ABSTRACT
The larval stage of fishes is critical in determining their dispersal, survival and recruitment, but little is known of the larval behaviours and tolerances of amphidromous fishes, particularly in New Zealand. We report the results of a series of observational and experimental studies on bluegill bully (Gobiomorphus hubbsi), including spawning sites and behaviours, larval characteristics at hatch, phototactic responses of larvae, and larval survival at different salinity levels. Spawning primarily occurred in the lower reaches of the river, and larvae from different nests exhibited marked differences in, and trade-offs between, larval characteristics at hatch, potentially affecting larval success. Larvae were positively phototactic to intense light, an unexpected result based on diel drift patterns and international research. Finally, larvae exhibited markedly higher survival rates when reared at intermediate salinities compared to freshwater or seawater, suggesting estuaries may play an important role as nursery grounds for bluegill bully and other amphidromous fish.
Introduction
Success during the larval period is widely recognised as being critical to the persistence of fish populations, with changes in larval growth and survival having a profound effect on subsequent recruitment (Bailey & Houde Citation1989; Chambers & Trippel Citation1997). Newly hatched larvae are highly vulnerable to external factors such as predation, starvation, contaminants and adverse environmental conditions (e.g. Pepin Citation1991; Iguchi & Mizuno Citation1999; Sfakianakis et al. Citation2015), and intrinsic characteristics of the fish themselves such as metabolism and assimilation efficiency may also influence survival (Letcher et al. Citation1996). Understanding larval behaviours and their tolerances of various environmental conditions is therefore of great importance both in understanding species’ distributions, and if their conservation and management is to be successful (Keith et al. Citation2008; Iida et al. Citation2010).
New Zealand’s freshwater fish fauna is dominated by amphidromous species (McDowall Citation1998), that live and spawn in freshwater, but migrate to the sea at hatch for a period of larval feeding before returning to freshwater as late-stage larvae or juveniles (McDowall Citation2007; Closs & Warburton Citation2016). Due to their complex migratory life-history, the larvae of amphidromous fish may be exposed to a range of environmental conditions, not all of which are conducive to development, growth and survival (e.g. Valade et al. Citation2009, Iida et al. Citation2010, Ellien et al. Citation2016). As such, amphidromous larvae may have specific environmental tolerances, as well as both adults and larvae exhibiting behaviours such as spawning site selection and diel vertical migration, that may maximise larval survival. However, the early life-history of much of New Zealand’s amphidromous fish fauna remains largely unstudied.
A small number of studies exist examining the early life-stages of some of New Zealand’s amphidromous galaxiid species (e.g. Mitchell Citation1989, Hickford et al. Citation2010, Jellyman & Harding Citation2014, Wylie et al. Citation2016), but New Zealand’s other amphidromous species (three Gobiomorphus eleotrids and torrentfish, Cheimarrichthys fosteri) have received comparatively little attention. Few studies exist, with these primarily being descriptive field studies identifying drifting larvae (e.g. Jarvis et al. Citation2017), and examining diel and spatial distribution patterns of larvae in lakes (e.g. Rowe et al. Citation2001), estuaries (Sutherland & Closs Citation2001) and streams (Jarvis & Closs Citation2015). The spawning habits and locations of many species remain unknown, and no experimental studies examining larval behaviours, survival or growth rates exist.
The aim of this study was to improve our understanding of the larval ecology and environmental tolerance of an understudied component of New Zealand’s freshwater fish fauna, the bluegill bully (Gobiomorphus hubbsi Stokell, 1959). To examine the reproductive ecology of bluegill bully, we conducted qualitative field surveys of spawning locations and behaviours. To better understand the behaviour, ecology and habitat requirements of newly hatched larvae during their seaward migration, we also measured several ecologically relevant larval traits at hatch (e.g. Jones & Closs Citation2016), and conducted experiments examining both the phototactic behaviours of larvae, and the survival of larvae at varying salinity levels (e.g. Iida et al. Citation2010).
Methods
Study species
Bluegill bully are an amphidromous eleotrid (sleeper goby) species endemic to New Zealand. Being a riffle specialist, the species mainly inhabits swiftly flowing broken water in shallow gravel-bed streams and rivers (McDowall Citation1990). Bluegill bully are widespread throughout New Zealand, but are listed as ‘At risk: declining’ by the New Zealand Department of Conservation, who also acknowledge a lack of data available on the species (Goodman et al. Citation2014). Newly hatched larvae are known to drift downstream immediately following sunset during the austral summer (November to February; Jarvis & Closs2015), but little else is known about the larval ecology and behaviours of the species.
Nest searches and larval collection
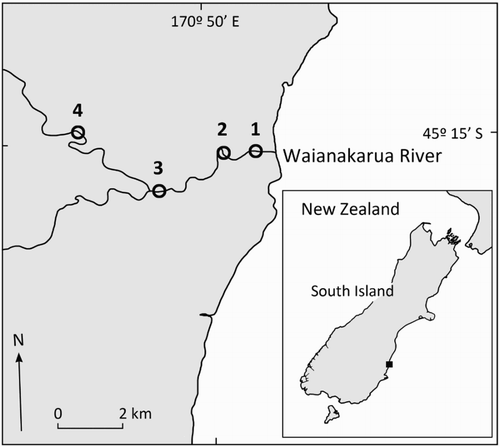
Nest searches were conducted during the austral summer at four sites on the Waianakarua River, South Island, New Zealand (), selected due to its status as a population stronghold for bluegill bully on the eastern South Island (Jarvis & Closs Citation2015). The Waianakarua is a relatively short river (c. 33 km) which drains a catchment of c. 260 km2, mostly consisting of native tussock and forest in the upper reaches, and forestry, sheep and dairy farming in the middle and lower reaches. The most downstream site was located immediately upstream of the upper extent of the Waianakarua’s small estuary swamp, c. 1 km inland. Qualitative searches for nests were conducted, examining every potential nesting location at a site (i.e. all available spawning substrate, regardless of flow or depth). Because many amphidromous fishes lay adhesive nests on the underside of unembedded cobbles (e.g. Teichert et al. Citation2013; Ellien et al. Citation2016), these areas were targeted in our searches. Nests of common bully (G. cotidianus) and upland bully (G. breviceps), distinguished by differences in egg size, were also discovered during our searches, but were excluded for the present study. To collect larvae for observation, measurement and experimental trials, several cobbles bearing nests were immediately transferred to individual buckets containing river water, inducing immediate hatch (Jarvis & Closs Citation2015). Nests and larvae were then either immediately preserved for genetic or morphological analysis (see below), or returned to the laboratory within c. 2 h of hatch for use in experiments.
Genetic analyses of nests
During nest searches, many nests appeared to contain multiple distinct patches of bluegill bully eggs (though several instances of apparent multi-species nests were also noted), distinguished from each other by apparent degree of development, with the most developed eggs exhibiting a darker colour. To examine this phenomenon, as well as confirm the nests as those of bluegill bully (the Waianakarua also supports a number of other Gobiomorphus species (Jarvis et al. Citation2017)), three nests containing a single egg plaque, and a single nest with three distinct patches of eggs, were collected for genetic analyses. Thirty eggs from each nest (ten eggs from each of the three patches for the multi-plaque nest) were collected via pipette and transferred to microtubes containing 95% ethanol prior to DNA extraction. DNA was extracted using a Chelex digestion (Walsh et al. Citation1991). Purified DNA was stored at 4°C prior to PCR amplification and DNA sequencing.
Approximately 700 bp of the mitochondrial DNA COI was amplified by PCR using Fish F1 [5'-TCAACCAACCACAAAGACATTGGCAC-3'] and Fish R1 [5'-TAGACTTCTGGGTGGCCAAAGAATCA-3'] primers (Ward et al. Citation2005). PCR amplification was carried out in 25 µl volumes consisting of 1.5 mM MgCl2, 200 µM of dNTPs, 0.5 µM of the forward primer, 0.6 µg/ml bovine serum albumin, 0.5 U of Taq polymerase (Bioline, London, UK) and c. 15 ng of DNA. PCR thermocycling was performed on an Eppendorf Mastercycler ep Gradient S (Eppendorf AG, Hamburg, Germany), under the following regime: 95°C for 2 min, then followed by 30 cycles of 95°C for 30 s, 53°C for 30 s, 72°C for 30 s with a final extension of 72°C for 10 min. PCR products were purified with ExoSAP-IT (Affymetrix, Inc., Santa Clara, CA) following the manufacturer’s instructions. Purified PCR products were sequenced on an ABI 3730xl DNA Analyser (Genetic Analysis Services, Department of Anatomy, University of Otago) using primer FishF1 (Ward et al. Citation2005). DNA sequences were edited and aligned in Sequencher version 5.1 (Gene Codes Corporation, Ann Arbor, MI).
Larval characteristics at hatch
Ten larvae from each of 12 nests were randomly sampled using a pipette, and immediately fixed in 10% formalin for two days before being transferred to 70% ethanol. A dissecting microscope (Olympus SZ51; Olympus Corporation, Tokyo, Japan) and compound microscope (Nikon Alphaphot 2; Nikon Corporation, Tokyo, Japan) were used in conjunction with a stage micrometer to measure a number of ecologically relevant morphological features (). Notochord length and eye diameter were measured, which influence the swimming and prey capture ability of larvae (Miller et al. Citation1988). Yolk sac diameter was also taken as a measure of energy reserves, critical for amphidromous fish in resisting starvation during downstream migration (Iguchi & Mizuno Citation1999). To test for differences in these characteristics at hatch between nests, ANOVA analyses and Tukey’s post-hoc tests were conducted. Additionally, potential trade-offs in various traits at hatch were examined through regression analyses. All analyses were conducted in R version 3.2.2 (R Development Core Team Citation2015).
Phototaxis experiment
To elucidate the mechanism behind distinct diel drift patterns in bluegill bully and other amphidromous larvae (e.g. Jarvis & Closs Citation2015), a phototaxis experiment was conducted in a glass aquaria (30 × 25 × 25 cm) located in a dark room, using larvae sourced from a single nest. An adjustable light source and light-blocking black plastic were used to illuminate a discrete area representing 10.5% of the aquaria. The area of the aquaria illuminated was determined randomly for each trial to control for potential bias related to other factors such as depth. Trials were conducted using different larvae each time, at a range of light intensities selected to represent different stages of day, determined using a light meter (Sekonic Multimaster L-408, Sekonic Corporation, Tokyo): 100, 500 (sunrise/sunset), 2000, 8000 (overcast days and shade), and 15500 lux (bright sunlight). Tentrials were conducted at each illumination level. Fifty newly hatched (<2 h old) larvae were released into the aquaria, and left to acclimate in darkness for 3 min, after which the light source was turned on for a further 3 min. At the end of the trial, the number of larvae in the illuminated area was estimated based on the counts of three observers. The percentage of larvae present in the illuminated area of the aquaria were compared with those expected under a random distribution (10.5% of larvae in the light) using a one-sample t-test.
Survival experiment
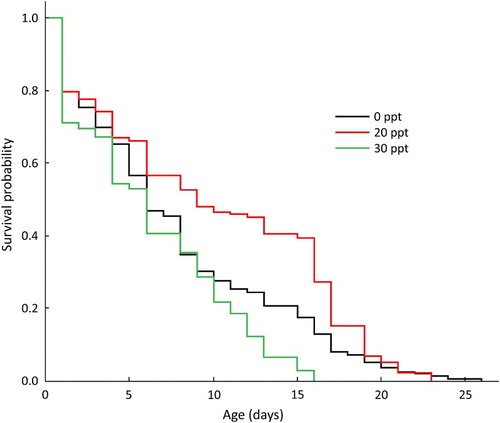
To examine the effects of freshwater retention and reaching the sea on bluegill bully larvae, an experiment examining the survival of newly hatched larvae at different salinities was also conducted, based on the methods of Valade et al. (Citation2009) and Iida et al. (Citation2010). In an attempt to control for potential between-nest differences, trials were conducted using larvae from eight different nests, all collected from the same general location (site 2). For each nest, two replicate trials were conducted at each of three salinity levels, selected to represent freshwater, brackish water, and seawater (0, 20, and 30 ppt, respectively). For each trial, c. 50 newly hatched larvae (<2 h old) were transferred to 2 L aquaria. Dead fish in each aquaria were counted and removed daily until all larvae had died. For analysis, data from all nests were pooled, and Kaplan–Meier survival curves were used to examine survivorship as a function of time for each treatment (Morrongiello et al. Citation2013). Differences between survival curves were evaluated using pairwise Tarone–Ware tests between treatment levels.
Results
Observations on spawning habitat and behaviour
Bluegill bully spawning sites were predominantly located in the lower reaches of the stream, on the undersides of flat unembedded cobbles lying in shallow broken water. Male bluegill bullies were often seen holding positon under the stones with nests on them. High densities of nests were found at the sites located furthest downstream (sites 1 and 2; ), where egg plaques were found on the underside of nearly every suitable cobble. The middle site (site 3) had lower numbers of nests than those located further downstream, and no nests were found at the site located furthest upstream (site 4). In regards to the nests collected for genetic analysis, A BLAST (basic local alignment search tool) search of the NCBI GenBank database (Altschul et al. Citation1990) confirmed all sequences as those of G. hubbsi. Additionally, mixed base peaks of approximately equal height were observed at four sites in the sequence from the pooled egg sample (see supplementary information; Figure S1), while the profile was otherwise clean, with single peaks for the remaining positions, indicating that multiple sequences were present in the amplified product.
Differences between nests at hatch
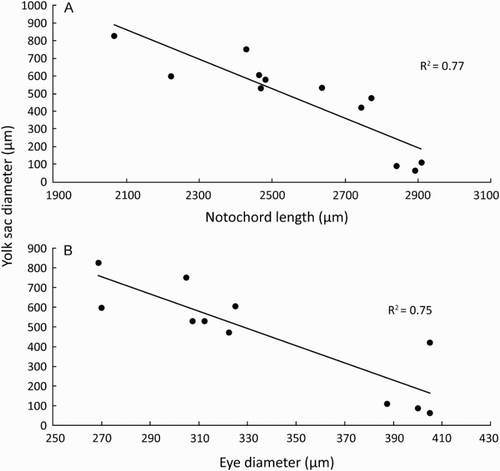
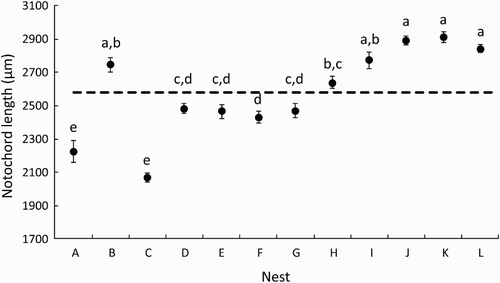
Larvae hatched from different nests exhibited significant differences in morphological characteristics at hatch ( and ). Mean (±SEM) length at hatch varied by as much as 40%, ranging from 2068 (±100.5) to 2910 (±69.5) µm. Mean yolk sac diameter at hatch ranged from 420 (±69.5) to 597.5 (±82) µm. Mean eye diameter at hatch ranged from 270 (±68.5) to 407.5 (±37.4) µm. Regression analysis indicated strong and significant negative relationships between yolk reserves at hatch and both length (p < .01, R2 = 0.63) and eye size (p < .001, R2 = 0.96) of larvae at hatch.
Figure 3. Notochord length (mean ± SEM; µm) of larval bluegill bully at hatch from 12 nests collected from the Waianakarua River, South Island, New Zealand. Nests marked with the same lowercase letter did not differ significantly (Tukey’s HSD test, alpha = 0.05). Dashed line represents mean notochord length of drifting larvae caught in situ (Jarvis & Closs Citation2015).
Phototaxis experiment
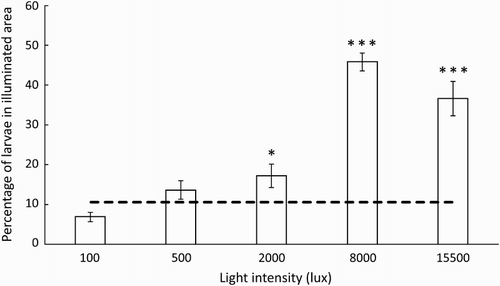
At weak light intensities (100 and 500 lux), the percentage of larvae in the illuminated area (6.8 and 13.6%, respectively) did not differ significantly from those expected (10.5%) against a random distribution (100 lux: t = −3.1831, df = 9, p = .11; 500 lux: t = 1.3121, df = 9, p = .222; ). At more intense light levels, numbers of larvae in the illuminated area were significantly higher than expected against a random distribution. At 2000 lux this association was relatively small (17.2% of larvae) yet significant (t = 2.678, df = 9, p < .05), but was far more pronounced at 8000 lux (45.8% of larvae; t = 15.621, df = 9, p < .001; ) and 15500 lux (36.6% of larvae; t = 5.9976, df = 9, p < .001; ).
Figure 5. Percentage of larvae (mean ± SEM; N = 10 trials of 50 larvae each) present in illuminated area (10.5%) of an experimental aquaria at different light intensities after 3 min. Dashed line indicates expected percentage (10.5) based on a random distribution of larvae throughout the aquaria. Significance codes shown if percentage differed significantly from that expected at random as follows: * = p < .05, ** = p < .01, *** = p < .001.
Survival experiment
Pairwise Tarone–Ware tests indicated that all survival functions differed significantly from each other (: 0 and 30 ppt: Chisq = 3.8, p < .01; 0 and 20 ppt: Chisq = 15.4, p < .001; 20 and 30 ppt: Chisq = 51.1, p < .001). Mortality for all treatments was similar for the first few days post-hatch, but after c. 6 days, larvae reared at intermediate salinity levels exhibited markedly higher survival than those reared at 0 or 30ppt. Survival functions for larvae reared in freshwater and brackish water converged again at c. 20 days, with a small number of larvae surviving for up to 27 and 23 days, respectively. Conversely, larvae reared in seawater exhibited a relatively constant mortality rate throughout the experiment, with no larvae surviving beyond 16 days. Median time to death was similar for larvae reared in freshwater (0ppt; n = 483, median = 6 days, 95% CI = 6–8 days) and seawater (30 ppt; n = 508, median = 6 days, 95% CI = 5–6 days). Larvae reared in water of an intermediate salinity (20 ppt) survived considerably longer, with a median time to death of 9 days (n = 508, 95% CI = 8–12 days).
Discussion
Observations on spawning sites and behaviours
Bluegill bully spawning sites were found predominantly at the locations furthest downstream (<2–5 km stream distance from the sea). This aligns with the spatial patterns of larval drift observed in the Waianakarua by Jarvis and Closs (Citation2015), though they also caught limited numbers of bluegill bully larvae at the upstream site, where no nests were found in the present study. Taken together, these resultsindicate that while some reproduction does occur >10 km inland, these fish contribute little to the overall reproductive output of the system. In regards to the nest with multiple egg patches, the presence of multiple signatures within the amplified mitochondrial DNA suggests distinct layings from different females. Together with our behavioural observations, McDowall’s assertion that males defend nests (McDowall Citation1990) is supported, with males observed to defend spawning sites that may contain the eggs of multiple females.
Taken together, these results suggest strong competition between males for preferred spawning sites. This seems intuitive for amphidromous fish, as sites located closest to the sea would seem to have a clear advantage over those further upstream (Bell Citation2007), maximising chances of larvae reaching the pelagic environment before starvation due to retention in pools and eddies (Moriyama et al. Citation1998). However, these results are at odds with the findings of Atkinson and Joy (Citation2009), who concluded based on longitudinal size distributions of bluegill bully in the Hutt and Raikaia rivers that bluegill bully continuously migrate upstream throughout their life. Such a strategy would seem at odds with the need to expedite larval transport to the marine environment, and may suggest a downstream migration undertaken by adults prior to spawning, as has been described in a number of other amphidromous species (Scrimgeour & Eldon Citation1989; Augspurger et al. Citation2017).
Larval characteristics at hatch
The three morphological variables measured varied markedly between different nests, though intra-nest variation was minimal. Our data suggest a clear trade-off occurring in newly hatched larvae between yolk reserves and both length and eye size. Such trait trade-offs are well-known in fish (Winemiller & Rose Citation1992), but have rarely been examined in amphidromous fishes, where yolk provisioning plays a critical role in surviving their vulnerable period of downstream migration. It is possible these differences relate to the spontaneous hatching of nests upon disturbance (Jarvis & Closs Citation2015), with nests being disturbed at various stages of development. However, the size of larvae measured in the present study correspond well with those caught naturally drifting downstream in terms of average size and range (see supplementary information, Figure S2), hence we are confident this is not the case. Potential explanations for these differences include maternal effect, whereby factors such as female age, size and environmental history influence larval characteristics (e.g. Berkeley et al. Citation2004), or different environmental conditions experienced by different egg batches during incubation (e.g. Braun et al. Citation2013). Another proposed explanation is that under natural conditions, the nest-guarding male stimulates his eggs to hatch at an optimal time (Maeda & Tachihara Citation2010), and so the male may play an active role in determining the yolk reserves/larval size of his offspring at hatch. Whatever the case, there are a number of interesting hypotheses that could be tested regarding trait trade-offs in amphidromous larvae in future.
Larval behaviours and phototaxis
During acclimation, newly hatched larvae were observed to alternately sink down and swim upwards in the water column. This behaviour is thought to expedite larval transport downstream, and has previously been reported in several amphidromous fishes (e.g. Keith Citation2003; Iida et al. Citation2010; Ellien et al. Citation2011), though our observations represent the first report from a family other than Gobiidae. Further, we observed that this behaviour lasts only for a short period of time before larvae appeared to tire and sank to the bottom to rest, which may explain the short period of peak downstream drift (c. 2 h per day) reported for bluegill bully (Jarvis & Closs Citation2015).
The results of our phototaxis experiment show clear evidence for positive phototaxis to strong light in larval bluegill bully, but weak light yielded little or no response. These results are counter to the known pattern of diel drift of bluegill bully larvae, which is highest at sunset, and lowest throughout the day (Jarvis & Closs Citation2015). These results are also at odds with those of previous studies examining phototaxis in amphidromous larvae, which either show no phototactic response (Iida et al. Citation2010) or a positive reaction to weak light, and negative reaction to strong light (Iguchi & Mizuno Citation1991). Of interest is the developmental stage at which bluegill bully hatch relative to those species previously studied. Relative to the bluegill bully, gobies such as Rhinogobius brunneus (Keith Citation2003) and Sicyopterus japonicus (Yamasaki et al. Citation2011) are much smaller (c. 1.5 mm compared to c. 2.5 mm) and less developed at hatch, and appear to require reaching the sea before development begins (e.g. Valade et al. Citation2009). It may then be that while the phototactic behaviours of these gobies are directly related to expediting downstream drift, those of bluegill bully may serve a different purpose, such as enhancing dispersal or feeding opportunities during their marine pelagic phase (Jékely Citation2009). Additional study is required to elucidate the possible benefits of positive phototaxis and migratory mechanisms in this species, and could include the examination of diel changes in larval specific gravity relative to other amphidromous larvae (see Iida et al. Citation2017).
Effect of salinity on early larval survival
All larval batches had high initial mortality regardless of treatment, supporting the idea that the first few days post-hatch are a critical period during which high mortality rates are experienced (McCasker et al. Citation2014). Following this period, larvae reared at intermediate salinity levels exhibited markedly higher survival rates than those retained in freshwater or transferred to seawater. Reaching the sea has been shown to be a requirement for larval survival and development in some amphidromous fishes (e.g. Valade et al. Citation2009; Ellien et al. Citation2011), but increased mortality might also be expected at high salinities due to the metabolic stresses and increased energy requirements for osmosis encountered when transitioning from freshwater to seawater, accelerating starvation (Iguchi & Takeshima Citation2011). Overall, the results of our survival experiment support the idea that areas of intermediate salinity such as estuaries or river mouths may play an important role in the early survival of amphidromous fish, as suggested by Iguchi and Takeshima (Citation2011). A reliance on estuaries or freshwater influenced near-shore habitats would explain the limited long distance dispersal found in some amphidromous taxa (e.g. Hughes et al. Citation2014). A focus on the use of these habitats by amphidromous larvae would likely prove a fruitful area of future research, and could be accomplished through the use of direct methods such as light traps and plankton nets (Hickford & Schiel Citation1999), or indirectly through otolith microchemistry (Hughes et al. Citation2014) or stable isotope analyses (Sorensen & Hobson Citation2005).
Another key finding from our rearing experiment was that median time to death in freshwater was around 6 days, indicating bluegill bully larvae have <1 week to complete their downstream migration. This would appear to be plenty of time to make the relatively short journey, but the time taken for larvae to reach the sea is currently unknown. Some studies have estimated migration time assuming larvae are passive particles, but distinct diel variation in larval drift density indicates larvae only migrate for a few hours per day (e.g. Iguchi & Mizuno Citation1990; Jarvis & Closs Citation2015). Additionally, the presence of clear behavioural traits that would act to either accelerate or resist migration suggest that assuming larvae are passively transported downstream is inaccurate. It should also be noted that we measured survival and not irreversible starvation, defined as the point at which larvae would be unable to successfully feed and develop even if they reached their pelagic feeding habitat (Iguchi & Mizuno Citation1999). As such, our freshwater survival results likely overestimate the length of time larvae may spend emigrating through freshwater before failing to survive and develop. Quantifying the time taken for amphidromous larvae to migrate downstream thus represents a critical area of future research, and could be accomplished through laboratory swimming trials (e.g. Jones & Closs Citation2016) or mark-recapture field studies using fluorescent otolith markers (e.g. Wilson et al. Citation1987; Beckman & Schulz Citation1996).
Captions for Supplementary Figures
Download MS Word (12.6 KB)Figure S1.
Download TIFF Image (347 KB)Figure S2.
Download TIFF Image (99.8 KB)Acknowledgements
Ken Miller assisted with preparing figures, and in supplying equipment for the phototaxis experiment. Tania King assisted with genetic analyses. Jason Augspurger and Bastien Bonvalet assisted with the phototaxis experiment. Associate editor: Professor Kendall Clements.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Matt G. Jarvis http://orcid.org/0000-0001-8276-1097
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ 1990. Basic local alignment search tool. Journal of Molecular Biology. 215:403–410. doi: 10.1016/S0022-2836(05)80360-2
- Atkinson NK, Joy MK 2009. Longitudinal size distributions of bluegill bullies (Gobiomorphus hubbsi) and torrentfish (Cheimarrichthys fosteri) in two large New Zealand rivers. New Zealand Journal of Marine and Freshwater Research. 43:643–651. doi: 10.1080/00288330909510030
- Augspurger JM, Warburton M, Closs GP. 2017. Life-history plasticity in amphidromous and catadromous fishes: a continuum of strategies. Reviews in Fish Biology and Fisheries. 27:177–192 doi: 10.1007/s11160-016-9463-9
- Bailey KM, Houde ED 1989. Predation on eggs and larvae of marine fishes and the recruitment problem. Advances in Marine Biology. 25:1–83. doi: 10.1016/S0065-2881(08)60187-X
- Beckman DW, Schulz RG 1996. A simple method for marking fish otoliths with alizarin compounds. Transactions of the American Fisheries Society. 125:146–149. doi: 10.1577/1548-8659(1996)125<0146:ASMFMF>2.3.CO;2
- Bell KNI 2007. Opportunities in stream drift: methods, goby larval types, temporal cycles, in situ mortality estimation, and conservation implications. Bishop Museum Bulletin in Cultural and Environmental Studies. 3:35–61.
- Berkeley SA, Chapman C, Sogard SM 2004. Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecology. 85:1258–1264. doi: 10.1890/03-0706
- Braun DC, Patterson DA, Reynolds J 2013. Maternal and environmental influences on egg size and juvenile life-history traits in Pacific salmon. Ecology and Evolution. 3:1727–1740. doi: 10.1002/ece3.555
- Chambers EA, Trippel RC 1997. Early life history and recruitment in fish populations. London: Chapman and Hall.
- Closs GP, Warburton M. 2016. Life histories of amphidromous fishes. In: Morais P & Daverat F, editors. An introduction to fish migration. Boca Raton, FL: CRC Press; p. 102–122.
- Ellien C, Valade P, Bosmans J, Tellebois L, Teichert N, Keith P 2011. Influence of salinity on larval development of Sicyopterus lagocephalus (Pallas, 1770) (Gobioidei). Cybium. 35:381–390.
- Ellien C, Werner U, Keith P 2016. Morphological changes during the transition from freshwater to sea water in an amphidromous goby, Sicyopterus lagocephalus (Palas 1770) (Teleostei). Ecology of Freshwater Fish. 25:48–59. doi: 10.1111/eff.12190
- Goodman JM, Dunn NR, Ravenscroft PJ, Allibone RM, Boubee JAT, David BO, Giffiths M, Ling N, Hitchmough RA, Rolfe JR. 2014. Conservation status of New Zealand freshwater fish, 2013. Department of Conservation, Wellington, New Zealand. New Zealand Threat Classification Series 7. 12 pp.
- Hickford MJH, Cagnon M, Schiel DR 2010. Predation, vegetation and habitat-specific survival of terrestrial eggs of a diadromous fish, Galaxias maculatus. Journal of Experimental Marine Biology and Ecology. 385:66–72. doi: 10.1016/j.jembe.2010.01.010
- Hickford MJH, Schiel DR 1999. Evaluation of the performance of light traps for sampling fish larvae in inshore temperate waters. Marine Ecology Progress Series. 186:293–302. doi: 10.3354/meps186293
- Hughes JM, Schmidt DJ, Macdonald JI, Huey JA, Crook DA 2014. Low interbasin connectivity in a facultatively diadromous fish: evidence from genetics and otolith chemistry. Molecular Ecology. 23:1000–1013. doi: 10.1111/mec.12661
- Iguchi K, Mizuno N 1990. Diel changes of larval drift among amphidromous gobies in Japan, especially Rhinogobius brunneus. Journal of Fish Biology. 37:255–264. doi: 10.1111/j.1095-8649.1990.tb05857.x
- Iguchi K, Mizuno N 1991. Mechanisms of embryonic drift in the amphidromous goby, Rhinogobius brunneus. Environmental Biology of Fishes. 31:295–300. doi: 10.1007/BF00000694
- Iguchi K, Mizuno N 1999. Early starvation limits survival in amphidromous fishes. Journal of Fish Biology. 54:705–712. doi: 10.1111/j.1095-8649.1999.tb02027.x
- Iguchi K, Takeshima H 2011. Effect of saline water on early success of amphidromous fish. Ichthyological Research. 58:33–37. doi: 10.1007/s10228-010-0191-1
- Iida M, Kondo M, Tabouret H, Maeda K, Pecheyran C, Hagiwara A, Keith P, Tachihara K 2017. Specific gravity and migratory patterns of amphidromous gobioid fish from Okinawa Island, Japan. Journal of Experimental Marine Biology and Ecology. 486:160–169. doi: 10.1016/j.jembe.2016.09.011
- Iida M, Watanabe S, Yamada Y, Lord C, Keith P, Tsukamoto K 2010. Survival and behavioural characteristics of amphidromous goby larvae of Sicyopterus japonicus (Tanaka, 1909) during their downstream migration. Journal of Experimental Marine Biology and Ecology. 383:17–22. doi: 10.1016/j.jembe.2009.11.006
- Jarvis MG, Closs GP 2015. Larval drift of amphidromous Gobiomorphus spp. in a New Zealand coastal stream: a critical spatial and temporal window for protection. New Zealand Journal of Marine and Freshwater Research. 49:439–447. doi: 10.1080/00288330.2015.1072569
- Jarvis MG, Warburton ML, Vivancos A, Closs GP. 2017. First capture and description of larval torrentfish (Cheimarrichthys fosteri) during their seaward migration. New Zealand Journal of Marine and Freshwater Research. doi:10.1080/00288330.2017.1310116.
- Jékely G 2009. Evolution of phototaxis. Philosophical Transactions of The Royal Society B. 364:2795–2808. doi: 10.1098/rstb.2009.0072
- Jellyman PH, Harding JS 2014. Variable survival across low pH gradients in freshwater fish species. Journal of Fish Biology. 85:1746–1752. doi: 10.1111/jfb.12497
- Jones PE, Closs GP 2016. Interspecific differences in early life-history traits in a species complex of stream-resident galaxiids. Ecology of Freshwater Fish. 25:211–224. doi: 10.1111/eff.12203
- Keith P 2003. Biology and ecology of amphidromous Gobiidae of the Indo-Pacific and the Caribbean regions. Journal of Fish Biology. 63:831–847. doi: 10.1046/j.1095-8649.2003.00197.x
- Keith P, Hoareau TB, Lord C, Ah-Yane O, Gimonneau G, Robinet T, Valade P 2008. Characterisation of post-larval to juvenile stages, metamorphosis and recruitment of an amphidromous goby, Sicyopterus lagocephalus (Pallas) (Teleostei: Gobiidae: Sicydiinae). Marine and Freshwater Research. 59:876–889. doi: 10.1071/MF08116
- Letcher BH, Rice JA, Crowder LB, Rose KA 1996. Variability in survival of larval fish: disentangling components with a generalized individual-based model. Canadian Journal of Fisheries and Aquatic Science. 53:787–801. doi: 10.1139/f95-241
- Maeda K, Tachihara K 2010. Diel and seasonal occurrence patterns of drifting fish larvae in the Teima stream, Okinawa Island. Pacific Science. 64:161–176. doi: 10.2984/64.2.161
- McCasker N, Humphries P, Meredith S, Klomp N. 2014. Contrasting patterns of larval mortality in two sympatric riverine fish species: a test of the critical period hypothesis. PloS One. 9(10):e109317. doi: 10.1371/journal.pone.0109317
- McDowall RM 1990. New Zealand freshwater fishes: a natural history and guide. Auckland: Heinemann Reed.
- McDowall RM 1998. Driven by diadromy: its role in the historical and ecological biogeography of the New Zealand freshwater fish fauna. Italian Journal of Zoology. 65:73–85. doi: 10.1080/11250009809386799
- McDowall RM 2007. On amphidromy, a distinct form of diadromy in aquatic organisms. Fish and Fisheries. 8:1–13. doi: 10.1111/j.1467-2979.2007.00232.x
- Miller TJ, Crowder LB, Rice JA, Marschall EA 1988. Larval size and recruitment mechanisms in fishes: towards a conceptual framework. Canadian Journal of Fisheries and Aquatic Sciences. 45:1657–1670. doi: 10.1139/f88-197
- Mitchell CP 1989. Laboratory culture of Galaxias maculatus and potential applications. New Zealand Journals of Marine and Freshwater Research. 23:325–336. doi: 10.1080/00288330.1989.9516369
- Moriyama A, Yanagisawa Y, Mizuno N, Omori K 1998. Starvation of drifting goby larvae due to retention of free embryos in upstream reaches. Environmental Biology of Fishes. 52:321–329. doi: 10.1023/A:1007333302864
- Morrongiello JR, Bond NR, Crook DA, Wong BBM 2013. Intraspecific variation in the growth and survival of juvenile fish exposed to Eucalyptus leachate. Ecology and Evolution. 3:3855–3867. doi: 10.1002/ece3.757
- Pepin P 1991. Effect of temperature and size on development, mortality, and survival rates of the pelagic early life history stages of marine fish. Canadian Journal of Fisheries and Aquatic Sciences. 48:503–518. doi: 10.1139/f91-065
- R Development Core Team. 2015. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- Rowe DK, Nichols S, Kelly GR 2001. Depth distribution and abundance of the common bully, Gobiomorphus cotidianus (Eleotridae), in three oligotrophic New Zealand lakes, one of which is turbid. Environmental Biology of Fishes. 61:407–418. doi: 10.1023/A:1011675602774
- Scrimgeour GJ, Eldon GA 1989. Aspects of the reproductive biology of torrentfish, Cheimarrichthys fosteri, in two braided rivers of Canterbury, New Zealand. New Zealand Journal of Marine and Freshwater Research. 23:19–25. doi: 10.1080/00288330.1989.9516336
- Sfakianakis DG, Renieri E, Kentouri M, Tsatsakis AM 2015. Effects of heavy metals on fish larvae deformities: a review. Environmental Research. 137:246–255. doi: 10.1016/j.envres.2014.12.014
- Sorensen PW, Hobson KA 2005. Stable isotope analysis of amphidromous Hawaiian gobies suggests their larvae spend a substantial period of time in freshwater river plumes. Environmental Biology of Fishes. 74:31–42. doi: 10.1007/s10641-005-3212-6
- Sutherland DL, Closs GP 2001. Spatial and temporal variation in the abundance and composition of Ichthyoplankton in a large South Island, New Zealand river Estuary. New Zealand Journal of Marine and Freshwater Research. 35:1061–1069. doi: 10.1080/00288330.2001.9517063
- Teichert N, Valade P, Bosc P, Richarson M, Gaudin P 2013. Spawning-habitat selection of an Indo-Pacific amphidromous gobiid fish, Sicyopterus lagocephalus (Pallas). Marine and Freshwater Research. 64:1058–1067. doi: 10.1071/MF13035
- Valade P, Lord C, Grondin H, Bosc P, Tallebois L, Iida M, Tsukamoto K, Keith P 2009. Early life history of larval stages of an amphidromous goby, Sicyopterus lagocephalus (Gobioidei: Sicydiinae). Cybium. 33:309–319.
- Walsh PS, Metzger DA, Higuchi R 1991. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 10:506–513.
- Ward RD, Zemlak TS, Innes BH, Last PR, Herbert PD 2005. DNA barcoding Australia’s fish species. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 360:1847–1857. doi: 10.1098/rstb.2005.1716
- Wilson CA, Beckman DW, Dean JM 1987. Calcein as a fluorescent marker of otoliths of larval and juvenile fish. Transactions of the American Fisheries Society. 116:668–680. doi: 10.1577/1548-8659(1987)116<668:CAAFMO>2.0.CO;2
- Winemiller KO & Rose KA 1992. Patterns of life-history diversification in North American fishes: implications for population regulation. Canadian Journal of Fisheries and Aquatic Sciences. 49:2196–2218. doi: 10.1139/f92-242
- Wylie MJ, Closs GP, Damsteegt EL, Lokman PM 2016. Effects of salinity and temperature on artificial cultivation and early ontogeny of giant kokopu, Galaxias argenteus (Gmelin 1789). Aquaculture Research. 47:1472–1148. doi: 10.1111/are.12605
- Yamasaki N, Kondo M, Maeda K, Tachihara K 2011. Reproductive biology of three amphidromous gobies, Sicyopterus japonicus, Awaous melanocephalus, and stenogobius sp., on Okinawa Island. Cybium. 35:345–359.