ABSTRACT
As a tool to understand Tierra del Fuego’s basic ecology and detect changes due to human pressures, this study develops habitat bioindicators. We compared the freshwater benthic macroinvertebrates at 61 study sites in six habitat types: grassland streams, urbanised streams, forested streams, beaver ponds, lakes and peat bog ponds. Forty-nine taxa were identified; insects were the most diverse group. Beaver pond, lake and grassland stream assemblages were similar, as were those from lakes, grassland streams and peat bog ponds. Fourteen taxa were habitat-specific. In forests, these included mayfly scrapers (Andesiops, Meridialaris) and blackfly filterers (Gigantodax). In lakes, two copepod filterers were indicators, and in urban streams, one shredder (Aphroteniella) and three collector-gatherers (springtail, earthworm, aquatic worm). Predators (Corixa, Aeshna) were characteristic of peat bog ponds. Beaver ponds had no indicator species. Establishing links between species and ecosystems constitutes the beginning of a broader effort to understand anthropogenic impacts to Fuegian watersheds.
Introduction
Ecological studies of biotic assemblages and their spatial patterns allow us to understand how biodiversity responds to physico-chemical and biological attributes of the landscape. Stream ecologists have long studied the relationship of benthic macroinvertebrate community structure and function with environmental factors that control ecological processes in fluvial ecosystems (e.g. River Continuum Concept: Vannote et al. Citation1980). On a practical level, stream benthos also have been used to develop rapid assessments and environmental policies to promote the linkage of habitat and water quality (Kenney et al. Citation2009). Indeed, these taxa are considered to be good biotic indicators of ecosystem health and land use change because they are widely distributed and diverse, have a range of sensitivities to contamination and disturbance, display different feeding and habitat requirements and respond over periods of time that go beyond point-specific sampling (Rosenberg & Resh Citation1993; Pizzolón & Miserendino Citation2001).
In northern Patagonia, stream ecologists began to describe the benthic community in the 1980s (e.g. Wais Citation1987, Citation1990). Later, in the 1990s, species assemblages were related to habitat factors, such as land use (e.g. Miserendino & Pizzolón Citation2003). In addition, biotic indicators of stream health were developed (e.g. Biotic Monitoring Patagonia Stream, Miserendino & Pizzolón Citation1999) to detect pollution and disturbances from natural resource exploitation. Meanwhile, southern Patagonian stream ecology has been less studied, and most research has focused on understanding the ecological role of invasive North American beavers (Castor canadensis). As an ecosystem engineer, the introduced beaver has pervasive effects not only reducing stream benthic diversity at the habitat scale (Anderson & Rosemond Citation2007), but also impacting ecosystem dynamics like carbon flow at the landscape level (Anderson & Rosemond Citation2010; Anderson et al. Citation2014).
In southern Patagonia, the emphasis towards studies on the effects of beavers also has generated a bias in the basic information on the structure and function of sub-Antarctic streams in the face of both natural and anthropogenic drivers. In this context, there continues to be a need to understand the relationship of stream biota to habitat conditions, which has both conceptual and practical applications. Therefore, the objective of this study was to establish the relationships of freshwater macroinvertebrates to a suite of habitat types that are characteristic in the Tierra del Fuego (TDF) Archipelago and to determine habitat-specific indicator species. Overall, we predicted that there would be significant differences in community assemblage between habitat types. Previous research in TDF found that benthic macroinvertebrate diversity was related to substrate heterogeneity (Anderson & Rosemond Citation2007). Therefore, habitats with lotic hydrological regimes were expected to be more diverse than those with lentic conditions, due to the reduced substrate diversity in the latter. At the same time, we anticipated that natural habitat types (i.e. forested streams, grassland streams, peat bog ponds, lakes) would have a greater number of indicators species, than recently created ones (i.e. beaver ponds, urbanised streams).
Methods
Study area
This study was conducted in the TDF Archipelago, which is shared between Argentina and Chile. It is part of two ecoregions: the Magellanic sub-Antarctic forest and the Patagonia steppe. The former has been identified as one of the last remaining pristine wilderness areas on the planet, given its extensive size, low human population density and largely intact native vegetation cover (Mittermeier et al. Citation2003). The latter is part of the Global 200 Priory Biomes for conservation (Olson et al. Citation1995). Notwithstanding the conservation status of this region, it confronts various anthropogenic drivers of ecosystem change, including habitat fragmentation for productive purposes, such as livestock and forestry (Martínez Pastur et al. Citation2010); alterations due to invasive exotic species (Anderson et al. Citation2006); and also the effects associated with economic development, particularly tourism, global warming and ozone depletion (Rozzi et al. Citation2012).
Data collection and analysis
We constructed a database from samples collected at 61 study sites between 2003 and 2015 in habitats with lotic (urbanised streams: 4; grassland streams: 10, forested streams: 18) and lentic (beaver ponds: 14, peat bog ponds: 7, lakes: 11) hydrological conditions (see also Anderson et al. Citation2014). At each site, three benthic core samples (0.07 m2) were taken by agitating the substrate and passing contents through a 250-μm sieve for three minutes. The material collected was preserved in 70% ethanol. In the laboratory, benthic macroinvertebrates were separated and identified to the lowest possible taxonomic level, using Merritt and Cummins (Citation1996), Fernández and Domínguez (Citation2001), Domínguez and Fernández (Citation2009) and McLellan and Zwick (Citation2007). The taxa identified were classified into functional feeding groups (FFG: shredders, predators, collector-gatherers, scrappers and filterers), according to Merritt and Cummins (Citation1996) and Dominguez and Fernández (Citation2009).
To identify differences between habitat-specific community assemblages, we conducted an analysis of variance (ANOVA) with Student’s t test, using JMP 12 (SAS Institute Inc.), to compare the means of benthic macroinvertebrate taxa richness (# of taxa m−2), diversity (Shannon–Weiner, H′), and density (# individuals m−2). The assumptions of normality and homogeneity of variances for all cases were tested using the Shapiro–Wilks test and the Levene test, respectively. To evaluate community similarity between habitats, a non-metric multidimensional scaling (NMDS) process was performed, and subsequently, we applied a multi-response permutation procedure (MRPP) to detect significant differences within and between communities per habitat type (McCune & Grace Citation2002). Finally, we applied an indicator species analysis (ISA) to measure relative abundance and relative frequency for a species with the Monte Carlo test for significance to determine taxa that were indicators of each habitat. All multivariate analyses were conducted in PCOrd v. 5.0.
Results
The database of 61 sampling sites throughout TDF included 49 taxa (Appendix 1). Insects were the most diverse class (36 taxa). Richness was not significantly different between habitats, but density in urban streams was higher than other habitat types at a marginally significant level (). Diversity was significantly different between habitats; forest streams were the most diverse and urban streams the least (). FFG richness was significantly different between habitats. Peat bog ponds had greater predator richness, and forest streams had greater richness of scrappers, shredders and filterers. Additionally, shredder density and diversity was significantly higher in forest streams ().
Table 1. Benthic macroinvertebrate community variables were measured for six habitat types with lotic (forest, grassland and urbanised streams) and lentic hydrological conditions (peat bog ponds, lakes and beaver ponds).
Table 2. Mean values (±SE) for benthic density, richness and diversity of each FFG in each habitat type with lotic (forest, grassland and urban) and lentic (peat bog, lake and beaver pond) hydrological conditions.
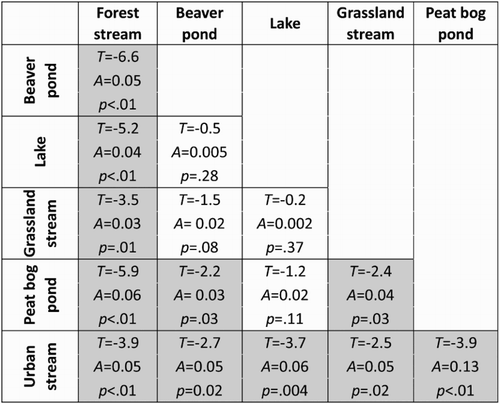
NMDS analysis showed that the three habitat types with lotic hydrological conditions (forest, grassland and urbanised streams) had distinct community assemblages, while the three habitats with lentic conditions (beaver and peat bog ponds and lakes) had overlapping and less distinct assemblages (). Nonetheless, MRPP confirmed that most habitats have significantly different community assemblage, with the exception of two groups: (a) beaver ponds, lakes and grassland streams and (b) lakes, grassland streams and peat bog ponds ().
Figure 1. A graphic representation of the NMDS analysis that demonstrates the overall similarity of freshwater macroinvertebrate assemblages between sample sites (each point) categorised by habitat type (see legend for symbols).
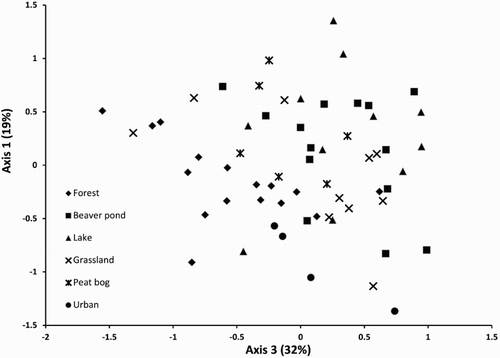
Figure 2. Shaded cells indicate statistically significant differences in biotic community assemblage between the two compared habitat types, based on NMDS analysis of Bray–Curtis similarity results and an MRPP to detect differences within and between sites, using PCOrd 5.0.
ISA identified 12 taxa as biotic indicators for 5 of our 6 habitat types: forest streams, grassland streams, urbanised streams, lakes and peat bog ponds (). In forests, two mayfly scrapper genera (Andesiops and Meridialaris) and a filtering blackfly genus (Gigantodax) were identified as indicators. In lakes, two subclass of copepods (Cyclopoida and Calanoida) were characteristic. In the urbanised area, one genus of shredder (the midge Aphroteniella) plus three collectors-gathering organisms (the collembola Entomobryomorpha, Oligochaetes and earthworms). Predatory taxa (the waterboatman Corixa and the dragonfly Aeshna variegata) were indicators of peat bogs. Indeed, dragonflies were only found in this habitat. Finally, in grassland streams, amphipods of the genus Hyalella were indicative. Beaver ponds were the only habitat without characteristic benthos. The indicator species identified represented all of the FFGs in our list.
Table 3. Macroinvertebrate taxa identified as indicators (p < 0.05) for habitat, using Indicator Species Analysis with a Monte Carlo test of significance of observed maximum indicator value for taxa (PCOrd 5.0).
Discussion
Understanding Fueguian biodiversity and ecosystems
Based on previous research (Anderson & Rosemond Citation2007), we expected that the benthic communities of habitats with lentic conditions would be less diverse and more homogenous than those with flowing water. Indeed, we observed that lakes, beaver ponds and peat bogs have a similar community assemblage, but grassland streams also clustered with this group. In addition, there was no significant relationship between species richness or diversity and substrate diversity, as we had predicted. Therefore, while substrate conditions could determine benthic diversity at smaller spatial scales (e.g. reach) and in some ecosystem types (e.g. forested streams) (see Anderson & Rosemond Citation2007), at broader scales and in different habitats, other factors seemed to play a more crucial role.
For example, vegetation does not only represent the surrounding physical habitat conditions of water bodies, but also constitutes basal resources for many benthic macroinvertebrates, which in TDF depend heavily on allochthonous organic matter (Anderson & Rosemond Citation2010). It has been shown, for instance, that when pastures are established for livestock grazing, the amount of allochthonous leaf material entering the stream can be reduced significantly and affect the streams trophic structure (Miserendino Citation2005). The other two habitats with lotic hydrological conditions (i.e. forest and urbanised streams) had a distinct community assemblage. It is widely shown that human impacts via urbanisation, such as deforestation, river channelisation and pollution, reduce benthic taxonomic diversity (e.g. Miserendino et al. Citation2011). In agreement with these expectations, the urbanised streams in TDF also presented the lowest diversity found in any Fueguian freshwater habitat type and the highest density of individuals.
In forested streams, higher diversity was found. On the one hand, this finding corresponds to the expectation that greater substrate heterogeneity offers more variety of niches for the different benthic organisms. However, functionally, it is important to note that these streams also hosted the highest richness of shredders, scrappers and filterers. The functional structure of the community is expected to reflect the food availability in the stream (Vannote et al. Citation1980). For example, a high proportion of filterers should be found when there is a higher relative amount of fine particulate organic matter in transport in the water column (Strand & Merritt Citation1999). Therefore, we expect that in these streams the proportions of litter, fine sediment and periphyton would be high, but more detailed studies are necessary to confirm such relationships.
Developing bioindicators in TDF
Southern Patagonia and particularly TDF present various opportunities and challenges for ecological research and environmental management. On the one hand, it is a relatively unstudied part of the planet, but at the same time it faces various anthropogenic impacts that require attention (Rozzi et al. Citation2012). We propose that the indicator species identified here serve two functions. On the one hand, they help improve our knowledge of biotic relationships between species and ecosystems. At the same time, on a practical level, they can be used as a baseline to aid in environmental monitoring in the future. To date, however, the only such research that has been conducted on TDF is in terrestrial ecosystems, where foresters have shown that logging affects the insect community (Lencinas et al. Citation2014) and proposed that some species like the pseudoscorpian Neochelanops michaelseni can be indicative of impacts in Nothofagus pumilio forests (Lencinas et al. Citation2015). Similar work in streams, however, has only been developed farther north in Patagonia (Miserendino & Pizzolón Citation1999) or in sub-tropical and tropical regions (Moya et al. Citation2011).
In general, it is known that land uses in a watershed are major determinants of in-stream processes; numerous studies have documented the associations between land use and measures of stream condition, using multisite comparisons and empirical models (e.g. Miserendino et al. Citation2016). These negative impacts to watersheds can have severe implications for the ability of these ecosystems to continue to provide quality ecosystem services. Zavaleta et al. (Citation2010), for example, showed that minimum levels of biological richness were required to provide multiple levels of ecosystem functions. Furthermore, since ecosystem goods and services result from multiple ecosystem functions, the reduction in diversity that was shown here may over time result in decreased service quality and quantity, thereby negatively impacting the social well-being of communities interacting with these ecosystems (Díaz et al. Citation2015). Previously, we have shown that watersheds in southern Patagonia are valued in multiple ways by local residents (Zagarola et al. Citation2014). By providing data on the links between habitat and species assemblage, as well as habitat bioindicators, we are advancing the development of the tools needed to monitor these impacts and ultimately reduce their effects on these ecosystems and the human and non-human biotic communities that depend on them.
Acknowledgements
Associate editor: Dr Marc Schallenberg.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Christopher B. Anderson http://orcid.org/0000-0001-8120-5689
Additional information
Funding
References
- Anderson CB, Lencinas MV, Valenzuela AEJ, Simononok MP, Wallem PK, Martinez Pastur G. 2014. Ecosystem engineering by an invasive species, the beaver, increases landscape-level ecosystem function but does not affect biodiversity in Tierra del Fuego’s freshwater systems. Diversity and Distributions. 20:214–222. doi: 10.1111/ddi.12147
- Anderson CB, Rosemond AD. 2007. Ecosystem engineering by invasive exotic beavers reduces in-stream diversity and enhances ecosystem function in Cape Horn Chile. Oecologia. 154:141–153. doi: 10.1007/s00442-007-0757-4
- Anderson CB, Rosemond AD. 2010. Beaver invasion alters terrestrial subsidies to sub-Antarctic stream food webs. Hydrobiologia. 652:349–361. doi: 10.1007/s10750-010-0367-8
- Anderson CB, Rozzi R, Torres-Mura JC, McGehee SM, Sherriffs MF, Schüttler E, Rosemond AD. 2006. Exotic vertebrate fauna in the remote and pristine sub-Antarctic Cape Horn Archipelago, Chile. Biodiversity and Conservation. 15:3295–3313. doi: 10.1007/s10531-005-0605-y
- Díaz S, Demissew S, Carabias J, Joly C, Lonsdale M, Ash N, Larigauderie A, Adhikari JR, Arico S, Báldi A. 2015. The IPBES conceptual framework – connecting nature and people. Current Opinion in Environmental Sustainability. 14:1–16. doi: 10.1016/j.cosust.2014.11.002
- Domínguez E, Fernández HR. 2009. Macroinvertebrados Bentónicos Sudamericanos: Sistemática y Biología. Tucumán, Argentina: Fundación Miguel Lillo; p. 307.
- Fernández HR, Domínguez E. 2001. Guía para la Determinación de los Artrópodos Bentónicos Sudamericanos. Tucumán, Argentina: Universidad Nacional de Tucumán; p. 282.
- Kenney MA, Sutton-Grier AE, Smith RF, Gresens SE. 2009. Benthic macroinvertebrates as indicators of water quality: the intersection of science and policy. Terrestrial Arthropod Reviews. 2:99–128. doi: 10.1163/187498209X12525675906077
- Lencinas MV, Kreps G, Soler R, Peri P, Porta A, Ramirez M, Martinez Pastur G. 2015. Neochelanops michaelseni (Pseudoscorpiones: Chernetidae) as a potencial bioindicator in managed and unmanaged Nothofagus forests of Tierra del Fuego. Journal of Arachnology. 43:406–412. doi: 10.1636/0161-8202-43.3.406
- Lencinas MV, Martinez Pastur G, Gallo E, Cellini JM. 2014. Decreasing negative impacts of harvesting over insect diversity using variable retention silviculture in southern Patagonian forests. Journal of Insect Conservation. 18:479–495. doi: 10.1007/s10841-014-9661-5
- Martínez Pastur G, Lencinas MV, Peri PL, Cellini JM, Moretto A. 2010. Long-term forest management research in South Patagonia – Argentina: lessons from the past, challenges from the present. Revista Chilena Historia Natural. 30:159–169.
- McCune B., Grace JB. 2002. Analysis of ecological communities. Oregon, USA: MjM Software Design; p. 300.
- McLellan ID, Zwick P. 2007. New species of and keys to South American Gripopterygidae (Plecoptera). Illiesia. 3:20–42.
- Merritt RW, Cummins KW. 1996. An introduction to the aquatic insects of North America. 3rd ed. Dubuque, Iowa: Kendall/Hunt; p. 862
- Miserendino ML. 2005. Interacción bosque-río: implicaciones para los ecosistemas acuáticos. Patagonia Forestal. 11:14–17.
- Miserendino ML, Casaux R, Archangelsky M, Di Prinzio C, Brand C, Kutschker A. 2011. Assessing land-use effects on water quality, in-stream habitat, riparian ecosystems and biodiversity in Patagonian northwest streams. Science of the Total Environment. 409:612–624. doi: 10.1016/j.scitotenv.2010.10.034
- Miserendino ML, Kutschker A, Brand C, La Manna L, Di Prinzio C, Papazián G, Bava J. 2016. Ecological status of a Patagonian mountain river: usefulness of environmental and biotic metrics for rehabilitation assessment. Environmental Management. 57:1166–1187. doi: 10.1007/s00267-016-0688-0
- Miserendino ML, Pizzolon L. 1999. Rapid assessment of river water quality using macroinvertebrates: a family level biotic index for the Patagonic Andean zone. Acta Limnologica Brasiliensia. 11:137–148.
- Miserendino ML, Pizzolón LA. 2003. Distribution of macroinvertebrate assemblages in the Azul-Quemquemtreu river basin, Patagonia, Argentina. New Zealand Journal of Marine and Freshwater Research. 37:525–539. doi: 10.1080/00288330.2003.9517187
- Mittermeier RA, Mittermeier CG, Brooks J, Pilgrim J, Konstant J, da Fonseca GAB, Kormos C. 2003. Wilderness and biodiversity conservation. Proceedings of the National Academy of Sciences. 100:10309–10313. doi: 10.1073/pnas.1732458100
- Moya N, Hugues RM, Domínguez E, Gibon FM, Goitia E, Oberdorff T. 2011. Macroinvertebrate-based multimetric predictive models for evaluating the human impact on biotic condition of Bolivian streams. Ecological Indicators. 11:840–847. doi: 10.1016/j.ecolind.2010.10.012
- Olson DM, Graham DJ, Webster AL, Primm SA, Bookbinder MP, Ledec G, Young KR. 1995. A conservation assessment of the terrestrial ecoregions of Latin America and the Caribbean (No. P01 47). Washington, DC: World Bank.
- Pizzolón L, Miserendino ML. 2001. The performance of two regional biotic indices for running water quality in northern patagonian Andes. Acta Limnologica Brasiliensia. 13:11–27.
- Rosenberg DM, Resh VH. 1993. Freshwater biomonitoring and freshwater invertebrates. New York, NY, USA: Chapman & Hall; p. 488.
- Rozzi R, Armesto J, Gutiérrez J, Massardo F, Likens GE, Anderson CB, Poole A, Moses K, Hargrove E, Mansilla A, et al. 2012. Integrating ecology and environmental ethics: earth stewardship in the southern end of the Americas. BioScience. 62:226–236. doi: 10.1525/bio.2012.62.3.4
- SAS Institute. 2015. JMP®, Version 12. Cary, NC: SAS Institute Inc.
- Strand M, Merritt RW. 1999. Impacts of cattle grazing activities on stream insect communities and the riverine environment. American Entomologist. 45:13–29. doi: 10.1093/ae/45.1.13
- Vannote RL, Minshall GW, Cummins KW, Sedell JR, Gushing E. 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences. 37:130–137. doi: 10.1139/f80-017
- Wais IR. 1987. Macrozoobenthos of Negro river basin, Argentine, Patagonia. Studies on Neotropical Fauna and Environment. 22:73–91. doi: 10.1080/01650528709360721
- Wais IR. 1990. A checklist of the benthic macroinvertebrates of the Negro river basin, Patagonia, Argentina, including an approach to their functional feeding groups. Acta Limnologica Brasiliensia. 3:829–845.
- Zagarola JP, Anderson CB, Veteto J. 2014. Perceiving Patagonia – an assessment of social values and perspectives regarding watershed ecosystem services and management in Southern South America. Environmental Management. 53:769–782. doi: 10.1007/s00267-014-0237-7
- Zavaleta ES, Pasaria JR, Hulveya KB, Tilman GD. 2010. Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity. Proceedings of the National Academy of Sciences. 107:1443–1446. doi: 10.1073/pnas.0906829107
