ABSTRACT
While many of New Zealand’s freshwater fishes undertake larval migrations as part of their amphidromous life-history, little is known of the larval stages of these fish. Torrentfish (Cheimarrchthys fosteri), a New Zealand endemic, amphidromous, riffle specialist are particularly enigmatic; their spawning sites and behaviours are unknown, and larvae have never been collected either emigrating from freshwater or during their marine feeding phase. During summer drift sampling, we captured unidentified fish larvae emigrating downstream in the Waianakarua River, South Island, New Zealand. Based on multiple lines of evidence (meristic comparisons with adults, morphology, time of capture, and adult fish populations of the Waianakarua) we identify these larvae as torrentfish. This represents the first time torrentfish larvae have been captured or identified, laying the foundations for future studies into the early life-history and ecology of this unique and threatened fish.
Introduction
Knowledge of the reproductive habits and early life-history of fish is critical for their conservation and management, allowing for the monitoring and targeted management of early life stages and their habitats (Chambers & Trippel Citation1997). Unfortunately, the early life-histories of many of New Zealand’s freshwater fishes remain largely unknown or unconfirmed, with much of our understanding based on estimates and assumptions (e.g. McDowall Citation1995). For example, despite contributing to the highly valued whitebait fishery, the timing and location of spawning of New Zealand’s largest and perhaps most iconic freshwater fish, the giant kokopu (Galaxias argenteus), was only recently described (Franklin et al. Citation2015), and the timing of events such as spawning and larval/juvenile migration for many species is either unknown or based on speculation (McDowall Citation1995).
The torrentfish (Cheimarrichthys fosteri), endemic to New Zealand, is a unique freshwater fish species, being the sole member of the family Cheimarrichthyidae (though this classification has been disputed; see McDowall Citation2010). A riffle specialist, torrentfish are widespread throughout the country, but are recognised as threatened, being listed as ‘declining’ by the New Zealand Department of Conservation (Goodman et al. Citation2014) and ‘vulnerable’ by the IUCN (Allibone et al. Citation2014). Little is known of the life-history of the torrentfish, particularly their reproductive and early life-history.
Adult torrentfish often penetrate considerable distances inland (nearly 300 km in some low-gradient river systems (McDowall Citation2000; NIWA Citation2016)), but undertake a downstream migration towards the sea prior to spawning (Scrimgeour & Eldon Citation1989; Warburton Citation2016), although the actual timing, mode, and location of spawning remains unknown. Trace element analyses of torrentfish otoliths have confirmed an amphidromous life-history (Tana Citation2009; Warburton Citation2016), with larvae spending a short feeding period in the sea before returning to freshwater as juveniles. Torrentfish have been observed during their adult spawning migration (Scrimgeour & Eldon Citation1989; Warburton Citation2016), as well as returning to rivers as juveniles (McDowall Citation1994), but have never been described as larvae, either during their period of downstream drift, or their marine larval phase. In the absence of these descriptions, the study of torrentfish early life-history and the implementation of management actions to protect them during their vulnerable migration (e.g. Jarvis & Closs Citation2015) is impossible.
Here, we report the first capture of newly hatched torrentfish larvae emigrating to sea, collected during summer drift sampling of the Waianakarua River, South Island, New Zealand (see Jarvis & Closs Citation2015). We describe the key characteristics which differentiate torrentfish larvae from New Zealand’s other amphidromous fishes, allowing for their identification and study in future.
Materials and methods
Field sampling
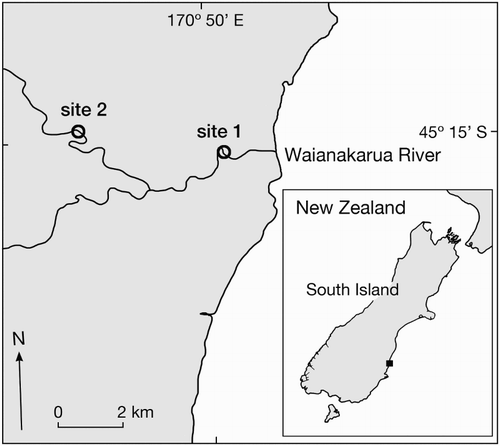
Larval fish were collected from the Waianakarua River, South Island, New Zealand (). Rectangular drift nets (mouth = 60 cm × 30 cm, length = 1.2 m, mesh = 250 µm) were deployed at two sites during the austral summer (January 2014). At each site, three nets were deployed simultaneously for c. 20 min, spaced evenly from the main channel to the bank. Samples were collected at six time periods (before sunrise, after sunrise, midday, before sunset after sunset, midnight) over three 24-h periods (9–10 January, 20–21 January, and 25–26 January) and immediately preserved in 5% formalin. Larval fish were isolated from each sample, identified (described below), counted, and measured for various morphological characteristics. For a full description of sampling and subsequent sample processing, see Jarvis and Closs (Citation2015).
Larval identification
Fish larvae were identified based on multiple lines of evidence. Adult fish assemblages (determined via electro-fishing surveys (M. Warburton and M. Jarvis pers. obs.) and the New Zealand freshwater fish database (NIWA Citation2016)) were combined with existing (albeit incomplete) larval keys (McCarter Citation1994) to identify fish larvae. Where incomplete information in existing keys did not allow for morphological identification, meristic comparisons of larval myomere counts and adult vertebral counts were used for identification (e.g. McDowall & Suren Citation1995). Because the original intention of the sampling was to determine the spatial and temporal drift patterns of larval bluegill bully (Gobiomorphus hubbsi), larvae were preserved in formalin, which best maintains their size and shape for morphological measurement and identification (Cunningham et al. Citation2000), and so genetic identification was impossible. For a full rationale of identification as torrentfish, see the discussion.
Results
One hundred and eight drift samples were processed, containing a total of 22,253 fish larvae of 5 different species. The larval catch was dominated (>99%) by amphidromous Gobiomorphus species (bluegill bully G. hubbsi and common bully G. cotidianus; for further information, see Jarvis & Closs Citation2015). The other three species identified contributed <1% of total numbers; and included (in order of abundance): torrentfish (n = 56), upland bully (Gobiomorphus breviceps; n = 22), and pouched lamprey (Geotria australis; n = 1). This represents the first collection and identification of larval torrentfish.
Description of torrentfish larvae
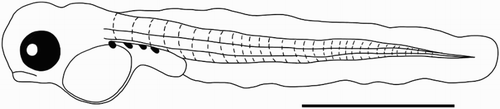
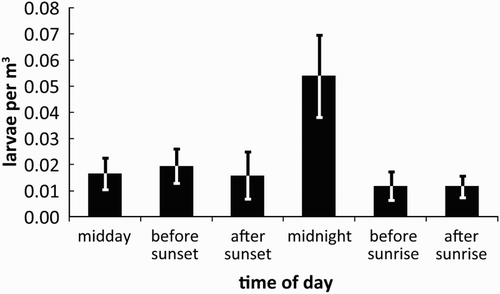
Torrentfish larvae (n = 56; ) had a mean notochord length of 2892 ± 27 μm. A large yolk sac (mean depth = 447 μm) was present on all larvae. Myomere counts ranged between 32 and 33. All larvae were also characterised by a row of melanophores above the yolk sac and gut, which is short (compared to Galaxias) and relatively deep and straight (as opposed to Gobiomorphus larvae, in which the gut is curved and more slender at a similar developmental stage, or kinked due to the location of the swim bladder at a similar size (McCarter Citation1994; Jarvis & Closs Citation2015)). Larval torrentfish were captured only at the downstream site, located c. 1.5 km from the sea. Drifting larvae were captured during all time periods sampled, but exhibited a small peak in abundance during the ‘midnight’ sampling ().
Discussion
Identification of torrentfish larvae
The Waianakarua supports a diverse fish fauna, but is dominated by bluegill bully (G. hubbsi) and torrentfish (M. Warburton & M. Jarvis pers. obs., NIWA Citation2016). Also present, however, are multiple species of galaxiid (inanga Galaxias maculatus, koaro G. brevipinnis, and Canterbury galaxias G. vulgaris), additional Gobiomorphus species (common bully G. cotidianus, redfin bully G. huttoni, and upland bully G. breviceps), smelt (Retropinna retropinna), black flounder (Rhombosolea retiaria), brown trout (Salmo trutta), eels (shortfin Anguilla australis and longfin A. dieffenbachii), and pouched lamprey (G. australis). The larvae in question are definitely not trout, eels, or lamprey, which can all immediately be excluded based on size, morphology, and spawning habits. The larvae are also definitely not those of a Rhombosolea flounder (Robertson & Raj Citation1971). Further, morphology and myomere counts (32–33) clearly exclude the larvae from both Galaxias and Retropinna, which are long (c. 6–8 mm+) and slender, with the gut extending much further along the body, and have c. 50+ vertebrae (Charteris et al. Citation2003; McDowall Citation2003).
The larvae are also morphologically and meristically distinct from Gobiomorphus, which were also captured during the present sampling (see Jarvis & Closs Citation2015). Compared to G. hubbsi and G. cotidianus, the larvae differ in size, pigmentation, morphology, and meristics. The larvae (c. 2.9 mm) were slightly larger than drifting G. hubbsi (c. 2.6 mm), but much smaller than G. cotidianus (c. 3.5 mm). The similarity to bluegill bully makes sense, as the two species share an amphidromous life-history, and live in similar adult habitats (Atkinson & Joy Citation2009). Morphologically, the larvae feature the distinctly undershot jaw and tadpole-like shape identified in post-larval/juvenile torrentfish returning from the sea (McDowall Citation1994), with a much deeper head relative to body, a more pronounced fin fold, and short and unkinked gut. Most important for our classification, larval myomere counts (corresponding to non-hypural vertebrae counts in adults) are equal to those of adult torrentfish (32–33, McDowall Citation1973), and not Gobiomorphus (28–29, Bleackley et al. Citation2009). Finally, the timing and location of capture fits with the findings of Warburton (Citation2016), who found evidence of summer adult spawning migrations occurring in the Waianakarua River. Based on the combined weight of the multiple lines of evidence outlined above, we are confident in our identification of torrentfish larvae despite the lack of genetic identification.
Drift patterns, future directions, and concluding remarks
While we successfully captured and identified larval torrentfish for the first time, the numbers caught (c. 50 larvae) were extremely low, especially considering torrentfish are highly fecund (c. 3000–50,000+ eggs per female depending on size, Scrimgeour & Eldon Citation1989). These low numbers limit our ability to draw meaningful conclusions about torrentfish spawning and larval migration, for example, spatial or diel patterns in larval drift (Jarvis & Closs Citation2015). Our data suggest a peak in migration throughout the night, yet many other studies have indicated the vast majority of amphidromous fish tend to migrate just after sunset (e.g. Maeda & Tachihara Citation2010; Jarvis & Closs Citation2015; Lagarde et al. Citation2016). However, the pattern we observed may be due to the extremely low numbers of larvae caught in the present study, and further research is required for definitive identification of spatial or temporal patterns in the migration of larval torrentfish. There are a number of reasons we may have caught so few larvae. Firstly, it is likely that our sampling sites were located upstream of prime torrentfish spawning sites, as torrentfish are known to undertake an extensive downstream spawning migration, and are thought to spawn as close to the ocean as possible (Scrimgeour & Eldon Citation1989; Warburton Citation2016), which would be beneficial in reducing larval transport time and associated risk of starvation (Iguchi & Mizuno Citation1999). Additionally, our sampling may not have encompassed the main spawning season, as torrentfish are thought to spawn from January to April in nearby Canterbury rivers (Scrimgeour & Eldon Citation1989).
The capture and description of torrentfish larvae provides a critical piece of information on torrentfish life-history, allowing future researchers to identify and target emigrating torrentfish larvae. In future, our sampling methods and locations could be adapted to better target torrentfish larvae (i.e. sampling later in the year and lower in the river), allowing key ecological questions concerning factors such as the spatial and temporal patterns of larval migration to be addressed.
Acknowledgements
Sincere thanks to M. Holland for kindly providing land access, K. Miller (University of Otago) for aiding in figure preparation, and J. Augspurger (University of Otago) for providing larval koaro. C. Burridge and A. Stewart’s suggestions and comments during the review stage greatly improved the final paper. Associate Editor: Professor Kendall Clements.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Matt G. Jarvis http://orcid.org/0000-0001-8276-1097
References
- Allibone R, David B, Franklin P, Ling N, Crow S, West D. 2014. Cheimarrichthys fosteri [Internet]. The IUCN Red List of Threatened Species 2014: e.T197330A2482366; [cited 2016 Aug 24]. Available from: http://dx.doi.org/10.2305/IUCN.UK.2014-3.RLTS.T197330A2482366.en
- Atkinson NK, Joy MK. 2009. Longitudinal size distributions of bluegill bullies (Gobiomorphus hubbsi) and torrentfish (Cheimarrichthys fosteri) in two large New Zealand rivers. New Zealand Journal of Marine and Freshwater Research. 43:643–651. doi: 10.1080/00288330909510030
- Bleackley NA, Landman MJ, Ling N. 2009. Ecology of common bully (Gobiomorphus cotidianus) in the Tarawera and Rangitaiki rivers: isolation by inland distance or anthropogenic discharge? New Zealand Journal of Marine and Freshwater Research. 43:889–899. doi: 10.1080/00288330909510047
- Chambers CR, Trippel E, editors. Early life history and recruitment in fish populations. Fish and Fisheries Series 21. London: Chapman & Hall.
- Charteris SC, Allibone RM, Death RGD. 2003. Spawning site selection, egg development, and larval drift of Galaxias postvectis and G. fasciatus in a New Zealand stream. New Zealand Journal of Marine and Freshwater Research. 37:493–505. doi: 10.1080/00288330.2003.9517184
- Cunningham MK, Granberry WF, Pope KL. 2000. Shrinkage of inland silverside larvae preserved in ethanol and formalin. North American Journal of Fisheries Management. 20:816–818. doi: 10.1577/1548-8675(2000)020<0816:SOISLP>2.3.CO;2
- Franklin PA, Smith J, Baker CF, Bartels B, Reeve K. 2015. First observations on the timing and location of giant kokopu (Galaxias argenteus) spawning. New Zealand Journal of Marine and Freshwater Research. 49:419–426. doi: 10.1080/00288330.2015.1045004
- Goodman JM, Dunn NR, Ravenscroft PJ, Allibone RM, Boubee JAT, David BO, Giffiths M, Ling N, Hitchmough RA, Rolfe JR. 2014. Conservation status of New Zealand freshwater fish, 2013. Wellington: Department of Conservation. New Zealand Threat Classification Series 7; 12 pp.
- Iguchi K, Mizuno N. 1999. Early starvation limits survival in amphidromous fishes. Journal of Fish Biology. 54:705–712. doi: 10.1111/j.1095-8649.1999.tb02027.x
- Jarvis MG, Closs GP. 2015. Larval drift of amphidromous gobiomorphus spp. in a New Zealand coastal stream: a critical spatial and temporal window for protection. New Zealand Journal of Marine & Freshwater Research. 49:439–447. doi: 10.1080/00288330.2015.1072569
- Lagarde R, Teichert N, Grondin H, Magalon H, Pirog A, Ponton D. 2016. Temporal variability of larval drift of tropical amphidromous gobies along a watershed in Reunion Island. Canadian Journal of Fisheries and Aquatic Sciences. 1–10. doi:10.1139/cjfas-2016-0101.
- Maeda K, Tachihara K. 2010. Diel and seasonal occurrence patterns of drifting fish larvae in the Teima stream, Okinawa Island. Pacific Science. 64:161–176. doi: 10.2984/64.2.161
- McCarter N. 1994. A key to some larval fish from New Zealand fresh water. NIWA ecosystem publication no. 10. Hamilton, NIWA. 32 p.
- McDowall RM. 1973. Relationships and taxonomy of New Zealand torrent fish, Cheimarrichthys fosteri Haast (Pisces: Mugilouididae). Journal of the Royal Society of New Zealand. 3:199–217. doi: 10.1080/03036758.1973.10430602
- McDowall RM. 1994. Distinctive form and colouration of juvenile torrentfish, Cheimarrichthys fosteri (Pisces: Pinguipedidae). New Zealand Journal of Marine and Freshwater Research. 28:385–390. doi: 10.1080/00288330.1994.9516628
- McDowall RM. 1995. Seasonal pulses in migrations of New Zealand diadromous fish and the potential impacts of river mouth closure. New Zealand Journal of Marine and Freshwater Research. 29:517–526. doi: 10.1080/00288330.1995.9516684
- McDowall RM. 2000. Biogeography of the New Zealand torrentfish, Cheimarrichthys fosteri (Teleostei: Pinguipedidae): a distribution driven mostly by ecology and behaviour. Environmental Biology of Fishes. 58:119–131. doi: 10.1023/A:1007666014842
- McDowall RM. 2003. Variation in vertebral number in galaxiid fishes (Teleostei: Galaxiidae): a legacy of life history, latitude and length. Environmental Biology of Fishes. 66:361–381. doi: 10.1023/A:1023902922843
- McDowall RM. 2010. New Zealand freshwater fishes: an historical and ecological biogeography. Dordrecht: Springer.
- McDowall RM, Suren AM. 1995. Emigrating larvae of koaro, Galaxias brevipinnis Gunther (Teleostei: Galaxiidae), from the Otira River, New Zealand. New Zealand Journal of Marine & Freshwater Research. 29:271–275. doi: 10.1080/00288330.1995.9516660
- NIWA. 2016. NZ freshwater fish database [Internet]. [Cited 2016 Sep 16]. Available from: http://nzffdms.niwa.co.nz
- Robertson DA, Raj U. 1971. Egg and yolk sac stages of the sand flounder (Teleostei: Pleuronectidae). New Zealand Journal of Marine and Freshwater Research. 5:404–414. doi: 10.1080/00288330.1971.9515394
- Scrimgeour GJ, Eldon GA. 1989. Aspects of the reproductive biology of torrentfish, Cheimarrichthys fosteri, in two braided rivers of Canterbury, New Zealand. New Zealand Journal of Marine and Freshwater Research. 23:19–25. doi: 10.1080/00288330.1989.9516336
- Tana R. 2009. Population dynamics and migrational history of torrentfish (Cheimarrichthys fosteri, Haast 1874), in two Waikato streams on the North Island of New Zealand [dissertation]. Hamilton, New Zealand: University of Waikato.
- Warburton ML. 2016. Migratory movements of torrentfish (Cheimarrichthys fosteri, Haast 1874) [dissertation]. Dunedin, New Zealand: University of Otago.