle and depend on marine recruitment (Zwick Citation1992).
The New Zealand native fish fauna are broadly separated into two groups: diadromous species which migrate to and from the sea, and those that are permanent residents of freshwater (McDowall Citation1998; McIntosh & McDowall Citation2004). Diadromy is exhibited by 17 New Zealand species and their inland distributions are strongly influenced by this characteristic. Physical barriers such as waterfalls, intermittent reaches or impoundments have been shown to prevent fish from migrating upstream and reaching suitable habitats (McIntosh & McDowall Citation2004; Eikaas & McIntosh Citation2006). Once a diadromous fish has negotiated downstream obstacles, its survival in a stream is dictated by factors such as availability of habitat, water quality, disturbance and interactions with other biota.
The response of New Zealand fish species to AMD has not been well documented (Greig et al. Citation2010; Jellyman & Harding Citation2014). One aspect of survival in AMD is tolerance to acidity. Some invertebrate taxa show a degree of tolerance to naturally low pH, which is considered to convey some resistance to the effects of anthropogenic acidification and metal contamination (Collier et al. Citation1990; Winterbourn & McDiffett Citation1996; Hickey & Clements Citation1998). Similarly, New Zealand fish taxa have been found to occur in naturally low pH streams (Collier et al. Citation1990; Olsson et al. Citation2005; Greig et al. Citation2010) although they are generally intolerant of values <4 (West et al. Citation1997; Greig et al. Citation2010; Jellyman & Harding Citation2014). In a survey of fish communities on the West Coast, South Island, Greig et al. (Citation2010) found that common bully (Gobiomorphus cotidanus), redfin bully (G. huttoni) and bluegill bully (G. hubbsi) did not occur at pH below 5.9, but kōaro (Galaxias brevipinnis), banded kokopū (Galaxias fasciatus) and eels (Anguilla spp.) were present down to pH of 4.4. West et al. (Citation1997) used laboratory experiments on nine species of adult and juvenile fish to show that six species avoided pH less than 6.5. The exceptions were shortfin eel elvers, kōaro and banded kokopū. Jellyman & Harding (Citation2014) found similar variation in experimental species mortality after 14 days, although no species survived below pH 4. West et al. (Citation1997) found adult fish showed stronger pH preferences than the juveniles. However, Jellyman & Harding (Citation2014) found that juvenile īnanga (Galaxias maculatus) showed a higher mortality than adults at low pH. Thus, different species may have variable tolerances to pH and variable adaptation for short-term tolerance during their migratory stage.
AMD typically consists of low pH waters and a cocktail of heavy metals. Thus, fish toxicity in these systems is dependent on tolerance to multiple stressors. Greig et al. (Citation2010) also found a strong negative relationship between fish community and metal concentrations. They attributed this pattern to several mechanisms. First, in streams with high concentrations of certain metals, particularly aluminium (Al) and iron (Fe), acute mortality is highly likely (Baker & Schofield Citation1982; Peuranen et al. Citation1994). These toxic effects occur via electrolyte loss from inhibition of ion exchange, and adherence of metal hydroxides to gill membranes resulting in respiratory stress (Baker & Schofield Citation1982). Chronic exposure to metal concentrations below levels required for acute mortality can still result in mortality or non-lethal effects such as impaired predator avoidance, feeding, migration and fecundity (Pane et al. Citation2004). Finally, iron hydroxide precipitation and iron flocculation can result in lethal and non-lethal effects on fish and indirect effects on food resources and habitat (Vuori Citation1995; Niyogi et al. Citation2002).
Although not strictly the result of AMD, high turbidity in streams draining active mines can occur due to coal fines, road and excavation works. Studies on the effects of turbidity have shown variable response thresholds for New Zealand fish species. In trials to establish lethal sediment thresholds, banded kokopū and redfin bully showed no response to very high levels of suspended sediment (Rowe et al. Citation2009). However, banded kokopū exhibited a strong behavioural avoidance of sediment (Boubée et al. Citation1997). Juvenile banded kokopū in particular are highly sensitive to turbidity >25 NTU which reduces migration into a stream (Richardson et al. Citation2001). Kōaro avoid elevated suspended sediments, although to a lesser degree than banded kokopū, while neither longfin nor shortfin eel species showed avoidance behaviour even at 1100 NTU (Boubée et al. Citation1997). In addition, fish respond to the siltation of habitats and consequent alteration to invertebrate food resources (Richardson & Jowett Citation2002).
The impacts of AMD may extend upstream of affected reaches through isolation of migration dependent populations. In a study of fish communities in Monday Creek, Ohio, USA, AMD eliminated many taxa from an impacted reach (Stoertz et al. Citation2002). In addition, isolated headwaters, which had good water and habitat quality, had depauperate fish communities. A chemical barrier to the migration of bluegill (Lepomis macrochirus) and largemouth bass (Micropterus salmoides) was inferred from acute toxicity testing at the confluence of AMD and pH neutral streams in Walker County, Alabama, USA (Henry et al. Citation2012). Fish mortality in the mixing zone reached 96%–100% after a maximum of 10 h. However, Cravotta et al. (Citation2010) observed the return of fish to AMD impacted upper Swatara Creek, Pennsylvania, USA after water treatment in the catchment. In a similar study in Racoon Creek, Ohio, Kruse et al. (Citation2012) observed an improvement in both macroinvertebrate and fish communities over a 5 year period during which lime dosing was used to raise pH. However, due to a brief cessation of dosing (c. 2 weeks) fish populations were severely impacted and had not recovered 18 months later.
In this study we investigate the response of fish populations, particularly a native diadromous fish, kōaro, to the remediation of an AMD impacted tributary which joined the mid-reaches of a 5th order river. Fish community response to changes in water quality were assessed using a before-and-after control impact design (Quinn & Keough Citation2002).
Methods
Study catchment
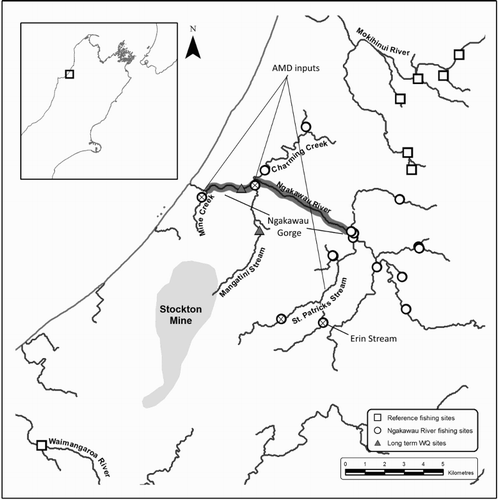
Fish and water quality sampling occurred primarily in the Ngakawau River which drains west into the Tasman Sea from the Glasgow Range and Stockton Plateau, West Coast, South Island, New Zealand (). The Ngakawau River is a 5th order river with a catchment area of 197.5 km² and mean flow of 24.7 m³/s. Annual precipitation at the coast is c. 3000 mm/year, increasing to c. 6000 mm/year 4 km inland. Daily rainfall frequently exceeds 200 mm (Davies et al. Citation2011) and the Ngakawau River is prone to large and unpredictable floods. The majority of the catchment is vegetated in native podocarp forest, although areas of fire damage have reverted vegetation to scrub and grassland (Norton & Roper-Lindsay Citation1997). There are areas of naturally acidic Pakihi bog wetland (Mew Citation1980). The upper catchment forms a large basin, before the river descends into a steep sided, 7 km long gorge (). There are no other significant human activities in this catchment except coal mining. Several tributaries are naturally brown-water formed by leaching of humic acids from slow draining organic soils (Collier et al. Citation1990). These streams are characterised by low pH (4–6), high dissolved organic carbon (>10 mg/L) and low bioavailable toxic metals (Collier & Winterbourn Citation1987; Winterbourn & Collier Citation1987). Approximately 30% of the catchment forms the Stockton Plateau (700–1100 m asl), dominated by Stockton opencast coal mine. The Stockton mine is the largest opencast mine in New Zealand and mining has occurred on this plateau since about 1880. Several major tributaries drain the mine and old workings, including Mine Creek, Mangatini and St Patrick’s streams (). Mangatini Stream enters the Ngakawau River via a >20 m waterfall which would be a significant physical barrier to any fish attempting to colonise this stream. Coal measures on the plateau are high in pyrite (max 5 wt %) which when exposed to rainfall and oxygen result in AMD (Weber et al. Citation2004).
Figure 1. Fish survey streams and water quality monitoring sites within the Ngakawau, Mohikinui and Waimangaroa rivers. The Stockton mine and major waterways are shown along with the location of long-term water quality monitoring stations. Ngakawau sites devoid of fish are marked with an X. AMD discharges to to the Ngakawau River are labelled and the extent of the Ngakawau Gorge is highlighted.
AMD from Stockton mine contains high concentrations of dissolved Al and Fe, elevated concentrations of trace metals, low pH and high sediment compared with natural streams in the area (Alarcón León & Anstiss Citation2002; Harding & Boothroyd Citation2004; Black et al. Citation2005). Consequently, streams receiving mine drainage have severely impaired ecological communities. Upstream of the confluence with the AMD impacted St Patrick’s Stream, the Ngakawau catchment is an unimpacted 4th order stream with high water quality.
We also sampled two reference rivers to provide comparisons. The Mokihinui River is immediately north of the Ngakawau River and is a 5th order river dominated by native podocarp forest. Water quality is generally high throughout the catchment although several tributaries contain abandoned coal mines. The Waimangaroa River is a 4th order stream immediately south of the Ngakawau and drains southern Stockton Plateau and adjacent Denniston Plateau. The Waimangaroa River receives AMD from several historic mines and has impaired fish and invertebrate communities, but high water quality in the sampled coastal tributary (Harding et al. Citation2006; Gray & Harding Citation2012). Both of these catchments also have no other significant human land use activities.
Water quality remediation
Remediation began in 2005 in the Mangatini Stream with the aim of achieving water quality targets by 2010. Remediation treatments included sedimentation ponds, a water treatment plant, an active and continual limestone dosing of the Mangatini Stream and the Mangatini sump (a 900,000 m³ settling pond). Full details of remediation are described by Pizey et al. (Citation2012). Although the Ngakawau River receives AMD from St Patrick’s Stream, Mangatini Stream and Mine Creek, Mangatini Stream drains currently active mining sites and is the main source of AMD to the Ngakawau River (Pizey et al. Citation2012).
Water quality and fish sampling
Daily monitoring of pH, Al and turbidity occurred in the Mangatini Stream and lower Ngakawau River at long-term water quality monitoring stations between 2005 and 2012 (). pH was measured using a Hach HQ30d meter. Total dissolved aluminium was analysed according to APHA 21st ed. Citation2005 standard methods (APHA Citation2005) after sample preservation with nitric acid using an ICP-MS at the SGS laboratory in Waihi, New Zealand. Turbidity (NTU) was measured using a Hach Turbidity model 2100 N meter. During fish surveys, spot measurements of water quality (pH, conductivity, temperature and dissolved oxygen) were made at each site using a YSI ProPlus multimeter that was calibrated at the start of each day.
Fish surveys were conducted in 2005 and 2011–2012. Populations were assessed at 25 sites: 18 within the Ngakawau catchment of which five had water chemistry indicative of AMD, while 13 did not (). Seven reference sites with unimpacted water chemistry were selected on tributaries of the Mokihinui River (six sites) and the Waimangaroa River (one site). Sites were selected based on accessibility and presumed water quality with the intention of providing a symmetrical comparison of fish communities relative to AMD impact and distance inland. Unfortunately, there are no suitable coastal (i.e. downstream of physical barriers) non-AMD impacted tributaries of the Ngakawau. Thus, the effect of AMD remediation on non-climbing/coastal species could not be assessed. Fish community sampling occurred along a stream reach up to 50 m in length, using single pass electric fishing with a Kainga 300 electrofishing backpack machine (NIWA). Fish were captured using a single downstream push net and handheld dip nets. In 2005, all sites were sampled during November in the austral spring, whereas during the latter survey, Ngakawau River sites were sampled in November 2011 and reference streams in March 2012. The later sampling of reference sites may have resulted in a lesser density of smaller, young-of-the-year fish compared with the Ngakawau River, but was unavoidable. Stream reaches were chosen to include multiple habitats (e.g. riffle, run, pool) and fishing effort at each site was similar between occasions. Fish were identified, measured to the nearest mm (fork length) and released.
Statistical analysis
Differences in spot water quality and kōaro density between 2005 and 2012 were assessed using two-way repeated measures ANOVA (rep-ANOVAs). The between-subjects factor (treatment) for the comparison of water quality were the three stream types, and the within-subjects factor (time) were the two measurements taken at each site in 2005 and 2010–2011. Differences between spot water quality treatments were assessed using Tukey HSD tests. Kōaro were absent from the majority of AMD impacted Ngakawau sites and so the kōaro density rep-ANOVA compared only two stream types: Ngakawau and reference. Within treatment differences between occasions for kōaro density were assessed using paired t-tests. Both the water quality and kōaro rep-ANOVAs used type III sums of squares to allow for unequal sample sizes between site categories (Quinn & Keough Citation2002). Data were assessed for normality and heteroscedasticity, and log transformations were deemed necessary for temperature and conductivity (Quinn & Keough Citation2002). Analyses were conducted in SPSS version 21 (International Business Machines Corporation).
Results
Long-term water quality
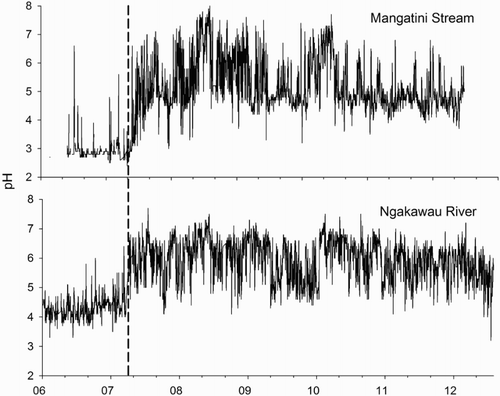
Between 2007 and 2012, water quality in Mangatini Stream and lower Ngakawau River showed a marked improvement in pH, Al and turbidity. Before remediation, pH in the Mangatini Stream had a median value of 2.9 (range 2.4–6.6) (). After remediation began in early 2007, the median value increased to 5 (3.0–8.0). The lower Ngakawau River (below the Mangatini Stream confluence) responded to this improvement. Median pH increased from 4.3 (3.3–6.7) to 6 (3.2–7.7) post remediation. The pH minima in both rivers remain similar at each site irrespective of remediation. These minima typically occurred during high-flow events due to flushes of organic acids in stream water but also due to temporary failure of the remediation equipment to deal with excessively high flows.
Figure 2. Water pH recorded in the Mangatini Stream and lower Ngakawau River between 2006–2012. The vertical black line indicates the start date of limestone dosing.
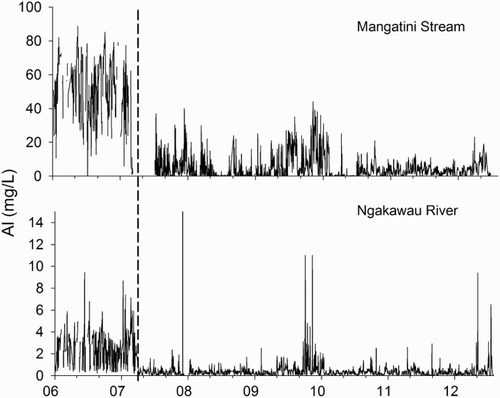
Dissolved aluminium concentrations in the Mangatini Stream prior to remediation were elevated (). At April 2007, median Al was 51.3 mg/L (0.008–88). After remediation, the median value declined markedly to 4.6 mg/L (0.0016–44). In the lower Ngakawu River, prior to April 2007, Al concentrations had a median value 2.48 mg/L (0.082–9.46). Subsequently, the value fell to 0.41 mg/L (0.001–52). Occasional spikes in Al concentrations are also assumed to be linked to failures in the remediation equipment.
Figure 3. Dissolved Al (mg/L) concentrations in the Mangatini Stream and Ngakawau River. The vertical black line indicates the start date of limestone dosing.
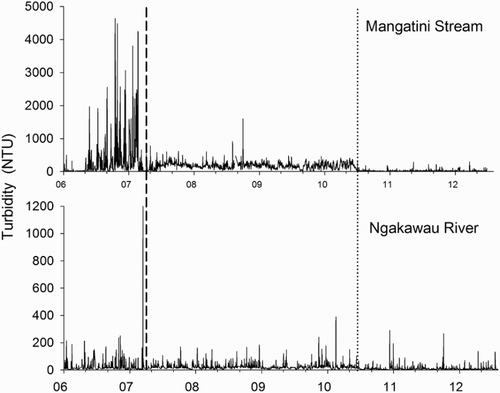
Prior to remediation, turbidity in the Mangatini Stream had a median value of 113 NTU (1–4640) which increased to a median of 180 NTU (1–1600) between April 2007 and July 2010 due to lime dosing (). However, from July 2010, the Mangatini sump was constructed and from that time limestone dosing sludge was captured in this trap (Pizey et al. Citation2012). This resulted in a decline in turbidity to a median value of 18 NTU (2–292). In the lower Ngakawau River, turbidity showed a similar, if attenuated, pattern. Prior to April 2007, the median value was 12 NTU (2–1233), whereas between April 2007 and July 2010 the value increased to 18 NTU (3–390). Subsequent to July 2010, turbidity declined to 7 NTU (1–290).
Survey water quality
Spot water chemistry at individual sampling sites showed little change between 2005 and 2012 (). The exception was the remediated Mangatini Stream where pH increased from 3.1 to 4.7 and conductivity declined from 834 to 442 μS25/cm. Overall, AMD sites in the Ngakawau River were characterised by significantly lower pH and higher conductivity, but were also warmer than unimpacted and reference sites (). The difference in temperature is likely due to the lack of riparian vegetation along many of the AMD impacted streams. Repeated measure ANOVA showed no overall changes over time for temperature or pH, but conductivity was shown to be significantly lower in 2011–2012 than 2005 (F(1) = 13.997, P = 0.001). In unimpacted Ngakawau and reference streams, water quality was very similar between occasions in terms of temperature, dissolved oxygen and conductivity. However, while pH in the reference streams was circum-neutral, unimpacted streams within the Ngakawau River catchment showed a considerable range, 4.8–7.8. This range is within natural variation and due to brown-water streams containing high concentrations of organic acids (Greig et al. Citation2010).
Table 1. Comparison of water quality at reference sites and within the Ngakawau River system in 2005 and 2011–2012.
Fish communities
A total of 11 fish species were identified during the surveys. Seven species in 2005 and nine in 2011–2012. The majority of taxa were from diadromous families; Anguillidae (two species), Galaxiidae (three species), Gobiidae (three species) and Pinguipedidae (one species). Non-diadromous Gobiidae, upland bully and an introduced salmonid, brown trout (Salmo trutta), were also recorded.
Despite sampling 17 sites in the Ngakawau River, fish diversity was low compared with the overall survey with four species, kōaro, longfin eel, shortfin eel and upland bully (Gobiomorphus breviceps) observed. This is most likely due to the paucity of unimpacted coastal tributaries in the lower Ngakawau River that could support and act as a refuge for migratory juvenile fish. Eels were represented by three longfins in 2005 and a single shortfin eel in 2011. Upland bully were found in low abundance; 15 individuals (mean 0.08 fish/10 m²) across six sites in 2005 and 20 individuals (0.31 fish/10 m²) across five sites in 2012. Three AMD impacted tributaries; Mine Creek, Mangatini and St Patrick’s Stream were devoid of fish (). Erin Stream also contained no fish, although water quality did not indicate AMD effects.
In contrast, the seven reference streams contained a greater diversity of fish species (nine species). Many of the reference sites were close to the coast and downstream of any significant coastal barriers that might prevent colonisation by non-climbing species. Kōaro were present at all references sites in 2005 and five sites in 2012. Longfin eel were common in 2005, but occurred at fewer sites in 2012. Despite greater overall fish diversity in reference streams, no single site contained more than four taxa.
In 2005, 34 kōaro were present across 10 of the 14 Ngakawau River sites which contained any fish. However, in 2011, 134 kōaro were found in 12 of those 14 sites. The additional sites that contained kōaro in 2011 were upstream of the likely chemical barrier that existed in 2005. In 2005, St David’s Stream was devoid of fish, while Frank Creek, a tributary of Charming Creek, contained a single longfin eel. Charming Creek has a waterfall at its confluence with the Ngakawau River, which probably acts as a partial physical barrier. In the Ngakawau River, overall kōaro density in 2005 averaged 0.16 fish/10 m², but increased to 1.13 fish/10 m² in 2011 (). A significant interaction was found between time and treatment (rep-ANOVA [within-subjects factor] time*treatment, F(1) 30.459, P < 0.001) and this was due to both the increase in kōaro density between 2005 and 2011 in the Ngakawau River (paired t test, t(11) = −5.473, P < 0.001) and a decrease in kōaro density in the reference streams over the same period (paired t test, t(5) = 4.690, P = 0.005) () (). In reference streams, the decline in kōaro density between 2005 and 2012 from 0.81 to 0.39 fish/10 m² may have been partially driven by the fewer young-of-the-year fish observed in March 2012 compared with November 2005. Young-of-the-year fish are likely to have experienced natural rates of mortality in the 2012 populations prior to the March sampling. Nonetheless, the reference streams show a reduction in kōaro density compared with an increase in the Ngakawau River over the same time period indicating that non-AMD related fluctuations in recruitment are unlikely to be the cause of the change observed in the Ngakawau River.
Figure 5. The standard error of the mean (±1 SEM) density of kōaro at sites within and adjacent to the Ngakawau River system in 2005 and 2012. Significant (P < 0.05) differences between groups derived from within treatment pairwise t-tests are indicated by letters.
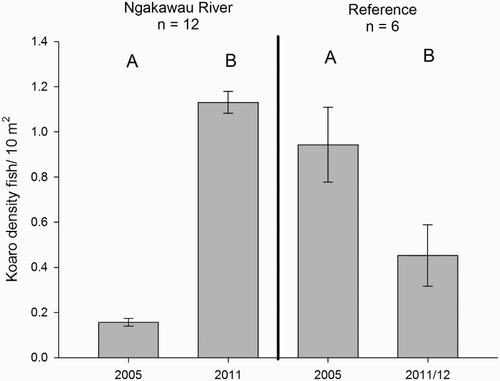
Table 2. Repeated measures ANOVA of kōaro density at sites within and adjacent to the Ngakawau River system in 2005 and 2012.
Length frequency distributions of kōaro in the Ngakawau River and reference streams in 2005 and 2011–2012 showed some distinct differences (). In the Ngakawau River, overall size range of kōaro remained fairly constant between occasions. However, in 2005, the median length was 166 mm, but 93 mm in 2011. In 2005, the Ngakawau River kōaro population was dominated by a few mature fish and only 9% of fish were <100 mm. However by 2011, there were more kōaro overall and a greater number of juvenile fish (58% <100 mm). Kōaro ≤100 mm in length are presumed to be recent recruits from the sea that have entered the river in spring of 2011 or 2010.
Figure 6. Length frequency distributions of kōaro within and adjacent to the Ngakawau River system in 2005 and 2011–2012.
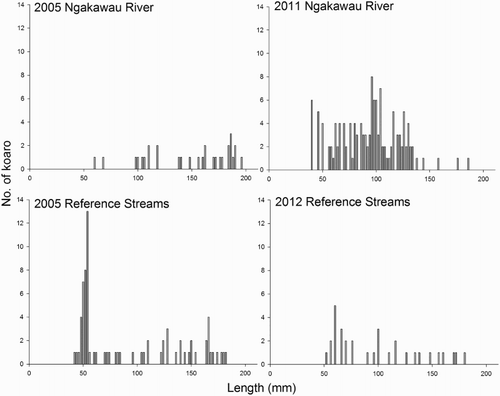
In reference streams, the size range and demographics of kōaro remained fairly constant between occasions, although the large cohort of migrating juvenile fish observed in 2005 were less apparent in 2012. Sampling in 2005 occurred in November as opposed to March in 2012, which may have influenced the numbers of migrating juvenile fish recorded. However, the kōaro populations were largely unchanged having a median length of 66 mm in 2005 and 93 mm in 2012.
Discussion
Numerous studies of migratory fish in New Zealand have indicated the importance of distance inland and ability of species to surmount downstream barriers such as waterfalls or drying reaches (McDowall Citation1998). In this study, we present evidence that an AMD discharge composed of low pH (e.g. 3.3), high dissolved metals and sediment concentrations constituted a chemical barrier for migratory fish. Fish populations in unimpacted upstream reaches became isolated and recruitment limited. Isolated populations had low densities and were dominated by larger (and presumably older) fish. The occurrence of some fish suggests limited recruitment into these upper reaches was possible. This may have occurred during floods when water quality improved sufficiently for juveniles to swim 7–8 km of impacted waters. Remediation resulted in a marked reduction in the frequency and magnitude of the chemical barrier and restored connection between headwaters and the sea for much of the time. These findings suggest that with adequate treatment of discharge to waterways, the impacts of AMD on fish migration can be effectively mitigated.
Fish population dynamics
Despite changes to water quality, fish diversity in the Ngakawau River changed little between 2005 and 2011. The only difference was the occurrence of a single shortfin eel in 2011 and the absence of any longfin eels, of which three were recorded in 2005. Neither species was abundant nor were we able to determine trends in eel occurrence. Non-migratory upland bullies remained sporadic and at low abundances on both sampling occasions. Conversely, kōaro occurred at 20% more sites in 2011 than 2005, and were more abundant (294% increase). Most sites, except those furthest upstream, contained young-of-the-year fish in 2011, but also cohorts indicative of successful colonisation over successive recent years. Although we are currently unable to estimate the age of fish, it seems likely that significant numbers of kōaro navigated the lower Ngakawau River during the previous three migratory seasons coinciding with water quality improvements.
Fish populations fluctuate naturally, being prone to mortality due to flooding and fluctuations in recruitment from the sea (McIntosh & McDowall Citation2004). However, the change in kōaro population structure in the Ngakawau River between occasions contrasted with that in adjacent reference streams. Reference streams were relatively unimpacted by mining and other land use and assumed to provide an indication of natural fluctuations in recruitment and extirpation by disturbance. There was no difference between kōaro densities and little change in demographics in reference streams. This provides further evidence that AMD discharges to the Ngakawau River were a chemical barrier to fish migration which was subsequently largely remediated.
The absence in 2011 of mature kōaro observed in 2005 is likely due to natural senescence. Kōaro are known to be a relatively long-lived fish which grow slowly once mature and may be faithful to a location. Twenty-seven percent of Kōaro tagged in 1988 in a North Island, New Zealand, stream were recaptured at the initial tagging site in 1997 and showed growth rates between 0.2 and 4.7 mm per year (R Allibone, pers. comm. June 2012). The small population of mature fish observed in 2005 in the Ngakawau River would have been vulnerable to stochastic events such as those caused by floods. The greater abundance and size range of fish found in 2011 represents a more resilient population.
Potential fish diversity
Fish populations in the upper Ngakawau River appear species depauperate, being composed of only three diadromous taxa and two sparse non-diadromous species: the upland bully and a population of brown mudfish, Neochanna apoda, in the upper Mangatini Stream. This survey did not include non-mine impacted streams close to the coast, i.e. below potential physical barriers. However, small drains feeding into the Ngakawau River mouth contain eel, īnanga, kōaro, banded kokopū and common bully (J Harding, unpubl. data) and nearby lowland streams contain shortjaw kokopū (Galaxias postvectis), giant kokopū (Galaxias argenteus), redfin and bluegill bully, torrentfish (Cheimarrichthys fosteri), and brown trout (Harding et al. Citation2006). Thus, all of these species could potentially occur throughout the Ngakawau River.
The Ngakawau Gorge is likely to be a significant physical barrier to fish passage, irrespective of water quality. A galaxiid fish distribution model, based on effects of physical obstacles (but not AMD) was modified from Eikaas & McIntosh (Citation2006) and applied to the Ngakawau River (Harding et al. Citation2006). The model predicted that the only galaxiid species able to migrate the Ngakawau Gorge would be kōaro. Banded kokopū, proficient climbers, were not predicted to occur upstream. Several non-galaxiid species might breach the gorge. For example, we recorded eels in low numbers.
Introduced brown trout are a significant predator of many native New Zealand fish to the extent that populations have entirely disjunct distributions in some river systems (McIntosh Citation2000). The potential for brown trout to access the Upper Ngakawau River after further improvements to water quality remains unknown. The absence of brown trout is an unusual feature for a river of this size and location. There are occasional tributaries of rivers where waterfalls create refuges for native fish. However, a trout-free catchment is rare and provides an opportunity to observe a subset of the native fish fauna without the constraints of salmonid predation. While other examples occur on both Stewart and the Chatham Islands (Chadderton & Allibone Citation2000) potential further loss of native fish habitat to trout should be viewed as a negative consequence of AMD remediation. Although the relative contributions of physical obstacles and chemical barriers to the failure of colonisation by brown trout are currently unknown it seems likely that a combination of low pH, elevated turbidity and dissolved metals may be a significant obstacle.
Conclusion
Very poor water quality in the lower Ngakawau River has in the past almost certainly prevented colonisation by several fish species and reduced the recruitment of kōaro (Greig et al. Citation2010; Jellyman & Harding Citation2014). However, water quality, particularly pH, dissolved metals and turbidity have improved such that we might expect increased recruitment by kōaro and eels and recolonisation by the other common fish taxa found on the West Coast. If water quality continues to improve, it is possible that several more fish species, including brown trout, may colonise at least the lower Ngakawau River and potentially navigate the gorge into the upper reaches.
The environmental impacts of mining extend well beyond the boundaries of mining activities, particularly when discharges to waterways are considered. Impacts extend downstream, but also have ramifications for the upstream catchment when populations require access to and from the ocean. However, this study shows the environmental gains that can be achieved through appropriate treatment or remediation of AMD discharges to waterways and provides a useful example for current and future mining developments.
Acknowledgements
The Department of Conservation granted permission to sample fish in 2005 under permit 11/589 and in 2011–2012 under permit WC-3241-FAU. In 2005, sampling was conducted under permit 2005/30R from the University of Canterbury Animal Ethics Committee. We thank Justin Kitto, Pete McHugh, Phil Jellyman, Simon Howard, Annabel Barnden, Katherine Muchna and Richard Allibone for assistance in the field and Glenn Rutter of Solid Energy (New Zealand) for water quality data. We thank Richard Allibone for comments on a draft of this manuscript.
Associate Editor: Dr Joanne Clapcott.
References
- Alarcón León E, Anstiss RG. 2002. Selected trace elements in Stockton, New Zealand waters. New Zeal J Mar Freshw Res. 36:81–87. doi: 10.1080/00288330.2002.9517072
- APHA. 2005. Standard methods for the examination of water and wastewater. 21st ed. Washington, DC: American Public Health Association.
- Baker JP, Schofield CL. 1982. Aluminium toxicity to fish in acidic waters. Water Air Soil Pollut. 18:289–309. doi: 10.1007/BF02419419
- Black A, Trumm D, Lindsay P. 2005. Impacts of coal mining on water quality and metal mobilisation: case studies from West Coast and Otago. In: Moore TA, Black A, Centeno J, Harding J, Trumm D, editors. Metals in New Zealand. Christchurch, New Zealand: Resolutionz Press Ltd; p. 247–260.
- Boubée JAT, Dean TL, West DW, Barrier RFG. 1997. Avoidance of suspended sediment by the juvenile migratory stage of six New Zealand native fish species. New Zeal J Mar Freshw Res. 31:61–69. doi: 10.1080/00288330.1997.9516745
- Chadderton WL, Allibone RM. 2000. Habitat use and longitudinal distribution patterns of native fish from a near pristine Stewart Island, New Zealand, stream. New Zeal J Mar Freshw Res. 34:487–499. doi: 10.1080/00288330.2000.9516951
- Collier KJ, Ball OJ, Graesser AK, Main MR, Winterbourn MJ. 1990. Do organic and anthropogenic acidity have similar effects on the aquatic fauna. Oikos. 59:33–38. doi: 10.2307/3545119
- Collier KJ, Winterbourn MJ. 1987. Faunal and chemical dynamics of some acid and alkaline New Zealand streams. Freshw Biol. 18:227–240. doi: 10.1111/j.1365-2427.1987.tb01310.x
- Cravotta CA, Brightbill RA, Langland MJ. 2010. Abandoned mine drainage in the Swatara creek basin, southern anthracite coalfield, Pennsylvania, USA: 1 stream water quality trends coinciding with the return of fish. Mine Water Environ. 29:176–199. doi: 10.1007/s10230-010-0112-6
- Davies H, Weber P, Lindsay P, Craw D, Peake B, Pope J. 2011. Geochemical changes during neutralisation of acid mine drainage in a dynamic mountain stream, New Zealand. Sci Total Environ. 409:2971–2980. doi: 10.1016/j.scitotenv.2011.04.034
- Eikaas HS, McIntosh AR. 2006. Habitat loss through disruption of constrained dispersal networks. Ecol Appl. 16:987–998. doi: 10.1890/1051-0761(2006)016[0987:HLTDOC]2.0.CO;2
- Gray DP, Harding JS. 2012. Acid Mine Drainage Index (AMDI): a benthic invertebrate biotic index for assessing coal mining impacts in New Zealand streams. New Zeal J Mar Freshw Res. 46:335–352. doi: 10.1080/00288330.2012.663764
- Greig HS, Niyogi DK, Hogsden KL, Jellyman PG, Harding JS. 2010. Heavy metals: confounding factors in the response of New Zealand native freshwater fish assemblages to natural and anthropogenic acidity. Sci Total Environ. 408:3240–3250. doi: 10.1016/j.scitotenv.2010.04.006
- Harding JS. 2005. Impacts of metals and mining on stream communities. In: Moore TA, Black A, Centeno JA, Harding JS, Trumm D, editors. Metal contaminants in New Zealand. Christchurch: Caxton Press; p. 343–357.
- Harding JS, Boothroyd IKG. 2004. Impacts of mining. In: Harding JS, Mosley MP, Pearson C, Sorrell B, editors. Freshwaters of New Zealand. Christchurch: New Zealand Limnological Society; p. 36.1–36.10.
- Harding JS, McIntosh AR, Gray DP, Jellyman P, Eikaas H. 2006. Distribution of native fish in the Ngakawau and Waimangaroa rivers. Report for Solid Energy, School of Biological Sciences. Christchurch: University of Canterbury.
- Henry TB, Urwin ER, Grizzle JM, Wildhaber ML, Brumbaugh WG. 2012. Acute toxicity of an acid mine drainage mixing zone to juvenile bluegill and largemouth bass. T Am Fish Soc. 128:919–928. doi: 10.1577/1548-8659(1999)128<0919:ATOAAM>2.0.CO;2
- Hickey CW, Clements WH. 1998. Effects of heavy metals on benthic macroinvertebrate communities in New Zealand streams. Environ Toxicol Chem. 17:2338–2346. doi: 10.1002/etc.5620171126
- Jellyman P, Harding JS. 2014. Variable survival across low pH gradients in freshwater fish species. J Fish Biol. 85:1746–1752. doi: 10.1111/jfb.12497
- Kruse NA, Bowman JR, Mackey AL, McCament B, Johnson KS. 2012. The lasting impacts of offline periods in lime dosed streams: a case study of Racoon Creek, Ohio. Mine Water Environ. 31:266–272. doi: 10.1007/s10230-012-0194-4
- McDowall RM. 1998. Fighting the flow: downstream-upstream linkages in the ecology of diadromous fish faunas in West Coast New Zealand Rivers. Freshw Biol. 40:111–122. doi: 10.1046/j.1365-2427.1998.00336.x
- McIntosh AR. 2000. Habitat- and size-related variations in exotic trout impacts on native galaxiid fishes in New Zealand streams. Can J Fish Aquat Sci. 57:2140–2151. doi: 10.1139/f00-188
- McIntosh AR, McDowall R. 2004. Fish communities in rivers and streams. In: Harding JS, Mosley P, Pearson C, Sorrell B, editors. In: Freshwaters of New Zealand. Christchurch: New Zealand Limnological Society; Chapter 17.
- Mew G. 1980. West Coast South Island. In: Lee R, editors. Soil groups of New Zealand Part 5. Podzols and gley podzols. Lower Hutt: New Zealand Soil Society; p. 238–248.
- Niyogi DK, Lewis WM, McKnight DM. 2002. Effects of stress from mine drainage on diversity, biomass, and function of primary producers in mountain streams. Ecosystems. 5:554–567.
- Norton D, Roper-Lindsay J. 1997. Ngakawau ecological area–Burma Road–Mangatini Stream boundary. Report to the Department of Conservation. West Coast Land Conservancy.
- Olsson K, Stenroth P, Nystrom P, Holmqvist N, McIntosh AR, Winterbourn MJ. 2005. Does natural acidity mediate interactions between introduced brown trout, native crayfish and other invertebrates in West Coast New Zealand streams? Biol Conserv. 130:255–267. doi: 10.1016/j.biocon.2005.12.019
- Pane EF, Haque A, Goss GG, Wood CM. 2004. The physiological consequences of exposure to chronic, sub-lethal waterborne nickel in rainbow trout (Oncorhynchus mykiss): exercise vs. resting physiology. J Exp Biol. 207:1249–1261. doi: 10.1242/jeb.00871
- Peuranen S, Vuorinen PJ, Vuorinen PM, Hollender A. 1994. The effects of iron, humic acids and low pH on the gills and physiology of brown trout (Salmo trutta). Ann Zoologici Fennici. 31:389–396.
- Pizey M, Weber P, Lindsay P, Rutter G, Rossiter P, Thomas D, Crombie F, Gray DP. 2012. Management of mine-impacted waters on the Ngakawau River. In: Proceeding of the Australasian Institute of Mining and Metallurgy New Zealand Branch Annual Conference; 2012 August 26-29; Rotorua, New Zealand. AusIMM, New Zealand Branch; p. 351–368.
- Quinn GP, Keough MJ. 2002. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press; 527p.
- Richardson J, Jowett IK. 2002. Effects of sediment on fish communities in East Cape streams, North Island, New Zealand. New Zeal J Mar Freshw Res. 36:431–442. doi: 10.1080/00288330.2002.9517098
- Richardson J, Rowe DK, Smith JP. 2001. Effects of turbidity on the migration of juvenile banded kokopū (Galaxias fasciatus) in a natural stream. New Zeal J Mar Freshw Res. 35:191–196. doi: 10.1080/00288330.2001.9516989
- Rowe DK, Hicks M, Smith JP, Williams E. 2009. Lethal concentrations of suspended solids for common native fish species that are rare in New Zealand rivers with high suspended sediment loads. New Zeal J Mar Freshw Res. 43:1029–1038. doi: 10.1080/00288330.2009.9626526
- Stoertz MW, Bourne H, Knotts C, White MW. 2002. The effects of isolation and acid mine drainage on fish and macroinvertebrate communities of Monday Creek, Ohio, USA. Mine Water Environ. 21:60–72. doi: 10.1007/s102300200021
- Vuori KM. 1995. Direct and indirect effects of iron on river ecosystems. Ann Zoologici Fennici. 32:317–329.
- Weber PA, Stewart WA, Skinner WM, Weisener CG, Thomas JE, Smart R. 2004. Geochemical effects of oxidation products and framboidal pyrite oxidation in acid mine drainage prediction techniques. Appl Geochem. 19:1953–1974. doi: 10.1016/j.apgeochem.2004.05.002
- West DW, Boubée JAT, Barrier RFG. 1997. Responses to pH of nine fishes and one shrimp native to New Zealand freshwaters. New Zeal J Mar Freshw Res. 31:461–468. doi: 10.1080/00288330.1997.9516779
- Winterbourn MJ, Collier KJ. 1987. Distribution of benthic invertebrates in acid, brown water streams in the South Island of New Zealand. Hydrobiologia. 153:277–286. doi: 10.1007/BF00007214
- Winterbourn MJ, McDiffett WF. 1996. Benthic faunas of streams of low pH but contrasting water chemistry in New Zealand. Hydrobiologia. 341:101–111. doi: 10.1007/BF00018114
- Zwick P. 1992. Stream habitat fragmentation—a threat to biodiversity. Biodivers Conserv. 1:80–97. doi: 10.1007/BF00731036