ABSTRACT
The threat posed by ocean acidification (OA) to the diversity and productivity of New Zealand marine ecosystems is assessed in a synthesis of published trends and impacts. A 20-year time series in Subantarctic water, and a national coastal monitoring programme, provide insight into pH variability, and context for experimental design, modelling and projections. A review of the potential impact of changes in the carbonate system on the major phyla in New Zealand waters confirms international observations that calcifying organisms, and particularly their early life-history stages, are vulnerable. The synthesis considers ecosystem and socio-economic impacts, and identifies current knowledge gaps and future research directions, including mechanistic studies of OA sensitivity. Advanced ecosystem models of OA, that incorporate the indirect effects of OA and interactions with other climate stressors, are required for robust projection of the future status of New Zealand marine ecosystems.
Introduction
Since the start of the Industrial Revolution, an additional 555 Pg C of carbon dioxide (CO2) have been released into the atmosphere, of which approximately 30% has entered the ocean (Ciais et al. Citation2013). This ocean sink for was initially viewed as a beneficial buffer to the increasing anthropogenic input to the atmosphere and its impact on Earth’s climate. However, it is now apparent that this transfer of anthropogenic
alters ocean chemistry, with potentially serious ramifications for marine biota and ecosystems (Orr et al. Citation2005; Fabry Citation2008). This process, known as ocean acidification (OA), is defined as the ‘global-scale long-term decrease in seawater pH, that is currently primarily due to the human-driven increase in atmospheric CO2 (Secretariat of the Convention on Biological Diversity Citation2014). However, OA encompasses not just a decline in seawater pH, but also changes in the concentration of different species in the marine carbonate system (). The increase in CO2 transfer from the atmosphere causes an increase in dissolved CO2 (pCO2), which reacts with water to form carbonic acid (H2CO3). This acid then dissociates to produce bicarbonate ions (
), and hydrogen ions (H+), with the latter reacting with carbonate ions
. Consequently, OA is characterised by an increase in pCO2 and
, and corresponding decreases in pH (i.e. an increase in H+) and
(). It also results in a decrease in the carbonate saturation state (Ω, the ratio of dissolved calcium and carbonate ions to their solubility product constants), which differs between the two main polymorphs of carbonate, calcite (ΩC) and aragonite (ΩA), due to their differing crystal structure and stability.
Figure 1. Conceptual diagram showing the reactions and chemical species of the marine carbonate system (black arrows), with the direction of the vertical red arrows indicating the net change in response to increasing atmospheric CO2, and resulting in ocean acidification.
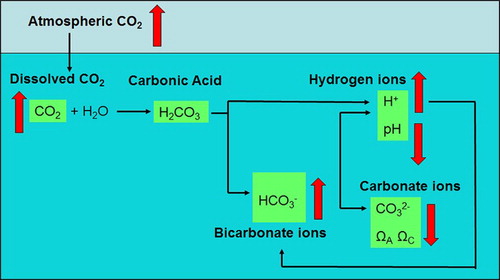
This fundamental shift in ocean chemistry may influence a variety of biotic, as well as abiotic, processes. International studies on the impact of OA initially focussed upon the decline in availability, and the potential detrimental effect on calcifying species that produce carbonate shells (Orr et al. Citation2005; Langer et al. Citation2006). Although it has subsequently been established that some calcifiers use CO2 and
to produce
during calcification (Roleda, Boyd, et al. Citation2012), this remains a topic of debate (Waldbusser, Hales, et al. Citation2016). Although potential benefits of OA may accrue for some non-calcifying phytoplankton and macroalgae, in the form of increased pCO2 for photosynthesis, OA may affect the physiology of a broad range of marine organisms via alteration of their acid–base balance in response to changing [H+] (Pörtner Citation2002; Kroeker et al. Citation2013). The resulting additional metabolic cost in physiological compensation may then influence species survival (mortality, reproduction, fitness), and condition (growth, biomass, fecundity), with potential ramifications for foodwebs, ecosystem stability, services and economic value (Doney et al. Citation2009).
New Zealand has a large Exclusive Economic Zone (EEZ) incorporating Subtropical, Subantarctic and frontal waters that support a variety of ecosystems, with high biodiversity and endemism (Gordon et al. Citation2010). The coast includes a variety of ecotypes, that range from anthropogenically impacted to pristine, and includes unique ecosystems such as the fjords in the south-west, and CO2 seeps in the Bay of Plenty. The deep sea in the EEZ contains numerous submarine topographical features, including seamounts, guyots, ridges, methane seeps and canyons, that are often sites of elevated faunal biomass and diversity. As with many island nations, New Zealand is dependent upon its coastal and marine resources for a variety of socio-economic benefits, such as shellfish aquaculture, fisheries and broader ecosystem services (MacDiarmid et al. Citation2013). These biological and geophysical factors, combined with socio-economic imperatives, have directed national marine research activities.
Although OA is a global phenomenon, its rate and impacts will vary regionally, dependent upon geographical location, ocean dynamics and associated biogeochemistry. The current global surface pH mean of c. 8.1 (range 7.9–8.2; Takahashi et al. Citation2014), is estimated to have decreased by c. 0.1 (equivalent to a 30% increase in H+ concentration), over the last 250 years (Turley et al. Citation2006). This is similar to the mean pH of c. 8.088 (range 8.04–8.13) recorded in a 13-year time series in Subantarctic waters, east of New Zealand, during which pH declined by 0.0013 per annum (Bates et al. Citation2014). Projected changes in pH in the New Zealand region are also consistent with those for the global ocean, with a decline of 0.33 by 2100 (Law et al., Citationforthcoming), estimated using the RCP8.5 emission scenario (Representative Concentration Pathway 8.5, where 8.5 is the radiative forcing expected by the year 2100; van Vuuren et al. Citation2011). Critically, these projections indicate the lowest global pH value, and the fastest rate of pH change, over the last 25 M years (Turley et al. Citation2006). Consequently, New Zealand waters are already exposed to OA, and will be subject to further pH stress in the future. Although research has been steadily increasing over the last decade, there is still much to learn regarding the impacts of OA on New Zealand marine ecosystem structure and function, and the services they support. This synthesis reviews the current understanding of OA and its impacts in New Zealand waters, focussing initially on the marine carbonate system and regional and temporal trends in pH, followed by an assessment of the sensitivity of different biotic groups using all currently published information. The synthesis concludes by identifying current knowledge gaps, and recommendations for future research in New Zealand waters.
The carbonate system in New Zealand waters
A prerequisite to establishing the regional extent and magnitude of OA is an understanding of the carbonate system of the water masses around New Zealand. Although carbonate parameters (Total Alkalinity and Dissolved Inorganic Carbon (DIC)) have been routinely analysed in the S.W. Pacific, as part of the World Ocean Circulation Experiment (WOCE) in the 1990s, the complex bathymetry has until recently restricted extrapolation to waters around New Zealand. However, a relationship between carbonate parameters and hydrographic data has now been developed for the Southern Ocean south of 25° (Bostock, Mikaloff-Fletcher, et al. Citation2013), using a multiple linear regression (MLR) approach and the CARS (CSIRO Atlas of Regional Seas Citation2009) ocean climatology. This was used to map ΩA and ΩC in the deep waters of the S.W. Pacific, including around New Zealand (; Bostock, Mikaloff-Fletcher, et al. Citation2013), with validation using recent carbonate measurements in New Zealand waters (2011–2014; Bostock et al. Citation2015).
Figure 2. Maps of the depth (metres) of the calcite saturation horizon (left) and aragonite saturation horizon (right) for the New Zealand region, developed from MLR algorithms (Bostock, Mikaloff-Fletcher, et al. Citation2013; Bostock et al. Citation2015). Key to currents: AAIW, Antarctic Intermediate Water; PF, Polar Front; NPDW, North Pacific Deep Water; CPDW, Circumpolar Deep Water. (Reprinted by permission from Elsevier Deep Sea Research)
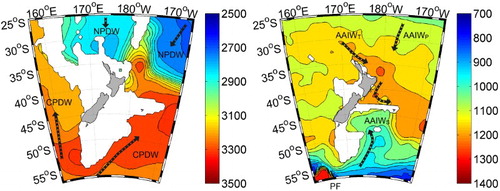
Although much of the surface ocean is supersaturated with respect to carbonate, the lower temperature and higher pCO2 of polar waters result in carbonate undersaturation (ΩA, ΩC <1.0). This is also the case in the deep ocean, where the elevated pressure further enhances carbonate dissolution. The Ω = 1.0 threshold, below which dissolution of solid carbonate begins, is referred to as the Saturation Horizon. The WOCE transects and MLR carbonate maps show significant regional variation in the depths of the Calcite and Aragonite Saturation Horizons (CSH and ASH, respectively) around New Zealand (), reflecting the different water masses and their circulation around the complex bathymetry. To the south and west of New Zealand the deep waters that enter the region from the Southern Ocean are relatively young, and have experienced less respiratory production of CO2 and have higher carbonate concentrations. Conversely, the North Pacific Deep Waters (NPDW) that flow into the basins north-northeast of New Zealand are older and have low carbonate concentration (Bostock et al. Citation2011; Chiswell et al. Citation2015). This results in the CSH being considerably shallower in the north (c. 2800 m) relative to the south (c. 3100 m; Bostock et al. Citation2011, ). This trend is interrupted by a tongue of younger, higher carbonate, deep water from the Southern Ocean that flows north around the Chatham Rise along the Kermadec Ridge as part of the Circumpolar Deep Water (CPDW), and slowly mixes with the more corrosive NPDW flowing south (; Bostock, Mikaloff-Fletcher, et al. Citation2013; Bostock et al. Citation2015).
In contrast to the CSH, the ASH is shallow (<1000 m), south of New Zealand at the Polar Front (PF), but deeper in the north (c. 1200 m, ). The variability in ASH depth across the Chatham Rise is due to the different Antarctic Intermediate Water (AAIW) inputs; AAIWS flowing in from the south of New Zealand has higher DIC and alkalinity relative to the AAIWP and AAIWT (Tasman AAIW) which circulate around the South Pacific gyre before entering the New Zealand region from the north and northwest, respectively (Bostock, Sutton, et al. Citation2013). The shoaling of the ASH in the Southern Ocean reflects upwelling of deep, cold carbonate-deplete waters along the PF (Bostock, Mikaloff-Fletcher, et al. Citation2013), although these surface waters are subsequently subducted to form AAIW (Bostock, Sutton, et al. Citation2013). Consequently, the ventilation age and circulation patterns of the different water masses, and their resulting carbonate concentrations, influence the distribution of deep water carbonate organisms and sediments in the S.W. Pacific (Bostock et al. Citation2011, Citation2015). Carbonate sediments start to dissolve below the CSH, while the depth at which carbonate dissolves rapidly, termed the lysocline, is coincident with the modern ocean calcite saturation of ΩC = 0.8 (Milliman et al. Citation1999; Bostock et al. Citation2011).
Several of the WOCE transect lines have been revisited over the past decade, with the results indicating an increase in anthropogenic carbon at depths <1000 m (Feely et al. Citation2012). This effect is most apparent in the cold surface waters of the Southern Ocean that are subducted as AAIW (Feely et al. Citation2004; Murata et al. Citation2007). The increased uptake in the deep ocean has reduced aragonite saturation over the last 10–20 years, with a shoaling of the ASH by up to 2 m yr−1 in the South Pacific (Sabine et al. Citation2008; Feely et al. Citation2012), suggesting that deep-sea organisms may already be experiencing OA stress. Recent projections for the New Zealand region using an Earth System Model (GFDL-ESM2G) and current emission scenarios (RCP8.5; van Vuuren et al. Citation2011), indicate shoaling of the ASH to 820–950 m across much of the EEZ by 2060. This shoaling is greatest in the colder Subantarctic water, with a projected ASH depth of c. 350–400 m by 2060, and c. 100–200 m by 2100 (Law et al. Citation2016). Continued monitoring of the carbonate system and calcifying biota are required to confirm these projections.
Temporal and spatial trends in pH and the carbonate system
Although OA is driven primarily by atmospheric CO2, there is considerable spatial and temporal variation in pH and the carbonate system. pH is a function of temperature, salinity, pressure, alkalinity and CO2, which in turn are influenced by geological, physical and biological processes. Consequently, characterizing the spatial and temporal variability, in addition to the rate, of pH change is critical to establishing the potential threat of OA. New Zealand maintains the longest running time-series measurements of carbonate chemistry and associated parameters in the Southern Hemisphere, on a transect that has been sampled bimonthly since January 1998 (Currie et al. Citation2011). The 65-km long Munida transect extends from coastal neritic water, across the Otago Shelf through modified Subtropical water, and terminates at a station in Subantarctic water (45.83°S 171.50°E). In general, pHT (total scale at in situ temperature; from here on identified as pH) is higher nearer the coast, and decreases offshore into Subantarctic water. Subantarctic surface pH exhibits a seasonal cycle that is dominated by variability in water temperature and biological uptake (Brix et al. Citation2013). These two factors operate antagonistically – in spring and summer the warmer water temperature causes a decrease in pH, whereas the uptake of CO2 during daytime photosynthesis causes a pH increase – with the net result being a summer maximum in pH. From 1998 to 2012 Subantarctic surface water pH declined by an annual average of 0.0013 (±0.0003 standard error) per year (equivalent to 25 pmol kg−1 [H+]) (Bates et al. Citation2014). This decrease in pH is consistent with the increase in atmospheric CO2 over the same time-period at the NIWA Atmospheric Station at Baring Head, Wellington (A; Brailsford et al. Citation2012), confirming atmospheric CO2 as the main driver of the pH decline. The Munida time series also identified a decreasing trend in carbonate availability, with an annual decline in ΩA of −0.0085 relative to a mean value of 2.3 (1.99–2.77) for this period (Bates et al. Citation2014).
Figure 3. A, Time series of atmospheric CO2 at Baring Head (North Island) (red line, Brailsford et al. Citation2012), and surface water pCO2 (dark blue triangles) and pH (light blue circles) at the Subantarctic water station (location identified in inset map) on the Munida transect (K. Currie, pers. comm.); B, pH time series at three coastal stations; Chatham Islands (blue squares and line) Wellington (black circles and line); Jackson Bay (red triangles and line) in the NZOA-ON; C, surface water pH in the Firth of Thames, Hauraki Gulf, and inner continental shelf on 9–12 March 2010. Figure created using Ocean Data View (Schlitzer, R., Ocean Data View, odv.awi.de)
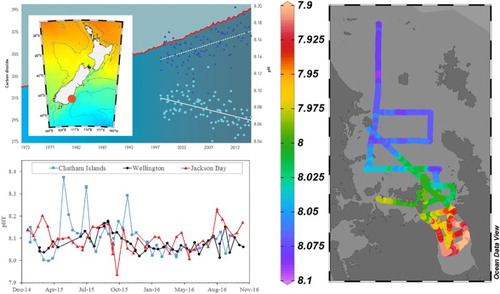
Projecting future pH in the ocean around New Zealand is currently limited by the lack of a regional hydrodynamic model that incorporates biogeochemistry. Application of an Earth System Model (GFDL-ESM2G) to the New Zealand region, indicates a decrease in surface water pH to 7.95 by 2050, and 7.75 by 2100 (Law et al., Citationforthcoming, Citation2016), and so a 0.33 decrease from the current mean of 8.088 (Bates et al. Citation2014). The spatial variability of pH in surface waters around New Zealand is low, with a meridional gradient of 0.03 pH units, reflecting the increased solubility of CO2 at lower temperatures (Law et al. Citation2016). Of course, pH projections are sensitive to future CO2 emissions; for example, under the mitigation scenario RCP2.6 (van Vuuren et al. Citation2011), surface water pH does not decrease below the current pH minimum in Subantarctic waters recorded on the Munida time series whereas it falls below this pH threshold around 2040 under the highest emission scenario RCP8.5 (Law et al., Citationforthcoming, Citation2016).
The pH of coastal waters is inherently more variable due to a complex interplay of factors that include temperature, biological uptake and respiration, terrestrial run-off and pollution, with diel variations of up to 0.94 (7.46–8.7) pH units reported in New Zealand kelp beds (Cornwall, Hepburn, McGraw, et al. Citation2013). Although water quality monitoring occurs routinely in some New Zealand coastal regions, there is currently a paucity of pH data of sufficient quality to determine spatial variability and establish a baseline for assessing future change. The New Zealand Ocean Acidification Observing Network (NZOA-ON) was initiated in 2015 to address this deficiency, by determining the rate and magnitude of acidification at 11 coastal stations including a variety of pristine and impacted locations (Figure S1). A variety of stakeholders, including government agencies, councils, industry and citizens, provide alkalinity and DIC samples on a fortnightly basis for analysis at a centralised facility (NIWA/University of Otago Research Centre for Oceanography). Internationally recognised protocols and reference materials are used to ensure international comparability with the Global Ocean Acidification Network (GOA-ON), with pH, carbonate ion concentration and Ω calculated from the DIC and alkalinity measurements. In addition, SeaFET sensors (Martz et al. Citation2010), which collect pH measurements every 30 min, are deployed at some NZOA-ON sites.
The first year of sample collection by NZOA-ON shows coastal pH at the different sites spanning a range of 7.8–8.3. The seasonal pH cycle is controlled by the temperature at most sites, with a maximum pH in late winter and minimum in late summer–autumn, as at Wellington Harbour (B). Local factors also influence pH, as at the Chatham Islands where phytoplankton blooms along the Subtropical Front result in periods of elevated pH, and in Jackson Bay (West Coast, South Island) where freshwater input causes sharp pH declines. Freshwater input also supplies excess nutrients and organic matter in some regions, which enhance acidification of coastal waters. The Firth of Thames, an embayment of the Hauraki Gulf, has been monitored over an 18-year time series to establish the factors influencing primary production. High nutrient loading, originating from the intensively farmed catchment, results in phytoplankton blooms in spring and early summer, that are followed by heterotrophic respiration and oxygen depletion in late summer and autumn (Zeldis et al. Citation2015). Seasonal surveys of surface water show a coincident pH decline to 7.9 in autumn in the innermost Firth (C; Zeldis et al. Citation2015; K. Currie, pers. comm.), with subsequent monitoring with SeaFET sensors detecting pH events of less than 7.7 (J. Zeldis, pers. comm.). This pH is lower than that projected for the surface open ocean around New Zealand by the year 2100 (Law et al., Citationforthcoming, Citation2016), indicating that some coastal waters already experience periods of intense acidification. Consequently, these regions may be highly vulnerable to further decreases in pH.
Impact of OA on taxa in New Zealand waters
In addition to the spatial and temporal variation in pH and the carbonate system, marine organisms also show a wide variety of responses to OA. This variability arises due to a broad range of factors including exposure, life history and physiology, which manifest as different responses both between and within phyla and taxa (Doney et al. Citation2009; Meyer and Riebesell Citation2015). In the following section, the impacts of OA on the major taxa in New Zealand waters are assessed and compared with international findings, with the experimental details and responses detailed in Supplementary Table S1. Most of these are laboratory manipulation experiments on single species, although some are observational studies. New Zealand researchers have developed advanced experimental systems for response studies, that include automated pH control in trace metal-free conditions (McGraw et al. Citation2010; Hoffmann et al. Citation2013), and under-ice chambers (Barr et al. Citation2017), and optimised methodologies for measurement of different parameters under low pH (MacLeod et al. Citation2015; Burrell et al. Citation2016). In most of the experiments discussed below, the response to OA was determined experimentally by comparison of a lower pH (pH 7.6–7.8) or elevated CO2 (700–1000 µatm) treatment, with a present-day control (pH 8.0–8.1, or CO2 380–420 µatm).
Microbes
Marine bacteria play a critical role in the decomposition of organic matter and recycling of nutrients, during which respiratory CO2 is produced that may exacerbate OA, particularly in coastal waters (Breitburg et al. Citation2015). Experiments on blooms of different phytoplankton species in frontal waters on the Chatham Rise have shown that bacterial composition, production and abundance are not significantly altered under lower pH (−0.3, from initial values of 8–8.16; Burrell et al. Citation2017). However, bacterial extracellular enzyme activity increased under lower pH, as also observed in coastal waters, and in water over CO2 seeps (Burrell et al. Citation2015, Citation2016, Citation2017), consistent with reports from other regions (Piontek et al. Citation2010; Maas et al. Citation2013). In the New Zealand studies the protein-degrading enzyme, leucine aminopeptidase, showed the strongest and most consistent stimulation under lower pH, inferring that this pH may be more optimal for the activity of this enzyme. This observation suggests that proteins may be more rapidly degraded in the future ocean, with potential implications for food quality and nutrient availability; furthermore, this may enhance the microbial loop with positive feedback on atmospheric CO2 and a reduction in carbon export to the deep ocean. However, bacterial extracellular enzyme activity is just one component of the complex web of pelagic microbial processes, and more research is required to determine the net effects of OA (Riebesell et al. Citation2009).
Phytoplankton are critical to the productivity and functioning of ocean ecosystems, as they provide the energy, via photosynthesis, that supports marine foodwebs. They also play an important role in the ocean carbon sink, as the primary source of the particulate carbon that sinks into the deep ocean and remains isolated from the atmosphere over century-millennial timescales. The New Zealand region is characterised by significant phytoplankton blooms along the Subtropical Front at 45–50°S, south of the Tasman Sea, and along the Chatham Rise, east of New Zealand, where elevated phytoplankton biomass supports productive fisheries and benthic ecosystems. Conversely, the main water bodies, the Subtropical and Subantarctic surface water, that meet in these frontal systems generally exhibit low productivity due to nitrogen and iron limitation, respectively. As phytoplankton utilise CO2 and bicarbonate as a substrate for photosynthesis, an increase in DIC (CO2 + ) may be beneficial to productivity and biomass (Riebesell Citation2004). However, the response to OA will vary between phytoplankton groups and species, depending on physiology. A decrease in pH may also alter both macro- and micronutrient availability, and the decrease in carbonate ion saturation may affect the ability of calcifying phytoplankton to produce and maintain a carbonate shell (Riebesell Citation2004).
Picoplankton (<2 µm), such as the cyanobacteria Synechococcus, are the dominant plankton in low productivity oligotrophic systems, such as the Subtropical waters north of New Zealand. In incubations of northern Tasman Sea waters Synechococcus abundance increased under elevated CO2 (780 vs. 380 µatm), coincident with a decline in larger (2–20 µm) phytoplankton (Hoffmann et al. Citation2013). Synechococcus is a non-calcifier, and this result suggests some benefit from an increase in pCO2. However, an increase in Synechococcus was not observed in complimentary experiments in frontal waters over the Chatham Rise (Law et al. Citation2014), suggesting that other factors, such as nutrient availability or grazing, may moderate response to OA. Diazotrophic plankton, which fix dinitrogen (N2), have been identified as potential ‘winners’ from OA, following observations of significant increases in nitrogen and carbon fixation by the large colonial cyanobacteria Trichodesmium under elevated (Hutchins et al. Citation2009). However, an increase in pCO2 to 780 µatm (pH decreased from 8.05–8.1 to 7.86–7.89) had no effect on nitrogen fixation rate, or abundance of nitrogen fixers, in incubations of northern Tasman Sea surface water (Law et al. Citation2012). This lack of response to increased pCO2 was attributed to differences in sensitivity of diazotroph species, as the Tasman Sea diazotrophs were dominated by unicellular cyanobacteria, as opposed to Trichodesmium. Incubations of North Pacific surface waters have shown a similar lack of response (Gradoville et al. Citation2014), suggesting that CO2 stimulation may be restricted to specific diazotroph species or strains, or that a positive response to increased CO2 by diazotrophs may be suppressed or obscured by other processes or interactions in natural population communities (Law et al. Citation2012).
Diatoms are a group of larger (2–500 µm) non-calcifying phytoplankton that dominate in nutrient-replete waters, and play an important role in silicate cycling and carbon export to the deep ocean. Photosynthesis by diatoms is saturated under current dissolved CO2 levels (Riebesell Citation2004), and so there is unlikely to be stimulation of growth under future increased CO2. Nevertheless, elevated pCO2 alters diatom community size structure and nutrient cycling in Southern Ocean waters (Hoppe et al. Citation2013). In a study of the factors controlling the growth of a Subantarctic diatom, Pseudonitzschia multiseries, temperature and iron were identified as the most important drivers, with elevated CO2 (747 µatm, pH 7.69–7.73), being less important (Boyd et al. Citation2015). This study assessed the individual and interactive effects of the different drivers, which is an important step towards determining in situ responses to climate change. In addition, indirect effects of OA, such as influence on nutrient availability, may be important. For example, lower pH may alter dissolved iron concentration and speciation (Hoffmann et al. Citation2012), and nitrate availability (Hutchins et al. Citation2009), and influence phytoplankton growth in New Zealand Subtropical and Subantarctic waters, where these nutrients are limiting (Boyd et al. Citation1999; Law et al. Citation2011). Projecting the status of future plankton populations may be complicated by other indirect effects, such as the interaction and competition between species within natural communities. For example, a taxa or species that benefits from an increase in pCO2 may outcompete another. However, studies to date in New Zealand waters have found no effect of elevated CO2 on competition between coastal diatom species (Tatters et al. Citation2013), or between benthic diatoms and coralline algae at lower pH (7.6 relative to 8.05 present day; James et al. Citation2014; Roleda et al. Citation2015).
Foraminifera are calcifying protozoa that are potentially susceptible to OA, although there have been no experimental studies to date in New Zealand waters. However, the sensitivity of the coccolithophores, calcifying phytoplankton that produce an external layer of calcite plates or liths, has been assessed. Coccolithophores occur globally, often in high abundance in large blooms, and play a major role in both the soft tissue carbon pump and the carbonate-counter pump (Ziveri et al. Citation2007). As carbonate lith production involves the release of CO2, coccolithophore photosynthesis and calcification also influence the alkalinity of the surface ocean and the air–sea CO2 gradient (Meyer and Riebesell Citation2015). Coccolithophores are well-studied in the context of OA, following early reports of lith dissolution under reduced pH (Riebesell Citation2004). There are over 45 coccolithophore species in New Zealand waters (Chang and Northcote Citation2016), with the globally ubiquitous Emiliania huxleyi being the dominant species. In a study of the different controls of physiology and cell composition in a strain of E. huxleyi from New Zealand waters (NIWA1108; Feng Citation2015; Feng et al. Citation2016), nitrate concentration was identified as a critical factor for growth, photosynthesis and calcification. Conversely, elevated CO2 (733–1080 µatm) only altered the calcification:photosynthesis ratio, as reflected by a decrease in cellular Particulate Inorganic Carbon (PIC) relative to Particulate Organic Carbon (POC). A decrease in PIC:POC under elevated CO2 is also apparent in other strains of E. huxleyi from the New Zealand region and South Pacific (), and is a common response in international studies (Findlay et al. Citation2011; Meyer and Riebesell Citation2015). However, the decrease in PIC:POC reflects differing responses in cellular PIC and POC between strains (), as also reported in a meta-analysis of 48 culture experiments on coccolithophores (Meyer and Riebesell Citation2015). There is still debate regarding the role of calcification in coccolithophores, with proposed functions that include stabilisation of external pH and protection from grazing (Flynn et al. Citation2016; Monteiro et al. Citation2016). Consequently, if natural coccolithophore populations reflect experimental studies, then the decline in PIC:POC in response to OA has implications for foodwebs and biodiversity, via changes in grazing susceptibility. Furthermore, a reduction in the carbonate ‘ballast’ that is considered to accelerate organic carbon transfer to the deep ocean (Ziveri et al. Citation2007), may reduce the ocean carbon sink.
Table 1. Response of cellular PIC and POC content in different strains of E. huxleyi from the South Pacific (adapted from Meyer and Riebesell Citation2015), where ↑ indicates an increase, ↔ no significant change and ↓ a decrease under elevated  (700–1080 µatm), relative to ambient CO2 (380–400 µatm).
(700–1080 µatm), relative to ambient CO2 (380–400 µatm).
As with other groups and phyla, calcifying plankton may be more impacted by OA than non-calcifiers in New Zealand waters; however, there is currently limited understanding of the indirect effects of OA, such as via nutrient availability, on which to base projections of community change. This is further confounded by the predominance of single-species studies to date, with little consideration of how OA influences interactions within natural plankton communities. Further mixed species studies are required, to determine whether OA impacts on species or groups are obscured or over-ridden by interactions (e.g. Law et al. Citation2012), and also to provide insight into the net effects of OA on pelagic microbial processes and associated implications for the ocean carbon sink and foodweb (Riebesell Citation2004). In addition, experiments to date have generally been relatively short-term (<3 weeks), whereas ongoing long-term incubations (300+ generations) of E. huxleyi strain NIWA1108 suggest potential for adaptation to elevated CO2 (E. Armstrong, pers. comm.), as reported in international studies of coccolithophores (Lohbeck et al. Citation2012).
Macroalgae
Seaweeds fulfil crucial roles as primary producers and habitat formers in coastal ecosystems throughout New Zealand (Shears and Babcock Citation2007; Rodgers and Shears Citation2016), and are particularly vulnerable to the impacts of OA (Hall-Spencer et al. Citation2008), although responses may vary among species and habitats. Species-specific physiology varies with respect to DIC (Hurd et al. Citation2009; Hepburn et al. Citation2011; Roleda and Hurd Citation2012), and differential susceptibility between algal groups and species (Hurd et al. Citation2011; Fabricius et al. Citation2015) may influence competition, and alter the structure and function of macroalgal communities. These factors, combined with the buffering capacity and hydrodynamic complexity of coastal waters (Denny et al. Citation2003; Gaylord et al. Citation2007), confound prediction of how macroalgae communities will respond to future OA.
Coralline algae (calcifying Rhodophyta) are recognised globally as ecologically crucial on rocky and coral reefs and in soft sediment environments. They may be present as rhodoliths or growing on shell debris and cobbles (Nelson Citation2009), and extend to depths beyond that of large macroalgae species (Hepburn et al. Citation2011; Nelson et al. Citation2015). Coralline algae play an important role in facilitating settlement of invertebrate larvae (Roberts Citation2001), and bind together, and even create, new reefs (Richards and O’Leary Citation2015). Meta-analyses reveal that coralline algae may be amongst the most vulnerable calcifying organisms to reduced pH in a future ocean (Kroeker et al. Citation2013), although the mechanisms responsible for the decline in coralline algal calcification due to OA have not been identified, despite intensive research effort (McCoy and Kamenos Citation2015). In addition, little is known about the geographic and ecological distribution of coralline species in New Zealand (Harvey et al. Citation2005; Farr et al. Citation2009), which precludes prediction of their future status.
Research on selected New Zealand coralline species suggests OA will result primarily in a reduction in net calcification rates and growth, possibly due to increased dissolution of calcium carbonate at lower pH (Cornwall, Hepburn, McGraw, et al. Citation2013, Cornwall et al. Citation2014). Laboratory studies of recruitment in New Zealand coralline algae showed little effect of reduced pH (7.65; Cornwall, Hepburn, McGraw, et al. Citation2013; Roleda et al. Citation2015), in contrast to field studies along volcanic vent sites in Papua New Guinea where recruitment was reduced to <20% at pH <7.8 (Fabricius et al. Citation2015). This difference in settlement response may reflect different experimental protocols or alternatively, that recruitment in temperate corallines is less susceptible to OA than in the tropics. The effects of OA may be habitat-dependent, as corallines exert greater metabolic influence at lower seawater velocities, increasing the pH at their surface and so moderating the impact of OA (Hurd et al. Citation2011; Cornwall, Hepburn, Pilditch, et al. Citation2013, Cornwall et al. Citation2014, Cornwall, Pilditch, et al. Citation2015).
For non-calcareous (fleshy) macroalgae, research on south-eastern New Zealand kelp forests suggests that macroalgae sensitivity reflects the variable DIC physiology of different species (Hepburn et al. Citation2011). The majority of macroalgae use CO2 concentrating mechanisms (CCMs) for energised uptake of (Hepburn et al. Citation2011; Diaz-Pulido et al. Citation2016), and also utilise CO2 via diffusion (Cornwall, Revill, et al. Citation2015; Fernández et al. Citation2015). However, CCMs are energetically expensive, and so species with the capacity to reduce the requirement for CCMs in favour of CO2 uptake may benefit from OA (Hepburn et al. Citation2011; Cornwall et al. Citation2012). Research to date suggests these potential benefits of increased CO2 uptake may be greater under low light, such as in deeper habitats and within the low-light environments of kelp forests (Hepburn et al. Citation2011; Diaz-Pulido et al. Citation2016). Conversely, larger canopy-forming species in high-light areas, such as on New Zealand rocky reefs, are less likely to benefit from OA (Roleda and Hurd Citation2012; Fernández et al. Citation2014, Citation2015), when CCM are active (Hepburn et al. Citation2011). Similar responses would be expected from shallow-water bloom-forming macroalgae, such as Ulva spp. (Rautenberger et al. Citation2015).
OA research on seaweeds has predominately focussed on the conspicuous macroscopic phases of their life history; however, some seaweeds have a microscopic stage or alternative life-history stage, which may be more sensitive to environmental stress than the adult (Schiel and Foster Citation2006; Hurd et al. Citation2014). Roleda et al. (Citation2015) identified reduced growth rates of newly recruited New Zealand corallines under low pH (7.65) relative to present-day pH, and there was evidence that the new recruits grew better in low pH compared to the adult thalli from which they were derived. Conversely, spore germination in the kelp Macrocystis pyrifera is little-affected, with Leal et al. (Citation2017a, Citation2017b), reporting no effect of pH in the range of 7.2–8.4, and Roleda, Morris, et al. (Citation2012) finding only a slight reduction in germination (9–13%) under reduced pH (7.6). Similarly, spore germination of the invasive kelp, Undaria pinnatifida, was unaffected by pH (7.2–8.4; Leal et al. Citation2017a, Citation2017b). Subsequent development of micro-stages of M. pyrifera and U. pinnatifida is generally positively affected by reduced pH, with higher germling growth rate at pH 7.2 relative to higher pH treatments (Roleda, Morris, et al. Citation2012; Leal et al. Citation2017a, Citation2017b).
While it is established that macroalgae modify pH at their surface, and also at larger scales (Hurd et al. Citation2011; Cornwall, Hepburn, McGraw, et al. Citation2013; Cornwall, Hepburn, Pilditch, et al. Citation2013), the ramifications of this are poorly understood (Boyd et al. Citation2016). For example, kelp beds exhibit large diel pH variability (Cornwall, Hepburn, McGraw, et al. Citation2013; Britton et al. Citation2016, ), the range of which exceeds the pH change projected for the open ocean around New Zealand by 2100 (Law et al., Citationforthcoming). The attenuation of flow by dense kelp and fucoid beds in New Zealand coastal waters (Cornwall, Pilditch, et al. Citation2015), combined with an increase in pH during the daytime, may buffer the impact of OA on calcifying species within the kelp bed (Cornwall, Hepburn, McGraw, et al. Citation2013; Hurd Citation2015). Conversely, pH fluctuations may enhance the negative impacts of reduced pH on growth and calcification by juvenile and adult coralline algae (ambient pH variation of 7.65–8.05 compared with low pH variation of 7.25–8.05; Cornwall, Hepburn, Pilditch, et al. Citation2013; Roleda et al. Citation2015). Consequently, the large variability in magnitude and direction of changes in the carbonate chemistry may have differential impacts upon the macroalgae, and also associated organisms (Cornwall et al. Citation2014; Boyd et al. Citation2016).
Figure 4. A kelp forest and associated community on a rocky reef (Tavora, North Otago) that shows understory species including Landsburgia quercifolia and coralline algae (left panel), and a plot of pH variability in a kelp bed at Karitane (South Island East coast; Cornwall, Hepburn, McGraw, et al. Citation2013, right panel), showing large pH variation up to 0.94 units under high light and low swell conditions.
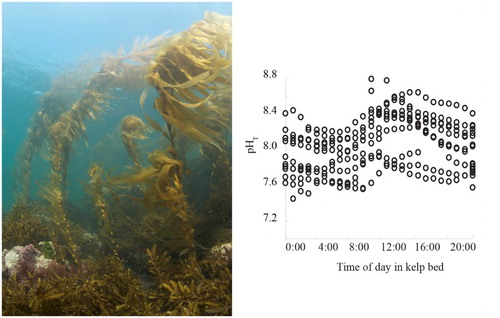
Understanding the dynamics and impacts of changing conditions within an ecologically relevant context is an important research priority, given the high biomass of macroalgae on New Zealand’s rocky reefs (Shears and Babcock Citation2007; Desmond et al. Citation2015). Although general patterns in the response of macroalgae to OA have been proposed (Hepburn et al. Citation2011), these trends have not been tested experimentally. It is likely that communities will display differing degrees of perturbation by OA, depending on their natural composition and the prevalence of other anthropogenic drivers. There has also been little research into the indirect effects of OA on macroalgal communities, for example, whether competition amplifies change (Diaz-Pulido et al. Citation2011; Hepburn et al. Citation2011), or if there are strong impacts on higher trophic levels (Cornwall and Eddy Citation2015).
Mineralogy of calcifying organisms
As previously discussed, a reduction in the carbonate saturation state has a major impact on organisms that produce carbonate structures (Kroeker et al. Citation2013). Many invertebrate phyla have the capacity for calcification and use one or both of the two carbonate polymorphs, calcite and aragonite, in the formation of endo- or exo-skeletal elements. Passive calcifiers, such as algae and corals, produce calcium carbonate that is generally in equilibrium with the seawater around them (Kamenos et al. Citation2016), whereas active calcifiers, such as some marine molluscs (McConnaughey and Gillikin Citation2008), and bryozoans, are more determinate, with their skeletal composition less closely tied to seawater chemistry (Ries et al. Citation2009; Roleda, Boyd et al. Citation2012; Thomsen et al. Citation2015). The skeletal carbonate mineralogy of temperate marine invertebrates in New Zealand was determined in studies during the 1990s, that established differences in composition between temperate and tropical carbonates and also hemispheric differences (Nelson Citation1978, Citation1988; Nelson et al. Citation1988, ). Mineralogical studies of temperate invertebrates and calcareous algae have shown that many are characterised by greater variability in skeletal mineralogy than previously reported ( and references within). Consequently, this variability in composition contributes to differences in the relative susceptibility of invertebrate phyla to OA, as detailed below for New Zealand species.
Table 2. Summary of skeletal carbonate mineralogy studies for New Zealand and global species.
Lophophorates
Lophophorates are a biotic clade that possess a unique lophophore feeding structure, and include the Bryozoa and Brachiopoda phyla which produce extensive calcified structures that are potentially sensitive to seawater pH. Bryozoans are colonial marine invertebrates that produce a very wide range of carbonate mineralogies and morphologies. Laboratory studies of biogenic carbonate dissolution have shown that the relationship between Bryozoan skeletal composition and surface area: volume is complex (Smith et al. Citation1992), but also that morphology is, in general, a more important determinant of dissolution rate than mineralogy (Smith and Garden Citation2013). Bryozoans in high-CO2 vent waters have been shown to produce different colony morphologies relative to control groups (Lombardi et al. Citation2013), although whether this change occurs in New Zealand shelf bryozoans is currently under investigation. Temperate marine carbonate sediments are dominated worldwide by bivalve and gastropod molluscs, with sub-dominant components such as echinoids, barnacles and serpulid worms, whereas Bryozoa are often the dominant, or co-dominant, carbonate producers in the Southern Hemisphere (Nelson et al. Citation1988). As bryozoan sediments cover New Zealand’s southern shelves, they have potential as in situ indicators of carbonate dissolution (Smith Citation2009).
Another Lophophorate group that is potentially sensitive to OA are the brachiopods, or lamp shells. New Zealand is renowned for its diversity of brachiopods, with ∼10% of all extant identified species in these waters, and they can be an important component of certain ecosystems, such as the New Zealand fiords. However, few studies have examined their sensitivity to OA, despite this group having one of the highest levels of calcification of marine invertebrates, with carbonate representing >90% body mass. A 12-week study by Cross et al. (Citation2016) demonstrated that the New Zealand species, Callioria inconspicua, could continue shell production and repair of damaged surfaces under reduced pH (7.62, 7.79), and that process rates were not significantly different from control conditions. These results were consistent with those reported for an Antarctic brachiopod at projected future pH levels (7.54, 7.75; Cross et al. Citation2015).
Sponges
Sponges are an important component of hard substratum benthic ecosystems throughout New Zealand, and play a significant role in transferring carbon from pelagic to benthic environments (Perea-Blazquez et al. Citation2012). While there has been considerable interest in the impacts of OA on sponges in tropical environments, since they appear to be less sensitive to changes in pH compared to corals (Bell et al. Citation2013; Bennett et al. Citation2016), there is still little information on the OA sensitivity of temperate sponges, including those found in New Zealand. Sponges might be expected to be particularly vulnerable to variations in water chemistry, including pH, as only a single cell layer separates a sponge from its external environment (Bergquist Citation1978), and they also have limited capacity for acid–base regulation (Pörtner Citation2008). Furthermore, different sponge taxonomic classes might be impacted disproportionately in relation to skeletal composition. For example, calcareous sponges have a skeleton made of calcium carbonate, and differences in mineralogy may make some species potentially more susceptible (Smith, Berman, et al. Citation2013; ). Conversely, demosponges have a silica and spongin skeleton, and are less likely to be influenced by OA. In the only New Zealand study to date, Bates (Citation2015) reported that a reduction from pH 8 to 7.6 had a small, but significant effect on the respiration rate of two demosponge species from the Wellington region (Tethya bergquistae and Crella incrustans). In contrast, increased temperature (20°C and 22°C) had a negative impact on respiration and survival rate for both species with elevated mortality, compared to sponges kept at average annual and maximum summer temperatures (13.5°C and 18°C). These results are consistent with studies of tropical sponges (see Bennett et al. Citation2016), where there appears to be minimal effects of reduced pH, but potentially greater threat from future warming (Bell et al. Citation2013). Importantly, there is no available information on the potential sensitivity of calcareous sponges to OA, despite their abundance around New Zealand (Berman and Bell Citation2016), and their reliance on calcium carbonate for skeletal composition.
Corals
Globally, most experimental work on the potential impact of OA on corals has been on shallow-water tropical reef species. Although coral reefs are not found in New Zealand waters, there are many temperate and subtropical shallow-water coral species that are an important component of benthic communities in northern regions, such as the Kermadec Islands. The response to OA of these corals may be significant; for example, in shallow-water Pacific corals calcification rates may decrease and carbonate dissolution rates increase when pH is reduced only slightly (pH 7.85–7.95), with substantial impacts when the pH is reduced to 7.60–7.70 (Anthony et al. Citation2008). The response of central Pacific shallow-water corals to OA has also been reported at a cellular level (Gibbin et al. Citation2014), and the sensitivity of corals that host endosymbiotic dinoflagellates (Symbiodinium spp.), to rising ocean temperatures and lower pH is well documented (Gori et al. Citation2016). However, it is currently unclear how other varieties of corals, such as the stylasterid hydrocorals and black corals that inhabit the unique environments in Fiordland, will respond to OA.
New Zealand waters support a broad diversity of deep-sea sea coral fauna (Sánchez Citation2005; Gordon Citation2009), most of which live between depths of 200 and 1200 m (Tracey, Anderson, et al. Citation2011; Tracey, Rowden, et al. Citation2011). The skeletons of cold-water scleractinian corals are composed of aragonite, the more soluble polymorph of carbonate, which makes them vulnerable to OA-induced dissolution. Consequently, the shoaling of the ASH represents a potential threat to the abundance and distribution of deep-sea cold-water corals, as it will become challenging to construct and maintain their skeletons in water undersaturated with respect to aragonite (Guinotte et al. Citation2006; Tracey et al. Citation2013; Bostock et al. Citation2015). Cold-water corals are an important biogenic habitat for fish and invertebrates on slope margins, ridges and seamounts (Clark and Rowden Citation2009; Clark and Koslow Citation2012) with fish seen on, or in close proximity to, stony and other habitat forming corals (Purser et al. Citation2013). Changes in deep-sea coral distribution arising from OA may then indirectly affect the future distribution of deep-sea fish, with potential economic implications for fisheries.
Deep water coral species, such as Lophelia pertusa and the New Zealand species Madrepora oculata, exhibit lower calcification rates and metabolic depression, after 21 days under lower pH (−0.3 decrease to 7.76; Maier et al. Citation2009; Hennige et al. Citation2014). However, the respiration and growth rate of Solenosmilia variabilis, a dominant New Zealand species, showed no significant difference between colonies maintained under ambient pH (7.88, ΩA = 0.93) and lower pH (7.65 ΩA = 0.78) for two years (Gammon Citation2016; Tracey et al. Citation2016; B). There was a decrease in skeletal colouration in the low pH treatment, which was attributed to a loss of coenochyme, the tissue connecting neighbouring polyps and covering the outer skeleton, indicating reallocation of energy under lower pH, to physiological processes such as growth and respiration.
Figure 5. A, S. variabilis corals on a seamount on the Chatham Rise, New Zealand (NIWA Deep Towed Imaging System Image). B, Fragment of the deep-sea coral S. variabilis in a respiration chamber.

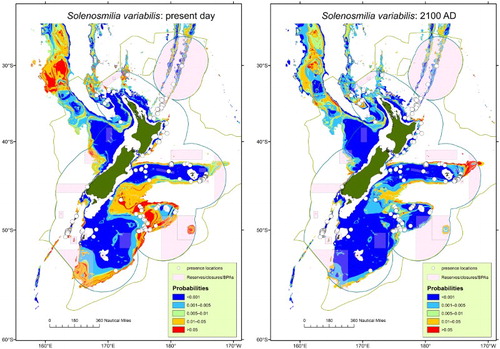
From a broad survey of New Zealand cold-water coral species and carbonate saturation, Tracey et al. (Citation2013) identified a strong dependency of coral distribution on ΩA and ΩC. However, many cold-water coral species can cope with some degree of aragonite undersaturation (ΩA c. 0.8–0.9), with some species tolerant of 0.7 (Bostock et al. Citation2015). Consequently, a mechanistic understanding of the maintenance of calcification at depths below the ASH is required. Projected changes in marine environmental conditions between the present day and 2100, derived from global ESMs, have been incorporated into habitat suitability models to predict the future distribution of S. variabilis and other protected deep-sea coral species in the New Zealand region (Anderson et al. Citation2016). A general decline in suitable habitat was identified for S. variabilis in most regions by 2100, although there was an increase on the fringes of the Chatham Rise and other seafloor features in the west and north, and in deeper Campbell Plateau waters south-east of New Zealand (; Anderson et al. Citation2016). This suggests that seamounts and other topographic features around New Zealand could represent important future refugia for cold-water corals (Tittensor et al. Citation2010; Thresher et al. Citation2015).
Figure 6. Habitat suitability maps for S. variabilis based upon present-day distribution from bottom tow data (left panel), and projected future distribution derived using an ESM (right panel, Anderson et al. Citation2016).
Molluscs
Molluscs are recognised as one of the most susceptible taxa to OA, with studies documenting negative impacts on survival, calcification, growth and development (Kroeker et al. Citation2013). It is generally considered that larval stages (particularly during initial shell development), and species with shells comprised of aragonite, will be most susceptible to dissolution under lower pH (Ries et al. Citation2009; Kroeker et al. Citation2013). Examples of New Zealand mollusc species with aragonitic shells include cockles (Austrovenus stutchburyi) and green-lipped mussels (Perna canaliculus), whereas shells of pāua (black footed abalone, Haliotis iris) contain both calcite and aragonite, the combination of which varies with location (Gray and Smith Citation2004). Molluscs have some biological control over the shell generation and calcification processes (e.g. Cummings et al. Citation2011), but maintaining or intensifying these processes under OA may result in increased metabolic cost (Gazeau et al. Citation2013), with concomitant impacts on growth and development (Thomsen and Melzner Citation2010; Navarro et al. Citation2013; Waldbusser, Gray, et al. Citation2016).
There are over 3600 species of molluscs in New Zealand waters, most of which are gastropods (>3000 species) and bivalves (>400 species), and so, consequently, OA research has focussed on these two groups. Many NZ species, including pāua, cockles and flat oysters (Ostrea chilensis) play important roles in maintaining healthy ecosystem function and seafloor diversity via biogeochemical processing, mineralisation, nutrient recycling, control of phytoplankton biomass and provision of habitat structure. These species are also culturally and recreationally important, as well as commercially valuable, with the export value of bivalve and gastropod molluscs (including mussels, oysters, pāua, scallops, tuatua and cockles) at NZD$339M for 2016 (Seafood NZ Citation2016).
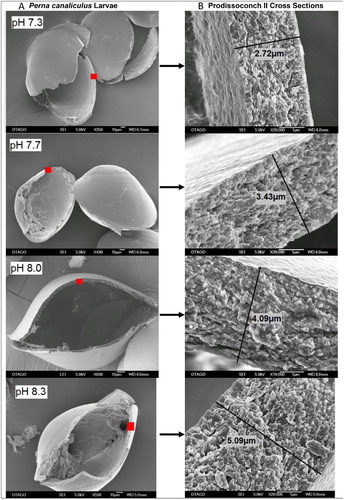
The green-lipped (Greenshell™) mussel supports New Zealand’s largest aquaculture export industry, exceeding USD$190 m p.a. in value (Anon. Citation2014). Despite their importance to New Zealand ecosystems and the economy, and their potential sensitivity to OA, there are few published studies investigating their susceptibility to future high-CO2 conditions. Preliminary investigation into the impacts on early life stages of P. canaliculus in commercial hatchery tanks, has shown that initial larval development and formation of the first shell (Prodissoconch I), which occurs 24–48 h post-fertilization, are severely impacted by extreme pH levels (pH 7.42 and 7.27, ΩA 0.7 and 0.5). All the larvae exhibited malformation or arrested development, with none surviving beyond veliger stage (N. Ragg, unpubl. data). Larvae more than two days old showed greater resilience to this extreme low pH; shell growth and calcification decreased (; Ericson Citation2010), and the survival rate was reduced by 60–85%, although the surviving larvae could metamorphose and recruit to the juvenile population. Ongoing studies are now exploring the implications of near-future pH on the entire life cycle of the green-lipped mussel.
Figure 7. Representative SEM micrograph images showing Prodissoconch II shell structure and thickness in 13-day-old P. canaliculus larvae reared under extreme OA (pH 7.3), moderate OA (pH 7.7) and ambient seawater (pH 8.0, 8.3). Red squares indicate the areas on the shell where the corresponding cross sections are located; the shells showed consistent thickness and microstructure across the umbo region (reproduced with permission from Ericson Citation2010).
Pāua are by far the most valuable wild-caught mollusc species harvested in New Zealand, with an asset value of >NZ$300 million per year, and export revenue of over NZ$50 million (2009 figures; Statistics New Zealand Citation2010). Growth and shell deposition of juvenile pāua were significantly reduced under low pH (pH 7.8 and 7.6) in 100-day incubations, particularly in smaller juveniles and during spring/summer (Cunningham et al. Citation2016), with the smaller size classes showing reduced shell weight and severe dissolution of the outer shell at lower pH. However, survival was only affected for large juveniles in autumn/winter, being significantly lower at pH 7.6 (Cunningham et al. Citation2016). Conversely, a separate investigation found no influence of pH over 120 days on survival, growth, physiological condition or respiration rate in juvenile pāua (7.65 vs. 8.00, 15°C; Cummings et al. Citation2016). However, empty pāua shells in the same experiment lost significantly more weight in the lowest pH treatment relative to control conditions (Cummings et al. Citation2016), inferring that live pāua expend additional metabolic energy to maintain their shell under lower pH. The live shells also showed increased etching of the dorsal shell surface at the lower pH, indicative of a decline in shell strength and integrity (Cummings et al. Citation2016). The growth of the flat oyster, O. chilensis, was slightly reduced at pH 7.65 relative to pH 8.00, but the form of the oyster was too variable to detect impacts on shell characteristics (Cummings et al. Citation2016). The intertidal gastropod, Zeacumantus subcarinatus was also affected following 90 days’ exposure to low pH (pH 7.6 and 7.4), with enhanced mortality, respiration rates and shell dissolution, and reductions in shell growth, length and tensile strength (MacLeod Citation2015; MacLeod and Poulin Citation2015b, Citation2016a, Citation2016b).
Pelagic molluscs with carbonate shells are also vulnerable to OA in open ocean waters (Orr et al. Citation2005). Pteropods (Sea Butterflies) are found in surface waters around New Zealand, and represent an important foodweb component in some regions of the Southern Ocean. As their shells are composed of aragonite they are likely to be seriously affected by decreasing pH in polar and subpolar waters (Bednaršek et al. Citation2016), with increased shell dissolution and reduced survival at aragonite saturation ≤1.0 reported for Arctic pteropods (Comeau et al. Citation2010). Although experiments have not been carried out on New Zealand pteropods, their temporal abundance over a 12-year period has been determined by analysis of sediment trap material from 1500 m depth in both Subtropical and Subantarctic waters (Nodder et al. Citation2016). Although both sites displayed considerable intra-annual variation, neither showed a statistically significant decline in pteropod abundance (Law et al. Citation2016). This result contrasts with a comparable sediment trap time series in Subantarctic waters south of Australia, which showed a decline in pteropod abundance over the same time-period (Roberts et al. Citation2011). This different response between locations may reflect environmental differences (water mass, temperature, food availability, pH) between the two sites, and also logistical factors (sampling interval, sample depths, sediment trap design). The sensitivity of calcification in Arctic pteropods to aragonite saturation (Comeau et al. Citation2010), combined with the projected decrease in ΩA in New Zealand Subantarctic waters (Law et al. Citation2016), suggests a future decline in pteropods, although further research is required to establish sensitivity and the potential impact on pelagic foodwebs.
The Paper Nautilus, Argonauta nodosa, is a pelagic cephalopod in which the females produce a calcium carbonate brood chamber. The shell of this chamber is thin, comprised of calcite with c. 5% weight MgCO3 (considered an ‘intermediate’ Mg calcite content), and lacks an organic surface layer, making it potentially susceptible to dissolution under lower pH. In a study using fragments of A. nodosa shell from Australian waters, Wolfe et al. (Citation2012) found significantly lower weight under low pH (7.8) compared to ambient pH, with surface etching of the shell at pH < 7.6. This susceptibility to OA of a structure associated with reproduction may represent a barrier to future species success.
In summary, from the few studies conducted to date, the response of New Zealand molluscs to OA is consistent with international studies, particularly with respect to the greater impact on early life-history stages. Although this may be detrimental to survival to the adult stage, and therefore species success, the subsequent impact of this larval ‘bottleneck’ on later life-history stages is not well-studied. Consequently, further research is required to establish effects across the entire life cycle, and beyond to multiple generations, to determine the implications for food web, ecosystem and socio-economic implications (Cooley et al. Citation2009).
Echinoderms
New Zealand has a diverse echinoderm fauna (623 described species), and many of these, such as the sea urchin Evechinus chloroticus, are ecologically and culturally important. As a group, echinoderms are thought to be relatively sensitive to changes in seawater pH, as they are heavily calcified, and have an intermediate to high-Mg calcite skeleton (; Smith et al. Citation2016), a poor capacity for acid–base regulation (Miles et al. Citation2007), and typically a complex lifecycle that generally includes a relatively vulnerable calcifying planktonic larval stage (). The mineralogy of a number of New Zealand echinoderm species has been established as part of a global study of present-day and future carbonate saturation states for marine calcifiers in their natural environment (Lebrato et al. Citation2016). Currently c. 25% of the studied species are found in undersaturated conditions (ΩC <1), with Ω projections indicating an increase to 57% over the next 200–3000 years. In a study of the mineralogy of echinoids across polar to tropical latitudes, Smith et al. (Citation2016) showed that Mg content in skeletal elements is a function of latitude, with New Zealand species fitting the global trend, and averaging between 5.7% and 9.3% weight MgCO3 in calcite (). In addition to a wider understanding of the impacts of reduced carbonate saturation states on echinoderms, this information will inform the assessment of the relationship between the Mg2+ content of skeletal elements, ΩC and calcite dissolution.
Figure 8. A, Adult sea urchin E. chloroticus, B, E. chloroticus planktonic pluteus larval stage. The larvae have a calcitic endoskeleton, consisting of fine rods extending along the larva, which can be visualised using polarised light. Experiments have shown loss of skeletal integrity in larvae in the region identified by the red square in B when reared under D, lower seawater pH relative to C, normal pH (from Clark et al. Citation2009). E, Adult cushion star P. regularis, and F, its early developmental stages (0–24 h old, Byrne, Gonzalez-Bernat, et al. Citation2013) showing slower and abnormal development under experimentally reduced pH 7.6 (F/ E-H) compared with development under ambient pH 8.16 (F/ A-D). Scale bar = 100 µm for all larval pictures and 5 µm for SEM images (Permission to reproduce this figure provided courtesy of Inter-Research).
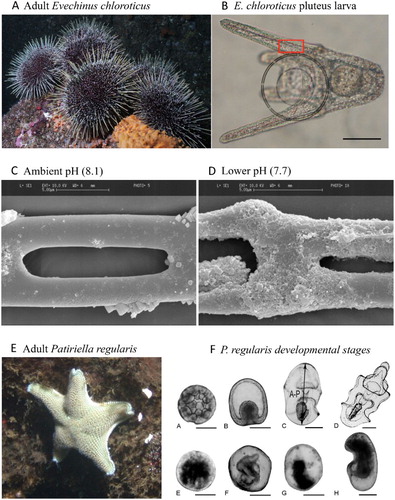
Research into the sensitivity of New Zealand echinoderms to pH has mainly focussed on the early developmental stages. Clark et al. (Citation2009) examined the effects of seawater pH across a broad range (6.5 to 8.1) on the development, calcification and survival of two echinoid species (E. chloroticus and Pseudechinus huttoni), and noted that, although larval development and calcification were reduced at pH levels projected for 2100, survival was unaffected (). Pecorino et al. (Citation2014) examined the interaction of pH and temperature on fertilisation in the northern New Zealand sea urchin Centrostephanus rodgersi, finding fertilisation to be robust, or only slightly reduced, under future pH levels (7.8 and 7.6). Embryological development was progressively slowed by reducing pH from 8.1 to 7.6, and the proportion of abnormally developed embryos (i.e. asymmetry, morphological disparity, irregular cell division), increased from c. 5% in ambient pH to c. 25% in pH 7.6. Warming counteracted some of the reduced development rates induced by pH, but enhanced the effects of pH on abnormality rate.
Similar responses to reduced pH and warming were also reported in New Zealand and Australian populations of the cushion star, Patiriella regularis (B; Byrne, Berman, et al. Citation2013), with reduced survival, smaller larvae and slower development under low pH (7.8, 7.6), all of which were intensified by the effects of warming (+4°C). These observations, of stunted or abnormal larvae, under reduced pH in New Zealand species are consistent with responses in larvae of 13 echinoid species ranging from polar to tropical latitudes (Byrne, Lamare, et al. Citation2013). The interaction between temperature and pH on larval development and survival was assessed in detail by Karelitz et al. (Citation2016), who examined development thermal windows (the temperature range within which normal development proceeds), in ambient and reduced seawater pH for a range of echinoderm species, including the New Zealand sand dollar Fellaster zelandiae and P. regularis. Although thermal windows of developmental stages are an important life-history trait, and it has been hypothesised that OA may reduce their breadth (Pörtner and Farrell Citation2008), Karelitz et al. (Citation2016) found that, while pH 7.8 had a negative effect on development, there was no evidence that thermal windows were altered.
Much less is known about the response of adult echinoderms to OA. Hurd et al. (Citation2011) determined pH within the boundary layer, the ≤1 cm region between the organism and the overlying water where exchange is dominated by molecular diffusion, under different flow rates. With the sea urchin, E. chloroticus, seawater pH was reduced by 0.35 units at its surface, due to respiration and/or calcification. These results suggest that some marine species may be exposed to a wider range of pH at their surface due to metabolic modification of their environment, and that this may provide a mechanism to cope with, or adapt to, future OA.
Research on New Zealand echinoderms has provided valuable insight into the effects of OA on marine invertebrates, and contributed to global reviews of the future effects of elevated pCO2 on marine invertebrates (Dupont et al. Citation2010; Byrne, Lamare, et al. Citation2013; Kroeker et al. Citation2013). There are, however, a number of gaps in our knowledge, with few studies on adult echinoderms, deeper water species, and species within the Classes Ophiuroidea (brittle stars) and Holothuroidea (sea cucumbers). While the suite of experiments quantifying the effects of reduced seawater pH on echinoderms has provided useful models of OA impacts on life histories, the focus is now turning to adaptation potential, with New Zealand researchers using echinoderms to quantify adaptive capacity in marine populations. This incorporates a quantitative genetics approach, as applied with polar species (i.e. Foo et al. Citation2016), and long-term experiments to examine transgenerational plasticity.
Fish
Fish were provisionally considered to be relatively insensitive to OA, as they do not possess a calcareous exoskeleton and their physiology is generally able to withstand high pCO2 (Melzner et al. Citation2009). However, it has subsequently been recognised that the neurosensory and behavioural systems of fish may be affected by elevated pCO2. Juvenile and adult fish actively regulate the concentration of acid–base relevant ions in the blood and tissues to prevent acidosis in a high pCO2 environment (Heuer and Grosell Citation2014). However, early life stages, such as eggs and larvae, may be more susceptible to high pCO2 as they are still developing their physiological regulatory processes (Brauner Citation2008). Furthermore, beyond survival, significant effects of elevated pCO2 on fish are frequently observed, such as for otolith deposition (Checkley et al. Citation2009), growth and survival (Baumann et al. Citation2011), reproduction (Miller et al. Citation2013) and metabolic rate (Enzor et al. Citation2013). When exposed to elevated pCO2 for more than a few days, changes are observed in olfactory (Munday et al. Citation2009), auditory (Simpson et al. Citation2011) and visual function (Chung et al. Citation2014). Phototaxis, response to chemical cues and startle behaviour are also affected in larval fish (Ferrari et al. Citation2011; Allan et al. Citation2013; Forsgren et al. Citation2013). The disruption of such a broad range of sensory systems indicates that elevated pCO2 influences the central nervous system rather than individual sensory systems. This is supported by recent experiments that demonstrated the involvement of the central nervous system neurotransmitter receptor GABAA in the disruption of sensory functions, such as olfaction and lateralisation, under elevated pCO2 (c. 900 µatm; Nilsson et al. Citation2012). These changes in behaviour may affect the outcome of predator–prey interactions (Ferrari et al. Citation2011; Allan et al. Citation2013), leading to increased mortality (Munday et al. Citation2010), with potential far-reaching consequences for marine ecosystems and fisheries (Heenan et al. Citation2015; Nagelkerken and Munday Citation2016).
It is currently unclear how fish species and stocks around New Zealand will respond to OA, as few empirical studies have been carried out. Munday et al. (Citation2015) investigated the effect of elevated CO2 on the early life history of Kingfish, Seriola lalandi, with eggs and larvae exposed to control and two elevated pCO2 treatments (880 and 1700 µatm; pH 7.75 and 7.49), until larvae reached three day’s post-hatch. Growth, survival, morphometric traits and behaviours were all assessed, with the only significant change being a reduced oil globule diameter. As the oil globule of larval fish is a rich source of lipids that are catabolized during development (Heming and Buddington Citation1988), this suggests that pre-feeding larval kingfish may tolerate future OA; however, the inferred increase in use of endogenous energy reserves also implies increased vulnerability at times when food availability is suboptimal. Contrary to studies on tropical fish, no behavioural effects were observed, although these may not occur until later stages, when gill-based acid–base regulation is initiated. Longer duration experiments with kingfish are currently ongoing to address this hypothesis, with future studies examining adaptation potential to elevated CO2 via differential genotype performance. Similar investigations with snapper, Chrysophrys auratus, are also planned in the near future.
The only other investigation into the impacts on New Zealand fish utilised a seafloor CO2 seep as a potential natural laboratory for OA (Nagelkerken et al. Citation2015). This approach was first used to study OA impacts in coastal waters of volcanic islands in the Mediterranean (Hall-Spencer et al. Citation2008), and has led to subsequent insights into the role of pH in shaping benthic communities at different locations worldwide (Fabricius et al. Citation2011). In the White Island study (Bay of Plenty, North Island), adult Common Triplefin (Forsterygion lapillum) showed an increase in risk-taking behaviour and slower escape speed in lower pH (7.72–7.86) water associated with CO2 vents, relative to those in water of ambient pH (8.05–8.14; Nagelkerken et al. Citation2015). Although this supports the response observed in other fish species to elevated pCO2, fish abundances were higher at the vent sites, and the slower escape responses were modulated by habitat availability and shelter near the high-CO2 vents. The potential use of New Zealand CO2 seeps for OA studies has also been investigated by Brinkman and Smith (Citation2015), with a focus on coralline algae on White Island, and by Burrell et al. (Citation2015) who examined the impact of deeper CO2 seeps (40–190 m) on water column pH and biogeochemistry in the Bay of Plenty. The latter study confirmed that bacterial extracellular activity is enhanced at lower pH (7.68–7.81), relative to ambient pH (8.03), but also identified potential confounding factors associated with CO2 seeps, including elevated trace metals, sulphides and methane. A recent interdisciplinary study was carried out at White island to further assess the impact of CO2 from seeps on the microbiota, water chemistry, benthic community structure, mineralogy and fish (A. Smith, pers. comm.).
Knowledge gaps and recommendations for future research
The potential vulnerability to OA, and the relative state of knowledge on sensitivity to low pH/high CO2, for each phylum in New Zealand waters are detailed in Table S1, and summarised in . This summary is based on the level of confidence in the overall trend and/or the number of published papers. Non-calcifying microbial groups, such as phytoplankton and bacteria, appear relatively resilient, whereas calcifying invertebrates, such as molluscs and echinoderms, show greater vulnerability to lower pH (), as also identified in international studies (Kroeker et al. Citation2013). Although sensitivity to OA has been studied for different New Zealand groups, such as the macroalgae and molluscs, there is often no consistent trend in response to OA, as described above. This is due to a broad range of factors, including species differences in habitat (e.g. pelagic, benthic), and associated differential exposure to low pH and other stressors, as well as life history and behaviour. Indeed, variations in response may occur in different strains of the same species, as shown for the coccolithophore E. huxleyi (), and in populations of the same species (Waldbusser et al. Citation2010; Parker et al. Citation2011). This variability in sensitivity makes it challenging to predict the composition and status of future ecosystems, and highlights the importance of determining the response of species that are endemic to New Zealand, as opposed to assuming consistency in sensitivity between analogous or related global species and functional groups (Law et al. Citation2012).
Table 3. Qualitative assessment of a) the vulnerability, and b) the current state of knowledge of different aspects of OA research for the major biotic groups in New Zealand waters, based upon publications cited in the respective sections in the text and detailed in Supplementary . Vulnerability is classified on a Low to High scale, with “?” indicating where vulnerability is currently unknown. Current knowledge is also classified on a Low to High scale, and based upon the number and results of published studies (see key), with “-“ indicating that no studies have been carried out. Ongoing New Zealand studies are indicated by “+”, with those in the CARIM project indicated by “*”.
A consistent feature of the studies on different New Zealand phyla is that different life-history stages are not equally susceptible to OA. This is apparent in macroalgae, molluscs and echinoderms (see above), and is also reflected in global studies (Kroeker et al. Citation2013). This needs to be considered in OA response studies for other phyla, and in population models, as enhanced vulnerability in early life-history stages are a potential ‘bottleneck’ to development of the adult stage. Studies of macroalgal sensitivity to OA also emphasise the significance of identifying the physiological mechanisms that determine response (Hepburn et al. Citation2011), which may potentially strengthen extrapolation of responses between species and groups with similar physiology. However, mechanistic studies of response to low pH/high CO2 are currently rare or absent for other New Zealand phyla (), and an increased focus on understanding mechanisms and traits, for example in cold-water corals and other calcifying invertebrates, will assist prediction of future ecosystem states. In addition, investigations of the mechanisms determining responses to OA as part of longer term studies will add value to adaptation studies. Preliminary evidence of the potential to adapt to low pH in groups such as the coccolithophores is encouraging (Lohbeck et al. Citation2012), and more long-term studies are required for key New Zealand organisms. Although long-term studies present considerable logistic challenges this information is essential to determining future success under prolonged exposure to OA.
The interaction between an organism and its environment may play a significant role in its overall response to OA, although this is often challenging to determine experimentally, and has been relatively overlooked in New Zealand studies to date (). It is increasingly recognised that this interaction is two-way; for example, a significant proportion of pH variability in coastal waters arises from primary production and respiration (Cornwall, Hepburn, Pilditch, et al. Citation2013; Hurd Citation2015), and this natural variability needs to be factored into experimental studies, so that responses can be interpreted in the context of the pH the organism experiences in situ. It should also be considered that potential indirect effects of OA, such as the change in prey food quality, predation or substrate availability for settlement, may be more critical to the future success of an organism than the direct physiological impacts of OA. New Zealand studies on macroalgae and sponges detailed above illustrate the importance of considering how interactions between species and phyla may be altered by OA. For example, novel studies have examined the influence of OA on the relationship between New Zealand molluscs and amphipods with their trematode parasites. Shell growth, strength and dissolution were significantly modified in individuals of the gastropod Z. subcarinatus infected with trematode parasites by extreme low pH (7.4, 7.6), relative to uninfected individuals (MacLeod and Poulin Citation2015a, Citation2015b). Differences in trematode species also influenced the response to low pH (MacLeod and Poulin Citation2016b), whereas the pH altered the transmission success of trematode parasites (Harland et al. Citation2015; Guilloteau et al. Citation2016). These results emphasise that biotic interactions may be further confounding factors to establishing an organism’s overall response to OA (Macleod and Poulin Citation2012; MacLeod Citation2016).
The resilience of marine foodwebs in New Zealand waters is critical from both ecological and economic perspectives, yet there has been limited research to date in this field. OA may alter the food quality of the lower foodweb, as for example with the New Zealand coastal diatom, Cylindrotheca fusiformis, in which amino and fatty acid content decreased by 3–6% under elevated pCO2 (750 μatm, Bermúdez et al. Citation2015). The potential impact of this change on consumers, such as zooplankton and other crustaceans that occupy a critical role in the transfer of energy and carbon to higher consumers, has yet to be considered (). Consequently, potential impacts of OA on groups such as cephalopods, cetaceans and seabirds remain unclear (Kawaguchi et al. Citation2013; ), despite their importance as top consumers and, in some cases, their protected status in New Zealand waters (Baker et al. Citation2016; Robertson et al. Citation2013). Ecosystem modelling is a critical requirement for understanding how OA will impact foodwebs and the transfer of energy between trophic groups, and also the sustainability of New Zealand fish stocks and fisheries (Griffith et al. Citation2012; Pinkerton Citation2017). There has been only one ecosystem model study to date that has examined the potential effect of OA on trophic interactions in New Zealand waters. This model of the Wellington marine reserve suggests that an OA-induced reduction in calcification in higher trophic levels, such as lobsters, could have positive effects on lower trophic levels via a reduction in predation pressure (Cornwall and Eddy Citation2015). This example highlights the need for advanced ecosystem models of OA, that incorporate both trophic interactions and indirect effects, for robust projection of the future status of New Zealand marine ecosystems.
OA will not occur in isolation, and will increase coincident with other climate-induced changes in New Zealand marine ecosystems (Lundquist et al. Citation2011; Law et al., Citationforthcoming, Citation2016). Warming and acidification, and associated factors, will have individual and interactive impacts on organisms and ecosystems. As temperature determines reaction rates, warming will directly affect the metabolism and physiology of many organisms, and consequently may have a greater overall effect on biomass and distribution than OA (Poloczanska et al. Citation2013). The interaction of temperature and pH has been considered for some New Zealand phyla (), with groups such as bacteria, sponges and echinoderms, showing greater sensitivity to warming. A synthesis of global results suggests synergistic effects are the dominant interactive response between warming and OA (Gunderson et al. Citation2016), although antagonistic responses dominated in a study of bacterial responses to warming and low pH in New Zealand waters (Burrell et al. Citation2017). Although assessment of multiple stressors requires increasing experimental complexity (Boyd et al. Citation2016), identifying these interactive effects is essential for prediction of future ecosystems.
There is currently little information to assess whether OA will significantly alter broader ecosystem services, such as carbon sequestration, nutrient regeneration and water purification around New Zealand (MacDiarmid et al. Citation2013). For example, there are no studies to date on the influence of OA on sediments, despite their importance in determining the productivity and biogeochemistry of New Zealand coastal waters, although preliminary experiments indicate they may be affected (C. Pilditch, pers. comm.). International assessments of the socio-economic impacts of OA have focussed on shellfish aquaculture and coral reefs (Cooley et al. Citation2009), but there have been no comparative studies in New Zealand (). However, following the experience of the oyster industry on the northwest coast of the USA (Barton et al. Citation2015), the potential impacts of OA on the seafood industry in New Zealand are now receiving attention. Coastal Acidification: Rate, Impacts and Management (CARIM) is a New Zealand project that will determine current pH variability and its influence on coastal ecosystems, with a focus on key species of ecological and socio-economic importance. This includes assessment of OA sensitivity in larval stages of snapper (C. auratus), a highly valued inshore fish species, to inform an assessment of New Zealand stocks (Parsons et al. Citation2014). Population models for the green-lipped mussel and pāua will include the direct effects of OA on all life-history stages, as well as indirect impacts on food and habitat availability, via impacts upon coastal plankton, periphyton and calcifying coralline algae. Species transgenerational effects (Parker et al. Citation2012, Citation2015), and the capacity to adapt to lower pH, will also be investigated for both molluscs using different aquaculture families. A regional hydrodynamic-biogeochemistry model is under development in CARIM, that will assist management of acidification by determining the relative contributions to coastal pH variability of different drivers, such as terrestrial run-off, atmospheric and ocean input, and other project results will inform Taiāpure (Māori reserve) management. A related project is assisting the aquaculture industry, by assessing different technical approaches to elevate pH at the spatial scale of mussel farms.
In summary, New Zealand policy-makers and scientists are relatively well-informed on the potential direct impacts of OA for a range of marine phyla (excluding vertebrates), but currently lack insight into the mechanistic controls of organism responses, and indirect ecosystem interactions. Scaling up the responses of organisms from laboratory experiments to natural communities and ecosystems, where multiple stressors and interactions add considerable complexity, represents a major challenge. As the mean pH of Subantarctic surface waters in the New Zealand EEZ will decline below the current pH minimum in the next ten to twenty years (Law et al., Citationforthcoming, Citation2016), addressing these knowledge gaps is critical for the development of models and projections that will inform mitigation and adaptation to the acidification of New Zealand waters.
Figure S1. Location of New Zealand coastal time-series stations, with CARIM sites indicated by green circles and NZOA-ON sites by red circles. Sites mentioned in the text are also labelled.
Download MS Word (219 KB)Table S1. Details, response and citation for published OA response experiments for New Zealand species.
Download MS Word (44.7 KB)Acknowledgements
We thank John Zeldis for information on the Firth of Thames, Jessica Ericson for permission to publish electron micrographs of P. canaliculus larvae and Mike Williams and Mark Tucker for revisions to figures. The NZOA-ON water samples were collected by John Zeldis (Firth of Thames), Cecilia Clarke (Chatham Islands) and Barry Horne (Jackson Bay). We also acknowledge the contribution of Keith Hunter for initiating carbonate science at the University of Otago, and involvement with the Munida time series from its inception. Finally, we thank two unidentified reviewers for comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Cliff S. Law http://orcid.org/0000-0002-7669-2475
Simon K. Davy http://orcid.org/0000-0003-3584-5356
Miles Lamare http://orcid.org/0000-0001-8656-7240
Sara E. Mikaloff-Fletcher http://orcid.org/0000-0003-0741-0320
Wendy A. Nelson http://orcid.org/0000-0003-0014-5560
Abigail M. Smith http://orcid.org/0000-0001-6468-9124
Additional information
Funding
References
- Allan BJM, Domenici P, McCormick MI, Watson SA, Munday PL. 2013. Elevated CO2 affects predator-prey interactions through altered performance. PLoS One. 8:e58520. doi: 10.1371/journal.pone.0058520
- Anderson OF, Guinotte JM, Rowden AA, Tracey DM, Mackay KA, Clark MR. 2016. Habitat suitability models for predicting the occurrence of vulnerable marine ecosystems in the seas around New Zealand. Deep Sea Research Part I: Oceanographic Research Papers. 115:265–292. doi: 10.1016/j.dsr.2016.07.006
- Anon. 2014. New Zealand seafood exports. Report 7: Seafood exports by product type. Calendar year to December 2014. Seafood New Zealand. p. 118.
- Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proceedings of the National Academy of Sciences. 105(45):17442–17446. doi: 10.1073/pnas.0804478105
- Baker CS, Chilvers BL, Childerhouse S, Constantine R, Currey R, Mattlin R, Van Helden A, Hitchmough R, Rolfe J. 2016. Conservation status of New Zealand marine mammals, 2013. (New Zealand threat classification series; 14).
- Barr NG, Lohrer AM, Cummings VJ. 2017. An in situ incubation method for measuring the productivity and responses of under-ice algae to ocean acidification and warming in polar marine habitats. Limnology and Oceanography: Methods. 15(3):264–275. doi: 10.1002/lom3.10154
- Barton A, Waldbusser GG, Feely RA, Hales B, Langdon CJ. 2015. Impacts of coastal acidification on the Pacific Northwest shellfish industry and adaptation strategies implemented in response. Oceanography. 28(2):146–159. doi: 10.5670/oceanog.2015.38
- Bates T. 2015. Sponge physiology and function in a changing ocean: responses to ocean acidification and increased sea surface temperature [MSc thesis]. Wellington: Victoria University of Wellington.
- Bates N, Astor Y, Church M, Currie K, Dore J, Gonaález-Dávila M, Lorenzoni L, Muller-Karger F, Olafsson J, Santa-Casiano M. 2014. A time-series view of changing ocean chemistry due to ocean uptake of anthropogenic CO2 and ocean acidification. Oceanography. 27(1):126–141. doi: 10.5670/oceanog.2014.16
- Baumann H, Talmage SC, Gobler CJ. 2011. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nature Climate Change. 2(1):38–41. doi: 10.1038/nclimate1291
- Bednaršek N, Harvey CJ, Kaplan IC, Feely RA, Možina J. 2016. Pteropods on the edge: cumulative effects of ocean acidification, warming, and deoxygenation. Progress in Oceanography. 145:1–24. doi: 10.1016/j.pocean.2016.04.002
- Bell JJ, Davy SK, Jones T, Taylor MW, Webster NS. 2013. Could some coral reefs become sponge reefs as our climate changes? Global Change Biology. 19:2613–2624. doi: 10.1111/gcb.12212
- Bennett HM, Altenrath C, Woods L, Davy SK, Webster NS, Bell JJ. 2016. Interactive effects of temperature and pCO2 on sponges: from the cradle to the grave. Global Change Biology. 23: 2031–2046. doi: 10.1111/gcb.13474
- Bergquist PR. 1978. Sponges. Berkeley: University of California Press.
- Berman J, Bell JJ. 2016. Short-term temporal variability in a temperate sponge assemblage. Marine Biology. 163:241–249. doi: 10.1007/s00227-016-2825-y
- Bermúdez R, Feng Y, Roleda MY, Tatters AO, Hutchins DA, Larsen T, Boyd PW, Hurd CL, Riebesell U, Winder M. 2015. Long-term conditioning to elevated pCO2 and warming influences the fatty and amino acid composition of the diatom Cylindrotheca fusiformis. PLoS One. 10(5):e0123945. doi: 10.1371/journal.pone.0123945
- Bostock HC, Hayward BW, Neil HL, Currie KI, Dunbar GB. 2011. Deep-water carbonate concentrations in the southwest Pacific. Deep Sea Research Part I: Oceanographic Research Papers. 58:72–85. doi: 10.1016/j.dsr.2010.11.010
- Bostock HC, Mikaloff-Fletcher SE, Williams MJM. 2013. Estimating carbonate parameters from hydrographic data for the intermediate and deep waters of the Southern Hemisphere oceans. Biogeosciences. 10:6199–6213. doi: 10.5194/bg-10-6199-2013
- Bostock HC, Sutton PJ, Williams MJM, Opdyke BN. 2013. Reviewing the circulation and mixing of Antarctic intermediate water in the South Pacific using evidence from geochemical tracers and Argo float trajectories. Deep Sea Research Part I: Oceanographic Research Papers. 73:84–98. doi: 10.1016/j.dsr.2012.11.007
- Bostock HC, Tracey DM, Currie KI, Dunbar GB, Handler MR, Mikaloff-Fletcher SE, Smith AM, Williams MJM. 2015. The carbonate mineralogy and distribution of habitat forming deep-sea corals in the southwest Pacific region. Deep Sea Research Part I: Oceanographic Research Papers. 100:88–104. doi: 10.1016/j.dsr.2015.02.008
- Boyd PW, Cornwall CE, Davison A, Doney SC, Fourquez M, Hurd CL, Lima ID, McMinn A. 2016. Biological responses to envrionmental heterogeneity under future ocean conditions. Global Change Biology. 22:2633–2650. doi: 10.1111/gcb.13287
- Boyd PW, Dillingham PW, McGraw CM, Armstrong EA, Cornwall CE, Feng YY, Hurd CL, Gault-Ringold M, Roleda MY, Timmins-Schiffman E, et al. 2015. Physiological responses of a Southern Ocean diatom to complex future ocean conditions. Nature Climate Change. 6:207–213.
- Boyd P, LaRoche J, Gall M, Frew R, McKay RML. 1999. Role of iron, light, and silicate in controlling algal biomass in Subantarctic waters SE of New Zealand. Journal of Geophysical Research: Oceans. 104(C6):13395–13408. doi: 10.1029/1999JC900009
- Brailsford GW, Stephens BB, Gomez AJ, Riedel K, Mikaloff Fletcher SE, Nichol SE, Manning MR. 2012. Long-term continuous atmospheric CO2 measurements at Baring Head, New Zealand. Atmospheric Measurement Techniques. 5(12):3109–3117. doi: 10.5194/amt-5-3109-2012
- Brauner CJ. 2008. Acid-base balance. In: Finn RN and Kapoor BG, editors. Fish larval physiology. Enfield: Science Publishers, 724 p.; p. 185–198.
- Breitburg DL, Salisbury J, Bernhard JM, Cai WJ, Dupont S, Doney SC, Kroeker KJ, Levin LA, Long WC, Milke LM, et al. 2015. And on top of all that … coping with ocean acidification in the midst of many stressors. Oceanography. 28(2):48–61. doi: 10.5670/oceanog.2015.31
- Brinkman TJ, Smith AM. 2015. Effect of climate change on crustose coralline algae at a temperate vent site, White Island, New Zealand. Marine and Freshwater Research. 664:360–370. doi: 10.1071/MF14077
- Britton D, Cornwall CE, Revill AT, Hurd CL, Johnson CR. 2016. Ocean acidification reverses the positive effects of seawater pH fluctuations on growth and photosynthesis of the habitat-forming kelp, Ecklonia radiata. Scientific Reports. 6:26036. DOI:10.1038/srep26036.
- Brix H, Currie KI, Mikaloff Fletcher S. 2013. Seasonal variability of the carbon cycle in Subantarctic surface water in the South West Pacific. Global Biogeochemical Cycles. 27:200–211. doi: 10.1002/gbc.20023
- Burrell TJ, Maas EW, Hulston DA, Law CS. 2015. Bacterial abundance, processes and diversity responses to acidification at a coastal CO2 vent. FEMS Microbiology Letters. 362. DOI:101093/femsle/fnv154. doi: 10.1093/femsle/fnv154
- Burrell TJ, Maas EW, Hulston DA, Law CS. 2017. Variable response to warming and ocean acidification by bacterial processes in different plankton communities. Applied Environmental Microbiology. 79:49–62.
- Burrell TJ, Maas EW, Teesdale-Spittle PH, Law CS. 2016. Assessing approaches to determine the effect of ocean acidification on bacterial processes. Biogeosciences. 13: 4379–4388. doi: 10.5194/bg-13-4379-2016
- Byrne M, Gonzalez-Bernat M, Doo S, Foo S, Soars N, Lamare M. 2013. Effects of ocean warming and acidification on embryos and non-calcifying larvae of the invasive sea star Patiriella regularis. Marine Ecology Progress Series. 473:235–246. doi: 10.3354/meps10058
- Byrne M, Lamare M, Winter D, Dworjanyn SA, Uthicke S. 2013. The stunting effect of a high CO2 ocean on calcification and development in sea urchin larvae, a synthesis from the tropics to the poles. Philosophical Transactions of the Royal Society B: Biological Sciences. 368:20120439. doi: 10.1098/rstb.2012.0439
- Cairns SD, Macintyre IG. 1992. Phylogenetic implications of calcium carbonate mineralogy in Stylasteridae (Cnidaria: Hydrozoa). Palaios. 7:96–107. doi: 10.2307/3514799
- Chang FH, Northcote L. 2016. Species composition of extant coccolithophores including twenty six new records from the southwest Pacific near New Zealand. Marine Biodiversity Records. 9(1):75. doi: 10.1186/s41200-016-0077-7
- Checkley DM Jr, Dickson AG, Takahashi M, Radich JA, Eisenkolb N, Asch R. 2009. Elevated CO2 enhances otolith growth in young fish. Science. 324:1683. doi: 10.1126/science.1169806
- Chiswell SM, Bostock HC, Sutton PJH, Williams MJM. 2015. Physical oceanography of the deep seas around New Zealand: a review. New Zealand Journal of Marine and Freshwater Research. 49: 286–317. doi: 10.1080/00288330.2014.992918
- Chung WS, Marshall NJ, Watson SA, Munday PL, Nilsson GE. 2014. Ocean acidification slows retinal function in a damselfish through interference with GABAA receptors. Journal of Experimental Biology. 217:323–326. doi: 10.1242/jeb.092478
- Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, et al. 2013. Carbon and other biogeochemical cycles. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change]. Cambridge (UK): Cambridge University Press. p. 465–570.
- Clark D, Lamare M, Barker M. 2009. Response of sea urchin pluteus larvae (Echinodermata: Echinoidea) to reduced seawater pH: a comparison among a tropical, temperate, and a polar species. Marine Biology. 156:1125–1137. doi: 10.1007/s00227-009-1155-8
- Clark MR, Koslow JA. 2012. Impacts of fishing on seamounts In: Pitcher TJ; Hart PJB; De’ath G, Fabricius K E, Sweatman H, Puotinen M, editors. The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proceedings of the National Academy of Sciences 109:17995–17999.
- Clark MR, Rowden AA. 2009. Effects of deepwater trawling on the macro-invertebrate assemblages of seamounts on the Chatham rise, New Zealand. Deep Sea Research Part I: Oceanographic Research Papers. 56:1540–1554. doi: 10.1016/j.dsr.2009.04.015
- Comeau S, Jeffree R, Teyssie JL, Gattuso JP. 2010. Response of the Arctic pteropod Limacina helicina to projected future environmental conditions. PLoS One. 5:e11362. doi: 10.1371/journal.pone.0011362
- Cooley SR, Kite-Powell HL, Doney SC. 2009. Ocean acidification’s potential to alter global marine ecosystem services. Oceanography. 22(4):172–181. doi: 10.5670/oceanog.2009.106
- Cornwall CE, Boyd PW, McGraw CM, Hepburn CD, Pilditch CA, Morris JN, Smith AM, Hurd CL. 2014. Diffusion boundary layers ameliorate the negative effects of ocean acidification on the temperate coralline macroalga Arthrocardia corymbosa. PLoS One. 9:e97235. doi: 10.1371/journal.pone.0097235
- Cornwall CE, Eddy TD. 2015. Effects of near-future ocean acidification, fishing, and marine protection on a temperate coastal ecosystem. Conservation Biology. 29:207–215. doi: 10.1111/cobi.12394
- Cornwall CE, Hepburn CD, McGraw CM, Currie KI, Pilditch CA, Hunter KA, Boyd PW, Hurd CL. 2013. Diurnal fluctuations in seawater pH influence the response of a calcifying macroalga to ocean acidification. Proceedings of the Royal Society Biological Sciences Series B. 280:20132201. doi: 10.1098/rspb.2013.2201
- Cornwall CE, Hepburn CD, Pilditch CA, Hurd CL. 2013. Concentration boundary layers around complex assemblages of macroalgae: implications for the effects of ocean acidification on understory coralline algae. Limnology and Oceanography. 58:121–130. doi: 10.4319/lo.2013.58.1.0121
- Cornwall CE, Hepburn CD, Pritchard DW, McGraw CM, Currie KI, Hunter KA, Hurd CL. 2012. Carbon-use strategies in macroalgae: differential responses to lowered pH and implications for ocean acidification. Journal of Phycology. 48:137–144. doi: 10.1111/j.1529-8817.2011.01085.x
- Cornwall CE, Pilditch CA, Hepburn CD, Hurd CL. 2015. Canopy macroalgae influence understorey corallines’ metabolic control of near-surface pH and oxygen concentration. Marine Ecology Progress Series. 525:81–95. doi: 10.3354/meps11190
- Cornwall CE, Revill AT, Hurd CL. 2015. High prevalence of diffusive uptake of CO2 by macroalgae in a temperate subtidal ecosystem. Photosynthesis Research. 124(2):181–190. doi: 10.1007/s11120-015-0114-0
- Cross EL, Peck LS, Harper EM. 2015. Ocean acidification does not impact shell growth or repair of the Antarctic brachiopod Liothyrella uva (Broderip, 1833). Journal of Experimental Marine Biology and Ecology. 462:29–35. doi: 10.1016/j.jembe.2014.10.013
- Cross EL, Peck LS, Lamare MD, Harper EM. 2016. No ocean acidification effects on shell growth and repair in the New Zealand brachiopod Calloria inconspicua (Sowerby, 1846). ICES Journal of Marine Science. 73(3):920–926. doi: 10.1093/icesjms/fsv031
- CSIRO Atlas of Regional Seas. 2009. http://wwwmarinecsiroau/c.dunn/cars2009/2009.
- Cummings V, Hewitt J, Van Rooyen A, Currie K, Beard S, Thrush S, Norkko J, Barr N, Heath P, Halliday J, et al. 2011. Ocean acidification at high latitudes: potential effects on functioning of the Antarctic bivalve Laternula elliptica. PLoS One. 6(1):e16069. doi: 10.1371/journal.pone.0016069
- Cummings VJ, Marriott PM, Halliday NJ, Smith AM, Peebles B. 2016. Assessment of shellfish mineralogy and structure. Final research report prepared for the Ministry of Primary Industries project ZBD2013-06. 55 p.
- Cunningham SC, Smith AM, Lamare MD. 2016. The effects of elevated pCO2 on growth, shell production and metabolism of cultured juvenile abalone, Haliotis iris. Aquaculture Research. 47:2375–2392. doi: 10.1111/are.12684
- Currie KI, Reid MR, Hunter KA. 2011. Interannual variability of carbon dioxide drawdown by Subantarctic surface water near New Zealand. Biogeochemistry. 104:23–34. doi: 10.1007/s10533-009-9355-3
- Denny MW, Miller LP, Stokes MD, Hunt LJH, Helmuth BST. 2003. Extreme water velocities: topographic amplification of wave-induced flow in the surf zone of rocky shores. Limnology and Oceanography. 48:1–8. doi: 10.4319/lo.2003.48.1.0001
- Desmond MJ, Pritchard DW, Hepburn CD. 2015. Light limitation within southern New Zealand kelp forest communities. PLoS ONE. 10:e0123676. doi: 10.1371/journal.pone.0123676
- Diaz-Pulido G, Cornwall CE, Gartrell P, Hurd CL, Tran DV. 2016. Strategies of dissolved inorganic carbon use in macroalgae across a gradient of terrestrial influence: implications for the Great Barrier Reef in the context of ocean acidification. Coral Reefs. 35(4):1327–1341. doi: 10.1007/s00338-016-1481-5
- Diaz-Pulido G, Gouzezo M, Tilbrook B, Dove S, Anthony K. 2011. High CO2 enhances the competitive strength of seaweeds over corals. Ecology Letters. 14:156–162. doi: 10.1111/j.1461-0248.2010.01565.x
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. 2009. Ocean acidification: the other CO2 problem. Marine Science. 1:169–192. doi: 10.1146/annurev.marine.010908.163834
- Dupont S, Ortega-Martínez O, Thorndyke M. 2010. Impact of near-future ocean acidification on echinoderms. Ecotoxicology. 19:449–462. doi: 10.1007/s10646-010-0463-6
- Enzor LA, Zippay ML, Place SP. 2013. High latitude fish in a high CO2 world: synergistic effects of elevated temperature and carbon dioxide on the metabolic rates of Antarctic notothenioids. Comparative Biochemistry and Physiology Part A: Molecular Integrative Physiology. 164:154–161. doi: 10.1016/j.cbpa.2012.07.016
- Ericson J. 2010. Effects of ocean acidification on fertilisation and early development in polar and temperate marine invertebrates [MSc thesis]. Dunedin: University of Otago; p. 117.
- Fabricius K, Kluibenschedl A, Harrington L, Noonan S, De’ath G. 2015. In situ changes of tropical crustose coralline algae along carbon dioxide gradients. Scientific Reports 5, 9537. DOI:10.1038/srep09537.
- Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De’ath G, Okazaki R, Muehllehner N, Glas MS, Lough JM. 2011. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Climate Change. 1(3):165–169. doi: 10.1038/nclimate1122
- Fabry VJ. 2008. Marine calcifiers in a high-CO2 ocean. Science. 320:1020–1022. doi: 10.1126/science.1157130
- Farr T, Broom J, Hart D, Neill K, Nelson WA. 2009. Common coralline algae of northern New Zealand: An identification guide. NIWA.
- Feely RA, Sabine CL, Byrne RH, Millero FJ, Dickson AG, Wanninkhof R, Murata A, Miller LA, Greeley D. 2012. Decadal changes in the aragonite and calcite saturation state of the Pacific Ocean. Global Biogeochemical Cycles. 26, GB3001. doi: 10.1029/2011GB004157
- Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ. 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science. 305:362–366. doi: 10.1126/science.1097329
- Feng Y. 2015. Environmental controls on the marine coccolithophore Emiliania huxleyi strain NIWA1108 [PhD thesis]. Dunedin: University of Otago.
- Feng Y, Roleda MY, Armstrong E, Boyd PW, Hurd CL. 2016. Environmental controls on the growth, photosynthetic and calcification rates of a Southern Hemisphere strain of the coccolithophore Emiliania huxleyi. Limnology and Oceanography. 62:519–540. doi: 10.1002/lno.10442
- Fernández PA, Hurd CL, Roleda MY. 2014. Bicarbonate uptake via an anion exchange protein is the main mechanism of inorganic carbon acquisition by the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae) under variable pH. Journal of Phycology. 50:998–1008. doi: 10.1111/jpy.12247
- Fernández PA, Roleda MY, Hurd CL. 2015. Effects of ocean acidification on the photosynthetic performance, carbonic anhydrase activity and growth of the giant kelp Macrocystis pyrifera. Photosynthesis Research. 124:293–304. doi: 10.1007/s11120-015-0138-5
- Ferrari MCO, McCormick MI, Munday PL, Meekan MG, Dixson DL, Lonnstedt O, Chivers DP. 2011. Putting prey and predator into the CO2 equation – qualitative and quantitative effects of ocean acidification on predator-prey interactions. Ecology Letters. 14:1143–1148. doi: 10.1111/j.1461-0248.2011.01683.x
- Findlay HS, Calosi P, Crawfurd K. 2011. Determinants of the PIC:POC response in the coccolithophore Emiliania huxleyi under future ocean acidification scenarios. Limnology and Oceanography. 56:1168–1178. doi: 10.4319/lo.2011.56.3.1168
- Flynn KJ, Clark DR, Wheeler G. 2016. The role of coccolithophore calcification in bioengineering their environment. Proceedings of the Royal Society B: Biological Sciences. 283:20161099. doi: 10.1098/rspb.2016.1099
- Foo SA, Sparks KM, Uthicke S, Byrne M, Lamare M. 2016. Contributions of genetic and environmental variance in early development of the Antarctic sea urchin Sterechinus neumayeri in response to increased ocean temperature and acidification. Marine Biology. 163(6):130. DOI:10.1007/s00227-016-2903-1.
- Forsgren E, Dupont S, Jutfelt F, Amundsen T. 2013. Elevated CO2 affects embryonic development and larval phototaxis in a temperate marine fish. Ecology and Evolution. 3:3637–3646. doi: 10.1002/ece3.709
- Gammon M. 2016. The physiological response of a protected deep sea coral (Solenosmilia variabilis) to ocean acidification [Unpublished Master’s thesis]. Wellington: Victoria University. 117 p.
- Gaylord B, Rosman JH, Reed DC, Koseff JR, Fram J, MacIntyre S, Arkema K, McDonald C, Brzezinski MA, Largier JL, Monismith SG. 2007. Spatial patterns of flow and their modification within and around a giant kelp forest. Limnology and Oceanography. 52:1838–1852. doi: 10.4319/lo.2007.52.5.1838
- Gazeau F, Parker LM, Comeau S, Gattuso J, O’Connor WA, Martin S, Pörtner H, Ross PM. 2013. Impacts of ocean acidification on marine shelled molluscs. Marine Biology. 160:2207–2245. doi: 10.1007/s00227-013-2219-3
- Gibbin EM, Putnam HM, Davy SK, Gates RD. 2014. Intracellular pH and its response to CO2-driven seawater acidification in symbiotic versus non-symbiotic coral cells. Journal of Experimental Biology. 217:1963–1969. doi: 10.1242/jeb.099549
- Gordon DP, editor. 2009. New Zealand inventory of biodiversity. Vol. 1, Kingdom Animalia Radiata, Lopotrochozoa, Deuteromstomia. Christchurch: Canterbury University Press. 648 p.
- Gordon DP, Beaumont J, MacDiarmid A, Robertson DA, Ahyong ST. 2010. Marine biodiversity of Aotearoa New Zealand. PLoS One. 5(8):1–17. doi: 10.1371/journal.pone.0010905
- Gori A, Ferrier-Pagès C, Hennige SJ, Murray F, Rottier C, Wicks LC, Roberts JM. 2016. Physiological response of the cold-water coral Desmophyllum dianthus to thermal stress and ocean acidification. PeerJ. 4:e1606. doi: 10.7717/peerj.1606
- Gradoville MR, White AE, Böttjer D, Church MJ, Letelier RM. 2014. Diversity trumps acidification: lack of evidence for carbon dioxide enhancement of Trichodesmium community nitrogen or carbon fixation at Station ALOHA. Limnology and Oceanography. 59(3):645–659. doi: 10.4319/lo.2014.59.3.0645
- Gray BE, Smith AM. 2004. Mineralogical variation in shells of the blackfoot abalone Haliotis iris (Mollusca: Gastropoda: Haliotidae), in southern New Zealand. Pacific Science. 58:47–64. doi: 10.1353/psc.2004.0005
- Griffith GP, Fulton EA, Gorton R, Richardson AJ. 2012. Predicting interactions among fishing, ocean warming, and ocean acidification in a marine system with whole-ecosystem models. Conservation Biology. 26(6):1145–1152. doi: 10.1111/j.1523-1739.2012.01937.x
- Guilloteau P, Poulin R, MacLeod CD. 2016. Impacts of ocean acidification on multiplication and caste organisation of parasitic trematodes in their gastropod host. Marine Biology. 163(5):96. doi: 10.1007/s00227-016-2871-5
- Guinotte JM, Orr J, Cairns S, Freiwald A, Morgan L, George R. 2006. Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals? Frontiers in Ecology and the Environment. 4(3):141–146. doi: 10.1890/1540-9295(2006)004[0141:WHCISC]2.0.CO;2
- Gunderson AR, Armstrong EJ, Stillman JH. 2016. Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Annual Review of Marine Science. 8:357–378. doi: 10.1146/annurev-marine-122414-033953
- Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia MC. 2008. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature. 454:96–99. doi: 10.1038/nature07051
- Harland H, MacLeod CD, Poulin R. 2015. Non-linear effects of ocean acidification on the transmission of a marine intertidal parasite. Marine Ecology Progress Series. 536:55–64. doi: 10.3354/meps11416
- Harvey A, Woelkerling W, Farr T, Neill K, Nelson W. 2005. Coralline algae of central New Zealand: an identification guide to common ‘crustose’ species. (NIWA Information Series). 145 p.
- Heenan A, Pomeroy R, Bell J, Munday PL, Cheung W, Logan C, Brainard R, Yang Amri A, Aliño P, Armada N, et al. 2015. A climate-informed, ecosystem approach to fisheries management. Marine Policy. 57:182–192. doi: 10.1016/j.marpol.2015.03.018
- Heming, TA, Buddington RK. 1988. Yolk-absorption in embryonic and larval fishes. In: Hoar WS, Randall DJ, editors. Fish physiology , Vol. 11, Part A. San Diego (CA): Academic Press; p. 407–446.
- Hennige SJ, Wicks LC, Kamenos NA, Bakker DCE, Findlay HS, Dumousseaud C, Roberts JM. 2014. Short-term metabolic and growth responses of the cold-water coral Lophelia pertusa to ocean acidification. Deep Sea Research Part II: Topical Studies in Oceanography. 99:27–35. doi: 10.1016/j.dsr2.2013.07.005
- Hepburn CD, Pritchard DW, Cornwall CE, McLeod RJ, Beardall J, Raven JA, Hurd CL. 2011. Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Global Change Biology. 17:2488–2497. doi: 10.1111/j.1365-2486.2011.02411.x
- Heuer RM, Grosell M. 2014. Physiological impacts of elevated carbon dioxide and ocean acidification on fish American Journal of Regulatory. Integrative and Comparative Physiology. 307:R1061–R1084. doi: 10.1152/ajpregu.00064.2014
- Hoffmann L, Breitbarth E, Boyd PW, Hunter KA. 2012. Influence of ocean warming and acidification on trace metal biogeochemistry. Marine Ecology Progress Series. 470:191–205. doi: 10.3354/meps10082
- Hoffmann LJ, Breitbarth E, McGraw CM, Law CS, Currie KI, Hunter KA. 2013. A trace-metal clean, pH-controlled incubator system for ocean acidification incubation studies. Limnology and Oceanography: Methods. 11(1):53–61. doi: 10.4319/lom.2013.11.53
- Hoppe CJ, Hassler CS, Payne CD, Tortell PD, Rost B, Trimborn S. 2013. Iron limitation modulates ocean acidification effects on Southern Ocean phytoplankton communities. PLoS One. 8(11):e79890. doi: 10.1371/journal.pone.0079890
- Hoppe CJM, Langer G, Rost B. 2011. Emiliania huxleyi shows identical responses to elevated pCO2 in TA and DIC manipulations. Journal of Experimental Marine Biology and Ecology. 406:54–62. doi: 10.1016/j.jembe.2011.06.008
- Hurd CL. 2015. Slow-flow habitats as refugia for coastal calcifiers from ocean acidification. Journal of Phycology. 51(4):599–605. doi: 10.1111/jpy.12307
- Hurd CL, Cornwall CE, Currie KI, Hepburn CD, McGraw CM, Hunter KA, Boyd P. 2011. Metabolically-induced pH fluctuations by some coastal calcifiers exceed projected 22nd century ocean acidification: a mechanism for differential susceptibility? Global Change Biology. 17:3254–3262. doi: 10.1111/j.1365-2486.2011.02473.x
- Hurd CL, Harrison PJ, Bischof K, Lobban CS. 2014. Seaweed ecology and physiology. Christchurch: Cambridge University Press. 551 p.
- Hurd CL, Hepburn CD, Currie KI, Raven JA, Hunter KA. 2009. Testing the effects of ocean acidification on algal metabolism: considerations for experimental designs. Journal of Phycology. 45(6):1236–1251. doi: 10.1111/j.1529-8817.2009.00768.x
- Hutchins DA, Mulholland MR, Fu F. 2009. Nutrient cycles and marine microbes in a CO2-enriched ocean. Oceanography. 22(4):128–145. doi: 10.5670/oceanog.2009.103
- Iglesias-Rodriguez MD, Halloran PR, Rickaby RE, Hall IR, Colmenero-Hidalgo E, Gittins JR, Green DRH, Tyrrell T, Gibbs SJ, von Dassow P, et al. 2008. Phytoplankton calcification in a high-CO2 world. Science. 320:336–340. doi: 10.1126/science.1154122
- James RK, Hepburn CD, Cornwall CE, McGraw CM, Hurd CL. 2014. Growth response of an early successional assemblage of coralline algae and benthic diatoms to ocean acidification. Marine Biology. 161:1687–1696. doi: 10.1007/s00227-014-2453-3
- Kamenos NA, Perna G, Gambi MC, Micheli F, Kroeker KJ. 2016. Coralline algae in a naturally acidified ecosystem persist by maintaining control of skeletal mineralogy and size. Proceedings of the Royal Society B: Biological Sciences. 283:20161159. doi: 10.1098/rspb.2016.1159
- Karelitz SE, Uthicke S, Foo SA, Barker MF, Byrne M, Pecorino D, Lamare MD. 2016. Ocean acidification has little effect on developmental thermal windows of echinoderms from Antarctica to the tropics. Global Change Biology. 23: 657–672. doi: 10.1111/gcb.13452
- Kawaguchi S, Ishida A, King R, Raymond B, Waller N, Constable A, Nicol S, Wakita M, Ishimatsu A. 2013. Risk maps for Antarctic krill under projected Southern Ocean acidification. Nature Climate Change. 3:843–847. doi: 10.1038/nclimate1937
- Kroeker KJ, Kordas RL, Crim R, Singh GG. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Global Change Biology. 19:1884–1896. doi: 10.1111/gcb.12179
- Langer G, Geisen M, Baumann KH, Kläs J, Riebesell U, Thoms S, Young JR. 2006. Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochemistry, Geophysics, Geosystems. 7:Q09006. doi: 10.1029/2005GC001227
- Langer G, Nehrke G, Probert I, Ly J, Ziveri P. 2009. Strain-specific responses of Emiliania huxleyi to changing seawater carbonate chemistry. Biogeosciences. 6:2637–2646. doi: 10.5194/bg-6-2637-2009
- Law CS, Breitbarth E, Hoffmann LJ, McGraw CM, Langlois RJ, LaRoche J, Marriner A, Safi KA. 2012. No stimulation of nitrogen fixation by non-filamentous diazotrophs under elevated CO2 in the South Pacific. Global Change Biology. 18:3004–3014. doi: 10.1111/j.1365-2486.2012.02777.x
- Law CS, Ellwood M, Woodward EMS, Marriner A, Bury S, Safi K. 2011. Response of surface nutrient inventories and nitrogen fixation to a tropical cyclone in the South-West Pacific. Limnology and Oceanography. 56(4):1372–1385. doi: 10.4319/lo.2011.56.4.1372
- Law CS, Rickard GJ, Mikaloff-Fletcher SE, Pinkerton MH, Behrens E, Chiswell SM, Currie K. Forthcoming. Climate change projections for the surface ocean around New Zealand. New Zealand Journal of Marine and Freshwater Research.
- Law CS, Rickard GJ, Mikaloff-Fletcher SE, Pinkerton MH, Gorman R, Behrens E, Chiswell SM, Bostock HC, Anderson O, Currie K. 2016. The New Zealand EEZ and South West Pacific. Synthesis report RA2, marine case study. Climate changes, impacts and implications (CCII) for New Zealand to 2100. MBIE contract C01X1225. 41 p.
- Law CS, Schwarz JN, Chang FH, Nodder SD, Northcote LC, Safi KA, Marriner A, Langlois RJ, LaRoche J, Amosa P, et al. 2014. Predicting changes in plankton biodiversity and productivity of the EEZ in response to climate change induced ocean acidification. Final research report for the NZ Ministry of Primary Industries on project ZBD200811.
- Leal PP, Hurd CL, Fernández PA, Roleda MY. 2017a. Meiospore development of the kelps Macrocystis pyrifera and Undaria pinnatifida under ocean acidification and ocean warming: independent effects are more important than their interaction. Marine Biology. 164. DOI:10.1007/s00227-016-3039-z.
- Leal PP, Hurd CL, Fernández PA, Roleda MY. 2017b. Ocean acidification and kelp development: reduced pH has no negative effects on meiospore germination and gametophyte development of Macrocystis pyrifera and Undaria pinnatifida. Journal of Phycology. DOI:10.1111/jpy.12518.
- Lebrato M, Andersson AJ, Ries JB, Aronson RB, Lamare MD, Koeve W, Oschlies A, Iglesias-Rodriguez MD, Thatje S, Amsler M, et al. 2016. Benthic marine calcifiers coexist with CaCO3-undersaturated seawater worldwide. Global Biogeochemical Cycles. 30: 1038–1053. doi: 10.1002/2015GB005260
- Lohbeck KT, Riebesell U, Reusch TB. 2012. Adaptive evolution of a key phytoplankton species to ocean acidification. Nature Geoscience. 5(5):346–351. doi: 10.1038/ngeo1441
- Lombardi C, Taylor PD, Cocito S. 2013. Bryozoan constructions in a changing Mediterranean Sea. In: Goffredo S, Dubinski Z, editors. The Mediterranean Sea: its history and present challenges. Dordrecht: Springer Science; p. 373–384.
- Lundquist CJ, Ramsay D, Bell R, Swales A, Kerr S. 2011. Predicted impacts of climate change on New Zealand’s biodiversity. Pacific Conservation Biology. 17:179–191. doi: 10.1071/PC110179
- Maas EW, Hall JA, Law CS, Pickmere S, Currie KI, Chang FH, Voyles KM, Caird D. 2013. Effect of ocean acidification on bacterial abundance, activity and diversity in the Ross Sea, Antarctica. Aquatic Microbial Ecology. 70:1–15. doi: 10.3354/ame01633
- MacDiarmid AB, Law CS, Pinkerton M, Zeldis J. 2013. New Zealand marine ecosystem services In Dymond JR, editor. Ecosystem services in New Zealand – conditions and trends. Lincoln: Manaaki Whenua Press. p. 238–253.
- MacLeod CD. 2015. The effects of ocean acidification on host–parasite associations [PhD thesis]. Dunedin: University of Otago.
- MacLeod CD. 2016. Parasitic infection: a missing piece of the ocean acidification puzzle – ICES. Journal of Marine Science. 74: 929–933.
- MacLeod CD, Poulin R. 2012. Host–parasite interactions: a litmus test for ocean acidification? Trends in Parasitology. 28(9):365–369. doi: 10.1016/j.pt.2012.06.007
- MacLeod CD, Poulin R. 2015a. Differential tolerances to ocean acidification by parasites that share the same host. International Journal for Parasitology. 457:485–493. doi: 10.1016/j.ijpara.2015.02.007
- MacLeod CD, Poulin R. 2015b. Interactive effects of parasitic infection and ocean acidification on the calcification of a marine gastropod. Marine Ecology Progress Series. 537:137–150. doi: 10.3354/meps11459
- MacLeod CD, Poulin R. 2016a. Parasitic infection: a buffer against ocean acidification? Biology Letters. 12:20160007. doi: 10.1098/rsbl.2016.0007
- MacLeod CD, Poulin R. 2016b. Parasitic infection alters the physiological response of a marine gastropod to ocean acidification. Parasitology. 143:1397–1408. doi: 10.1017/S0031182016000913
- MacLeod CD, Doyle HL, Currie KI. 2015. Technical note: maximising accuracy and minimising cost of a potentiometrically regulated ocean acidification simulation system. Biogeosciences. 12:713–721. doi: 10.5194/bg-12-713-2015
- Maier C, Hegeman J, Weinbauer MG, Gattuso JP. 2009. Calcification of the cold-water coral Lophelia pertusa under ambient and reduced pH. Biogeosciences. 6:1671–1680. doi: 10.5194/bg-6-1671-2009
- Martz TR, Connery JG, Johnson KS. 2010. Testing the Honeywell Durafet (R) for seawater pH applications. Limnology and Oceanography: Methods. 8:172–184. doi: 10.4319/lom.2010.8.172
- McConnaughey TA, Gillikin DP. 2008. Carbon isotopes in mollusk shell carbonates. Geo-Marine Letters. 28:287–299. doi: 10.1007/s00367-008-0116-4
- McCoy SJ, Kamenos NA. 2015. Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological, and geochemical responses to global change. Journal of Phycology. 51:6–24. doi: 10.1111/jpy.12262
- McGraw CM, Cornwall CE, Reid MR, Currie KI, Hepburn CD, Boyd PW, Hunter KA. 2010. An automated pH-controlled culture system for laboratory-based ocean acidification experiments. Limnology and Oceanography: Methods. 8(12):686–694. doi: 10.4319/lom.2010.8.0686
- Melzner F, Göbel S, Langenbuch M, Gutowska MA, Pörtner HO, Lucassen M. 2009. Swimming performance in Atlantic cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater. Aquatic Toxicology. 92(1):30–37. doi: 10.1016/j.aquatox.2008.12.011
- Meyer J, Riebesell U. 2015. Reviews and syntheses: responses of coccolithophores to ocean acidification: a meta-analysis. Biogeosciences. 12:1671–1682. doi: 10.5194/bg-12-1671-2015
- Miles H, Widdicombe S, Spicer JI, Hall-Spencer JM. 2007. Effects of anthropogenic seawater acidification on acid-base balance in the sea urchin Psammechinus miliaris. Marine Pollution Bulletin. 54:89–96. doi: 10.1016/j.marpolbul.2006.09.021
- Miller GM, Watson SA, McCormick MI, Munday PL. 2013. Increased CO2 stimulates reproduction in a coral reef fish. Global Change Biology. 19:3037–3045. doi: 10.1111/gcb.12259
- Milliman JD, Troy PJ, Balch WM, Adams AK, Li Y-H, Mackenzie FT. 1999. Biologically mediated dissolution of calcium carbonate above the chemical lysocline? Deep Sea Research Part I: Oceanographic Research Papers. 46:1653–1669. doi: 10.1016/S0967-0637(99)00034-5
- Monteiro FM, Bach LT, Brownlee C, Bown P, Rickaby RE, Poulton AJ, Tyrrell T, Beaufort L, Dutkiewicz S, Gibbs S, et al. 2016. Why marine phytoplankton calcify. Science Advances. 2(7):e1501822. doi: 10.1126/sciadv.1501822
- Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Doving KB. 2009. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proceedings of the National Academy of Sciences of the United States of America. 106:1848–1852. doi: 10.1073/pnas.0809996106
- Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MCO, Chivers DP. 2010. Replenishment of fish populations is threatened by ocean acidification. Proceedings of the National Academy of Sciences of the United States of America. 107:12930–12934. doi: 10.1073/pnas.1004519107
- Munday PL, Watson SA, Parsons DM, King A, Barr NG, McLeod IM, Allan BJM, Pether SMJ. 2015. Effects of elevated CO2 on early life history development of the yellowtail kingfish, Seriola lalandi, a large pelagic fish. ICES Journal of Marine Science. 73(3):641–649. doi: 10.1093/icesjms/fsv210
- Murata A, Kumamoto Y, Watanabe S, Fukasawa M. 2007. Decadal increases of anthropogenic CO2 in the south Pacific subtropical ocean along 32°S. Journal of Geophysical Research. 112:C05033.
- Nagelkerken I, Russell BD, Gillanders BM, Connell SD. 2015. Ocean acidification alters fish populations indirectly through habitat modification. Nature Climate Change. 6:89–93. doi: 10.1038/nclimate2757
- Nagelkerken I, Munday PL. 2016. Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Global Change Biology. 22(3):974–989. doi: 10.1111/gcb.13167
- Navarro JM, Torres R, Acuna K, Duarte C, Manriquez PH, Lardies M, Lagos NA, Vargas C, Aguilera V. 2013. Impact of medium-term exposure to elevated pCO2 levels on the physiological energetics of the mussel Mytilus chilensis. Chemosphere. 90:1242–1248. doi: 10.1016/j.chemosphere.2012.09.063
- Nelson CS. 1978. Temperate shelf carbonate sediments in the Cenozoic of New Zealand. Sedimentology. 25:737–771. doi: 10.1111/j.1365-3091.1978.tb00328.x
- Nelson CS. 1988. An introductory perspective on non-tropical shelf carbonates. Sedimentary Geology. 60:3–12. doi: 10.1016/0037-0738(88)90108-X
- Nelson CS, Keane SL, Head PS. 1988. Non-tropical carbonate deposits on the modern New Zealand shelf. Sedimentary Geology. 60:71–94. doi: 10.1016/0037-0738(88)90111-X
- Nelson WA. 2009. Calcified macroalgae – critical to coastal ecosystems and vulnerable to change: a review. Marine and Freshwater Research. 60:787–801. doi: 10.1071/MF08335
- Nelson WA, Neill KF, D’Archino R, Anderson T, Beaumont J, Dalen J. 2015. Beyond diving depths: deepwater macroalgae in the New Zealand region. Marine Biodiversity. 45:797–818. doi: 10.1007/s12526-014-0293-5
- Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sorensen C, Watson SA, Munday PL. 2012. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nature Climate Change. 2(3):201–204. doi: 10.1038/nclimate1352
- Nodder SD, Chiswell SM, Northcote LC. 2016. Annual cycles of deep-ocean biogeochemical export fluxes in subtropical and Subantarctic waters, southwest Pacific Ocean. Journal of Geophysical Research: Oceans. 121(4):2405–2424.
- Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 437:681–686. doi: 10.1038/nature04095
- Parker LM, O’Connor WA, Raftos DA, Pörtner HO, Ross PM. 2015. Persistence of positive carryover effects in the Oyster, Saccostrea glomerata, following transgenerational exposure to ocean acidification. PLoS One. 10(7):e0132276. doi: 10.1371/journal.pone.0132276
- Parker LM, Ross PM, O’Connor WA. 2011. Populations of the Sydney rock oyster, Saccostrea glomerata, vary in response to ocean acidification. Marine Biology. 158:689–697. doi: 10.1007/s00227-010-1592-4
- Parker LM, Ross PM, O’Connor WA, Borysko L, Raftos DA, Pörtner HO. 2012. Adult exposure influencesoffspring response to ocean acidification in oysters. Global Change Biology. 18:82–92. doi: 10.1111/j.1365-2486.2011.02520.x
- Parsons DM, Sim-Smith CJ, Cryer M, Francis MP, Hartill B, Jones EG, Le Port A, Lowe M, McKenzie J, Morrison M, Paul LJ. 2014. Snapper (Chrysophrys auratus): a review of life-history and key vulnerabilities in New Zealand. New Zealand Journal of Marine and Freshwater Research. 48:256–283. doi: 10.1080/00288330.2014.892013
- Pecorino D, Barker MF, Dwarjanyn SA, Byrne M, Lamare MD. 2014. Impacts of near future sea surface pH and temperature conditions on fertilisation and embryonic development in Centrostephanus rodgersii from northern New Zealand and northern New South Wales, Australia. Marine Biology. 161:101–110. doi: 10.1007/s00227-013-2318-1
- Peebles BA, Smith AM, Spencer HG. 2017. Valve microstructure and phylomineralogy of New Zealand chitons. Journal of Structural Biology. 197:250–259. doi: 10.1016/j.jsb.2016.12.002
- Perea-Blazquez A, Davy SK, Bell JJ. 2012. Estimates of particulate organic carbon flowing from the pelagic environment to the benthos through sponge assemblages. PLoS One. e29569. doi: 10.1371/journal.pone.0029569
- Pinkerton, MH. Forthcoming 2017. Impacts of climate change on New Zealand fisheries and aquaculture. In: Phillips BF, Pérez-Ramírez M, editors. The impacts of climate change on fisheries and aquaculture. Wiley/Blackwell.
- Piontek J, Lunau M, Händel N, Borchard C, Wurst M, Engel A. 2010. Acidification increases microbial polysaccharide degradation in the ocean. Biogeosciences. 7:1615–1624. doi: 10.5194/bg-7-1615-2010
- Poloczanska ES, Brown CJ, Sydeman WJ, Kiessling W, Schoeman DS, Moore PJ, Brander K, Bruno JF, Buckley LB, Burrows MT, et al. 2013. Global imprint of climate change on marine life. Nature Climate Change. 3:919–925. doi: 10.1038/nclimate1958
- Pörtner HO. 2002. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 132:739–761. doi: 10.1016/S1095-6433(02)00045-4
- Pörtner HO. 2008. Ecosystem effects of ocean acidification in times of ocean warming: a physiologists view. Marine Ecology Progress Series. 373:203–217. doi: 10.3354/meps07768
- Pörtner HO, Farrell AP. 2008. Physiology and climate change. Science. 322:690–692.
- Purser A, Orejas C, Gori A, Tong R, Unnithan V, Thomsen L. 2013. Local variation in the distribution of benthic megafauna species associated with cold-water coral reefs on the Norwegian margin. Continental Shelf Research. 54:37–51. doi: 10.1016/j.csr.2012.12.013
- Rautenberger R, Fernandez PA, Strittmatter M, Heesch S, Cornwall CE, Hurd CL, Roleda MY. 2015. Saturating light and not increased carbon dioxide under ocean acidification drives photosynthesis and growth in Ulva rigida (Chlorophyta). Ecology and Evolution. 5(4):874–888. doi: 10.1002/ece3.1382
- Richards ZT, O'Leary MJ. 2015. The coralline algal cascades of Tallon Island (Jalan) fringing reef, NW Australia. Coral Reefs. 34:595. doi: 10.1007/s00338-015-1262-6
- Riebesell U. 2004. Effects of CO2 enrichment on marine phytoplankton. Journal of Oceanography. 60(4):719–729. doi: 10.1007/s10872-004-5764-z
- Riebesell U, Körtzinger A, Oschlies A. 2009. Sensitivities of marine carbon fluxes to ocean change. Proceedings of the National Academy of Sciences. 106(49):20602–20609. doi: 10.1073/pnas.0813291106
- Ries JB, Cohen AL, McCorkle DC. 2009. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology. 37:1131–1134. doi: 10.1130/G30210A.1
- Roberts D, Howard WR, Moy AD, Roberts JL, Trull TW, Bray SG, Hopcroft RR. 2011. Interannual pteropod variability in sediment traps deployed above and below the aragonite saturation horizon in the Subantarctic Southern Ocean. Polar Biol. 34:1739–1750. doi: 10.1007/s00300-011-1024-z
- Roberts RD. 2001. A review of settlement cues for larval abalone (Haliotis spp). Journal of Shellfish Research. 20:571–586.
- Robertson HA, Dowding JE, Elliott GP, Hitchmough RA, Miskelly CM, O’Donnell CF, Powlesland RG, Sagar PM, Scofield RP, Taylor GA. 2013. Conservation status of New Zealand birds, 2012. New Zealand Threat Classification Series 4. Wellington: Department of Conservation. 22 p.
- Rodgers KL, Shears NT. 2016. Modelling kelp forest primary production using in situ photosynthesis, biomass and light measurements. Marine Ecology Progress Series. 553:67–79. doi: 10.3354/meps11801
- Rokitta SD, Rost B. 2012. Effects of CO2 and their modulation by light in the life-cycle stages of the coccolithophore Emiliania huxleyi. Limnology and Oceanography. 57:607–618. doi: 10.4319/lo.2012.57.2.0607
- Roleda MY, Boyd PW, Hurd CL. 2012. Before ocean acidification: calcifier chemistry lessons. Journal of Phycology. 48(4):840–843. doi: 10.1111/j.1529-8817.2012.01195.x
- Roleda MY, Cornwall CE, Feng Y, McGraw CM, Smith AM, Hurd CL. 2015. Effect of ocean acidification and pH fluctuations on the growth and development of coralline algal recruits, and an associated benthic algal assemblage. PLoS One. 10:e0140394. doi: 10.1371/journal.pone.0140394
- Roleda MY, Hurd CL. 2012. Seaweed responses to ocean acidification. In: Wiencke C, Bischof K, editors. Seaweed Biology, Ecological Studies 219. Springer-Verlag. p. 407–431.
- Roleda MY, Morris JN, McGraw CM, Hurd CL. 2012. Ocean acidification and seaweed reproduction: increased CO2 ammeliorates the negative effect of lowered pH on meiospore germination in the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae). Global Change Biology. 18:854–864. doi: 10.1111/j.1365-2486.2011.02594.x
- Sabine CL, Feely RA, Millero FJ, Dickson AG, Langdon C, Mecking S, Greeley D. 2008. Decadal changes in Pacific carbon. Journal of Geophysical Research. 113:C07021. doi: 10.1029/2007JC004577
- Sánchez JA. 2005. Systematics of the bubblegum corals (Cnidaria: Octocorallia: Paragorgiidae) with description of new species from New Zealand and the eastern Pacific. Zootaxa. 1014:1–72. doi: 10.11646/zootaxa.1014.1.1
- Schiel DR, Foster MS. 2006. The population biology of large brown seaweeds: ecological consequences of multiphase life histories in dynamic coastal environments. Annual Review of Ecology, Evolution, and Systematics. 37:343–372. doi: 10.1146/annurev.ecolsys.37.091305.110251
- Seafood New Zealand, Export Statistics for December. 2016. http://www.seafood.org.nz/publications/export-information/export-statistics.
- Secretariat of the Convention on Biological Diversity. 2014. An updated synthesis of the impacts of ocean acidification on marine biodiversity. In Hennige S, Roberts JM, Williamson P, editors. (Montreal, Technical Series no. 75). 99 p.
- Shears NT, Babcock RC. 2007. Quantitative description of mainland New Zealand’s shallow subtidal reef communities. Science for Conservation 280. Wellington: Science & Technical Publishing, Department of Conservation. 21 p.
- Shi D, Xu Y, Morel FMM. 2009. Effects of the pH/pCO2 control method on medium chemistry and phytoplankton growth. Biogeosciences. 6:1199–1207. doi: 10.5194/bg-6-1199-2009
- Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY. 2011. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biology Letters. 7:917–920. doi: 10.1098/rsbl.2011.0293
- Smith AM. 2009. Bryozoans as southern sentinels of ocean acidification: a major role for a minor phylum. Marine and Freshwater Research. 605:475–482. doi: 10.1071/MF08321
- Smith AM, Berman J, Key MM, Winter DJ. 2013. Not all sponges will thrive in a high-CO2 ocean: review of the mineralogy of calcifying sponges. Palaeogeography Palaeoclimatology Palaeoecology. 392:463–472. doi: 10.1016/j.palaeo.2013.10.004
- Smith AM, Byrne M, Clark DE, Lamare MD, Winter DJ. 2016. Risk and resilience: variations in magnesium in echinoid skeletal calcite. Marine Ecology Progress Series. 561:1–16. doi: 10.3354/meps11908
- Smith AM, Garden CJ. 2013. Being a bimineralic bryozoan in an acidifying ocean. In: Bryozoan studies 2010. Berlin: Springer; p. 327–337.
- Smith AM, Key MM Jr, Gordon DP. 2006. Skeletal mineralogy of bryozoans: taxonomic and temporal patterns. Earth-Science Reviews. 78:287–306. doi: 10.1016/j.earscirev.2006.06.001
- Smith AM, Nelson CS, Danaher PJ. 1992. Dissolution behavior of bryozoan sediments: taphonomic implications for non-tropical carbonates. Palaeogeography Palaeoclimatology Palaeoecology. 93:213–226. doi: 10.1016/0031-0182(92)90098-P
- Smith AM, Nelson CS, Spencer HG. 1998. Skeletal mineralogy of New Zealand bryozoans. Marine Geology. 151:27–46. doi: 10.1016/S0025-3227(98)00055-3
- Smith AM, Riedi MA, Winter DJ. 2013. Temperate reefs in a changing ocean: skeletal carbonate mineralogy of serpulids. Marine Biology. 160:2281–2294. doi: 10.1007/s00227-013-2210-z
- Smith AM, Spencer HG. 2015. Skeletal mineralogy of scaphopods: an unusual uniformity. Journal of Molluscan Studies. 2015:1–5.
- Smith AM, Sutherland JE, Kregting L, Farr TJ, Winter DJ. 2012. Phylomineralogy of the coralline red algae: correlation of skeletal mineralogy with molecular phylogeny. Phytochemistry. 81:97–108. doi: 10.1016/j.phytochem.2012.06.003
- Smith AM, Wolfe K, Byrne M. 2012. Argonauta at risk: dissolution and carbonate mineralogy of egg cases. Proceedings of the 12th International Coral Reef Symposium, James Cook University. ICRS2012_8A_1. http://www.icrs2012.com/.
- Statistics New Zealand. 2010. Fish monetary stock account 1996–2009. Wellington: Statistics New Zealand. ISSN 1177-5440 (online), 63 p.
- Takahashi T, Sutherland SC, Chipman DW, Goddard JG, Ho C, Newberger T, Sweeney C, Munro DR. 2014. Climatological distributions of pH, pCO2, total CO2, alkalinity, and CaCO3 saturation in the global surface ocean, and temporal changes at selected locations. Marine Chemistry. 164:95–125. doi: 10.1016/j.marchem.2014.06.004
- Tatters AO, Roleda MY, Schnetzer A, Fu F, Hurd CL, Boyd PW, Caron DA, Lie AA, Hoffmann LJ, Hutchins DA. 2013. Short- and long-term conditioning of a temperate marine diatom community to acidification and warming. Phil Trans R Soc B. 368(1627):20120437. doi: 10.1098/rstb.2012.0437
- Thresher RE, Guinotte JM, Matear RJ, Hobday AJ. 2015. Options for managing impacts of climate change on a deep-sea community. Nature Climate Change. 5:635–639. doi: 10.1038/nclimate2611
- Thomsen J, Haynert K, Wegner K, Melzner F. 2015. Impact of seawater carbonate chemistry on the calcification of marine bivalves. Biogeosciences Discussions. 12:1543–1571. doi: 10.5194/bgd-12-1543-2015
- Thomsen J, Melzner F. 2010. Moderate seawater acidification does not elicit long-term metabolic depression in the blue mussel Mytilus edulis. Marine Biology. 157:2667–2676. doi: 10.1007/s00227-010-1527-0
- Tittensor DP, Baco AR, Hall-Spencer JM, Orr JC, Rogers AD. 2010. Seamounts as refugia from ocean acidification for cold-water stony corals. Marine Ecology – An Evolutionary Perspective. 31:212–225. doi: 10.1111/j.1439-0485.2010.00393.x
- Tracey DM, Anderson OF, Naylor JR, Comps. 2011. A guide to common deepsea invertebrates in New Zealand waters. (New Zealand Aquatic Environment and Biodiversity report no. 86). 317 p.
- Tracey D, Bostock H, Currie K, Mikaloff-Fletcher S, Williams M, Hadfield M, Neil H, Guy C, Cummings V. 2013. The potential impact of ocean acidification on deep-sea corals and fisheries habitat in New Zealand waters. (New Zealand Aquatic Environment and Biodiversity report no. 117). 101 p. Manuscript 2695 ISBN 978-0-478-42099-9.
- Tracey D, Gammon M, Marriott P, Cummings V, Davy S. 2016. Live deepwater coral experiments phase 2. Final research report for the NZ Ministry of Primary Industries on project ZBD2014-01.
- Tracey DM, Rowden AA, Mackay KA, Compton T. 2011. Habitat-forming cold-water corals show affinity for seamounts in the New Zealand region. Marine Ecology Progress Series. 430:1–22. doi: 10.3354/meps09164
- Turley C, Blackford J, Widdicombe S, Lowe D, Nightingale P D, Rees AP. 2006. Reviewing the impact of increased atmospheric CO2 on oceanic pH and the marine ecosystem. Avoiding Dangerous Climate Change. 8:65–70.
- van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, Hibbard K, Rose SK. 2011. The representative concentration pathways: An overview. Climatic Change. 109:5–31. doi: 10.1007/s10584-011-0148-z
- Waldbusser GG, Bergschneider H, Green MA. 2010. Size-dependent pH effect on calcification in post-larval hard clam Mercenaria spp. Marine Ecology Progress Series. 417:171–182.
- Waldbusser GG, Hales B, Haley BA. 2016. Calcium carbonate saturation state: on myths and this or that stories. ICES Journal of Marine Science. 73:563–568. doi: 10.1093/icesjms/fsv174
- Waldbusser GG, Gray MW, Hales B, Langdon CJ, Haley BA, Gimenez I, Smith SR, Brunner EL, Hutchinson G. 2016. Slow shell building, a possible trait for resistance to the effects of acute ocean acidification. Limnol. Oceanogr. 61:1969–1983. doi: 10.1002/lno.10348
- Wolfe K, Smith AM, Trimby P, Byrne M. 2012. Vulnerability of the paper nautilus (Argonauta nodosa) shell to a climate-change ocean: potential for extinction by dissolution. The Biological Bulletin. 223 (2):236–244. doi: 10.1086/BBLv223n2p236
- Zeldis J, Swales A, Currie K, Safi K, Nodder S, Depree S, Elliott F, Pritchard M, Gall M, O’Callaghan J, et al. 2015. Firth of THAMES water quality and ecosystem health – data report. (Waikato Regional Council Technical report: TR 2015/23). http://www.waikatoregion.govt.nz/tr201523/
- Ziveri P, de Bernardi B, Baumann KH, Stoll HM, Mortyn PG. 2007. Sinking of coccolith carbonate and potential contribution to organic carbon ballasting in the deep ocean. Deep Sea Research II. 54:659–675. doi: 10.1016/j.dsr2.2007.01.006
