ABSTRACT
The black-footed abalone Haliotis iris is an economically important shellfish species in New Zealand. We successfully amplified, sequenced and analysed the complete nuclear ribosomal DNA (nrDNA) of H. iris. The length of the nrDNA was determined to be around 9.6 kb and included, in order, small subunit ribosomal RNA (nrSSU, 1858bp), internal transcribed spacer (ITS, 749 bp), large subunit ribosomal RNA (nrLSU, 3412bp) and an intergenic spacer (IGS, 3560–3662 bp). The nrLSU genes were identical in two individuals, whereas the nrSSU and ITS regions existed at three and four base differences, respectively. The IGS was more variable than the other nrDNA regions. A phylogenetic tree was constructed based on the ITS sequence datasets, which revealed that Haliotidae has two major subclades, mainly distributed in the North Pacific, Europe and Australia. The complete nrDNA sequence will be useful for the classification, phylogeny and breeding of this shellfish.
Introduction
Abalones are marine molluscs that have been studied extensively because of their increased economic value and high nutritional value among shellfish. The genus includes 56 extant species distributed along the coastlines of most continents and islands in the Pacific, Indian and Atlantic oceans, of which more than 10 species are commercially cultured (Geiger Citation2000; Hernandez-Ibarra et al. Citation2008; van der Merwe and Roodt-Wilding Citation2008). The black-footed abalone (H. iris), the largest and most common abalone species in New Zealand and known locally as pāua, is cultivated for the export market (Chew et al. Citation2013; Hadi et al. Citation2014). H. iris has enormous value and is a cultural keystone and treasured species of the Mäori. The production of H. iris reached 86.85 tonnes in 2014 (FAO Citation2014) and helped to support a commercial fishery that was worth $57 million to the New Zealand economy in exports in 2011 (Seafood Industry Council Citation2012).
In recent years, research on H. iris has focused on its aquaculture (Tung and Alfaro Citation2011; James and Barr Citation2012; Hadi et al. Citation2014; Cunningham et al. Citation2016), immunology (Grandiosa et al. Citation2016), nutrition (Tung Citation2012), ecology (Aguirre and McNaught Citation2011; Dyck et al. Citation2011; Chew et al. Citation2013; Somerville et al. Citation2014) and molecular biology (Bryant et al. Citation2006; Will et al. Citation2011, Citation2015). The molecular studies of H. iris are major in population genetics and functional gene expression, which are relatively poor compared to those of other abalone species, e.g. H. discus hannai (Li et al. Citation2003; Liu et al. Citation2006; Yang et al. Citation2015; Yu et al. Citation2015; Nam et al. Citation2016).
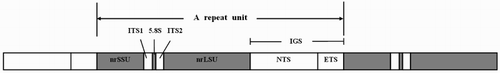
In eukaryotes, the nuclear ribosomal DNA (nrDNA) is a multigene family that comprises a transcribed rRNA gene and adjacent non-transcribed genes. The rDNA transcription units consist of the small subunit ribosomal RNA gene (nrSSU), internal transcribed spacer (ITS), large subunit ribosomal RNA gene (nrLSU) and an intergenic spacer (IGS), which are arrayed in this order and then clustered in large tandems located on certain chromosomes and form what is known as nucleolus organising regions (Li et al. Citation2016) (). The ITS region contains internal transcribed spacer 1 (ITS1), the 5.8S rDNA gene and ITS2, with the 5.8S rDNA located between ITS1 and ITS2 (Bianciardi et al. Citation2012). The IGS region contains a non-transcribed spacer (NTS) region and an external transcribed spacer (ETS) region.
Figure 1 . Schematic diagram of the tandemly repeated ribosomal DNA in eukaryotes, nrSSU: small subunit ribosomal RNA; nrLSU: large subunit ribosomal RNA; ITS1: internal transcribed spacer 1; ITS2: internal transcribed spacer 2; NTS: non-transcribed spacer; ETS: external transcribed spacer; IGS: intergenic spacer.
The rDNA clusters present concerted evolution and do not evolve independently from each other, but each region of the rDNA units evolves at a different rate (Hillis & Dixon Citation1991). Thus, nrDNA regions have always been used as molecular markers for analysing phylogeny and identifying germplasm (Li et al. Citation2016). The nrSSU and nrLSU genes evolve relatively conserved and are more valuable for phylogenetic comparisons at the family and order levels. Passamaneck et al. (Citation2004) used nrSSU and nrLSU genes to determine the relationships between the major lineages within the Mollusca. The species H. discus and H. diversicolor have the same nrSSU (18S rRNA) sequences but differ in their ITS1 sequences (Wang et al. Citation2004). The 5.8S rRNA gene is rarely used by itself because of its shortness and is generally combined with ITS regions to study intraspecific or interspecific evolution. The non-coding regions including IGS, ITS1 and ITS2 evolve rapidly and have been used for phylogenetic comparison at the genus, species and subspecies levels, and even in individuals (Parvaresh et al. Citation2014; Xu et al. Citation2016). Li et al. (Citation2010) revealed that the IGS sequence could be used as the critical genetic marker in intraspecies of Pyropia haitanensis. The ITS sequence of H. iris was used to explore the phylogeny of Haliotis spp. as reported by Coleman and Vacquier (Citation2002), who showed that H. iris was quite distant from the remaining Haliotis spp. species, almost as much as the more obvious outgroup, the keyhole limpet. Van Wormhoudt et al. (Citation2011) supposed that the position of H. iris might be confusing and suggested that a new analysis of this species was necessary. Meanwhile, no related research has been performed on the remaining rDNA regions of H. iris.
In this paper, the complete nrDNA sequence of the black-footed abalone H. iris was analysed for the first time, to our knowledge, and the features of each region were revealed. These findings will provide more data for classification, identification, phylogeny, germplasm management and heredity breeding in the Haliotis species. In addition, the method applied in this study can be used to amplify the complete rDNA unit in other marine mollusc species.
Materials and methods
Sampling and DNA extraction
Two individuals of H. iris were collected from Wellington located on a latitude of 41.337139° and longitude of 174.792826°, and the average shell length was 15.35 ± 0.21 cm. The foot muscles of black-footed abalone were extracted, transferred into 75% alcohol and stored at −20°C until use. The total genomic DNA was extracted using a TIANamp Marine Animals DNA Kit (TIANGEN Biotech Co., Ltd.) according to the manufacturer’s instructions.
PCR amplification
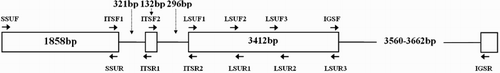
The rDNA unit genes were amplified by using the primers listed in , and the schematic diagram is shown in . The nrSSU gene was amplified using the primers SSUF and SSUR reported by Huang et al. (Citation2007). The primers to amplify the ITS region and nrLSU gene were designed based on the complete ITS sequence of H. discus (AY146403) and H. discus (AY145418.1), respectively. The primers of the IGS region were designed based on the sequences of the 3′ end of the nrLSU gene and the 5′ end of the nrSSU gene of the H. iris studied in this work. All primers were synthesised by Sangon Biotech Co., Ltd. (Shanghai, China).
Table 1. Primers used in this study.
The reactions of the PCR amplification were 25-μl mixtures containing 12.5 μl 2 × TransTaq High Fidelity (HiFi) PCR SuperMix (TransGen Biotech Co., Ltd.), 0.5 μl of each primer (20 μmol/L), 2 μl DNA template and 9.5 μl ddH2O. The PCR amplification protocol is listed in . The PCR products were electrophoresed on 1% agarose gels and purified using the SanPrep Column DNA Gel Extraction Kit (Sangon Biotech Co., Ltd.). The purified fragments were ligated into the pUCm-T vector, and the plasmid was extracted using the pUCm-T Vector Cloning Kit and SanPrep Column Plasmid Mini-Preps Kit (Sangon Biotech Co., Ltd.) according to the manufacturer’s instructions. Three positive recombinant colonies of each of the amplification products were picked and cultivated, their plasmids were extracted by the alkaline lysis method, and the plasmids were sequenced by Shanghai Sangon Biotech Co., Ltd.
Table 2. PCR reaction protocols for amplifying H. iris ribosomal DNA fragments.
Sequencing and analysis
The obtained sequences were ascertained using BLAST software by comparison with those data available from the National Center for Biotechnology Information (NCBI) GenBank database and then aligned with Clustal X (Larkin et al. Citation2007). Variable regions of the aligned dataset were removed using the program Gblocks (Hassan et al. Citation2015). The pairwise distances of the rDNA gene sequences were calculated with MEGA 6.0 according to the two-parameter model of Kimura (Tamura et al. Citation2013). The phylogenetic analysis of the ITS sequence dataset based on the maximum likelihood (ML) method was conducted using PhyML (Guindon et al. Citation2010) with 1000 bootstrap replicates. This program utilised the ‘General Time Reversible+I+G’ model, which was selected as the ‘best-fit’ for the ITS dataset in jmodeltest 0.1 (Posada Citation2009). Repeat fragments of the IGS region were sought using the Tandem Repeats Finder (Benson Citation1999).
Results
Length of nrDNA
The target gene fragments were successfully sequenced after amplification and cloning. After comparison, alignment and assembly, the lengths of the nrDNA regions were determined. The lengths of the nrSSU, ITS, nrLSU and IGS regions were 1858, 749, 3412 and 3560–3662 bp, respectively. The total length of the nrDNA was around 9.6 kb. The GenBank accession numbers, the GC contents and the length of each region of our newly sequenced nrDNA genes are listed in .
Table 3. GenBank accession number, the GC contents and the length of each region of the ribosomal DNA.
nrSSU
The complete nrSSU gene was amplified and sequenced from two black-footed abalone individuals, and the sequence identity between them was 99.84%, with just three base differences. We searched the information on SSU sequences from the NCBI and found that 14 kinds of abalone have been studied (). Among these species, only H. tuberculata (AF120511.1), H. diversicolor (AY698072.1) and H. discus (AF082177.1) had complete sequences; the other abalones had only partial sequences that varied from 547 to 1809bp. The pairwise distances among them showed 0.000–0.842 sequence divergence, and the genetic distance of H. rufescens was large (0.670–0.842) compared with that of the other abalones listed in . Except for H. rufescens, the values ranged from 0.000 to 0.094.
Table 4. Collection information, GenBank accession numbers and the lengths of each ribosomal region for Haliotis species used in the present study.
Table 5. Pairwise distances of the nrSSU gene sequences.
ITS
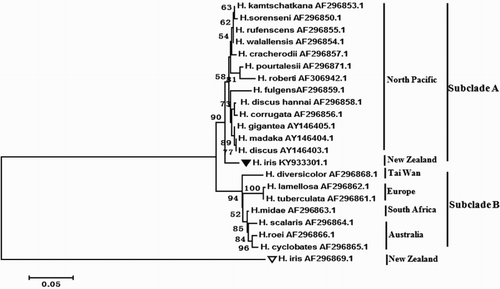
The ITS region was successfully sequenced, and the boundaries of the region were confirmed by comparison with H. discus (AY146403.1). 5.8S was used to compare with the gastropod Arion rufus (X00131.1) according to its highly conservative feature. The lengths of ITS, ITS1, 5.8S and ITS2 were 749, 321, 132, and 296 bp, respectively. The Genbank accession numbers of ITS1 and ITS2 were KY936886–KY936887 and KY936888–KY936889, respectively. The average homology value of the samples was 99.47%, with just four base differences. Up to now, ITS genes have been studied in 22 Haliotidae species (). The genetic distances among them varied from 0.000 to 0.063 (), and an ML phylogenetic tree was constructed to reveal the evolutionary relationships of abalone with 1000 bootstrap replications. The tree had two major subclades, which were mainly distributed in the North Pacific, Australia and Europe (), and the phylogeny of Haliotidae showed that it was easy to cluster into one clade when the ITS region was distributed in a nearby area and on the same chromosome.
Table 6. Pairwise distances of ITS gene sequences.
nrLSU
The nrLSU gene from H. iris was sequenced and assembled successfully, and the length (3412 bp) was confirmed by comparison with the ITS region studied in this work and the nrLSU region of H. discus (AY145418.1). Among the five kinds of abalone recorded in the NCBI database, the nrLSU and ITS regions are partial sequences, which vary from 300 to 3430 bp. The nrLSU sequence obtained in this study had greater than 98% identity with the other five Haliotis species published in the GenBank database. The pairwise distances between them showed 0.011–0.042 sequence divergences, as shown in . H. tuberculata and H. asinine, H. corrugate and H. iris, and H. discus and H. iris exhibited minimum genetic distances (0.011). The largest genetic distances occurred between H. corrugata and H. diversicolor supertexta and between H. discus and H. diversicolor supertexta (0.042).
Table 7. Pairwise distances of the nrLSU gene sequences.
IGS
Because there was no relevant research on intergenic spacers in shellfish, according to the repeat tandem feature of nrDNA, the IGS primers were designed with the 3′ end of the nrLSU and the 5′ end of the nrSSU of H. iris studied in this work, and the complete length of the IGS region was determined to be 3560 and 3662 bp, respectively. Compared with two sequences (KY978225–KY978226) obtained in this work, they had 95.66% sequence identity, which existed in three insertions located between bp 1136 and bp 1198, bp 1668 and bp 1691 and bp 1776 and bp 1798, respectively. The three insertions were mainly repeat fragments and were, in turn, ‘GGCTA’, ‘TGTA’ and ‘GA’. In addition, some repeat fragments were also found in the IGS upstream sequence within the scope of 1078 to 1833 bp except the three insertions.
Discussion
The ribosome is one of most important organelles found in cells because it is responsible for the coding of proteins. A complete nrDNA unit comprises six different genes, and identical copies of these genes are encoded multiple times. The process that is believed to maintain similarities between different copies of the repeated sequences is known as concerted evolution (Ohta Citation2000), which is considered to be standard among multigene families arranged in tandem arrays (Graur and Li Citation2000). However, evolutionary rates differ in each gene of the nrDNA. The IGS and ITS sequences have been found to evolve more quickly than the nrSSU and nrLSU regions. Thus, IGS and ITS regions are useful molecular markers for classification and identification at the species and subspecies levels and even individuals (Danica and Baldwin Citation2007).
This is the first report of the complete nrDNA sequence in a mollusc. The complete nrDNA of H. iris was around 9.6 kb in length, which is smaller than that of the Chinese mitten crab Eriocheir sinensis (11.66 Kb; Yu et al. Citation2010), the algae Bangia (13 Kb; Xu et al. Citation2016) and Pyropia yezoensis (12.65 Kb; Li et al. Citation2016). The most significant differences are in the lengths of the IGS and ITS regions.
The nrSSU and nrLSU genes are efficient and reliable molecular markers for phylogenetic investigations at the higher taxonomic levels. Geiger and Thacker (Citation2005) analysed the nrSSU (18S rRNA) of 38 ingroup vetigastropods and two neritimorph outgroups. Using a combined analysis with seven molecular loci that included the nrSSU and nrLSU genes, Aktipis and Giribet (Citation2010) confirmed that Gastropoda, Caenogastropoda, Neritimorpha, Neomphalina and Patellogastropoda were monophyletic and that Neomphalina and Pleurotomariidae fell outside of the remaining vetigastropods. The nrSSU and nrLSU genes are extremely conserved. In the present study, the nrSSU sequences of H. iris differed by only three bases and the nrLSU sequences were identical. These results correspond to those of Wang et al. (Citation2004), Huang et al. (Citation2007), Van Wormhoudt et al. (Citation2009), and Yang et al. (Citation2012). All of these reports indicated that the nrSSU gene differed very little among the species studied and was almost identical in some closely related abalone species. Comparing the distance values of the nrSSU and nrLSU genes ( and , respectively) indicated that the mutation rate of the nrLSU gene was slightly faster than that of the nrSSU gene. The pairwise distances of the nrSSU and nrLSU genes were both less than the interspecific genetic distance (0.1) except in H. rufescens. Thus, the nrSSU and nrLSU genes are not suitable for identifying abalone species.
The evolution of the ITS sequence has been reported to be more rapid than that of the conserved nrSSU and nrLSU genes. The ITS region has been a useful molecular marker for animal and plant classification and identification at the species and subspecies levels (Ma et al. Citation2000; Wang et al. Citation2004; Li et al. Citation2009; Van Wormhoudt et al. Citation2009). The present study found three base differences in ITS1 and one base difference in ITS2 and no second type existed, whereas two ITS1 types were detected in H. tuberculata (Van Wormhoudt et al. Citation2009). The phylogenetic tree based on the ITS sequence was similar to the result of Coleman and Vacquier (Citation2002) except for H. iris. Comparison of the ITS sequence of H. iris (GenBank accession No. AF296869.1) with the datasets studied in present study showed just 53.62% homology. Coleman and Vacquier (Citation2002) proposed that this could have been caused by rapid evolution producing a long branch, or it may reflect ancient isolation of the lineage leading to H. iris. The genetic distance between H. iris and other abalone species varies from 0.025 to 0.063, and it conforms with the phylogenetic relationship of Haliotidae. Therefore, we suppose that two types of ITS may exist in H. iris, with the two ITS clusters localised on different chromosomes. Our results indicated that the separation of the two sets of sequences might have occurred before the speciation of the recent Haliotidae (Geiger Citation1999). Van Wormhoudt et al. (Citation2009), Guo et al. (Citation2017) and Wang et al. (Citation2017) constructed phylogeny tree of abalone based on mitochondrial genes, 16S rRNA and CoI. Which also mainly contained Pacific subclade, Australia subclade, and Europe subclade, and H. iris was grouping with the Pacific group. It indicated that species distributed in the same region tended to cluster together in the same clade.
The IGS region is suitable for determining the genetic relationships of different subspecies and individuals because it has more informational sites than the ITS sequences do (Dai et al. Citation2008; Jiang et al. Citation2008; Li et al. Citation2010). The genetic distance between individuals was only 0.012, which indicated the close relationship within the same species. There was no way to compare and analyse the IGS sequence with other species because of the lack of IGS sequences in other Haliotis species, so the IGS sequences of H. iris reported here provide pioneering data for GenBank and significant molecular information for the further study of Haliotidae. The location of the NTS and ETS of the IGS region could not be distinguished because of a lack of reference sequences from closely related species. The IGS region had several repeat fragments, both of which were insertions covering 2, 4, 6, 12, 14, 20 and 32 bp within the scope of 1078–1833 bp. The repeat fragments of IGS, well known as enhancers, could promote ribosomal transcription and were also reported to relate to biological growth characteristics (Weider et al. Citation2004). Besides, the repeat units also had species specificity: Xenopus laevis (Reeder Citation1984) had repeat fragments of 60 and 81 bp, and Mus musculus (Kuhn et al. Citation1990), Eriocheir sinensis (Yu et al. Citation2010) and P. yezoensis (Li et al. Citation2016) had lengths of 140, 136–139 and 74 bp, respectively.
This is the first time that the complete nrDNA cistron sequence of H. iris has been studied. This sequence will enrich GenBank and provide additional information for phylogenetic analyses. Moreover, the approach used in this study will be useful in future studies of molluscs at various taxonomic levels.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aguirre JD, McNaught DC. 2011. Habitat modification affects recruitment of abalone in central New Zealand. Marine Biology. 158(3):505–513. doi: 10.1007/s00227-010-1576-4
- Aktipis SW, Giribet G. 2010. A phylogeny of Vetigastropoda and other ‘‘archaeogastropods’’: reorganizing old gastropod clades. Invertebrate Biology. 129(3):220–240. doi: 10.1111/j.1744-7410.2010.00198.x
- Benson G. 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Research. 27(2):573–580. doi: 10.1093/nar/27.2.573
- Bianciardi A, Boschi M, Swanson EE, Belloni M, Robbins LG. 2012. Ribosomal DNA organization before and after magnification in Drosophila melanogaster. Genetics. 191(3):703–723. doi: 10.1534/genetics.112.140335
- Bryant MJ, Flint HJ, Sin FYT. 2006. Isolation, characterization, and expression analysis of three actin genes in the New Zealand black-footed abalone, Haliotis iris. Marine Biotechnology. 8(2):110–119. doi: 10.1007/s10126-005-5139-5
- Chew CA, Hepburn CD, Stephenson W. 2013. Low-level sedimentation modifies behaviour in juvenile Haliotis iris and may affect their vulnerability to predation. Marine Biology. 160:1213–1221. doi: 10.1007/s00227-013-2173-0
- Coleman AW, Vacquier VD. 2002. Exploring the phylogenetic utility of ITS sequences for animals: a test case for abalone (Haliotis). Journal of Molecular Evolution. 54:246–257. doi: 10.1007/s00239-001-0006-0
- Cunningham SC, Smith AM, Lamare MD. 2016. The effects of elevated pCO2 on growth, shell production and metabolism of cultured juvenile abalone, Haliotis iris. Aquaculture Research. 47(8):2375–2392. doi: 10.1111/are.12684
- Dai XJ, Ou LJ, Li WJ, Liang MJ, Chen LB. 2008. Analysis of rDNA intergenic spacer (IGS) sequences in Oryza sativa L. and their phylogenetic implications. Acta Agronomica Sinica. 34(9):1569–1573. (in Chinese with English Abstract). doi: 10.3724/SP.J.1006.2008.01569
- Danica TH, Baldwin GB. 2007. Phylogeny and biogeography of the sandalwoods (Santalum, Santalaceae): repeated dispersals throughout the Pacific. American Journal of Botany. 94:1028–1040. doi: 10.3732/ajb.94.6.1028
- Dyck M, Roberts R, Jeffs A. 2011. Assessing alternative grazing-tolerant algae for nursery culture of abalone, Haliotis iris. Aquaculture. 320(1–2):62–68. doi: 10.1016/j.aquaculture.2011.07.033
- Food and Agriculture Organization of the United Nations (FAO). 2014. Fishstatj. Rome.
- Geiger DL. 1999. A total evidence cladistic analysis of the Haliotidae (Gastropoda Vetigastropoda) [dissertation]. South California: University of South California.
- Geiger DL. 2000. Distribution and biogeography of the Haliotidae (Gastropoda: Vetigastropoda) world-wide. Bollettino Malacologico. 35:57–120.
- Geiger DL, Thacker CE. 2005. Molecular phylogeny of Vetigastropoda reveals non-monophyletic Scissurellidae, Trochoidea, and Fissurelloidea. Molluscan Research. 25(1):47–55.
- Grandiosa R, Merien F, Pillay K, Alfaro A. 2016. Innovative application of classic and newer techniques for the characterization of haemocytes in the New Zealand black-footed abalone (Haliotis iris). Fish & Shellfish Immunology. 48:175–184. doi: 10.1016/j.fsi.2015.11.039
- Graur D, Li WH. 2000. Fundamentals of molecular evolution. Sinauer associates. Sunderland: Sinauer Associates Inc.
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology. 59(3):307–321. doi: 10.1093/sysbio/syq010
- Guo ZS, Zhang JW, Li L, Hou XG, Zhang HT, Zhang XJ. 2017. Sequence analysis of mitochondrial 16S rRNA gene and molecular phylogeny of three species of abalones. Journal of Shandong University(Natural Science). 52(1):1–8 (in Chinese with English Abstract).
- Hadi JA, Gutierrez N, Alfaro AC, Roberts RD. 2014. Use of probiotic bacteria to improve growth and survivability of farmed New Zealand abalone (Haliotis iris). New Zealand Journal of Marine and Freshwater Research. 48(3):405–415. doi: 10.1080/00288330.2014.909857
- Hassan KA, Liu Q, Henderson PJF, Paulsena IT. 2015. Homologs of the Acinetobacter baumannii aceI transporter represent a new family of bacterial multidrug efflux systems. MBio. 6 (1):e01982–14. doi: 10.1128/mBio.01982-14
- Hernandez-Ibarra NK, Leitch AR, Cruz P, Ibarra AM. 2008. Fluorescent in situ hybridization and characterization of the SalI family of satellite repeats in the Haliotis L. species (abalone) of the Northeast Pacific. Genome. 51:570–579. doi: 10.1139/G08-041
- Hillis DM, Dixon MT. 1991. Ribosomal DNA: molecular evolution and phylogenetic inference. The Quaterly Review of Biology. 66:411–453. doi: 10.1086/417338
- Huang B, Fang ZG, Liu JL, Zhou Z, Wang XB. 2007. Cloning and sequence of genes encoding Haliotis asinina 18S rRNAs. Oceanologia Et Limnologia Sinica. 38(3):212–216. (in Chinese with English Abstract).
- James PJ, Barr NG. 2012. The effects of elevated concentrations of dissolved inorganic phosphate in seawater on the growth and survival of juvenile abalone, Haliotis iris. Aquaculture Research. 43(3):438–446. doi: 10.1111/j.1365-2109.2011.02847.x
- Jiang SH, Meng ZY, Chen XQ, Li GJ. 2008. Application of rDNA sequence analysis in insect systematics. Entomotaxonomia. 30(3):225–238. (in Chinese with English Abstract).
- Kuhn A, Deppert U, Grummt I. 1990. A 140-base-pair repetitive sequence element in the mouse rRNA gene spacer enhancer transcription by RNA polymerase I in a cell-free system. Proceedings of the National Academy of Sciences of the United States of America. 87(19):7527–7531. doi: 10.1073/pnas.87.19.7527
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics. 23:2947–2948. doi: 10.1093/bioinformatics/btm404
- Li Q, Park C, Kobayashi T, Kijima A. 2003. Inheritance of microsatellite DNA markers in the Pacific abalone Haliotis discus hannai. Marine Biotechnology. 5(4):331–338. doi: 10.1007/s10126-002-0116-8
- Li XC, Xu JJ, He Y, Shen SS, Zhu JY, Shen ZG. 2016. The complete nuclear ribosomal DNA (nrDNA) cistron sequence of Pyropia yezoensis (Bangiales, Rhodophyta). Journal of Applied Phycology. 28:663–669. doi: 10.1007/s10811-015-0522-8
- Li YY, Shen SD, He LH, Xu P, Lu S. 2010. Sequence analysis of rDNA intergenic spacer (IGS) of Porphyra haitanensis. Journal of Applied Phycology. 22:187–193. doi: 10.1007/s10811-009-9441-x
- Li YY, Shen SD, He LH, Xu P, Wang GC. 2009. Sequence analysis of the ITS region and 5.8S rDNA of Porphyra haitanensis. Chinese Journal of Oceanology and Limnology. 27(3):493–501. doi: 10.1007/s00343-009-9168-1
- Liu XD, Liu X, Guo XM, Guo QK, Zhao HE, Zhang GF. 2006. A preliminary genetic linkage map of the pacific abalone Haliotis discus hannai Ino. Marine Biotechnology. 8(4):386–397. doi: 10.1007/s10126-005-6133-7
- Ma XJ, Wang XQ, Xiao PG, Hong DY. 2000. Comparison of ITS sequences between wild ginseng DNA and garden ginseng DNA. China Journal of Chinese Materia Medica. 25:206–209. (in Chinese with English Abstract).
- Nam BH, Jung M, Subramaniyam S, Yoo SI, Markkandan K, Moon JY, Kim YO, Kim DG, An CM, Shin Y, et al. 2016. Transcriptome analysis revealed changes of multiple genes involved in Haliotis discus hannai innate immunity during Vibrio parahemolyticus infection. PLOS ONE. 11(4):e0153474. doi: 10.1371/journal.pone.0153474
- Ohta T. 2000. Evolution of gene families. Gene. 259:45–52. doi: 10.1016/S0378-1119(00)00428-5
- Parvaresh M, Talebi M, Sayed-Tabatabaei BE. 2014. Molecular characterization of ribosomal DNA intergenic spacer (IGS) region in pomegranate (Punica granatum L.). Plant Systematics and Evolution. 300(5):899–908. doi: 10.1007/s00606-013-0928-1
- Passamaneck YJ, Schander C, Halanych KM. 2004. Investigation of molluscan phylogeny using large-subunit and small-subunit nuclear rRNA sequences. Molecular Phylogenetics and Evolution. 32:25–38. doi: 10.1016/j.ympev.2003.12.016
- Posada D. 2009. Selection of models of DNA evolution with jModelTest. Bioinformatics for DNA sequence analysis. Methods in Molecular Biology. 537:93–112. doi: 10.1007/978-1-59745-251-9_5
- Reeder RH. 1984. Enhancers and ribosomal gene spacers. Cell. 38(2):349–351. doi: 10.1016/0092-8674(84)90489-6
- Seafood Industry Council. 2012. Exports of Seafood Produce for 12 months ending December 2011. NZ SeaFIC.
- Somerville GJ, Krkosek M, Hepburn CD. 2014. A matrix model and elasticity analysis for New Zealand’s blackfoot paua Haliotis iris. Fisheries Research. 151:158–168. doi: 10.1016/j.fishres.2013.11.008
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA 6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 30(12):2725–2729. doi: 10.1093/molbev/mst197
- Tung CH, Alfaro AC. 2011. Initial attachment, metamorphosis, settlement, and survival of black-footed abalone, Haliotis iris, on microalgal biofilms containing different amino acid compositions. Journal of the World Aquaculture Society. 42(2):167–183. doi: 10.1111/j.1749-7345.2011.00454.x
- Tung CH, Alfaro AC. 2012. Alternative protein sources in artificial diets for New Zealand’s black-footed abalone, Haliotis iris, Martyn 1784, Juveniles. Journal of the World Aquaculture Society. 43(1):1–29. doi: 10.1111/j.1749-7345.2011.00545.x
- van der Merwe M, Roodt-Wilding R. 2008. Chromosome number of the South African abalone Haliotis midae. African Journal of Marine Science. 30(1):195–198. doi: 10.2989/AJMS.2008.30.1.21.471
- Van Wjormhoudt A, Gaume B, Le Bras Y, Roussel V, Huchette S. 2011. Two different and functional nuclear rDNA genes in the abalone Haliotis tuberculata: tissue differential expression. Genetica. 139:1217–1227. doi: 10.1007/s10709-011-9623-8
- Van Wormhoudt A, Le Bras Y, Huchette S. 2009. Haliotis marmorata from Senegal; a sister species of Haliotis tuberculata: morphological and molecular evidence. Biochemical Systematics and Ecology. 37:747–755. doi: 10.1016/j.bse.2009.12.020
- Wang LS, Xue YJ, Fang SS, Liu X. 2017. Comparison of COI gene complete sequence and phylogenetic analysis in eight kinds of economic abalone. Journal of Biology. 34(2):32–40. (in Chinese with English Abstract).
- Wang ZY, Ho KC, Yu KC, Yu DH, Ke CH, Mak WY, Chu KH. 2004. Lack of genetic divergence in nuclear and mitochondrial DNA between subspecies of two Haliotis species. Journal of Shellfish Research. 23(4):1143–1146.
- Weider LJ, Glenn KL, Kyle M, Elser JJ. 2004. Associations among ribosomal rDNA intergenic spacer length variation, growth rate, and C:N: P stoichiometry in the genus Daphnia. Limnology and Oceanogr. 49(4):1417–1423. doi: 10.4319/lo.2004.49.4_part_2.1417
- Will M, Hale ML, Schiel DR. 2011. Low to moderate levels of genetic differentiation detected across the distribution of the New Zealand abalone, Haliotis iris. Marine Biology. 158(6):1417–1429. doi: 10.1007/s00227-011-1659-x
- Will M, McCowan T, Gemmell NJ. 2015. Broad-scale genetic patterns of New Zealand abalone, Haliotis iris, across a distribution spanning 13A degrees latitude and major oceanic water masses. Genetica. 143(4):487–500. doi: 10.1007/s10709-015-9847-0
- Xu JJ, Jiang B, Chai SM, He Y, Zhu JY, Shen ZG, Shen SD. 2016. Complete nuclear ribosomal DNA sequence amplifi cation and molecular analyses of Bangia (Bangiales, Rhodophyta) from China. Chinese Journal of Oceanology and Limnology. 34(5):1044–1053. doi: 10.1007/s00343-016-5033-1
- Yang EC, Nam BH, Noh SJ, Kim YO, Kim DG, Jee YJ, Park JH, Noh JH, Yoon HS. 2015. Complete mitochondrial genome of Pacific abalone (Haliotis discus hannai) from Korea. Mitochondrial DNA. 26(6):917–918. doi: 10.3109/19401736.2013.863289
- Yang WJ, Huang B, Wang RE, Zhang Y. 2012. Cloning and sequence analysis of Haliotis ovina 18S rDNA in the different geographical populations of Hainan. Journal of Anhui Agriculture Science. 40(20):10370–10373. (in Chinese with English Abstract).
- Yu JH, Li HX, Ge XP, Li JL, Tang YK. 2010. Characteristics analysis of complete ribosomal DNA sequence in Chinese mitten crab (Eriocheir sinensis). Journal of Fisheries of China. 34(5):698–703. (in Chinese with English Abstract). doi: 10.3724/SP.J.1231.2010.06659
- Yu Q, Li YP, Xing Q, Li X, Zhao L, Wang YF, Hu XL, Bao ZM. 2015. Forty-nine SNP markers developed from the genome dataset of Pacific abalone (Haliotis discus hannai) and their application in population genetic analysis. Conservation Genetics Resources. 7(4):781–787. doi: 10.1007/s12686-015-0482-y